Biopharmaceuticals made using current production systems are prohibitively expensive and are not affordable for a large majority of the global population. The cost of protein drugs ($140 billion in 2013) exceeds GDP of >75% of countries around the globe (Walsh, 2014), making them unaffordable in these countries. The one-third of the global population that earns <$2 per day cannot afford any protein drugs. This is because of their production in prohibitively expensive fermenters, purification, cold storage/transportation, short shelf life and sterile delivery methods. Simpler methods of delivery such as oral dosing could obviate much of the expense: however, oral delivery of protein drugs has been elusive for decades because of their degradation in the digestive system, inability to cross the gut epithelium and delivery to target cells/tissues.
Mechanism of oral drug delivery
An advantage of using plants to produce biologics is that the plant cell wall protects expressed protein drugs from acids and enzymes in the stomach via bioencapsulation. However, gut microbes have evolved to break down every component of plant cell walls—so that when intact plant cells containing protein drugs reach the gut, commensal microbes digest the cell walls and release the proteins. When tags (receptor binding proteins) are fused to protein drugs, they efficiently cross the intestinal epithelium (largest mucosal area of the body—1.8–2.7 m2) and are delivered to the circulatory or immune system (Jin and Daniell, 2015). The use of CTB and LTB as transmucosal carriers facilitates transportation of conjugated proteins into circulation by binding to GM1 receptors (~15 000 per cell). After crossing the epithelium, tags can be cleaved at engineered sites by proteases.
Oral delivery of plant-made biopharmaceuticals currently advanced to the clinic
Recombinant glucocerebrosidase expressed in carrot cells via the nuclear genome and used as a replacement therapy to treat Gaucher's disease is the first and most recent FDA approved biopharmaceutical made in plant cells (Walsh, 2014). But this system still requires expensive purification, cold storage/transportation and sterile delivery. However, recent studies from the same group demonstrate significant potential for oral delivery of this important drug (Shaaltiel et al., in this special issue). Glucocerebrosidase released from carrot cells retained activity up to 120 min in simulated intestinal fluid mimicking fasting conditions. When compared to intravenous injection of glucocerebrosidase with peak activity in plasma after 30 min, an oral formulation showed an extended and slower release of between 6 and 8 h; however, the efficiency of oral delivery of carrot cells was 10-fold less. This may be due to the low-level expression of glucocerebrosidase via the carrot nuclear genome.
One approach to increase transgene expression is to engineer the chloroplast genome: each plant cell contains up to 10 000 copies of the chloroplast genome; therefore, transgenes inserted into chloroplast genomes are expressed at high levels (up to 70% of the total leaf protein). A wide range of proteins, ranging from very small antimicrobial peptides or hormones to very large proteins encoded by bacterial, viral, fungal, animal and human genes, have been successfully expressed in plant chloroplasts (Jin and Daniell, 2015). Most importantly, therapeutic proteins are expressed at exceptionally high levels and lyophilized plant cells can be stored for several months or years without a decrease in their functionality, thereby eliminating costs of cold storage and transportation (Kwon et al., 2013).
