Abstract
Objective
To examine relationships between peak expiratory (cough) airflow rate (PEFR) and swallowing symptom severity in participants with Parkinson Disease
Design
Participants were cued to cough into an analog peak flow meter then swallowed three, 20 mL thin liquid barium boluses. Analyses were directed at detecting potential relationships among disease severity, swallowing symptom severity and PEFR.
Participants
Sixty eight male and females with PD.
Interventions
Not applicable
Main outcome measures
PEFR and swallow symptom severity
Results
PEFR varied significantly across swallowing severity classifications. Participants with more severe disease displayed a significant, linear decrease in PEFR compared to those participants with earlier stage, less severe disease. Swallowing symptom severity varied significantly across groups when comparing participants with less severe PD to those with more severe PD. Participants with early-stage PD demonstrated little to no swallowing symptoms and had the highest measures of PEFR. In contrast, participants with the most severe swallowing symptoms also displayed the lowest measures of PEFR.
Conclusions
Relationships existed among PD severity, swallowing symptom severity and PEFR in participants with PD. PEFR may eventually stand as a non-invasive predictor of aspiration risk in those with PD, particularly later-stage disease. Inclusion of PEFRs into existing clinical swallowing assessments may increase the sensitivity and predictive validity of these assessments.
Keywords: voluntary cough, Parkinson’s disease, dysphagia, respiratory, aspiration, airway protection, cough
Parkinson’s Disease (PD) 1 is neurodegenerative disorder primarily affecting adults. Cardinal symptoms associated with PD include rigidity, bradykinesia, resting tremor and postural instability.2 There is no cure for PD and although medications alter its progression,3 significant morbidities remain, particularly as the disease evolves. This evolution initially targets and destroys subcortical grey matter regions prior to progressing to cortical regions, presenting first within the temporal mesocortex.4–6 Later on, larger regions of the neocortex yield to the disease, including high-order sensory association and prefrontal areas. Ultimately, the degenerative disease effects reach first-order sensory association areas and premotor fields, in some patients terminating within the primary sensorimotor cortex.4–6 Later on, progressive degeneration of ascending and descending neural pathways impairs a range of physiological functions including life-sustaining mechanisms of airway protection, including breathing, coughing and swallowing, that arise from a set of shared subcortical substrates.7–9
With regard to breathing, several investigations have shown involvement of upper airway musculature and resultant airflow limitation10–15 (secondary to increased resistance), progressive declines in respiratory muscle strength16 and diaphragmatic instability and tremor.17 Cough impairment in PD is typically attributed to disease-related rigidity within chest wall structures18–21 which, coupled with reduced respiratory muscle strength, precludes the generation of sufficiently high-velocity, high-volume airflows for airway clearance. Early in the disease, motoric components of cough are primarily affected with later decline in cough sensory capabilities, reducing cough sensory thresholds in response to stimulation.22–24 Functional associations between cough and swallowing function further complicate the clinical presentation.25–27
Swallowing dysfunction or dysphagia is a key clinical feature, particularly among patients in the mid to later stages of PD,28–30 and varies relative to numerous factors such as age, gender, disease duration and the presence or absence of dementia.32 The incidence of dysphagia in individuals is estimated at 18.5%-100% with aspiration pneumonia being the leading cause of death.13,33–34 Dysphagia in PD results from disrupted motor function secondary to rigidity, hypokinesia and tremor similar to those mechanisms that impair cough.35–36 The motor dysfunction affects every stage of swallowing, potentially causing lingual tremor, difficulty with bolus manipulation, delayed onset of the pharyngeal swallowing response, reduced rate of spontaneous swallowing, increases in post-swallow oral and pharyngeal residue, decreased range of motion of the epiglottis, slowing of laryngeal elevation and excursion during pharyngeal swallowing, laryngeal penetration, aspiration, incoordination of upper esophageal sphincter opening and disruption in swallowing-respiratory coordination.35–39
Clear relationships exist between cough and aspiration risk in individuals with PD22–23, 40–45 and can be characterized through analysis of cough waveform measures and swallowing physiology.42–45 Past studies used high-tech methods for cough collection and measurement, consisting of a pneumotachograph and associated equipment, followed by digitization and computerized analysis of cough waveforms. In contrast to these methods, a low-tech method involving simple, one-step collection of voluntary peak cough expiratory airflow rate (PEFR), using a hand held analog PEFR meter, has emerged as a sensitive and non-invasive means of estimating cough strength. Phase one of this two-part study (previously published) 40 established the validity and sensitivity of an analog PEFR meter for (1) discriminating individuals with PD from healthy controls and (2) detecting differences in PEFR relative to gender. However, if this process is to transition to routine clinical use, additional information is needed regarding the relationships among breathing, coughing and swallowing in order to enhance the sensitivity and predictive validity of existing assessments of airway protection in those with PD. This Phase two report examines potential relationships between PEFR and swallowing severity as measured by the penetration-aspiration scale score (PAS). 46 Additional objectives were to examine differences in voluntary PEFR (at various perceived cough strengths) as a function of age, gender and disease severity; and compare PEFRs from our cohort of participants with PD to those previously (during Phase one) obtained from a cohort of healthy controls. We hypothesized that, for participants with moderate-severe PD would demonstrate significant impairment in both PEFR and measures of swallowing severity compared to participants with mild, early stage PD.
