Abstract
Objective
To describe longitudinal changes in plasma lipid levels and pubertal stage in youths from age 8-18 years, in Project HeartBeat!
Study design
Fasting blood samples and pubertal stage, using physical assessment of secondary sex characteristics, were obtained every 4 months for up to 4 years in a mixed longitudinal study of 633 children (49.1% female, 20.1% black), initially aged 8, 11, and 14 years. Total cholesterol, low density lipoprotein-cholesterol, high density lipoprotein-cholesterol, triglycerides (TG), and nonhigh density lipoprotein-cholesterol measurements were obtained. Data were collected from 1991-1995.
Results
Pubertal stage correlations with age varied among all race-sex groups (range, r = 0.61-0.70), and a given pubertal stage could represent a range of 5 years or more of chronological age. Throughout puberty, levels of total cholesterol, low density lipoprotein-cholesterol, and nonhigh density lipoprotein-cholesterol decreased, TG in males increased, and high density lipoprotein-cholesterol and TG in females showed no changes. Within a given pubertal stage, plasma lipid levels tended to differ by race, sex, or both.
Conclusions
Lipid levels change markedly by pubertal stage, and patterns differ by sex and race. Chronological age ranges widely within a given pubertal stage and is an insensitive indicator of pubertal stage and the related changes in lipid levels. Pubertal development should be considered when determining screening criteria to identify youths with adverse blood lipid levels.
Adverse levels of blood lipids constitute a well-established cardiovascular disease risk factor.1 Studies in children and young adults have documented tracking of lipid levels over time,2-4 and their association with early atherosclerotic lesions.5-7 Several cross-sectional and longitudinal studies have described the changes of lipid levels by age during childhood and adolescence8-21; fewer studies have taken pubertal stage into account.15-21 Recently, the Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents recommended universal screening with nonfasting nonhigh density lipoprotein-cholesterol (non-HDL-C) at ages 9-11 and 17-21 years.22 These ages are within the time of the greatest changes of lipid levels, after infancy, where hormonal changes and associated physical and sexual maturation occur.23 The 9- to 11-year-old age group has the greatest variation in sexual maturation.24
Children of the same age, sex, and race vary considerably in their degree of sexual and somatic maturation. The influence of hormonal changes associated with puberty on lipid levels is documented, and several studies have described lipid levels during these pubertal changes.17,19,20,25,26 However, the pattern of changes of blood lipid components varied among these studies and changes of non-HDL-C were not described. These data are derived from studies where sexual maturation was assessed once in cross-sectional studies and annually or semiannually, at most, in longitudinal studies. Sexual maturation data were based on the age of menarche, self-assessment using photographs for pubertal stage, or by physical examination. Observations have commonly been reported in terms of 2 or 3 categories (eg, early, middle, and late puberty). This report describes the changes in blood lipid levels (total cholesterol [TC], low density lipoprotein-cholesterol [LDL-C], high density lipoprotein-cholesterol [HDL-C], and non-HDL-C, and triglycerides [TG]) in relation to changes in pubertal stage during puberty in a primarily normal weight cohort of black and nonblack youths, aged 8-18 years. Pubertal staging, using physical assessment of secondary sex characteristics, was obtained every 4 months for up to 4 years in 633 participants.
Methods
Project HeartBeat! was designed to evaluate dynamics of change in cardiovascular disease risk factors among children and adolescents. Design and methods of the study have been described in detail elsewhere.27,28 Briefly, 3 cohorts of children, initially aged 8,11, and 14 years, were enrolled between October 1991 and July 1993 from The Woodlands and Conroe, Texas. The total study sample consisted of 678 participants: 49.1% female, 74.6% white, 20.1% black, and 5.3% other (Hispanic, Asian, and American Indian). For data analysis, race/ethnicity was categorized as black or nonblack based on questionnaire responses provided by the participants’ parents because separate analysis for Hispanic, Asian, and American Indian respondents was not advisable because of small sample size. A participant's exact age was calculated on each occasion of data collection. Participants were examined 3 times per year through August 1995 (mean number of 8.3 examinations per participant). Because the study design included overlapping ages between cohorts, it was possible to estimate a consecutive 10-year developmental pattern (for ages 8-18 years) over the period of 4 years. For the primary analysis, 633 participants who had pubertal stage, lipids, and body mass index (BMI) z-score for at least 1 encounter were included. Analysis excluded observations when age and sex specific BMI was ≥95th percentile (BMI z-score of 1.645) leaving 587 participants and a total of 4229 observations. Secondary analysis was performed excluding all participants who ever had BMI ≥95th percentile leaving 533 participants and 3986 observations. The differences between lipid levels calculated in the primary and secondary analyses were compared at each pubertal stage for all sex-race groups. Clinically important difference of lipid levels was arbitrarily set to be equal or greater than 3 mg/dL. The study protocol was approved by the institutional review boards of the University of Texas at Houston Health Science Center and of Baylor College of Medicine; the University of Utah gave an exemption to the current secondary data analysis. For each participant, informed consent or assent and parental consent were obtained.
