Abstract
Objective
Despite the association of obesity with incident cardiovascular disease, obese patients with acute coronary syndrome (ACS) appear to have more favourable short-term outcomes. A study was undertaken to determine whether this ‘obesity paradox’ persists in the long term and to examine the specific relationship of central obesity with outcomes after ACS.
Methods
The relationship was investigated between two measures of obesity—body mass index (BMI) and waist circumference (WC)—and 30-day and 1-year outcomes after ACS. 6560 patients with non-ST elevation ACS in the MERLIN-TIMI 36 trial were followed for 1 year. Patients were stratified into three BMI groups (<25, 25–30, ≥30 kg/m2) and gender-specific tertiles of WC. The primary endpoint was cardiovascular death, myocardial infarction or recurrent ischaemia.
Results
Patients with BMI ≥30 kg/m2 had a significantly lower risk of the primary endpoint than those with BMI <25 kg/m2 (HR 0.64; 95% CI 0.51 to 0.81, p<0.0001) at 30 days. However, after the 30-day acute phase, landmark analysis from 30 days to 1 year showed no difference in risk between BMI groups (HR 1.09; 95% CI 0.92 to 1.29, p=0.34). WC tertiles demonstrated a similar relationship. When BMI groups were stratified by WC there was a trend towards more adverse outcomes in higher WC groups among those in lower BMI groups. The group with the lowest BMI and highest WC had the highest risk (HR 2.8; 95% CI 0.93 to 8.3; p=0.067).
Conclusions
Obesity is associated with more favourable short-term outcomes after ACS. However, in the longer term the obesity paradox is no longer present and may reverse. Those with WC out of proportion to BMI suggestive of significant central adiposity may be at highest risk following ACS.
INTRODUCTION
The epidemic of obesity presents a major challenge for global healthcare. The prevalence of obesity continues to rise; by 2015, 75% of US adults are projected to be overweight and 41% obese.1 Moreover, obesity is associated with a greater prevalence of cardiovascular (CV) risk factors and a higher risk of first CV events, contributing to the rise in CV morbidity and mortality worldwide.
Increased body mass index (BMI) is established as an independent risk factor for myocardial infarction.2,3 Despite the association of obesity with incident CV disease, in patients who have presented with acute MI obesity appears to have an unexpected ‘protective effect’ on outcome—the so-called ‘obesity paradox’.4–12 The aetiology of this paradoxical association remains largely unexplained. Moreover, little is known about how this association evolves over time. At the same time, attention has recently been drawn to alternative measures of adiposity—such as waist circumference (WC) and waist-to-hip ratio—that provide information regarding body fat distribution and visceral fat. WC and waist-to-hip ratio may enhance risk prediction of incident CV disease compared with BMI.3,13–19 However, little is known about how WC is related to outcomes after acute coronary syndrome (ACS).
Give this uncertainty, we sought to evaluate the relationship of BMI with death and CV outcomes after non-ST elevation (NSTE)-ACS during the acute phase of the first 30 days and during longer term follow-up from 30 days to 1 year in a well-characterised large multinational population of patients presenting with NSTE-ACS. We further sought to explore the role of central adiposity and its influence on the risk relationship of obesity with CV outcomes.
METHODS
Patient population
The details of the MERLIN (Metabolic Efficiency with Ranolazine for Less Ischaemia in NSTE-ACS)-TIMI 36 trial design and study population have been published previously.20,21 Between 2004 and 2006, 6560 patients underwent randomisation at 442 sites in 17 countries. Eligible patients were aged 18 years or older, had symptoms consistent with myocardial ischaemia at rest and had at least one indicator of moderate to high risk of death or recurrent ischaemic events. Major exclusion criteria relevant to this analysis included cardiogenic shock, clinically significant hepatic disease, end-stage renal disease requiring dialysis or a life expectancy of <12 months. Race and ethnicity were self-reported using categories defined by the investigators.
Study protocol
The protocol specified that patients were to receive standard treatment for NSTE-ACS and secondary prevention. Patients underwent early invasive or conservative management strategies at the discretion of the responsible physician. Patients were randomised to receive either ranolazine or placebo, continued for the duration of the study.
Height, weight and WC were obtained at the initial intake. WC was measured by the research coordinator at the top of the iliac crest using supplied non-stretchable measuring tape, parallel to the floor, with the patient standing after normal exhalation. Patients returned for study visits at 14 days, 4 months and every 4 months thereafter until the end of the study. Baseline characteristics, including demographics, historical factors and presentation were collected. Additionally, detailed information regarding medications and therapies used during both the initial and chronic management were collected using a case report form.