Methods
Sample size and power calculations were performed using NCSS-PASS (Power Analysis and Sample Size, 2008) and SAS (macro) based on a one-way analysis of variance for the primary dependent variable, peak cough airflow, with level of significance set at 0.05. Our preliminary data of participants evaluated for cough response (strong) using a pneumotachagraph, reported mean peak cough airflow for healthy adults as 7.58 L/s (2.5) and mean peak cough airflow for participants with PD participants as ranging from 6.93 L/s (1.8) to severe = 5.98 L/s (2.4). These data assumed cough response would show a linear trend in both healthy and participants with PD and that . the difference between the healthy and groups with PD can reach ≥ 1.6 l/sec (difference between the healthy and PD participants on pneumotachagraph readings). Given an estimated event rate of 0.32 for laryngeal penetration or aspiration in participants with PD obtained from unpublished preliminary data, it was estimated that the number of individuals in this study (where P1>P0 or Odds Ratio>1; odds ratio of 1.7, ,1-β =80%, α = 0.05) would be 128. Given an anticipated attrition rate of slightly greater than 2%, our targeted enrollment for the Phase two (R33 phase) was 132 participants with PD.
Participants were recruited from regional support groups for inclusion in this study. All study-related activities were completed within the radiology departments of affiliated hospitals (UF Health Center for Movement Disorders and Neurorestoration in Gainesville, FL and Memorial Hospital in Jacksonville, FL). Those interested in participating were considered for inclusion based on the following criteria: (1) diagnosis of PD by a neurologist (all severity levels accepted); (2) 30- 80 years of age; (3) nonsmoking or no smoking within the previous 5 years; (4) no history of head and neck cancer, asthma, chronic obstructive pulmonary disease, or untreated hypertension; (5) sufficient facial muscle strength so as to achieve and maintain adequate lip closure around a circular mouthpiece; (6) cognition within normal limits as determined by informal interactions between the researchers and participants. If a participant’s cognitive status was called into question at any point, the Mini Mental Status Examination47 was administered and a score of 27 or higher required in order to continue on in the study. No participants demonstrated overt signs of cognitive impairment and additional cognitive assessment was not indicated for any participant. Finally, all participants were to have (7) no neurologic (other than PD) condition that adversely affects respiratory muscles or gas exchange system. All participants taking medication for PD were tested while on the ‘on-medication response curve’ (determined through manual recording of the time of last medication administration).
Following informed consent (University of Florida IRB-01 Protocol #367–2010; Jacksonville University IRB Protocol Number 2013–32) all participants underwent measurement of PEFR during voluntary cough. Each participant completed nine coughs (three voluntary coughs at three perceived strengths of weak, moderate and strong) into an analog PEFR meter (Mini Wright Peak Flow Meter; www.miniwrightpeakflowmeter.com; Figure 1). Further details regarding the elicitation of the various cough strengths is presented within the methods section. This device was selected based on phase one of this study40 which found the analog meter to be superior to a similar, digital, peak flow meter and more closely aligned with the “gold standard” pneumotachograph with regard to discrimination of disease state (those with PD versus healthy controls) and severity of Parkinson’s disease as defined by Hoehn and Yahr (H&Y) Scale Score as well as a superior likelihood value. The meter features a high visibility scale that ranges from 60 to 900 liters per minute. Prior to measurement, the meter was fitted with a disposable pulmonary function test filter (Creative Biotech, Inc., CBI 1501) with 99.99% + bacterial filtration efficiency and 99.90%+ viral filtration efficiency. This filter was discarded after use by each participant.
Figure 1.

Mini Wright Analog Peak Flow Meter www.miniwrightpeakflowmeters.com
Each participant was provided with the following verbal instructions prior to voluntary PEFR sampling: (1) Take a breath in and give me a “soft” cough. (2) Take a breath in and cough “as hard as you can.” (3) Produce a cough that is midway between your “soft” and “hardest” cough. In other words, produce a “medium” cough. Following instruction and cued practice at each of the perceived cough intensities, participants were prompted to produce a series of nine coughs. The first three coughs were carried out in order for all participants: soft, hard, medium. The following six coughs were cued, by a research clinician (ES, AJ or FS), in computer randomized order. Cough airflow values were manually recorded by an investigator at the time of collection and later entered into a computer database spreadsheet. Each participant was questioned throughout the PEFR collection about possible task-related fatigue. No fatigue was reported by any participant and no participant requested discontinuation of the trials.
