Abstract
Purpose
To characterize the frequency of and clinical indications for which experts treat retinopathy of prematurity (ROP) milder than Type 1 disease, the recommended threshold for treatment from established consensus guidelines.
Design
Descriptive analysis
Methods
Setting: Multicenter. Study Population: A database of 1444 eyes generated prospectively from all babies screened for ROP at one of 6 major ROP centers whose parents provided informed consent. Intervention: Retrospective review of the database and charts to identify all patients treated for ROP milder than Type 1. Main Outcome Measure: Indication(s) for treatment.
Results
137 eyes of 70 infants were treated for ROP. Of these 137 eyes, 13 (9.5%) were treated despite a clinical diagnosis milder than Type 1 ROP. Indications for treatment included: active ROP with the fellow eye being treated for Type 1 ROP (2 eyes, 15.4%); concerning structural changes (9 eyes, 69.2%), including tangential traction with temporal vessel straightening concerning for macular dragging (8 eyes, 61.5%) and thick stage 3 membranes with anteroposterior traction concerning for progression to stage 4 ROP (3 eyes, 23.1%); persistent ROP at an advanced postmenstrual age (4 eyes, 30.8%); and/or vitreous hemorrhage (3 eyes, 23.1%).
Conclusion
Experts in this study occasionally recommended treatment in eyes with disease less than Type 1 ROP. This study has important clinical implications and highlights the role of individual clinical judgment in situations not covered by evidence-based treatment guidelines.
Retinopathy of prematurity (ROP) is a disorder of retinal vascular development in premature and low birth weight infants that is a leading cause of childhood blindness in the United States and throughout the world. However, timely screening and treatment can dramatically improve outcomes in patients with ROP and prevent blindness in children with treatment-requiring disease. 1–5 Close adherence to published consensus ROP screening and treatment guidelines has been recommended by experts not only to ensure evidence-based ROP care and improve outcomes, but also to reduce medicolegal liability.1–8 ROP is classified using a standard international scheme describing the stage of pathology, the zone of retina affected, and the presence or absence of plus disease.
Based on the multicenter Early Treatment for Retinopathy of Prematurity (ET-ROP) study, ROP is now classified as mild ROP; Type 2 ROP, defined as zone I, stage 1 or 2 without plus disease or zone II, stage 3 without plus; or Type 1 ROP, defined as zone I, stage 3; zone I, any stage with plus disease; or zone II, stage 2 or 3, with plus disease.1–4 In 2013, a consensus policy statement by the American Academy of Pediatrics, American Academy of Ophthalmology, and the American Association for Pediatric Ophthalmology and Strabismus recommended treatment for Type 1 or worse ROP, based on the ET-ROP study findings.1–4,9
Despite these well-defined criteria for treatment, it has been our anecdotal experience that clinicians and ROP experts may occasionally recommend treatment for patients with ROP milder than Type 1. This has important implications for the role of clinical judgment in individual cases not covered by evidence-based treatment guidelines. The purpose of this study is to characterize the frequency and nature of cases of ROP that were treated for disease milder than Type 1 by retrospectively evaluating a large, prospectively collected, multicenter database of neonates screened for ROP.
Methods
This is a descriptive analysis from a prospectively collected multicenter i-ROP (Imaging & Informatics in ROP research consortium) database of all neonates screened for ROP at any of 6 major ROP centers in the United States whose parents provided informed written consent for inclusion in the database and associated studies. The study was performed in accordance with protocols approved by the Institutional Review Board of all institutions involved, and all analyses were performed in a fashion compliant with the Health Insurance Portability and Accountability Act.
Participating centers included Oregon Health & Science University, Weill Cornell Medical College, Bascom Palmer Eye Institute (University of Miami), Children’s Hospital Los Angeles, William Beaumont Hospital, and Columbia University Medical Center. Study examinations were performed between 4/4/2006 and 1/21/2015.
All children who were screened for ROP and whose parents consented for inclusion in the database were included in this study. Screening criteria were birth weight ≤1500 grams, gestational age at birth ≤30 weeks, or unstable clinical course or high risk of ROP as determined by the pediatrician or neonatologist. Clinical examinations were performed at the bedside by a retina specialist or pediatric ophthalmologist using indirect ophthalmoscopy. Wide-angle retinal images were also captured using a commercially available camera (RetCam; Clarity Medical Systems, Pleasanton, CA) if they were felt by the examiner to contribute diagnostic value. Posterior, temporal, nasal, superior, inferior, and iris images of each eye were taken, along with additional images as needed.
The i-ROP database was reviewed for all infants treated for ROP with either laser photocoagulation or intravitreal anti-vascular endothelial growth factor (VEGF) agent. Infants who were treated based on a clinical diagnosis milder than Type 1 ROP were identified. The treating center was documented and is presented here in a coded format. Medical records of these subjects, including all examination and operative notes, were reviewed by a coauthor (MPG) to confirm the clinical diagnosis and determine the treating clinician’s reasoning for treatment. Each case was subsequently reviewed by the study authors (MFC, RVPC, MPG). Indications for treatment for each case were reviewed and determined to fall into one or more of the following categories: (1) Active ROP with fellow eye being treated for Type 1 ROP; (2) Concerning structural changes; (3) Persistent active ROP at an advanced postmenstrual age; (4) Vitreous hemorrhage.
When available, fundus photographs from the examination prior to treatment were reviewed and subjected to a previously reported method for generating a “reference standard” diagnosis. Briefly, the “reference standard” diagnosis was derived by review of fundus photographs by 3 readers experienced in image-based ROP diagnosis and incorporation of the clinical diagnosis by the examining clinician and arbitration as necessary when discrepancies between these 4 diagnoses arose.10 The “reference standard” diagnosis was evaluated as a quality check to assess likelihood of accurate diagnosis by the treating clinician. However, because this study sought to determine the treating clinician’s rationale for treatment, indications for treatment were determined strictly from the medical documentation and review of the case with the treating clinician, rather than our retrospective review of the imaging or the “reference standard” diagnosis.
