Abstract
Introduction
Primary open angle glaucoma (POAG) is a progressive optic neuropathy characterized by impaired aqueous outflow and extensive remodeling in the trabecular meshwork (TM). The aim of this study was to characterize and compare the expression patterns of selected proteins belonging to the tissue remodeling, inflammation and growth factor pathways in ex vivo glaucomatous and post-mortem TMs using protein-array analysis.
Methods
TM specimens were collected from 63 white subjects, including 40 patients with glaucoma and 23 controls. Forty POAG TMs were collected at the time of surgery and 23 post-mortem specimens were from non-glaucomatous donor sclerocorneal tissues. Protein profiles were evaluated using a chip-based array consisting of 60 literature-selected antibodies.
Results
A different expression of some factors was observed in POAG TMs with respect to post-mortem specimens, either in abundance (interleukin [IL]10, IL6, IL5, IL7, IL12, IL3, macrophage inflammatory protein [MIP]1δ/α, vascular endothelial growth factor [VEGF], transforming growth factor beta 1 [TGFβ1], soluble tumor necrosis factor receptor I [sTNFRI]) or in scarcity (IL16, IL18, intercellular adhesion molecule 3 [ICAM3], matrix metalloproteinase-7 [MMP7], tissue inhibitor of metalloproteinase 1 [TIMP1]). MMP2, MMP7, TGFβ1, and VEGF expressions were confirmed by Western blot, zymography, and polymerase chain reaction. No difference in protein profile expression was detected between glaucomatous subtypes.
Conclusion
The analysis of this small TM population highlighted some proteins linked to POAG, some previously reported and others of new detection (IL7, MIPs, sTNFαRI). A larger POAG population is required to select promising disease-associated biomarker candidates.
Funding
This study was partially supported by the Fondazione Roma, the Italian Ministry of Health and the “National 5xMille 2010 tax donation to IRCCS-G.B. Bietti Foundation”.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-016-0285-x) contains supplementary material, which is available to authorized users.
Keywords: Extracellular matrix, Intraocular pressure, Primary open angle glaucoma, Trabecular meshwork
Introduction
Primary open angle glaucoma (POAG), the most common form of glaucoma, is a multifactorial disease with unclear pathogenesis, characterized by retinal ganglion cell death and irreversible damage of the optic nerve [1, 2]. Elevated intraocular pressure (IOP) represents the main risk factor involved in the onset and progression of POAG, due to an imbalance between aqueous humor production and outflow [3, 4]. POAG trabecular meshwork (TM) displays chronic inflammation and long-standing tissue remodeling [5, 6]. These molecular events are mainly of endogenous origin and related to the long-term accumulation of oxidative damages arising from mitochondrial failure and endothelial dysfunction [7]. Proteins deriving from the damage occurring in endothelial TM cells underwent dramatic variation, reflecting oxidative damage, mitochondrial damage, neural degeneration, and apoptosis [8, 9]. Structural changes encompass extensive extracellular matrix (ECM) deposition, typified by increased collagen, fibronectin and elastin deposition (thickness of trabecular sheets), impaired growth factors release, unbalance in ECM enzymes and unusual myofibroblast development [10–15]. Apoptosis of endothelial cells and targets of oxidative stress (glyceraldehyde-3-phosphate dehydrogenase [GAPDH], a glycolytic enzyme; heat shock 70 kDa protein 1 [HSP72], a stress protein; and glutamine synthetase, an excitotoxicity-related protein) were detected in POAG TMs, supporting the presence of a continuous inflammation, remodeling and immune system activation [16, 17]. While many components of TM ECM have been identified, only few studies address the entire TM proteome. Cumulating data point to impaired synthesis/release of transforming growth factor beta (TGFβs), nerve growth factor (NGF), vascular endothelial growth factor (VEGF), connective tissue growth factor (CTGF), tumor necrosis factor alpha (TNFα), interleukin (IL)1α, IL2, IL8, fibroblast growth factor (FGF), and their receptors, detected by single conventional approaches [18–22]. Some pro-inflammatory/fibrogenic factors have been detected in the aqueous humor, suggestive of future applications [23–25].
To date, the mechanism and the molecular basis responsible for structural, biochemical, and functional changes occurring in glaucomatous TM are poorly understood [26, 27]. Several studies have been performed to identify systemic and local biochemical risk factors and some novel protein/antibody profiles have been prospected as potential targets to counteract the degenerative effects in POAG, in addition to the IOP [3, 4, 28]. Recently, the protein microarray approach has highlighted, most resourceful than conventional approach, some candidates (biomarkers) for POAG, pertinent to routine diagnostic applications/monitoring of treatment efficacy or to target therapy [18, 29, 30].
The aim of the present study was to explore the expression of some factors in TMs from patients with POAG.
Methods
Ethics Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national), with the Helsinki Declaration of 1964, as revised in 2013, and was reviewed/approved by the institutional Ethic Committee (IRCCS IFO—Fondazione Bietti). A written informed consent was signed by patients joining the study and undergoing surgical therapy.
POAG Population
A total of 40 POAG and 23 age/sex-matched post-mortem TM specimens were used for the study (Table 1). Both slit lamp and clinical data were collected the day before surgery from each patient including age, gender, ocular history, diagnosis and duration of disease, previous medications, IOP, visual acuity and other ocular surgical interventions. The inclusion criteria comprised patients who needed to undergo trabeculectomy for uncontrolled IOP under maximal tolerated topical medical therapy, while the exclusion criteria comprised patients with systemic (either inflammatory, autoimmune and/or immunosuppressive) or pre-existing ocular (retinal vein/artery occlusion, diabetic retinopathy and age-related macular degeneration) diseases, or previous trabeculectomy or other ocular surgery or use of topical or systemic corticosteroids. All POAG TM samples were further grouped according to the IOP-lowering topical therapy ongoing before glaucoma surgery [Group 1 (n = 14): prostaglandin analogs (PGE) + carbonic anhydrase inhibitors (CAI); Group 2 (n = 26): PGE + CAI + beta-blockers (BB)].
Table 1.
Patient’s main characteristics
| Characteristics | POAGa | Control |
|---|---|---|
| Population | 40 | 23 |
| Male/female | 17/23 | 15/8 |
| Mean age (years) | 69.25 ± 9.49 | 74.25 ± 7.94 |
| Mean (±range) IOP, mm Hg | 19.83 ± 4.55 | – |
| Surgery: trabeculectomy/phacoemulsification | 11/29 | Noneb |
| Previous therapyc | IOP-lowering eye drop | None |
All data are shown as mean ± standard deviation
IOP intraocular pressure, POAG primary open angle glaucoma
aBoth inclusion and exclusion criteria are detailed in the “Methods” section
bThe non-glaucomatous control TMs were dissected out by mimicking trabeculectomy and phaco-trabeculectomy surgery on sclerocorneal specimens (EyeBank)
cMedications are listed in the “Methods” section
All analytical grade reagents and sterile plastic-ware were from SERVA (Weidelberg, Germany), ICN (Costa Mesa, CA, USA), Euroclone (Milan, Italy), Sigma-Aldrich (St. Louis, MO, USA) and NUNC (Roskilde, Denmark), unless otherwise specified. Ultrapure Milli-Q Grade Water was daily provided by the DirectQ 5 apparatus (Millipore, Vimodrone, Italy).
