Abstract
Background
Organophosphate flame retardants (OPFRs) have grown in usage since concerns about the health effects of the previously used polybrominated flame retardants led to their being phased out. The potential for OPFRs to cause adverse health effects of their own is still unexamined. Because of their structural similarities to organophosphate pesticides, which have themselves been heavily researched and shown to be neurobehavioral teratogens, we investigated the possibility that developmental exposure to two OPFRs, triphenyl phosphate (TPHP), and tris(1,3-dichloroisopropyl)phosphate (TDCIPP) might lead to behavioral impairment across the lifespan, as has been observed with the organophosphate pesticide chlorpyrifos.
Methods
Zebrafish were exposed to 0.03 or 0.3 μM of TPHP, TDCIPP, or chlorpyrifos from 0 to 5 days post fertilization. Vehicle control consisted of 0.03% solution of DMSO. At 6 days post fertilization, larvae were tested on a locomotor assay. Separate cohorts of 6 day old larvae that were not tested on the larval assay were allowed to grow to adulthood. At 12 weeks post fertilization, these adult zebrafish were tested on a battery of behavioral assays that included tests of novel environment exploration, startle habituation, social affiliation, and predator escape.
Results
Developmental exposure altered zebrafish behavior across the lifespan. Larval zebrafish exposed to the 0.03 μM doses of chlorpyrifos or TDCIPP exhibited significant (p < 0.05) hyperactivity in the locomotor assay. Organophosphate exposure significantly (p < 0.05) altered the time course of adult zebrafish behavior in the novel environment, startle habituation, and social affiliation assays. Predator escape behavior was significantly (p < 0.05) reduced in fish exposed to the 0.3 μM dose of TDCIPP. Exposure also caused hyperactivity in adult fish, with fish exposed to the 0.3 μM dose of TDCIPP exhibiting significantly (p < 0.05) elevated locomotor behavior in the novel environment assay.
Discussion
Early developmental exposure to OPFRs produced behavioral impairment that persisted into adulthood. These findings support broader research investigating the role of organophosphate compounds, including the OPFRs used here, in developmental neurotoxicity.
Keywords: Zebrafish, Organophosphate, Flame retardant, Behavior, Cognition, Developmental
1. Introduction
Recently, growing concern regarding the safety of common flame retardants has led to phase outs of the older polybrominated flame retardants and replacement with several new categories of flame retardants. Among these replacements are organophosphate flame retardants, or OPFRs. It has become apparent, since then, that exposure to OPFRs is widespread. Two common OPFRs, tris(1,3-dichloro-2-propyl)phosphate (TDCIPP) and triphenylphosphate (TPHP), have been found in over 96% of samples of house dust and furniture foam, with levels as high as 1.8 mg/g (Stapleton et al., 2009; Meeker and Stapleton, 2010), and in elementary schools at levels as high as 0.27 mg/g (Mizouchi et al., 2015). TDCIPP has similarly been found in foam inside of infant products (Stapleton et al., 2011) and in handwipe samples of children (Hoffman et al., 2015; Stapleton et al., 2014). Correspondingly, metabolites of TPHP, TDCIPP, and other OPFRs can be found in the urine of adults (Carignan et al., 2013; Meeker et al., 2013), including pregnant women and paired mothers and children (Butt et al., 2014; Hoffman et al., 2014).
Despite the growing consensus regarding widespread OPFR exposure, there has been little research concerning whether or not these compounds are actually safer than their predecessors. Specifically, the structural similarities of OPFRs to organophosphate (OP) pesticides, which have been extensively researched and shown to cause persisting toxicity, might point to a reason to be concerned regarding possible toxic effects of OPFRs. OP pesticides such as chlorpyrifos have been revealed to be especially risky with early-life exposures causing persisting neurobehavioral impairments (see Eaton et al., 2008 for a review). Rodents exposed developmentally to OP insecticides display abnormal sensorimotor response emotional dysfunction, and cognition as adults (Aldridge et al., 2005, Roegge et al., 2008, Timofeeva et al., 2008a; Timofeeva et al., 2008b). In zebrafish, a rapidly growing model for studies of neurobehavioral toxicities (Bailey et al., 2013, Bailey et al., 2015), early-life exposures to chlorpyrifos causes deficits in numerous domains of sensorimotor behavior, emotional function, and cognition (Levin et al., 2003; Levin et al., 2004; Eddins et al., 2010; Sledge et al., 2011), mirroring the findings found in rodents.
With the evidence for the neurobehavioral teratology of organophosphates spanning species and compounds, several of the existing studies of OPFR toxicity have started to investigate similar endpoints. Zebrafish larvae exposed developmentally to doses of OPFRs such as TDCIPP and TPHP below those that cause overt physiological toxicities display abnormal locomotor behavior in light and dark environments (Dishaw et al., 2014; Noyes et al., 2015). One study found that, although developmental exposure to TDCIPP did not cause behavioral effects or alter monoamine levels in larval zebrafish, females exposed through to adulthood showed depressed levels of both dopamine and serotonin later in life (Wang et al., 2015). Correspondingly, in vivo work has shown that TDCIPP can affect cellular function in the PC12 cell line and increase its differentiation into both cholinergic and dopaminergic cell types (Dishaw et al., 2011).
