Abstract
Solid tumors have acidic extracellular pH (pHe) but near neutral intracellular pH (pHi). Because acidic pHe milieu is conducive to tumor growth and builds resistance to therapy, simultaneous mapping of pHe inside and outside the tumor (i.e., intratumoral-peritumoral pHe gradient) fulfills an important need in cancer imaging. We used Biosensor Imaging of Redundant Deviation in Shifts (BIRDS), which utilizes shifts of nonexchangeable protons from macrocyclic chelates (e.g., 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis(methylene phosphonate) or DOTP8−) complexed with paramagnetic thulium (Tm3+) ion, to generate in vivo pHe maps in rat brains bearing 9L and RG2 tumors. Upon TmDOTP5− infusion, MRI identified the tumor boundary by enhanced water transverse relaxation and BIRDS allowed imaging of intratumoral-peritumoral pHe gradients. The pHe measured by BIRDS was compared with pHi measured with 31P-MRS. In normal tissue pHe was similar to pHi, but inside the tumor pHe was lower than pHi. While the intratumoral pHe was acidic for both tumor types, peritumoral pHe varied with tumor type. The intratumoral-peritumoral pHe gradient was much larger for 9L than RG2 tumors, because in RG2 tumors acidic pHe was found in distal peritumoral regions. Increased presence of Ki-67 positive cells beyond the RG2 tumor border suggested that RG2 was more invasive than 9L tumor. These results indicate that extensive acidic pHe beyond the tumor boundary correlates with tumor cell invasion. In summary, BIRDS has sensitivity to map in vivo intratumoral-peritumoral pHe gradient, thereby creating preclinical applications in monitoring cancer therapeutic responses (e.g., with pHe-altering drugs).
Keywords: metastasis, CEST, magnetic susceptibility, permeability, extravasation, SPIO
INTRODUCTION
Glioblastoma multiforme (GBMs) are the most lethal of brain cancers. In solid tumors extracellular pH (pHe) is more acidic than intracellular pH (pHi) which is neutral to alkaline (1). An acidic pHe seems to favor tumor growth and metastasis by activating matrix metalloproteases and cathepsins (2). Rapidly growing tumor cells possess elevated rates of glucose uptake but reduced rates of oxidative phosphorylation (i.e., Warburg effect), thereby causing acidity in the extracellular space (3). Since acidic pHe milieu is conducive to tumor cell propagation and builds resistance to therapy (4), imaging the intratumoral and peritumoral gradient of pHe is an important, but yet unmet need in cancer imaging (5).
It has been shown that the acidic tumor microenvironment is related to the size and altered vascularity of tumors, which in turn stimulates hypoxia (6,7). Hypoxia induces gene expression changes that result in increased glycolysis and accumulation of carboxylic and lactic acids in the extracellular microenvironment. However, hypoxia is not the only contributor to acidification. Poor vascularity also hinders the elimination of protons and lactic acid contributing to lower pHe. Low pHe increases metastatic potential, angiogenesis, induction of genomic instability, and decreases immune function (8–11). Low pHe also leads to resistance to radiotherapy and certain forms of chemotherapy (12). Since proton movement across the cell membrane is coupled to movement of other ions, pHe is an important readout (13). Thus quantitative and simultaneous mapping of pHe inside and outside the tumor (i.e., intratumoral-peritumoral pHe gradient) can be useful for tumor localization and following response to cancer therapy (14).
Non-invasive magnetic resonance imaging (MRI) and spectroscopy (MRS) methods are mainstays for clinical cancer imaging (15). Smart MRI contrast agents enable physiochemical parameters like pHe to be measured with lanthanide III ion (Ln3+) derivatives of 1,4,7,10-tetraazacyclododecane (cyclen) (16), where most agents are built on the 1,4,7,10-tetraacetic acid (DOTA) backbone, similar to FDA-approved Gd3+ agents. While non-Gd3+ agents have yet to be tested in humans, molecular imaging with MRI has been demonstrated with a variety of DOTA-based macrocyclics in preclinical studies (17): relaxation-based methods and Chemical Exchange Saturation Transfer (CEST). The relaxation-based methods rely on the paramagnetic effect induced by the Ln3+ ion on inner sphere of water protons, whereas CEST detects exchange between bulk water protons and an inner sphere of bound water that is shifted by the paramagnetic Ln3+ ion or exchangeable protons on the ligand (e.g., -OH or -NHx).
Recently, we established a method that combines high spatial resolution MRI with high molecular specificity MRS into a 3D chemical shift imaging (CSI) platform called Biosensor Imaging of Redundant Deviation in Shifts (BIRDS), where paramagnetically-shifted nonexchangeable protons (i.e., -CHy) on DOTA-based macrocyclic complexes are directly detected (18–24). Longitudinal (T1) and transverse (T2) relaxation times of non-exchangeable protons on BIRDS agents being very short (in ms range), allow high-resolution and high-speed CSI (18). BIRDS agents, similar to clinically-used DOTA, could potentially be translated across a range of magnetic fields because the T2/T1 ratio remains high and the proton shift sensitivities to the molecular event in the environment are largely unaffected. The readout of the physicochemical environment does not depend on diffusion or blood flow and BIRDS signals can be detected beyond MRI contrast (i.e., we see BIRDS signals when the MRI images are darkened by T2 shortening) (18,19,21,22). Moreover, although the distribution of BIRDS agents may depend on vessel permeability (e.g. normal versus tumor tissue), the shift-based reporting is independent of agent’s dose. Previously we showed that DOTA containing phosphonate agents (e.g., TmDOTP5− and TmDOTA-4AmP5−) can provide pHe maps with BIRDS in vivo (18,19).
In this study we used BIRDS to measure pHe in the brains of 9L and RG2 tumor-bearing rats. We used TmDOTP5− because of its commercial availability, but other BIRDS agents can also be used. Specifically, our goal was to see whether BIRDS can help determine if tumor invasiveness can be mapped by the intratumoral-peritumoral pHe gradient, defined as the absolute pHe difference between values inside and outside the tumor.
MATERIALS and METHODS
All experiments were performed according to NIH guidelines. Yale University’s animal care and user committee (IACUC) approved the protocol. All scans were conducted on an 11.7T Agilent (Santa Clara, CA) horizontal-bore spectrometer with a bore size of 21 cm and maximum gradient strength of 400 mT/m, using 1H and 31P surface radio-frequency (RF) probes (each 1.4 cm diameter).
Animal preparation for rats bearing 9L and RG2 tumors
Adult male Fischer 344 rats (200–250 g; n = 29) were obtained from Yale University vendors and maintained according to approved animal care protocols. RG2 (n = 16) and 9L (n = 13) tumors were studied (25). Rats were anesthetized with isoflurane (2–3%) and positioned in a stereotaxic instrument with the incisor bar set at 3.0 mm below the interaural line. RG2 and 9L cells at high confluence were harvested, washed, and suspended in appropriate media. Intrathalamic injections were made with a 25 µL Hamilton syringe, fitted with a 26 gauge beveled needle, into the right thalamus at coordinates 3 mm to the right from bregma and 3 mm ventral to the dura. A 5 µL volume of the cell suspension was injected over the course of 5 minutes and the needle was left in place for an additional 5 minutes before slow withdrawing. After injection the scalp was closed, treated with antibiotic, and non-steroidal anti-inflammatory drug (meloxicam, 1 mg/kg) was injected subcutaneously to prevent pain and inflammation.
On the day of the scanning, rats were anesthetized with isoflurane (<2%), tracheotomized and artificially ventilated (70% N2O/30% O2). A femoral vein was cannulated (PE-10) to administer TmDOTP5− (0.4 mmol/kg). A femoral artery was cannulated (PE-50) for monitoring of physiological parameters (pCO2, pO2, pH, blood pressure) throughout the experiment. An intraperitoneal line was inserted for administration of α-chloralose (40 mg/kg/hr). α-chloralose provides stable physiology over long periods for terminal rat experiments. However for longitudinal experiments isoflurane would be more ideal, but the exposure duration has to be kept short since vasomotion effects are induced by prolonged exposure of this anesthetic. Because the body temperature decreases in animals under anesthesia, we used a water-heating pad to maintain the body temperature in the physiological range (36–37 °C).
