Abstract
Significantly simplified work flows were developed for rapid analysis of various types of cosmetic and foodstuff samples by employing a miniature mass spectrometry system and ambient ionization methods. A desktop Mini 12 ion trap mass spectrometer was coupled with paper spray ionization, extraction spray ionization and slug-flow microextraction for direct analysis of Sudan Reds, parabens, antibiotics, steroids, bisphenol and plasticizer from raw samples with complex matrices. Limits of detection as low as 5 μg/kg were obtained for target analytes. On-line derivatization was also implemented for analysis of steroid in cosmetics. The developed methods provide potential analytical possibility for outside-the-lab screening of cosmetics and foodstuff products for the presence of illegal substances.
Keywords: Ambient ionization, Slug-flow microextraction, Paper Spray, Extraction Spray, Miniature ion trap mass spectrometer, Cosmetics, Foodstuffs, Steroids, Sudan Red, Parabens, Plasticizer
Graphical Abstract
INTRODUCTION
In this study, we developed methods using a miniature mass spectrometer and ambient ionization for direct analysis of prohibited substances in food and cosmetic products. Food safety has been drawing public attention due to its relevance to the public health. Although restrictive regulations are enforced worldwide, highly publicized incidents occur from time to time [1, 2]. Safety of cosmetic products typically is not getting much public attention, but it is also of a significant concern for public health, simply because of the wide and routine use of these products. Cosmetics cover a wide range of materials that are applied in contact with the human body for cleansing purposes or for altering appearance. Cosmetic products worldwide play an important role in people’s daily lives. In addition to the use by adults, cosmetics have also been increasingly used in the care of infants and toddlers. The cosmetic industry represents a tremendous global market, with total sales of about 168 billion Euros in 2014 in the European Union, the United States, China, and Japan [3].
Voluntary addition of illicit substances to lower the costs of production is the most common problem in both the food and cosmetics industry [4–9]. For cosmetic products, illicit substances are also added to enhance short-term cosmetic effectiveness. Common banned additives include antibiotics [10, 11], corticosteroids [12–15], sexual hormones (oestrogens [16], progestogens [16], androgen [17]), pharmacologically active substances [16–18], prohibited preservatives (parabens [19–22], methyldibromoglutaronitrile [23]), whitening agents [13, 24], phthalates [25–28], and nitromusk fragrances [25, 28]. Long-term exposure to these substances could cause adverse effects such as skin irritation, allergic reactions, and antibiotic resistance, which represents a severe risk to public health.
The need to enforce product safety and regulatory compliance in both the food and cosmetics industries, calls for the development of effective and convenient methods to identify illicit ingredients with high molecular specificity and sensitivity. The analytical techniques that have been reported for the chemical analysis of foods and cosmetic products include thin layer chromatography [29, 30], capillary electrophoresis [22, 31], gas chromatography (GC) with flame ionization detection [21, 32] or coupled with various types of mass spectrometers [19, 20, 23, 25, 26, 28, 33–36], high-performance liquid chromatography (HPLC) using ultraviolet [10, 12, 16, 17, 24, 37], electrochemical detection [38] or coupled with various types of mass spectrometers [1, 11–17, 26, 35, 39]. These methods are typically implemented in analytical laboratories and performed by experienced chemists using bench-top equipment. Sample preparation is usually achieved through multi-step, laborious and time-consuming processes, which also require laboratories procedures such as solvent extraction, dilution, reagent mixing, sonication, heating, centrifugation, and filtration. Although these analytical processes work well for analysis of large numbers of samples at centralized locations, it is also highly desirable to develop fast and easy-to-use methods for on-site screening, especially for situations when rapid decision making is required by inspectors in the field [40, 41].
As already demonstrated, MS is a highly sensitive and selective technology which is suitable for both qualitative and quantitative chemical analysis. Conventional laboratory-scale mass spectrometers are bulky and typically used in combination with GC or HPLC, which limits their usage for in-field applications. As opposed to traditional chemical analysis work flow, where samples are brought to the laboratory for analysis, miniaturized mass spectrometers can now be brought to the samples in the field [42, 43]. A wide variety of small mass spectrometers have been developed, with a weight as low as 4 kg [44]. However, not all of them are suitable for analysis of food or cosmetic products, for which a majority of the target analytes are non-volatile. The miniature MS instruments with internal ionization sources, which relies on sample introduction through GC, [45] membrane, [46] solid-phase microextraction (SPME) [45, 47] or sorbents, [48] typically can only analyze non- or semi-volatile compounds. To enable the coupling with in-air ionization methods, such as electrospray ionization that is suitable for ionizing non-volatile compounds, atmospheric pressure interface (API) is required for transferring the ions into the mass analyzer under vacuum. Small mass spectrometers with APIs have been developed, among which the Mini 10/11/12 series of instruments [44, 49, 50] used the discontinuous APIs [51] to achieve the ion transfer without requiring additional pumping capacity. The miniature ion trap instruments also have an advantage of performing MS/MS analysis, which provides additional confirmation of the chemical identity and improved sensitivity for analysis of complex mixtures. [42, 52]
Sample pretreatment is typically required prior to MS analysis, which would also need to be done quickly in the field to minimize the matrix effects. Ambient ionization has been developed for direct MS analysis of the analytes in untreated samples, and this represents a promising solution for simplification or elimination of sample preparation procedures in on-site analysis [53]. Since desorption electrospray ionization (DESI) [54] and direct analysis in real time (DART) [55] were reported in 2004 and 2005, respectively, more than 40 ambient ionization methods have been developed [53, 56]. Sample pretreatment and chromatographic separation, traditionally required for MS-based analysis, can now be bypassed. Notably, a set of ambient ionization methods, e.g., paper spray [57, 58], extraction spray [59], or low temperature plasma [60], have been coupled with miniature mass spectrometers, with promising results obtained for on-site applications in food safety [61, 62], product authentication [44], environmental monitoring [48, 63, 64], biomolecule analysis [65, 66], homeland security [67]and biomedical diagnosis as well as in forensic investigations [68].
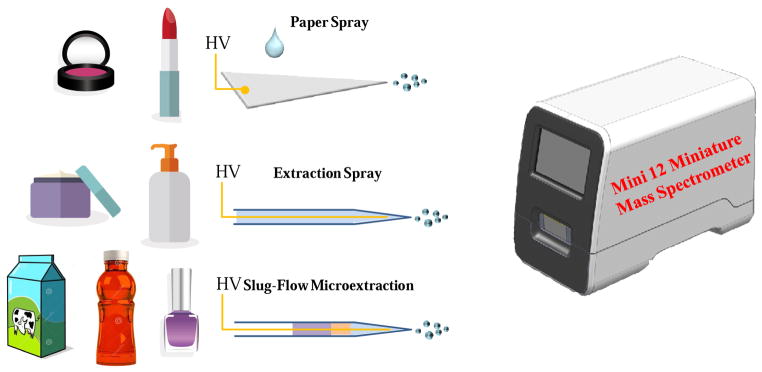
In this study, direct identification of illicit ingredients in food and cosmetic products has been explored by coupling a miniature ion trap mass spectrometry system with ambient ionization methods (Figure 1). Versatile procedures using paper spray [57, 58], extraction spray [59, 69], and slug-flow microextraction [70] were developed for direct analysis of a wide variety of cosmetic products. In comparison with traditional methods requiring sample pretreatment and separation steps, the methods reported here enable a research to identify illicit ingredients in cosmetics with significantly improved throughput.
Figure 1.