Results
Description of Study Population
During the study period, there were 722 infants in the i-ROP database (1444 eyes, 4795 eye exams). Within this study database, 137 eyes (9.5%) of 70 infants were treated for ROP with laser photocoagulation or intravitreal anti-VEGF agents. Of these 70 infants, 3 underwent unilateral treatment and 67 underwent bilateral treatment.
Indications for Treatment of ROP Milder than Type 1 ROP
Of 137 eyes treated for ROP, 13 eyes (9.5%) of 9 infants were treated despite a clinical diagnosis of less than Type 1 disease (Table). These 9 cases were from 4 of the 6 ROP centers included in the database. All of these eyes were treated with laser photocoagulation. Of the 137 eyes treated, 134 were cases of bilateral treatment and 3 were cases of unilateral treatment – all 3 of the unilaterally treated eyes (Cases 6, 8, and 9 in the Table) were outliers. Indications for treatment are described below. Only a few cases are described in detail below in order to describe a representative case for each treatment indication. Details for all cases studied are included in the Table. Of note, some eyes were treated for more than one indication.
Table.
Indications for treatment of retinopathy of prematurity for clinical diagnosis milder than Type 1 retinopathy of prematurity
| Subject: Eye | Center (coded) | Gestational age at birth (weeks) | Birth weight (grams) | Gestational age at time of treatment (weeks) | Clinical diagnosis | “Reference standard” diagnosis (when available) | Indication(s) for treatment |
|---|---|---|---|---|---|---|---|
| 1: OS | A | 25 | 600 | 42 | Zone II, Stage 2, Pre-plus (mild ROP) | Zone II, Stage 2, No plus (mild ROP) |
|
| 2: OS | A | 25 | 740 | 39 | Zone II, Stage 2, No plus (mild ROP) | Zone II, Stage 2, Pre-plus (mild ROP) |
|
| 3: OD | B | 27 | 990 | 47 | Zone II, Stage 3, No Plus (Type 2 ROP) | Zone II, Stage 3, Pre plus (Type 2 ROP) |
|
| 3: OS | B | 27 | 990 | 47 | Zone II, Stage 3, No Plus (Type 2 ROP) | Zone II, Stage 3, Pre-plus (Type 2 ROP) |
|
| 4: OD | C | 23 | 534 | 38 | Zone II, Stage 3, No Plus (Type 2 ROP) | N/A |
|
| 4: OS | C | 23 | 534 | 38 | Zone II, Stage 3, No Plus (Type 2 ROP) | N/A |
|
| 5: OD | B | 23 | 510 | 40 | Zone II, Stage 3, Pre-plus (Type 2 ROP) | N/A |
|
| 5: OS | B | 23 | 510 | 40 | Zone II, Stage 3, Pre-plus (Type 2 ROP) | N/A |
|
| 6: OS | D | 26 | 950 | 42 | Zone II, Stage 3, No Plus (Type 2 ROP) | Zone II, Stage 3, No plus (Type 2 ROP) |
|
| 7: OD | B | 25 | 840 | 41 | Zone II, Stage 3, No Plus (Type 2 ROP) | Zone II, Stage 3, Pre-Plus (Type 2 ROP) |
|
| 7: OS | B | 25 | 840 | 41 | Zone II, Stage 3, No Plus (Type 2 ROP) | Zone II, Stage 3, Pre-Plus (Type 2 ROP) |
|
| 8: OD | C | 26 | 1090 | 43 | Zone III, Stage 3, No Plus (mild ROP) | N/A |
|
| 9: OS | B | 28 | 1077 | N/A (12 months of age) | Zone III, Stage 1, No plus; peripheral ischemia on fluorescein angiography (mild ROP) | Zone II, Stage 2, No Plus (mild ROP) |
|
A prospectively collected database of all neonates screened for retinopathy of prematurity (ROP) from 6 major ROP centers in the United States was retrospectively reviewed to identify cases of treatment with laser or anti-vascular endothelial growth factor agents for ROP milder than Type 1 ROP. The charts of these patients were reviewed to determine the clinical diagnosis and indication for treatment. Table 1 describes each subject and eye treated for ROP milder than Type 1, the center where the patient was diagnosed (coded), gestational age and birth weight, clinical diagnosis and indication for treatment, as well as the “reference standard” diagnosis determined based on review of fundus photographs, when available. Abbreviations: OD: right eye, OS: left eye; ROP: retinopathy of prematurity. Center names have been de-identified and coded A–D
Active ROP with fellow eye being treated for Type 1 ROP
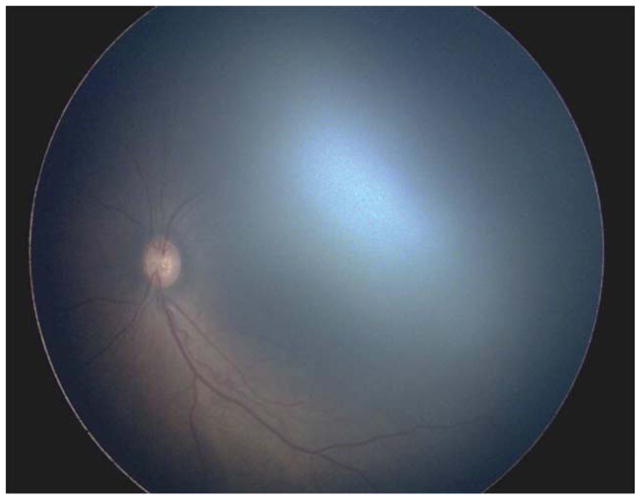
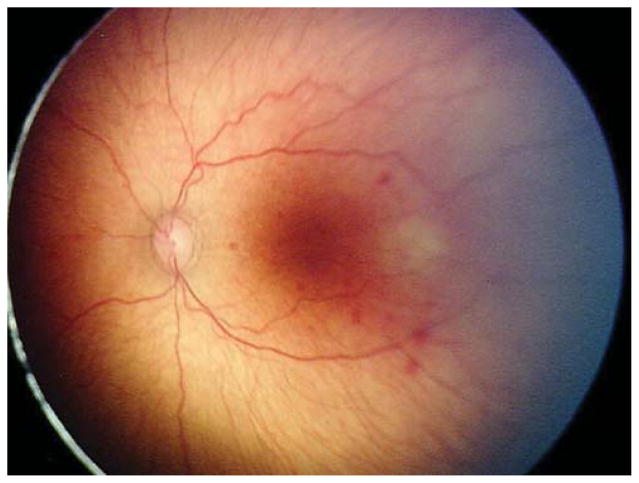
Two (15.4%) of the 13 eyes were treated for active Type 2 or less ROP in the setting of the fellow eye being treated for Type 1 ROP. Case 1 was born at 25 weeks gestational age, with birth weight 600 grams. Examination at 42 weeks postmenstrual age revealed zone II, stage 3 with plus disease in the right eye (OD) (Figure 1 top left and top right) and zone II, stage 2 with pre-plus disease in the left eye (OS) (Figure 1 bottom left and bottom right). Of the 137 treated eyes evaluated in this study, these 3 cases were only ones in which only one of the two eyes met treatment guidelines, and both of these were treated in a single session, bilaterally. Given the presence of Type 1 disease OD meeting guidelines for treatment, the option of treatment OS at the same time versus close observation OS was discussed with the parents. Informed consent was obtained, and laser photocoagulation was performed in both eyes (OU).