The anesthetized rats were prepared with renal ligation (18,26) to maintain a high TmDOTP5− concentration gradient between the blood and extracellular space. Since renal ligation stops clearance of the agent, with slow infusion (~0.5 mL/hr) of the agent (19), the agent concentration builds up in the microvasculature sufficiently high enough for slow extravasation, which subsequently leads to agent accumulation in the extracellular space. Based on prior observations in rats without renal ligation (27), we expect higher agent accumulation in tumors (vs. normal tissue) because of porous immature blood vessels. Fenestrated vessels in circumventricular organ of normal brain may also play a role in agent accumulation in extracellular space (28–30). Previous 31P MRS experiments in normal rats showed that brain pH with and without renal ligation are similar (18,26). Ventilation was adjusted to maintain normal physiology. Body temperature was measured with a rectal temperature probe and no further adjustments in the water-heating pad were made for the entire duration of the experiment (~2 hrs). After experiments some rats were perfused and their brains were harvested for histopathological assessments.
Given that relaxation rates of agents like TmDOTP5− or GdDOTA-4AmP5− are possibly pH-dependent (18,23,27), we directly measured the TmDOTP5− concentrations in normal and tumor tissues. A few rats (n = 4) were euthanized with focused microwave irradiation (31) and brain tissue (i.e., normal and tumor) was removed, weighed, and homogenized for agent concentration analysis ex vivo by 1H NMR. Blood plasma samples were also collected to measure the agent in the plasma for correction of agent concentration in the tissue using tissue-plasma volumes (32). NMR solutions of each organ suspensions were prepared and 0.1 mM TmDOTMA− was used as internal reference. NMR spectra were acquired using standard pulse-acquire experiments on an 11.7T vertical bore magnet using short TR and resonance intensities of H6 proton of TmDOTP5− were measured. Final tissue concentrations of TmDOTP5− were calculated after reference and plasma corrections.
Histological comparisons between 9L and RG2 tumors
Rats used for histochemical analysis were perfuse-fixed with 4% paraformaldehyde and embedded in paraffin. Brain tissue sections (4 µm) were stained with hematoxylin and eosin (H&E) for determination of tissue structure followed by immunohistochemical stains for monocarboxylate transporter 4 (MCT4), glial fibrillary acidic protein (GFAP) and Ki-67. Immunostaining was performed as previously described (33). Primary antibody diluted in 0.1% BSA/PBS was applied for 90 min at room temperature for GFAP (1:500) (DAKO, Z0334) and MCT4 (1:50) (H-90, Santa Cruz Biotechnology, sc-50329) and overnight at 4 °C for Ki-67 (1:100, Abcam, ab66155). The sections were incubated with a goat anti-rabbit secondary antibody (1:500, Pierce, 31460) for 60 minutes followed by incubation with metal enhanced 3,3-diaminobenzidine (DAB; Life technologies, 34065) for 10 min. Expression of various markers was quantified by evaluating the amount of DAB staining. The percentage of the positive stained cells (PP) in each section was estimated by manually counting the cells as observed under ×50 magnification and graded in a scale ranging from 0–100%. The DAB color intensity (IDAB) was graded as non-staining (0), faint (1), medium (2) and intense (3). The staining score (SS) representing the expression of the markers was defined as follows
| (1) |
BIRDS for pHe measurements
Prior to BIRDS experiments, spin-echo MRI datasets were obtained. 11 coronal slices of 128×128 resolution and 1 mm thickness were acquired using a field of view (FOV) of 25×25 mm2, a recycle time (TR) of 6s and twelve different echo time (TE) values from 10 to 120 ms. Transverse relaxation time (T2) and rate (R2) maps were obtained in Matlab (MathWorks, Inc., Natick, MA) by fitting the absolute MRI intensity versus TE to a single exponential function (34). Following TmDOTP5− infusion, the same MRI data were acquired to compare the T2 changes induced by the paramagnetic agent (27).
The in vivo CSI acquisition for BIRDS was optimized for a 3D sampling of 1 µL effective resolution, where the effective volume of a voxel was estimated by calculating the point spread function for gaussian-weighted spherical encoding (21,35). A dual-banded refocused 90° Shinnar-Le Roux (SLR) RF pulse of 35 kHz bandwidth and 90 kHz separation with 205 µs duration was used for selective excitation of the H2/H3 and H6 protons of TmDOTP5−. The datasets were acquired with a TR of 5 ms and a FOV of 25×25×25 mm3. The phase encode gradient duration was 160 µs, the spectral window was 250000 Hz and the acquisition time was 4.1 ms. The 1H spectrum for each encoding step was line broadened (300 Hz), phased (zero order), and baseline corrected (first order). The pH was calculated from the chemical shifts of the H2, H3, and H6 protons of TmDOTP5− (δ2, δ3 and δ6) according to
| (2) |
where the coefficients a0, and were calculated from linear least-squares fit of pH as a function of chemical shifts δ2, δ3 and δ6, as previously described (18). The pH readout by eq. 2 is unaffected by TmDOTP5− concentration below 10 mM, which covers the TmDOTP5− concentration range we observe in vivo (18,26). Within the pH range of 6.6–7.6, the changes in the chemical shift for the H2, H3, and H6 protons at 35 °C are 4.3, 3.4 and 4.3 ppm, respectively.
31P-MRS for pHi measurements
Prior to BIRDS experiments, 6 glioma-bearing rats underwent single voxel 31P-MRS acquisitions for intratumoral pHi and peritumoral pHi measurements, where the localized volumes were determined by the tumor size. 31P spectra were acquired with ISIS localization (36) using a 1000 µs adiabatic half-passage pulse and the following acquisition parameters: TR = 16 s; number of averages = 96; dummy scans = 16. The pHi was calculated by
| (3) |
where Δδ is the chemical shift difference between inorganic phosphate (Pi) and phosphocreatine (PCr) resonances, δ1 = 3.23 ppm and δ2 = 5.70 ppm are the corresponding chemical shift differences at low and high pH, respectively (35), and pKa = 6.915±0.002 was calculated by linear least-squares regression of Pi-PCr data for the different conditions.
Analysis of pHe maps
Regional variations of pHe measured by BIRDS were assessed for voxels inside the tumor, at the tumor margin, and well outside the tumor. The tumor margin was defined by MRI contrast. Intratumoral and peritumoral voxels were defined as all voxels inside and outside the tumor boundary defined by MRI respectively. The voxels at the tumor margin were defined as one voxel that were either partly inside or outside of the tumor (i.e., right on the tumor boundary defined by MRI contrast). A parameter f that represents the fraction of voxels with pH values smaller than a threshold value pHth was calculated and a sigmoidal function (describing a cumulative distribution) was used to fit f versus pHth according to the equation:
| (4) |
where pHm represents the distribution midpoints (given by pHth value for which f = 0.5) while SpH represents the slope of the sigmoidal curve at pHth = pHm.
Statistics
All staining (eq. 1) and pHe (eqs. 2 and 3) results were expressed as mean ± standard deviation (SD) and comparisons were assessed by Student’s t test with two tails. Significance between pHm measured intratumorally, within the tumor margin and peritumorally (eq. 4) were assessed by Student’s t test, where p-values less than 0.05 were considered to be significant.