Following PEFR measurement, each participant was seated in an upright position and instructed to self-administer and swallow three (3), 20 mL thin liquid boluses (Varibar; E-Z-Em; Lake Success, NY) in a continuous manner during a videofluoroscopic examination of swallowing function (VFSS) which was digitally recorded using the Digital Swallow Station Model 7200 (Kay Elemetrics Corp; Lincoln Park, NJ). A total of three PAS scores (one per bolus) were generated for each participant (see Table 1). During the examinations, a minimum of two experienced research clinicians with expertise in VFSS swallowing studies (ES and/or AJ, FS), independently determined the degree of laryngeal penetration or aspiration after reviewing the VFSS recording of the swallowing of each bolus. In the event that different PAS scores were produced, the digital recording was reviewed, by both clinicians, until a consensus score was obtained. For each participant, both the mean and median PAS score over all recorded swallows was calculated and descriptively reviewed, then manually recorded and entered into a computer database spreadsheet for later analysis. Following this participants were grouped, according to median PAS Score and placed into swallowing severity categories: “mild” (PAS = 1–2), “moderate” (PAS 3–5) and “severe” (PAS 6–8). No adverse events were reported at the time of the experiment or during the 1 month period following consent of the final participant.
Table 1.
Penetration Aspiration Scale (PAS)46
| Score | Interpretation |
|---|---|
| 1 | Material does not enter the airway. |
| 2 | Material enters the airway, remains above the vocal folds, and is ejected from the airway. |
| 3 | Material enters the airway, remains above the vocal folds, and is not ejected from the airway. |
| 4 | Material enters the airway, contacts the vocal folds, and is ejected from the airway. |
| 5 | Material enters the airway, contacts the vocal folds, and is not ejected from the airway. |
| 6 | Material enters the airway, passes below the vocal folds, and is ejected from the airway. |
| 7 | Material enters the airway, passes below the vocal folds, and is not ejected from the airway in spite of effort. |
| 8 | Material enters the airway, passes below the vocal folds, and no effort is made to eject. |
Statistical Analysis: For the purposes of all analysis, participants were grouped by age (<60, 60–69 and ≥ 70), gender and disease severity (“mild”= H&Y 1–2; “moderate-severe”= H&Y 3–5). All data were visually evaluated using matrix plots prior to statistical analysis in order to identify extreme outliers within the datasets. Variances in the datasets were evaluated for equivalency before ANOVA tests were completed thereby allowing for use of pooled standard deviations versus individual standard deviations. (Table 2). Comparison values from healthy control (HC) participants were used to satisfy Aim 2 and were obtained from the previously-collected phase one HC dataset.40 All calculations were completed using SPSS 21.0 and Minitab Version 17.
Table 2.
Patient demographics by gender
| Male (n= 55) | Female (n=13) | Significance | |
|---|---|---|---|
| Mean Age (SD) | 68.1 (8.1) | 71.3 (6.14) | NS |
| Mean age at diagnosis (SD) | 59.8 (10.4) | 64.5 (8.21) | NS |
| H&Y score | 2.41 (.95) | 2.3 (.59) | NS |
| Prior reported swallowing issues (%) ** | 47% | 77% | p<.05 |
| Hours since medication to assessment(SD) | 2.97 (2.5) | 2.55 (2.4) | NS |
| Analog ( mean L/sec)** | 3.55 (1.11) | 2.81 (.75) | p<.04 |
non parametric
Results
Participants: Sixty-eight participants completed all study tasks. Age groupings were as follows: <60 (n=10), 60–69 (n=18), and ≥ 70 (n=39). There were 13 females (mean age 71.3 years, SD=6.14 years) and 55 males (mean 68.1 years, SD=8.1 years). The H&Y ratings for all participants ranged from 1 to 5 with a mean HY score of 2.4 (SD=0.90). There were 36 participants in the “mild” PD disease severity group (H&Y 1–2) and 32 participants in the “moderate-severe” PD disease severity group. Mean years since diagnosis of PD was 7.82 years (SD=8.2 years; Table 2).
Prior reported swallowing concerns were identified in 52.9% of participants. Significantly (p<0.05) more swallowing concerns were self-reported from female participants (Table 2). At the time of testing 97% of subjects were on medication to treat PD symptoms, with mean hours since medication of 2.9 (SD=2.4). No participants displayed signs of dyskinesia during the study tasks.
Within each dataset variances were equivalent, with the exception of perceived PEFR strength wherein “hard” coughs demonstrating statistically (p<0.05) greater variance than “soft” or “medium.” Mean cough strength correlated significantly (p<0.05) at each of the three perceived PCF strengths. The combined means of all three perceived PEFR strengths was not statistically different to the perceived “moderate” PEFR strength, therefore moderate PEFR values were used for all subsequent analyses.