Paper spray ionization, extraction spray ionization, slug-flow microextraction, and Mini 12 desktop ion trap mass spectrometer for the rapid identification of prohibited substances in cosmetics, milk, and beverage samples
EXPERIMENTAL
Chemicals and reagents
Sudan Red I (1), Sudan Red II (2), Sudan Red III (3), Sudan Red IV (4), phenylparaben (8), chloramphenicol (10), metronidazole (11), and bis(2-ethylhexyl)phthalate(14) were purchased from Sigma-Aldrich (St. Louis, MO, USA); isopropylparaben (5), isobutylparaben (6), benzylparaben (9), and bisphenol A (13)were purchased from AccuStandard (New Haven, CT, USA); pentylparaben (7) was purchased from Alfa Aesar (Ward Hill, MA, USA); epitestosterone (12) was purchased from Steraloids (Newport, RI, USA). All reference standards had purities greater than 96%, except for Sudan Red II and IV (both 90%). The chemical information for the analytes is listed in Table S1. Ethanol, dichloromethane, methanol, and ethyl acetate of HPLC grade were purchased from Merck (Darmstadt, Germany). Ultrapure water was obtained from a Millipore Milli-Q integral water purification system (Bedford, MA, USA). Other chemicals used in the experiment were purchased from Sigma-Aldrich (St. Louis, MO, USA). The Grade 1 Chr (0.18 mm thickness), Grade 3MM Chr (0.34 mm thickness), Grade 4 Chr (0.21 mm thickness), Grade 31ET Chr (0.50 mm thickness) cellulose chromatography papers and the Grade SG81 (0.27 mm thickness) ion exchange paper were purchased from Whatman (Piscataway, NJ, USA) and used without further chemical treatment to prepare sample substrates for paper spray ionization. Stock standard solutions were prepared by dissolving each analyte with solvents. Sudan Red I, II, III and IV were dissolved in dichloromethane:ethanol (40:60); all other reference standards were dissolved in methanol. A variety of untreated cosmetic samples of different categories were collected from local stores, including the face powder (L.A. COLORS® Mineral Blush), lipstick (L.A. COLORS® Purely Matte Lipstick), cream (April Bath & Shower Cold Cream), shampoo (PANTENE® Pro-V Classic All Hair Types Shampoo), and lotion (LANCOME® Redefining Lifting Beauty Lotion). Stock solutions of the analytes were further diluted and spiked into 0.1 g of raw cosmetic samples, which were weighed into 2.0-mL Eppendorf Safe-Lock tubes. The mixtures were vortex mixed thoroughly and let dry overnight for subsequent analysis.
Instrumentation
In this study, rapid analyses for cosmetic and food samples were carried out using a Mini 12 desktop miniature mass spectrometry system [50]. The integrated Mini 12 system had a weight of 25 kg, an outer dimension of 19.6×22.1×16.5 in., and consumed less than 100 W of power. The pumping system consisted of a two-stage diaphragm roughing pump (KNF Neuberger, Trenton, NJ, USA) and a HiPace 10 turbomolecular pump (Pfeiffer Vacuum, Nashua, NH, USA). The manifold pressure could be pumped down to below 1×10−5 Torr. Ions from the ambient ionization source were drawn in a pulsed fashion through the inlet capillary of the discontinuous atmospheric pressure interface (DAPI) [49, 51] into a rectilinear ion trap (RIT) located inside the vacuum manifold. For each scan, the DAPI was opened briefly for about 15 ms for ion introduction and closed during the rest of the time in each scan cycle. Due to the special mode of ion introduction using the DAPI, residual air was used as the collision gas instead of helium, which is typically used for commercial lab-scale ion trap instruments. The portability of the miniature MS system, however, was improved by eliminating the need for helium gas cylinders. Mass analysis was performed in a mass-selective instability scan mode to generate mass spectra using an rf of 1 MHz. A supplementary dipolar AC excitation at 350 kHz with its amplitude being ramped with the rf scan was applied to achieve resonance ejection. The frequency and the amplitude of the excitation signal used for CID are shown in Table 2. A scan speed of 10,000 m/z per second was used. The user interface for instrument control and data acquisition was developed in-house.
Table 2.
Source voltage, SWIFT notch, CID AC voltage and AC frequency for analytes
| Compound | Source voltage (kV) | SWIFT notch (kHz) | CID AC voltage (V) | AC frequency (kHz) |
|---|---|---|---|---|
| Sudan Red I | 4.0 | 232–240 | 0.42 | 124.8 |
| Sudan Red II | 4.0 | 204–212 | 0.36 | 111.7 |
| Sudan Red III | 4.0 | 155–163 | 0.72 | 87.0 |
| Sudan Red IV | 4.0 | 142–150 | 0.72 | 80.4 |
| Isopropylparaben | 2.5 | 382–390 | 0.65 | 178.2 |
| Isobutylparaben | 2.5 | 332–340 | 0.62 | 164.0 |
| Pentylparaben | 2.5 | 297–305 | 0.65 | 152.0 |
| Phenylparaben | 2.5 | 285–293 | 0.75 | 147.4 |
| Benzylparaben | 2.5 | 262–270 | 0.55 | 137.7 |
| Chloramphenicol | 2.5 | 171–179 | 0.46 | 95.6 |
| Metronidazole | 2.5 | 442–450 | 0.42 | 188.8 |
| Epitestosterone | 2.5 | 183–191 | 0.68 | 101.4 |
| 3-Oxime-epitestosterone | 2.5 | 194–202 | 0.68 | 106.9 |
| Bisphenol A | 2.5 | 262–270 | 0.72 | 137.7 |
| Bis(2-ethylhexyl)phthalate | 2.5 | 138–146 | 0.65 | 78.1 |
RESULTS AND DISCUSSION
Analysis of Powders and Lipstick Samples using Paper Spray
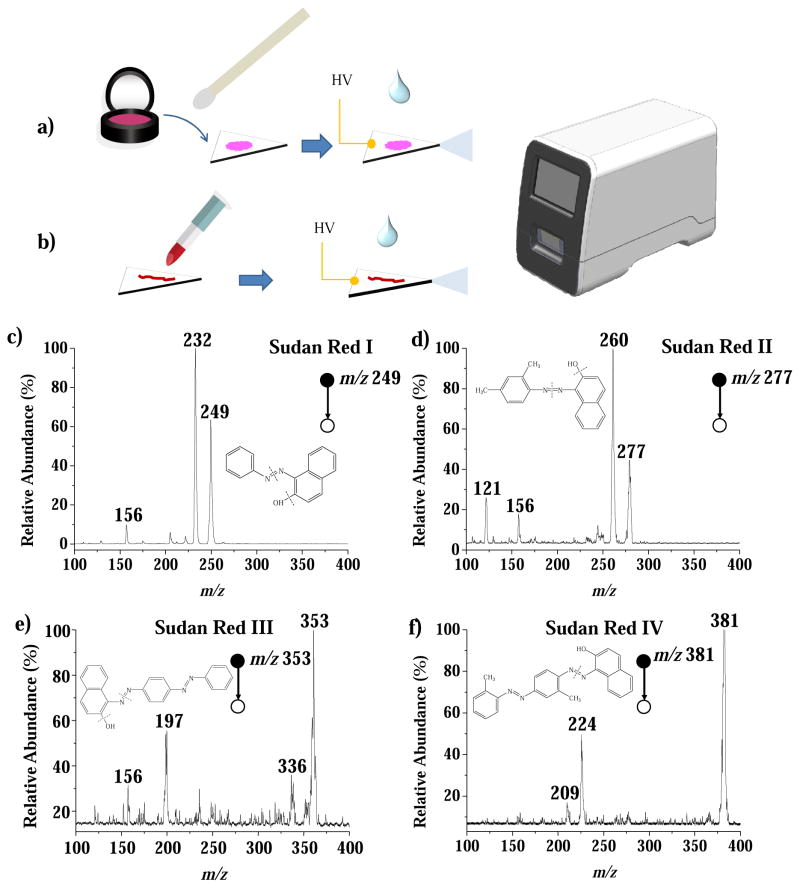
A significantly simplified protocol for direct analysis of powders and lipstick samples demonstrated the use of the miniature mass spectrometer system with paper spray ionization. Sudan Red dyestuffs (Sudan Red I, II, III and IV), an exemplary class of prohibited substances of great concern for regulatory control [71], were selected for method development and validation. Each sample of face powder, makeup, eye shadow or lipstick was approximately 1 mg and contained Sudan Red spiked at a concentration of 50 μg/kg. Each sample was loaded simply by smudging or swabbing it onto a piece of Grade 31ET chromatography paper substrate, which was then cut into a small triangle with a sharp tip (0.5 cm base width, 0.75 cm height and top angle 30°). A solid copper mini-alligator clip (Mueller, Ballinger, TX, USA) was used to hold the paper triangle with its tip about 5 mm from the DAPI inlet of the Mini 12. The metal clip was connected to a Bertan 205B-05R high voltage power supply (Hauppauge, New York, USA); 20 μL of dichloromethane:ethanol:formic acid (40:60:0.1)was applied as the wetting and spray solvent to induce a spray from the tip of the paper triangle. The MS analysis was performed in positive ion mode with a spray voltage at 4.0 kV. Based on the observed signal-to-noise ratio (S/N) of the most intense MS/MS fragment peaks and using the S/N = 3 as a criterion, the limits of detection (LODs) are estimated to be between 5 to 30 μg/kg for the four prohibited Sudan Red dyestuffs in powders and lipstick samples (Figure 2).