RESULTS
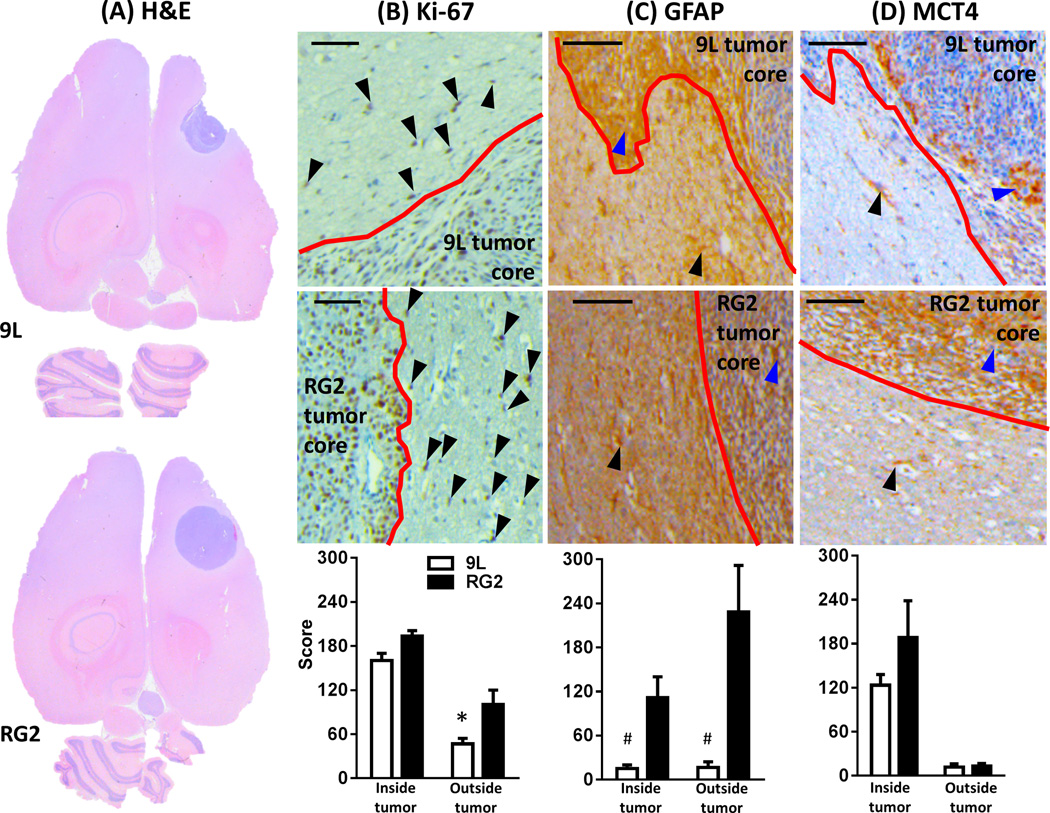
Figure 1 shows comparison of histological stains for 9L and RG2 tumors. At macroscopic scale the H&E staining of both tumors showed a greater extent of hematoxylin staining (i.e., blue pigmentation) in the parenchyma (vs. parenchyma of normal brain tissue), which at microscopic scale represented the tumoral mass within the inoculation zone (Figure 1A). The tumors were stained (eq. 1) for markers of proliferation (Ki-67), gliosis (GFAP) and glycolysis/lactate transport (MCT4). Ki-67 (Figure 1B) and MCT4 (Figure 1D) showed nuclear and membrane localization, respectively. Higher expression of Ki-67 (Figure 1B) and GFAP (Figure 1C) was detected in cells beyond the RG2 tumor boundary. Increased expression of MCT4 and GFAP was observed in RG2 tumor when compared to 9L, and in both 9L and RG2 tumors cells migrating from the tumor boundary showed MCT4 positive cells. Examples of immunohistochemistry images for Ki-67, GFAP, and MCT4 in the same rat are shown in Figure S1, which are presented at different magnifications indicating locations of the images.
Figure 1.
Evaluation of 9L and RG2 tumors by immunohistochemistry. Examples of staining results for (A) H&E, (B) Ki-67, (C) GFAP, and (D) MCT4 in individual rats. Black and blue arrows show positive DAB staining outside and inside tumor boundary, respectively (scale bar = 100 µm; red lines in B to D indicate tumor boundary) for 9L (top) and RG2 (middle) tumors. Averaged staining scores (eq. 1) inside and outside tumor boundary (bottom) for a group of rats (n = 3 for each tumor type) are shown in graphs. Ki-67 expression outside tumor core in RG2 was significantly higher than in 9L (*, p < 0.031); GFAP expression was higher in RG2 tumors than in 9L both inside and outside tumor boundary (#, p < 0.025 and 0.027, respectively). While MCT4 staining was higher in RG2 tumors than 9L tumors, the difference did not reach significance.
Figure S2 shows that MRI could identify the tumor with T2 contrast upon TmDOTP5− infusion, similar to Gd3+-induced longitudinal relaxation time (T1) contrast. Due to the Tm3+-induced paramagnetic effect, tumors could be identified either by darkening caused by T2 decrease (Figure S2A) or R2 enhancement (Figure S2B) within intratumoral regions in relation to peritumoral regions. The TmDOTP5− induced contrast in tumoral tissue is likely due to increased tumor vascular permeability allowing more TmDOTP5− in the extracellular space of tumors compared to neighboring tissue.
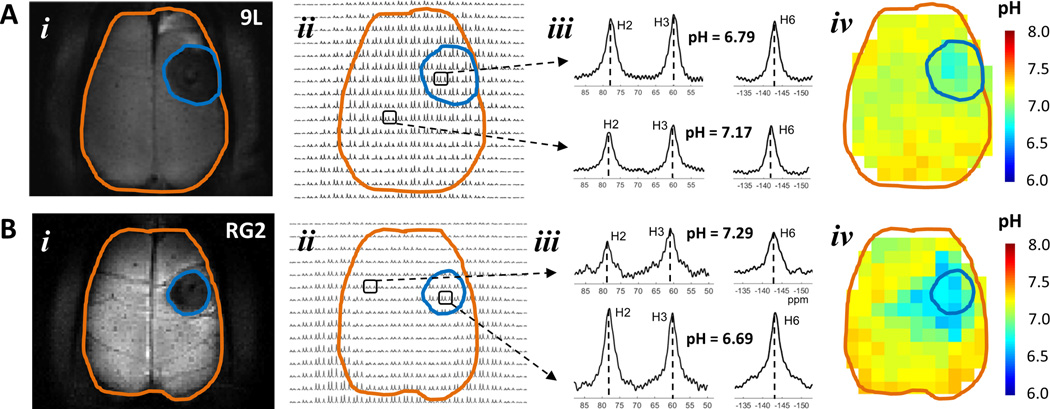
Figure 2A and 2B show representative BIRDS data from 9L and RG2 bearing rats, respectively. The intratumoral regions of both tumors were localized similarly by T2-based contrast. The intensities of TmDOTP5− protons were somewhat variable across different regions, with muscle signals being usually larger than those originating from the brain (Figure 2Bii). Since TmDOTP5− clearance rates from and permeability into various tissues are likely to be different, in some cases muscle and brain (i.e., both normal and tumor tissue) signal intensities were quite similar (Figure 2Aii). While TmDOTP5− proton intensities varied across brain regions, only the shift variations of H2, H3, and H6 peaks allowed pH to be determined independent of the agent concentration using eq. 2, thus enabling pHe maps to be compared across all voxels both within and beyond the tumor boundary. The intratumoral pHe values were distinctly lower than peritumoral pHe values. For example, in the 9L and RG2 tumors shown in Figure 2A and 2B the intratumoral pHe values were 6.8±0.1 and 6.7±0.1, respectively, whereas a large majority of voxels in the peritumoral regions had pHe values of 7.2 or higher. But the brains with RG2 tumor showed patterns of diffuse pHe reductions several mm beyond the tumor boundary. In the brain with RG2 tumor shown the pHe values were quite similar in intratumoral regions vs. regions immediately adjacent to the tumor boundary, whereas in the brain with 9L tumor shown the pHe values were distinctly smaller in intratumoral regions vs. regions immediately adjacent to the tumor boundary.
Figure 2.
Representative pHe maps from BIRDS data of rats bearing (A) 9L and (B) RG2 tumors. In (i) the T2-weighted image shows the tumor localization identified by darkening (tumor = blue outline; brain = brown outline), which is likely due to increased tumor vascular permeability allowing more TmDOTP5− in the interstitial space of the tumor compared to neighboring non-tumor tissue (see Figure S2). In (ii) a portion of the 3D CSI data for the slice in (i) shows varying TmDOTP5− levels throughout the brain. In (iii) examples of spectra from intratumoral and peritumoral voxels. In (iv) quantitative maps of pHe were obtained using multiple TmDOTP5− peaks and their respective pH sensitivities (eq. 2). (A) Specific voxels in a brain bearing 9L tumor shows significantly shifted TmDOTP5− peaks indicating different pHe inside (pHe = 6.79) than outside (pHe = 7.17) the tumor boundary. The 9L tumor was identified by significantly lower pHe within the tumor boundary. (B) Specific voxels in a brain bearing RG2 tumor shows significantly shifted TmDOTP5− peaks indicating different pHe inside (pHe = 6.69) than outside (pHe = 7.29) the tumor boundary. The RG2 tumor was clearly identified by lower pHe within the tumor boundary. While the most significantly low pHe voxels were found inside the tumor margin, there were low pHe voxels that were spread well beyond the tumor margin.