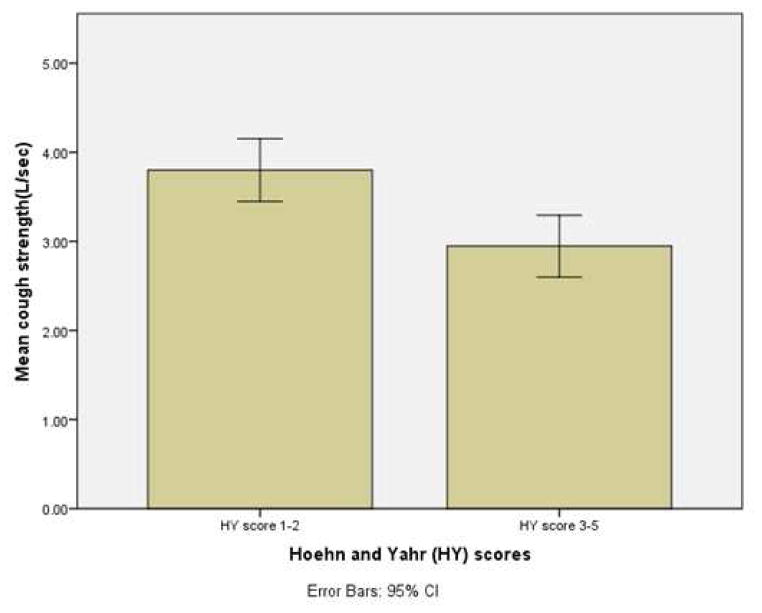
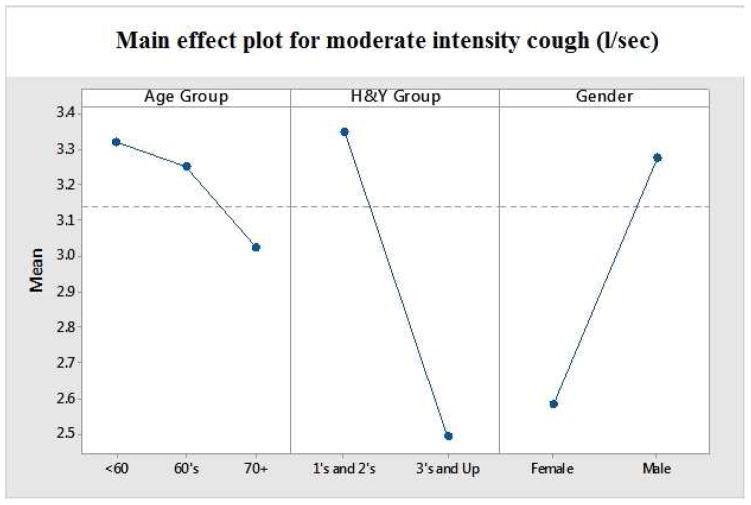
Aim 1 of this study sough to evaluate the differences in voluntary cough peak airflow during soft, moderate and forceful productions in individuals with PD as a function of age, gender and disease severity. Peak cough airflow values descriptively trended downward among the three age brackets (<60, 60–69 and ≥ 70), although these results did not rise to the level of statistical significance. Males displayed mean PEFRs which were significantly greater than females (F (1, 58) =6.869, p<.01). A significant relationship was identified between mean peak cough airflow and disease severity [r =-0.312, df 68, p<0.01; Figure 2 and Table 3].
Figure 2.
Mean PEFR in early (H&Y 1–2) and later stage (H&Y 3+) participants with PD.
Table 3.
Cough strength by H&Y score
| Cough strength (mean L/Sec) | H&Y (low <3) (n=37) | H&Y (high ≥3 ) (n = 31) | Significance |
|---|---|---|---|
| Weak cough | 2.79 (.94) | 2.09 (.76) | p<.001 |
| Moderate cough | 3.49 (1.04) | 2.66 (1.01) | p<.002 |
| Strong cough | 5.11 (1.5) | 4.08 (1.35) | p<.005 |
| Mean total cough strength | 3.79 (1.05) | 2.94 (.94) | p <.001 |
A significant linear decrease in mean PEFR was found for participants with moderate-severe (H&Y 3–5) disease severity ratings compared to mild (H&Y 1–2), [F (1,58)=7.859, p <.007; Figure 3].
Figure 3.
Main effects for PEFR as a function of age, gender and disease severity.
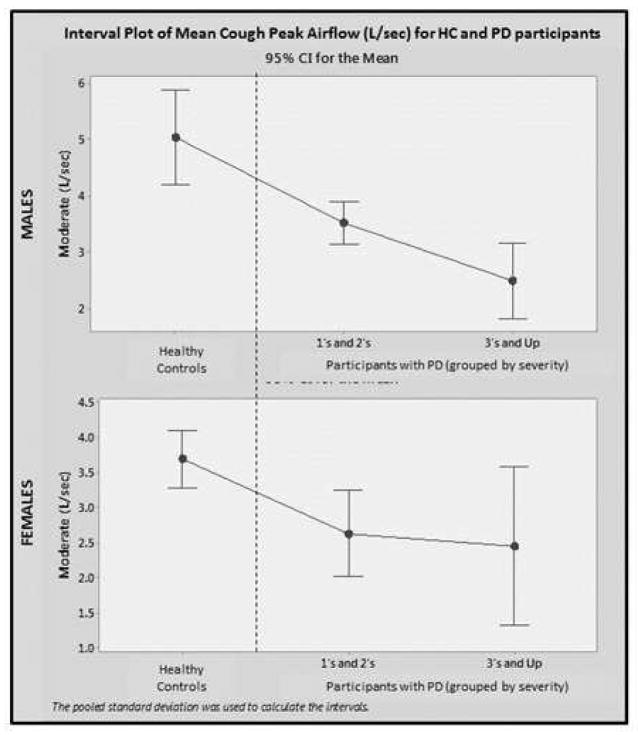
Aim 2 of this study sought to compare voluntary mean PEFR measures, obtained from those with PD, to identical measures obtained from HCs.40 Both groups demonstrated significantly higher cough peak airflow values for men versus women (p<.05) and no statistical difference in mean cough peak airflow values across age groups was demonstrated by either group. Participants with PD, at all severity levels, demonstrated significantly (p<.05) lower mean PEFR values compared to age matched HCs (Figure 4).