Figure 2.
Procedures for the analysis of (a) powder and (b) lipstick samples by paper spray ionization. MS/MS spectra for (a) Sudan Red I (precursor m/z 249) and (d) Sudan Red II (precursor m/z 277) spiked in powder and (e) Sudan Red III (precursor m/z 353) and (f) Sudan Red IV (precursor m/z 381) spiked in lipstick, each at 50 μg/kg.
As discussed in previous studies [72], paper spray involves three-steps: the extraction of analytes from the deposited sample by the spray solvent, the transport of extracted analytes across the paper substrate, and the generation of charged droplets due to the high voltage applied. The improvement in sensitivity with optimized paper spray conditions is more important for miniature mass spectrometers than for the commercial lab-scale instruments, since the performance of miniature instruments could be compromised due to the less sophisticated atmospheric pressure interfaces supported by their much smaller vacuum systems.
The selection of proper spray solvent and paper substrate is critical to the analytical performance of the paper spray MS analysis. The composition of the solvent not only affects the efficiency of extracting analytes from the sample matrix on paper, but also the efficiencies for their transfer across the paper and of ion formation during the spray event. In an attempt to optimize the overall elution/ionization efficiency for Sudan Red dyestuffs spiked in powdery and lipstick samples, the composition effect of the spray solvent was first evaluated by testing a series of pure solvents of different polarities, including hexane, tetrahydrofuran, ethyl acetate, chloroform, dichloromethane, acetone, acetonitrile, isopropanol, ethanol, methanol, and water. With a spray voltage of 4.0 kV, the highest signal intensities were observed with dichloromethane and ethanol, which have moderate solvent polarities (Figure S1a, b).
Solvent properties, such as the polarity, volatility and surface tension, also have a significant impact on the electrospray process. Typically, relative concentrations of the components in a mixture of solvents are adjusted to optimize the spray process for analysis of the target class of analytes [73]. As paper spray is expected to share similar spray characteristics with electrospray, a follow-up experiment for spray optimization was performed using a set of dichloromethane/ethanol mixture solvents at different mixing ratios. It was found that a mixture of dichloromethane:ethanol at 40:60 gave the maximum signal responses (Figure S1c, d). Addition of formic acid or acetic acid was also found to enhance the sensitivity in the positive ionization mode, with a most significant improvement obtained with formic acid (Figure S1e, f). The actual optimum concentration of the formic acid was determined to be 0.3%, through experimental testing of different concentrations between 0.1% and 2.0% (Figure S1g, h).
The nature of the paper substrate also plays a key role in the process of paper spray, since analyte elution and migration on paper are also dependent on the interactions between the analyte and the paper substrate, which acts somewhat like a stationary phase. As a porous and hydrophilic material, cellulose paper allows liquid flow over the surface and wicking through the inside microchannels by capillary action. Both the physical and chemical nature of the paper substrate might impact the migration behavior of the analytes, the ionization efficiency, and ultimately the analysis results. A variety of paper substrates, including cellulose chromatography papers (Grade 1 Chr, Grade 3MM Chr, Grade 4 Chr, Grade 31ET Chr) and ion exchange paper (Grade SG81), have been investigated in this study (Figure S2a, b). Upon the application of the optimized elution solvent of dichloromethane:ethanol:formic acid (40:60:0.3) and a spray voltage of 4.0 kV, Grade 31ET chromatography paper was identified as the best paper substrate with the highest signal intensities delivered.
Analysis of Shampoo and Cream Samples Using Extraction Spray
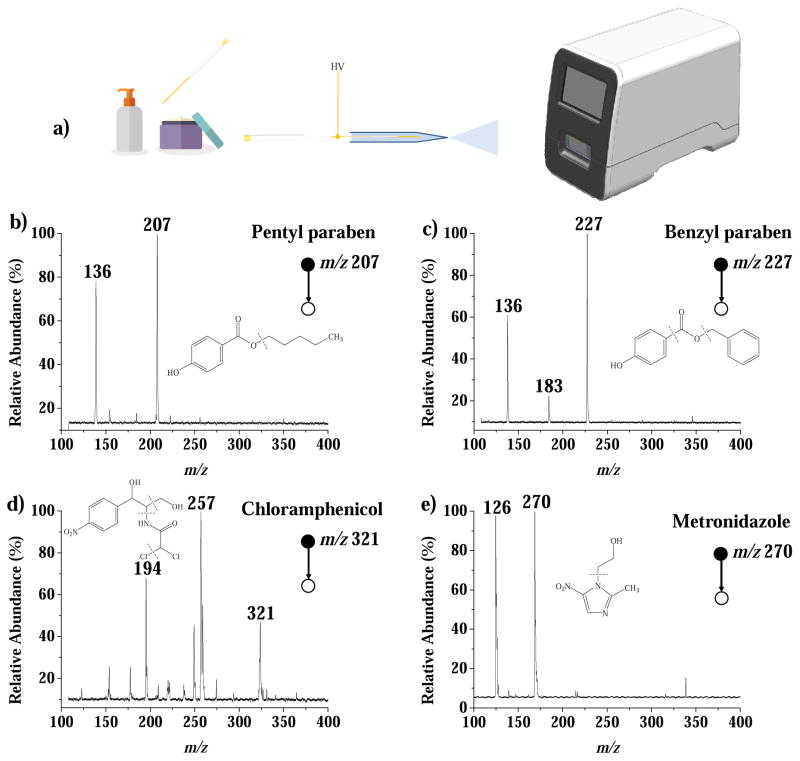
Another ambient ionization method, extraction spray, was used to develop a simple sampling/ionization method for the analysis of the prohibited paraben preservatives and antibiotics in the shampoo and cream products. Five paraben preservative (isopropyl-, isobutyl-, pentyl-, phenyl- and benzyl-) and two antibiotics [71] (chloramphenicol and metronidazole) were spiked into the samples. The paraben preservatives have been forbidden for use in cosmetics because of their doubtful toxicological profiles [74]. Samples were gently picked up using a metal wire probe (approximately 1 mg loaded), and then inserted into a borosilicate glass capillary (1.5 mm o.d., 0.86 mm i.d., 5 cm length) with a pulled tip, which had been pre-filled with 10 μL methanol:water:ammonia solution (60:40:0.5) for parabens) for analysis of parabens or 10 μL methanol:water:ammonia solution (80:20:1.0) for antibiotics analysis. The nanoESI capillary was then placed in front of the DAPI inlet of Mini 12 with a voltage of 2.5 kV applied via the metal wire, to produce the spray ionization for immediate MS analysis. The MS/MS spectra recorded for samples spiked with analytes at 50 μg/kg are shown in Figure 3. Based on the observed signal-to-noise ratios of the most abundant fragment peaks in MS/MS spectra and using the S/N = 3 as a criterion, the LODs are estimated to be between 5 and 25 μg/kg for the five parabens in shampoo and 20 or 10 μg/kg for chloramphenicol or metronidazole in cream, respectively.