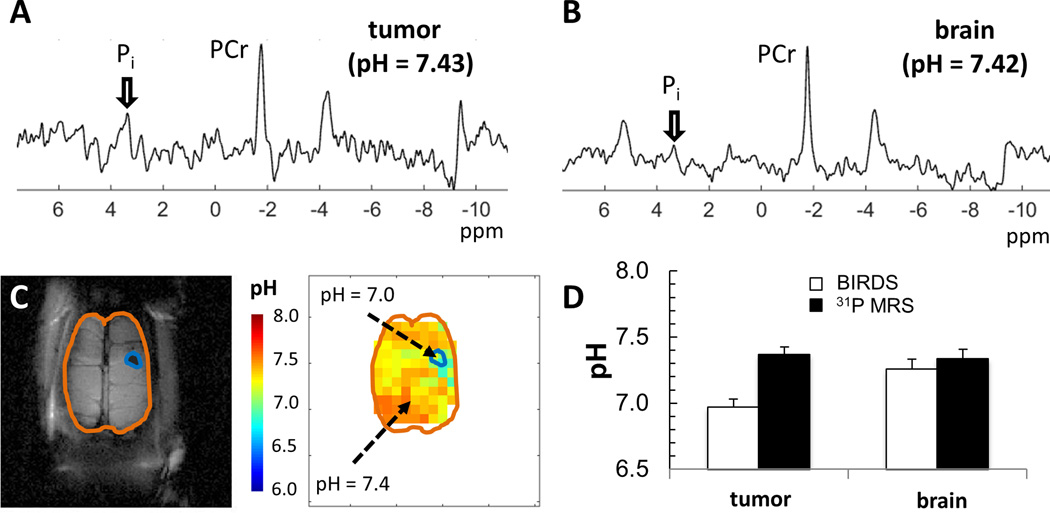
Figure 3 shows comparison of pHe measured by BIRDS and pHi measured using 31P-MRS from the same animals bearing small gliomas. For BIRDS and 31P-MRS, the pH-induced shifts are ~4 and ~1 ppm/pH unit, respectively, with measurement error <0.01 pH units (18). 31P spectra inside a tumor (Figure 3A) and outside a tumor (Figure 3B) show pHi values that were neutral in both locations. In the same rat, after TmDOTP5− infusion, the BIRDS data show lower pHe values inside vs. outside the tumor (Figure 3C). Overall, there was good agreement between pHi and pHe in normal tissue outside the tumor, whereas inside the tumor pHe was significantly lower than pHi (Figure 3D).
Figure 3.
Comparison of pH measured by 31P-MRS (pHi) and BIRDS (pHe) in small gliomas (~2 mm). The spectroscopic data were sequentially acquired in the same rats, first with 31P-MRS and then with BIRDS. The pH measured by 31P-MRS was calculated from the PCr and Pi shifts (eq. 3), which reflects the intracellular compartment, whereas the pH measured by BIRDS (eq. 2) represents the extracellular compartment. (A)31P spectrum from a voxel localized inside the tumor showed a pH value of 7.43. (B)31P spectrum from a voxel localized outside the tumor (i.e., inside normal brain tissue) showed a pH value of 7.42. (C) MRI and BIRDS data from the same rat, where the pH inside and outside the tumor were approximately 7.0 and 7.4, respectively. The tumor is indicated with the blue outline, whereas the brain is shown with brown outline. (D) Averaged pH measured by 31P-MRS (black bars) and BIRDS (white bars) across a group of rats (n = 6). Good agreement between pHi (by 31P-MRS) and pHe (by BIRDS) in normal brain tissue (p > 0.5), whereas inside the tumor pHi (by 31P-MRS) was significantly higher than pHe (by BIRDS). Abbreviations: PCr, phosphocreatine; Pi, inorganic phosphate.
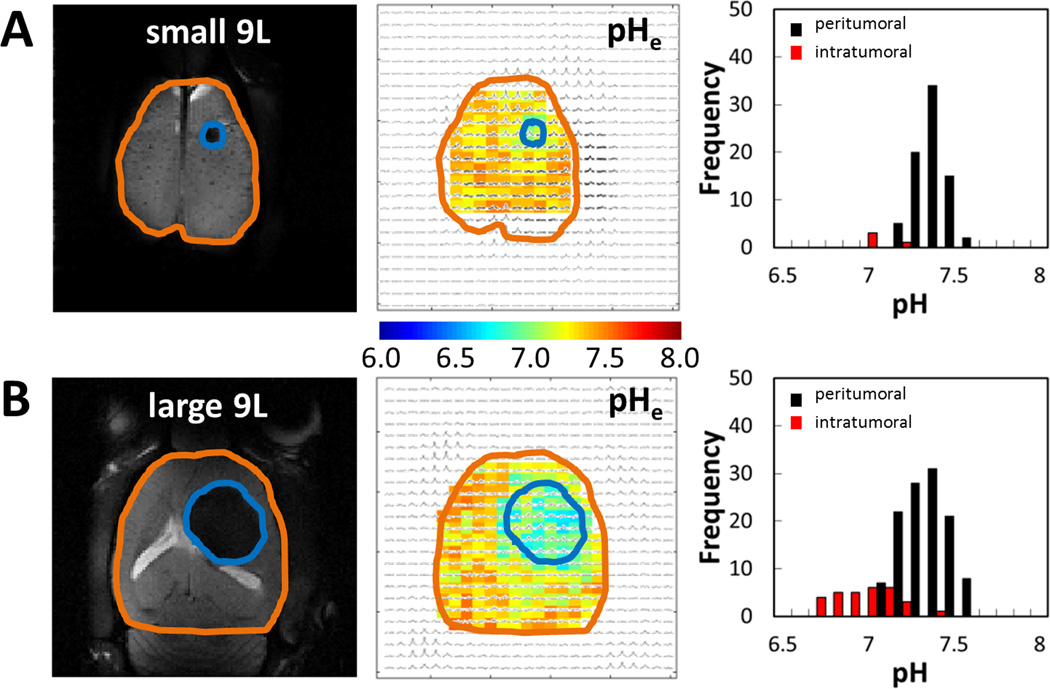
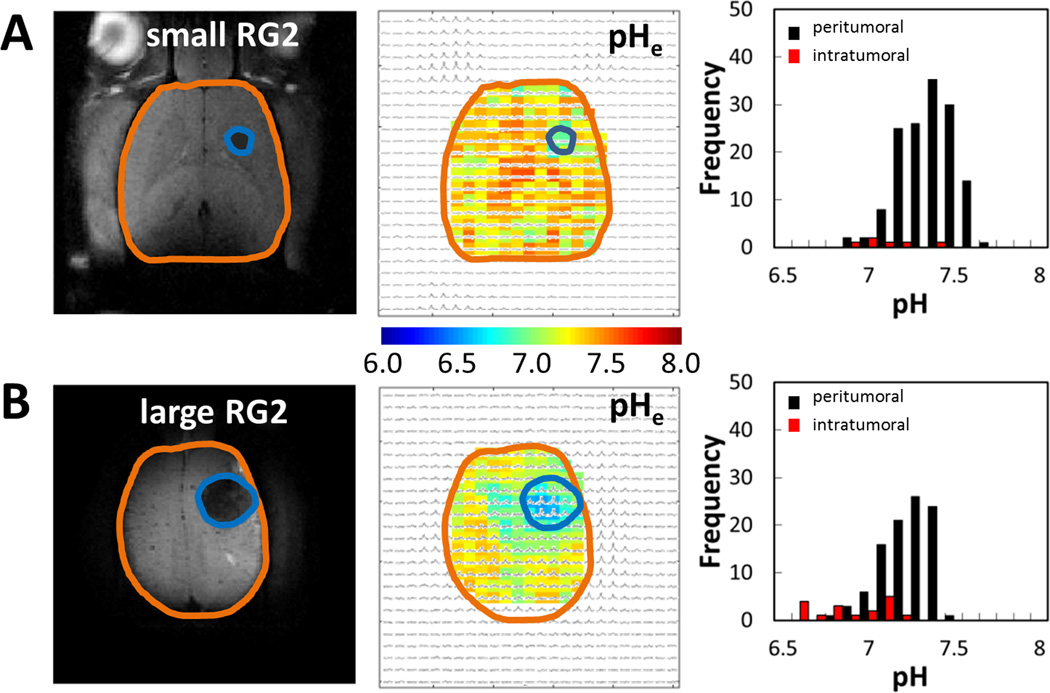
BIRDS had sufficient sensitivity to distinguish different sizes of 9L (Figure 4) and RG2 (Figure 5) tumors. However, in case of small tumors (1–2 mm diameter) the partial contribution from non-tumor tissue (based on tumor boundary defined by T2-based contrast) could become significant. To minimize this contribution, for intratumoral pHe calculation we used only the voxels with a partial volume of more than 50% occupied by tumor tissue. Thus, we estimated the error in pHe gradient between tumor and non-tumor tissue assuming 50% partial volume for voxels positioned at the tumor boundary and a nominal pHe gradient of 0.1 within each boundary voxel. Since for small tumors (≤6 voxels) all voxels touch the boundary and experience partial volume effects, then the calculated error in pHe gradient reported would be less than 0.05 units. In contrast, however, for larger tumors (40–50 voxels) only about 25% of the voxels touch the boundary and experience partial volume effects. Therefore, in larger tumors, the error in pHe gradient estimation would be less than 0.013 units. Since this suggests that the error in pHe gradient between the tumor and its boundary scales inversely with the tumor size, we analyzed the larger 9L and RG2 tumors to find some differences in terms of intratumoral vs. peritumoral pHe measurements. Overall, the intratumoral pHe values of both tumor types became more acidic with tumor growth and changes in the peritumoral pHe depended on the tumor type (i.e., more acidic peritumoral pHe for tumors that invade).