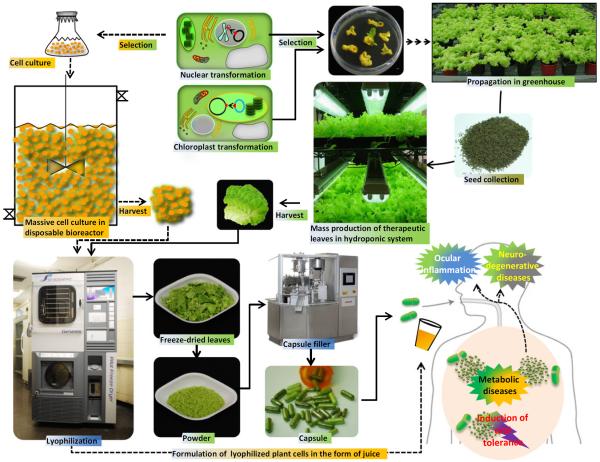
In a recent study, lettuce plants expressing clotting factor IX fused to cholera nontoxin subunit B (CTB-FIX) were grown in the Fraunhofer research facility under growth conditions that are directly translatable to its GMP pilot plant (Figure 1). Although only the native human gene was expressed, up to ~1 mg/g CTB-FIX was observed in lyophilized lettuce cells. CTB-FIX was stable, with proper folding, disulphide bonds and pentamer assembly when stored for ~2 years at ambient temperature. Feeding lettuce cells to haemophilia B mice delivered CTB-FIX efficiently to the gut immune system, induced LAP+ regulatory T cells and suppressed inhibitor/IgE formation and anaphylaxis against FIX. Ten-fold dose escalation studies showed similar tolerance efficacy facilitating oral delivery of appropriate doses for patients of different age groups. Using the Fraunhofer cGMP hydroponic system, ~870 kg fresh or 43.5 kg dry weight can be harvested per 1000 ft2 in 3–4 months yielding 24 000–36 000 doses for 20 kg paediatric patients, enabling the first commercial development of an oral drug and addressing the prohibitively expensive purification, cold storage/transportation and short shelf life of current protein drugs (Su et al., 2015a,b). The concept of oral delivery of protein drugs expressed in plant cells is illustrated in Figure 1. Also, recent studies on oral delivery of biopharmaceuticals and atuoantigens produced in plant cells and their efficacy in animal testing are summarized in Table 1 and discussed briefly below.
Figure 1.
Engineering plant cells to express protein drugs and lyophilization for oral delivery. Plant cells are engineered by transforming either the nuclear or chloroplast genome. After a selection process, plant cells are grown in sterile suspension cell bioreactors or in a hydroponic cGMP facility. Harvested plant cells are lyophilized to facilitate long-term storage and cold chain-free delivery. The lyophilized leaves are powdered and capsulated or formulated in the form of juice for oral administration to treat or prevent human diseases.
Table 1.
Recent studies on oral delivery of biopharmaceuticals and autoantigens produced in plant cells to treat human diseases
| Disease | Protein | Transformation system | Expression levels | Tested animals | Functional evaluation | References |
|---|---|---|---|---|---|---|
| Gaucher's disease | Glucocerebrosidase | Nucleus | Not reported | Pig and rat | The activity of orally delivered glucocerebrosidase bioencapsulated in carrot cells was detected in the digestive tract, sera, liver/spleen. Oral formulation showed extended release of prGCD and peaked between 6 and 8 h. | Shaaltiel (2015) in this issue |
| The prGCD activity was detected even 24 h in plasma after oral feeding but at 10-fold lower than injected prGCD | ||||||
| Pulmonary hypertension (PH) | CTB-ACE2 and CTB-Ang-(1-7) | Chloroplasts | 86 μg/g (CTB-ACE2, FW) and 584 μg/g [CTB-Ang-(1-7), FW] | Rat | Oral administration of bioencapsulated ACE2 or Ang-(1-7) to rats prevented the development of monocrotaline-induced PH and improved associated cardiopulmonary pathophysiology. Also, the disease progression was significantly arrested as well as recovery of right heart function. Combination therapy with ACE2 and Ang-(1-7) demonstrated the beneficial effects against monocrotaline-induced lung injury | Shenoy et al. (2014) |