Endpoints
The primary endpoint of the trial was the composite of CV death, myocardial infarction (MI) or recurrent ischaemia. MI had to be distinct from the index event and was defined by symptoms suggestive of ischaemia/infarction in association with ECG, cardiac biomarker or pathological evidence of infarction using criteria adapted from the definition developed by the American College of Cardiology. Recurrent ischaemia included any of the following: (1) recurrent ischaemia with ECG changes; (2) recurrent ischaemia leading to hospitalization; (3) recurrent ischaemia prompting revascularization; and (4) worsening of angina/ischaemia by at least one Canadian CV Society class of angina that prompted intensification of anti-anginal therapy.20,21 All elements of the primary composite endpoint were adjudicated by a blinded clinical events committee.
Statistical analyses
Patients were divided into three groups based on BMI (<25 kg/m2, 25–30 kg/m2 and ≥30 kg/m2 for both men and women) using the WHO definitions of normal, overweight or obese. Patients were further divided into gender-specific tertiles of WC. Baseline characteristics were compared using the Wilcoxon rank sum test for continuous variables and the χ2 test for categorical variables. Likelihood ratios, adjusted for age and gender, for various treatment strategies was assessed. Event rates at 30 days and 1 year were determined using the Kaplan–Meier method. In addition, a landmark analysis was performed to assess the cumulative incidence of events occurring from 30 days to the end of follow-up. Event rates were calculated for the entire population, with sex included as a covariate in the multivariable analysis. Multivariable analysis was performed using Cox regression, adjusting for sex and clinical risk indicators including age, risk factors for coronary artery disease (hypertension, hyperlipidaemia, diabetes, family history of coronary artery disease or current tobacco use), history of known coronary artery disease, aspirin use in the past 7 days, the pace of angina within the 24 h prior to presentation, ST changes ≥0.5 mm and elevated cardiac troponin or creatine kinase-MB, as defined by the TIMI risk score.22 To specifically address the effect of age, an interaction term between age (stratified by age ≥65 or age <65) and BMI was also tested. BMI was also evaluated as a continuous variable. Additionally, in each BMI group, patients were further stratified by gender-specific WC tertiles and the analysis was repeated for these nine groups to assess the effect of WC within a given BMI group.
All analyses were performed by the Thrombolysis in Myocardial Infarction study group using STATA V.9.2; p<0.05 (two-tailed) was considered to indicate statistical significance.
RESULTS
Baseline characteristics
Of the 6560 participants in MERLIN-TIMI 36, 6470 (98.6%) patients had BMI data and 5910 (90.1%) patients had WC data. Of these patients, 5884 (89.7%) had data available for both BMI and WC. Among patients who had BMI measured, 2293 (35.4%) were obese (BMI ≥30 kg/m2), 2804 (43.3%) were overweight (BMI 25–30 kg/m2) and 1373 (21.2%) had normal BMI. The median BMI was 28.3 kg/m2 (25th, 75th percentiles 25.5, 31.5) and median WC was 101 (25th, 75th percentiles 92, 110). When BMI groups were stratified by WC tertiles, a majority of patients (58.6%) were in groups with concordant BMI and WC (ie, subjects with high BMI also had high WC). However, there was a sizeable group of patients with discordant BMI and WC (41.4%). There were some differences in BMI distribution by region in this multinational population. In North America there was a larger proportion of subjects in the BMI ≥30 kg/m2 group (44.7%) compared with Western Europe (28.1%) or Eastern Europe (32.0%).
Clinical characteristics and treatments
The baseline characteristics of the study population are shown in table 1. Individuals with higher BMI had a higher incidence of risk factors for atherosclerosis including hypertension, dyslipidaemia and diabetes. Age and the incidence of smoking were inversely correlated with BMI. In contrast, those with a higher BMI were less likely to present with indicators of higher risk of complications of ACS. Specifically, they had a lower incidence of ≥1 mm ST depression, elevated troponin, elevated B-type natriuretic peptide and impaired renal function. There were no significant differences in the extent of coronary disease seen on angiography (table 1).
Table 1.
