Abstract
Rationale
In response to injury, the rodent heart is capable of virtually full regeneration via cardiomyocyte proliferation very early in life. This regenerative capacity, however, is diminished as early as one week post-natal and remains lost in adulthood. The mechanisms that dictate post injury cardiomyocyte proliferation early in life remain unclear.
Objective
To delineate the role of miR-34a, a regulator of age-associated physiology, in regulating cardiac regeneration secondary to myocardial infarction (MI) in neonatal and adult mouse hearts.
Methods and Results
Cardiac injury was induced in neonatal and adult hearts through experimental MI via coronary ligation. Adult hearts demonstrated overt cardiac structural and functional remodeling, whereas neonatal hearts maintained full regenerative capacity and cardiomyocyte proliferation, and recovered to normal levels within one week time. As early as one week post-natal, miR-34a expression was found to have increased and was maintained at high levels throughout the lifespan. Intriguingly, seven days following MI, miR-34a levels further increased in the adult but not neonatal hearts. Delivery of a miR-34a mimic to neonatal hearts prohibited both cardiomyocyte proliferation and subsequent cardiac recovery post-MI. Conversely, locked nucleic acid-based anti-miR-34a treatment diminished post-MI miR-34a upregulation in adult hearts and significantly improved post-MI remodeling. In isolated cardiomyocytes, we found that miR-34a directly regulated cell cycle activity and death via modulation of its target genes, including Bcl2, Cyclin D1, and Sirt1.
Conclusions
miR-34a is a critical regulator of cardiac repair and regeneration post-MI in neonatal hearts. Modulation of miR-34a may be harnessed for cardiac repair in adult myocardium.
Keywords: miR-34a, cardiac regeneration, myocardial infarction, cell death, proliferation
INTRODUCTION
Amphibians and lower vertebrates, such as zebrafish, exhibit a high capacity for cardiac regeneration throughout their lifespan and are capable of full recovery from significant injury, in part, through proliferation of existing cardiomyocytes.1, 2 Recently, studies have shown that mice also maintain a significant capacity for cardiac repair and regeneration following injury such as apex resection or myocardial infarction (MI), particularly if the injury occurs within the first days of life.3, 4 Such endogenous repair mechanisms, however, are largely lost within 7 days after birth and remain absent in adult hearts, with evidence of limited regeneration of unclear functional significance following injury.5, 6 Understanding the mechanisms that give rise to cardiac repair and regeneration early in neonatal mouse hearts may reveal new therapeutic strategies to enhance endogenous regenerative mechanisms in the adult heart following MI.
MicroRNAs (miRs) are small non-coding RNA molecules that are critical to a large array of cellular processes, such as survival and growth in health, aging and disease.7–9 miRs act in part through coordinated modulation of multiple genes and subsequent downstream gene networks to bring about complex changes in cellular behavior.10–12 miR-34a, one of the three members of the miR-34 family, has been found to be a suppressor of cellular proliferation, including in cancer cells. It also regulates normal functions including cell differentiation and organ development.13–15 Moreover, miR-34a has been noted to be upregulated over time in endothelial cells and cardiomyocytes,16, 17 with cardiac injury,17–19 and in patients with heart failure.20 While inhibition of miR-34a may protect against the decline in cardiac function with aging,17 it is less clear whether this may be beneficial in models of post-MI cardiomyocyte proliferation and regeneration.17, 19 Furthermore, the role of miR-34a in regulating endogenous cardiac regeneration in the early post-natal heart remains largely unknown.
In this study, we reveal that cardiac miR-34a levels are low in the early post-natal period, in conjunction with preserved regenerative capacity, and soon rise to adult levels within one week following birth. Overexpression of miR-34a in early post-natal mice was found to limit cardiomyocyte proliferation and cardiac regeneration with injury. Conversely, antagonism of miR-34a improved post-MI cardiac function in adult mice in part through modulation of cell cycle and survival genes, including Bcl2, Cyclin D1 and Sirt1. Collectively, our findings support miR-34a as a key parameter regulating endogenous post-injury cardiac repair and regeneration.
METHODS
Animal models
Eight week old male C57BL/6J and pregnant female mice (E16) (#027) were purchased from Charles River Laboratories and housed in a temperature-controlled environment with 12 hours light/dark cycles with food and water available ad libitum. MI was generated by ligating the main branch coronary artery in mice one day post birth (neonatal) or at 8 weeks of age (adult) as described previously.21 For surgical MI, adult mice were anesthetized with isofluorane (2%). A rodent ventilator (model 845; Harvard Apparatus Inc.) was used to supply oxygen with 2% isofluorane during the surgical procedure. Neonatal mice were anesthetized on ice for 6 minutes and kept on ice during the surgical procedure. Adult and neonatal chests were opened by a horizontal incision through the muscle between the fourth and the fifth intercostal space. The pericardium was then removed in adult mice only. The descending coronary artery was permanently ligated with a suture: 8-0 prolene (BV175-7) for adults and 10-0 nylon (BV100-4) for neonates. After surgery, the thoracic wall and skin were closed with sutures: 5-0 absorbable (CV-23) for adult and 8-0 prolene for neonates. Sham operated animals underwent an identical surgical operation without occlusion of the coronary artery. To prevent any post-operative discomfort, adult animals received buprenorphine (0.03–0.06 mg/kg). All animal procedures were performed under the guidelines of Harvard Medical School, the Institutional Animal Care and Use Committee (IACUC), and the National Society for Medical Research.
miRNA mimic and LNA miRNA inhibitor delivery
Immediately after neonatal MI, a miR-34a mimic (miRIDIAN) or control miRNA mimic was injected intra-myocardially into each mouse (5mg/kg). Six hours and 2 days after MI in adults, a miR-34a LNA inhibitor (miRCURY) or control LNA inhibitor was injected intravenously via tail vein into each mouse (5mg/kg). Non-invasive transthoracic echocardiography, performed as previously described,22 was used to determine the extent of initial injury four hours following MI. Structural and functional alterations were also evaluated through non-invasive transthoracic echocardiography at one, three and seven days following cardiac injury.
An expanded Methods section is available in the Online Data Supplement.
