Abstract
Objective
To assess the relationship between prenatal methamphetamine exposure (PME) and behavior problems at age 7.5 years, and the extent to which early adversity mediated this relationship.
Study design
The multicenter, longitudinal IDEAL study enrolled 412 mother-infant pairs at 4 sites. Methamphetamine-exposed participants (n= 204) were identified by self-report and/or gas chromatography/mass spectrometry confirmation of amphetamine and metabolites in infant meconium. Matched participants (n = 208) denied methamphetamine use and had a negative meconium screen. At the 7.5 year follow-up, 290 children with complete Child Behavior Checklist (CBCL) data and an early adversity index score were available for analysis (n=146 exposed).
Results
PME was significantly associated with an increased early adversity index score (P<0.001) and with increased externalizing, rule-breaking behavior, and aggressive behavior (P<0.05). Early adversity was also associated with higher externalizing behavior scores. Early adversity significantly mediated the relationship between PME and behavioral problems. After adjusting the mediation model for sex, prenatal tobacco, alcohol, and marijuana exposures, and study site, the association of PME with early adversity remained significant.
Conclusion
Though PME is associated with behavioral problems, early adversity may be a strong determinant of behavioral outcome for children exposed to methamphetamine in utero. Early adversity significantly mediated the relationship between PME and behavioral problems.
Keywords: Central Nervous System Stimulants, Substance Exposure, Childhood Behavioral Checklist (CBCL)
Methamphetamine use continues to be prevalent in the United States, especially in young adults including women of child bearing age. The number of recent new users of methamphetamine among persons aged 12 or older was 133,000 in 2011 which was greater than the 2010 estimate (107,000). The average age of new users of methamphetamine was 17.8 years.[1] Illicit drug use, including methamphetamine, among women during pregnancy continues to be a persistent problem. Among women aged 15 to 44 who were pregnant, 5% were current illicit drug users.[1] Further, the prevalence of methamphetamine abuse during pregnancy in women seeking treatment tripled from 1994 to 2006, rising to 24% of all pregnant women admitted to federally funded treatment centers[2].
Many of the initial studies evaluating prenatal methamphetamine exposure (PME) in children have been cross-sectional rather than longitudinal and very few address the influence of exposure on behavioral concerns. An exception is a small study (n=66) of Swedish children exposed to methamphetamines who were followed from birth to age 14 years. These children demonstrated higher levels of aggression and behavioral problems, poorer psychosocial well-being and lower academic achievement.[3–6] Limitations to these findings included lack of a control/comparison group and a high rate of polydrug exposure.
The Infant Development, Environment, and Lifestyle Study (IDEAL Study) is a prospective, multicenter study of children exposed to methamphetamine designed to address some of the limitations of previous investigations. Neurodevelopmental findings from the IDEAL study have demonstrated increased scores for emotional reactivity and anxious/depressed problems at ages 3 and 5 years, and externalizing and attention-deficit/hyperactivity disorder problems by age 5 years[7]. Heavy PME exposure was associated with attention problems and withdrawn behavior at both ages 3 and 5 years with no effects of PME on the internalizing or total behavior problems scales. Children with PME at 5.5 years demonstrated no differences in cognition, but did exhibit indicators of risk for Attention Deficit Hyperactivity Disorder warranting closer monitoring[8].
Less is known regarding the associations between PME and long term neurodevelopmental outcomes in children in the context of adverse environmental conditions. Previous work seeking to determine the extent early adversity mediated the relationship between PME and neurobehavioral disinhibition[9] utilized an early adversity index score created using data collected on the children and families between 0 and 3 years of age. Initial work using this adversity index score reported PME was predictive of childhood neurobehavioral disinhibition with early adversity mediating this relationship. Specifically, emotional regulation and behavior control issues at age 5 years and deficits in executive cognitive functioning at age 6.5 years [10] associated with PME was mediated by early adversity. [11] Additionally, the question of other predictors of gains in neurodevelopmental outcomes remains. Manley et al[12] evaluated cognitive scores in infants from the Caffeine for Apnea of Prematurity Study (CAP Study) at 18 months and 5 years. They found that higher maternal and paternal education as well as caregiver employment were independent and additive social variables that predicted gains in cognitive scores in these children.