It is not yet understood whether early-life exposure to OPFRs can cause behavioral or cognitive abnormalities that persist into adulthood, as seen with OP pesticides. The present study seeks to examine whether developmental exposure to the OPFRs TDCIPP and TPHP leads to changes in behavior in larval and adult zebrafish. These exposures were done at doses equimolar to those of chlorpyrifos previously shown to cause lasting behavioral effects in zebrafish (Levin et al., 2003; Levin et al., 2004; Eddins et al., 2010; Sledge et al., 2011). Zebrafish exposed to two doses of chlorpyrifos, TDCIPP, or TPHP (Appendix Fig. 1) for the first 5 days post fertilization were tested on behavioral endpoints both as larvae and as adults.
2. Methods
2.1. Subject housing and husbandry
Zebrafish (AB* strain) were bred from a colony originating with progenitors obtained from the Zebrafish International Resource Center (ZIRC, Eugene, OR, USA). Breeding tanks of N = 12–15 were maintained with a male-to-female ratio of 2:1. Eggs were collected via in-tank inserts approximately 1–2 h after the lights-on phase of a 14:10 h light:dark cycle. Eggs from approximately 6 such tanks were combined and rinsed with 10,000× diluted solution of bleach for 1 min, followed by 3 likewise rinses in fresh aquarium water. Eggs were inspected under a dissection microscope and unfertilized or otherwise abnormal eggs were discarded. Approximately 5 h post fertilization, eggs (N = 60) were randomly distributed into glass Petri dishes corresponding to differing exposures and placed in an incubator held at 29 °C and illuminated with an identical 14:10 light cycle until 6 days post fertilization.
Fish aged 6 days post fertilization and older were housed in 3 L tanks on a circulating aquarium rack system (Aquatic Habitats/Pentair Aquatic Eco-Systems, Apopka, FL, USA). Aquarium water was made from a mixture of sea salt (Instant Ocean, 0.5 parts per thousand) and buffer (Seachem Neutral Regulator, 2.5-g/19 L H2O) dissolved in de-ionized water and was maintained at 26 °C. Water chemistry, salinity, and temperature were monitored biweekly. Fish were fed twice per day: a suspension of 24-h-old brine shrimp raised in-house (origin Brine Shrimp Direct, Ogden, UT, USA) in the morning and solid food (TetraMin Tropical Flakes, Blacksburg, VA, USA) in the evening. Younger fish (until 3–4 weeks post fertilization) were supplemented with smaller-particle solid food (Brine Shrimp Direct Golden Pearl). All adult behavioral testing was conducted between 11:00 AM (2 h post lights-on) and 6:00 PM, with testing time counterbalanced across experimental groups. On days of behavioral testing, the evening feeding was withheld until testing was complete. All larval testing was run between 3:00 PM and 5:00 PM.
2.2. Chemical exposures
At 5 h post fertilization, zebrafish eggs (N = 60) were placed in separate glass Petri dishes in 40 ml of solutions of chlorpyrifos, tris(1,3-dichloro-2-propyl) phosphate (TDCIPP), or triphenyl phosphate (TPHP) (Sigma–Aldrich, St. Louis, MO, USA), at either 0.03 or 0.3 μM. DMSO 0.03% in aquarium water served as a vehicle control. These solutions were renewed every 24 h, through 5 days post fertilization. Hatched larvae were then placed into fresh aquarium water for 24 h, at which point they were examined under a dissecting microscope. Larvae exhibiting arrested development or malformations were discarded. All other larvae were transferred to the aquarium rack system or prepared for the larval motility assay.
2.3. Larval motility assay
After 6-day-old larvae were inspected, they were placed into 96-well plates with glass well inserts each with 0.5 ml of aquarium water (n = 23–28 per exposure condition, over two exposure replicates). Exposure conditions were all represented within each plate and across multiple plates. Plates were then returned to the incubator for an hour before being placed into a DanioVision™ lightbox running EthoVision XT® tracking software (Noldus, Wageningen, The Netherlands). Locomotor activity was tracked during a paradigm in which an initial 10-min acclimation period in the dark (0% illumination) was followed by 2 cycles of 10 min at 100% illumination (5000 lx) and 10 min at 0% illumination. An infrared camera tracked larval locomotion across the 50-min trial. EthoVision XT® was used to calculate the average distance moved in cm per minute for each subject.
2.4. Adult behavioral test battery
6-day-old larvae distinct from those used in the larval motility assay were transferred to the aquarium rack systems and allowed to age normally to 12 weeks of age (n = 24–31 per exposure condition over two exposure replicates). Over approximately the following 2 weeks, the adult fish were run through a series of behavioral tests assessing various behavioral and cognitive functions.
2.5. Novel environment exploration
Adult fish were assessed for novel environment exploration in a test described previously (Bencan and Levin, 2008; Levin et al., 2007). Briefly, fish were placed into 1.5-L tanks filled 10 cm high with aquarium water. A camcorder feeding into EthoVision XT® software was used to track the position of the fish across a 5-min trial and to calculate both the average distance moved in cm per minute and the average distance from the floor of the tank in cm per min of the test.