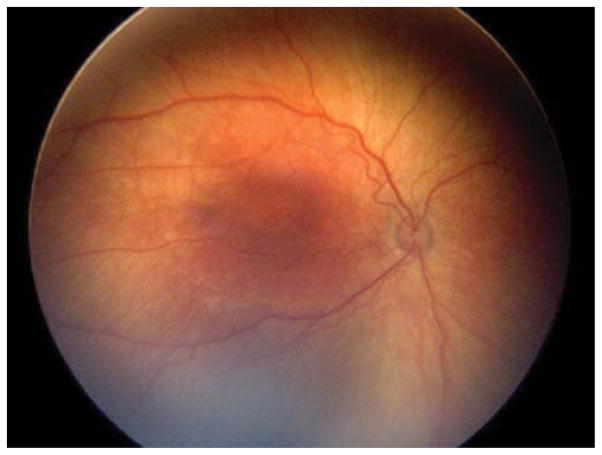
Figure 1. Representative color fundus photograph of a patient (Case 1) who was treated for active retinopathy of prematurity milder than Type 1 disease because the fellow eye was simultaneously being treated for Type 1 retinopathy of prematurity.
Fundus photos reveal zone II, stage 3 retinopathy of prematurity with plus disease in the right eye (top left: posterior, top right: temporal) and zone II, stage 2 retinopathy of prematurity with pre-plus disease in the left eye (bottom left: posterior, bottom right: temporal).
Concerning structural changes
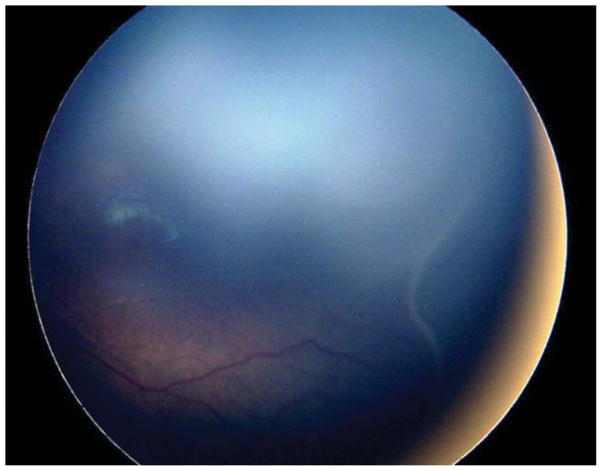
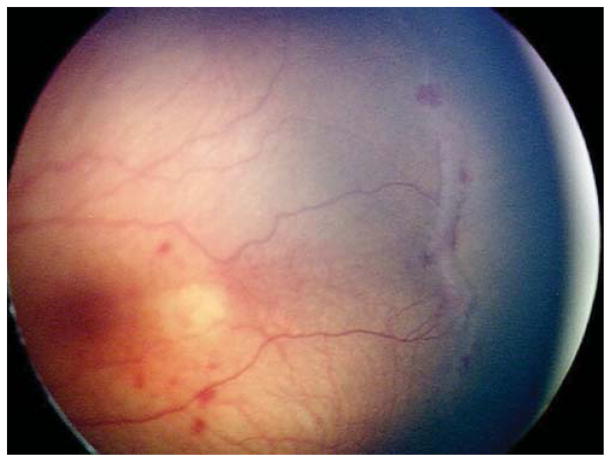
Nine (69.2%) of the 13 eyes were treated for concerning structural changes. Of these, 8 eyes (61.5%) were treated for tangential traction with temporal vessel straightening concerning for macular distortion and dragging. Case 3, for example, was born at 27 weeks gestational age, with birth weight 990 grams. At 47 weeks postmenstrual age, examination revealed zone II, stage 3, no plus disease with temporal vascular straightening (Figure 2) concerning for future macular distortion and dragging. The option of laser versus close observation was discussed with the parents. Informed consent was obtained, and laser photocoagulation was performed OU.
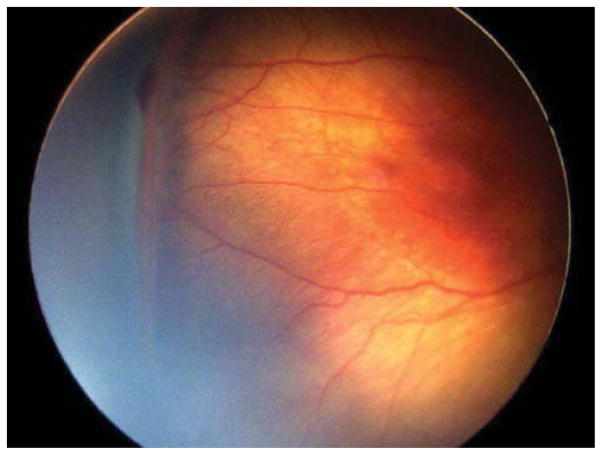
Figure 2. Representative color fundus photograph of a patient (Case 4) who was treated for retinopathy of prematurity milder than Type 1 disease due to concerning structural changes of tangential traction with temporal vessel straightening concerning for macular distortion and ectopia.