RESULTS
Cardiac regeneration in neonatal hearts is associated with increased cell cycle activity
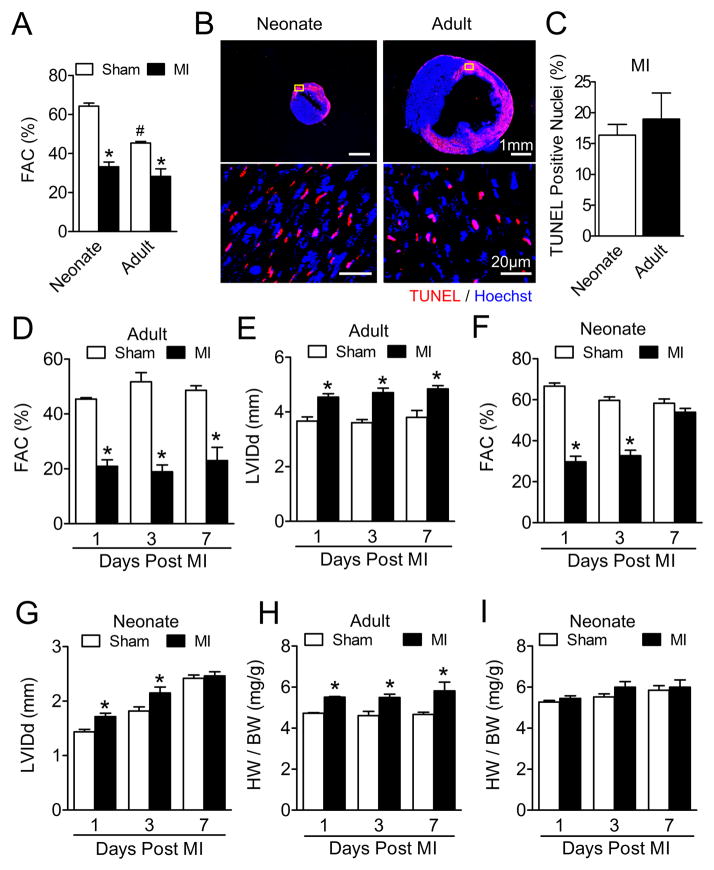
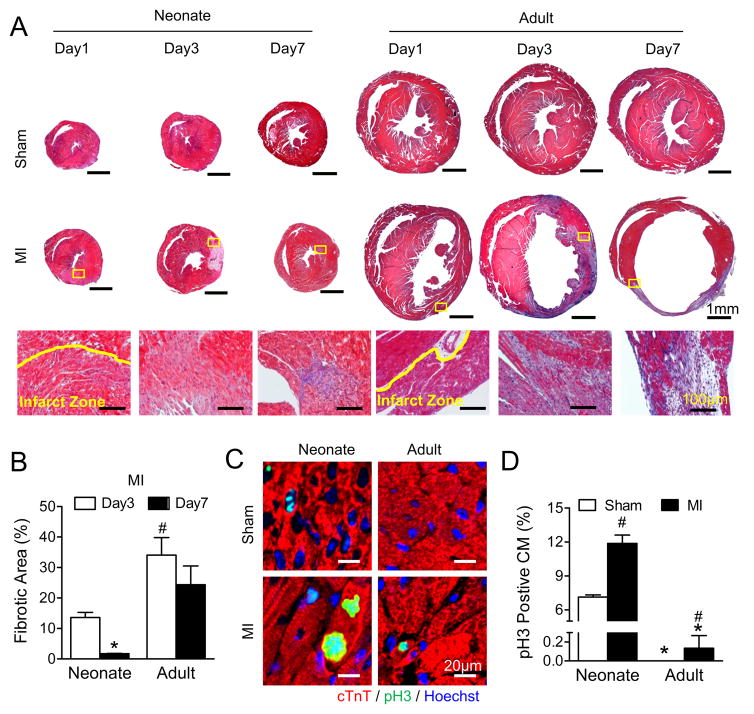
To examine cardiac regenerative capacity in neonatal and adult mouse hearts, MI was induced in 1-day-old neonatal and 8-week-old adult mice by coronary ligation. The degree of cardiac injury was determined by decrements in contractile function using non-invasive transthoracic echocardiography four hours post-surgery, and was comparable with MI in neonatal and adult animals (Figure 1A) at similar heart rates (Online Figure I). Similarly, early post-MI cell death was comparable between neonatal and adult hearts, with similar degrees of TUNEL staining (Figures 1B and 1C). Following MI, cardiac performance progressively declined in adult hearts relative to sham-operated counterparts, with reduction in cardiac function (Figure 1D) and dilatation of ventricular chambers (Figure 1E). In contrast, while early indices of cardiac performance were reduced in neonatal hearts with injury, cardiac function and chamber dimensions were restored to baseline sham levels within seven days post-MI, consistent with significant cardiac recovery (Figures 1F and 1G). Likewise, whereas adult hearts underwent cardiac remodeling following injury with increased heart weight to body weight ratios (HW/BW) (Figure 1H), neonatal HW/BW ratios were comparable to sham operated controls (Figure 1I). Consistent with cardiac recovery, neonatal hearts post-MI exhibited limited fibrotic tissue replacement (Figures 2A and 2B) and increased cardiomyocyte cell cycle activity (Figures 2C and 2D). In contrast, adult hearts post-MI exhibited very limited cardiomyocyte proliferation. Note that collagen fibril formation was not mature at 1 day post-MI, and was therefore not quantified using staining with Masson’s Trichrome.
Figure 1. Myocardial recovery following myocardial infarction in neonatal hearts.
A, Fractional area change (FAC%) in neonatal and adult hearts 4 hours post-myocardial infarction (MI) compared to Sham controls measured by echocardiographic analysis. * P<0.05 vs. Sham. # P<0.05 vs. Neonate. N=9 for Neonate Sham; N=7 for Neonate and Adult MI; N=6 for Adult Sham. B, TUNEL assay (red) in ventricular cross sections of neonatal and adult hearts 8 hours post-MI; samples were counterstained with Hoechst (blue) dye. Representative images of whole ventricular cross sections (Upper panels), yellow boxes indicate representative infarcted areas shown at higher magnification (Lower panels). C, Quantification of TUNEL positive nuclei (N=3 for Neonate and Adult) shown in (B). D to G, FAC% measurements of adult (D) and neonatal (F) post-MI hearts by echocardiographic analysis. * P<0.01 vs. Sham. Left ventricular diameter at end diastole (LVIDd/mm) measurements of adult (E) and neonatal (G) post-MI hearts by echocardiographic analysis. * P<0.05 vs. Sham. N=6 for Day 1 and Day 3 Sham; N=3 for Day 7 Sham and MI; N=7 for Day 1 and Day 3 MI. H to I, Postmortem measurements of heart weight (mg) and body weight (g) ratios of adult (H) and neonatal (I) post-MI hearts. * P<0.05 vs. Sham. N=3 for Adult; N=5 for Neonate.