2.6. Startle habituation
Habituation to a startling stimulus was tested in adult fish via a protocol used previously (Eddins et al., 2010; Sledge et al., 2011). Adult fish were placed into 40 ml of water in one of 8 translucent plastic cups (5.7 cm in diameter) arranged in a 4 × 2 array. Below each cup was a centrally located push solenoid that could be controlled to deliver a sudden tap to the bottom of the cup. A camcorder positioned above the cups was used to record the locomotion of the fish in all 8 cups at once. Following a 5-min acclimation period after the fish were placed in the cups, 10 taps were delivered once per minute over 10 min. EthoVision XT® software was used to control the solenoids and to calculate the average distance moved in cm during the 5 s preceding and following each of the 10 taps.
2.7. Social affiliation
The third test in the behavioral battery, an assessment of social affiliation, utilized a type of behavioral response called shoaling, in which a zebrafish will approach a group of conspecifics (a “shoal”) and engage in a dart/pause locomotor behavior alongside the conspecific group (“shoaling”). Adult fish were singly isolated in 1.5-L tanks surrounded by opaque dividers for 1 h. After the hour, they were placed in a large transparent rectangular tank 45 cm long by 13 cm wide by 12.5 cm deep. On both ends of the tank was positioned a computer monitor for stimulus display. The fish was recorded using a digital video recorder placed above the tank. During the first minute of the 6-min test, each monitor screen displayed a background of static ovals approximately the size of an adult zebrafish and displaying the pattern and colors of a typical zebrafish. At the end of the first minute, one of the two monitors began to display a video recording of a zebrafish shoal for the remaining 5 min. The side of the tank displaying the shoal video was counterbalanced among exposure groups. EthoVision XT® was used to control the two displays, to record the locomotion of the fish, and to calculate both the average distance traveled in cm per minute and the average distance from the tank wall through which the shoal video was visible in cm for each subject across the 6 min of the test.
2.8. Predator escape
The final test involved an assessment of escape and avoidance behavior following exposure to a predator-like stimulus. Adult fish were placed in a 1.5 L tank facing a computer monitor. The 5-min test involved an initial minute of acclimation, followed by 2 rounds of a cycle including a 1-min presentation of a small (1.3 cm diameter) circle appearing in the center of the screen, growing to 30.5 cm in diameter, disappearing, and repeating, to simulate a larger fish swimming toward the tank, followed by 1 min of a blank screen. The test was performed first with a blue dot that grew slowly (4 s), and again several days later with a red dot that grew quickly (1 s). A camcorder positioned above the tank tracked the fish. EthoVision XT® was used to control the display, to record the fish's activity, and to calculate both the average distance traveled in cm per minute and the average distance from the tank wall through which the screen was visible in cm for each subject over the 5 min. The escape/flee response was then defined as the difference between the distance from the tank wall during the minute of stimulus presentation and the distance from the wall during the preceding “blank” 1-min period.
2.9. Statistical analysis
The dependent measures corresponding to each assessment are described above. All statistical analyses were performed with Supernova/Statview (SAS, Cary, NC, USA) and all graphs were produced with SigmaPlot (SYSTAT Software Inc., Richmond, VA, USA). Type 1 error rate (α) was set at 0.05 for all tests. A mixed-design repeated-measures of analysis (RMANOVA) with organophosphate exposure and dose as the between-subject factor and tap number, session minute, or time condition as the repeated measures was used for all behavioral tests. Dunnett's test was used for all post hoc comparisons of controls to exposed groups. Survival and malformation data were analyzed using an ANOVA. A Huynh–Feldt adjustment was used to control for possible deviations from sphericity. Log transformations of the raw data were performed (as noted) when the distribution of the data was positively or negatively skewed.
3. Results
3.1. Survival
During the developmental exposures, from 5 h post fertilization (hpf) to 5 days post fertilization (dpf), the viability and survival of the embryos were tracked and logged daily. The majority of dead or nonviable eggs were identified within the first 24 h after plating and were likely due to misidentification of fertilized or healthy eggs at the time of egg selection plating at 5 hpf. These eggs regularly accounted for approximately 30% of the eggs plated and death across the remainder of the exposure was negligible (n = 0–3 eggs). At 6 dpf, hatched embryos were examined for physical malformations under a dissecting scope. Abnormal spinal curvature or edema of the pericardium or yolk sac qualified an embryo for exclusion from all further testing, occurring in 0–4% of embryos. Survival and malformation rates did not differ significantly across exposure conditions (p > 0.05).
3.2. Larval motility assay
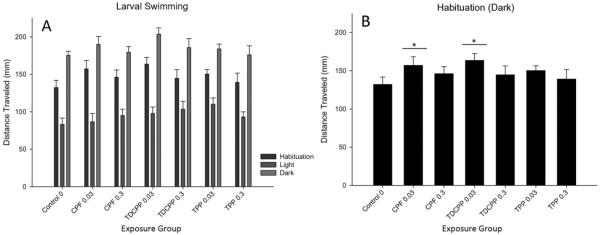
A main effect of light condition (F(1230) = 819.71, p < 0.001) on locomotion was observed, indicating the larval fish swam more in the dark than in the light. There was a main effect of OP exposure (F(6230) = 2.22, p < 0.05) on locomotor activity during the initial dark acclimation period, with fish exposed to the 0.03 μM doses of chlorpyrifos and TDCIPP swimming significantly more than control fish (p < 0.05) (Fig. 1).