Plasma lipid concentrations were determined in the Lipid Research Laboratory of Baylor College of Medicine. At each examination, the participant's blood was drawn, after an overnight fast, into powdered ethylenediaminetetraacetic acid-containing tubes by a trained phlebotomist at the participant's home. The blood was kept at 4°C and was separated within 1 hour of collection. Aliquots were held at −70°C until laboratory testing. TC, HDL-C, and TG levels were determined using standard enzymatic methods.29,30 The Cobas Fara II analyzer (Roche Diagnostics, Switzerland) was used for the determination. Standards of performance for the inter- and intra-assay required that coefficients of variation not exceed 3%. LDL-C was calculated as LDL-C = (TC – [TG/5 + HDL-C]).31 Non-HDL-C was calculated as non-HDL-C = TC – HDL-C.32
Physical assessment of secondary sex characteristics, was performed through direct visual assessment of pubic hair and breast or testicular volume/scrotal texture/penile growth according to the method of Tanner,33,34 based on earlier work by Reynolds and Wines.35,36 The Project HeartBeat! protocol was implemented under evaluation by James M. Tanner, co-investigator on the project. The observed pubertal stage ranged from 1 (prepubescent) to 5 (adult) for each characteristic. For this report, we used breast staging for females and genital staging for males to reflect pubertal development.
Statistical Analyses
We fit linear mixed models of serum lipids on pubertal stage, sex, and race using SAS Proc Mixed (SAS Institute Inc, Cary, North Carolina)37 to account for repeated measurements across visits for the same subject. We report least squares means and SEs derived from the fitted models. A heterogeneous variance autoregressive covariance structure was found to be appropriate for all measurements considered in this report. For each measurement, we considered main effects and 2-way interactions for sex, race (black vs nonblack), and pubertal stage treated as a 5-level categorical variable. We retained interactions statistically significant at α = 0.05 level in final models along with the corresponding main effects. The interaction pubertal stage-race was not significant for any model, which may be due to smaller numbers of black females. Notably, after the addition of pubertal stage to stepwise models, age at examination was no longer significant.
Concordance between breast/genital and pubic hair staging was 81.2%, with the highest concordance at pubertal stage 1 and pubertal stage 5 (95.3% and 92.4%, respectively). The correlation of age with pubertal stage, within the same individual observed over time because there are repeated measurements for each individual over time, was calculated using the approach of Bland and Altman.38 Correlation calculations were also performed stratifying by sex and race.
We adjusted the type 1 error using Bonferroni correction, where α = 0.05 was divided by the number of tests (0.05/225 = 0.00022), and the level of significance was conservatively set to 0.0001. We arrived at 225 tests by conducting 5 pair wise comparisons between 4 race-sex categories at each of the 5 time points (pubertal stage) for each of the 5 outcomes (lipid components). For each race-sex category, we compared means at adjacent pubertal stage and between pubertal stage 1 and pubertal stage 5. Thus, the total number of comparisons is 5 × [(5 × 5) + (4 × 5)] = 225.
Results
Table I addresses the composition of the study population and the relation between pubertal stage and chronological age. For every race-sex group, pubertal changes started in some participants before age 9 years; within each group other than black males, some individuals completed puberty before age 12 years. In each group, the majority of participants attained pubertal stage 5 by age 17 years. Correlation coefficients for age and pubertal stage were 0.65 (P < .05) for the entire cohort, 0.61 (P < .05) for nonblack males, 0.66 (P < .05) for black males, 0.67 (P < .05) for nonblack females, and 0.70 (P < .05) for black females (data not shown). A wide range of ages is apparent within each pubertal stage for 1 or more of the race-sex groups. Table II presents baseline data on plasma lipid levels for each of the 8-, 11-, and 14-year-old cohorts. Plasma lipid levels varied across the 3 cohorts. For example, TC, LDL-C, and non-HDL-C levels were highest among the 11-year-old males and among the 8-year-old females. Among females, HDL-C decreased in successive older cohorts.