POAG and Post-mortem TM Specimens
Pathological TMs were collected from patients who underwent surgical procedures (n = 10 from trabeculectomy and n = 25 from phacotrabeculectomy) and quickly stabilized in a storage buffer (50 mM Tris-buffer, 150 mM NaCl, 1 mM EDTA, 10% glycerol) supplemented with protease inhibitors (#78410; Pierce Biotechnology, Rockford, IL) and stored at −70 °C. Delivery to the Laboratory Unit was performed according to a standardized procedure, avoiding sample thawing and protein degradation.
Control samples, made available by the Eye Bank of Rome (Italy), were obtained from organ donors undergoing removal of eyes for corneal transplant, within 6 h of death and provided in Eusol-C medium (herein shorten as post-mortem samples). The absence of any eye diseases in these donors was ascertained in agreement with Italian national law (No. 301 of 12 August 1993). Briefly, four representative TMs were collected for each specimen under a dissector microscope (SMZ645; Nikon, Tokyo, Japan) equipped with cold-light optic fibers (PL2000 photonic; Axon, Vienna, Austria), reproducing accurately the procedure of trabeculectomy and quickly stored in inhibitor-supplemented storage buffer at −70 °C until using in parallel with POAG samples. All post-mortem TMs were quickly stabilized in appropriate buffer, similar to POAG specimens.
Additional 5 glaucomatous (n = 2 from trabeculectomy and n = 3 from phacotrabeculectomy) and 5 post-mortem specimen were sampled in Thin Prep (Cytyc Corp., Milan, Italy) and subjected to molecular analysis.
Total Protein Extraction and Sodium Dodecyl Sulphate—Polyacrylamide Gel Electrophoresis, (SDS-PAGE) Analysis
Enzymatic pre-digestion was used to extract protein from each specimen (n = 35 POAG and n = 18 post-mortem samples). Briefly, TM specimens were treated with dispaseII (2 µg/mL in HBSS without Ca2+ and Mg2+) for 15 min at 37 °C, diluted 1:1 (v/v) with 2× Lysis buffer (25 mM Tris-buffer, 150 mM NaCl, 0.1% Tween20, 1 mM EDTA, 10% glycerol, 0.1% SDS, 10 mM NaF and 1 mM PMSF; pH 7.5), homogenized with a Polytron (25 s/0 °C at 21500 rev./min; Ultra Turrax T25 basic; IkaWerke, Staufen, Germany) and briefly sonicated to shear DNA/RNA (VibraCell, Sonics, Newtown, CT, USA). Tissue lysates were clarified by centrifugation and protein amount/quality was analyzed using the Nanodrop Spectrophotometer (A1000; Celbio, Milan, Italy). Protein amounts were detected with the A280-Nanodrop option by means of common internal standards, after “blank” options against water and extraction buffer. Data (µg/µL) were used to normalize protein extracts before loading in the specific assay.
SDS-PAGE was performed under reducing (electrophoretic profile) conditions on a 4–12% precasted-SDS mini gel (MiniProtean III apparatus; Biorad Laboratories Inc, Hercules, CA, USA). Separated bands were transblotted to Hybond membranes (Amersham Biosciences; GE Healthcare, Buckinghamshire, England) under semidry conditions (Transblot apparatus; Biorad). Membranes were stained with the Ponceau S solution (SERVA). Whenever required, protein extracts were concentrated according to the 500 VivaSpin manufacturers’ recommendations (Sartorius AG, Goettingen, Germany). Some samples were subject to IgG removal according to a standard procedure (GE Healthcare) [31].
Chip-Based Arrays for Proteome Analysis
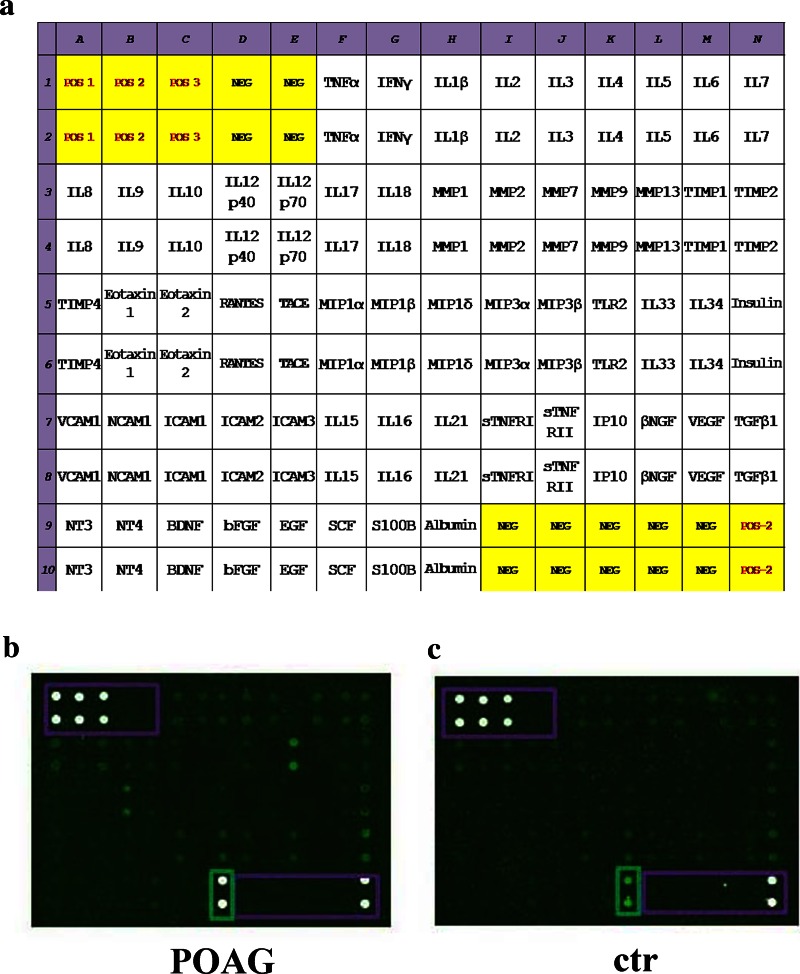
The proteome array was performed on customized chip-based arrays provided on glass-slides, according to the manufacturers’ procedures with minor modifications (RayBio technology; Norcross, CA). Each glass-slide (array) comprised 14 identical subarrays containing 60 biomarkers assembled on demand. The scheme of each chip is reported in Fig. 2a. Inter-assay normalization was conducted by including multiple positive markers and negative controls for each sub-array. The minimum sensitivity range for detection of each protein varied, ranging from 3.8 to 56 pg/mL.