The current study extends our followup findings by evaluating the association between PME and behavioral and emotional control at 7.5 years as determined by the Child Behavior Checklist (CBCL). These findings are evaluated within the context of the early adversity index score based on lifestyle and family conditions from 0 to 3 years to better determine the relationship these factors have on PME and behavioral problems.
Methods
The Infant Development, Environment, and Lifestyle (IDEAL) study is a multisite, longitudinal study investigating the effects of PME on childhood outcomes. The recruitment methods have been previously reported for the IDEAL study in detail.[13] Briefly, from September 2002 to November 2004, participants were recruited at the time of delivery from 7 hospitals in 4 geographically diverse, collaborating centers in Los Angeles, CA; Des Moines, IA; Tulsa, OK; and Honolulu, HI. 34,833 mother-infant pairs were screened. Of this population, 26,999 were available to be approached; of which, 17,961 (67%) were eligible for the study. Of the eligible population, 3705 (21%) mother-infant pairs were consented for participation (n=204 PME; n=3701 comparisons). Methamphetamine use was confirmed with meconium tests on all consented infants. Exposure to methamphetamine was determined by self-reported use during this pregnancy and/or a positive meconium screen and gas chromatography/mass spectroscopy (GC/MS) confirmation. Of the 204 subjects in the PME group, 8 subjects denied methamphetamine use but were identified as exposed by toxicology results only; 196 subjects reported amphetamine use with 146 by self-report only (toxicology was negative) and 50 by self-report and positive toxicology. No exposure to methamphetamine was defined as those denying methamphetamine use during this pregnancy and a negative GC/MS for amphetamine and metabolites. The institutional review boards at all the participating sites approved the study, and all subjects signed an informed consent. Confidentiality of information regarding the mothers' drug use was assured by obtaining a National Institute on Drug Abuse Certificate of Confidentiality, which superseded mandatory reporting of illegal substance use.
A post-partum mother was excluded if she met the following criteria: younger than 18 years; used opiates, lysergic acid diethylamide, phencyclidine, or cocaine only during her pregnancy; institutionalized for developmental delay or emotional disorders; was overtly psychotic or had a documented history of psychosis; or was non-English speaking. Exclusion criteria for the infants included critically ill and unlikely to survive, multiple birth delivery, major life-threatening congenital anomaly, documented chromosomal abnormality associated with mental or neurologic deficiency, overt clinical evidence of an intrauterine infection, and sibling previously enrolled in the IDEAL study.
For longitudinal follow up beginning at 1 month of age, a total of 204 infants were classified as PME (as described in the Study Design) and 208 mother-infant dyads were matched within site on maternal race, infant birth weight, private verses public insurance and maternal educational status. At the 7.5 year follow-up, 290 (70.4%) subjects with complete CBCL data and an early adversity index score were available for analysis. There were no differences in maternal or newborn characteristics of those included versus those not included in the analysis (Table I; available at www.jpeds.com).
Table 1.
Comparison of dyads included and not included at 7.5 year evaluation.