| Ocular inflammation | CTB-ACE2 and CTB-Ang-(1-7) | Chloroplasts | 86 μg/g (CTB-ACE2, FW) and 584 μg/g [CTB-Ang-(1-7), FW] | Mice | Oral feeding of bioencapsulated ACE2 and Ang-(1-7) significantly reduced endotoxin-induced uveitis (EIU) and dramatically decreased cellular infiltration, retinal vasculitis, damage and folding in experimental autoimmune uveoretinitis (EAU) | Shil et al. (2014) |
| Alzheimer's disease | CTB-MBP | Chloroplasts | 2% TLP (31.2 μg/300 μl/dose) | Mice | Orally delivered lyophilized CTB-MBP crossed the blood-brain barrier and significantly reduced amyloid loads in hippocampus and cortex brain regions, along with decreased Aβ42 accumulation in retinae. Also it prevented loss of retinal ganglion cells | Kohli et al. (2014) |
| Type II diabetes | CTB-EX4 | Chloroplasts | 498 μg/g (FW), 6300 μg/g (DW) | Mice | The treatment of purified CTB-EX4 to pancreatic cell line showed increased level of insulin secretion similar to commercial EX4. Oral feeding of lyophilized CTB-EX4 lowered blood glucose levels similar to the injection of commercial EX4 | Kwon et al. (2013) |
| Type II diabetes | EX4-Tf | Nucleus | 37 μg/g (FW) | Mice | Partially purified EX4-Tf stimulated insulin release from pancreatic β-cells, promoted β-cell proliferation and retained the ability to induce internalization of human intestinal cell membrane. Oral administration of EX4-Tf significantly enhanced glucose tolerance | Choi et al. (2014) |
| Pompe | GAA | Chloroplasts | 6.38 μg/g (FW), 190 μg/g (DW) | Mice | Oral administration of bioencapsulated GAA significantly suppressed IgG1 and IgG2a inhibitory antibody formation against GAA in treatment of Pompe mice | Su et al. (2015a,b) |
| Haemophilia A | CTB-hFVIII | Chloroplasts | 370 μg/g (FVIII-C2, FW) and 80 μg/g (FVIII-HC, FW) | Mice | Oral delivery of a mixture of bioencapsulated hFVIII heavy chain and C2 domain antigens substantially suppressed T helper cell responses and inhibitor formation against FVIII in haemophilia A mice. | Sherman et al. (2014) |
| Substantial reduction of inhibitor titres in pre-immune mice demonstrated that the protocol could also reverse inhibitor formation |
FW, fresh weight; DW, dry weight; TLP, total leaf protein.
Delivery of protein drugs across the blood–brain and blood–retinal barriers (BBB/BRB)
Alzheimer's disease (AD) is one of the major concerns of modern human society with an anticipated increase in the affected population to 65.7 million by 2030. There is no cure or treatment for this dreadful neurodegenerative disorder. Most of all, the tightly sealed blood–brain barrier has hampered the translocation of drugs. In 2014, phase 3 trials with bapineuzumab and solanezumab failed to meet primary endpoints in patients or to improve cognitive and functional performance. Therefore, we developed a new approach to deliver protein drugs across the BBB. MBP has been known to bind and degrade Aβ peptide and inhibit Aβ fibril formation and it is a major structural component of the central nervous system (CNS). CTB facilitated translocation of MBP through the intestinal epithelial membrane to the circulation and across the BBB by binding to GM1 receptors at both sites. In this study, bioencapsulated myelin basic protein (MBP) fused with CTB and expressed in chloroplasts, when orally delivered, significantly reduced Aβ42 aggregates (Kohli et al., 2014). The MBP level in brains of triple transgenic Alzheimer's disease (3 × TgAD) mice was increased after oral delivery of lyophilized plant cells containing CTB-MBP, which was accompanied by a reduction of the Aβ level and of Aβ42 aggregates in the hippocampus and cortex. In addition, amyloid deposit was dramatically reduced in the inner retina of AD mice, which indicated that MBP passed through the blood–retinal barrier.