Table I.
Age means and ranges in years and the number of observations of each sexual maturation stage for participants – Project HeartBeat!
| Sexual maturation ratings |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Nonblack males | |||||
| Age mean (SD) | 9.17 (1.3) | 11.54 (1.12) | 13.26 (1.06) | 14.11 (0.80) | 14.69 (0.91) |
| Age range | 8.13-12.02 | 8.48-14.81 | 10.42-15.43 | 11.76-15.75 | 11.22-16.57 |
| N of observations | 884 | 362 | 168 | 153 | 490 |
| Black males | |||||
| Age mean (SD) | 9.30 (1.02) | 11.19 (1.38) | 12.11 (0.48) | 14.02 (0.87) | 14.46 (0.75) |
| Age range | 8.28-11.74 | 8.96-13.05 | 11.71-12.77 | 12.45-14.94 | 13.46-15.53 |
| N of observations | 192 | 106 | 40 | 28 | 53 |
| Nonblack females | |||||
| Age mean (SD) | 8.79 (0.98) | 10.81 (0.94) | 12.44 (1.21) | 13.35 (1.26) | 14.43 (1.04) |
| Age range | 8.13-12.15 | 8.36-13.01 | 10.14-14.78 | 10.49-15.72 | 11.61-17.31 |
| N of observations | 672 | 423 | 225 | 213 | 569 |
| Black females | |||||
| Age mean (SD) | 8.80 (0.36) | 9.99 (1.25) | 11.72 (1.68) | 11.95 (0.83) | 14.05 (1.72) |
| Age range | 8.16-9.51 | 8.22-12.96 | 9.54-14.16 | 10.95-12.89 | 10.87-16.05 |
| N of observations | 100 | 120 | 77 | 44 | 158 |
N, number.
Table II.
Study population (n = 633) by age, sex, and race, and baseline plasma lipids (means and SDs) by age, and sex, Project HeartBeat!
| 8-year-old cohort |
11-year-old cohort |
14-year-old cohort |
||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| Age (y) | 8.5 (0.3) | 8.5 (0.3) | 11.5 (0.3) | 11.5 (0.4) | 14.4 (0.2) | 14.4 (0.2) |
| Sex (n, %) | 149 (49.5) | 152 (50.5) | 95 (51.1) | 91 (48.9) | 73 (50.0) | 73 (50.0) |
| Black (n, %)* | 37 (12.3) | 41 (13.6) | 20 (10.8) | 17 (9.1) | 7 (4.8) | 11 (7.5) |
| Nonblack (n, %)* | 112 (37.2) | 111 (36.9) | 75 (40.3) | 74 (39.8) | 66 (45.2) | 62 (42.5) |
| TC (mg/dL) | 163.1 (24.4) | 163.6 (27.7) | 165.0 (28.9) | 159.4 (24.5) | 149.3 (22.6) | 159.4 (29.1) |
| LDL-C (mg/dL) | 95.0 (22.2) | 97.7 (25.5) | 96.8 (25.5) | 91.52 (23.9) | 85.2 (20.6) | 93.4 (27.7) |
| HDL-C (mg/dL) | 54.2 (11.2) | 50.7 (10.2) | 51.2 (12.4) | 50.7 (12.2) | 45.2 (10.0) | 48.9 (10.7) |
| TG (mg/dL) | 69.3 (28.9) | 75.5 (34.8) | 84.8 (53.4) | 85.8 (43.2) | 93.7 (57.2) | 85.3 (40.5) |
| Non-HDL-C (mg/dL) | 108.9 (24.3) | 112.85 (26.5) | 113.8 (30.2) | 108.7 (24.9) | 104.0 (25.0) | 110.5 (29.6) |
Percentages are for the 4 race-sex groups within each cohort and add to 100% per cohort.