| N (%) or Mean (SD) | Included (n = 290) |
Not Included (n= 122) |
P-Value |
|---|---|---|---|
| Race | 0.644 | ||
| White | 117 (40.3%) | 43 (35.2%) | |
| Hispanic | 59 (20.3%) | 33 (27.0%) | |
| Pacific Islander | 51 (17.6%) | 20 (16.4%) | |
| Asian | 41 (14.1%) | 16 (13.1%) | |
| Black | 14 (4.8%) | 8 (6.6%) | |
| American Indian | 8 (2.8%) | 2 (1.6%) | |
| Low SES (Hollingshead Index=5) | 64 (22.1%) | 29 (24.2%) | 0.644 |
| Partner at birth | 162 (55.9%) | 65 (53.3%) | 0.630 |
| Education <12 years | 120 (41.5%) | 52 (43.0%) | 0.786 |
| Maternal Age | 24.9 (5.5) | 25.8 (5.9) | 0.167 |
| Prenatal MA use | 146 (50.3%) | 58 (47.5%) | 0.603 |
| Heavy prenatal MA use (>=3 days/week) | 27 (9.4%) | 8 (6.8%) | 0.619 |
| Prenatal tobacco use | 156 (53.8%) | 62 (50.8%) | 0.581 |
| Prenatal alcohol use | 69 (23.8%) | 37 (30.3%) | 0.166 |
| Prenatal marijuana use | 51 (17.6%) | 25 (20.5%) | 0.488 |
| Male | 153 (52.8%) | 67 (54.9%) | 0.688 |
| Gestational age | 38.6 (2.2) | 38.7 (1.8) | 0.689 |
| Birth weight | 3234 (602) | 3279 (593) | 0.488 |
| Birth length | 50.4 (3.5) | 50.4 (3.1) | 0.966 |
| Birth head circumference | 33.8 (1.8) | 34.2 (1.9) | 0.038 |
Measures
PME was defined by maternal self-reported prenatal methamphetamine use and/or gas chromatography/mass spectroscopy confirmation of methamphetamine metabolites in infant meconium.
Procedures and indicators similar to those used by Fisher et al[14] and Flaherty et al[15] were used to create a single index score to represent early adversity. Postnatal visits occurred at 1 month, 1 year, 2 years, 2.5 years, and 3 years such that cumulative measures of adversity were available. In the current study, the early adversity index was the sum of a set of binary indicators, including: (a) any self-reported maternal postnatal substance use through 3 years (i.e., tobacco, alcohol, marijuana, methamphetamine); (b) any extreme poverty experienced between birth and 3 years, as indicated by annual household income less than $10,000 (representing approximately 50% of the U.S. Department of Health and Human Services poverty line for families with two to five members during the years data were collected);(c) any primary caregiver changes through 3 years; (d) any reported caregiver sexual or physical abuse through 3 years; (e) any maternal subscale score on the Brief Symptom Inventory above the clinical cut point [16] through 3 years; (f) maternal depression one standard deviation or greater above the mean from birth through 3 years as indicated by the Beck Depression Inventory [17]; (g) quality of the living environment one standard deviation or greater below the mean at 2.5 years as indicated by the HOME Inventory [18]; (h) community violence one standard deviation or greater above the mean from birth through 3 years as indicated by the Neighborhood Problems section of the Lifestyle Interview and (i) social position one standard deviation or greater below the sample mean from birth through 3 years as indicated by the Index of Social Position, which represents a weighted average of parental occupational status and educational.[19,20]
The Child Behavior Checklist (CBCL) for ages 6 to 18 utilized in this study has been widely used as a method of identifying problem behavior in children.[21] The CBCL was read to the caregiver by a certified interviewer then computer scored to yield measures of internalizing, externalizing and total problems and syndrome scores that aggregate co-occurring problems and are the basis for internalizing(anxious/depressed, somatic complaints, or withdrawn) and externalizing (rule-breaking behavior and aggressive behavior) scores. Higher scores indicate more problems. Following scale developers' recommendations, internalizing, externalizing and total problem scores were standardized (T scores) and raw scores were used for the syndrome scales. Some items on the CBCL are consistent with Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-V) diagnostic categories.
Statistical Analyses
The PROCESS macro for SPSS[22] was used to test for mediation effects. Direct and indirect effects are tested using a regression-based approach. Mediation in this study is defined as a statistically significant indirect effect. A priori infant characteristics included as covariates in the mediation models were sex, prenatal tobacco, alcohol and marijuana exposures, and study site. All analyses were performed using SPSS v17.0.3 (Chicago, Illinois).