Demographic data and prior history of study participants
| BMI <25 kg/m2 | BMI 25–30 kg/m2 | BMI ≥30 kg/m2 | p Value | |
|---|---|---|---|---|
| Age (years) | 67 (57–74) | 65 (56–72) | 61 (54–69) | <0.001 |
| % Women | 499 (36.3) | 827 (29.5) | 932 (40.6) | <0.001 |
| Waist circumference (cm) | 89 (82–95) | 99 (92–105) | 110 (104–119) | <0.001 |
| Region | ||||
| North America | 180 (13.1) | 392 (14) | 518 (22.6) | <0.001 |
| Eastern Europe | 487 (35.5) | 1093 (39) | 901 (39.3) | <0.001 |
| Western Europe | 706 (51.4) | 1319 (47) | 874 (38.1) | <0.001 |
| Risk factors | ||||
| Hypertension | 875 (64.4) | 1991 (71.6) | 1881 (82.3) | <0.001 |
| Dyslipidaemia | 770 (61) | 1705 (66.9) | 1525 (72.5) | <0.001 |
| Diabetes | 318 (23.2) | 812 (29) | 1059 (46.2) | <0.001 |
| Current smoker | 407 (29.7) | 719 (25.6) | 534 (23.3) | 0.01 |
| Prior CVD | ||||
| Congestive heart failure | 207 (15.1) | 453 (16.2) | 429 (18.7) | 0.31 |
| Peripheral vascular disease | 139 (10.2) | 248 (9) | 173 (7.6) | 0.04 |
| Cerebrovascular disease | 162 (11.8) | 294 (10.5) | 243 (10.6) | 0.79 |
| Prior stroke | 81 (5.9) | 137 (4.9) | 110 (4.8) | 0.63 |
| Index diagnosis | ||||
| Unstable angina | 598 (43.6) | 1283 (45.8) | 1154 (50.3) | 0.03 |
| NSTEMI | 748 (54.5) | 1461 (52.1) | 1078 (47) | 0.03 |
| Other | 27 (2) | 60 (2.1) | 61 (2.7) | 0.03 |
| Presentation | ||||
| ST depression ≥1 mm | 534 (38.9) | 1018 (36.3) | 725 (31.6) | <0.001 |
| Troponin ≥0.04 mcg/l | 647 (70.5) | 1269 (66.6) | 964 (59) | <0.001 |
| BNP >80 pg/ml | 466 (50.4) | 825 (43) | 608 (37) | <0.001 |
| eGFR <60 ml/min | 565 (41.3) | 578 (20.7) | 241 (10.5) | <0.001 |
| Fasting blood glucose (mg/dl) | 101 (88–124) | 105 (92–128) | 114 (97–148) | <0.001 |
| Heart rate (bpm) | 70 (61–78) | 70 (62–78) | 70 (63–80) | <0.001 |
| LVEF (%)* | 55 (47–61) | 55 (47–60) | 55 (47–60) | 0.67 |
| Disease extent (≥50%)† | ||||
| LM or 3VD | 286 (35.2) | 597 (35.4) | 470 (34.6) | 0.34 |
| 2VD | 190 (23.4) | 440 (26.1) | 340 (25) | 0.34 |
| 1VD | 232 (28.6) | 473 (28.1) | 373 (27.5) | 0.34 |
| None | 104 (12.8) | 176 (10.4) | 175 (12.9) | 0.34 |
Data are reported as median (25th, 75th percentiles) for continuous variables and N (%) for categorical variables.
Among 4383 patients with LVEF available.
Among 3856 patients with angiographic data available.
1VD, one vessel disease; 2VD, two vessel disease; 3VD, three vessel disease; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; LM, left main; LVEF, left ventricular ejection fraction.
Treatments also varied among groups defined by BMI (table 2). Patients with increased BMI more often received some guideline-recommended medical treatments such as β-blockers and ACE inhibitors or angiotensin receptor blockers. Patients with higher BMI were also more likely to receive unfractionated heparin and less likely to receive a low-molecular weight heparin, although overall anticoagulant and GP IIb/IIIa use did not differ between groups. In addition, there were no significant qualitative differences in who was managed invasively or received statins or dual anti-platelet therapy (clopidogrel or aspirin), particularly after adjusting for age and sex.
Table 2.