Figure 2. Less fibrotic area and more mitotic cardiomyocytes in neonatal than adult hearts.
A, Fibrosis was assessed by Trichrome staining in both neonatal and adult hearts following myocardial infarction (MI). Representative images of whole sections (Upper panels); and higher magnification of infarct areas (Lower panels), indicated by yellow boxes on the whole sections, are shown. B, Fibrotic area quantifications from day 3 and day 7 post-MI, * P<0.05 vs. Day 3, # P<0.05 vs. Neonate. N=8 for Neonate Day 3; N=6 for Neonate Day 7and Adult Day 3; N=7 for Adult Day 3. C, Immunostaining of neonatal and adult ventricular cross sections against phospho-histone H3 (pH3). D, Quantification of pH3 positive myocytes (CM) at the border areas 7 days post-MI. Note that the total count for pH3 positive CM for adult-sham was 0. * P<0.05 vs. Neonate. # P<0.05 vs. Sham. N=4 for Adult; N=3 for Neonate.
miR-34a expression is increased in adult but not in neonatal mouse hearts post-MI
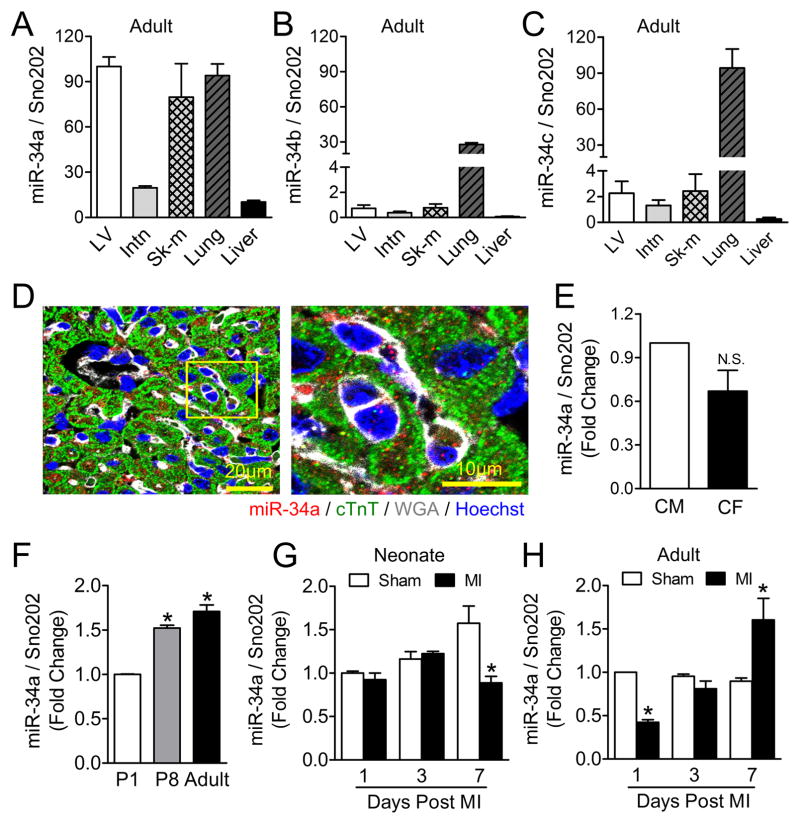
Expression of miR-34 family members (miR-34a, miR-34b, and miR-34c) was examined in the mouse left ventricle (LV), intestine (Intn), skeletal muscle (Sk-m), lung and liver tissues, with miR-34a found to be most highly expressed in heart tissue (Figures 3A–C). Using in situ hybridization, we found that miR-34a is expressed in both cardiomyocytes and non-cardiomyocytes within the heart (Figure 3D, U6 and scrambled miRNA control in Online Figure II). Real time PCR revealed a similar degree of miR-34a expression between neonatal rat cardiomyocytes and neonatal rat cardiac fibroblasts (Figure 3E), with increasing expression over the first week of life, reaching adult levels at one week post-birth (Figure 3F). With cardiac injury, miR-34a levels remained low in early post-natal hearts (Figure 3G), whereas in adult hearts, miR-34a levels initially declined within 24 hours post-cardiac injury and then increased progressively within one week post-MI (Figure 3H).
Figure 3. miR-34a expression levels in mouse cardiac tissue.
A to C, Real-time-PCR analysis of miR-34a (A), miR-34b (B) and miR-34c (C) levels in various organs in adult mice. N=4. D, Postnatal day 8 mouse ventricular cross sections were subjected to in situ hybridization using DIG-labeled miR-34a probes (red), anti-cardiac Troponin T (cTnT, green) antibodies as well as wheat germ agglutinin (WGA, white) and Hoechst (blue) showing cardiomyocytes, membranes and nuclei respectively. Representative image of a cross section (Left) followed by a higher magnification picture (Right) of the region indicated by the yellow box. E, Real-time-PCR analysis of miR-34a expression levels in cultured neonatal rat ventricular myocytes (CM; N=5) and neonatal rat cardiac fibroblast (CF; N=5). F, Real-time-PCR analysis of miR-34a expression levels in postnatal day 1 (P1), day 8 (P8) and day 60 (Adult) left ventricles. * P<0.01 vs. P1. N=3 for P1; N=5 for P8; N=6 for Adult. G and H, Real-time-PCR analysis of miR-34a levels at the border area of 1 day, 3 days and 7 days post-myocardial infarction (MI) neonatal (G) and adult (H) mouse hearts. *P<0.05 vs. Sham. N=3 for Neonate Day 1, Neonate Day 3, and Adult Sham; N=4 for Neonate Day 7 and Adult MI. In each of the above real-time PCR analyses, Sno202 RNA was used as an internal normalization control for small RNA.