Figure 4.
PEFR measures obtained from early stage (H&Y 1–2) and later stage (H&Y 3+) participants with PD compared to HCs.
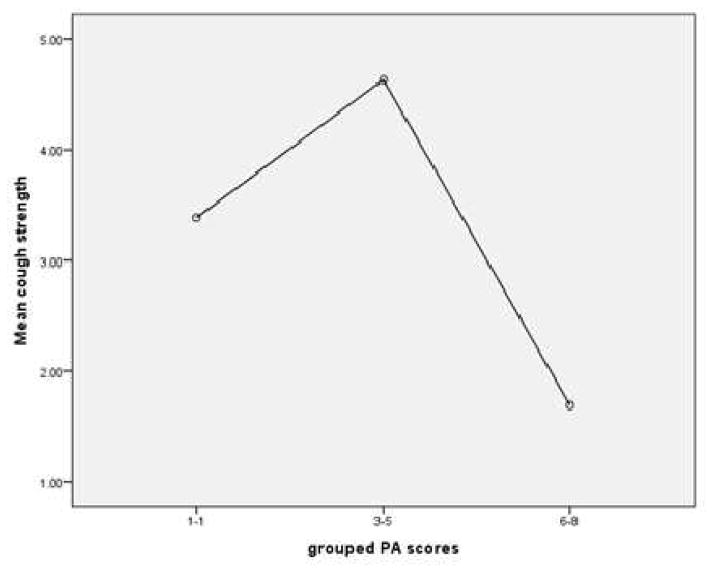
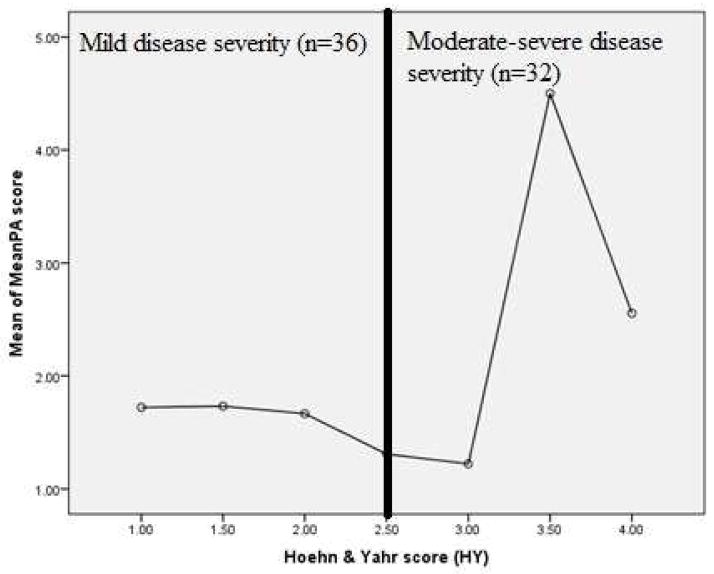
Aim 3 of this study sought to examine the relationship between PEFR and PAS Score during a sequential swallowing challenge task in those with PD. Results demonstrated that mean PEFR was significantly different across PAS score groupings [(PAS 1–2, PAS 3–5, PAS 6–8) for participants with PD (F (2, 65) =6.15, p<.004) Figure 5]. Additionally, participants with PAS scores classified as “severe” demonstrated the lowest mean PEFR. Similarly, PAS scores were significantly higher in patients presenting with the higher H&Y scores [F (6, 60) = 2.898, p <.015; Figure 6].
Figure 5.
Cough strength (PEFR) as a function of swallow severity (PAS) in participants with PD.
Figure 6.
Swallow severity (PAS) as a function of disease severity (H&Y) in participants with PD.
Discussion
The results of this investigation have revealed relationships among PEFR, PAS score and disease severity. Prior work established correlative relationships among PEFR, overall disease severity and age.40 Other investigations have revealed a correlative relationship between PAS and overall disease severity.22–24,31 The current findings stand as a novel addition to the literature, capturing for the first time relationships among PEFR, PAS and disease severity.
Strengths and Limitations
The strengths of this study lie with its expanded two phase approach to evaluating cough performance on PD subjects. Inclusion of both normative comparisons and the use of a simple robust measure of cough performance align it strongly with actual clinical practice. Data obtained support existing evidence of hypothesized physiological relationships between cough and swallow and will serve as the preliminary bases for future, larger scale investigations.
Limitations of this work are acknowledged as our participant pool was restricted in size, gender distribution and disease severity (the majority of participants classified as mild-moderate disease severity). Participants were not asked about their perceived level of baseline (e.g pre-testing) fatigue at the time of testing and it is reasonable to assume that, if a participant was experiencing fatigue as a result of overexertion, poor sleep, poor nutrition, illness, or any number of other factors, that this may have an influenced PEFR values that were obtained that day. Additionally, laryngeal penetration and aspiration are frequently an intermittent phenomenon. This assumption seems to reasonably to extend to our participant pool, all of whom were tolerating a total oral diet at the time of testing. A comprehensive VFSS of swallowing status, consisting of multiple boluses of varying sizes and consistencies administered over a longer examination period, would have provided more opportunities to observe disordered swallowing behavior.