Fig. 2.
Representative chip-based arrays. Chip are grids that contain small amounts of purified proteins in high density, hybridized to sample and detected by fluorescent technique. a The map showing the selected factors in a 14 × 10 grid. Each subarray contains 60 antibodies and specific positive/negative referring spots. b, c Representative GenePix acquired arrays from POAG (b) and post-mortem (c) TM specimens, both loaded as normalized extracts. White points are positive technique controls (framed), dark points are negative technique controls and green points are POAG/post-mortem TMs (cy3-labeled spots). Abbreviations of the main factors were according to International Classification. EGF epidermal growth factor, IL interleukin, MIP macrophage inflammatory protein, MMP matrix metalloproteinase, POAG primary open angle glaucoma, SCF stem cell factor, SDS sodium dodecyl sulphate, TIMP tissue inhibitor of metalloproteinase, TLR toll-like receptor, TM trabecular meshwork, TNF tumor necrosis factor
For hybridization, an equal amount of protein extracts (350 ng/mL in Lysis Buffer) was applied to each sub-array (70 µL/subarray). Both POAG (n = 25 POAG) and control (n = 13) extracts were processed in parallel. After an overnight incubation at 4 °C, the array slides were washed and exposed to a biotinylated antibody mixture followed by a cy3-streptAvidin labeling solution. All steps were performed under orbital shaking (CertomaxII, Sartorius AG) and all the hybridization/washing solutions were provided by the kit. As a final point, the glass-slides were washed once with MilliQ water, spin-dried and acquired with a GenePix 4400 Microarray scanner (Molecular Devices LLC, Sunnyvale, Silicon Valley, CA). To obtain appropriate Cy5 (background signal) and Cy3 (specific signal) images, the slides were scanned over previously validated acquisition parameters and the images/arrays (blocks) were uniformly adjusted for size, brightness and contrast at the moment of acquisition. Using the SPOT tool, the specific area (corresponding to each cytokine on the array) was manually spotted and automatically adjusted, according to prefixed acquisition parameters applied to all glass-slides of the study. The fluorescent intensity data (FI) of each spot was calculated by the GenePix Pro 6.0 pro software (Molecular Devices), that provide background-subtracted FI data (F532-B532, N factor) as of a value for spot volume representing the product of the area and the highest pixel value contained in that area.
Confirmation Experiments by Western Blot, Zymography, and Real-Time PCR
Western Blotting
The membranes from electrophoretic analysis were equilibrated in 0.5% Triton, blocked in 3% BSA, incubated with the primary antibodies (0.2–0.8 μg/mL, 18 h; MMP2 (sc-10736) and MMP7 (sc-130819) were from Santa Cruz; TGFβ1 (mab240) and VEGF (AF-293-NA) were purchased from R&D Systems, Minneapolis, Il) and labeled with secondary specie-specific POD-conjugated antibodies (1/7000, 90 min; Jackson Laboratories, West Grove, PA). The specific signals were visualized by SuperSignal West Pico Trial (Pierce).
Zymography
To study MMP2 and MMP9 functional activity, tissue extract were three fold-concentrated (VivaSpin) and pre-mixed with 5X Loading buffer under non-reducing conditions and incubated at 37 °C over 30 min. Normalized protein extracts (20 µL/lane) were pre-incubated at 37 °C over 30 min. Samples were then fractioned on 7–10% SDS-PAGE gels containing 0.1% gelatin (1 mg/mL gelatin), under non-reducing condition (frontline/130 V). Gels were washed in rinse-buffer (15 min; 50 mM Tris–HCl, 10 mM CaCl2 and 2.5% Triton X-100; pH 7.2) to promote recovery of protease activity and briefly rinsed with distilled water before incubating in substrate buffer (24 h/37 °C; 50 mM Tris–HCl, 150 mM NaCl, 10 mM CaCl2, 1% Triton-X100; pH 7.2). Gels were stained with 0.5% Coomassie blue R-250 in 30% isopropanol and 10% acetic acid for 30 min to 2 h and then de-stained in 10% isopropanol and 10% acetic acid until clear bands were visible. Internal controls and size marker were run in parallel. Molecular weights of the bands were estimated through the use of prestained molecular-weight markers MMP2 and MMP9 activity was identified as clear bands (white bands over a blue background) corresponding to degraded substrate.
Membranes and zymograms were captured in a 1D Kodak Image station (Kodak 550, Eastman Kodak Company, Sci. Imaging Systems, Rochester, NY, USA) and exported as 16-tiff converted images (1D Kodak Image Analysis Software). Single integrated optical density values (Intdensity) were registered for each group by means of 1D ImageJ software (ImageJ ver. 1.43; NIH-http://rsb.info.nih.gov/ij/) and assembled with minor modifications by Adobe photoshop 7.0 (Abacus concepts).
Real-Time PCR
RNA extraction was performed by Proteinase K predigestion and EuroGold TRIfast extraction, according to the manufactures’ procedure (EuroClone, Pero, Milan, Italy). Equivalent total RNAs (170 ng; 260/280 rate >1.8; Nanodrop) were used for cDNA synthesis that were carried out using 50 pM random primers and 200 U Reverse Transcriptase (IM-PROM kit, Promega, Milan, Italy) in a programmable PCR thermocycler (Primus 25 advanced-PCR; Peqlab LLC., Wilmington, USA). SYBR Green PCR amplifications (Applied Biosystems, Foster City, CA, USA) were run in a PCR Opticon2 (MJ Research, Watertown, MA), according to a standard protocol. The specific primers used were as follows: MMP2, MMP7, TGFβ1, VEGF, 18S, H3, and β2MG). Negative/positive controls and single-mode melting curves were used to validate the amplifications.
Statistical Analysis
POAG and post-mortem TMs were analyzed in duplicate (spots/bands) and mean values (±SD) were calculated from these replicates. Individual biomarker expression was provided by GenePix software. The row spot intensity data were entered into a Microsoft Excel database (Microsoft, Redmond, WA) and duplicate spots outside the 10% coefficient of variability were refused from the statistical analysis. FDR value of 0.01, as observed. Protein response ratios were defined as the variation in a given marker and a cut-off of two fold (changes) was used for differential expression, in accordance with previously published methods [32, 33]. The non-parametric Mann–Whitney U test was selected according to the small size population. Statistical significance for intra-pair POAG analysis was set at p < 0.00083 to control for multiple testing (p = 0.05/60 targets) and at p ≤ 0.05 for WB, Zymography and real-time PCR. The SPSS 15.0 statistic package (SPSS Inc, Chicago, IL, USA) was used for all comparisons. A specific REST/Mann–Whitney U test coupled analysis was carried out for PCR experiments.