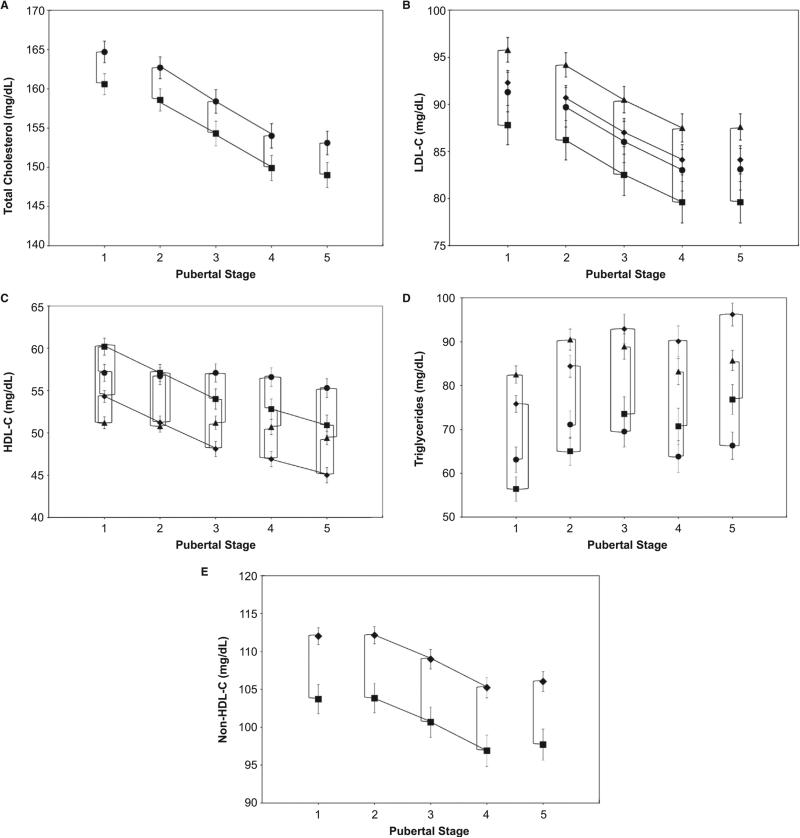
To present the several levels of statistical comparisons made for this analysis, Figure A-E show the data points corresponding to least squares means ± SEs for each lipid class for each significantly related subgroup (by race, sex, or both) at each pubertal stage. In general, for TC, LDL-C, and HDL-C, females had higher levels at most pubertal stages compared with those for males. For TG, males had higher levels at later pubertal stage. Non-HDL-C levels were similar for males and females at all pubertal stages.
Figure.
Longitudinal changes of plasma lipids in relation to pubertal stages. A, ■, males; ●, females; B, C, and D, ◆, nonblack males; ■, black males; ▲, nonblack females; ●, black females; and E, ◆, nonblack; ■, black. All data points are least squares means ± SE at each pubertal stage. Lines connecting any pair of data points (vertical or horizontal) indicate significant differences (P < .0001).
For both sex groups, TC (Figure, A) followed a similar pattern of change during puberty where mean levels significantly decreased in successive pubertal stage between pubertal stage 2 and pubertal stage 4, a total decline of about 11 mg/dL, then stabilized. At all pubertal stages, TC levels were significantly higher in females than in males. Data are presented for black and nonblack youths combined, as there was no significant race effect.
In all race-sex cohorts, LDL-C (Figure, B) levels significantly declined in successive pubertal stages between pubertal stage 2 and pubertal stage 4. At each pubertal stage, LDL-C for nonblack females was significantly higher (by about 3 mg/dL) than for black males.
Black youths had higher levels of HDL-C (Figure, C) at all pubertal stages compared with nonblacks. Black females had higher levels compared with nonblack males at each pubertal stage, whereas nonblack females had significantly lower levels than black males at pubertal stage 1 and pubertal stage 2. HDL-C levels significantly decreased in successive pubertal stages between pubertal stage 1 and pubertal stage 3 and between pubertal stage 4 and pubertal stage 5, and from pubertal stage 1 and 5 in males.
Nonblack females had significantly higher levels of TG (Figure, D) at pubertal stage 1 compared with those in black males. TG mean levels in females showed no significant changes between pubertal stage 1 and pubertal stage 5 whereas in males, levels significantly increased in successive pubertal stages between pubertal stage 1 and pubertal stage 3 and between pubertal stage 4 and pubertal stage 5 and were significantly higher than those in black females at pubertal stage 5. There was a significant difference in levels between black females and nonblack males at pubertal stage 3 to pubertal stage 5 and between nonblack females and black males at pubertal stage 1 and pubertal stage 2. There was a significant difference in levels between black and nonblack females and between black and nonblack males at all pubertal stages.