miR-34a overexpression prevents post-MI recovery in neonatal hearts
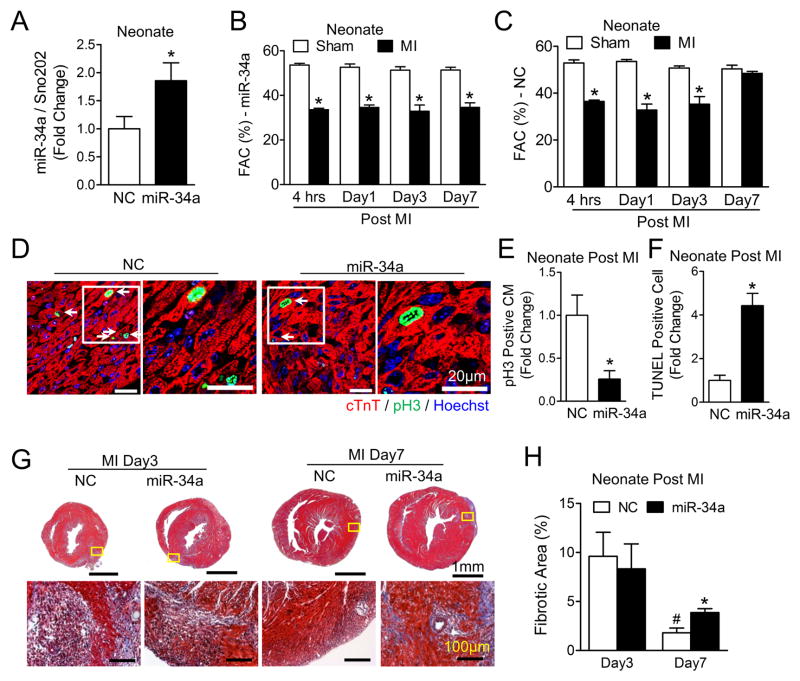
To determine if miR-34a modulates post-MI cardiac regeneration in early post-natal hearts, miR-34a levels were augmented by delivery of a miR-34a mimic to the myocardium at the time of MI with confirmed miR-34a sustained expression at 7 days post-injection (Figure 4A). Increased miR-34a levels inhibited functional post-MI recovery in neonatal mouse hearts (Figure 4B), whereas no effect was observed with delivery of a control miR mimic (Figure 4C). miR-34a overexpression reduced post-MI cardiomyocyte proliferation (Figures 4D and 4E), increased programmed cell death (Figure 4F and Online Figure III) and resulted in greater tissue fibrosis (Figures 4G and 4H). Collectively, these results suggest that low miR-34a levels in the early post-natal heart enable cardiac regeneration.
Figure 4. miR-34a overexpression inhibits neonatal post-myocardial infarction recovery.
A, Real-time-PCR analysis of miR-34a levels in a negative control miRNA mimic (NC) and in miR-34a mimic injected neonatal mouse hearts 7 days after injection. Sno202 RNA was used as an internal normalization control for small RNAs. * P<0.05 vs. NC. N=7. B and C, Echocardiographic analysis of FAC at designated time points on MI and sham neonatal mice following either miR-34a (B) or NC (C) mimic intramyocardial injection. * P<0.01 vs. Sham. N=3 for Sham-miR-34a; N=4 for MI-miR-34a and MI-NC; N=5 for Sham-NC. D, Neonatal ventricular cross sections immune-stained against phospho-histone H3 (pH3), representative images of cross sections at low magnification (Left) showing pH3 positive cardiomyoctyes (CM) (white arrows) and higher magnification images (Right) of regions indicated by white boxes on the low magnification sections. E, Quantification of pH3 positive CMs at the border areas 7 days post-MI, * P<0.05 vs. NC. N=9 for NC; N=6 for miR-34a. F, Quantification of TUNEL positive nuclei at the border area from neonatal ventricular cross sections 7 days post-MI, * P<0.01 vs. NC. N=6 for NC; N=4 for miR-34a. G, Trichrome staining for the assessment of fibrosis in neonatal hearts following MI; representative images of whole sections (Upper panels) with higher magnification images infarct areas (Lower panels) regions indicated by yellow boxes on the whole sections. H, Fibrotic area quantification is shown. N=3. * P<0.05 vs. NC. # P<0.05 vs. Day 3. N=4 for NC Day 3; N=3 for NC Day 7 and miR-34a.
Inhibition of miR-34a improves cardiac function in adult hearts post-MI
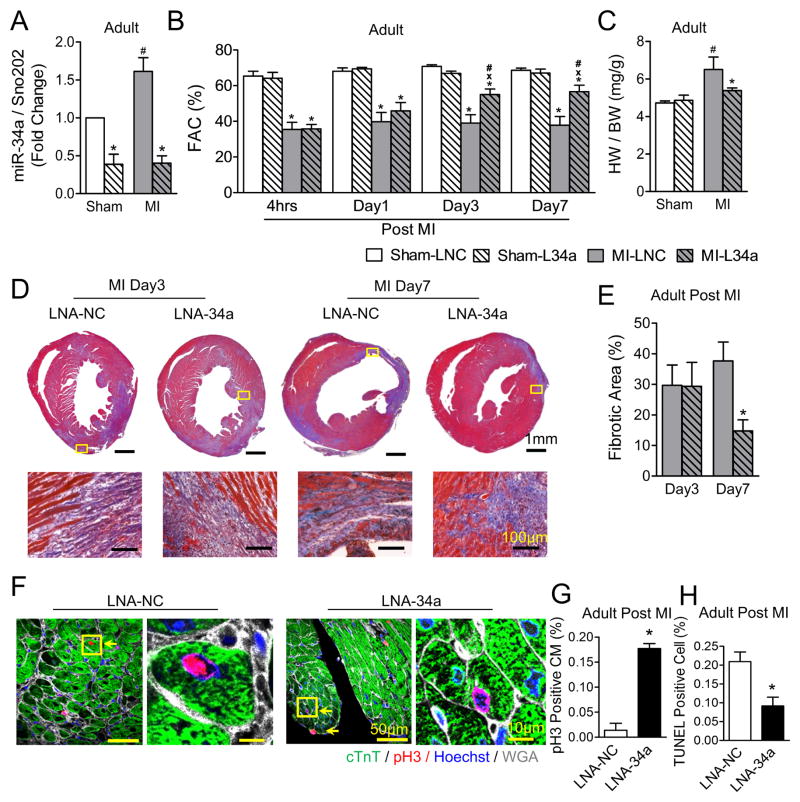
To determine if antagonizing miR-34a enhances cardiac regeneration in adult hearts, LNA based anti-miR-34a (LNA-34a) or scrambled negative control (LNA-NC) was delivered intravenously at six hours and two days following MI in adults (Online Figure IVA). LNA-34a effectively inhibited miR-34a expression levels (Figure 5A), with subsequent improvement in post-MI cardiac function (Figure 5B), diminished cardiac remodeling (Figure 5C) and reduced fibrosis (Figures 5D and 5E) in adult hearts. Inhibition of miR-34a levels also increased cardiomyocyte cell cycle activity (Figures 5F and 5G) and prevented cell death in adult hearts post-MI (Figure 5H and Online Figure IVB), suggesting that upregulation of miR-34a may contribute to the loss of endogenous regeneration in the adult heart.
Figure 5. Inhibition of miR-34a improves cardiac function in adult post-myocardial infarction hearts.