Fig. 1.
Larval Activity. Panel A is a plot of the total distance traveled (mm) during the three conditions of the larval activity assay (i.e. habituation, light, and dark), as a function of exposure group. Panel B plots behavior during habituation alone, for each exposure group. Error bars represent SEM. An “*” denotes significant difference from control (p < 0.05).
3.3. Novel environment exploration
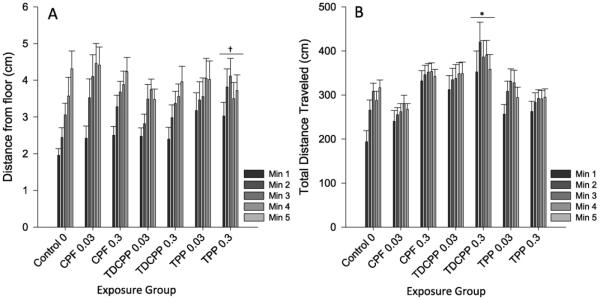
There was a main effect of minute (F(4628) = 40.30, p < 0.001) on distance from the bottom of the novel environment tank, with fish averaging a progressively farther distance from the bottom with each successive minute as is typically seen in this test. Additionally, there was a significant interaction of minute X OP exposure (F(24,628) = 1.76, p < 0.05) on distance from the bottom. Follow-up tests examined the effect of OPFR exposure on the progressive rise from the bottom over time that is normal control behavior. There was a significant effect of OP exposure (F(6157) = 2.16, p < 0.05) on the linear increase in distance from the bottom over the 5 min of the test. Dunnett's comparison showed that the fish exposed to the 0.3 μM dose of TPHP had a significantly different smaller rise than the control fish. There was also a main effect of OP exposure (F(6157) = 4.39, p < 0.001) on locomotor activity over the course of the assessment. Dunnett's comparisons showed that fish exposed to the 0.3 μM dose of TDCIPP swam significantly faster than controls (p < 0.05) (Fig. 2).
Fig. 2.
Novel Tank Exploration Assay. Panel A plots the tank location (i.e. distance from tank floor) of each exposure group for each min of the novel tank exploration trial. Panel B plots the total distance traveled for each min (i.e. swim speed) for each exposure group. Error bars represent SEM. An “*” denotes significant difference from control (p < 0.05). A “†” denotes significant linear trend (p < 0.05).
3.4. Startle habituation
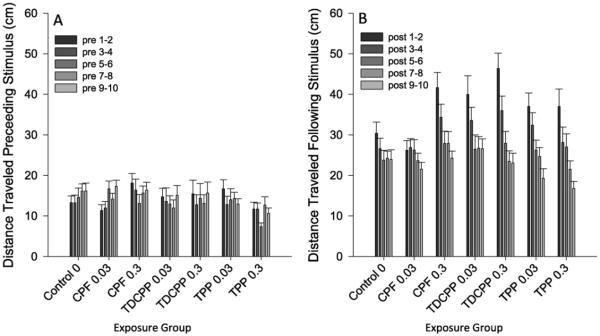
Data for the startle habituation were log-transformed prior to analysis. In the startle habituation test, there was a main effect of stimulus number on mean locomotion in the 5 s before (F(9,1341) = 3.43, p < 0.001) and after (F(9,1404) = 12.04, p < 0.001) each stimulus. Additionally, there was a significant interaction of OP exposure X stimulus number (F(54,1404) = 1.37, p < 0.05) on locomotion in the 5 s after each stimulus (Fig. 3). However, follow-up analysis did not detect any significant differences between individual OPFRs on the linear trend of stimulus response over successive trials.
Fig. 3.
Startle Habituation Assay. Panel A plots the total distance traveled in the 5″ following delivery of the tap (startle) stimulus for each exposure group for each bin of two stimulus presentations. Panel B plots the total distance traveled in the 5″ preceding delivery of the tap stimulus for each exposure group for each bin of two stimulus presentations. Error bars represent SEM.
3.5. Social affiliation
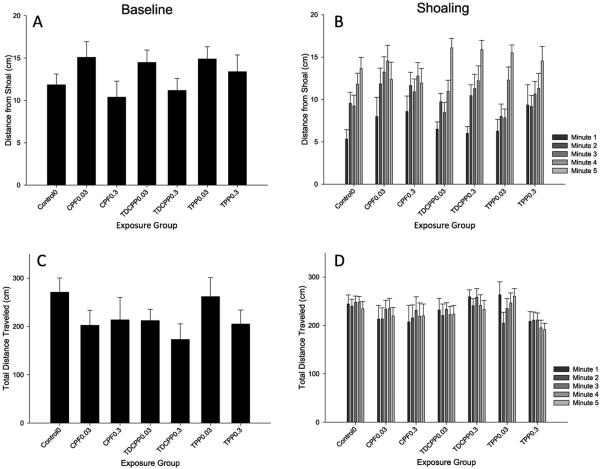
There was a main effect of minute (F(4388) = 39.44, p < 0.001) on distance from the tank wall through which the shoaling video is visible, with fish initially closest to the wall during the first minute of the video, and then averaging progressively farther distances with each subsequent minute. A significant interaction of OP exposure X minute (F(24,388) = 1.74, p < 0.05) was also observed. However, follow-up analysis did not detect any significant effects of individual OPFRs on the linear trend of distance from the shoal over time. There were no significant effects on locomotor activity (Fig. 4).