There was no significant difference in non-HDL-C levels (Figure, E) and their patterns of change between males and females; therefore, data are combined for sex groups and presented by race only. Nonblack youths had significantly higher levels (about 8 mg/dL) than those for black youths at all pubertal stages. In black and nonblack youths, levels significantly decreased in successive pubertal stages between pubertal stage 2 and pubertal stage 4.
Lipid levels based on secondary analysis were statistically significantly lower than those based on primary analysis. However, these differences were less than 3 mg/dL at all pubertal stages for all sex-race groups, except TG level was 3.3 mg/dL lower in nonblack males at pubertal stage 2, and non-HDL-C level was 3.1 mg/dL lower in black youths (there was no sex effect) at pubertal stage 1 and pubertal stage 2 (data not shown).
Discussion
Pubertal stage correlations with age varied among the 4 race-sex groups and a given pubertal stage could represent a range of 5 years or more of chronological age. A wide range of ages is apparent within each pubertal stage for 1 or more of the race-sex groups, further indicating limited correspondence between pubertal stage and chronological age.
During puberty, levels of plasma TC, LDL-C, and non-HDL-C in all groups, and HDL-C in males decreased, TG levels increased in males, and HDL-C and TG showed no changes in females. Within a given pubertal stage, plasma lipid levels tended to differ by race, sex, or both. For HDL-C, sex differences in the pattern of change emerge by pubertal stage 3. Females generally had higher lipid levels than males at each pubertal stage, except that males had similar levels of non-HDL-C and higher TG. Nonblack youths had higher lipid levels than blacks except for HDL-C.
In Project HeartBeat!, sexual maturation was based on the 5 pubertal stages defined by Tanner,33,34 and longitudinal lipid changes were described at each stage whereas none of the previous studies presented longitudinal lipid data using the 5-level pubertal staging. Males and females showed significant decreases in TC and LDL-C levels during puberty and that pattern was similar to those reported on TC changes in most of the previous studies.17,20,25,39 Other studies found a significant decrease in plasma TC in males only.12-15
In this analysis, for both black and nonblack youths, females had no changes in HDL-C and TG levels by pubertal stage; however, males with more advanced pubertal stage had lower levels of HDL-C and higher levels of TG than less mature males. Males with more advanced pubertal stage had also lower levels of HDL-C and higher levels of TG than females with the same pubertal stage. In earlier studies, Tell et al20 had similar findings among Finnish youths. In the Bogalusa Heart Study, Berenson et al23 reported similar observations only in white males but not in black males nor in females. Cobbaert et al26 reported significant decrease in HDL-C levels in males only and in TG in females only during puberty. Morrison et al17 reported no significant changes in TG levels during puberty in white boys and girls. The most important contribution of the Project HeartBeat! findings is that pubertal stage is clearly defined among each race-sex cohort and allows more precise comparison and description of the changes in lipids during puberty. None of the previous studies have described non-HDL-C pattern of changes by sexual maturation.
Because of the 2011 Integrated Pediatric Guidelines for Cardiovascular Risk Reduction in Children and Adolescents recommendation for universal screening with nonfasting non-HDL-C at ages 9-11 and 17-21 years,24 our findings warrant special emphasis. First, our study showed that puberty may start at age 8 years and end at age 11 years. This finding may not support the rationale for the universal screening for children ages 9-11 years. Secondly, demonstrating that non-HDL-C levels declined during puberty for all race-sex groups, diagnosis of dyslipidemia during this period using only age specific values without considering pubertal stage may underdiagnose dyslipidemia in some cases. Also, demonstrating variation of lipid level changes during puberty according to sex and race, using cut-off values for diagnosis of dyslipidemia without accounting for sex and race differences may not be ideal for all individuals. In addition, our data reconfirm the previously reported observation that a given pubertal stage can occur over several years of age.24 For example, we show that ages 10 and 11 years are included in every sexual maturation stage. This fact raises significant concern about use of chronological age to determine when lipid screening will be most useful and reliable and how results should be interpreted in relation to the individual course of change in lipid levels during childhood and adolescence.