Results
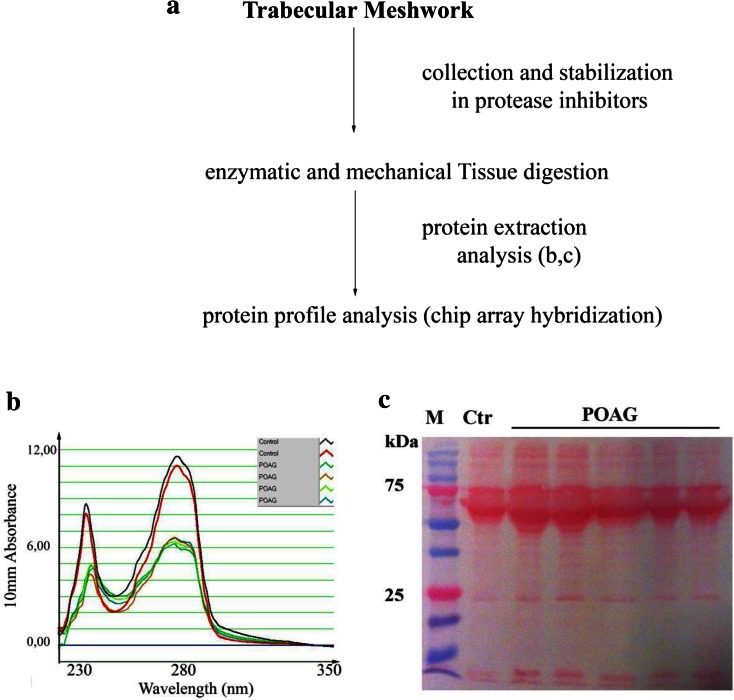
A total of 40 POAG and 23 post-mortem TM specimens were analyzed (Table 1). As detected in pilot studies, 17% (8/40 extracted specimens) of the total TM extracted samples was undetectable. The entire experimental procedure of this lower-case project, procedure, including TM stabilization to reduce protein degradation during transit, is shown in Fig. 1a. The total protein amount was detectable in all specimens included in the study, and representative spectrophotometer plots are shown in Fig. 1b. The electrophoretic analysis of POAG TM revealed the presence of abundant bands at the 100, 90, 70 and 40 kDa. Both heavy and light IgG chains were observed in several protein extracts (SDS-PAGE; Fig. 1c) and samples showing over-expressed heavy/light Ig chains were pretreated prior to hybridization (see M&M).
Fig. 1.
Experimental procedure and total protein analysis. a POAG and post-mortem TM specimens were simultaneously processed, according to the procedure reported in the “Methods” section. b Representative A280 plot showing the digital spectrophotometer outputs. Small peaks in the tract represent a small proportion of contaminants (solvents and salts; left side). c Comparative TM protein profiles by 1D SDS-PAGE analysis (representative membrane), from randomly selected POAG and post-mortem specimens generated after the enzymatic digestion procedure. Note the presence of bands resembling IgG proteins. POAG primary open angle glaucoma, SDS-PAGE sodium dodecyl sulphate-polyacrylamide gel electrophoresis, TM trabecular meshwork
To facilitate the reading/analysis of chip-array grids in Fig. 2a, appropriate clusters were defined. Two representative cy3-labeled fluorescence subarrays are reported in Fig. 2b, c, showing a significant increase of IF in POAG subarrays (B) with respect to post-mortem ones (C). The non-parametric Mann–Whitney two-sided U test was employed to select factors of significant value. The full data are available in Table 2, including single fold cut-offs and p values (>two fold changes and p ≤ 0.00083) provided in clusters as follows: cytokines; neurotrophins, fibrogenic and angiogenic factors; chemokines/adhesion molecules; ECM metabolism, metalloproteinases and tissue-inhibitory factors and other molecules, including soluble receptors and some referring proteins. A total of 32 out of 60 potential candidates were significantly associated with POAG. Most of them were represented by cytokines (IL10, IL6, IL5, IL7, IL12p40/70), chemokines (VCAM1, MIP1α/δ) and growth factors (TGFβ1, VEGF), showing high expression levels (>five fold) in POAG TMs, as compared to non-glaucomatous ones (p < 0.00083; Mann–Whitney U test). Moreover, IL16, IL18 and ICAM3 showed negative values, as compared to controls (p < 0.00083; Mann–Whitney U test). Regarding the ECM enzymes, a significant expression was detected for MMP2 while a low expression was quantified for MMP7 and TIMP1 (>two fold changes; p < 0.00083; Mann–Whitney U test). No difference in protein profile expression was observed between the two IOP-lowering subgroups (Group 1 and Group 2; p > 0.00083, Mann–Whitney U test), nor between the two different surgical approaches (trabeculectomy and phacotrabeculectomy; p > 0.00083, Mann–Whitney U test).
Table 2.
Protein profile expression
| Protein | Folds | p value |
|---|---|---|
| Cytokines | ||
| IL10 | 23.81 | 2.84E−07 |
| IL6 | 14.57 | 2.84E−07 |
| IL5 | 13.27 | 2.84E−07 |
| IL7 | 12.51 | 2.84E−07 |
| IL12p70 | 8.74 | 2.84E−07 |
| IL12p40 | 7.72 | 2.84E−07 |
| IL3 | 4.43 | 2.84E−07 |
| IL21 | 3.71 | 2.84E−07 |
| IL4 | 3.70 | 2.84E−07 |
| IL33 | 3.25 | 2.84E−07 |
| TNFα | 2.48 | 5.52E−07 |
| γIFN | 2.28 | 5.52E−07 |
| IL15 | 2.22 | 2.84E−07 |
| IL2 | 2.14 | 5.52E−07 |
| IL1β | 1.71 | 1.10E−04 |
| IL17 | 1.64 | 1.06E−05 |
| IL8 | 1.45 | 3.18E−06 |
| IL34 | 1.34 | 1.70E−03 |
| IL9 | 1.01 | NSS |
| IL18 | −11.79 | 2.16E−05 |
| IL16 | −43.65 | 5.03E−06 |
| Neurotrophins, fibrogenic, and angiogenic factors | ||
| VEGF | 6.10 | 9.01E−07 |
| TGFβ1 | 6.07 | 2.84E−07 |
| NT3 | 4.73 | 3.08E−07 |
| βFGF | 3.92 | 1.84E−04 |
| βNGF | 3.84 | 2.84E−07 |
| Insulin | 3.33 | 1.46E−06 |
| BDNF | 3.13 | 2.84E−07 |
| NT4 | 2.96 | 2.84E −07 |
| SCF | 1.32 | NSS |
| EGF | −1.51 | NSS |
| ECM metabolism | ||
| MMP2 | 3.18 | 2.84E−07 |
| MMP1 | 2.00 | 2.84E−07 |
| TIMP2 | 1.79 | 5.03E−06 |
| MMP13 | 1.27 | NSS |
| TIMP4 | −1.28 | NSS |
| MMP9 | −1.35 | NSS |
| MMP7 | −2.03 | 2.81E−04 |
| TIMP1 | −2.18 | 8.76E−04 |
| Chemokines/adhesion molecules | ||
| VCAM1 | 7.29 | 2.84E−07 |
| MIP1δ | 7.04 | 3.35E−07 |
| MIP1α | 5.51 | 2.84E−07 |
| Eotaxin1 | 2.61 | 3.18E−06 |
| ICAM1 | 2.16 | 1.51E−05 |
| TACE | 2.01 | 2.84E−07 |
| RANTES | 1.94 | 1.23E−03 |