Treatment strategies and adjusted likelihood ratios of receiving specific treatment strategies in study participants
| BMI <25 kg/m2 (%) | LR (p value) | BMI 25–30 kg/m2 (%) | LR (p value) | BMI ≥30 kg/m2 (%) | LR (p value) | |
|---|---|---|---|---|---|---|
| Medical therapies | ||||||
| Aspirin | 1172 (85.4) | 1.0 | 2476 (88.3) | 1.23 (0.03) | 1964 (85.7) | 0.93 (0.45) |
| Clopidogrel | 728 (53) | 1.0 | 1504 (53.6) | 0.99 (0.88) | 1172 (51.1) | 0.93 (0.33) |
| β-blocker | 1027 (74.8) | 1.0 | 2175 (77.6) | 1.16 (0.055) | 1827 (79.7) | 1.27 (0.003) |
| Statin | 1040 (75.7) | 1.0 | 2186 (78) | 1.08 (0.31) | 1782 (77.7) | 1.07 (0.38) |
| ACE-I or ARB | 886 (64.5) | 1.0 | 1973 (70.4) | 1.35 (<0.001) | 1757 (76.6) | 1.92 (<0.001) |
| UFH | 594 (43.3) | 1.0 | 1313 (46.8) | 1.14 (0.04) | 1149 (47.2) | 1.30 (<0.001) |
| LMWH | 872 (63.5) | 1.0 | 1783 (63.6) | 1.01 (0.08) | 1308 (57.0) | 0.79 (0.001) |
| UFH or LMWH | 1230 (89.6) | 1.0 | 2561 (91.3) | 1.22 (0.08) | 2063 (90.0) | 1.03 (0.77) |
| GP IIb/IIIa inhibitor | 202 (14.7) | 1.0 | 432 (15.4) | 1.01 (0.92) | 303 (13.2) | 0.85 (0.11) |
| Invasive therapies | ||||||
| Coronary angiography | 812 (59.1) | 1.0 | 1686 (60.1) | 0.98 (0.79) | 1358 (59.2) | 0.97 (0.64) |
| Any revascularisation | 523 (38.1) | 1.0 | 1164 (41.5) | 1.11 (0.14) | 867 (37.8) | 1.01 (0.85) |
| PCI | 404 (29.4) | 1.0 | 930 (33.2) | 1.14 (0.07) | 716 (31.2) | 1.08 (0.32) |
| CABG | 122 (8.9) | 1.0 | 244 (8.7) | 0.97 (0.80) | 158 (6.9) | 0.86 (0.23) |
Likelihood ratio adjusted for gender and age.
ACE-I, ACE inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; LMWH, low-molecular weight heparin; LR, adjusted likelihood ratio; PCI, percutaneous coronary intervention; UFH, unfractionated heparin.
BMI and 30-day outcomes
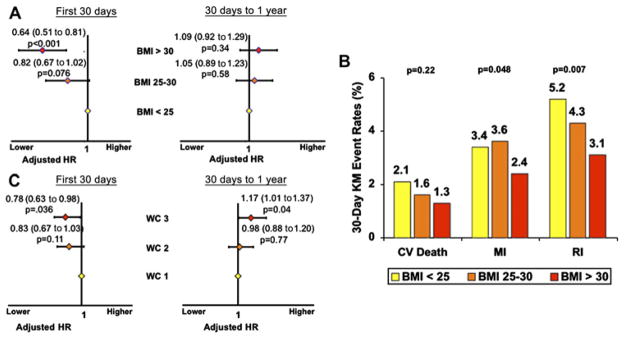
At 30 days there was a graded inverse association between BMI and the primary endpoint with a 36% lower incidence of the primary endpoint in the group with the highest BMI (adjusted HR 0.64; 95% CI 0.51 to 0.81; p<0.0001) relative to the group with BMI <25 kg/m2 (figure 1A). Notably, this pattern of inverse association was consistent for each component of the primary endpoint, although driven primarily by a reduction in recurrent ischaemia. Specifically, obese patients had a 29.4% lower risk of MI (p=0.048) and a 40.4% lower risk of recurrent ischemia (p=0.007) at 30 days (figure 1B). When BMI was examined as a continuous variable, each increase of 1 kg/m2 in BMI was associated with a 3.2% lower risk of developing the primary endpoint at 30 days (p=0.001). We found no evidence for an interaction between age and BMI and, although there was a relationship between BMI and age, the association of BMI and outcome was similar across all age groups (pinteraction=0.078).
Figure 1.
(A) 0–30-day and 30-day to 1-year landmark analysis for developing the primary endpoint by BMI group adjusted for TIMI Risk Score and gender. (B) Event rates of each component of the primary endpoint at 30 days among BMI groups adjusted by TIMI risk score. (C) 0–30-day and 30-day to 1-year landmark analysis for developing the primary endpoint by gender-specific WC tertile, adjusted for TIMI Risk Score. BMI, body mass index (kg/m2); CV, cardiovascular; MI, myocardial infarction; RI, recurrent ischaemia; WC, waist circumference.