Fundus photos in the left eye (left: posterior, right: temporal) reveal zone II, stage 3 retinopathy of prematurity without evidence of plus disease (left) with straightening of the vasculature temporally (right).
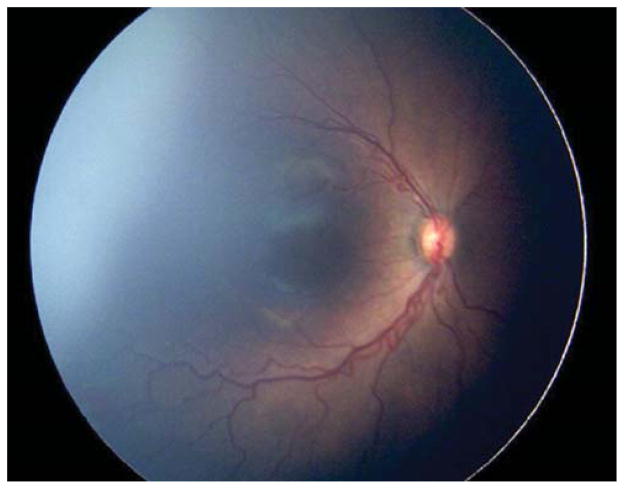
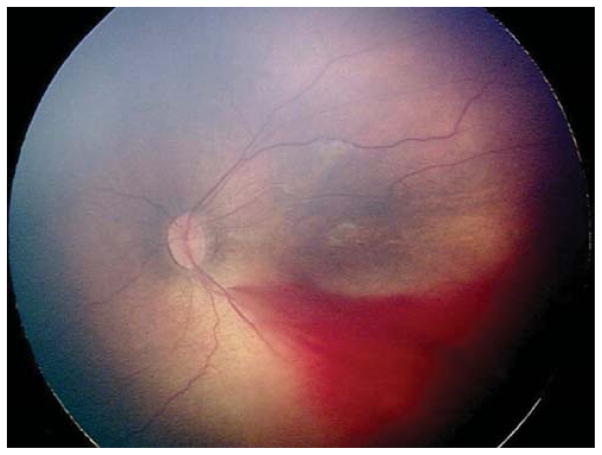
Three eyes (23.1%) were treated for thick stage 3 membranes with anteroposterior traction concerning for progression to stage 4 ROP. Case 6, for example, was born at 26 weeks gestational age, with birth weight 950 grams. At 42 weeks postmenstrual age, examination revealed zone II, stage 3, no plus disease with a thick stage 3 fibrovascular membrane OS causing significant anteroposterior traction concerning for progression to stage 4, as well as vitreous hemorrhage (Figure 3). Due to these concerning structural changes, as well as concern for further vitreous hemorrhage limiting visualization and/or treatment in the future (see below), the option of laser versus observation OS was discussed with the patient’s parents. Informed consent was obtained, and laser photocoagulation was performed OS.
Figure 3. Representative color fundus photograph of a patient (Case 6) treated for retinopathy of prematurity milder than Type 1 disease due to concerning structural change of thick stage 3 membrane with anteroposterior traction concerning for progression to stage 4 retinopathy of prematurity, as well as presence of vitreous hemorrhage.
Fundus photos of the left eye (left: posterior, right: temporal) reveal zone II, stage 3 disease with absence of plus disease. There is preretinal and vitreous hemorrhage posteriorly and temporally, as well as thick fibrovascular stage 3 with marked anteroposterior traction.
Persistent active ROP at an advanced postmenstrual age
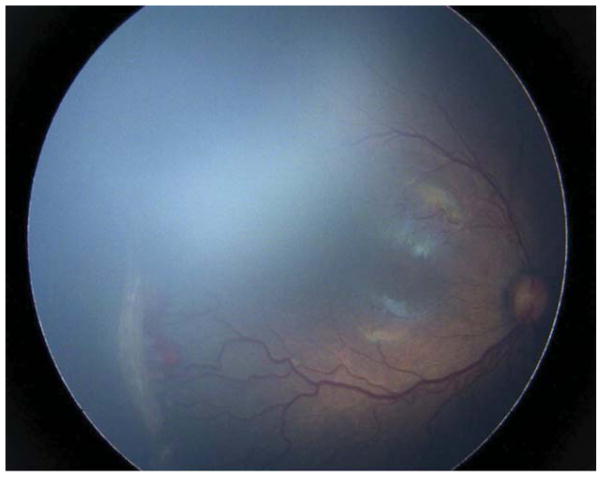
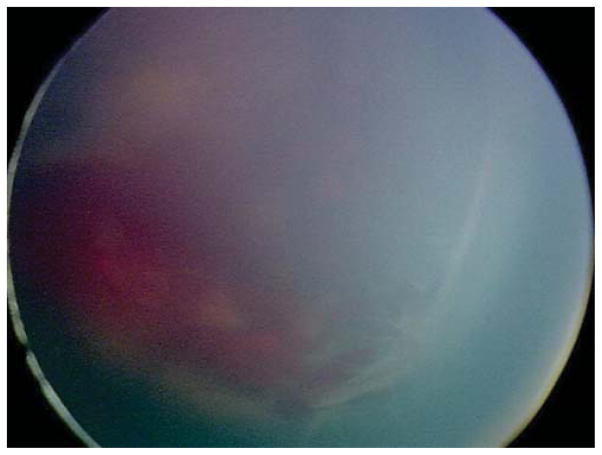
Four (30.8%) of the 13 eyes were treated for persistent active ROP at an advanced postmenstrual age. Of these, 3 (23.1%) were noted to have persistent active stage 3 ROP at a postmenstrual age >41 weeks. Case 7 was born at 25 weeks gestational age, with birth weight 840 grams. At 41 weeks postmenstrual age, the examination revealed zone II, stage 3 disease without plus OU (Figure 4). There was concern for the degree of active stage 3 ROP given the patient’s advanced postmenstrual age. Moreover, concerning structural changes were noted (as described above) including temporal vessel straightening and a thick neovascular ridge with significant anteroposterior vitreoretinal traction concerning for progression to stage 4 disease. Thus, the option of laser versus close observation was discussed with the parents. Informed consent was obtained, and laser photocoagulation was administered OU.