| ICAM2 | 1.91 | 1.88E−05 |
| Eotaxin2 | 1.69 | NSS |
| MIP3β | 1.50 | 5.83E−03 |
| NCAM1 | 1.46 | NSS |
| MIP3α | −1.14 | NSS |
| MIP1β | −1.73 | 1.84E−04 |
| ICAM3 | −8.90 | 6.50E−07 |
| Other molecules | ||
| sTNFR I | 4.41 | 4.68E−07 |
| Albumin | 1.84 | NSS |
| S100B | 1.80 | 5.83E−03 |
| IP10 | 1.77 | 1.41E−05 |
| sTNFR II | 1.03 | NSS |
| TLR2 | −1.21 | 3.26E−01 |
Mann–Whitney U test analysis with both POAG/ctr fold-changes (≥2) and p values (≤0.00083, see “Methods” section) for each biomarker in POAG TMs. NSS for results having p values >8.3E−04. The factors are listed for higher to lower expression. The ECM metabolism cluster included both metalloproteinases and their specific inhibitors, and the cluster other molecules comprised soluble receptors and some referring proteins
BDNF brain-derived neurotrophic factor, ECM extracellular matrix, EGF epidermal growth factor, FGF fibroblast growth factor, ICAM intercellular adhesion molecule, IFN interferon, IL interleukin, IP interferon gamma-induced protein, MIP macrophage inflammatory protein, MMP matrix metalloproteinase, NCAM neural cell adhesion molecule, NGF nerve growth factor, NSS not statistically significant, NT neurotrophin, POAG primary open angle glaucoma, RANTES regulated on activation, normal T cell expressed and secreted, SCF stem cell factor, sTNFR soluble tumor necrosis factor receptor, TACE TNF-α converting enzyme, TLR toll-like receptor, TIMP tissue inhibitor of metalloproteinase, TNF tumor necrosis factor, TM trabecular meshwork, VCAM vascular cell adhesion molecule, VEGF vascular endothelial growth factor
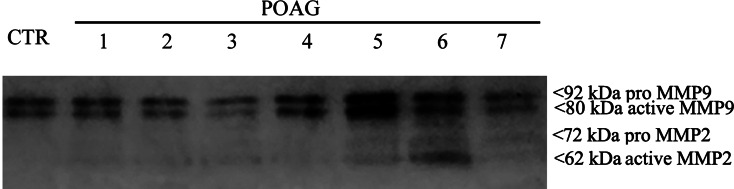
To better understand ECM metabolism and fibrosis/angiogenesis in glaucomatous TMs, MMPs 2/9/7 and TIMP1 as well as TGFβ1, VEGF were selected for Western Blot, Zymography and real-time PCR analysis. The Western Blot analysis showed as follows: increased expression for MMP2 (+1.39 ± 0.02 folds; p < 0.05), TGFβ1 (+2.00 ± 0.53 folds; p < 0.05) and VEGF (+2.13 ± 0.41 folds; p < 0.05); unchanged expression for MMP7 (+0.50 ± 0.39 folds; p > 0.05); and decreased expression for MMP9 (−3.71 ± 2.54 folds; p < 0.05), TIMP1 (−1.43 ± 0.33 folds; p < 0.05). Zymography (a functional test) identified bands of gelatinolytic activity, respectively, at 66 and 72 kDa, corresponding to the active and pro MMP2, respectively, at 80 and 92 kDa, corresponding to the active and pro MMP9 (Fig. 3). A high MMP9 gelatinolytic activity was also detected in non-glaucomatous control extracts (post-mortem specimens). Finally, the relative real-time PCR supported (p < 0.05) the increased expression of MMP2 (+2.53 ± 0.022log-ratio), TGFβ1 (+5.27 ± 0.032log-ratio) and VEGF (+2.84 ± 0.162log-ratio), and highlighted the deregulation of MMP7 (−3.77 ± 0.062log-ratio).
Fig. 3.
Confirmation of gelatinolytic activity of MMPs. Non-glaucomatous post-mortem and POAG TM (n = 7; lanes 1–7) extracts were electrophoresed on a gelatin-containing SDS gels (see the “Methods” section). Note the presence of gelatinolytic activity at 80/92 kDa (active/pro MMP9) and 62/72 (active/pro MMP2). Representative zymogram from three similar experiments. Both 80 and 92 kDa MMP9 bands are clearly visible in control TMs. Distinct 62 and 72 kDa bands specific for MMP2 were not detected in post-mortem extracts, while specific bands occur in POAG extracts. MMP matrix metalloproteinase, POAG primary open angle glaucoma, TM trabecular meshwork
Discussion
POAG represents one of the major leading causes of blindness worldwide, with poor early diagnosis and prognosis [1, 2, 28, 34]. Currently, no therapy is curative and an early diagnosis and/or a prompt intervention in “risk patients” would contribute significantly to reduce the progression of disease [1, 2, 24]. Sustained high-IOP levels or recurrent TM inflammation/remodeling or even long-standing homeostatic adjustments could trigger atypical cell–cell, cell–protein and/or protein–protein interactions, leading to TM structural changes in POAG [17, 35]. Several genes/proteins (soluble and stress-related mediators, growth factors, cytoskeletal and ECM-associated proteins) have been identified in POAG (mainly in TM-derived cells, aqueous humors, tears and blood) and proposed as potential candidates for the prognosis of disease and monitoring of therapy [24, 36].
Since any effort in the characterization of POAG-associated protein profile will be of great importance in understanding the mechanisms underlying POAG progression, a nonbiased chip-based microarray approach was carried out to discriminate 60 antibodies for potential proteins/factors relevant to pathological TM. The selected potential candidates are all known to be involved in chronic inflammation and tissue remodeling [16, 24, 28, 31, 36–39].