A parallel study demonstrated protein drug delivery across the BRB based on the strengthening protective axis of the renin–angiotensin system (RAS). Uveitis, a sight-threatening intraocular inflammatory disorder which is caused by various factors, including infectious agents, autoantigens and toxins, causes 5–15% of all cases of total blindness in the United States. Ocular inflammation is associated with hyperactivity of the RAS in which elevated angiotensin II (Ang II) acts as a potent pro-inflammatory effector. This deleterious axis of RAS, composed of angiotensin converting enzyme (ACE)/AngII/angiotensin type I receptor (AT1R), is counterbalanced by the protective axis of RAS involving ACE2/Ang-(1-7)/Mas. Oral delivery of plant cells expressing CTB fused to ACE2 and Ang-(1-7) increased their levels in the circulation and retina, enhanced systemic and local activity of the protective axis of RAS, reduced endotoxin-induced uveitis (EIU) and dramatically decreased cellular infiltration, retinal vasculitis, damage and folding in experimental autoimmune uveoretinitis (Shil et al., 2014).
Regulation of metabolic disorders by orally delivered protein drugs
Cardiovascular homoeostasis is also controlled by RAS. The WHO projects that >23 million people will die of cardiovascular disease annually by 2030. Pulmonary arterial hypertension (PAH) is a fatal disease characterized by remodelling of the pulmonary vasculature, resulting in elevated pulmonary vascular resistance and pulmonary artery pressure leading to increased right ventricular (RV) failure and death. This is of critical importance, as the long-term treatment of PAH is currently limited by expense ($100,000–$200 000/year), side effects and suboptimal survival of only 55% at 3 years. However, ACE2 in the lungs converts AngII to Ang-(1-7), which stimulates the Mas receptor, resulting in cardiopulmonary protective effects. Therefore, a decreased level of ACE2 in tissues and the circulation is linked to human lung diseases. When PH-induced rats were orally fed with plant cells expressing ACE2 or Ang-(1-7), significant improvements in cardiopulmonary structure and functions were observed in both the prevention and reversal protocols (Shenoy et al., 2014). Not only was the elevated right ventricular systolic blood pressure decreased, but the pulmonary blood flow was also improved. In addition, the authors found that oral ACE2/Ang-(1-7) feeding restored right heart function and attenuated maladaptive remodelling in diseased animals (Shenoy et al., 2014). Therefore, oral delivery of ACE2 or Ang-(−17) is effective against PAH and is suitable for clinical trials towards providing affordable PAH therapeutics.
Diabetes was the direct cause of 1.5 million deaths worldwide in 2012. Type 2 diabetes is responsible for 90% of the diabetic cases and affects a significant proportion of the global population. As described in two separate studies, exendin-4 (EX4) has excellent properties as an antidiabetes drug. Glucagon-like peptide-1 (GLP-1) is a peptide hormone secreted by intestine cells that stimulates secretion of insulin from the pancreas, but this has a short life because it is cleaved by dipeptidyl peptidase. EX4 is a DPP-resistant analogue of GLP-1, with higher binding efficacy to the mammalian GLP-1 receptor present on the pancreas than GLP-1 and functions as an effective agonist. EX4 modulates secretion of insulin to control the blood glucose level in a glucose-dependent manner and has shown promising biological activities in vivo for treating type 2 diabetes. EX4 is an injectable insulinotropic agent and demonstrates appreciable antidiabetic effects in clinical use among patients with type 2 diabetes but requires cold storage and abdominal injections, decreasing patient compliance. In two separate studies, EX4 fused to transferrin (Tf) or CTB was expressed via the nuclear or chloroplast genome, to facilitate translocation of EX4 across the epithelial cells. Both EX4 fusion proteins lowered the blood glucose level similarly to subcutaneous injections of commercial EX4, but EX4 delivered orally without bioencapsulation in plant cells failed to stimulate the pancreas to secrete insulin, due to its degradation in the stomach.