Figure 4. Representative color fundus photograph of a patient (Case 7) treated for retinopathy of prematurity milder than Type 1 disease due to concerning structural changes including tangential traction and temporal vessel straightening and thick stage 3 membrane with anteroposterior traction concerning for progression to stage 4 retinopathy of prematurity, as well stage 3 retinopathy of prematurity too active for his advanced postmenstrual age.
Fundus photos of the right eye (left: posterior, right: temporal) reveal zone II, stage 3 retinopathy of prematurity without evidence of plus disease. There was temporal vessel straightening as well as thick fibrovascular stage 3 with anteroposterior traction.
Case 9 was born at 28 weeks gestational age weighting 1077 grams. At initial presentation at 5 months of age, examination revealed stage 5 ROP OD and zone II, stage 2 disease without plus OS. The patient underwent vitrectomy OD, but vision subsequently reached no light perception OD. At 12 months of age, there was persistent ROP with zone III, stage 1 ROP without plus disease OS and peripheral ischemia on fluorescein angiography. Given persistent ROP at such a late stage in this monocular patient with history of stage 5 ROP in the fellow eye, the option of laser versus close observation OS was discussed with the parents. Informed consent was obtained, and laser photocoagulation was applied OS.
Vitreous hemorrhage
Three (23.1%) of the 13 eyes were treated because of vitreous hemorrhage. Of note, each of these eyes also demonstrated other concerning structural features that also contributed to the decision to treat. As detailed above, one eye (Case 6) also had thick stage 3 OS with anteroposterior traction concerning for progression to stage 4 (Figure 3), while 2 eyes (Case 5) also exhibited temporal vessel dragging and tangential traction on the retina.
Comparison of clinical diagnosis to “reference standard” diagnosis
Fundus photographs were available for 8 of 13 eyes that underwent treatment for clinical diagnosis of ROP milder than Type 1 ROP. The “reference standard” diagnosis of all 8 eyes matched the clinical diagnosis with regard to overall disease category (Type 1, Type 2, or mild ROP). The “reference standard” diagnosis eyes matched the clinician’s diagnosis for zone, stage, and presence of plus disease in 7 of 8 eyes (Table).
Discussion
Based on the ET-ROP study, published consensus guidelines currently recommend treatment for Type 1 ROP.1–4,9 However, it is our observation that ROP experts occasionally treat ROP in cases where the clinical diagnosis is milder than Type 1 ROP. To our knowledge, this is the first study to examine retinopathy of prematurity practice patterns by experts compared to these guidelines. The key findings of this study are: (1) In 9.5% of treated eyes in this study, experts recommended treatment for disease less than Type 1 ROP and (2) Underlying reasons included structural changes prompting concern about future anatomic complications (e.g. retinal traction), active ROP disease at an advanced postmenstrual age, logistical and systemic concerns (e.g. treatment in the fellow eye), and diagnostic concerns (e.g. vitreous hemorrhage).
The first key finding is that, from a retrospective review of a prospectively collected database of all patients screened for ROP at any of 6 major ROP centers in the United States, 9.5% of eyes that underwent treatment were treated despite a clinical diagnosis of ROP milder than Type 1 ROP. Treating clinicians in this study were all pediatric ophthalmologists or retinal specialists with extensive experience in ROP. Two of the clinicians had participated as certified ET-ROP study investigators, and 5 have each previously published >10 peer-reviewed journal papers related to ROP and speak regularly at national or international meetings. The 13 eyes treated for ROP milder than Type 1 ROP were from 4 of the 6 centers included in the database, demonstrating that this practice is not isolated to a single clinician or center. Of note, the cases of treatment for ROP milder than Type 1 disease in this study were not derived from a consensus but rather, reflect the management decisions of individual clinicians from a multicenter consortium of experts. Overall, however, these findings have implications for the importance of individual clinical judgment and for potential limitations of evidence-based guidelines.
The second key finding characterizes underlying reasons for treatment of ROP less than Type 1, and demonstrates that structural changes concerning for future anatomic complications, present in 69.1% of cases, were the most frequent indication for treatment. These structural changes included temporal vessel straightening and tangential traction concerning for progression to macular distortion or ectopia or presence of thick stage 3 membranes with anteroposterior traction concerning for progression to stage 4 ROP. Macular dragging has been associated with poor vision in ROP patients treated with either laser or cryotherapy.11–15 In the ET-ROP study, for example, on 6 year follow-up, only 13.6% of patients with macular ectopia and absence of unfavorable structural outcomes achieved best corrected vision of 20/40 or better, as compared to 40.7% of patients with normal retinal structure in zone I and the near periphery.14 Similar results were noted after the multicenter trial of cryotherapy for retinopathy of prematurity (CRYO-ROP) study.15 Whether treatment can reverse the progression of temporal vessel straightening to macular ectopia, however, remains to be demonstrated. In the CRYO-ROP study, on 10-year follow-up, the rate of vessel straightening and macular ectopia were similar between treated and control eyes.15 In the ET-ROP study, earlier laser treatment resulted in lower rates of temporal vessel straightening and macular ectopia than conventional threshold treatment, though this did not achieve statistical significance.2 In the absence of evidence to the contrary, it is also possible that laser therapy can worsen structural or visual outcomes in patients with temporal vessel straightening, although none of the cases in this study progressed after laser. Further studies are necessary to parse out the safety and efficacy, if any, of laser therapy for cases with temporal vessel straightening and risk of macular dragging.