To the best our knowledge in literature, this is the first study conducted in vivo on human normal and glaucomatous TMs. From this comparative analysis, 32 out of 60 explored candidates were significantly affected in POAG specimens, as compared to control specimens dissected out from non-glaucomatous sclerocorneal explants mimicking the surgical procedure (see Table 2). Particularly, some cytokines (IL10, IL6, IL7, IL12p40/70, VCAM1, MIP1δ/α, sTNFRI) as well as Th1/Th2- (IL2, IL3, IL4, IL5) and tissue remodeling- (MMP2, VEGF, TGFβ1) related proteins were significantly increased. This increase would suggest the presence of both chronic inflammatory and tissue remodeling process in these glaucomatous TM specimens. The observation of increased TNFα, γIFN, IL2, IL3, IL4, IL5, IL7, IL12p40/70 and VCAM1 expression in TM specimens is in line with previous single and multiparametric conventional approaches performed in human TM-derived primary cultures, aqueous and tears [17, 24, 35, 40]. During glaucoma, macrophages produce cytokines including IL6, IL1β and TNFα, leading to an acute inflammatory response. These cytokines can induce ECM remodeling and alter cytoskeletal interactions in the glaucomatous TM [20]. The increased expression of IL6 and IL10 in glaucomatous TMs is in line with the increased IL10 and IL6 levels detected, respectively, in glaucomatous fluids (sera and aqueous) and strongly support the hypothesis of IL10 and IL6 as potential biomarkers of prognosis of disease or therapy [25, 40–43]. The high expression of IL6 might be explained with the remodeling process occurring in glaucomatous TMs and the IL6-TGFβ1 cross talk observed in TM-derived primary cultures [40, 43, 44]. IL6 increased outflow facility in perfused anterior segments of porcine eyes and IL6 levels were increased in porcine TM cells as a result of oxidative challenge [45, 46]. Besides, IL6 and IL8 have been implicated in induction of cellular senescence [47]. TNFα and IL6 mediate human microvascular endothelial tight junction modulation, by their ability of cytoskeletal rearrangment [48]. Finally, IL8 was found to modulate the permeability of the Schlemm’s canal endothelial cells [49].
On the other hand, the increase of IL7 might be explained with the IL7 ability to counteract the TGFβ1 activity, as observed in other in vitro models [50]. By contrary, the high IL10 expression is actually missing of explanation, although in experimental models of fibrosis the IL10 triggered (i) the anti-inflammatory response through the inhibition of TNFα, IL1, IL6, IL8, IL12, MIP1α and MIP2α release from monocytes/macrophages (anti-inflammatory response), (ii) the macrophage activity (down-regulating response MMPs/TGFβ1) and (iii) the up-regulation of TIMPs [43, 51].
Of interest, the significant increase of MIP1α/δ and decrease of MIP1β as well as the increase of VCAM1 and decrease of ICAM3 might support the presence of an anti-inflammatory response, as observed in other systems [52]. As for IL10, the increase of MIPs might be involved in the modulation of macrophage migration, the activation of granulocytes and the modulation of IL5, IL3, IL4 and IL12 inflammatory/profibrogenic activity [52]. The high sTNFRI expression might represent a point of interest, since it has been recently reported that this soluble receptor might be involved in the regulation of TNFα receptor activity [53]. With respect to the significant low expression of IL18 and IL16 actually we have no explanation.
The observation of the overexpression of IgG bands in the electrophoretic profiles might suggest the contribution of B cell implying potential neuroprotective effects, as previously described [31, 54, 55].
Although cumulating data indicate that MMPs and their inhibitors are increased in TMs’ tissue and derived cells, our data show no significant variation in MMPs/TIMPs expression, except for MMP2 and TIMP1. This finding might be consistent with a steady-fibrotic status and/or the presence of some inhibitory feedbacks due to endogenous/exogenous factors [56]. Physiological ECM remodeling occurs via a tight cross talk between MMPs and their tissue-related inhibitors (TIMPs) [6]. The increased MMP2, the unchanged MMP9/MMP13 and the decreased MMP7/TIMP1 expression might be the result of an excessive ECM accumulation and a low proteolytic activity [6, 7, 10]. One explanation for this result might be the overexpression of VEGF/TGFβ1 (profibrogenic factors), IL7 (inhibitor of fibrosis) and/IL6/IL12/IL10/IL5/IL4/IL3 (profibrogenic cytokines), all directly/indirectly involved in the tissue remodeling process [20, 21, 57, 58]. Another explanation might lie in the eye-drop therapy, since in addition to endogenous factors and ECM-derived products, the exogenous medications might influence TM remodeling, representing a side-effect of the therapy [56]. Both endogenous (increased IOP) and exogenous-induced (medications) biomolecular changes might actively contribute to the biomechanical features of obstructive TM. It is noteworthy to highlight that the therapy usually starts with medications for reducing IOP in order to delay/prevent glaucoma progression [55]. Hence, our POAG population is represented by patients chronically treated with IOP-lowering eye-drops and not undergoing previous surgery. Therefore, the possibility that TM specimens collected at the time of trabeculectomy might express a protein profile potentially influenced by the topical therapy cannot be excluded. It should be also noted that in this study population no corticosteroids/antibiotics therapy was used before trabeculectomy. It is well known that the therapy can alter gene expression of the TM influencing the results of our research, but this is what really happens in all glaucoma patients who daily use antiglaucoma drugs to counteract the development of the disease [59]. Therefore, although it is not possible (in this study) to eliminate the influences and interference of therapy, the proteins found reflect the real profile of glaucomatous proteome.
Altogether the protein chip-based array indicates some new factors (IL7, MIPs, sTNFαRI) and confirm some old ones (IL10, IL6, IL5, IL12, VEGF, TGFβ1 and NGF), all indicative of an active inflammatory process, as previously described [60, 61]. Indeed, both protein and mRNA evaluations highlight the presence of an active process of tissue remodeling and angiogenesis (MMP2, MMP7, VEGF, TGFβ1). Current studies are underway to correlate this glaucomatous TM expression with those of some glaucomatous body fluids (aqueous, tears and blood).