A, Real-time-PCR analysis of miR-34a levels at the border area of day 7 MI or sham adult mice following either LNA-NC (LNC) or LNA-34a (L34a) intravenous injection; Sno202 RNA was used as an internal normalizing control for small RNAs. * P<0.05 vs. LNC. # P<0.05 vs. Sham. N=4 for all groups. B, Echocardiographic analysis at designated time points on MI or sham adult mice following either LNC or L34a injection. * P<0.01 vs. Sham. x P<0.05 vs. LNC. # P<0.05 vs. 4hrs. N=5 for all groups. C, Postmortem measurements of heart weight (mg) and body weight (g) ratios of adult post-MI hearts. * P<0.05 vs. LNC. # P<0.05 vs. Sham. N=5 for Sham; N=6 for MI-LNC; N=8 for MI-L34a. D, Trichrome staining for the assessment of fibrosis in adult hearts following MI. Representative images of whole cross sections (Upper panels) with higher magnification images of infarct areas (Lower panels) indicated by yellow boxes on the whole sections. E, Fibrotic area quantification is shown, * P<0.05 vs. LNC. N=3 for LNC; N=4 for Day 7 L34a. F, Adult ventricular cross sections immune-stained against phospho-histone H3 (pH3). Representative images of cross sections at low magnification (Left) showing pH3 positive cardiomyoctyes (CM) (yellow arrows) and high magnification images of regions (Right) indicated by yellow boxes on the low magnification sections. G, Quantification for pH3 positive CMs at the border areas 7 days post-MI is shown. * P<0.05 vs. LNA-NC. N=3 for all groups. H, Quantification of TUNEL positive nuclei at the border area from adult ventricular cross sections 7 days post-MI. # P<0.01 vs. LNA-NC. N=3 for all groups.
miR-34a overexpression inhibits neonatal cardiomyocyte cell cycle progression and survival
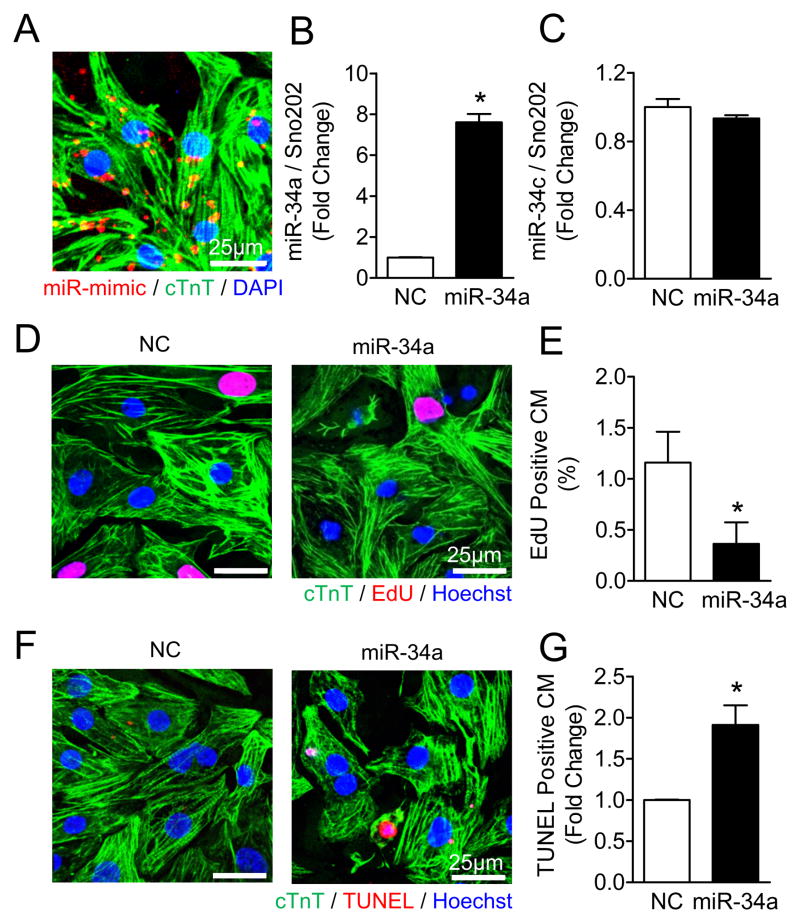
To determine whether miR-34a regulates cardiac regeneration in a cell autonomous manner, neonatal cardiomyocytes were directly transfected with a miR-34a mimic in vitro. Immunostaining of Dy547-labeled miRNA mimic suggested efficient entry into neonatal cells (Figure 6A) with a transfection yield of 97.4 ±1.6%, and specific upregulation of miR-34a expression (Figure 6B) without change in the expression of other miR-34 family members such as miR-34c (Figure 6C). Increasing expression levels of miR-34a reduced incorporation of the thymidine analogue, EdU, in neonatal cardiomyocytes suggesting inhibition of entry into S phase of the cell cycle (Figures 6D and 6E). Moreover, direct overexpression of miR-34a induced cell death in neonatal cardiomyocytes (Figures 6F and 6G). These data indicate that miR-34a directly modulates cardiomyocyte proliferation and survival in a cell autonomous manner.
Figure 6. miR-34a overexpression inhibits neonatal cardiomyocyte cell cycle progression and survival.
A, Neonatal rat ventricular myocytes (NRVMs) transfected with 20nM Dy547-labeled miRNA mimic were fixed 48h post-transfection and immunostained against cardiac Troponin T (cTnT, green). DAPI (blue) was used for nuclear staining. B to C, Real-time-PCR analysis of miR-34a (B) and miR-34c (C) levels in 20nM NC or miR-34a mimic transfected NRVMs. Sno202 RNA was used as an internal normalizing control for small RNAs. * P<0.05 vs. NC. N=3 for all groups. D, NRVMs transfected with 20nM NC or miR-34a mimic were treated with a thymidine analogue (50μM EdU) for 48 hours and stained with an EdU assay kit (red). E, Quantification of EdU positive cardiomyocytes (CM) shown in (D), * P<0.05 vs. NC. N=4 for NC; N=5 for miR-34a. F, NRVMs transfected with 20nM NC or miR-34a mimic for 48 hours were stained with a TUNEL assay kit (red). G, Quantification of TUNEL positive cardiomyocytes shown in (F). * P<0.05 vs. NC. N=5 for all groups.