Results
A comparison of children studied at 7.5 years of age when compared with those not studied demonstrates no significant differences in birth and maternal demographic characteristics with the exception of a slightly smaller head circumference in the group studied. (Table I)
Maternal demographic data for the groups (n= 146 (71.1%) PME and n=144 (69.2%) comparison subjects) are shown in Table II. Women in the PME group were more likely to have low socioeconomic status (SES) and be without a partner at birth. Further, mothers in the PME group were more likely to use tobacco, alcohol, and marijuana during pregnancy. There were no significant differences in maternal age, or education between the two groups of women. There also were no differences in sex, birth weight, and head circumference (Table II). Though gestational age was younger in the exposed group, both groups had a term mean gestational age. Children in the exposed group had shorter birth length relative to the comparison group.
Table 2. Maternal and neonatal characteristics by methamphetamine exposure.
| N (%)or Mean (SD) | Exposed (n = 146) | Comparison (n= 144) | P-Value |
|---|---|---|---|
| Maternal Characteristics | |||
|
| |||
| Race | |||
| White | 56 (38.4%) | 61 (42.4%) | 0.924 |
| Hispanic | 30 (20.5%) | 29 (20.1%) | |
| Pacific Islander | 27 (18.5%) | 24 (16.7%) | |
| Asian | 22 (15.1%) | 19 (13.2%) | |
| Black | 6 (4.1%) | 8 (5.6%) | |
| American Indian | 5 (3.4%) | 3 (2.1%) | |
| Low SES (Hollingshead Index=5) | 50 (34.2%) | 14 (9.7%) | <0.001 |
| Partner at birth | 64 (43.8%) | 98 (68.1%) | <0.001 |
| Education <12 years | 67 (45.9%) | 53 (37.1%) | 0.128 |
| Maternal age | 25.5 (5.6) | 24.4 (5.3) | 0.092 |
| Prenatal tobacco use | 120 (82.2%) | 36 (25.0%) | <0.001 |
| Prenatal alcohol use | 49 (33.6%) | 20 (13.9%) | <0.001 |
| Prenatal marijuana use | 45 (30.8%) | 6 (4.2%) | <0.001 |
|
| |||
| Neonatal Characteristics | |||
|
| |||
| Male | 77 (52.7%) | 76 (52.8%) | 0.995 |
| Birth weight (g) | 3171 (634) | 3298 (563) | 0.071 |
| Birth length (cm) | 49.7 (3.7) | 51.1 (3.1) | 0.001 |
| Birth head circumference (cm) | 33.6 (1.8) | 33.9 (1.8) | 0.130 |
| Gestational Age (weeks) | 38.2 (2.4) | 39.1 (1.8) | 0.001 |
Note: Exposed and Comparison groups were group matched on race, maternal education status, and infant birth weight
Children with PME were exposed to significantly more overall early adversity index than comparison children (Table III). Further, the PME group had a higher rate of any extreme poverty, any changes in the primary caregiver of the child and any low social position.
Table 3.
Overall Early Adversity Index by exposure group.