Conclusion
The possibility to define some factors in glaucomatous TMs potentially associated with disease and correlate this expression in glaucomatous body fluids represents a step forward in the management of glaucoma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Many thanks to Dr. Roberto Sacco (Laboratory of Molecular Psychiatry and Neurogenetics, UCBM, Rome, Italy) for microarray statistical support and Dr. Roberta Pastorelli (Protein and Gene Biomarkers Unit, Department of Environmental Health Sciences, IRCCS-Istituto di Ricerche Farmacologiche “Mario Negri”, Milan, Italy) for protein array critical suggestions. The study was partially supported by the Fondazione Roma, the Italian Ministry of Health and the “National 5xMille 2010 tax donation to IRCCS-G.B. Bietti Foundation”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have declared that no competing interests exist. This work is dedicated to the memory of our colleague and friend Prof. Marco Centofanti (M.C.; IRCCS- G.B. Bietti and Tor Vergata University) who recently passed away.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
A. Micera, L. Quaranta, G. Esposito, I. Floriani, A. Pocobelli, S. C. Saccà, I. Riva, G. Manni, F. Oddone have nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national), with the Helsinki Declaration of 1964, as revised in 2013, and was reviewed/approved by the institutional Ethic Committee (IRCCS IFO—Fondazione Bietti). A written informed consent was signed by patients joining the study and undergoing surgical therapy.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360:1113–1124. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 4.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 5.Luna C, Li G, Liton PB, et al. Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food Chem Toxicol. 2009;47:198–204. doi: 10.1016/j.fct.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddadin RI, Oh DJ, Kang MH, et al. Thrombospondin-1 (TSP1)-null and TSP2-null mice exhibit lower intraocular pressures. Invest Ophthalmol Vis Sci. 2012;53:6708–6717. doi: 10.1167/iovs.11-9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacca SC, Pulliero A, Izzotti A. The dysfunction of the trabecular meshwork during glaucoma course. J Cell Physiol. 2015;230:510–525. doi: 10.1002/jcp.24826. [DOI] [PubMed] [Google Scholar]
- 8.Izzotti A, Longobardi M, Cartiglia C, Sacca SC. Proteome alterations in primary open angle glaucoma aqueous humor. J Proteome Res. 2010;9:4831–4838. doi: 10.1021/pr1005372. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Iglesias H, Alvarez L, Garcia M, et al. Comparative proteomic study in serum of patients with primary open-angle glaucoma and pseudoexfoliation glaucoma. J Proteom. 2014;98:65–78. doi: 10.1016/j.jprot.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008;86:543–561. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diskin S, Kumar J, Cao Z, et al. Detection of differentially expressed glycogenes in trabecular meshwork of eyes with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:1491–1499. doi: 10.1167/iovs.05-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hann CR, Springett MJ, Wang X, Johnson DH. Ultrastructural localization of collagen IV, fibronectin, and laminin in the trabecular meshwork of normal and glaucomatous eyes. Ophthalmic Res. 2001;33:314–324. doi: 10.1159/000055687. [DOI] [PubMed] [Google Scholar]
- 13.Alexander JP, Samples JR, Acott TS. Growth factor and cytokine modulation of trabecular meshwork matrix metalloproteinase and TIMP expression. Curr Eye Res. 1998;17:276–285. doi: 10.1076/ceyr.17.3.276.5219. [DOI] [PubMed] [Google Scholar]
- 14.Wordinger RJ, Lambert W, Agarwal R, Talati M, Clark AF. Human trabecular meshwork cells secrete neurotrophins and express neurotrophin receptors (Trk) Invest Ophthalmol Vis Sci. 2000;41:3833–3841. [PubMed] [Google Scholar]
- 15.Alexander JP, Samples JR, Van Buskirk EM, Acott TS. Expression of matrix metalloproteinases and inhibitor by human trabecular meshwork. Invest Ophthalmol Vis Sci. 1991;32:172–180. [PubMed] [Google Scholar]
- 16.Liu T, Xie L, Ye J, Liu Y, He X. Screening of candidate genes for primary open angle glaucoma. Mol Vis. 2012;18:2119–2126. [PMC free article] [PubMed] [Google Scholar]
- 17.Babizhayev MA. Biomarkers and special features of oxidative stress in the anterior segment of the eye linked to lens cataract and the trabecular meshwork injury in primary open-angle glaucoma: challenges of dual combination therapy with N-acetylcarnosine lubricant eye drops and oral formulation of nonhydrolyzed carnosine. Fundam Clin Pharmacol. 2012;26:86–117. doi: 10.1111/j.1472-8206.2011.00969.x. [DOI] [PubMed] [Google Scholar]
- 18.Sacca SC, Centofanti M, Izzotti A. New proteins as vascular biomarkers in primary open angle glaucomatous aqueous humor. Invest Ophthalmol Vis Sci. 2012;53:4242–4253. doi: 10.1167/iovs.11-8902. [DOI] [PubMed] [Google Scholar]
- 19.de Kater AW, Shahsafaei A, Epstein DL. Localization of smooth muscle and nonmuscle actin isoforms in the human aqueous outflow pathway. Invest Ophthalmol Vis Sci. 1992;33:424–429. [PubMed] [Google Scholar]
- 20.Taurone S, Ripandelli G, Pacella E, et al. Potential regulatory molecules in the human trabecular meshwork of patients with glaucoma: immunohistochemical profile of a number of inflammatory cytokines. Mol Med Rep. 2015;11:1384–1390. doi: 10.3892/mmr.2014.2772. [DOI] [PubMed] [Google Scholar]
- 21.Kuchtey J, Kunkel J, Burgess LG, et al. Elevated transforming growth factor beta1 in plasma of primary open-angle glaucoma patients. Invest Ophthalmol Vis Sci. 2014;55:5291–5297. doi: 10.1167/iovs.14-14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Wang R, Thrimawithana T, et al. The nerve growth factor signaling and its potential as therapeutic target for glaucoma. Biomed Res Int. 2014;2014:759473. doi: 10.1155/2014/759473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chua J, Vania M, Cheung CM, et al. Expression profile of inflammatory cytokines in aqueous from glaucomatous eyes. Mol Vis. 2012;18:431–438. [PMC free article] [PubMed] [Google Scholar]
- 24.Kokotas H, Kroupis C, Chiras D, et al. Biomarkers in primary open angle glaucoma. Clin Chem Lab Med. 2012;50:2107–2119. doi: 10.1515/cclm-2012-0048. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Chen S, Gao X, et al. Inflammation-related cytokines of aqueous humor in acute primary angle-closure eyes. Invest Ophthalmol Vis Sci. 2014;55:1088–1094. doi: 10.1167/iovs.13-13591. [DOI] [PubMed] [Google Scholar]
- 26.Tezel G, Yang X, Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci. 2005;46:3177–3187. doi: 10.1167/iovs.05-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Z, Quigley HA, Pease ME, et al. Changes in gene expression in experimental glaucoma and optic nerve transection: the equilibrium between protective and detrimental mechanisms. Invest Ophthalmol Vis Sci. 2007;48:5539–5548. doi: 10.1167/iovs.07-0542. [DOI] [PubMed] [Google Scholar]
- 28.Borras T, Brandt CR, Nickells R, Ritch R. Gene therapy for glaucoma: treating a multifaceted, chronic disease. Invest Ophthalmol Vis Sci. 2002;43:2513–2518. [PubMed] [Google Scholar]