Each of these shortcomings can be remedied in future investigations through application of a longitudinal design, which would offer multiple observations of each participant, over time, throughout the progression of his or her disease. Application of such a longitudinal approach would enable the capture of a greater range of disease severities. Future studies should replace the brief swallowing examination used in this study (and singular use of PAS as means of measuring swallowing symptom severity) with a more comprehensive evaluation of swallowing function (e.g. both full clinical and VFSS) would also enhance the ability to capture and record instances of abnormal swallowing function when they occur and allow for all potential contributing factors to be evaluated. For example, it is possible that post-swallowing residue exerts an effect on overall pulmonary health. This effect may be lesser than, comparable to, or even of a greater magnitude than instances of laryngeal penetration or aspiration.
Hand held peak flow meter collection and measurement of PEFR may eventually emerge as a stand-alone means of screening for aspiration risk. However, the first step in addressing the type of cough measurement is to determine if the addition of PEFR measures to the bedside swallowing evaluation can increase the sensitivity and predictive validity of these procedures. At present, only one standardized clinical tool, the Mann Assessment of Swallowing Ability (MASA), 48 even addresses cough as a (subjectively measured) component of a clinical swallowing evaluation. To date, our team has successfully established correlative relationships among PEFR, PAS and disease severity. While current methodological limitations constrained our ability to extend these observations to females and males with earlier stage PD, we plan to apply prospective longitudinal designs to ameliorate these issues in future investigations. Further, our data remains suggestive that simple objective measures of PEFR may yet contribute to enhancing the sensitivity and predictive validity of existing measures of swallowing severity.
Conclusions
Voluntary peak cough airflow appears to reflect airway integrity. Patients with PD produce significantly lower PEFRs and, as disease severity worsens, the ability to clear an airway decreases. In participants with moderate-severe disease, swallowing severity and PEFR are markedly impaired when compared to those values obtained from participants with mild or early-stage disease. Future, longitudinal, studies that comprehensively assess swallowing function as well as PEFR may provide evidence of the ability to PEFR to improve the sensitivity and predictive validity of existing assessment procedures. If successful, this model could be readily generalized to other diverse patient populations.
Acknowledgments
Funding: This research was supported by NIH NIDCD R21/R33 Grant Number 5R33DC011131.03 “Accuracy of Examining Cough Function and Its Relationship to Airway Protection in Those with Parkinson’s Disease” (Christine Sapienza, Ph.D. PI) Project dates: 12/1/2010 – 11/30/2014
This research was supported by NIH NIDCD R21/R33 Grant Number 5R33DC011131.03 “Accuracy of Examining Cough Function and Its Relationship to Airway Protection in Those with Parkinson’s Disease” (CS, PI).
All authors serve as guarantors of this work and take full responsibility for all information contained herein.
The authors would like to thank Amy Obenour, M.A. CCC-SLP for her contributions toward this research.
All authors confirm that the study objectives and procedures are honestly disclosed. Additionally, all authors have met the following criteria for authorship: (1) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; (2) drafting or critically revising the final manuscript; (3) provided final approval of the version to be published; and (4) is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. In addition to this, ES and CS contributed to the design of this research, assisted with participant recruitment, ran participants, contributed significantly to the writing of the final manuscript and oversaw the statistical analyses.
ES and CS directed all activities relating to research compliance at the University of Florida and Jacksonville University. FS assisted with participant recruitment, assisted with the informed consent procedures and running of participants and coordinated activities relating to research compliance at Brooks Healthcare. BHR contributed substantially to the final manuscript and was also instrumental in the conceptualization (along with CS and ES) in the conceptual underpinnings of this project. JY conducted preliminary statistical analyses associated with this project. GCM conducted the final statistical analyses for this project. AJ, FS and ES ran all participants. AJ coordinated study activities with the Radiology department at Brooks Healthcare. CS assisted with participant recruitment and with the running of participants in addition to acting as PI with chief oversight of the project as a whole.
CS has an organized interest in Aspire Products, LLC; is an invited speaker, receiving reimbursement and honoraria; is a book author with Plural Publishing; and is the Primary Investigator of the grant that supported this project NIH (5R33DC011131.03).
ES is an invited speaker, receiving reimbursement and honoraria. The remaining authors report no financial disclosures.