| N (%) or Mean (SD) | PME | Comparison | Overall |
|---|---|---|---|
| Overall Early Adversity Index ** | 2.95 (1.47) | 1.97 (1.27) | 2.46 (1.45) |
| Maternal Postnatal Substance Use (n = 281) | 114 (80%) | 101 (73%) | 215 (77%) |
| Any Extreme Poverty (n = 290)* | 56 (38%) | 36 (25%) | 92 (32%) |
| Any Primary Caregiver Changes (n = 289) ** | 75 (52%) | 9 (6%) | 84 (29%) |
| Any Reported Caregiver Sexual or Physical Abuse (n = 256) | 7 (6%) | 5 (4%) | 12 (4%) |
| Any Positive Maternal Diagnosis of Psychological Distress (n = 290) | 82 (56%) | 66 (46%) | 148 (51%) |
| Any High Maternal Depression (n = 290) | 25 (17%) | 14 (10%) | 39 (13%) |
| Poor Quality Living Environment (n = 251) | 23 (19%) | 19 (15%) | 42 (17%) |
| High Community Violence (n = 290) | 22 (15%) | 25 (17%) | 47 (16%) |
| Any Low Social Position (n = 290)** | 26 (18%) | 8 (6%) | 34 (12%) |
Difference between PME and comparisons, p < 0.05
Difference between PME and comparisons, p < 0.001
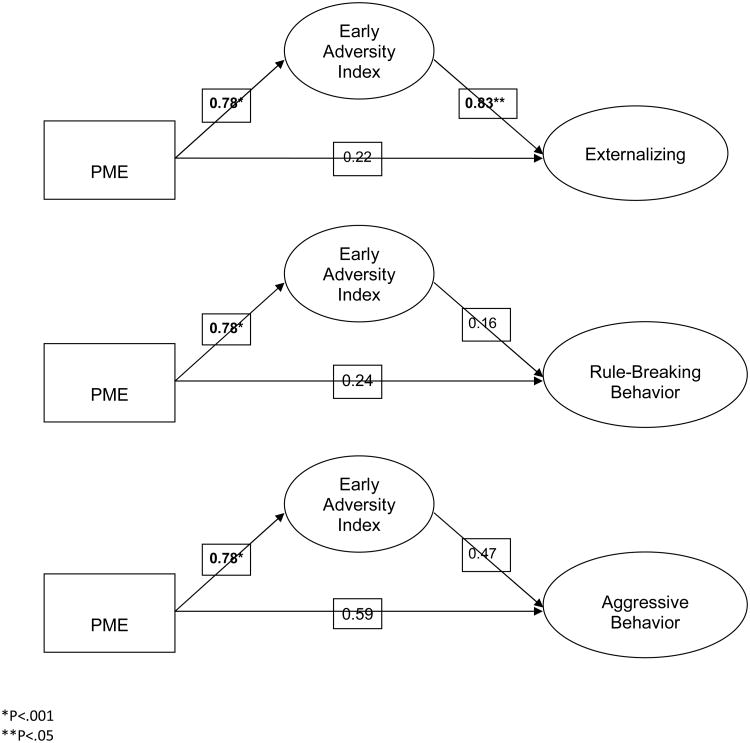
Table IV presents the results of the CBCL assessment. Children with PME at 7.5 years of age had increased externalizing, rule-breaking behavior, and aggressive behavior on the CBCL. The indirect effect of externalizing behavior mediated by early adversity was statistically significant and the total percent mediated was 34%. The indirect effect of rule breaking behavior mediated by early adversity was not significant and the total percent mediated was 26%. The indirect effect of aggressive behavior mediated by early adversity was not significant and the total percent mediated was 29%. PME was associated with increased early adversity (B=0.98, p<.001, R2=.11). Early adversity was associated with higher externalizing problems at 7.5 years (B=0.92, p<.05, overall R2=.03).and the direct effect of PME on externalizing problems was not significant (B=1.75, p>.05). Early adversity was associated with an increase in rule-breaking behavior at 7.5 years (B=0.64, p<.05, overall R2=.04) and the direct effect of PME on rule-breaking behavior was not significant (B=1.21, p>.05). Early adversity was associated with an increase in aggressive behavior at 7.5 years (B=0.77, p<.05, overall R2=.04) and the direct effect of PME on rule breaking behavior was not significant (B=1.81, p>.05). The association between PME and adversity remained significant with the covariates included in the models, as well as the relationship between early adversity and externalizing problems (Figure; available at www.jpeds.com).
Table 4.