Figure 4.
Representative BIRDS data from individual rats bearing 9L gliosarcomas of different sizes, shown in terms of pHe maps and their respective histograms for values inside (intratumoral) and outside (peritumoral) the tumor. The MRI depiction of the tumor size is shown on the left (tumor = blue outline; brain = brown outline). (A) Data for a small 9L (~2 mm) shows that the pHe were significantly separated for intratumoral voxels (pHe = 7.01±0.08) and peritumoral voxels (pHe = 7.34±0.09). (B) Data for a large 9L (~6 mm) shows also that the pHe values were significantly separated for intratumoral voxels (pHe = 6.91±0.17) and peritumoral voxels (pHe = 7.31±0.13). The distributions of peritumoral pHe values in small and large tumors were overlapping (e.g., 7.2–7.6). The distribution of intratumoral pHe values in large tumor (e.g., 6.7–7.2) was significantly shifted into acidic ranges compared to the intratumoral pHe values in small tumor (e.g., 7.0–7.2).
Figure 5.
Representative BIRDS data from individual rats bearing RG2 gliomablastomas of different sizes, shown in terms of pHe maps and their respective histograms for values inside (intratumoral) and outside (peritumoral) the tumor. The MRI depiction of the tumor size is shown on the left (tumor = blue outline; brain = brown outline). (A) Data for a small RG2 (~2 mm) shows that the pHe were significantly separated for intratumoral voxels (pHe = 6.84±0.22) and peritumoral voxels (pHe = 7.31±0.16). (B) Data for a large RG2 (~5 mm) shows also that the pHe values were significantly separated for intratumoral voxels (pHe = 6.91±0.17) and peritumoral voxels (pHe = 7.18±0.14). The distributions of peritumoral pHe values in small and large tumors were overlapping but the spread was quite large (e.g., 6.8–7.6). The distribution of intratumoral pHe values in large tumor (e.g., 6.6–7.2) was significantly shifted into acidic ranges compared to the intratumoral pHe values in small tumor (e.g., 6.9–7.2).
For 9L tumors (Figure 4), the distributions of peritumoral pHe values in a small (Figure 4A) and a large (Figure 4B) tumor were overlapping (e.g., 7.2–7.6), suggesting that peritumoral pHe is not significantly affected by 9L tumor growth. The distribution of intratumoral pHe values in a large tumor (e.g., 6.7–7.2) was significantly shifted into acidic ranges compared to the intratumoral pHe values in a small tumor (e.g., 7.0–7.2), suggesting that intratumoral pHe becomes more acidic with 9L tumor growth.
For RG2 tumors (Figure 5), the distributions of peritumoral pHe values in a small (Figure 5A) and a large (Figure 5B) tumor were overlapping but the spread was much larger than observed for 9L tumors (e.g., 6.8–7.6), suggesting that peritumoral pHe is already quite acidic in the earliest stages of RG2 tumor growth. The distribution of intratumoral pHe values in a large tumor (e.g., 6.6–7.2) was significantly shifted into acidic ranges compared to the intratumoral pHe values in a small tumor (e.g., 6.9–7.2), suggesting that intratumoral pHe becomes more acidic with RG2 tumor growth.
The intratumoral-peritumoral pHe gradients (Figure 6) measured by BIRDS were compared quantitatively. The sigmoidal relationships between pHe thresholds and percentage of voxels with pHe below these thresholds were represented by eq. 4. For brains with 9L tumors (Figure 6A) ~80% of intratumoral voxels had pHe lower than 7.0 (with values as low as 6.6) and ~80% of peritumoral voxels had pHe lower than 7.4 (with values as low as 7.1), whereas pHe trends for voxels within the tumor boundary were in between these two other trends. For brains with RG2 tumors (Figure 6B) ~80% of intratumoral voxels had pHe lower than 7.0 (with values as low as 6.6) and ~80% of peritumoral voxels had pHe lower than 7.3 (with values as low as 6.8), whereas pHe trends for voxels within the tumor boundary nearly matched pHe trends for peritumoral voxels. Hence, for 9L tumors the peritumoral pHe was clearly separated from intratumoral pHe and pHe within the tumor boundary (Figure 6C). But in RG2 tumors the peritumoral pHe was only clearly separated from intratumoral pHe because peritumoral pHe was quite similar to pHe within the tumor boundary (Figure 6C). These results indicate a significantly steeper intratumoral-peritumoral pHe gradient for brains with 9L tumors compared to brains with RG2 tumors (i.e., ~0.4 vs. ~0.2; Figure 6C). The histograms summarizing the results in terms of pHe distribution measured by BIRDS for intratumoral, margin, and peritumoral regions for 9L and RG2 tumors are shown in Figure S3. The most probable pHe values and the full width at half maximum (FWHM) obtained from the Gaussian fit are given in Table 1. As expected, for both 9L and RG2 tumors, the most probable pHe values obtained from the Gaussian fit of the pHe distribution (Figure S3 and Table 1) were the same (within the estimated error of the fit) as the distribution midpoints pHm obtained from the Sigmoidal fit (Figure 6), indicating that both types of analyses gave the same result.
Figure 6.
The intratumoral-peritumoral pHe gradient measured by BIRDS for 9L (n = 13) and RG2 (n = 16) tumors. Quantitative variations of pHe were obtained for intratumoral voxels (red circles), voxels within the tumor margin (blue squares), and peritumoral voxels (black diamonds). In (A) and (B) the vertical axis is a parameter f that represents the fraction of voxels with pHe values smaller than a threshold value (pHth), which is indicated on the horizontal axis. Variations of pHe for 9L and RG2 tumors show sigmoidal relationships for all voxels. The data points are the average, the error bars are the standard error, and the lines are the non-linear fit of f to eq. 4 for intratumoral voxels (red line), voxels within the tumor margin (blue line), and peritumoral voxels (black line). (A) For 9L tumors, pHe values of the margin were significantly different from peritumoral and intratumoral pHe values. (B) For RG2 tumors, pHe values of the margin were not significantly different from peritumoral pHe values, but significantly different from intratumoral pHe values. (C) The overall pHe differences between 9L and RG2 tumors are shown with regard to the distribution midpoint pHm, which represents the pHe at f = 0.5. Statistical group comparison using Student’s t-test with two tails for pHe shows that pHm was significantly different for 9L (p < 0.001) for all three possible comparisons (intratumoral vs. margin, peritumoral vs. margin, and intratumoral vs. peritumoral ), while for RG2 was significantly different (p < 0.0001) only for intratumoral vs. margin and intratumoral vs. peritumoral comparisons. When comparing pHm values for 9L versus RG2, only the peritumoral regions showed significant differences (p < 10−6).
Table 1.
Most probable pHe values and the FWHM obtained from the fit of the normal (Gaussian) distribution versus pHe shown in Figure S3 for intratumoral, margin and peritumoral regions in 9L and RG2 tumors.
| Region | 9L | RG2 | ||
|---|---|---|---|---|
| pHe | FWHM | pHe | FWHM | |
| intratumoral | 6.90 ± 0.01 | 0.38 ± 0.01 | 6.87 ± 0.01 | 0.44 ± 0.02 |
| tumor margin | 7.09 ± 0.01 | 0.41 ± 0.02 | 7.08 ± 0.01 | 0.50 ± 0.03 |
| peritumoral | 7.29 ± 0.01 | 0.32 ± 0.02 | 7.13 ± 0.01 | 0.35 ± 0.01 |
DISCUSSION
Because acidic pHe is believed to facilitate tumor cell invasion (2), quantitating pHe could have significant implications for diagnosis of tumor invasiveness and response to therapy (14,37). Since most MRI-based molecular imaging methods focus on the intratumoral pHe (27,38,39), the need for mapping the intratumoral-peritumoral pHe gradient has escaped attention. Thus we used BIRDS to measure intratumoral-peritumoral pHe gradient in rat brains bearing 9L and RG2 tumors to assess how this parameter changes with tumor invasiveness.