miR-34a regulates cardiomyocyte proliferation and survival through expression of Bcl2, Cyclin D1 and Sirt1
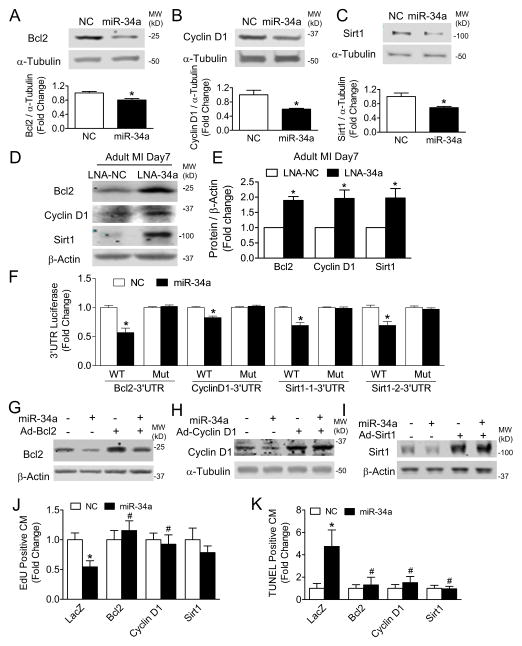
To identify potential miR-34a targets responsible for modulating proliferation and survival of cardiomyocytes post-MI, we examined genome wide microarray mRNA expression, and focused on those genes selectively downregulated in adult hearts relative to neonatal counterparts, with seven genes predicted as miR-34a targets. We further prioritized follow up investigations of four genes, Sirt1, Cyclin D1, Bcl2 and Jag1, previously implicated in cellular aging, cell cycle activity, survival and the Notch pathway, all potentially important components of cardiac regeneration. 23–27 Among these four genes, we found that miR-34a did not inhibit Jag1 3′UTR luciferase activity (Online Figure VA) or protein levels (Online Figures VB and VC) indicating that Jag1 is not a target of miR-34a in our experimental setting. miR-34a, however, effectively reduced protein levels for Sirt1, Cyclin D1 and Bcl2 in both cardiomyocytes in vitro and whole hearts in vivo (Figures 7A–C, Online Figures VIA and VIB). Conversely, inhibition of miR-34a by LNA in adult post-MI hearts increased protein levels of all three target genes (Figures 7D and 7E). 3′UTR luciferase assays confirmed miR-34a modulation of Bcl2, Cyclin D1 and Sirt1 expression, with loss of miR-34a dependency with selective mutation of the miR-34a binding site (Figure 7F). miR-34a mediated downregulation of target genes was rescued via adenoviral mediated overexpression of Bcl2, Cyclin D1 and Sirt1 (Figures 7G–I). Maintenance of Bcl2 and Cyclin D1 protein levels in neonatal cardiomyocytes preserved cardiomyocyte proliferation and cell cycle capacity as well as protected against cell death mediated by the miR-34a expression (Figures 7J, 7K and Online Figures VIIA and VIIB). Of note, Sirt1 was effective only in protecting against cell death but not loss of cell proliferation (Figures 7J and 7K). Collectively, these results suggest that the down-regulation of Bcl2, Cyclin D1 and Sirt1 may play important roles in mediating the effects of miR-34a on MI cardiomyocyte cell death and proliferation.
Figure 7. Bcl2, Cyclin D1 and Sirt1 are targets of miR-34a and mediate miR-34a’s function.
A to C, NRVMs transfected with 20nM NC or miR-34a mimic for 48 hours were harvested for immunoblot analyses using anti-Bcl2 (A), anti-Cyclin D1 (B), anti-Sirt1 (C) and anti-α-Tubulin antibodies. Results were quantified by densitometry. * P<0.05 vs. NC. N=3 for NC; N=4 for miR-34a. D, Immunoblot analyses in adult 7 days post-MI hearts using anti-Bcl2, anti-Cyclin D1, anti-Sirt1 and anti-β-Actin antibodies. E, Densitometry quantification of results shown in (D), * P<0.05 vs. LNA-NC. N=3 for LNA-NC; N=4 for LNA-34a. F, Luciferase assays performed with HEK-293 cells co-transfected with luciferase constructs harboring 3′UTR sequences from either wild-type (WT) or mutated (Mut) targets: Bcl2, Cyclin D1 and Sirt1 with or without miR-34a, * P<0.05 miR vs. NC. N=8 for all groups. G to I, 12 to 24 hours after transfection with NC or miR-34a mimic and transduced with adenoviruses: Ad-LacZ, Ad-Bcl2, Ad-Cyclin D1, or Ad-Sirt1, NRVMs were harvested for immunoblot analyses using anti-Bcl2 (G), anti-Cyclin D1 (H), anti-Sirt1 (I), anti-β-Actin or anti-α-Tubulin antibodies. J, 12 to 24 hours after transfection with NC or miR-34a mimic and transduction with adenoviruses: Ad-LacZ, Ad-Bcl2, Ad-Cyclin D1, or Ad-Sirt1, NRVMs were treated with 50μM EdU for 48 hours. Cells were stained with an EdU assay kit and subjected to EdU positive cardiomyocytes (CM) quantification. * P<0.05 vs. NC. # P<0.05 vs. LacZ. N=5 for all groups. K, 12 to 24 hours after transfection with NC or miR-34a mimic, NRVMs were transduced with Ad-LacZ, Ad-Bcl2, Ad-Cyclin D1, or Ad-Sirt1 for 48 hours. Cells were stained with a TUNEL assay and subjected to TUNEL positive CM quantification. * P<0.05 vs. NC. # P<0.05 vs. LacZ. N=5 for all groups.
DISCUSSION
Recent studies have revealed that neonatal rodent hearts maintain a significant regenerative capacity following cardiac injury, particularly if such injury is imposed within the first few days post-birth. 3, 4, 21, 28–30 The processes mediating the regenerative capacity of neonatal hearts and the mechanisms by which this regenerative capacity is lost early after birth, still remain unclear. Consistent with prior reports, we found that neonatal hearts less than two days old recovered almost fully from significant MI injury without loss of contractile function and with limited evidence of scarring/remodeling.4, 31, 32 Prior literature has identified miRNAs as critical regulators of cellular processes including senescence, proliferation and survival. miR-34a levels have been shown to increase in response to the aging process in a number of tissues, including the heart.16, 17 Our results demonstrate that the expression of miR-34a early post-birth is closely associated with the loss of regenerative potential. Consistent with this observation, antagonizing miR-34a expression in adult mouse hearts markedly improved cardiac repair and post-MI remodeling, whereas increasing miR-34a expression in early post-natal hearts impaired regeneration. Together, our results suggest that miR-34a may represent a critical key regulator of repair/regeneration capacity in the heart, and may be harnessed for enhancing endogenous repair mechanisms in the adult heart post-MI.