CBCL scores by exposure group.
| Mean (SD) | PME | Comparison | Overall |
|---|---|---|---|
| Externalizing* | 56.8 (10.9) | 54.2 (9.4) | 55.5 (10.3) |
| Rule-Breaking Behavior* | 2.9 (2.6) | 2.2 (2.0) | 2.6 (2.3) |
| Aggressive Behavior* | 8.4 (6.8) | 6.6 (5.2) | 7.5 (6.1) |
| Internalizing | 51.6 (9.6) | 50.1 (9.5) | 50.9 (9.6) |
| Anxious/Depressed | 3.3 (2.7) | 3.0 (2.8) | 3.1 (2.8) |
| Withdrawn | 1.5 (1.8) | 1.2 (1.6) | 1.4 (1.7) |
| Somatic Complaints | 1.3 (1.7) | 1.2 (1.6) | 1.3 (1.6) |
| Total Problems | 54.8 (10.6) | 53.3 (9.3) | 54.1 (10.0) |
Difference between PME and comparisons, p < 0.05
Figure 1.
Relationship between early adversity and behavioral problems adjusted for prenatal exposure to alcohol, marijuana, tobacco, gender, and study site.
Discussion
This followup study to age 7.5 years extends our understanding of the effects of PME on child behavior. We found that PME was associated with increased scores in the externalizing behavior domain of the CBCL. After adjusting for confounding variables associated with developmental outcome, early adversity appears to be a strong determinate of adverse behavioral outcomes in methamphetamine exposed children. These findings are consistent with previous work that demonstrated PME and early adversity were associated with behavioral and emotional control issues at age 5 years.[11]
The Child Behavior Checklist (CBCL) utilized in this study has been widely used as a method of identifying problem behavior in children[21]. Previous studies utilizing the CBCL have demonstrated that substance exposure was associated with externalizing and internalizing behavior problems in children as young as 3 years old.[23,24] These issues persisted through to school age with specific behavioral syndromes of attention problems at 4, 6, and 9 to 11 years, aggressive behavior at 3 and 7 years, anxiety/depression at 3 and 8 years and withdrawn behavior at 3 years[23]. Additionally, Linares et al[25] utilized the CBCL to demonstrate a probable clinical range for oppositional defiant disorder and attention deficit hyperactivity disorder at 6 years of age in children prenatally exposed to cocaine.
There are a limited number of prenatal drug exposure studies assessing the contribution of early adversity with postnatal outcome. Fisher et al[14] included early postnatal environmental adversity (described as postnatal drug exposure, unstable home and caregiver environment, low SES, caregiver experiences of abuse and psychopathology) as an additional predictor and mediator between maternal cocaine abuse and later negative neurodevelopmental outcomes in children with prenatal cocaine exposure.[14] These findings demonstrated that prenatal drug use predicted the emergence and growth in neurobehavioral disinhibition across adolescence (directly for behavioral dysregulation and indirectly for executive function difficulties via early adversity and behavioral dysregulation). [14] Early adversity uniquely predicted executive function difficulties. [14] Studies have shown that parental methamphetamine use is predictive of an adverse environment with parents reporting that they feel they have created an unsafe and poorly nurturing environment for their children as a result of their methamphetamine use.[26]
Further work has been done to evaluate childhood behavioral problems and early adversity in school- age children with PME. This growing body of literature has demonstrated cognitive and behavioral effects of PME on the growing child, specifically to their brain structure and neurochemistry.[27–29] There is evidence that the microstructural integrity of white matter is disrupted in PME children which coincides with impairment of motor function and aspects of executive function.[29] Other studies have demonstrated memory and attention deficits among children with PME. Chang et al (2004) linked smaller volume subcortical structures (i.e. putamen, globus pallidus, hippocampus, and caudate) with memory and attention deficits among children with PME from 3-16 years old[30]. In previous findings from the IDEAL study, Abar et al[11] examined the extent to which PME was predictive of childhood neurobehavioral disinhibition (ND) and the extent to which early adversity (birth through year 3) mediated this process. At age 6.5 years of age PME was associated with issues regarding behavioral and emotional control at 5 years of age, which was associated with executive function deficits at 6.5 years. Moreover, early adversity significantly mediated the relationship between PME and ND.