The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript
Abbreviations list
- PD
Parkinson’s disease
- PEFR
Peak expiratory (cough) airflow rate (l/min)
- VFSS
Video fluoroscopic swallowing study
- H&Y
Hoehn and Yahr score
- PAS
Penetration aspiration scale
Footnotes
Level of Evidence: 1b
Conflicts of Interest: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkinson J. An Essay On The Shaking Palsy. Chicago: American medical Association Press; 1932. [Google Scholar]
- 2.Goldman J, Postuma R. Premotor and nonmotor features of Parkinson3s disease. Current Opinion in Neurology. 2014;27(4):434–441. doi: 10.1097/wco.0000000000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goetz C, Pal G. Initial management of Parkinson's disease. BMJ. 2014;349(dec19 6):g6258–g6258. doi: 10.1136/bmj.g6258. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Rüb U, Gai W, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. Journal of Neural Transmission. 2003;110(5):517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 5.Phani S, Loike J, Przedborski S. Neurodegeneration and Inflammation in Parkinson's disease. Parkinsonism & Related Disorders. 2012;18:S207–S209. doi: 10.1016/s1353-8020(11)70064-5. [DOI] [PubMed] [Google Scholar]
- 6.Guo M. Molecular pathways to Parkinson’s disease. Molecular Neurodegeneration. 2012;7(Suppl 1):L13. doi: 10.1186/1750-1326-7-s1-l13. [DOI] [Google Scholar]
- 7.Troche M, Brandimore A, Godoy J, Hegland K. A framework for understanding shared substrates of airway protection. J Appl Oral Sci. 2014;22(4):251–260. doi: 10.1590/1678-775720140132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eccles R. Central Mechanisms IV: Conscious Control of Cough and the Placebo Effect. Pharmacology and Therapeutics of Cough. 2009:241–262. doi: 10.1007/978-3-540-79842-2_12. [DOI] [PubMed] [Google Scholar]
- 9.Hegland K, Bolser D, Davenport P. Volitional control of reflex cough. Journal of Applied Physiology. 2012;113(1):39–46. doi: 10.1152/japplphysiol.01299.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincken W, Gauthier S, Dollfuss R, Hanson R, Darauay C, Cosio M. Involvement of Upper-Airway Muscles in Extrapyramidal Disorders. New England Journal of Medicine. 1984;311(7):438–442. doi: 10.1056/nejm198408163110704. [DOI] [PubMed] [Google Scholar]
- 11.De Pandis M, Starace A, Stefanelli F, et al. Modification of respiratory function parameters in patients with severe Parkinson's disease. Neurological Sciences. 2002;23(0):s69–s70. doi: 10.1007/s100720200074. [DOI] [PubMed] [Google Scholar]
- 12.Seccombe L, Giddings H, Rogers P, et al. Abnormal ventilatory control in Parkinson's disease—Further evidence for non-motor dysfunction. Respiratory Physiology & Neurobiology. 2011;179(2–3):300–304. doi: 10.1016/j.resp.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Shill H, Stacy M. Respiratory Complications of Parkinson's Disease. Seminars in Respiratory and Critical Care Medicine. 2002;23(3):261–266. doi: 10.1055/s-2002-33034. [DOI] [PubMed] [Google Scholar]
- 14.Tzelepis G, McCool F, Friedman J, Hoppin F. Respiratory Muscle Dysfunction in Parkinson's Disease. American Review of Respiratory Disease. 1988;138(2):266–271. doi: 10.1164/ajrccm/138.2.266. [DOI] [PubMed] [Google Scholar]
- 15.Kolesnikova E. Changes in the control of external respiratory function in Parkinson’s disease. Neurophysiology. 2006;38(5–6):402–409. doi: 10.1007/s11062-006-0078-y. [DOI] [Google Scholar]
- 16.Wang Y, Shao W, Gao L, et al. Abnormal Pulmonary Function and Respiratory Muscle Strength Findings in Chinese Patients with Parkinson’s Disease and Multiple System Atrophy–Comparison with Normal Elderly. PLoS ONE. 2014;9(12):e116123. doi: 10.1371/journal.pone.0116123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estenne M, Hubert M, De Troyer A. Respiratory-Muscle Involvement in Parkinson's Disease. New England Journal of Medicine. 1984;311(23):1516–1517. doi: 10.1056/nejm198412063112314. [DOI] [PubMed] [Google Scholar]
- 18.Obenour WH, Stevens PM, Cohen AA, MuCutchen JJ. The Causes of Abnormal Pulmonary Function in Parkinson’s Disease. Am Rev Respir Dis. 1972;105(3):382–7. doi: 10.1164/arrd.1972.105.3.382. [DOI] [PubMed] [Google Scholar]
- 19.Pinnington L, Muhiddin K, Ellis R, Playford E. Non-invasive assessment of swallowing and respiration in Parkonson's disease. Journal of Neurology. 2000;247(10):773–777. doi: 10.1007/s004150070091. [DOI] [PubMed] [Google Scholar]
- 20.Fontana G, Lavorini F. Cough motor mechanisms. Respiratory Physiology & Neurobiology. 2006;152(3):266–281. doi: 10.1016/j.resp.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Robbins J, Logemann J, Kirshner H. Swallowing and speech production in Parkinson's disease. Annals of Neurology. 1986;19(3):283–287. doi: 10.1002/ana.410190310. [DOI] [PubMed] [Google Scholar]