BMI and 1-year outcomes
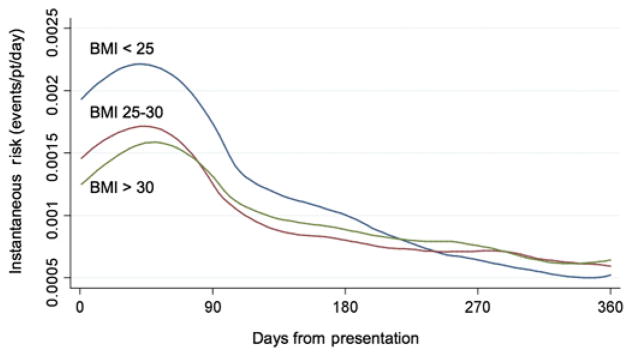
In contrast, at 1 year the relationship between BMI and outcome was substantially attenuated with no difference in the primary endpoint between groups defined by BMI. When BMI was examined as a continuous variable, there was no relationship between BMI and the primary endpoint at 1 year (adjusted HR 0.994; p=0.30). A landmark analysis starting at 30 days revealed no difference in risk of recurrent CV events among BMI groups for the period from 30 days to 1 year (figure 1A). Moreover, examination of the instantaneous HR over the study period showed that the pattern of risk appeared to change over time such that the higher RR of those with low body mass (or the low risk associated with obesity) showed a trend towards reversing over time (figure 2).
Figure 2.
Instantaneous risk over time of developing the primary endpoint by body mass index (BMI) group.
Integration of BMI and WC
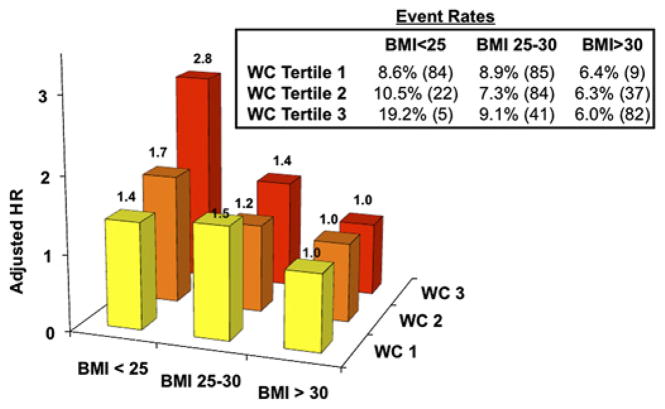
BMI groups were stratified further by WC tertiles. At 30 days there was a trend towards worse outcomes in higher WC groups in the lowest BMI group. The group with the lowest BMI and highest WC, consistent with central adiposity, trended to be the highest HR for risk of developing the primary endpoint (adjusted HR 2.8; 95% CI 0.93 to 8.3; p=0.067; figure 3).
Figure 3.
HR of occurrence of the primary endpoint at 30 days for groups stratified by body mass index (BMI) and waist circumference (WC) adjusted for TIMI risk score.
Notably, when considered alone, there was also an inverse relationship between WC and 30-day outcomes, although not as strong as for BMI. Nevertheless, the highest WC tertile was associated with a lower rate of the primary endpoint (adjusted HR 0.78; 95% CI 0.63 to 0.98, p=0.036) compared with the lowest WC tertile. Importantly, as in BMI groups, there was evidence of increased risk for those in the highest tertile of WC in the landmark analysis starting at 30 days (adjusted HR 1.17; 95% CI 1.00 to 1.37; p=0.042; figure 1C).
DISCUSSION
In this analysis of approximately 6000 patients with 1-year follow-up after NSTE-ACS, we found that the ‘obesity paradox’ was present in a contemporary population managed with standard therapy after ACS. However, we also showed that this paradoxical significant ‘protective’ association with obesity was limited to the short term after presentation. Our analysis indicates that this relationship changes over time, with the ‘obesity paradox’ no longer present between 30 days and 1 year and a possible pattern towards higher risk later during the phase of chronic secondary prevention. This was observed when using either BMI or WC as a marker of obesity. Additionally, we observed that central adiposity in those with low BMI may be associated with a particularly high risk.
Obesity and short-term outcomes
The ‘obesity paradox’ has been observed in both randomised trials and registries of patients with ACS including UA/NSTEMI4–6 and STEMI.6,8,9 However, many of these are based on single-centre or single-nation populations, limited to certain subgroups such as only those who underwent percutaneous coronary intervention (PCI) or limited only to in-hospital outcomes. Notably, we observed an inverse association between BMI and the risk of recurrent CV events at 30 days in a contemporary multinational population with UA/NSTEMI.
The explanations for this risk relationship have not been definitively determined. It has been suggested that the ‘obesity paradox’ may be related to younger age at presentation among obese individuals or the presence of chronic comorbid medical conditions in the severely underweight. However, we observed a seemingly protective relationship of obesity even after adjusting for age at presentation. In addition, in our study, patients with chronic illnesses such as renal or hepatic failure and life expectancy <12 months were excluded, thus also mitigating pairing of chronic illness and low BMI as a potential confounder. There is also the possibility that there are as yet undetermined direct pathobiological contributors for this inverse association.