As a generally consistent finding, TC levels decrease during sexual maturation. However, regarding TG and lipoprotein-cholesterol fractions, patterns of change during puberty have differed widely among studies, which may be attributable to differences in study design, population, and method of sexual maturation assessment. For example, findings from longitudinal studies3,12,13,17 can be confounded by time of the measurement effects vs cross-sectional studies,16,21,25,26 where results can be confounded by cohort effects. In previous studies, the time intervals between assessments varied between 112,17 and 3 years.2,3 Considering the average length of time for the completion of puberty is 3 years, details of changing patterns in lipid levels may be missed with longer follow-up intervals. Sexual maturation assessment was performed by physical examination4,16,17 in several studies, and by self-assessment25 or self-reported age of menarche7 in another. Correlation of self-reported assessment with physical examination varies from 0.61-0.82,40 and recall bias may affect self-reported age at menarche. The use of physical examination in a longitudinal study with frequent pubertal stage assessment strengthens the accuracy of the findings presented here.
Frequent assessment of sexual maturation rating, every 4 months, during the 4 years of follow-up by well-trained and certified examiners provided accurate measurements of each pubertal stage and its progression in individual study participants in the present study. The study included black and nonblack youth ages 8-18 years; this wide age range enabled us to capture most of the changes that are expected during puberty, including the variation in sexual maturation by chronological age by sex and race. Further, restriction of this analysis to observations without obesity at baseline and follow-ups may provide a description of lipid changes during puberty that is less affected by obesity. Furthermore, these findings were confirmed by the secondary analysis where all data on children who had obesity at any occasion were excluded. Data are derived from a synthetic cohort comprising 3 separate groups of individuals with overlapping ages who, together, span ages 8-18 years. This is a key aspect of the design such that the main age effect can be distinguished from cohort and time-of-measurement effects.41
There were some limitations to the study. Nonblack represents mainly white youths and patterns of change in lipid levels and their relations to pubertal stage for Hispanic, Asian, and American Indian participants could not be separately analyzed in this study but might be different from those for black and nonblack participants. Because multiple comparisons were performed, requiring substantial adjustment of the statistical significance level, power was limited to identify additional significant changes between sexual maturity rating stages or between race-sex subgroups. Finally, children with familial hypercholesterolemia and/or on lipid lowering therapy were not excluded. However, knowing the prevalence of heterozygous familial hypercholesterolemia is 1 every 200-500 individuals, only 2-3 participants, maximum, would fall in this category.
Plasma lipid levels change markedly by pubertal stage during puberty, and patterns differ by race and sex. Chronological age can range widely within a given pubertal stage and, in these instances, is an insensitive indicator of expected status of changing lipid levels. Pubertal development should be considered when determining screening and diagnostic criteria to identify children and adolescents with adverse blood lipid levels; pre-pubertal (ie, before age 9 years) and postpubertal (ie, after age 17 years) screening may be more informative and meaningful than currently recommended ages; alternatively, screening could be targeted by pubertal stage rather than chronological age.
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute (through Cooperative Agreement U01-HL-41166) and the Centers for Disease Control and Prevention through the Southwest Center for prevention Research (U48/CCU609653). N.M. was supported by the National Heart, Lung, and Blood Institute (HL#092069).
Glossary
- BMI
Body mass index
- HDL-C
High density lipoprotein-cholesterol
- LDL-C
Low density lipoprotein-cholesterol
- non-HDL-C
Nonhigh density lipoprotein-cholesterol
- TC
Total cholesterol
- TG
Triglycerides
Footnotes
The authors declare no conflicts of interest.