- 29.Cahill DJ. Protein and antibody arrays and their medical applications. J Immunol Methods. 2001;250:81–91. doi: 10.1016/S0022-1759(01)00325-8. [DOI] [PubMed] [Google Scholar]
- 30.Fan BJ, Wiggs JL. Glaucoma: genes, phenotypes, and new directions for therapy. J Clin Invest. 2010;120:3064–3072. doi: 10.1172/JCI43085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joachim SC, Pfeiffer N, Grus FH. Autoantibodies in patients with glaucoma: a comparison of IgG serum antibodies against retinal, optic nerve, and optic nerve head antigens. Graefes Arch Clin Exp Ophthalmol. 2005;243:817–823. doi: 10.1007/s00417-004-1094-5. [DOI] [PubMed] [Google Scholar]
- 32.Cui X, Churchill GA. Statistical tests for differential expression in cDNA microarray experiments. Genome Biol. 2003;4:210. doi: 10.1186/gb-2003-4-4-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Churchill GA. Using ANOVA to analyze microarray data. Biotechniques. 2004;37:173–175, 176. doi: 10.2144/04372TE01. [DOI] [PubMed] [Google Scholar]
- 34.Keller KE, Aga M, Bradley JM, Kelley MJ, Acott TS. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009;88:676–682. doi: 10.1016/j.exer.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 36.Stone EM, Fingert JH, Alward WL, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 37.Golubnitschaja O, Flammer J. What are the biomarkers for glaucoma? Surv Ophthalmol. 2007;52(Suppl 2):S155–S161. doi: 10.1016/j.survophthal.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Liton PB, Luna C, Challa P, Epstein DL, Gonzalez P. Genome-wide expression profile of human trabecular meshwork cultured cells, nonglaucomatous and primary open angle glaucoma tissue. Mol Vis. 2006;12:774–790. [PMC free article] [PubMed] [Google Scholar]
- 39.Hewitt AW, Craig JE, Mackey DA. Complex genetics of complex traits: the case of primary open-angle glaucoma. Clin Experiment Ophthalmol. 2006;34:472–484. doi: 10.1111/j.1442-9071.2006.01268.x. [DOI] [PubMed] [Google Scholar]
- 40.Liton PB, Luna C, Bodman M, et al. Induction of IL-6 expression by mechanical stress in the trabecular meshwork. Biochem Biophys Res Commun. 2005;337:1229–1236. doi: 10.1016/j.bbrc.2005.09.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, Yang P, Tezel G, et al. Induction of HLA-DR expression in human lamina cribrosa astrocytes by cytokines and simulated ischemia. Invest Ophthalmol Vis Sci. 2001;42:365–371. [PubMed] [Google Scholar]
- 42.Cvenkel B, Kopitar AN, Ihan A. Inflammatory molecules in aqueous humour and on ocular surface and glaucoma surgery outcome. Mediators Inflamm. 2010;2010:939602. doi: 10.1155/2010/939602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorkhabi R, Ghorbanihaghjo A, Javadzadeh A, Motlagh BF, Ahari SS. Aqueous humor hepcidin prohormone levels in patients with primary open angle glaucoma. Mol Vis. 2010;16:1832–1836. [PMC free article] [PubMed] [Google Scholar]
- 44.Liton PB, Li G, Luna C, Gonzalez P, Epstein DL. Cross-talk between TGF-beta1 and IL-6 in human trabecular meshwork cells. Mol Vis. 2009;15:326–334. [PMC free article] [PubMed] [Google Scholar]
- 45.Liton PB, Challa P, Stinnett S, et al. Cellular senescence in the glaucomatous outflow pathway. Exp Gerontol. 2005;40:745–748. doi: 10.1016/j.exger.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li G, Luna C, Liton PB, et al. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol Vis. 2007;13:2282–2288. [PMC free article] [PubMed] [Google Scholar]
- 47.Kuilman T, Michaloglou C, Vredeveld LC, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 48.Blum MS, Toninelli E, Anderson JM, et al. Cytoskeletal rearrangement mediates human microvascular endothelial tight junction modulation by cytokines. Am J Physiol. 1997;273:H286–H294. doi: 10.1152/ajpheart.1997.273.1.H286. [DOI] [PubMed] [Google Scholar]
- 49.Alvarado JA, Alvarado RG, Yeh RF, et al. A new insight into the cellular regulation of aqueous outflow: how trabecular meshwork endothelial cells drive a mechanism that regulates the permeability of Schlemm’s canal endothelial cells. Br J Ophthalmol. 2005;89:1500–1505. doi: 10.1136/bjo.2005.081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang L, Keane MP, Zhu LX, et al. Interleukin-7 and transforming growth factor-beta play counter-regulatory roles in protein kinase C-delta-dependent control of fibroblast collagen synthesis in pulmonary fibrosis. J Biol Chem. 2004;279:28315–28319. doi: 10.1074/jbc.C400115200. [DOI] [PubMed] [Google Scholar]
- 51.Clarke CJ, Hales A, Hunt A, Foxwell BM. IL-10-mediated suppression of TNF-alpha production is independent of its ability to inhibit NF kappa B activity. Eur J Immunol. 1998;28:1719–1726. doi: 10.1002/(SICI)1521-4141(199805)28:05<1719::AID-IMMU1719>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 52.Bell K, Gramlich OW, Hohenstein-Blaul NVTU, et al. Does autoimmunity play a part in the pathogenesis of glaucoma? Prog Retin Eye Res. 2013;36:199–216. doi: 10.1016/j.preteyeres.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Tezel G. TNF-alpha signaling in glaucomatous neurodegeneration. Prog Brain Res. 2008;173:409–421. doi: 10.1016/S0079-6123(08)01128-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Musch DC, Gillespie BW, Lichter PR, et al. Visual field progression in the Collaborative Initial Glaucoma Treatment Study the impact of treatment and other baseline factors. Ophthalmology. 2009;116:200–207. doi: 10.1016/j.ophtha.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quaranta L, Biagioli E, Riva I, Rulli E, Poli D, Katsanos A, Floriani I. Prostaglandin analogs and timolol-fixed versus unfixed combinations or monotherapy for open-angle glaucoma: a systematic review and meta-analysis. J Ocul Pharmacol Ther. 2013;29:382–389. doi: 10.1089/jop.2012.0186. [DOI] [PubMed] [Google Scholar]
- 56.Mosaed S, Dustin L, Minckler DS. Comparative outcomes between newer and older surgeries for glaucoma. Trans Am Ophthalmol Soc. 2009;107:127–133. [PMC free article] [PubMed] [Google Scholar]
- 57.Fuchshofer R, Tamm ER. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012;347:279–290. doi: 10.1007/s00441-011-1274-7. [DOI] [PubMed] [Google Scholar]
- 58.Bollinger KE, Crabb JS, Yuan X, et al. Quantitative proteomics: TGFbeta(2) signaling in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:8287–8294. doi: 10.1167/iovs.11-8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Izzotti A, La Maestra S, Micale RT, Longobardi MG, Sacca SC. Genomic and post-genomic effects of anti-glaucoma drugs preservatives in trabecular meshwork. Mutat Res. 2015;772:1–9. doi: 10.1016/j.mrfmmm.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Malvitte L, Montange T, Vejux A, et al. Measurement of inflammatory cytokines by multicytokine assay in tears of patients with glaucoma topically treated with chronic drugs. Br J Ophthalmol. 2007;91:29–32. doi: 10.1136/bjo.2006.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pieragostino D, Agnifili L, Fasanella V, et al. Shotgun proteomics reveals specific modulated protein patterns in tears of patients with primary open angle glaucoma naive to therapy. Mol BioSyst. 2013;9:1108–1116. doi: 10.1039/c3mb25463a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.