There are limitations to this study. First our findings may not generalize to all populations of women who use methamphetamine while pregnant and did not focus on mediators that can minimize the effects of adverse events on child outcomes. Because CBCL findings are based on caregiver report, there could be reporting bias. In addition, our measure of child abuse through caregiver report of Child Protective Services involvement likely underestimates true rates of abuse.
Though PME is associated with behavioral problems, early adversity may be a strong determinant of behavioral outcomes. Early adversity mediated the relationship between behavioral problems and PME. These findings are consistent with previous work that demonstrated PME and early adversity was associated with behavioral and emotional control at 5 years and early adversity mediated the relationship between PME and neurobehavioral disinhibition. The current study only follows behavioral outcomes from birth through 7.5 years; long-term follow-up is needed for a more complete understanding of the developmental, behavioral and social outcomes of PME infants. Further studies regarding the role of adversity in PME infants would need to be conducted to explicitly evaluate these associations.
Acknowledgments
Supported by the National Institute on Drug Abuse (R01DA014918), the National Institutes of Health/National Center for Advancing Translational Science at the University of California, Los Angeles (CTSI UL1TR000124), and the National Center on Research Resources (U54RR026136).
Abbreviations
- CBCL
Child Behavior Checklist
- PME
prenatal methamphetamine exposure
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Administration SA and MHS. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MA Rockville, MD: Off Appl Stud; 2012. NSDUH Ser H-44, HHS Publ No 12-4713. Rep No H-41 2012. [Google Scholar]
- 2.Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet Gynecol. 2009;113:1285–91. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- 3.Billing L, Eriksson M, Jonsson B, Steneroth G, Zetterström R. The influence of environmental factors on behavioural problems in 8-year-old children exposed to amphetamine during fetal life. Child Abuse Negl. 1994;18:3–9. doi: 10.1016/0145-2134(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 4.Billing L, Eriksson M, Larsson G, Zetterström R. Amphetamine addiction and pregnancy. III. One year follow-up of the children. Psychosocial and pediatric aspects Acta Paediatr Scand. 1980;69:675–80. doi: 10.1111/j.1651-2227.1980.tb07342.x. [DOI] [PubMed] [Google Scholar]
- 5.Billing L, Eriksson M, Steneroth G, Zetterström R. Pre-school children of amphetamine-addicted mothers. I. Somatic and psychomotor development. Acta Paediatr Scand. 1985;74:179–84. doi: 10.1111/j.1651-2227.1985.tb10946.x. [DOI] [PubMed] [Google Scholar]
- 6.Billing L, Eriksson M, Steneroth G, Zetterstrom R. Predictive indicators for adjustment in 4-year-old children whose mothers used amphetamine during pregnancy. Child Abus Negl. 1988;12:503–7. doi: 10.1016/0145-2134(88)90067-1. [DOI] [PubMed] [Google Scholar]
- 7.LaGasse LL, Derauf C, Smith LM, Newman E, Shah R, Neal C, et al. Prenatal methamphetamine exposure and childhood behavior problems at 3 and 5 years of age. Pediatrics. 2012;129:681–8. doi: 10.1542/peds.2011-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiblawi ZN, Smith LM, LaGasse LL, Derauf C, Newman E, Shah R, et al. The effect of prenatal methamphetamine exposure on attention as assessed by continuous performance tests: results from the Infant Development, Environment, and Lifestyle study. J Dev Behav Pediatr. 2013;34:31–7. doi: 10.1097/DBP.0b013e318277a1c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abar B, LaGasse LL, Derauf C, Newman E, Shah R, Smith LM, et al. Examining the Relationships Between Prenatal Methamphetamine Exposure, Early Adversity, and Child Neurobehavioral Disinhibition. Psychol Addict Behav. 2012;27:662–73. doi: 10.1037/a0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–85. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- 11.Abar B, LaGasse LL, Derauf C, Newman E, Shah R, Smith LM, et al. Examining the relationships between prenatal methamphetamine exposure, early adversity, and child neurobehavioral disinhibition. Psychol Addict Behav. 2013;27:662–73. doi: 10.1037/a0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manley BJ, Roberts RS, Doyle LW, Schmidt B, Anderson PJ, Barrington KJ, et al. Social Variables Predict Gains in Cognitive Scores across the Preschool Years in Children with Birth Weights 500 to 1250 Grams. J Pediatr. 2015 doi: 10.1016/j.jpeds.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, et al. The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118:1149–56. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- 14.Fisher PA, Lester BM, DeGarmo DS, Lagasse LL, Lin H, Shankaran S, et al. The combined effects of prenatal drug exposure and early adversity on neurobehavioral disinhibition in childhood and adolescence. Dev Psychopathol. 2011;23:777–88. doi: 10.1017/S0954579411000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flaherty EG, Thompson R, Litrownik AJ, Theodore A, English DJ, Black MM, et al. Effect of early childhood adversity on child health. Arch Pediatr Adolesc Med. 2006;160:1232–8. doi: 10.1001/archpedi.160.12.1232. [DOI] [PubMed] [Google Scholar]
- 16.Derogatis L. BSI Brief Symptom Inventory: Administration, scoring, and procedure manual. 4th. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- 17.Beck A, Steer R, Brown G. Beck Depression Inventory–II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 18.Caldwell B, Bradley R. Home inventory administration manual. Little Rock, AR: University of Arkansas at Little Rock; 2001. [Google Scholar]
- 19.Hollingshead A. Four Factor Index of Social Status. New Haven, CT: Department of Sociology, Yale University; 1975. [Google Scholar]
- 20.LaGasse LL, Seifer R, Wright LL, Lester BM, Tronick EZ, Bauer CR, Smeriglio V. The Maternal Lifestyle Study: The caretaking environment of infants exposed to cocaine/opiates. Pediatr Res. 1999;45 doi: 10.1203/00006450-199904020-01470. [DOI] [Google Scholar]
- 21.Achenbach TM, Rescorla LA. Manual for the ASEBA preschool Forms & Profiles. Burlington, VT: University of Vermont Research Center for Children, Youth & Families; 2000. [Google Scholar]
- 22.Hayes A. Introduction to mediation, moderation, and conditional process analysis. 2013 doi: 10.1017/SJP.2021.46. doi:978-1-60918-230-4. [DOI] [PubMed] [Google Scholar]
- 23.Bada HS, Das A, Bauer CR, Shankaran S, Lester B, LaGasse L, et al. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119:e348–59. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- 24.Richardson GA, Goldschmidt L, Leech S, Willford J. Prenatal cocaine exposure: Effects on mother- and teacher-rated behavior problems and growth in school-age children. Neurotoxicol Teratol. 2011;33:69–77. doi: 10.1016/j.ntt.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linares TJ, Singer LT, Kirchner HL, Short EJ, Min MO, Hussey P, et al. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol. 2006;31:85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown JA, Hohman M. The Impact of Methamphetamine Use on Parenting. J Soc Work Pract Addict. 2006;6:63–88. doi: 10.1300/J160v06n01_04. [DOI] [Google Scholar]
- 27.Lester BM, Lagasse LL. Children of addicted women. J Addict Dis. 2010;29:259–76. doi: 10.1080/10550881003684921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colby JB, Smith L, O'Connor MJ, Bookheimer SY, Van Horn JD, Sowell ER. White matter microstructural alterations in children with prenatal methamphetamine/polydrug exposure. Psychiatry Res. 2012;204:140–8. doi: 10.1016/j.pscychresns.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos A, Kwiatkowski Ma, Fouche JP, Narr KL, Thomas KGF, Stein DJ, et al. White matter integrity and cognitive performance in children with prenatal methamphetamine exposure. Behav Brain Res. 2015;279:62–7. doi: 10.1016/j.bbr.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, et al. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res - Neuroimaging. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]