Logistics and systemic concerns, accounting for 15.4% of eyes treated for ROP milder than Type 1 ROP in this study, prompted treatment for mild ROP when the contralateral eye had progressed to Type 1 ROP. Studies have previously demonstrated a high degree of symmetry between eyes of neonates with acute ROP.16–17 Given the risk of progression of the milder eye to Type 1 ROP when the fellow eye has already reached Type 1 ROP and the logistical challenges and systemic risks of multiple procedures under general anesthesia in premature neonates, these mild ROP eyes were treated at the same time that the fellow eye was undergoing laser for Type 1 ROP. In this study, only 2 of 70 cases had asymmetric disease such that one eye reached Type 1 ROP while the older remained milder, and both of these eyes were treated bilaterally. However, the sample size of this subgroup in our study is small. Further studies would be necessary to determine whether clinicians generally treat such cases unilaterally or bilaterally, as well as to determine the likelihood that the milder eye of such asymmetric cases will, indeed, eventually reach Type 1 ROP. Pending these studies, it is reasonable to treat bilaterally as was done in the two cases in this study, or to wait until the milder eye reaches Type 1 ROP before lasering it at a second session.
In a study reviewing 12 malpractice claims for ROP filed with the Ophthalmic Mutual Insurance Company between 1987 and 2009, 4 cases (25%) involved delay in follow-up involving poor compliance. 8 This appears to be a significant practical issue for ophthalmologists, although the current study found no cases treated for ROP milder than Type 1 ROP due to concerns about compliance with outpatient follow-up visits in the setting of borderline disease.
Although diagnostic challenges in the form of vitreous and preretinal hemorrhage were noted in 23.1% of eyes treated for ROP milder than Type 1 ROP, all of these eyes exhibited additional concerning structural features (described above) contributing to the decision to treat. Since no eye was treated for vitreous hemorrhage alone, it is unclear whether vitreous hemorrhage alone may be a factor prompting experts to treat for a clinical diagnosis milder than Type 1 ROP. In principle, in some cases, clinicians might consider treating infants with less than Type 1 ROP with significant vitreous hemorrhage while the view permits due to a concern that further hemorrhage would limit the ability to monitor for disease progression, contribute to vitreoretinal traction, or limit future treatment options. Intravitreal anti-VEGF agents are a potential treatment for active disease in this setting, as unlike laser, they do not require clear media.18–19
In this study, there were no cases in which anti-VEGF agents were used for treatment of ROP milder than Type 1 ROP. This may reflect the treatment pattern for anti-VEGF agents – often for zone I or posterior zone II disease or for aggressive posterior ROP (AP-ROP).20–21 This is partly by design, as AP-ROP cases by definition generally fall into the Type 1 ROP category. Additionally, none of the patients treated in this study outside of criteria exhibited zone I disease, and thus anti-VEGF agents may not typically have been considered. Additionally, given the absence of long-term data on the effect of anti-VEGF agents on ocular outcomes and overall development of the child,21 clinicians may have been reluctant to offer off-label anti-VEGF therapy for patients with ROP milder than that recommended in consensus guidelines. Nevertheless, whether the ever-growing role for anti-VEGF therapy in ROP might impact the decision of whether or not to apply laser in cases of ROP milder than Type 1 disease – for example, eyes with vitreous hemorrhage—remains to be shown.
Several eyes (30.8%) were treated due to active ROP disease at an advanced postmenstrual age. Case 8 exhibited active stage 3 that was persistent at 43 weeks gestational age, and Case 7 (2 eyes) exhibited multiple indication for treatment including concerning structural changes (thick stage 3 membrane with anteroposterior traction, as well as temporal vessel straightening) and the extent of disease was felt to be more active than the clinician felt was acceptable, at 41 weeks. The last such case (Case 9) was atypical both for ROP and for this series, with treatment at 12 months of age due to persistent Zone II, stage 3 disease, thus raising the possibility that the diagnosis was not ROP. There are no images available for this case to retrospectively review the diagnosis.
Gestational age at treatment was 38 to 47 weeks (average 41.4 weeks, excluding Case 9 treated at 12 months of age) in this study, somewhat later than the average age of ROP treatment (35–37 weeks). The reason for this is unclear. All of the cases treated for ROP milder than Type 1 disease were screened by the same clinicians who administered therapy, and thus there were no evident transfer-related delays in care. It is possible that the clinical findings that prompted therapy, especially structural changes, may not occur until an older gestational age. Alternatively, the presence of active ROP at a later gestational age may have contributed to a decision to treat in more eyes, although only 4 eyes’ clinical notes clearly indicated this as an indication for therapy (see limitation 4 below). Alternatively, it is possible that some of the eyes had progressed to Type 1 ROP at the earlier time point and then regressed.
Taken together, these study findings raise important questions regarding the role of individual clinical judgment in scenarios that are not precisely covered by previously published treatment guidelines. This limitation has been well recognized historically and was described by Sacket et al. in an editorial in the British Medical Journal: “Without clinical expertise, practice risks becoming tyrannized by evidence, for even excellent external evidence may be inapplicable to or inappropriate for an individual patient.”22 For example, the international classification of ROP, which has been the basis of multi-center clinical trials, does not incorporate any of the concerning structural or diagnostic features that prompted treatment for ROP milder than Type 1 ROP in this study.23–24 Similarly, published ROP management guidelines do not describe common real-world management concerns such as treatment of the fellow eye to modulate anesthesia risks or because of concerns about outpatient follow-up.8–9 The absence of large studies involving treatment of select cases of ROP milder than Type 1 disease such as those reported in this study suggest that there is a role for clinical judgment in situations that were previously untested in the literature. Moreover, this study suggests several features of mild or Type 2 ROP that might warrant further studies regarding natural history, future outcomes, and a potential role for expanded treatment indications.
This study may also have implications for medicolegal issues related to ROP care. A survey by the American Academy of Ophthalmology found that 67% of pediatric ophthalmologists and retina specialists who had stopped providing ROP care in the preceding decade cited medicolegal liability as the primary reason.7 Indeed, a review of national ophthalmology malpractice claims showed disproportionately high numbers of claims and indemnity payments for cases involving ROP.8 Studies reviewing ROP malpractice claims data recommend adherence to consensus ROP screening and treatment guidelines as a means to reduce liability.6,8 Yet, experts in this study frequently recommended treatment in eyes with disease milder than the Type 1 ROP in efforts to achieve the best outcome in individual clinical settings. This suggests the need for individual clinician judgment in ROP scenarios previously untested in the literature.