Fig. 4.
Shoaling Assay. Panels A–B plot the mean distance (cm) from the shoaling video as a function of exposure group. Panels C–D plot the distance traveled during the trial for each exposure group. Panels A and C correspond to behavior during baseline (when no shoaling image is present), and panels B and D plot behavior in the presence of the shoaling stimulus, for 5 consecutive mins. Error bars represent SEM.
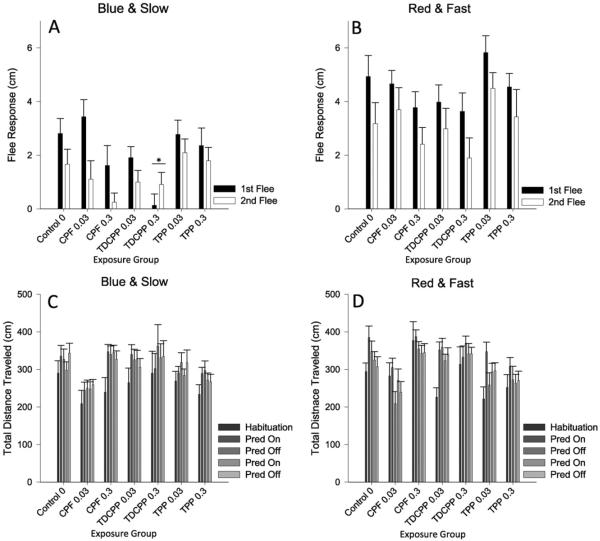
3.6. Predator escape
In the first iteration of the predator escape assessment, where the predator stimulus was a blue dot growing slowly, the fish fled the stimulus significantly less (F(1152) = 9.89, p < 0.01) the second time it was presented. Additionally, OP exposure decreased the flee response (F(6152) = 3.20, p < 0.01), with fish exposed to the 0.3-μM dose of TDCIPP fleeing significantly less than controls (p < 0.05). When the test was repeated with the predator stimulus consisting of a red dot that grew more quickly, the effect of OP exposure disappeared, although overall the fish still showed habituation upon the second presentation of the stimulus (F(1,92) = 14.00, p < 0.001). Though there was no effect of OP exposure on overall locomotor activity in the blue/slow escape test, there was in the second, red/fast iteration (F(6,92) = 3.31, p < 0.01) (Fig. 5).
Fig. 5.
Predator Escape/Avoidance Assay. Panels A–B plot the first and second flee response in the trial for each exposure group. Panels C–D plot the total distance traveled for each exposure group for each minute (or condition) of the trial. Data during the blue/slow stimulus presentation are plotted in panels A and C and data during the red/fast stimulus presentation are plotted in panels B and D. Error bars represent SEM. An “*” denotes significant difference from control (p < 0.05).
4. Discussion
Human exposure to organophosphate flame retardants (OPFRs) is very widespread—indeed, nearly ubiquitous in populations studied thus far, including pregnant women and children (Hoffman et al., 2015; Stapleton et al., 2014; Butt et al., 2014; Hoffman et al., 2014). Considering this widespread occurrence, it is concerning that so little is known about the possible effects of developmental exposures to OPFRs, especially given that the structurally similar OP pesticides cause well-characterized neurodevelopmental toxicities. This study was conducted to begin to address these concerns by examining whether early-life exposure to OPFRs can cause abnormal behavior and cognition throughout the lifespan and to compare these effects to those of the OP pesticide chlorpyrifos, using a zebrafish behavioral battery.
Developmental exposure to OPFR compounds produced widespread effects across all of the behavioral assays utilized. Locomotor activity was increased in both the larval assay and in the adult novel environment exploration test. Specifically, larvae exposed to the lower (0.03 μM) dose of either chlorpyrifos or TDCIPP showed increased activity during the initial dark habituation period in the locomotor assay. However, it was the high dose (0.3 μM) of TDCIPP that showed increased locomotion in the novel environment task during adulthood. OP exposure also impacted a number of cognitive endpoints, altering the temporal pattern of exploration in the novel environment assay, with the 0.3-μM dose of TPHP attenuating exploration in adult fish. While habituation to the repeated presentation of a predator stimulus was unaffected by OP exposure in either stimulus type, exposure did alter the degree to which the fish fled the blue stimulus, with the 0.3-μM dose of TDCIPP attenuating the flee response compared to controls. Significant interactions across time were observed for both reactions to repeated startling stimuli and prolonged exposure to a shoal of conspecifics; however, as further analyses of the linear trends found no significant differences, it is unclear what these significant interactions mean for cognition and behavior. Finally, these effects were all observed after exposure to doses that did not produce significantly different rates of physical abnormalities, reproducing previous findings (Dishaw et al., 2014) that demonstrated that, while OPFRs can cause overt toxicity at higher doses, the 0.3- and 0.03-μM doses used here are below the threshold for significant malformations.