Figure 3.
(a) Procedure for the analysis of cream and shampoo samples by extraction spray ionization. Product ion MS/MS spectra for (b) pentylparaben (precursor m/z 207) and (c) benzylparaben (precursor m/z 227) spiked in shampoo, and (d) chloramphenicol (precursor m/z 322) and (e) metronidazole (precursor m/z 170) spiked in cream, each at 50 μg/kg.
The solvent composition for the extraction spray ionization was also optimized. A range of solvents with different polarities, including tetrahydrofuran, acetone, acetonitrile, isopropanol, ethanol, methanol, water, were first compared. Among the solvents investigated, methanol gave the highest intensities as a pure solvent (Figure S3a, b). The experimental results demonstrated that the addition of water could help disperse shampoo or cream samples into the solvent and improve the extraction and ionization efficiency. The optimal proportions of water in methanol were identified to be 40% for the analysis of parabens in shampoo (Figure S3e, f) and 20% for the analysis antibiotics in cream (Figure S4e, f). Addition of ammonia was found to further improve the signal intensities in the negative ionization mode (Figure S3c, d and Figure S4c, d) with the best concentration of 0.5% for parabens (Figure S3g, h) and 1.0% for antibiotics (Figure S34g, h).
Analysis of Milk, Beverage and Lotion Samples Using Slug-Flow Microextraction
We used slug-flow microextraction (SFME) nanoESI [70], a newly developed method for direct analysis of liquid samples, such as milk and beverage, in a simple, one-step process. Ethyl acetate and an aqueous solution of sample, each of 10 μL, were sequentially injected into a borosilicate glass capillary (1.5 mm o.d., 0.86 mm i.d., 5 cm length) with a pulled tip. Liquid-liquid extraction of the analytes from the sample phase into the organic phase was expected, but at a fairly low efficiency because of the small interfacial area. However, as previously demonstrated [70], the analyte extraction speed could be significantly increased with slug flows induced by the movement of the two liquid plugs, which could be facilitated by tilting the capillary or applying a push-and-pull force through air pressure. Due to the friction with the capillary wall, internal circulations were formed inside each plug, transferring the analytes towards and away from the liquid-liquid interface, therefore significantly improving the extraction efficiency. After the extraction process, a stainless-steel wire was then inserted through the biofluid sample to reach the organic solvent plug and a high voltage applied to generate the nanoESI for MS analysis.
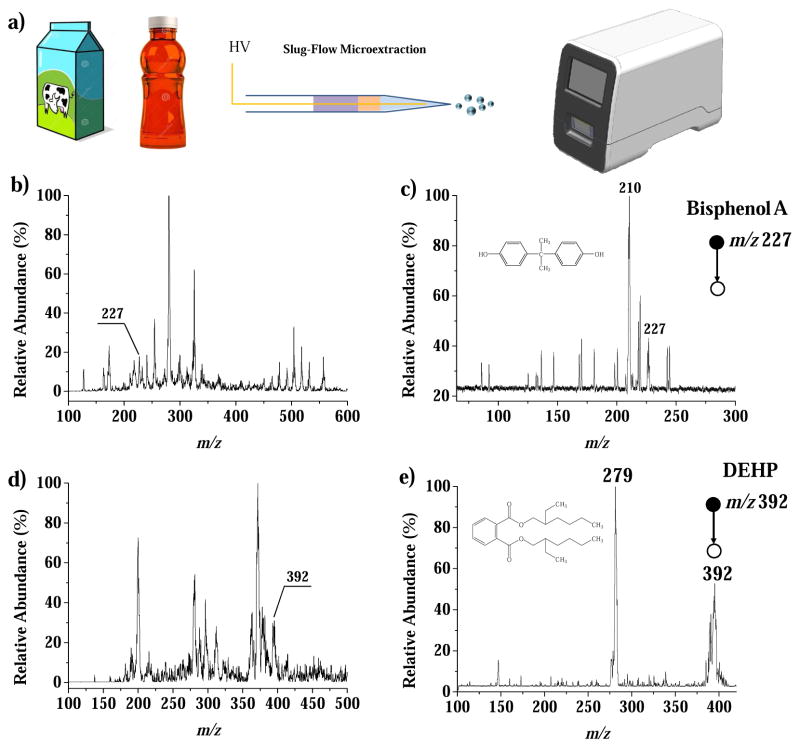
This method was applied to analyze milk samples spiked with bisphenol A (BPA) and beverage samples spiked with industrial plasticizer. BPA is an endocrine disruptor used as the monomer for the manufacture of polycarbonate plastics that is widely used for making containers and packaging materials; however, BPA has hormone-like properties and therefore is forbidden for use in making infant milk bottles [75]. Bis(2-ethylhexyl)phthalate is a plasticizer that has been used as a substitute for palm oil, the clouding agent in beverage, to lower the cost [7]. In this study, the BPA and bis(2-ethylhexyl)phthalate were spiked into the whole milk (Dean’s, Dean Dairy, Sharpsville, PA 16150) and Gatorade G2 Perform Fruit Punch (PepsiCoInc., Purchase, NY 10577), respectively, at a concentration of 50 μg/kg. The MS and MS/MS spectra recorded are shown in Figure 4. The LOD was estimated to be 10 μg/kg for BPA in milk and 5 μg/kg for bis(2-ethylhexyl)phthalate in the fruit punch.
Figure 4.
(a) Procedure for the analysis of milk and beverage samples using slug-flow microextraction nanoESI. MS and product ion MS/MS spectra for bisphenol A (precursor m/z 227) spiked in milk(b and c), and bis(2-ethylhexyl) phthalate(precursor m/z 392) spiked in beverage(d and e), each at 50 μg/kg.