Pros and cons of molecular imaging with BIRDS
BIRDS is a molecular imaging platform that detects paramagnetically-shifted nonexchangeable protons on the agent as opposed to water proton relaxation, which is the norm in molecular imaging with MRI. Because a paramagnetic CEST agent also has nonexchangeable protons, these CEST agents also show BIRDS properties and allow dual modality with the same agent (20,23). BIRDS is compatible with superparamagnetic iron oxide (SPIO) and the pHe readouts from BIRDS are unaffected by high dose SPIO (40), because the linewidths of TmDOTP5−resonances in rat brain are already large (1–2 ppm) and they do not change significantly after SPIO addition. Therefore it should be possible to apply SPIO nanoparticles to monitor drug delivery by MRI and then use BIRDS to monitor the physicochemical effects (e.g., pHe) of the drugs delivered.
Signal of agents like TmDOTP5− and TmDOTMA− can be observed in the brain of rats that have undergone renal ligation even after several hours following the start of the infusion (19), suggesting slow clearance from the extracellular space. We previously measured the presence of TmDOTP5− in the cerebral spinal fluid (26). We proposed that fenestrated vessels of circumventricular organs may also provide an additional route for these agents to gain access to the extracellular space, because fenestrated vessels in this location are present even in the adult brain (28–30). Additionally, using 1H MRS, at the end of 2 hours of infusion, we measured TmDOTP5− concentration in blood to be ~3 mM, while in extracted tissues from normal and tumor regions the TmDOTP5− concentrations were ~0.2 and ~0.5 mM, respectively. These signals contain ~13% contribution from blood (3% partial volume) and ~87% from extracellular space (20% partial volume) (18,19,26). Due to lack of reports on the volumes of extracellular and intravascular spaces in brain tumors, we assumed that the fractions of these compartments inside and outside tumors are the same. Thus TmDOTP5− in extracellular space of normal and tumor tissues was ~0.5 mM and ~2 mM, respectively.
Protonation/deprotonation of phosphonate groups of DOTP8− or DOTA-4AmP8− is fast on the NMR timescale, which can be observed either by the pH-dependent variations of the H2, H3 and H6 chemical shifts with BIRDS (18) or relaxation MRI (27). Thus, protonation/deprotonation of TmDOTP5− or other agents such as GdDOTA-4AmP5− is not diffusion limited in free solution, but it might be affected in normal or tumor tissue due to different water diffusion environments. However even for regions with restricted (slow) water diffusion, we and others expect that protonation/deprotonation equilibrium is reached rapidly because TmDOTP5− (or GdDOTA-4AmP5−) is a small molecule and is dilute in an otherwise aqueous medium (18,27). Moreover in vitro BIRDS measurements with large temperature variations do not affect the pH readout capability of DOTP8− (or DOTA-4AmP8−) (18,23), suggesting that the pHe measured with such probes represent protonation/deprotonation equilibrium over the observed diffusion range in vivo. However future studies with ADC and high-resolution BIRDS may indicate possible correlation (or lack thereof) between pHe and water diffusion.
The paramagnetic effect of TmDOTP5− on water protons provides sufficient T2 contrast for identification of the intratumoral volume, similar to enhanced T1 contrast with Gd3+ agents for tumor detection (26,27). As suggested by Martinez et al (27) and others (26), greater extent of these agents’ extravasation across porous tumor blood vessels is responsible for the superior MRI contrast between tumor and surrounding tissue (Figure S2). In addition, detection of the paramagnetically-shifted TmDOTP5− protons with BIRDS allows physicochemical imaging of the brain (Figure 2).
While the current study used TmDOTP5− for pH detection with BIRDS, in future studies methyl groups can be added in select regions of the ligand to improve sensitivity, thereby allowing reduced CSI voxel sizes and/or decreasing the amount of agent needed. At present, 1 µL voxel size in 3D CSI of whole brain is acquired in ~10 minutes with a minimum of ~0.2 mmol/kg dose using methylated agents (e.g., TmDOTMA−) for nominal SNR (21,22). In addition, preliminary results in our laboratory towards avoiding surgical intervention used to raise plasma levels of the agent (18,19), show that it is possible to co-infuse intravenously the agent with drugs that temporarily inhibit renal excretion (e.g., probenecid is used in humans to raise plasma concentration of drugs) (41). The co-infusion of the agent and probenecid should also lower the agent dose and allow repeated scans for longitudinal studies.
Morphology of 9L and RG2 tumors
Syngenic rat brain tumor models are widely used in preclinical cancer research (25). 9L and RG2 are considered gliosarcoma and glioblastoma models, respectively. A distinct advantage of such syngeneic models is that they are grafted into fully immune competent rats. 9L tumor margins are sharply delineated with little obvious invasion into the contiguous normal brain. The RG2 glioma has a relatively more infiltrative growth with foci of local collective invasion (25). 9L and RG2 tumors have different metabolite profiles and differences in energy demand (42). Microvessel density and angiogenesis seem to be higher in RG2 when compared to 9L by histological studies, however MRI methods could not reveal these differences (43).
Higher Ki-67 staining was detected in cells beyond the RG2 tumor boundary (Figure 1B) in agreement with prior observations (44). In addition, RG2 showed significantly higher GFAP (Figure 1C) and MCT4 (Figure 1D) staining inside the tumor compared to 9L, while significantly higher GFAP staining was observed outside the tumor in RG2 when compared to 9L. These results collectively imply morphological differences between 9L and RG2, where RG2 tumor cells seem to invade beyond the tumor boundary more than 9L tumor cells.
pHe mapping in tumors
Various factors contribute to the acidity of the extracellular space in peritumoral and intratumoral regions. It is generally believed that the Warburg effect mediates the acidosis within the tumor core, while beyond the tumor core inflammatory responses by lymphocytes and macrophage infiltrations could certainly affect pHe. However, it should be noted that poor perfusion in tumors hampers the ability of the microenvironment to remove tumor-derived acid through diffusion into blood vessels. Thus, H+ ions and lactic acid generated as a result of tumoral Warburg effect can diffuse through interstitial space along the concentration gradient due to lack of effective clearance by blood flow (45).
The low intratumoral pHe values in gliomas measured here are similar to prior MRI/MRS reports (27,46). We compared pHe measured by BIRDS with pHi measured by 31P-MRS. In both tumors, intratumoral pHe from BIRDS was significantly lower (~6.9) compared to intratumoral pHi from 31P-MRS (~7.4) and peritumoral pHe approached peritumoral pHi (Figure 3). In agreement with prior observations (1,14), our results indicate that the intratumoral pHe-pHi gradient is large, whereas the peritumoral pHe-pHi gradient is negligible. But the intratumoral-peritumoral pHe gradient was much smaller for brains bearing RG2 vs. 9L tumors, indicating tumor invasion relates to diffuse areas of low pHe (Figure 6).
A good example of pHe measurement in brain tumors is by Martinez et al, where they used a pH-sensitive T1 agent GdDOTA-4AmP5− injected intravenously into rats with C6 tumors with a pH-insensitive T2 agent DyDOTP5− (27). The T2 change caused by DyDOTP5−, similar to T2 darkening we observed with TmDOTP5− in this study (Figure S2), allowed [Gd3+] estimation to convert the T1 change of GdDOTA-4AmP5− into pHe. Alternate MRI methods using CEST, with either diamagnetic or paramagnetic agents (47), provide relative estimates of intratumoral pHe. Diamagnetic CEST has detected enhanced amide proton exchange (APT) in brain tumors (38,48), where the APT is related to pHi. Diamagnetic CEST has been applied for other types of tumors with low intratumoral pHe (39).