Another potential explanation may be differences in inhospital treatments in obese patients, as we and others have observed with higher rates of use of some evidence-based therapies in patients with high BMI.5 The fact that the ‘obesity paradox’ persisted in our study despite relatively modest differences in medical therapies suggests that other factors are also playing a role. For example, obese individuals may tolerate administered medical and procedural treatments with fewer complications, such as post-PCI bleeding which is more frequent in patients with lower BMI.7,9–12 This higher risk of bleeding may relate to dosing of anticoagulants, which are more likely to be overdosed in underweight and normal weight individuals.10,12 Moreover, biochemical factors have also been proposed to play a role in bleeding complications. For example, obese patients have increased concentrations of plasminogen activator inhibitor-1, which is a procoagulant molecule that may reduce bleeding.12,23
Obesity and longer-term outcome after ACS
Despite the consistent epidemiological observation of the ‘obesity paradox’, it is difficult to explain how patients with obesity and its accompanying risk factors for atherothrombosis (including hypertension and dysglycaemia) would remain at reduced risk in the longer term. Indeed, in a recent analysis by Zeller and colleagues in 2229 patients with STEMI or UA/NSTEMI, obesity was not related to outcome at 1 year after adjusting for covariates including age, gender and cardiac risk factors.24 However, in this study it was not clear whether the absence of a relationship was due to adequate adjustment for confounders or whether a change in the directionality of the risk relationship over time was contributing as a landmark analysis was not performed. Similarly, a prior report from the BARI registry showed more favourable in-hospital outcomes in obese patients but no difference in 5-year outcomes.11 Additionally, an analysis from the Superior Yield of the New strategy of Enoxaparin, Revascularisation and GlYcoprotein IIb/IIIa inhibitors (SYNERGY) trial showed that increasing BMI was associated with improved 1-year outcomes in those with BMI <30 kg/m2 but not in those with BMI >30 kg/m2.25
In contrast to these previous studies, we examined possible temporal changes in the relationship between obesity and CV risk after ACS using landmark analysis and examining the hazard functions over time. Through these analyses we uncovered potentially important differences in this relationship during the acute versus long-term periods. In the short term there was a protective effect of obesity; however, beyond the acute period there was no difference in outcomes. Plotting the instantaneous HR over time, we observed a point where obesity began to be associated with increased risk. This finding suggests that, if there are ‘protective’ effects of obesity, they are short-lived and, in the long run, the adverse health effects of obesity appear to predominate. This finding is consistent with one previous single-centre study of 1000 predominantly (>96%) Caucasian patients in which a 6-month landmark was used, after which no protective association was observed.8 While longer-term follow-up was not available in the MERLIN-TIMI 36 cohort, further work should focus on examining the longer-term effects of obesity.
Central adiposity
A shortcoming of BMI is that it measures total body mass, including both fat and fat-free mass which have opposing effects on health. It also does not measure fat distribution directly.26,27 As a result, efforts have been made to better capture the effects of more metabolically active abdominal obesity. Abdominal obesity is composed largely of subcutaneous and visceral fat. Visceral fat has been associated with the development of dyslipidaemia, insulin resistance, hypertension and secretion of proinflammatory cytokines, all of which can promote atherogenesis.28 However, this may vary among different ethnic groups.29
While imaging modalities such as CT or MRI are the most effective ways of assessing visceral fat,30 simpler alternatives such as WC measurement have been shown to be well correlated with overall visceral fat deposits.31,32 Moreover, abdominal obesity—assessed by WC and waist-to-hip ratio—has been implicated as a stronger risk factor for incident CV disease and first MI than BMI.3,13–19
In our study we found that both WC and BMI had similar relationships to outcomes following ACS. However, we observed that integrating these two measures of obesity suggested that patients with WC out of proportion to BMI (eg, high WC in a low BMI group) were at particularly high risk. The pattern is consistent with a previous study which also suggested an increased risk in patients with high WC despite low BMI.24 A similar analysis looking at all-cause mortality over 9 years also found an increased risk in higher WC groups after adjusting for BMI, although this analysis included the general population rather than just those with ACS.33 This intriguing finding supports additional investigation of the combined assessment of WC or waist-to-hip ratio along with BMI to elucidate more completely the relationship of central adiposity to outcomes in patients with ACS.
Limitations of the study
Our analysis has limitations. First, we were unable to assess the contribution of the very underweight and very overweight patients owing to small sample sizes of these groups.