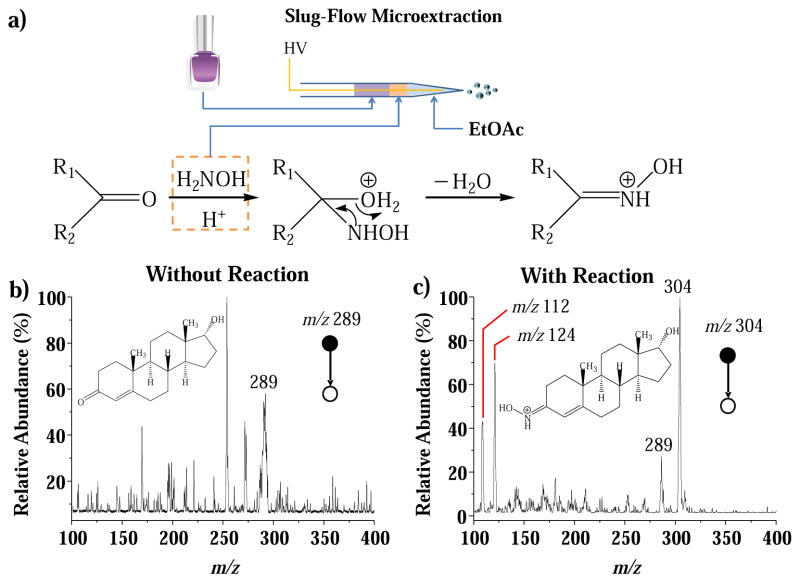
Chemical derivatization is an effective way of altering the properties of the target analytes to improve the efficiency of separation or ionization for MS analysis. For instance, epitestosterone is a type of corticosteroid forbidden in cosmetics [71], which can be expected to be extracted and preconcentrated into the organic phase using the SFME method; however, the efficiency of the subsequent ionization by nanoESI is relatively low due to the poor proton affinity of the epitestosterone. The reaction with hydroxylamine has previously been shown to improve the ionization efficiency of the steroids [76], and therefore was used in this study. A liquid plug of 10 μL water containing 100 mM hydroxylamine was injected between 10 μL ethyl acetate and 10 μL water-based lotion sample spiked with epitestosterone at 50 μg/kg. In comparison with the slug-flow microextraction and nanoESI without the derivatization, a significantly improved sensitivity was observed. The CID of the reaction product ion m/z 304 yielded characteristic fragment ions at m/z 112 and 124 (Figure 5). The LOD of this method is estimated to be about 5 μg/kg based on the S/N values of peaks attributed these two fragment ions.
Figure 5.
(a) Procedure for the analysis of (a) lotion samples using reactive slug-flow microextraction. Product ion MS/MS spectra for analysis of epitestosterone spiked in lotion at 50 μg/kg, (b) without and (c) with on-line derivatization using hydroxylamine.
CONCLUSIONS
In this study, three ambient ionization methods were used with a miniature mass spectrometer to develop simple procedures for direct analysis of illicit substances in food and cosmetic products. Paper spray is suitable for analysis of powders or dried sample spots, extraction spray can be used for lotion or gel samples, and slug-flow microextraction can be applied for direct analysis of aqueous samples. LODs at low parts-per-billion levels can be achieved with the miniature ion trap mass spectrometer using MS/MS experiments. Specialized analysis with target analytes is expected to be a major field of applications for the miniature MS systems. The future implementation of the ambient ionization methods for direct sampling ionization can be done through disposable kit containing a sample cartridge and small volumes of solvents. [50, 77, 78] The optimization of the solvent for sample extraction and spray ionization was shown to have significant impact on the overall analytical performance in this study. This information would be useful for future design of the sampling kits for on-site analysis of cosmetic and foodstuff samples.
Supplementary Material
Figure. S1. Optimization of solvents for the analysis of Sudan Red dyestuffs using paper spray, with the optimal conditions marked in red. Based on the observed intensities of the most intense MS/MS fragment peaks for Sudan Red I (m/z 232), Sudan Red II (m/z 260), Sudan Red III (m/z 197), and Sudan Red IV (m/z 224).
Figure. S2. Optimization of paper substrates for the analysis of Sudan Red dyestuffs using paper spray, with the optimal conditions marked in red. Based on the observed intensities of the most intense MS/MS fragment peaks for Sudan Red I (m/z 232), Sudan Red II (m/z 260), Sudan Red III (m/z 197), and Sudan Red IV (m/z 224).
Figure. S3. Optimization of solvents for the analysis of parabens preservatives using extraction spray, with the optimal conditions marked. Based on the observed intensities of the most intense MS/MS fragment peaks for isopropylparaben (m/z 136), isobutylparaben (m/z 136), pentylparaben (m/z 136), phenylparaben (m/z 93), and benzylparaben (m/z 136).
Figure. S4. Optimization of solvents for the analysis of antibiotics using extraction spray, with the optimal conditions marked in red. Based on the observed intensities of the most intense MS/MS fragment peaks for metronidazole (m/z 126), and chloramphenicol (m/z 257)
Table S1 Chemical information of the analytes
Table 1.
Experimental conditions for Analysis
| Compound | Matrix | Ambient Ionization | LODs (μg/kg) | Spray Solvent |
|---|---|---|---|---|
| Sudan Red I | Powder | Paper Spray | 5 | 6:4 EtOH-DCM containing 0.3% formic acid |
| Lipstick | Paper Spray | 5 | ||
| Sudan Red II | Powder | Paper Spray | 15 | 6:4 EtOH-DCM containing 0.3% formic acid |
| Lipstick | Paper Spray | 15 | ||
| Sudan Red III | Powder | Paper Spray | 30 | 6:4 EtOH-DCM containing 0.3% formic acid |
| Lipstick | Paper Spray | 30 | ||
| Sudan Red IV | Powder | Paper Spray | 20 | 6:4 EtOH-DCM containing 0.3% formic acid |
| Lipstick | Paper Spray | 20 | ||
| Isopropylparaben | Shampoo | Extraction Spray | 10 | 6:4 MeOH-water containing 0.3% ammonia |
| Isobutylparaben | Shampoo | Extraction Spray | 25 | 6:4 MeOH-water containing 0.3% ammonia |
| Pentylparaben | Shampoo | Extraction Spray | 25 | 6:4 MeOH-water containing 0.3% ammonia |
| Phenylparaben | Shampoo | Extraction Spray | 5 | 6:4 MeOH-water containing 0.3% ammonia |
| Benzylparaben | Shampoo | Extraction Spray | 20 | 6:4 MeOH-water containing 0.3% ammonia |
| Chloramphenicol | Cream | Extraction Spray | 20 | 8:2 MeOH-water containing 1.0% ammonia |
| Metronidazole | Cream | Extraction Spray | 10 | 8:2 MeOH-water containing 1.0% ammonia |
| Epistotesterone | Lotion | Slug-Flow Microextraction | 5 | EtOAc-100 mM Hydroxylamine |
| Bisphenol A | Milk | Slug-Flow Microextraction | 10 | EtOAc |
| Bis(2-ethylhexyl)phthalate | Beverage | Slug-Flow Microextraction | 5 | EtOAc |
HIGHLIGHTS.
Miniature ion trap analytical system with ambient ionization capability for direct chemical analysis
Direct analysis of condensed-phase samples in the forms of powder, aqueous mixtures, and cream
Optimal conditions identified for real-time sample extraction and analyte ionization
Acknowledgments
This work was supported by the Youth Talent Program of Chinese Academy of Inspection and Quarantine, the Science Research Program of General Administration of Quality Supervision, Inspection and Quarantine of China (2015IK314), the Science Research Program of Chinese Academy of Inspection and Quarantine (2014JK016), the National Key Technology Research and Development Program of China (2013BAK04B03), the National Natural Science Foundation of China (21307123), and National Institute of General Medical Sciences (1R01GM106016) from National Institutes of Health of USA. The authors thank Christina R. Ferreira, Karen E. Cesafsky, Xiaoxiao Ma, and Yuan Su for their helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Malik AK, Blasco C, Picó Y. Liquid chromatography–mass spectrometry in food safety. Journal of Chromatography A. 2010;1217:4018–4040. doi: 10.1016/j.chroma.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Lam H-M, Remais J, Fung M-C, Xu L, Sun SS-M. Food supply and food safety issues in China. The Lancet. 381:2044–2053. doi: 10.1016/S0140-6736(13)60776-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosmetics Europe Activity Report. 2014 https://www.cosmeticseurope.eu/publications-cosmetics-europeassociation/annual-reports.html.