One trend to emerge from these results is that developmental OPFR exposure can lead to hyperactivity throughout the lifetime. In contrast, another study using a dosing protocol similar to that used here and a variation of the larval activity assay did not find an effect of TDCIPP at doses tested in the current study. Instead, it was found that exposures an order of magnitude higher decreased larval activity in both the light and dark (Dishaw et al., 2014). Another study also failed to find that TPHP exposure during the same time period produced behavioral effects up to a dose two orders of magnitudes higher, at which point TPHP also depressed locomotion (Noyes et al., 2015). The same study, however, found that TDCIPP increased locomotion at doses near the higher dose tested here, but attenuated it at higher doses (Noyes et al., 2015). Variations between these results may partially be explained by differences in testing protocol (eg, beginning with a light phase as opposed to a dark phase) or differences in strain of zebrafish (5D in both Dishaw et al., 2014 and Noyes et al., 2015 compared to the AB* strain used here). There may also be a non-monotonic dose response, with the lower doses of chlorpyrifos and TDCIPP producing larval hyperactivity as seen in this study, and higher doses driving hypoactivity. Unfortunately, these other studies did not test adult behavior, so we cannot compare them to the adult hyperactivity caused by 0.3-μM TDCIPP seen here. It is possible that, since the manner and type of locomotion required in each test varied (eg, exploratory locomotion in the novel environment task compared to escape locomotion in the predator escape assay), the susceptibility of test-specific locomotion to OP exposure would also vary, accounting for the fact that hyperactivity was not uniformly observed. Nonetheless, in this and previous studies, it appears that OPFRs are likely to alter locomotion at various life stages, although the exact relationship between testing paradigm, dosing, and direction of effect remains to be fully elucidated.
OP exposure altered the pattern of behavior across the duration of the test in at least one of the adult assays. As noted above, exposed fish exhibited a generally attenuated exploratory response during the novel environment assay, displaying a smaller increase in their distance from the bottom of the tank across the duration of the test. And though the initial startle responses of exposed fish tended to be higher in the startle habituation assay, they quickly dropped down to control levels or lower. Similarly, though OP-exposed fish and control fish initially affiliated with the video of shoaling conspecifics to similar degrees, in subsequent minutes, the exposed fish showed an accelerated tendency to spend time away from the shoal. The extent of the effects of OP exposure on the startle and shoaling habituation, though, are unclear, as initial analyses showed altered interactions of the measures over time but follow-up investigations of the linear trends failed to show significant individual differences from control. Thus, it seems that developmental exposure to OP compounds, including OPFRs, might alter habituation to repeated or prolonged stimuli across the lifespan.
Another connection between the behavior of the OP-exposed fish across the assays is that developmental exposure to OP compounds appears to alter the saliency of both rewarding and aversive stimuli. For example, the iteration of the predator escape assay utilizing the blue predator stimulus showed a reduced fleeing response in OP-exposed fish. No such attenuation was found when the assay was performed with the faster red stimulus, which may have been so aversive that it overcame whatever neurobehavioral deficits were driving the abnormal behavior in other assays. This potent aversive stimulus serves as a behavioral control showing that the exposed fish are able to see visual stimuli quite well. Decreased aversive or rewarding saliency might explain the abnormal habituation observed in the other assays. Exposed fish might have altered exploratory behavior in the novel environment task because the normal initial aversion to exploration might be muted, leading to less time spent toward the bottom of the tank. Similarly, the startling tap stimulus might initially be more aversive to exposed fish than it is to controls but might lose its aversive qualities more rapidly, leading to an increased habituation (alternatively, the initially elevated startle responses observed in some of the exposed groups might be manifestations of OP-induced hyperactivity). In the same way, the shoaling video might not retain its rewarding salience in exposed fish as long as it does in controls, leading to a differential response over the course of the social affiliation assay. In this way, altered responses to stimuli might explain the majority of the behavioral abnormalities observed across the adult testing battery.
These results and explanations fit overall with what has been previously observed with developmental exposure to OP pesticides. Zebrafish exposed to 0.3-μM chlorpyrifos as they were in this study have previously been shown to exhibit hyperactivity and altered habituation in the startle habituation and novel environment assays (Sledge et al., 2011). Additionally, fish exposed developmentally to both 0.03 μM and 0.3 μM of chlorpyrifos showed significantly inhibited spatial discrimination (Levin et al., 2003), possibly explained by an attenuated salience of the aversive stimuli used to train the fish in the task. The altered locomotion seen in larval fish following chlorpyrifos exposure has also been previously observed (Levin et al., 2004), albeit in a different direction.
Furthermore, these findings fit into the literature regarding OP exposure behavioral abnormalities in rodents and possible underlying neurochemical mechanisms. Developmental OP exposure in rodents has been extensively linked to altered locomotion, habituation, and responses to rewarding and aversive stimuli or environments. Chlorpyrifos-exposed rats spend more time exploring the open arms of an elevated plus maze test (Aldridge et al., 2005), similar to the results seen in the novel environment assay in this study. Early-life exposure to two other OP pesticides, parathion and diazinon, produce varied responses that fit the theme of altered reward salience, including decreased preference for chocolate milk over water, reduced latencies to begin eating in an unfamiliar environment, greater time in the open arms of an elevated plus maze, reduced startle response, and decreased reward-motivated spatial discrimination learning (Roegge et al., 2008; Timofeeva et al., 2008a,b). All three compounds were also found to alter the normal development of serotonin systems in rodents (Slotkin et al., 2006; Slotkin et al., 2008; Slotkin et al., 2009). These neurochemical alterations have been partially mirrored in zebrafish, as developmental exposure to chlorpyrifos lowers both serotonin and dopamine levels in larval zebrafish, with the dopamine deficits lasting through adulthood (Eddins et al., 2010). More recently, developmental TDCIPP exposure was found to have no effect on dopamine or serotonin levels in larval fish, but a longer exposure lasting into adulthood led to lower levels of both serotonin and dopamine (Wang et al., 2015). Further research will be needed to link OPFR exposure to monoaminergic alterations to the extent that the link has been established with OP pesticides in rodents.