The therapeutic effects of miR-34a have been previously examined in the heart with mixed results.17,19 Consistent with our findings, miR-34a has been found to be highly expressed in cardiac tissue, and loss of miR-34a improves cardiac function and reduces cell death in aging hearts.17 Additionally, acute inhibition of miR-34a using LNA or an antagomir delivered intravenously within several hours post-MI also significantly improved cardiac function at two weeks.17 Other studies, however, have suggested that later delivery of LNA subcutaneously post-MI was only effective when targeting multiple miR-34 family members, but not miR-34a alone, even at a much higher concentration.19 Our findings are consistent with these reports, with early intravenous LNA targeting miR-34a found to improve post-MI cardiac function. It is likely that the mode of delivery, and subsequent kinetics of miR-34a inhibition, as well as the timing of miR antagonism, dictate the complex post-MI regenerative response. miR-34a was noted in our studies to be expressed in both cardiomyocytes and non-cardiomyocytes, and potential discordant effects in these cells with miR-34a inhibition may further be critical in dictating overall response. In settings of established cardiac injury, it is likely that antagonism of miR-34a alone is insufficient to promote cardiac regeneration, and a more broad inhibition of miR-34 family members is required.19, 33
Several molecular mediators and signaling pathways have been previously implicated as regulators of neonatal cardiomyocyte regeneration, including miR-15 family members and Yap.4, 32 Cardiac specific overexpression of miR-195 (a miR-15 family member) impaired neonatal post-MI regeneration with a reduced proportion of mitotic cardiomyocytes.4 Yap belongs to the evolutionarily conserved Hippo pathway that controls organ size,34 and has been implicated as a regulator of cardiomyocyte proliferation in both embryonic hearts35 and adult post-MI hearts.36 miR-34a shares a number of similarities with these mediators of neonatal cardiac regeneration. Both miR-34 and miR-15 family members have been identified as tumor suppressors, with co-regulation in cancer cells to control cell cycle progression in a synergistic and Rb-dependent fashion.37 Yap inhibits miR-34a processing by sequestering p72 from the microprocessor.38 Moreover, our data suggests that miR-34a levels are higher in adult than in neonatal hearts, and are further upregulated in adult post-MI hearts, with a pattern consistent with other negative regulators of cardiac regeneration, such as miR-15 family members.4, 39
To further assess the mechanism by which miR-34a regulates cardiomyocyte proliferation and cardiac regeneration, we specifically examined the expression of three miR-34a target genes which have previously been linked to cellular proliferation and/or survival, including Sirt1, Cyclin D1 and Bcl2. Sirt1, a class III histone deacetylase and an age-related protein, has been shown to prevent neonatal cells from death in vitro27 and to slow aging associated apoptosis and senescence.26 Cyclin D1 plays important roles in regulating cell proliferation in the developing heart. Overexpression of Cyclin D1 in the adult heart improves cardiac function following ischemic injury via promoting cardiomyocyte proliferation and survival.24 Bcl2, an anti-apoptotic protein, promotes cell survival in many organs, including the heart.40 More importantly, Bcl2 overexpression has been shown to promote cardiomyocyte cell cycle activity in postnatal mouse hearts.23 Our data suggest that the pro-survival functions of Bcl2, Cyclin D1 and Sirt1, as well as pro-proliferative nature of Bcl2 and Cyclin D1 are critical to miR-34a effects, and overexpression of these miR-34a target genes rescue cardiomyocyte cell cycle activity in miR-34a expressing cells.
While our work has suggested that miR-34a is an important regulator of endogenous cardiac regeneration and its inhibition may improve post-MI cardiac repair, the clinical implications remain to be determined. The therapeutic value of miR-34a is further complicated by the expression of miR-34a and potential differing effects in a number of cell types. For instance, given miR-34a’s tumor suppressive activity, intravenously delivered liposomal-based miR-34a mimics have entered into Phase I clinical trials as emerging cancer therapeutics. It remains to be seen whether chronic exposure to miR-34a may impact cardiac function in an adverse manner, as has been observed with other anti-cancer therapies.41
Supplementary Material
Novelty and Significance.
What Is Known?
Following myocardial infarction (MI), the regenerative capacity of adult mouse hearts is limited.
Neonatal mouse hearts, however, can mount a robust regenerative response to MI injury leading to cardiac recovery.
The mechanisms underlying robust cardiac regeneration in neonatal mouse hearts remain unknown.
What New Information Does This Article Contribute?
microRNA-34a (miR-34a) is highly expressed in adult hearts and is associated with the loss of post-injury regenerative capacity.
Overexpression of miR-34a in neonatal hearts inhibits cardiac regeneration.
Suppression of miR-34a in adult hearts increases post-MI cardiac regeneration and function.
miR-34a modulates cardiac regeneration through targeting of Bcl2, Cyclin D1 and Sirt1 in cardiomyocytes.
The capacity for robust cardiac regeneration in response to injury in neonatal hearts remains among the most promising avenues for understanding endogenous regenerative mechanisms. While microRNAs have been implicated in a number of cellular processes, their role in mediating cardiac regeneration in neonatal and adult hearts remains unknown. In this study, we find that miR-34a is increased in the myocardium soon after birth and is associated with the loss of regenerative capacity. Exogenous expression of miR-34a in neonatal hearts abrogates regeneration, and suppression of miR-34a in adult hearts improves post-injury regeneration and cardiac function. Finally, we find that miR-34a inhibits cardiomyocyte proliferation and induces cardiomyocyte cell death through targeting Bcl2, Cyclin D1 and Sirt1. These results suggest that inhibition of miR-34a may present a therapeutic option to enhance endogenous repair in adult post-MI hearts.
Acknowledgments
The authors thank Cardiovascular Physiology Core at Brigham and Women’s Hospital and Soeun Ngoy for surgical assistance and echocardiographic measurements. The authors also thank Dr. Junichi Sadoshima for providing reagents and Dr. Da-zhi Wang for his helpful suggestions and scientific insight.
SOURCES OF FUNDING
This work was supported in part by NIH grants (HL112831 and HL093148) to R.L. and American Heart Association postdoctoral fellowship award to K.U.
Nonstandard Abbreviations and Acronyms
- 3′UTR
3 prime untranslated region
- EdU
5-ethynyl-2′-deoxyuridine
- FAC
Fractional area change
- LNA
Locked nucleic acid
- MI
Myocardial infarction
- NC
Negative control miRNA
Footnotes
DISCLOSURES
None.