- 4.Desmedt B, Courselle P, De Beer JO, Rogiers V, Deconinck E, De Paepe K. Illegal cosmetics on the EU market: a threat for human health? Archives of Toxicology. 2014;88:1765–1766. doi: 10.1007/s00204-014-1317-4. [DOI] [PubMed] [Google Scholar]
- 5.Wang CC, Masi AN, Fernández L. On-line micellar-enhanced spectrofluorimetric determination of rhodamine dye in cosmetics. Talanta. 2008;75:135–140. doi: 10.1016/j.talanta.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 6.Wegener JW, Klamer JC, Govers H, Brinkman UAT. Determination of organic colorants in cosmetic products by high-performance liquid chromatography. Chromatographia. 1987;24:865–875. [Google Scholar]
- 7.Yang J, Hauser R, Goldman RH. Taiwan food scandal: The illegal use of phthalates as a clouding agent and their contribution to maternal exposure. Food and Chemical Toxicology. 2013;58:362–368. doi: 10.1016/j.fct.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 8.The L. Melamine and food safety in China. The Lancet. :373, 353. doi: 10.1016/S0140-6736(09)60114-8. [DOI] [PubMed] [Google Scholar]
- 9.He L, Su Y, fang B, Shen X, Zeng Z, Liu Y. Determination of Sudan dye residues in eggs by liquid chromatography and gas chromatography–mass spectrometry. Analytica Chimica Acta. 2007;594:139–146. doi: 10.1016/j.aca.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Jin W, Yang YJ, Wang WY, Ye JN. Simultaneous Determination of Antibiotics in Anti-Acne Cosmetics by Rapid LC with DAD. Chromatographia. 2009;69:1221–1226. [Google Scholar]
- 11.Liu SY, Huang XH, Wang XF, Jin Q, Zhu GN. Rapid determination of quinolones in cosmetic products by ultra high performance liquid chromatography with tandem mass spectrometry. Journal of Separation Science. 2014;37:1134–1140. doi: 10.1002/jssc.201301350. [DOI] [PubMed] [Google Scholar]
- 12.Nam YS, Kwon IK, Lee KB. Monitoring of clobetasol propionate and betamethasone dipropionate as undeclared steroids in cosmetic products manufactured in Korea. Forensic Science International. 2011;210:144–148. doi: 10.1016/j.forsciint.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 13.Desmedt B, Van Hoeck E, Rogiers V, Courselle P, De Beer JO, De Paepe K, Deconinck E. Characterization of suspected illegal skin whitening cosmetics. Journal of Pharmaceutical and Biomedical Analysis. 2014;90:85–91. doi: 10.1016/j.jpba.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Panusa A, Ottaviani M, Picardo M, Camera E, Gagliardi L, Chimenti P, Granese A, Tonelli D. Analysis of corticosteroids by high performance liquid chromatography-electrospray mass spectrometry. Analyst. 2004;129:719–723. doi: 10.1039/b402905d. [DOI] [PubMed] [Google Scholar]
- 15.Fiori J, Andrisano V. LC–MS method for the simultaneous determination of six glucocorticoids in pharmaceutical formulations and counterfeit cosmetic products. Journal of Pharmaceutical and Biomedical Analysis. 2014;91:185–192. doi: 10.1016/j.jpba.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 16.De Orsi D, Pellegrini M, Pichini S, Mattioli D, Marchei E, Gagliardi L. High-performance liquid chromatography–diode array and electrospray-mass spectrometry analysis of non-allowed substances in cosmetic products for preventing hair loss and other hormone-dependent skin diseases. Journal of Pharmaceutical and Biomedical Analysis. 2008;48:641–648. doi: 10.1016/j.jpba.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 17.De Orsi D, Pellegrini M, Marchei E, Nebuloni P, Gallinella B, Scaravelli G, Martufi A, Gagliardi L, Pichini S. High performance liquid chromatography-diode array and electrospray-mass spectrometry analysis of vardenafil, sildenafil, tadalafil, testosterone and local anesthetics in cosmetic creams sold on the Internet web sites. Journal of Pharmaceutical and Biomedical Analysis. 2009;50:362–369. doi: 10.1016/j.jpba.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Gagliardi L, De Orsi D, Chimenti P, Porra lsquo, Rita D Tonelli. HPLC Determination of Imidazole Antimycotis in Antidandruff Cosmetic Products. Analytical Sciences. 2003;19:1195–1197. doi: 10.2116/analsci.19.1195. [DOI] [PubMed] [Google Scholar]
- 19.Yang TJ, Tsai FJ, Chen CY, Yang TCC, Lee MR. Determination of additives in cosmetics by supercritical fluid extraction on-line headspace solid-phase microextraction combined with gas chromatography–mass spectrometry. Analytica Chimica Acta. 2010;668:188–194. doi: 10.1016/j.aca.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Prado L, Lamas JP, Lores M, Garcia-Jares C, Llompart M. Simultaneous In-Cell Derivatization Pressurized Liquid Extraction for the Determination of Multiclass Preservatives in Leave-On Cosmetics. Analytical Chemistry. 2010;82:9384–9392. doi: 10.1021/ac101985h. [DOI] [PubMed] [Google Scholar]
- 21.Wei H, Yang J, Zhang H, Shi Y. Ultrasonic nebulization extraction assisted dispersive liquid–liquid microextraction followed by gas chromatography for the simultaneous determination of six parabens in cosmetic products. Journal of Separation Science. 2014;37:2349–2356. doi: 10.1002/jssc.201400313. [DOI] [PubMed] [Google Scholar]
- 22.Cheng YC, Wang CC, Chen YL, Wu SM. Large volume sample stacking with EOF and sweeping in CE for determination of common preservatives in cosmetic products by chemometric experimental design. Electrophoresis. 2012;33:1443–1448. doi: 10.1002/elps.201100546. [DOI] [PubMed] [Google Scholar]
- 23.Pellegrini M, Bossù E, Rotolo MC, Pacifici R, Pichini S. Simple and rapid analysis of methyldibromo glutaronitrile in cosmetic products by gas chromatography mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2011;56:1112–1116. doi: 10.1016/j.jpba.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 24.Sheliya K, Shah K, Kapupara P. Development and validation of analytical method for simultaneous estimation of mometasone furoate, hydroquinone and tretinoin in topical formulation by RP-HPLC. Journal of Chemical and Pharmaceutical Research. 2014;6:934–940. [Google Scholar]
- 25.Sanchez-Prado L, Llompart M, Lamas JP, Garcia-Jares C, Lores M. Multicomponent analytical methodology to control phthalates, synthetic musks, fragrance allergens and preservatives in perfumes. Talanta. 2011;85:370–379. doi: 10.1016/j.talanta.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Wang L, Kannan K. Phthalates and Parabens in Personal Care Products From China: Concentrations and Human Exposure. Archives of Environmental Contamination and Toxicology. 2014;66:113–119. doi: 10.1007/s00244-013-9937-x. [DOI] [PubMed] [Google Scholar]
- 27.Su R, Zhao X, Li Z, Jia Q, Liu P, Jia J. Poly(methacrylic acid-co-ethylene glycol dimethacrylate) monolith microextraction coupled with high performance liquid chromatography for the determination of phthalate esters in cosmetics. Analytica Chimica Acta. 2010;676:103–108. doi: 10.1016/j.aca.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 28.Llompart M, Celeiro M, Pablo Lamas J, Sanchez-Prado L, Lores M, Garcia-Jares C. Analysis of plasticizers and synthetic musks in cosmetic and personal care products by matrix solid-phase dispersion gas chromatography–mass spectrometry. Journal of Chromatography A. 2013;1293:10–19. doi: 10.1016/j.chroma.2013.03.067. [DOI] [PubMed] [Google Scholar]