This study several limitations. (1) Clinical diagnosis in ROP is characteristically subjective, and prior studies have documented the lack of agreement between experts on ROP diagnosis.10,25 This raises the question of whether the clinical diagnoses evaluated in this study were “correct.” We have previously reported a method of incorporating image review by 3 experienced ROP image graders with the clinical diagnosis and arbitration to determine a “reference standard” diagnosis for ROP cases.10 Contemporaneous fundus photographs were available for 8 of the 13 eyes treated for ROP milder than Type 1 disease, and expert review of all of those photographs resulted in a “reference standard” diagnosis that matched the clinical diagnosis applied in this study. (2) Because of potential subjectivity in critical areas of ROP classification such as presence of plus disease or zone I disease,25,26 it is possible that clinicians were biased toward diagnosing borderline cases as being more severe in situations where they felt treatment was warranted. This would result in an underestimation of the true rate of treatment for ROP milder than Type 1 ROP. As fundus photographs were not available for a significant subset of all patients treated for ROP, we were unable to systematically review all treated eyes to confirm diagnosis of Type 1 ROP using the “reference standard” diagnosis. (3) Because a single examination, either clinically or photographically, does not provide a full understanding of how the patient is doing, especially with regard to the tempo or progression of the disease, we reviewed all clinical notes prior to the time of treatment for these patients to attempt to best determine the clinical features that prompted treatment in these atypical cases. However, this was a retrospective study, and thus, it is possible that additional clinical findings and other clinical or logistical nuances, such as tempo, progression, or lack of regression, may have influenced the decision to treat but were not incorporated into the documentation and therefore not captured in this study. (4) This study does not describe consensus opinion of experts regarding treatment of cases milder than Type 1 ROP, but rather describes the management decisions of individual clinicians from a multicenter consortium of experts. (5) This study was not designed to describe the long-term structural and visual outcomes of infants. All infants treated in this study for less than Type 1 disease had good structural outcomes, and further studies involving longer-term structural and visual outcomes for treatment of these cases of Type 2 or mild ROP may be important.
In summary, this study found that experts occasionally recommended ROP treatment in eyes with disease milder than Type 1, and we identified common indications for treatment such as structural features concerning for future anatomic complications, concerns about logistics and systemic care, diagnostic concerns, and active ROP disease at an advanced postmenstrual age. This has important implications for clinical reasons and more broadly for the application of individual clinical judgment in scenarios that are not precisely covered by previously published treatment guidelines.
Acknowledgments
Funding/Support: Michael F. Chiang are Susan Ostmo are supported by NIH grant EY19474 from the National Institutes of Health, Bethesda, MD. Michael F. Chiang, R.V. Paul Chan, Susan Ostmo, Karyn Jonas, and Mrinali Patel Gupta are supported by unrestricted departmental funding from Research to Prevent Blindness, New York, NY. R.V. Paul Chan was supported (at the time of this study) by the St. Giles Foundation, New York, NY. The following authors reports no funding/support for this study: Rachelle Anzures.
Acknowledgments such as Statisticians, Medical Writers, Expert contributions. There are no such additional acknowledgements.
Biographies

Mrinali Gupta, MD, is Assistant Professor of Ophthalmology in the Vitreoretinal Surgery Service at Weill Cornell Medical College. She graduated from Duke University School of Medicine and completed Ophthalmology Residency at Harvard Medical School/Mass Eye & Ear Infirmary. As a Vitreoretinal Surgery Fellow at Cornell, she was also a recipient of the Heed Fellowship and the Ronald G. Michels Fellowship Foundation Award. Mrinali is actively engaged in clinical and basic science research in retinal disease.

Michael F. Chiang is Knowles Professor of Ophthalmology & Medical Informatics at the Oregon Health & Science University (OHSU) Casey Eye Institute. He is a pediatric ophthalmologist, and leads an NIH-funded research group that studies telemedicine, image analysis, and biomedical informatics. He leads an NIH-funded visual science training program at OHSU. He serves on the Editorial Boards for Ophthalmology and JAAPOS, and is an Associate Editor for the Journal of the American Medical Informatics Association.
APPENDIX
This work is being published on behalf of the i-ROP (Imaging & Informatics in ROP research consortium) study group:
Oregon Health & Science University (Portland, OR): Michael F. Chiang, MD (Principal Investigator), Susan Ostmo, MS, Kemal Sonmez, PhD, J. Peter Campbell, MD, MPH. Weill Cornell Medical College (New York, NY): RV Paul Chan, MD, Karyn Jonas, RN. Columbia University (New York, NY): Jason Horowitz, MD, Osode Coki, RN, Cheryl-Ann Eccles, RN, Leora Sarna, RN. Bascom Palmer Eye Institute (Miami, FL): Audina Berrocal, MD, Catherin Negron, BA. William Beaumont Hospital (Royal Oak, MI): Kimberly Denser, MD, Kristi Cumming, RN, Tammy Osentoski, RN, Tammy Check, RN. Children’s Hospital Los Angeles (Los Angeles, CA): Thomas Lee, MD, Evan Kruger, BA, Kathryn McGovern, MPH. Cedars Sinai Hospital (Los Angeles, CA): Charles Simmons, MD, Raghu Murthy, MD, Sharon Galvis, NNP. LA Biomedical Research Institute (Los Angeles, CA): Jerome Rotter, MD, Ida Chen, PhD, Xiaohui Li, MD, Kaye Roll, RN. Massachusetts General Hospital (Boston, MA): Jayashree Kalpathy-Cramer, PhD. Northeastern University (Boston, MA): Deniz Erdogmus, PhD. Asociacion para Evitar la Ceguera en Mexico (APEC) (Mexico City): Maria Ana Martinez-Castellanos, MD, Samantha Salinas-Longoria, MD, Rafael Romero, MD, Andrea Arriola, MD.