Oral tolerance induction using plant cells
When the field of plant-made vaccines was pioneered by Prof. Charles Arntzen (see his memoir in this issue), the primary goal was to develop cold chain-free vaccines to treat global infectious diseases. This goal has not yet been realized, partly because of the inability to induce immunity without priming the host immune system with adjuvants via injections (see Chan and Daniell article in this issue for more details). In the presence of inflammatory stimuli (adjuvants), local dendritic cells (DCs) become activated and present antigens for T-cell priming, locally and in the peripheral lymphoid tissues where DCs can migrate. Immature DCs induce regulatory T cells (Tregs) that affect DC function and prevent stable DCs–effector T-cell contact, thereby priming the immune response. This is a very different scenario from the release of antigens into the gut immune system without priming, which is geared towards an anti-inflammatory response. When antigens are presented to T cells by immature DCs in the absence of inflammation or priming, they induce tolerance. Furthermore, by secreting cytokines such as IL-10 or by direct cell-to-cell contact, Tregs interfere with DC maturation, shifting DCs into tolerogenic function. Therefore, oral delivery of autoantigens is ideal for induction of tolerance rather than immunity. We describe below two recent examples of induction of oral tolerance using autoantigens expressed in plant chloroplasts.
Haemophilia is the X-linked bleeding disorder caused by mutations in clotting factor IX (FIX, haemophilia B) or its cofactor, factor VIII (FVIII, haemophilia A). The current clinical treatment for haemophilia patients is clotting factor replacement therapy via injection of plasma-derived or recombinant factor concentrate. However, formation of inhibitory antibodies (inhibitors) against FVIII or FIX seriously complicates treatment and increases morbidity and mortality of the disease. Patients with high titres of inhibitors have to be treated by immune tolerance induction (ITI) through administration of high-dose factor concentrate for a long period of time. The cost of the clinical ITI treatment is highly expensive. Furthermore, ~30% of the patients fail to respond to ITI treatment. We have developed an oral tolerance induction protocol by expression of blood clotting factor VIII (A1–A2 domains or heavy chain and C2 domain) fused to CTB in chloroplasts. After oral delivery of plant cells to male haemophilia A mice twice per week for 2 months, they were challenged with FVIII injections. Control mice fed with untransformed plant cells showed very a high titre of inhibitors. In contrast, inhibitor formation against FVIII was significantly suppressed (~sevenfold) in haemophilia A mice fed with FVIII-expressing plant cells. Most importantly, plant-made FVIII antigen-mediated oral tolerance induction could also reverse inhibitor formation (Sherman et al., 2014). These studies also identified a complex immune regulatory mechanism behind prevention of inhibitors. Induced latency-associated peptide expressing CD4+ regulatory T cells (CD4+CD25-LAP+) with increased expression levels of interleukin-10 (IL-10), transforming growth factor-β (TGF-β) and conventional CD4+CD25+ regulatory T cells were demonstrated to be crucial for suppressing the formation of pathogenic antibodies against clotting factors (Sherman et al., 2014).
In parallel studies, a similar suppression of antibody titres was observed in another disease model, facilitating broader application of this concept. Pompe disease (an autosomal recessive lysosome disorder) is caused by mutations in the gene encoding acid alpha-glucosidase (GAA). GAA is essential for the degradation of glycogen to glucose in lysosomes. Accumulation of glycogen in lysosomes damages muscle and nerve cells, causing a neuromuscular disease that impairs skeletal, cardiac and smooth muscles. Enzyme replacement therapy (ERT) with recombinant human GAA (rhGAA) is currently the only clinically available treatment. Without ERT, infantile-onset patients would not survive beyond 2 years of age. More than 80% of severely affected patients have been shown to form anti-GAA inhibitors, which not only neutralize the ERT but cause immunotoxicities. Therefore, costly clinical ITI treatment is required for these severe patients. We developed a cost-effective and efficient oral delivery protocol using plant chloroplast-made GAA antigen. The N-terminal 410 amino acids of GAA were selected through T-cell epitope mapping. Oral delivery of plant cells expressing CTB-GAA in chloroplasts, even at a 330-fold lower dose than infused rhGAA, significantly suppressed GAA-specific inhibitory antibody formation in Pompe mice with a threefold reduction in GAA-specific IgG1 titre and significant suppression of GAA-specific IgG2a (Su et al., 2015a,b), further demonstrating the high efficacy of oral tolerance induction by plant cells.