Since the focus in all previously reported pH-dependent maps with MRI and CEST methods was on intratumoral pHe, the intratumoral-peritumoral pHe gradient had not been quantitatively determined. Peritumoral pHe mapping has not received attention in past studies, and hence, in the absence of such data it could be inherently assumed that peritumoral pHe is close to normal tissue pHe. In this study we tested this assumption in two different tumor types and we showed that the peritumoral pHe of an aggressive tumor (RG2) is more acidic than normal tissue pHe. The MRI-measured intratumoral pHe reported by GdDOTA-4AmP5− for C6 tumors (27) is in general agreement with BIRDS-measured intratumoral pHe reported by TmDOTP5− for 9L and RG2 tumors. However in the present study we were able to compare the peritumoral pHe with intratumoral pHe. The ability to compare the intratumoral-peritumoral pHe gradient may be an important distinction as the diffuse nature of pHe reductions beyond the tumor boundary has been linked to tumor invasiveness (i.e., RG2 has higher extent of Ki-67 positive cells beyond the tumor boundary). These results are in good agreement with past studies, using primarily MRS techniques, that have shown that the intratumoral-peritumoral pHe gradient varies with tumor type (for a recent review see (2)).
SUMMARY
Our results show that intratumoral-peritumoral pHe gradient mapping by BIRDS can be used for high-resolution spatial evaluation of the tumor microenvironment. While acidic intratumoral pHe is a target for various cancer drugs (49), future chemotherapies could be further targeted to the diffuse areas of low peritumoral pHe because tumor cells invade (50).
Targeting the acidic pHe of the tumor microenvironment provides several therapeutic options. The proton pump seems to be a successful target for inhibitor designs to change the intratumoral pHe. For example, the V-ATPase inhibitor bafilomycin A1 inhibits tumor growth by increasing the intratumoral pHe without perturbing the intratumoral pHi (51). Another approach for increasing intratumoral pHe is systemic buffering by sodium bicarbonate and/or trisodium citrate (52,53).
Overall, a combination of pH regulating drugs with other cancer treatments (e.g., targeting glycolysis, chemotherapy and/or radiation) might help to reduce the tumor growth or prevent metastasis. One such example is the bicarbonate approach combined with dichloroacetate, which increases pyruvate dehydrogenase flux (52). In any event, if we can spatially localize the regions of low pHe, both within and beyond the tumor margin, we may detect tumor invasion, proliferation, angiogenesis, and response to therapy to determine the prognosis of the disease.
Supplementary Material
Acknowledgments
Supported by NIH grants (R01 EB-011968, R01 CA-140102, P30 NS-052519).
Abbreviations
- APT
amide proton exchange
- BIRDS
biosensor imaging of redundant deviation in shifts
- CEST
chemical exchange saturation transfer
- CSI
chemical shift imaging
- DAB
diaminobenzidine
- DOTP8-
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis methylene phosphonate
- FOV
field of view
- FWHM
full width at half maximum
- GFAP
glial fibrillary acidic protein
- GBM
glioblastoma multiforme
- H&E
hematoxylin and eosin
- Pi
inorganic phosphate
- MCT4
monocarboxylate transporter 4
- PP
percentage of positive stained cells
- PCr
phosphocreatine
- RF
radio-frequency
- SLR
Shinnar-Le Roux
- SD
standard deviation
- SS
staining score
- SPIO
superparamagnetic iron oxide
Footnotes
AUTHOR CONTRIBUTIONS
DC, YH, FH designed research. DC, YH, JUR, HMDF, CJ, FH performed research. DC, YH, JUR, FH analyzed data. DC, YH, JUR, DLR, FH wrote paper.
DISCLOSURE/CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Gerweck LE. The pH difference between tumor and normal tissue offers a tumor specific target for the treatment of cancer. Drug Resist Updat. 2000;3(1):49–50. doi: 10.1054/drup.2000.0122. [DOI] [PubMed] [Google Scholar]
- 2.Hashim AI, Zhang X, Wojtkowiak JW, Martinez GV, Gillies RJ. Imaging pH and metastasis. NMR in biomedicine. 2011;24(6):582–591. doi: 10.1002/nbm.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nature reviews Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 4.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nature reviews Cancer. 2011;11(9):671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 5.Kalpathy-Cramer J, Gerstner ER, Emblem KE, Andronesi OC, Rosen B. Advanced magnetic resonance imaging of the physical processes in human glioblastoma. Cancer Res. 2014;74(17):4622–4637. doi: 10.1158/0008-5472.CAN-14-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghunand N, Gatenby RA, Gillies RJ. Microenvironmental and cellular consequences of altered blood flow in tumours. The British journal of radiology. 2003;76(Spec No 1):S11–S22. doi: 10.1259/bjr/12913493. [DOI] [PubMed] [Google Scholar]
- 7.Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9(6):1957–1971. [PubMed] [Google Scholar]
- 8.Lardner A. The effects of extracellular pH on immune function. Journal of leukocyte biology. 2001;69(4):522–530. [PubMed] [Google Scholar]
- 9.Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ. Acidic pH enhances the invasive behavior of human melanoma cells. Clinical & experimental metastasis. 1996;14(2):176–186. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- 10.Morita T, Nagaki T, Fukuda I, Okumura K. Clastogenicity of low pH to various cultured mammalian cells. Mutation research. 1992;268(2):297–305. doi: 10.1016/0027-5107(92)90235-t. [DOI] [PubMed] [Google Scholar]
- 11.Shi Q, Le X, Wang B, Abbruzzese JL, Xiong Q, He Y, Xie K. Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene. 2001;20(28):3751–3756. doi: 10.1038/sj.onc.1204500. [DOI] [PubMed] [Google Scholar]
- 12.Song CW, Griffin R, Park HJ. Cancer drug resistance. Springer; 2006. Influence of tumor pH on therapeutic response; pp. 21–42. [Google Scholar]
- 13.Honasoge A, Sontheimer H. Involvement of tumor acidification in brain cancer pathophysiology. Frontiers in physiology. 2013;4:316. doi: 10.3389/fphys.2013.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillies RJ, Raghunand N, Garcia-Martin ML, Gatenby RA. pH imaging. A review of pH measurement methods and applications in cancers. IEEE Eng Med Biol Mag. 2004;23(5):57–64. doi: 10.1109/memb.2004.1360409. [DOI] [PubMed] [Google Scholar]
- 15.Bhujwalla ZM, Artemov D, Ballesteros P, Cerdan S, Gillies RJ, Solaiyappan M. Combined vascular and extracellular pH imaging of solid tumors. NMR in biomedicine. 2002;15(2):114–119. doi: 10.1002/nbm.743. [DOI] [PubMed] [Google Scholar]
- 16.Hingorani DV, Bernstein AS, Pagel MD. A review of responsive MRI contrast agents: 2005–2014. Contrast media & molecular imaging. 2014 doi: 10.1002/cmmi.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo B, Pagel MD. An overview of responsive MRI contrast agents for molecular imaging. Front Biosci. 2008;13:1733–1752. doi: 10.2741/2796. [DOI] [PubMed] [Google Scholar]
- 18.Coman D, Trubel HK, Rycyna RE, Hyder F. Brain temperature and pH measured by 1H chemical shift imaging of a thulium agent. NMR in Biomed. 2009;22(2):229–239. doi: 10.1002/nbm.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coman D, Trubel HK, Hyder F. Brain temperature by Biosensor Imaging of Redundant Deviation in Shifts (BIRDS): Comparison between TmDOTP5- and TmDOTMA- NMR in Biomed. 2010;23(3):277–285. doi: 10.1002/nbm.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coman D, Kiefer GE, Rothman DL, Sherry AD, Hyder F. A lanthanide complex with dual biosensing properties: CEST (chemical exchange saturation transfer) and BIRDS (biosensor imaging of redundant deviation in shifts) with europium DOTA-tetraglycinate. NMR in biomedicine. 2011;24(10):1216–1225. doi: 10.1002/nbm.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coman D, de Graaf RA, Rothman DL, Hyder F. In vivo 3D molecular imaging with BIRDS at high spatiotemporal resolution. NMR in biomedicine. 2013;26(11):1589–1595. doi: 10.1002/nbm.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coman D, Sanganahalli BG, Jiang L, Hyder F, Behar KL. Distribution of temperature changes and neurovascular coupling in rat brain following 3,4-methylenedioxymethamphetamine (MDMA,’ecstasy’) exposure. NMR in biomedicine. 2015;28(10):1257–1266. doi: 10.1002/nbm.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y, Coman D, Ali MM, Hyder F. Lanthanide ion (III) complexes of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraaminophosphonate for dual biosensing of pH with chemical exchange saturation transfer (CEST) and biosensor imaging of redundant deviation in shifts (BIRDS) Contrast media & molecular imaging. 2015;10(1):51–58. doi: 10.1002/cmmi.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Coman D, Hyder F, Ali MM. Dendrimer-based Responsive MRI Contrast Agents (G1-G4) for Biosensor Imaging of Redundant Deviation in Shifts (BIRDS) Bioconjugate chemistry. 2015 doi: 10.1021/acs.bioconjchem.5b00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barth RF, Kaur B. Rat brain tumor models in experimental neuro-oncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. Journal of neuro-oncology. 2009;94(3):299–312. doi: 10.1007/s11060-009-9875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trubel HK, Maciejewski PK, Farber JH, Hyder F. Brain temperature measured by 1H–NMR in conjunction with a lanthanide complex. J Appl Physiol. 2003;94(4):1641–1649. doi: 10.1152/japplphysiol.00841.2002. [DOI] [PubMed] [Google Scholar]