Previous data have suggested that these populations may skew the data and that both of them are at very high risk, creating more of a U-shaped pattern than a linear relationship.5,10 Second, although specific measurement instructions were given, there may be inter-site and inter-investigator variability in the measurement of WC, and waist-to-hip ratio was not assessed. In addition, while our population was multinational, it was still primarily Caucasian29 with few subjects of Asian descent. Finally, our data only includes 1-year follow-up and relationships may differ or change in the longer term.
CONCLUSION
Obesity is associated with more favourable short-term outcomes after ACS, confirming the ‘obesity paradox’ in the contemporary care of patients with ACS. In the long term the ‘obesity paradox’ is no longer significant and may reverse. Patients with higher BMI were more likely to receive guideline-recommended inhospital treatments. In patients with the lowest BMI, those with high WC tended to be at high risk, suggesting the need for additional investigation of the implications of central adiposity in this setting.
Acknowledgments
Funding MERLIN-TIMI 36 was supported by CV Therapeutics (now Gilead Science Inc).
Footnotes
Competing interests BMS has received grants for clinical research via the TIMI Study Group and Brigham and Women’s Hospital from CV Therapeutics, Novartis Pharmaceuticals Corporation, AstraZeneca Pharmaceuticals LP, Daiichi-Sankyo Inc, Merck & Co Inc, Johnson and Johnson Pharmaceutical Research & Development LLC, Bayer HealthCare Pharmaceuticals and Bristol-Myers-Squibb Company and has served as a consultant for AstraZeneca Pharmaceuticals LP, Novartis Pharmaceuticals Corporation, CV Therapeutics, Cogentus, Shionogi & Co Ltd, Gilead Sciences Inc, Merck & Co Inc and Schering-Plough Corporation. DAM has received grants for clinical research via the TIMI Study Group and Brigham & Women’s Hospital from CV Therapeutics, served as a consultant for Menarini Group and received honoraria for educational presentations from CV Therapeutics. MBK, CSF, MPB and SAM do not report any potential conflicts.
Ethics approval The protocol was approved by the relevant institutional review boards at all participating centres and written informed consent was obtained from all patients.
Contributors MK and DM participated in the design and execution of the study, data analysis and review of the manuscript, writing of the manuscript and have seen and approved the final version. SM participated in data analysis and review of the manuscript. CF participated in the design of the study, analysis of the data and review of the manuscript. BS and MB participated in review of the manuscript.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Wang Y, Beydoun MA. The obesity epidemic in the United States–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 2.Wolk R, Berger P, Lennon RJ, et al. Body mass index: a risk factor for unstable angina and myocardial infarction in patients with angiographically confirmed coronary artery disease. Circulation. 2003;108:2206–11. doi: 10.1161/01.CIR.0000095270.85646.E8. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 4.Buettner HJ, Mueller C, Gick M, et al. The impact of obesity on mortality in UA/non-ST-segment elevation myocardial infarction. Eur Heart J. 2007;28:1694–701. doi: 10.1093/eurheartj/ehm220. [DOI] [PubMed] [Google Scholar]
- 5.Diercks DB, Roe MT, Mulgund J, et al. The obesity paradox in non-ST-segment elevation acute coronary syndromes: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association Guidelines Quality Improvement Initiative. Am Heart J. 2006;152:140–8. doi: 10.1016/j.ahj.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Eisenstein EL, McGuire DK, Bhapkar MV, et al. Elevated body mass index and intermediate-term clinical outcomes after acute coronary syndromes. Am J Med. 2005;118:981–90. doi: 10.1016/j.amjmed.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Mehta L, Devlin W, McCullough PA, et al. Impact of body mass index on outcomes after percutaneous coronary intervention in patients with acute myocardial infarction. Am J Cardiol. 2007;99:906–10. doi: 10.1016/j.amjcard.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 8.Nigam A, Wright RS, Allison TG, et al. Excess weight at time of presentation of myocardial infarction is associated with lower initial mortality risks but higher long-term risks including recurrent re-infarction and cardiac death. Int J Cardiol. 2006;110:153–9. doi: 10.1016/j.ijcard.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 9.Nikolsky E, Stone GW, Grines CL, et al. Impact of body mass index on outcomes after primary angioplasty in acute myocardial infarction. Am Heart J. 2006;151:168–75. doi: 10.1016/j.ahj.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Ellis SG, Elliott J, Horrigan M, et al. Low-normal or excessive body mass index: newly identified and powerful risk factors for death and other complications with percutaneous coronary intervention. Am J Cardiol. 1996;78:642–6. doi: 10.1016/s0002-9149(96)00386-4. [DOI] [PubMed] [Google Scholar]