Future perspectives
Field trials were conducted a decade ago using transplastomic plants expressing biopharmaceuticals or vaccine antigens. Chloroplast genomes are maternally inherited, offering transgene containment via pollen (Jin and Daniell, 2015). Antigen expression in leaves offers the opportunity to harvest them prior to the appearance of any reproductive structures, facilitating complete transgene containment via both pollen and seeds. Most importantly, a USDA-APHIS certification (dated 1/30/2013 to Dr. Daniell) stated that transplastomic lines do not fit the definition of a regulated article under USDA-APHIS regulations 7 CFR part 340, because there are no plant pest components, which should further help in advancing this technology. In addition to potential regulatory advantages, recent advances in developing novel therapeutic proteins in chloroplasts for urgent unmet medical needs of several metabolic or genetic disorders, ability to express high levels of proteins in edible plant cells, store lyophilized cells at ambient temperature for several months/years without losing therapeutic efficacy, suitability for scale-up of production in cGMP facilities and support of major pharmaceutical companies augur well for advancing this low-cost and cold chain-free delivery system to lower the cost of protein drugs and make them affordable to populations in great need.
References
- Choi J, Diao H, Feng ZC, Lau A, Wang R, Jevnikar AM, Ma S. A fusion protein derived from plants holds promising potential as a new oral therapy for type 2 diabetes. Plant Biotechnol. J. 2014;12:425–435. doi: 10.1111/pbi.12149. [DOI] [PubMed] [Google Scholar]
- Jin S, Daniell H. Engineered chloroplast genome just got smarter. Trends Plant Sci. 2015 doi: 10.1016/j.tplants.2015.07.004. doi.org/10.1016/j.tplants.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli N, Westerveld DR, Ayache AC, Verma A, Shil P, Prasad T, Zhu P, Chan SL, Li Q, Daniell H. Oral delivery of bioencapsulated proteins across blood-brain and blood-retinal barriers. Mol. Ther. 2014;22:535–546. doi: 10.1038/mt.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KC, Nityanandam R, New JS, Daniell H. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stimulates insulin secretion in beta-TC6 cells. Plant Biotechnol. J. 2013;11:77–86. doi: 10.1111/pbi.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy V, Kwon KC, Rathinasabapathy A, Lin S, Jin G, Song C, Shil P, Nair A, Qi Y, Li Q, Francis J, Katovich MJ, Daniell H, Raizada MK. Oral delivery of Angiotensin-converting enzyme 2 and Angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension. 2014;64:1248–1259. doi: 10.1161/HYPERTENSIONAHA.114.03871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman A, Su J, Lin S, Wang X, Herzog RW, Daniell H. Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood. 2014;124:1659–1668. doi: 10.1182/blood-2013-10-528737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shil PK, Kwon KC, Zhu P, Verma A, Daniell H, Li Q. Oral delivery of ACE2/Ang-(1-7) bioencapsulated in plant cells protects against experimental uveitis and autoimmune uveoretinitis. Mol. Ther. 2014;22:2069–2082. doi: 10.1038/mt.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Zhu L, Sherman A, Wang X, Lin S, Kamesh A, Norikane JH, Streatfield SJ, Herzog RW, Daniell H. Biomaterials. 2015a. Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. doi:10.1016/j.biomaterials.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Sherman A, Doerfler PA, Byrne BJ, Herzog RW, Daniell H. Oral delivery of Acid Alpha Glucosidase epitopes expressed in plant chloroplasts suppresses antibody formation in treatment of Pompe mice. Plant Biotechnol. J. 2015b;13:1023–1032. doi: 10.1111/pbi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh G. Biopharmaceutical benchmarks, 2014. Nat. Biotechnol. 2014;32:992–1000. doi: 10.1038/nbt.3040. [DOI] [PubMed] [Google Scholar]