- 27.Martinez GV, Zhang X, Garcia-Martin ML, Morse DL, Woods M, Sherry AD, Gillies RJ. Imaging the extracellular pH of tumors by MRI after injection of a single cocktail of T1 and T2 contrast agents. NMR in biomedicine. 2011;24(10):1380–1391. doi: 10.1002/nbm.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida Y, Yamada M, Wakabayashi K, Ikuta F. Endothelial fenestrae in the rat fetal cerebrum. Brain Res Dev Brain Res. 1988;44(2):211–219. doi: 10.1016/0165-3806(88)90219-2. [DOI] [PubMed] [Google Scholar]
- 29.Kaya M, Chang L, Truong A, Brightman MW. Chemical induction of fenestrae in vessels of the blood-brain barrier. Exp Neurol. 1996;142(1):6–13. doi: 10.1006/exnr.1996.0174. [DOI] [PubMed] [Google Scholar]
- 30.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. Faseb J. 1993;7(8):678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 31.de Graaf RA, Chowdhury GM, Brown PB, Rothman DL, Behar KL. In situ 3D magnetic resonance metabolic imaging of microwave-irradiated rodent brain: a new tool for metabolomics research. Journal of neurochemistry. 2009;109(2):494–501. doi: 10.1111/j.1471-4159.2009.05967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lasher EP, Simmons BS, Everett NB. Effects of semistarvation on the distribution of erythrocytes and plasma in organs and tissues of the rat. The Journal of nutrition. 1958;65(2):317–326. doi: 10.1093/jn/65.2.317. [DOI] [PubMed] [Google Scholar]
- 33.van Berkel A, Rao JU, Kusters B, Demir T, Visser E, Mensenkamp AR, van der Laak JA, Oosterwijk E, Lenders JW, Sweep FC, Wevers RA, Hermus AR, Langenhuijsen JF, Kunst DP, Pacak K, Gotthardt M, Timmers HJ. Correlation Between In Vivo 18F–FDG PET and Immunohistochemical Markers of Glucose Uptake and Metabolism in Pheochromocytoma and Paraganglioma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55(8):1253–1259. doi: 10.2967/jnumed.114.137034. [DOI] [PubMed] [Google Scholar]
- 34.Strohbehn G, Coman D, Han L, Ragheb RR, Fahmy TM, Huttner AJ, Hyder F, Piepmeier JM, Saltzman WM, Zhou J. Imaging the delivery of brain-penetrating PLGA nanoparticles in the brain using magnetic resonance. Journal of neuro-oncology. 2015;121(3):441–449. doi: 10.1007/s11060-014-1658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Graaf RA. 2nd. Chichester, West Sussex, England: Wiley, John & Sons, Incorporated; 2007. In vivo NMR Spectroscopy - Principles and Techniques. [Google Scholar]
- 36.Ordidge RJ, Connelly A, Lohman JAB Image-selected in Vivo spectroscopy (ISIS) A new technique for spatially selective nmr spectroscopy. J Magn Reson. 1986;66:283–294. [Google Scholar]
- 37.McDaniel JR, Dewhirst MW, Chilkoti A. Actively targeting solid tumours with thermoresponsive drug delivery systems that respond to mild hyperthermia. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2013;29(6):501–510. doi: 10.3109/02656736.2013.819999. [DOI] [PubMed] [Google Scholar]
- 38.Sagiyama K, Mashimo T, Togao O, Vemireddy V, Hatanpaa KJ, Maher EA, Mickey BE, Pan E, Sherry AD, Bachoo RM, Takahashi M. In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(12):4542–4547. doi: 10.1073/pnas.1323855111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen LQ, Howison CM, Jeffery JJ, Robey IF, Kuo PH, Pagel MD. Evaluations of extracellular pH within in vivo tumors using acidoCEST MRI. Magn Reson Med. 2014;72(5):1408–1417. doi: 10.1002/mrm.25053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maritim S, Huang Y, Coman D, Hyder F. Characterization of a lanthanide complex encapsulated with MRI contrast agents into liposomes for biosensor imaging of redundant deviation in shifts (BIRDS) Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry. 2014;19(8):1385–1398. doi: 10.1007/s00775-014-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hedaya MA, Elmquist WF, Sawchuk RJ. Probenecid inhibits the metabolic and renal clearances of zidovudine (AZT) in human volunteers. Pharm Res. 1990;7(4):411–417. doi: 10.1023/a:1015835826114. [DOI] [PubMed] [Google Scholar]
- 42.Doblas S, He T, Saunders D, Hoyle J, Smith N, Pye Q, Lerner M, Jensen RL, Towner RA. In vivo characterization of several rodent glioma models by 1H MRS. NMR in biomedicine. 2012;25(4):685–694. doi: 10.1002/nbm.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tropres I, Pannetier N, Grand S, Lemasson B, Moisan A, Peoc’h M, Remy C, Barbier EL. Imaging the microvessel caliber and density: Principles and applications of microvascular MRI. Magn Reson Med. 2014 doi: 10.1002/mrm.25396. [DOI] [PubMed] [Google Scholar]
- 44.Wechsler W, Teske U, Reifenberger G, Deckert M, Seitz RJ, Mies G, Paschen W, Hossmann KA. Neuropathology and Regional Imaging of Microcirculation, Tissue pH, Metabolites and Necroses in Cerebral RG2 and F98 Anaplastic Rat Glioma Transplantation Tumors. In: Chatel MMD, Peeker J, editors. Brain Oncology. Vol. 52. Dordrecht, The Netherlands: Martinus Nijhoff Publishers; 1987. pp. 187–199. [Google Scholar]
- 45.Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, Bailey K, Balagurunathan Y, Rothberg JM, Sloane BF, Johnson J, Gatenby RA, Gillies RJ. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013;73(5):1524–1535. doi: 10.1158/0008-5472.CAN-12-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Martin ML, Herigault G, Remy C, Farion R, Ballesteros P, Coles JA, Cerdan S, Ziegler A. Mapping extracellular pH in rat brain gliomas in vivo by 1H magnetic resonance spectroscopic imaging: comparison with maps of metabolites. Cancer Res. 2001;61(17):6524–6531. [PubMed] [Google Scholar]
- 47.Sherry AD, Woods M. Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu Rev Biomed Eng. 2008;10:391–411. doi: 10.1146/annurev.bioeng.9.060906.151929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris RJ, Cloughesy TF, Liau LM, Prins RM, Antonios JP, Li D, Yong WH, Pope WB, Lai A, Nghiemphu PL, Ellingson BM. pH-weighted molecular imaging of gliomas using amine chemical exchange saturation transfer MRI. Neuro-oncology. 2015 doi: 10.1093/neuonc/nov106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evelhoch JL. pH and therapy of human cancers. Novartis Found Symp. 2001;240:68–80. doi: 10.1002/0470868716.ch5. discussion 80–64, 152–153. [DOI] [PubMed] [Google Scholar]
- 50.Gerweck LE, Richards B. Influence of pH on the thermal sensitivity of cultured human glioblastoma cells. Cancer Res. 1981;41(3):845–849. [PubMed] [Google Scholar]
- 51.McSheehy PM, Troy H, Kelland LR, Judson IR, Leach MO, Griffiths JR. Increased tumour extracellular pH induced by Bafilomycin A1 inhibits tumour growth and mitosis in vivo and alters 5-fluorouracil pharmacokinetics. European journal of cancer. 2003;39(4):532–540. doi: 10.1016/s0959-8049(02)00671-8. [DOI] [PubMed] [Google Scholar]
- 52.Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA, Gillies RJ. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69(6):2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silva AS, Yunes JA, Gillies RJ, Gatenby RA. The potential role of systemic buffers in reducing intratumoral extracellular pH and acid-mediated invasion. Cancer Res. 2009;69(6):2677–2684. doi: 10.1158/0008-5472.CAN-08-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.