Footnotes
Financial Disclosures: Michael F. Chiang is an unpaid member of the Scientific Advisory Board for Clarity Medical Systems (Pleasanton, CA). R.V. Paul Chan is a paid consultant for Allergan and Alcon. Rachelle Anzures received an honorarium for participation in the UNRAVEL study by Novartis. The following authors report no financial disclosures nor conflicts of interest in medicine: Mrinali Patel Gupta, Susan Ostmo, and Karyn Jonas.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Good WV Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233–250. [PMC free article] [PubMed] [Google Scholar]
- 2.The Early Treatment for Retinopathy of Prematurity Cooperative Group. Good WV, Hardy RJ, et al. Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol. 2010;128(6):663–671. doi: 10.1001/archophthalmol.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity. Arch Ophthalmol. 2003;121(12):1684–1696. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 4.Hardy RJ, Good WV, Dobson V, et al. Multicenter trial of early treatment for retinopathy of prematurity: study design. Control Clin Trials. 2004;25(3):311–325. doi: 10.1016/j.cct.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK. Childhood blindness. J AAPOS. 1999;3(1):26–32. doi: 10.1016/s1091-8531(99)70091-1. [DOI] [PubMed] [Google Scholar]
- 6.Mills MD. Retinopathy of prematurity malpractice claims. Arch Ophthalmol. 2009;127(6):803–804. doi: 10.1001/archophthalmol.2009.117. [DOI] [PubMed] [Google Scholar]
- 7.Altersitz K, Piechocki M. Survey: physicians being driven away from ROP treatment. [Accessed December 3, 2015];Ocular Surgery News. 2006 Aug 15; Available at: http://www.healio.com/ophthalmology/retina-vitreous/news/print/ocular-surgery-news/%7Bedfa784c-a2ac-4f93-b473-5be5d07d82ca%7D/survey-physicians-being-driven-away-from-rop-treatment.
- 8.Dey S, Menke AM, Abbott RL. Retinopathy of prematurity malpractice claims: the Ophthalmic Mutual Insurance Company Experience. Arch Ophthalmol. 2009;127(6):794–798. doi: 10.1001/archophthalmol.2009.97. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Pediatrics, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, and American Association of Certified Orthoptists. Policy statement: Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131(1):189–195. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 10.Ryan MC, Ostmo S, Jonas K, et al. Development and evaluation of reference standards for image-based telemedicine diagnosis and clinical research studies in ophthalmology. AMIA Annu Symp Proc. 2014;2014:1902–1910. [PMC free article] [PubMed] [Google Scholar]
- 11.Ng EY, Connolly BP, McNamara JA, Regillo CD, Vander JF, Tasman W. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years: part 1. Visual function and structural outcome. Ophthalmology. 2002;109(5):928–935. doi: 10.1016/s0161-6420(01)01017-x. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds J, Dobson V, Quinn GE, et al. Prediction of visual function in eyes with mild to moderate posterior pole residual of retinopathy of prematurity - Cryotherapy for Retinopathy of Prematurity Collaborative Group. Arch Ophthalmol. 1993;111(8):1050–1056. doi: 10.1001/archopht.1993.01090080046017. [DOI] [PubMed] [Google Scholar]
- 13.Soong GP, Shapiro M, Seiple W, Szlyk JP. Macular structure and vision of patients with macular heteropia secondary to retinopathy of prematurity. Retina. 2008;28(8):1111–1116. doi: 10.1097/IAE.0b013e3181744136. [DOI] [PubMed] [Google Scholar]
- 14.Wallace DK, Bremer DL, Good WV, et al. Correlation of recognition visual acuity with posterior retinal structure in advanced retinopathy of prematurity. Arch Ophthalmol. 2012;130(12):1512–1516. doi: 10.1001/archophthalmol.2012.2118. [DOI] [PubMed] [Google Scholar]
- 15.Dobson V, Quinn GE, Summers CG, Hardy RJ, Tung B Cryotherapy for Retinopathy of Prematurity Cooperative Group. Visual acuity at 10 years in cryotherapy for retinopathy of prematurity (CRYO-ROP) study eyes:effect of retinal residua on retinopathy of prematurity. Arch Ophthalmol. 2006;124(2):199–202. doi: 10.1001/archopht.124.2.199. [DOI] [PubMed] [Google Scholar]
- 16.Quinn GE, Dobson V, Biglan A, Evans J, Plotsky D, Hardy RJ. Correlation of retinopathy of prematurity in fellow eyes in the cryotherapy for retinopathy of prematurity study. Arch Ophthalmol. 1995;113(4):469–473. doi: 10.1001/archopht.1995.01100040089032. [DOI] [PubMed] [Google Scholar]
- 17.Fielder AR, Shaw DE, Robinson J, Ng YK. Natural history of retinopathy of prematurity: a prospective study. Eye. 1992;6(3):233–242. doi: 10.1038/eye.1992.46. [DOI] [PubMed] [Google Scholar]
- 18.Mintz-Hittner HA, Kennedy KA, Chuang AZ BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. New England Journal of Medicine. 2011;364(7):604–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law JC, Recchia FM, Morrison DG, Donahue SP, Estes RL. Intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. J AAPOS. 2010;14(1):6–10. doi: 10.1016/j.jaapos.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Mintz-Hitner HA, Kenna KA, Chuang AZ Beat-ROP Cooperative Group. N Engl J Med. 2011;364(7):603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds JD. Bevacizumab for retinopathy of prematurity. N Engl J Med. 2011;364(7):677–678. doi: 10.1056/NEJMe1100248. [DOI] [PubMed] [Google Scholar]
- 22.Sackett DJ, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71–72. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Committee for the Classification of Retinopathy of Prematurity. An International Classification of Retinopathy of Prematurity. Arch Ophthalmol. 1984;102:1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 24.Committee for the Classification of Retinopathy of Prematurity. International Classification of Retinopathy of Prematurity Revisited. Arch Ophthalmol. 2005;123(7):991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 25.Chiang MF, Jiang L, Gelman R, Du YE, Flynn JT. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol. 2007;125(7):875–880. doi: 10.1001/archopht.125.7.875. [DOI] [PubMed] [Google Scholar]
- 26.Chiang MF, Thyparampil PJ, Rabinowitz D. Interexpert agreement in the identification of macular location in infants at risk for retinopathy of prematurity. Arch Ophthalmol. 2010;128(9):1153–1159. doi: 10.1001/archophthalmol.2010.199. [DOI] [PubMed] [Google Scholar]