While it is difficult to translate the doses used in the present study to human exposure because of the lack of data on OPFR levels and kinetics in humans, it can be instructive to look at, and extrapolate from, the much more extensive research on chlorpyrifos exposure. Chlorpyrifos is readily absorbed both orally and via inhalation, crosses the blood–brain barrier, and can concentrate in the brain following chronic exposures (Eaton et al., 2008). It has been observed that comparable exposures to chlorpyrifos and TDCIPP lead to comparable absorption and kinetics in zebrafish (Dishaw et al., 2014), so it might be reasonable to hypothesize that TDCIPP and other OPFRs might be as easily absorbed as chlorpyrifos in humans. The findings that OPFRs are ubiquitous in house dust with levels reaching up to 1.8 mg/g (Stapleton et al., 2009) and child handwipe samples reach levels of 530 ng of TDCIPP (Stapleton et al., 2014) lead to a reasonable possibility of daily OPFR uptake levels in the μg/day range, comparable to those estimated for chlorpyrifos (Eaton et al., 2008) and that to lead to background blood levels around 20 pM (Whyatt et al., 2002; Eaton et al., 2008). As a 5-day exposure to 1 μM of TDCIPP and chlorpyrifos in zebrafish led to tissue levels of 10–20 pmol per fish (Dishaw et al., 2014), it is reasonable to extrapolate that the doses used here (0.03 and 0.3 μM) produced relatively lower tissue levels. Taken together, the doses used in the current study, which were insufficient to produce overt toxicity or structural malformation yet rendered the fish functionally impaired across a number of assessments, likely produced OPFR tissue levels within a range that can be plausibly linked to human exposures.
We have found that developmental exposures to organophosphate flame retardants, at levels equimolar to those of known neurotoxicant chlorpyrifos, produce behavioral abnormalities across a number of locomotor and cognitive endpoints in both larval and adult zebrafish. These findings suggest that further research into the possible health effects of OPFRs in humans, especially during critical times of neurodevelopment, are likely warranted. Additionally, it will be important to continue using animal models to look into the underlying neurobiological mechanisms of these behavioral effects, such as the possible role and extent of monoaminergic disruption.
Acknowledgement
Research was supported from the Duke University Superfund Research Center ES 010356.
Appendix A
Appendix Fig. 1.
Chemical structures of chlorpyrifos, triphenyl phosphate (TPHP), and tris(1,3-dichloroisopropyl)phosphate (TDCIPP)
Footnotes
Transparency document The Transparency document associated with this article can be found in the online version.
References
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to clorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ. Health Perspect. 2005;113(5):527–531. doi: 10.1289/ehp.7867. http://dx.doi.org/10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J, Oliveri A, Levin ED. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res. C Embryo Today. 2013;99(1):14–23. doi: 10.1002/bdrc.21027. http://dx.doi.org/10.1002/bdrc.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JM, Oliveri AN, Levin ED. Pharmacological analyses of learning and memory in zebrafish (Danio rerio) Pharmacol. Biochem. Behav. 2015 doi: 10.1016/j.pbb.2015.03.006. http://dx.doi.org/10.1016/j.pbb.2015.03.006 (epub ahead of print, PMID: 25792292) [DOI] [PMC free article] [PubMed]
- Bencan Z, Levin ED. The role of alpha7 and alpha4beta2 nicotinic receptors in the nicotine-induced anxiolytic effect in zebrafish. 2008;95(3):408–412. doi: 10.1016/j.physbeh.2008.07.009. doi: 10.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ. Sci. Technol. 2014;48(17):10432–10438. doi: 10.1021/es5025299. http://dx.doi.org/10.1021/es5025299. [DOI] [PubMed] [Google Scholar]
- Carignan CC, McClean MD, Cooper EM, Watkins DJ, Fraser AJ, Heiger-Bernays W, Webster TF. Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environ. Int. 2013;55:56–61. doi: 10.1016/j.envint.2013.02.004. http://dx.doi.org/10.1016/j.envint.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishaw LV, Powers CM, Ryde IT, Roberts SC, Seidler FJ, Slotkin TA, Stapleton HM. Is the PentaBDE replacement, tris (1,3-dichloro-2-propyl) phosphate (TDCIPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol. Appl. Pharmacol. 2011;256(3):281–289. doi: 10.1016/j.taap.2011.01.005. http://dx.doi.org/10.1016/j.taap.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishaw LV, Hunter DL, Padnos B, Padilla S, Stapleton HM. Developmental exposure to organophosphate flame retardants elicits overt toxicity and alters behavior in early life stage zebrafish (Danio rerio) Toxicol. Sci. 2014;142(2):445–454. doi: 10.1093/toxsci/kfu194. http://dx.doi.org/10.1093/toxsci/kfu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, Spencer PS. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol. 2008;38(Suppl. 2):1–125. doi: 10.1080/10408440802272158. http://dx.doi.org/10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol. Teratol. 