- 11.Gurm HS, Whitlow PL, Kip KE. The impact of body mass index on short- and long-term outcomes inpatients undergoing coronary revascularization. Insights from the bypass angioplasty revascularization investigation (BARI) J Am Coll Cardiol. 2002;39:834–40. doi: 10.1016/s0735-1097(02)01687-x. [DOI] [PubMed] [Google Scholar]
- 12.Powell BD, Lennon RJ, Lerman A, et al. Association of body mass index with outcome after percutaneous coronary intervention. Am J Cardiol. 2003;91:472–6. doi: 10.1016/s0002-9149(02)03252-6. [DOI] [PubMed] [Google Scholar]
- 13.Poirier P. Adiposity and cardiovascular disease: are we using the right definition of obesity? Eur Heart J. 2007;28:2047–8. doi: 10.1093/eurheartj/ehm321. [DOI] [PubMed] [Google Scholar]
- 14.Canoy D, Boekholdt SM, Wareham N, et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation. 2007;116:2933–43. doi: 10.1161/CIRCULATIONAHA.106.673756. [DOI] [PubMed] [Google Scholar]
- 15.Despres JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–49. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 16.de Koning L, Merchant AT, Pogue J, et al. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28:850–6. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- 17.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–20. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 18.Gruson E, Montaye M, Kee F, et al. Anthropometric assessment of abdominal obesity and coronary heart disease risk in men: the PRIME study. Heart. 2010;96:136–40. doi: 10.1136/hrt.2009.171447. [DOI] [PubMed] [Google Scholar]
- 19.Kaess BM, Jozwiak J, Mastej M, et al. Association between anthropometric obesity measures and coronary artery disease: a cross-sectional survey of 16,657 subjects from 444 Polish cities. Heart. 2010;96:131–5. doi: 10.1136/hrt.2009.171520. [DOI] [PubMed] [Google Scholar]
- 20.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al. Evaluation of a novel anti-ischemic agent in acute coronary syndromes: design and rationale for the Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-elevation acute coronary syndromes (MERLIN)-TIMI 36 trial. Am Heart J. 2006;151:1186, e1–9. doi: 10.1016/j.ahj.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297:1775–83. doi: 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- 22.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–42. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 23.Kockx M, Leenen R, Seidell J, et al. Relationship between visceral fat and PAI-1 in overweight men and women before and after weight loss. Thromb Haemost. 1999;82:1490–6. [PubMed] [Google Scholar]
- 24.Zeller M, Steg PG, Ravisy J, et al. Relation between body mass index, waist circumference, and death after acute myocardial infarction. Circulation. 2008;118:482–90. doi: 10.1161/CIRCULATIONAHA.107.753483. [DOI] [PubMed] [Google Scholar]
- 25.Mahaffey KW, Tonev ST, Spinler SA, et al. Obesity in patients with non-ST-segment elevation acute coronary syndromes: results from the SYNERGY trial. Int J Cardiol. 2010;139:123–33. doi: 10.1016/j.ijcard.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Lewis CE, McTigue KM, Burke LE, et al. Mortality, health outcomes, and body mass index in the overweight range: a science advisory from the American Heart Association. Circulation. 2009;119:3263–71. doi: 10.1161/CIRCULATIONAHA.109.192574. [DOI] [PubMed] [Google Scholar]
- 27.Allison DB, Faith MS, Heo M, et al. Hypothesis concerning the U-shaped relation between body mass index and mortality. Am J Epidemiol. 1997;146:339–49. doi: 10.1093/oxfordjournals.aje.a009275. [DOI] [PubMed] [Google Scholar]
- 28.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 29.Goel K, Misra A, Vikram NK, et al. Subcutaneous abdominal adipose tissue is associated with the metabolic syndrome in Asian Indians independent of intra-abdominal and total body fat. Heart. 2010;96:579–83. doi: 10.1136/hrt.2009.183236. [DOI] [PubMed] [Google Scholar]
- 30.van der Kooy K, Seidell JC. Techniques for the measurement of visceral fat: a practical guide. Int J Obes Relat Metab Disord. 1993;17:187–96. [PubMed] [Google Scholar]
- 31.Pouliot MC, Despres JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–8. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 32.Seidell JC, Bjorntorp P, Sjostrom L, et al. Regional distribution of muscle and fat mass in men–new insight into the risk of abdominal obesity using computed tomography. Int J Obes. 1989;13:289–303. [PubMed] [Google Scholar]
- 33.Jacobs EJ, Newton CC, Wang Y, et al. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med. 2010;170:1293–301. doi: 10.1001/archinternmed.2010.201. [DOI] [PubMed] [Google Scholar]