References
- 1.Labarthe DR. Epidemiology and prevention of cardiovascular diseases: a global challenge. Aspen; Gaithersburg, MD: 1998. [Google Scholar]
- 2.Freedman DS, Wang YC, Dietz WH, Xu JH, Srinivasan SR, Berenson GS. Changes and variability in high levels of low-density lipoprotein cholesterol among children. Pediatrics. 2010;126:266–73. doi: 10.1542/peds.2009-3454. [DOI] [PubMed] [Google Scholar]
- 3.Juhola J, Magnussen CG, Viikari JS, Kähönen M, Jula A, Lehtimäki T, et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159:584–90. doi: 10.1016/j.jpeds.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Clarke WR, Schrott HG, Leaverton PE, Connor WE, Lauer RM. Tracking of blood lipids and blood pressures in school age children: the Muscatine study. Circulation. 1978;58:626–34. doi: 10.1161/01.cir.58.4.626. [DOI] [PubMed] [Google Scholar]
- 5.Morrison KM, Dyal L, Conner W, Helden E, Newkirk L, Yusuf S, et al. Cardiovascular risk factors and noninvasive assessment of subclinical atherosclerosis in youth. Atherosclerosis. 2010;20:501–5. doi: 10.1016/j.atherosclerosis.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 6.Strong JP, Zieske AW, Malcom GT. Lipoproteins and atherosclerosis in children: an early marriage? Nutr Metab Cardiovasc Dis. 2001;11(Suppl 5):16–22. [PubMed] [Google Scholar]
- 7.Berenson GS, Srinivasan SR, Bao W, Newman WP, III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 8.Dai S, Fulton JE, Harrist RB, Grunbaum JA, Steffen LM, Labarthe DR. Blood lipids in children: age-related patterns and association with body-fat indices: Project HeartBeat!. Am J Prev Med. 2009;37:S56–64. doi: 10.1016/j.amepre.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Kit BK, Carroll MD, Lacher DA, Sorlie PD, DeJesus JM, Ogden C. Trends in serum lipids among US youths aged 6 to 19 years, 1988-2010. JAMA. 2012;308:591–600. doi: 10.1001/jama.2012.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellerio H, Alberti C, Druet C, Capelier F, Mercat I, Josserand E, et al. Novel modeling of reference values of cardiovascular risk factors in children aged 7 to 20 years. Pediatrics. 2012;129:e1020–9. doi: 10.1542/peds.2011-0449. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Mokdad AH, Ajani UA. Trends in risk factors for cardiovascular disease among children and adolescents in the United States. Pediatrics. 2004;114:1534–44. doi: 10.1542/peds.2004-0674. [DOI] [PubMed] [Google Scholar]
- 12.Twisk JW, Kemper HC, Mellenbergh GJ. Longitudinal development of lipoprotein levels in males and females aged 12-28 years: the Amsterdam Growth and Health Study. Int J Epidemiol. 1995;24:69–77. doi: 10.1093/ije/24.1.69. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan SR, Wattigney W, Webber LS, Berenson GS. Race and gender differences in serum lipoproteins of children, adolescents, and young adults—emergence of an adverse lipoprotein pattern in white males: the Bogalusa Heart Study. Prev Med. 1991;20:671–84. doi: 10.1016/0091-7435(91)90063-a. [DOI] [PubMed] [Google Scholar]
- 14.Lauer RM, Lee J, Clarke WR. Factors affecting the relationship between childhood and adult cholesterol levels: the Muscatine Study. Pediatrics. 1988;82:309–18. [PubMed] [Google Scholar]
- 15.Porkka KV, Viikari JS, Rönnemaa T, Marniemi J, Akerblom HK. Age and gender specific serum lipid and apolipoprotein fractiles of Finnish children and young adults. The Cardiovascular Risk in Young Finns Study. Acta Paediatr. 1994;83:838–48. doi: 10.1111/j.1651-2227.1994.tb13155.x. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong N, Balding J, Gentle P, Kirby B. Serum lipids and blood pressure in relation to age and sexual maturity. Ann Hum Biol. 1992;19:477–87. doi: 10.1080/03014469200002312. [DOI] [PubMed] [Google Scholar]
- 17.Morrison JA, Laskarzewski PM, Rauh JL, Brookman R, Mellies M, Frazer M, et al. Lipids, lipoproteins, and sexual maturation during adolescence: the Princeton maturation study. Metabolism. 1979;28:641–9. doi: 10.1016/0026-0495(79)90017-9. [DOI] [PubMed] [Google Scholar]
- 18.Altwaijri YA, Day RS, Harrist RB, Dwyer JT, Ausman LM, Labarthe DR. Sexual maturation affects diet-blood total cholesterol association in children: Project HeartBeat!. Am J Prev Med. 2009;37:S65–70. doi: 10.1016/j.amepre.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Srinivasan SR, Elkasabany A, Berenson GS. Distribution and correlates of serum high-density lipoprotein subclasses (LpA-I and LpA-I: A-II) in children from a biracial community. The Bogalusa Heart Study. Metabolism. 1998;47:757–63. doi: 10.1016/s0026-0495(98)90042-7. [DOI] [PubMed] [Google Scholar]