2010;32(1):99–108. doi: 10.1016/j.ntt.2009.02.005. http://dx.doi.org/10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Daniels JL, Stapleton HM. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ. Int. 2014;63:169–172. doi: 10.1016/j.envint.2013.11.013. http://dx.doi.org/10.1016/j.envint.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM. Monitoring indoor exposure to organophosphate flame retardants : hand wipes and house dust. Environ. Health Perspect. 2015;160(2):160–165. doi: 10.1289/ehp.1408669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol. Teratol. 2003;25(1):51–57. doi: 10.1016/s0892-0362(02)00322-7. http://dx.doi.org/10.1016/S0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Swain HA, Donerly S, Linney E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol. Teratol. 2004;26(6):719–723. doi: 10.1016/j.ntt.2004.06.013. http://dx.doi.org/10.1016/j.ntt.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 2007;90(1):54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Cooper EM, Stapleton HM, Hauser R. Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environ. Health Perspect. 2013;121(5):580–585. doi: 10.1289/ehp.1205907. http://dx.doi.org/10.1289/ehp.1205907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Stapleton HM. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ. Health Perspect. 2010;118(3):318–323. doi: 10.1289/ehp.0901332. http://dx.doi.org/10.1289/ehp.0901332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizouchi S, Ichiba M, Takigami H, Kajiwara N, Takamuku T, Miyajima T, Ueno D. Exposure assessment of organophosphorus and organobromine flame retardants via indoor dust from elementary schools and domestic houses. Chemosphere. 2015;123:17–25. doi: 10.1016/j.chemosphere.2014.11.028. http://dx.doi.org/10.1016/j.chemosphere.2014.11.028. [DOI] [PubMed] [Google Scholar]
- Noyes PD, Haggard DE, Gonnerman GD, Tanguay RL. Advanced morphological - behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol. Sci. 2015;145(1):177–195. doi: 10.1093/toxsci/kfv044. http://dx.doi.org/10.1093/toxsci/kfv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED. Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res. Bull. 2008;75(1):166–172. doi: 10.1016/j.brainresbull.2007.08.008. http://dx.doi.org/10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledge D, Yen J, Morton T, Dishaw L, Petro A, Donerly S, Levin ED. Critical duration of exposure for developmental chlorpyrifos-induced neurobehavioral toxicity. Neurotoxicol. Teratol. 2011;33(6):742–751. doi: 10.1016/j.ntt.2011.06.005. http://dx.doi.org/10.1016/j.ntt.2011.06. 005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T.a., Levin ED, Seidler FJ. Developmental neurotoxicity of parathion: progressive effects on serotonergic systems in adolescence and adulthood. Neurotoxicol. Teratol. 2009;31(1):11–17. doi: 10.1016/j.ntt.2008.08.004. http://dx.doi.org/10.1016/j.ntt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Levin ED, Seidler FJ. Developmental neurotoxicity of low dose diazinon exposure of neonatal rats: effects on serotonin systems in adolescence and adulthood. Brain Res. Bull. 2008;75(5):640–647. doi: 10.1016/j.brainresbull.2007.10.008. http://dx.doi.org/10.1016/j.brainresbull.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T.a., Tate C.a., Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ. Health Perspect. 2006;114(10):1542–1546. doi: 10.1289/ehp.9337. http://dx.doi.org/10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ. Sci. Technol. 2009;43(19):7490–7495. doi: 10.1021/es9014019. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2782704&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Blum A. Identification of flame retardants in polyurethane foam collected from baby products. Environ. Sci. Technol. 2011;45(12):5323–5331. doi: 10.1021/es2007462. http://dx.doi.org/10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Flame retardant associations between children's handwipes and house dust. Chemosphere. 2014;116:54–60. doi: 10.1016/j.chemosphere.2013.12.100. http://dx.doi.org/10.1016/j.chemosphere.2013.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva O.a., Roegge CS, Seidler FJ, Slotkin TA, Levin ED. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organ-ophosphate pesticide, diazinon. Neurotoxicol. Teratol. 2008a;30(1):38–45. doi: 10.1016/j.ntt.2007.10.002. http://dx.doi.org/10.1016/j.ntt.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, Levin ED. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res. Bull. 2008b;77(6):404–411. doi: 10.1016/j.brainresbull.2008.08.019. http://dx.doi.org/10.1016/j.brainresbull.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Lam JC-W, Man Y-C, Lai NL-S, Kwok KY, Guo YY, Zhou B. Bioconcentration, metabolism and neurotoxicity of the organophorous flame retardant 1,3-dichloro 2-propyl phosphate (TDCPP) to zebrafish. Aquat. Toxicol. 2015;158:108–115. doi: 10.1016/j.aquatox.2014.11.001. http://dx.doi.org/10.1016/j.aquatox.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, Perera FP. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ. Health Perspect. 2002;111(5):749–756. doi: 10.1289/ehp.5768. http://dx.doi.org/10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]