- 29.Siddique S, Parveen Z, Ali Z, Zaheer M. Qualitative and Quantitative Estimation of Hydroquinone in Skin Whitening Cosmetics. Journal of Cosmetics, Dermatological Sciences and Applications. 2012;2:224–228. [Google Scholar]
- 30.Sherma J. Thin-layer chromatography in food and agricultural analysis. Journal of Chromatography A. 2000;880:129–147. doi: 10.1016/s0021-9673(99)01132-2. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Xue F, Wang Y, Xue Y, Sun CJ. Simultaneous Determination of Seven Adulterants in Functional Foods by High-Performance Capillary Electrophoresis. Chinese Journal of Analytical Chemistry. 2011;39:1716–1720. [Google Scholar]
- 32.Sarafraz-Yazdi A, Abbasian M, Amiri A. Determination of furan in food samples using two solid phase microextraction fibers based on sol–gel technique with gas chromatography–flame ionisation detector. Food Chemistry. 2012;131:698–704. [Google Scholar]
- 33.Sanchez-Prado L, Alvarez-Rivera G, Lamas JP, Llompart M, Lores M, Garcia-Jares C. Content of suspected allergens and preservatives in marketed baby and child care products. Analytical Methods. 2013;5:416–427. [Google Scholar]
- 34.Alvarez-Rivera G, Vila M, Lores M, Garcia-Jares C, Llompart M. Development of a multi-preservative method based on solid-phase microextraction–gas chromatography–tandem mass spectrometry for cosmetic analysis. Journal of Chromatography A. 2014;1339:13–25. doi: 10.1016/j.chroma.2014.02.075. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Wang S, Cai Z. The latest developments and applications of mass spectrometry in food-safety and quality analysis. TrAC Trends in Analytical Chemistry. 2013;52:170–185. [Google Scholar]
- 36.Hernández F, Portolés T, Pitarch E, López FJ. Gas chromatography coupled to high-resolution time-of-flight mass spectrometry to analyze trace-level organic compounds in the environment, food safety and toxicology. TrAC Trends in Analytical Chemistry. 2011;30:388–400. [Google Scholar]
- 37.Ito Y, Ikai Y, Oka H, Kagami T, Takeba K. Application of ion-exchange cartridge clean-up in food analysis: II. Determination of benzylpenicillin, phenoxymethylpenicillin, oxacillin, cloxacillin, nafcillin and dicloxacillin in meat using liquid chromatography with ultraviolet detection. Journal of Chromatography A. 1999;855:247–253. doi: 10.1016/s0021-9673(99)00695-0. [DOI] [PubMed] [Google Scholar]
- 38.Rastogi SC, Zachariae C, Johansen JD, Devantier C, Menné T. Determination of methyldibromoglutaronitrile in cosmetic products by high-performance liquid chromatography with electrochemical detection: Method validation. Journal of Chromatography A. 2004;1031:315–317. doi: 10.1016/j.chroma.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 39.Hird SJ, Lau BPY, Schuhmacher R, Krska R. Liquid chromatography-mass spectrometry for the determination of chemical contaminants in food. TrAC Trends in Analytical Chemistry. 2014;59:59–72. [Google Scholar]
- 40.Zhang X, Liu Y, Zhang J, Hu Z, Hu B, Ding L, Jia L, Chen H. Neutral desorption extractive electrospray ionization mass spectrometry for fast screening sunscreen agents in cream cosmetic products. Talanta. 2011;85:1665–1671. doi: 10.1016/j.talanta.2011.06.070. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Zhang X, Ouyang Y, Hu Z, Ma L, Zhang J, Lin J, Chen H. Trace detection of hormones and sulfonamides in viscous cosmetic products by neutral desorption extractive electrospray ionization tandem mass spectrometry. Journal of Mass Spectrometry. 2011;46:794–803. doi: 10.1002/jms.1944. [DOI] [PubMed] [Google Scholar]
- 42.Ouyang Z, Cooks RG. Miniature Mass Spectrometers. Annual Review of Analytical Chemistry. 2009;2:187–214. doi: 10.1146/annurev-anchem-060908-155229. [DOI] [PubMed] [Google Scholar]
- 43.Xu W, Manicke NE, Cooks GR, Ouyang Z. Miniaturization of Mass Spectrometry Analysis Systems. Journal of the Association for Laboratory Automation. 2010;15:433–439. doi: 10.1016/j.jala.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao L, Sugiarto A, Harper JD, Cooks RG, Ouyang Z. Design and Characterization of a Multisource Hand-Held Tandem Mass Spectrometer. Analytical Chemistry. 2008;80:7198–7205. doi: 10.1021/ac801275x. [DOI] [PubMed] [Google Scholar]
- 45.Contreras JA, Murray JA, Tolley SE, Oliphant JL, Tolley HD, Lammert SA, Lee ED, Later DW, Lee ML. Hand-Portable Gas Chromatograph-Toroidal Ion Trap Mass Spectrometer (GC-TMS) for Detection of Hazardous Compounds. Journal of the American Society for Mass Spectrometry. 2008;19:1425–1434. doi: 10.1016/j.jasms.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 46.Gao L, Song QY, Patterson GE, Cooks RG, Ouyang Z. Handheld rectilinear ion trap mass spectrometer. Analytical Chemistry. 2006;78:5994–6002. doi: 10.1021/ac061144k. [DOI] [PubMed] [Google Scholar]
- 47.Riter LS, Meurer EC, Cotte-Rodriguez I, Eberlin MN, Graham Cooks R. Solid phase micro-extraction in a miniature ion trap mass spectrometer. Analyst. 2003;128:1119–1122. doi: 10.1039/b308292j. [DOI] [PubMed] [Google Scholar]
- 48.Keil A, Hernandez-Soto H, Noll RJ, Fico M, Gao L, Ouyang Z, Cooks RG. Monitoring of Toxic Compounds in Air Using a Handheld Rectilinear Ion Trap Mass Spectrometer. Analytical Chemistry. 2008;80:734–741. doi: 10.1021/ac070906o. [DOI] [PubMed] [Google Scholar]
- 49.Gao L, Cooks RG, Ouyang Z. Breaking the pumping speed barrier in mass spectrometry: Discontinuous atmospheric pressure interface. Analytical Chemistry. 2008;80:4026–4032. doi: 10.1021/ac800014v. [DOI] [PubMed] [Google Scholar]
- 50.Li L, Chen T-C, Ren Y, Hendricks PI, Cooks RG, Ouyang Z. Mini 12, Miniature Mass Spectrometer for Clinical and Other Applications—Introduction and Characterization. Analytical Chemistry. 2014;86:2909–2916. doi: 10.1021/ac403766c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu W, Charipar N, Kirleis MA, Xia Y, Ouyang Z. Study of Discontinuous Atmospheric Pressure Interfaces for Mass Spectrometry Instrumentation Development. Analytical Chemistry. 2010;82:6584–6592. [PubMed] [Google Scholar]
- 52.Ouyang Z, Noll RJ, Cooks RG. Handheld Miniature Ion Trap Mass Spectrometers. Analytical Chemistry. 2009;81:2421–2425. doi: 10.1021/ac900292w. [DOI] [PubMed] [Google Scholar]
- 53.Monge ME, Harris GA, Dwivedi P, Fernández FM. Mass Spectrometry: Recent Advances in Direct Open Air Surface Sampling/Ionization. Chemical Reviews. 2013;113:2269–2308. doi: 10.1021/cr300309q. [DOI] [PubMed] [Google Scholar]