- 20.Tell GS, Mittelmark MB, Vellar OD. Cholesterol, high density lipoprotein-cholesterol and triglycerides during puberty: the Oslo Youth Study. Am J Epidemiol. 1985;122:750–61. doi: 10.1093/oxfordjournals.aje.a114158. [DOI] [PubMed] [Google Scholar]
- 21.Sprecher DL, Morrison JA, Simbartl LA, Schreiber GB, Sabry ZI, Biro FM, et al. Lipoprotein and apolipoprotein differences in black and white girls. The National Heart, Lung, and Blood Institute Growth and Health Study. Arch Pediatr Adolesc Med. 1997;151:84–90. doi: 10.1001/archpedi.1997.02170380088014. [DOI] [PubMed] [Google Scholar]
- 22.Kavey RW, Simons-Morton DG, de Jesus JM, editors. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary reportIn. Pediatrics. 2011;128:S213–56. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee PA. Normal ages of pubertal events among American males and females. J Adolesc Health Care. 1980;1:26–9. doi: 10.1016/s0197-0070(80)80005-2. [DOI] [PubMed] [Google Scholar]
- 24.Berenson GS, Srinivasan SR, Cresanta JL, Foster TA, Webber LS. Dynamic changes of serum lipoproteins in children during adolescence and sexual maturation. Am J Epidemiol. 1981;113:157–70. doi: 10.1093/oxfordjournals.aje.a113080. [DOI] [PubMed] [Google Scholar]
- 25.Katon JG, Flores YN, Salmeron J. Sexual maturation and metabolic profile among adolescents and children of the Health Worker Cohort Study in Mexico. Salud Publica Mex. 2009;51:219–26. doi: 10.1590/s0036-36342009000300012. [DOI] [PubMed] [Google Scholar]
- 26.Cobbaert C, Deprost L, Mulder P, Rombaut K, Gijsels G, Kesteloot H. Pubertal serum lipoprotein (a) and its correlates in Belgian schoolchildren. Int J Epidemiol. 1995;24:78–87. doi: 10.1093/ije/24.1.78. [DOI] [PubMed] [Google Scholar]
- 27.Labarthe DR, Nichaman MZ, Harrist RB, Grunbaum JA, Dai S. Development of cardiovascular risk factors from ages 8 to 18 in Project Heart-Beat! Study design and patterns of change in plasma total cholesterol concentration. Circulation. 1997;95:2636–42. doi: 10.1161/01.cir.95.12.2636. [DOI] [PubMed] [Google Scholar]
- 28.Mueller WH, Harrist RB, Doyle SR, Ayars CL, Labarthe DR. Body measurement variability, fatness, and fat-free mass in children 8, 11, and 14 years of age: Project HeartBeat! Body measurement variability, fatness, and fat-free mass in children 8, 11, and 14 years of age: Project Heart-Beat!. Am J Hum Biol. 1999;11:69–78. doi: 10.1002/(SICI)1520-6300(1999)11:1<69::AID-AJHB7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 29.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–88. [PubMed] [Google Scholar]
- 30.Siedel J, Hägele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29:1075–80. [PubMed] [Google Scholar]
- 31.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 32.Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 33.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds EL, Wines JV. Individual differences in physical changes associated with adolescence in girls. Am J Dis Child. 1948;75:329–50. doi: 10.1001/archpedi.1948.02030020341006. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds EL, Wines JV. Physical changes associated with adolescence in boys. AMA Am J Dis Child. 1951;82:529–47. doi: 10.1001/archpedi.1951.02040040549002. [DOI] [PubMed] [Google Scholar]
- 37.Base SAS. Procedures Guide Program. Version 9.1.3. 2006 [Google Scholar]
- 38.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 1–correlation within subjects. BMJ. 1995;310:446. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo S, Beckett L, Chumlea WC, Roche AF, Siervogel RM. Serial analysis of plasma lipids and lipoproteins from individuals 9-21 years of age. Am J Clin Nutr. 1993;58:61–7. doi: 10.1093/ajcn/58.1.61. [DOI] [PubMed] [Google Scholar]
- 40.Brooks-Gunn J, Warren M, Rosso J, Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child Develop. 1987;58:829–41. [PubMed] [Google Scholar]
- 41.Kemper HCG, Van't Hof MA. Design of a multiple longitudinal study of growth and health in teenagers. Eur J Pediatr. 1978;129:147–55. doi: 10.1007/BF00442158. [DOI] [PubMed] [Google Scholar]