References
- 1.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 2.Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN, Sadek HA. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci U S A. 2013;110:187–192. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 6.Gerdes AM, Kellerman SE, Moore JA, Muffly KE, Clark LC, Reaves PY, Malec KB, McKeown PP, Schocken DD. Structural remodeling of cardiac myocytes in patients with ischemic cardiomyopathy. Circulation. 1992;86:426–430. doi: 10.1161/01.cir.86.2.426. [DOI] [PubMed] [Google Scholar]
- 7.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 8.Wu G, Huang ZP, Wang DZ. MicroRNAs in cardiac regeneration and cardiovascular disease. Sci China Life Sci. 2013;56:907–913. doi: 10.1007/s11427-013-4534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeger FH, Zeiher AM, Dimmeler S. MicroRNAs in stem cell function and regenerative therapy of the heart. Arterioscler Thromb Vasc Biol. 2013;33:1739–1746. doi: 10.1161/ATVBAHA.113.300138. [DOI] [PubMed] [Google Scholar]
- 10.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 12.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26:5017–5022. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- 15.Rokavec M, Li H, Jiang L, Hermeking H. The p53/miR-34 axis in development and disease. J Mol Cell Biol. 2014;6:214–230. doi: 10.1093/jmcb/mju003. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Yagi S, Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun. 2010;398:735–740. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Treguer K, Carmona G, Bonauer A, Horrevoets AJ, Didier N, Girmatsion Z, Biliczki P, Ehrlich JR, Katus HA, Muller OJ, Potente M, Zeiher AM, Hermeking H, Dimmeler S. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 18.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernardo BC, Gao XM, Winbanks CE, Boey EJ, Tham YK, Kiriazis H, Gregorevic P, Obad S, Kauppinen S, Du XJ, Lin RC, McMullen JR. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci U S A. 2012;109:17615–17620. doi: 10.1073/pnas.1206432109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M, Kitamura T, Hamasaki T, Nanto S, Kawahara Y, Komuro I. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res. 2013;113:322–326. doi: 10.1161/CIRCRESAHA.113.301209. [DOI] [PubMed] [Google Scholar]
- 21.Mahmoud AI, Porrello ER, Kimura W, Olson EN, Sadek HA. Surgical models for cardiac regeneration in neonatal mice. Nat Protoc. 2014;9:305–311. doi: 10.1038/nprot.2014.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer M, Cheng S, Jain M, Ngoy S, Theodoropoulos C, Trujillo A, Lin FC, Liao R. Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res. 2011;108:908–916. doi: 10.1161/CIRCRESAHA.110.239574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Limana F, Urbanek K, Chimenti S, Quaini F, Leri A, Kajstura J, Nadal-Ginard B, Izumo S, Anversa P. Bcl-2 overexpression promotes myocyte proliferation. Proc Natl Acad Sci U S A. 2002;99:6257–6262. doi: 10.1073/pnas.092672899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamamori-Adachi M, Takagi H, Hashimoto K, Goto K, Hidaka T, Koshimizu U, Yamada K, Goto I, Maejima Y, Isobe M, Nakayama KI, Inomata N, Kitajima S. Cardiomyocyte proliferation and protection against post-myocardial infarction heart failure by cyclin D1 and Skp2 ubiquitin ligase. Cardiovasc Res. 2008;80:181–190. doi: 10.1093/cvr/cvn183. [DOI] [PubMed] [Google Scholar]
- 25.Soonpaa MH, Koh GY, Pajak L, Jing S, Wang H, Franklin MT, Kim KK, Field LJ. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J Clin Invest. 1997;99:2644–2654. doi: 10.1172/JCI119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 27.Alcendor RR, Kirshenbaum LA, Imai S, Vatner SF, Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circ Res. 2004;95:971–980. doi: 10.1161/01.RES.0000147557.75257.ff. [DOI] [PubMed] [Google Scholar]
- 28.Strungs EG, Ongstad EL, O’Quinn MP, Palatinus JA, Jourdan LJ, Gourdie RG. Cryoinjury models of the adult and neonatal mouse heart for studies of scarring and regeneration. Methods Mol Biol. 2013;1037:343–353. doi: 10.1007/978-1-62703-505-7_20. [DOI] [PubMed] [Google Scholar]
- 29.Haubner BJ, Adamowicz-Brice M, Khadayate S, Tiefenthaler V, Metzler B, Aitman T, Penninger JM. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging (Albany NY) 2012;4:966–977. doi: 10.18632/aging.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jesty SA, Steffey MA, Lee FK, Breitbach M, Hesse M, Reining S, Lee JC, Doran RM, Nikitin AY, Fleischmann BK, Kotlikoff MI. c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc Natl Acad Sci U S A. 2012;109:13380–13385. doi: 10.1073/pnas.1208114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497:249–253. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernardo BC, Gao XM, Tham YK, Kiriazis H, Winbanks CE, Ooi JY, Boey EJ, Obad S, Kauppinen S, Gregorevic P, Du XJ, Lin RC, McMullen JR. Silencing of miR-34a attenuates cardiac dysfunction in a setting of moderate, but not severe, hypertrophic cardiomyopathy. PLoS One. 2014;9:e90337. doi: 10.1371/journal.pone.0090337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Re DP, Yang Y, Nakano N, Cho J, Zhai P, Yamamoto T, Zhang N, Yabuta N, Nojima H, Pan D, Sadoshima J. Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury. J Biol Chem. 2013;288:3977–3988. doi: 10.1074/jbc.M112.436311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bandi N, Vassella E. miR-34a and miR-15a/16 are co-regulated in non-small cell lung cancer and control cell cycle progression in a synergistic and Rb-dependent manner. Mol Cancer. 2011;10:55. doi: 10.1186/1476-4598-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori M, Triboulet R, Mohseni M, Schlegelmilch K, Shrestha K, Camargo FD, Gregory RI. Hippo signaling regulates microprocessor and links cell-density-dependent miRNA biogenesis to cancer. Cell. 2014;156:893–906. doi: 10.1016/j.cell.2013.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam YJ, Matkovich SJ, Dorn GW, 2nd, van Rooij E, Olson EN. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res. 2011;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gustafsson AB, Gottlieb RA. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol. 2007;292:C45–51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- 41.Force T, Kolaja KL. Cardiotoxicity of kinase inhibitors: the prediction and translation of preclinical models to clinical outcomes. Nat Rev Drug Discov. 2011;10:111–126. doi: 10.1038/nrd3252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.