- 54.Takáts Z, Wiseman JM, Gologan B, Cooks RG. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 55.Cody RB, Laramée JA, Durst HD. Versatile New Ion Source for the Analysis of Materials in Open Air under Ambient Conditions. Analytical Chemistry. 2005;77:2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 56.Huang MZ, Yuan CH, Cheng SC, Cho YT, Shiea J. Ambient Ionization Mass Spectrometry. Annual Review of Analytical Chemistry. 2010;3:43–65. doi: 10.1146/annurev.anchem.111808.073702. [DOI] [PubMed] [Google Scholar]
- 57.Wang H, Liu J, Cooks RG, Ouyang Z. Paper Spray for Direct Analysis of Complex Mixtures Using Mass Spectrometry. Angewandte Chemie International Edition. 2010;49:877–880. doi: 10.1002/anie.200906314. [DOI] [PubMed] [Google Scholar]
- 58.Liu J, Wang H, Manicke NE, Lin JM, Cooks RG, Ouyang Z. Development, Characterization, and Application of Paper Spray Ionization. Analytical Chemistry. 2010;82:2463–2471. doi: 10.1021/ac902854g. [DOI] [PubMed] [Google Scholar]
- 59.Ren Y, Liu J, Li L, McLuckey MN, Ouyang Z. Direct mass spectrometry analysis of untreated samples of ultra low amounts using extraction nano-electrospray. Analytical Methods. 2013;5:6686–6692. doi: 10.1039/C3AY41149D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harper JD, Charipar NA, Mulligan CC, Zhang X, Cooks RG, Ouyang Z. Low-Temperature Plasma Probe for Ambient Desorption Ionization. Analytical Chemistry. 2008;80:9097–9104. doi: 10.1021/ac801641a. [DOI] [PubMed] [Google Scholar]
- 61.Huang G, Xu W, Visbal-Onufrak MA, Ouyang Z, Cooks RG. Direct analysis of melamine in complex matrices using a handheld mass spectrometer. Analyst. 2010;135:705–711. doi: 10.1039/b923427f. [DOI] [PubMed] [Google Scholar]
- 62.Soparawalla S, Tadjimukhamedov FK, Wiley JS, Ouyang Z, Cooks RG. In situ analysis of agrochemical residues on fruit using ambient ionization on a handheld mass spectrometer. Analyst. 2011;136:4392–4396. doi: 10.1039/c1an15493a. [DOI] [PubMed] [Google Scholar]
- 63.Huang G, Gao L, Duncan J, Harper J, Sanders N, Ouyang Z, Cooks RG. Direct detection of benzene, toluene, and ethylbenzene at trace levels in ambient air by atmospheric pressure chemical ionization using a handheld mass spectrometer. Journal of the American Society for Mass Spectrometry. 2010;21:132–135. doi: 10.1016/j.jasms.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 64.Sokol E, Edwards KE, Qian K, Cooks RG. Rapid hydrocarbon analysis using a miniature rectilinear ion trap mass spectrometer. Analyst. 2008;133:1064–1071. doi: 10.1039/b805813j. [DOI] [PubMed] [Google Scholar]
- 65.Sokol E, Noll RJ, Cooks RG, Beegle LW, Kim HI, Kanik I. Miniature mass spectrometer equipped with electrospray and desorption electrospray ionization for direct analysis of organics from solids and solutions. Int J Mass Spectrom. 2011;306:187–195. [Google Scholar]
- 66.Janfelt C, Talaty N, Mulligan CC, Keil A, Ouyang Z, Cooks RG. Mass spectra of proteins and other biomolecules recorded using a handheld instrument. Int J Mass Spectrom. 2008;278:166–169. [Google Scholar]
- 67.Dalgleish JK, Hou K, Ouyang Z, Cooks RG. In Situ Explosive Detection Using a Miniature Plasma Ion Source and a Portable Mass Spectrometer. Analytical Letters. 2012;45:1440–1446. [Google Scholar]
- 68.Ma Q, Bai H, Li W, Wang C, Graham Cooks R, Ouyang Z. Rapid analysis of synthetic cannabinoids using a miniature mass spectrometer with ambient ionization capability. Talanta. 2015;142:190–196. doi: 10.1016/j.talanta.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mandal MK, Yoshimura K, Saha S, Ninomiya S, Rahman MO, Yu Z, Chen LC, Shida Y, Takeda S, Nonami H, Hiraoka K. Solid probe assisted nanoelectrospray ionization mass spectrometry for biological tissue diagnostics. Analyst. 2012;137:4658–4661. doi: 10.1039/c2an36006c. [DOI] [PubMed] [Google Scholar]
- 70.Ren Y, McLuckey MN, Liu J, Ouyang Z. Direct Mass Spectrometry Analysis of Biofluid Samples Using Slug-Flow Microextraction Nano-Electrospray Ionization. Angewandte Chemie International Edition. 2014;53:14124–14127. doi: 10.1002/anie.201408338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:EN:PDF.
- 72.Ren Y, Wang H, Liu J, Zhang Z, McLuckey M, Ouyang Z. Analysis of Biological Samples Using Paper Spray Mass Spectrometry: An Investigation of Impacts by the Substrates, Solvents and Elution Methods. Chromatographia. 2013;76:1339–1346. doi: 10.1007/s10337-013-2458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Z, Xu W, Manicke NE, Cooks RG, Ouyang Z. Silica Coated Paper Substrate for Paper-Spray Analysis of Therapeutic Drugs in Dried Blood Spots. Analytical Chemistry. 2012;84:931–938. doi: 10.1021/ac202058w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R0358&from=EN.
- 75.Hoekstra EJ, Simoneau C. Release of Bisphenol A from Polycarbonate—A Review. Critical Reviews in Food Science and Nutrition. 2013;53:386–402. doi: 10.1080/10408398.2010.536919. [DOI] [PubMed] [Google Scholar]
- 76.Huang G, Chen H, Zhang X, Cooks RG, Ouyang Z. Rapid Screening of Anabolic Steroids in Urine by Reactive Desorption Electrospray Ionization. Analytical Chemistry. 2007;79:8327–8332. doi: 10.1021/ac0711079. [DOI] [PubMed] [Google Scholar]
- 77.Manicke NE, Yang QA, Wang H, Oradu S, Ouyang Z, Cooks RG. Assessment of paper spray ionization for quantitation of pharmaceuticals in blood spots. Int J Mass Spectrom. 2011;300:123–129. [Google Scholar]
- 78.Yang Q, Wang H, Maas JD, Chappell WJ, Manicke NE, Cooks RG, Ouyang Z. Paper spray ionization devices for direct, biomedical analysis using mass spectrometry. Int J Mass Spectrom. 2012;312:201–207. doi: 10.1016/j.ijms.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure. S1. Optimization of solvents for the analysis of Sudan Red dyestuffs using paper spray, with the optimal conditions marked in red. Based on the observed intensities of the most intense MS/MS fragment peaks for Sudan Red I (m/z 232), Sudan Red II (m/z 260), Sudan Red III (m/z 197), and Sudan Red IV (m/z 224).
Figure. S2. Optimization of paper substrates for the analysis of Sudan Red dyestuffs using paper spray, with the optimal conditions marked in red. Based on the observed intensities of the most intense MS/MS fragment peaks for Sudan Red I (m/z 232), Sudan Red II (m/z 260), Sudan Red III (m/z 197), and Sudan Red IV (m/z 224).
Figure. S3. Optimization of solvents for the analysis of parabens preservatives using extraction spray, with the optimal conditions marked. Based on the observed intensities of the most intense MS/MS fragment peaks for isopropylparaben (m/z 136), isobutylparaben (m/z 136), pentylparaben (m/z 136), phenylparaben (m/z 93), and benzylparaben (m/z 136).
Figure. S4. Optimization of solvents for the analysis of antibiotics using extraction spray, with the optimal conditions marked in red. Based on the observed intensities of the most intense MS/MS fragment peaks for metronidazole (m/z 126), and chloramphenicol (m/z 257)
Table S1 Chemical information of the analytes