- 22.Hegland K, Okun M, Troche M. Sequential Voluntary Cough and Aspiration or Aspiration Risk in Parkinson’s Disease. Lung. 2014;192(4):601–608. doi: 10.1007/s00408-014-9584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebihara S. Impaired Efficacy of Cough in Patients With Parkinson Disease. Chest. 2003;124(3):1009–1015. doi: 10.1378/chest.124.3.1009. [DOI] [PubMed] [Google Scholar]
- 24.Troche M, Brandimore A, Okun M, Davenport P, Hegland K. Decreased Cough Sensitivity and Aspiration in Parkinson Disease. Chest. 2014;146(5):1294. doi: 10.1378/chest.14-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitts T, Rose M, Mortensen A, et al. Coordination of cough and swallow: A meta-behavioral response to aspiration. Respiratory Physiology & Neurobiology. 2013;189(3):543–551. doi: 10.1016/j.resp.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolser D, Gestreau C, Morris K, Davenport P, Pitts T. Central Neural Circuits for Coordination of Swallowing, Breathing and Coughing. Otolaryngologic Clinics of North America. 2013;46(6):957–964. doi: 10.1016/j.otc.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitts T. Airway Protective Mechanisms. Lung. 2013;192(1):27–31. doi: 10.1007/s00408-013-9540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barichella M, Cereda E, Pezzoli G. Major nutritional issues in the management of Parkinson's disease. Movement Disorders. 2009;24(13):1881–1892. doi: 10.1002/mds.22705. [DOI] [PubMed] [Google Scholar]
- 29.Walker R, Dunn J, Gray W. Self-Reported Dysphagia and Its Correlates Within a Prevalent Population of People with Parkinson’s Disease. Dysphagia. 2010;26(1):92–96. doi: 10.1007/s00455-010-9317-x. [DOI] [PubMed] [Google Scholar]
- 30.Umemoto G, Tsuboi Y, Kitashima A, Furuya H, Kikuta T. Impaired Food Transportation in Parkinson’s Disease Related to Lingual Bradykinesia. Dysphagia. 2010;26(3):250–255. doi: 10.1007/s00455-010-9296-y. [DOI] [PubMed] [Google Scholar]
- 31.Kalf J, de Swart B, Bloem B, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: A meta-analysis. Parkinsonism & Related Disorders. 2012;18(4):311–315. doi: 10.1016/j.parkreldis.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Cereda E, Cilia R, Klersy C, et al. Swallowing disturbances in Parkinson's disease: A multivariate analysis of contributing factors. Parkinsonism & Related Disorders. 2007;20(12):1382–1387. doi: 10.1016/j.parkreldis.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 33.Hoehn M, Yahr M. Parkinsonism: Onset, progression and mortality. Neurology. 2011;77(9):874–874. doi: 10.1212/01.wnl.0000405146.06300.91. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez HH, Lapane KL. Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s Disease. Med Sci Monit. 2002;8(4):CR241–6. [PubMed] [Google Scholar]
- 35.Leopold N, Kagel M. Pharyngo-Esophageal Dysphagia in Parkinson's Disease. Dysphagia. 1997;12(1):11–18. doi: 10.1007/pl00009512. [DOI] [PubMed] [Google Scholar]
- 36.Lieberman AN, Horowitz L, Redmond P, Pachter L, Lieberman I, Leibowitz M. Dysphagia in Parkinson’s Disease. Am J Gastroenterol. 1980;74(2):157–60. [PubMed] [Google Scholar]
- 37.Coates C, Bakheit A. Dysphagia in Parkinson's Disease. Eur Neurol. 1997;38(1):49–52. doi: 10.1159/000112902. [DOI] [PubMed] [Google Scholar]
- 38.Lim A, Leow L, Huckabee M, Frampton C, anderson T. A Pilot Study of Respiration and Swallowing Integration in Parkinson’s Disease: “On” and “Off” Levodopa. Dysphagia. 2007;23(1):76–81. doi: 10.1007/s00455-007-9100-9. [DOI] [PubMed] [Google Scholar]
- 39.Brodsky M, Abbott K, McNeil M, Palmer C, Grayhack J, Martin-Harris B. Effects of Divided Attention on Swallowing in Persons with Idiopathic Parkinson’s Disease. Dysphagia. 2011;27(3):390–400. doi: 10.1007/s00455-011-9381-x. [DOI] [PubMed] [Google Scholar]
- 40.Silverman E, Carnaby-Mann G, Pitts T, Davenport P, Okun M, Sapienza C. Concordance and Discriminatory Power of Cough Measurement Devices for Individuals With Parkinson Disease. Chest. 2014;145(5):1089. doi: 10.1378/chest.13-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leow L, Huckabee M, anderson T, Beckert L. The Impact of Dysphagia on Quality of Life in Ageing and Parkinson’s Disease as Measured by the Swallowing Quality of Life (SWAL- QOL) Questionnaire. Dysphagia. 2009;25(3):216–220. doi: 10.1007/s00455-009-9245-9. [DOI] [PubMed] [Google Scholar]
- 42.Pitts T, Troche M, Mann G, Rosenbek J, Okun MS, Sapienza C. Using Voluntary Cough To Detect Penetration and Aspiration During Oropharyngeal Swallowing in Patients With Parkinson Disease. CHEST. 2010;138(6):1426. doi: 10.1378/chest.10-0342. [DOI] [PubMed] [Google Scholar]
- 43.Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary Cough Production and Swallow Dysfunction in Parkinson’s Disease. Dysphagia. 2008;23(3):297–301. doi: 10.1007/s00455-007-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fusillo J. 5-30-08 Silent aspiration in Parkinson’s disease. Journal of the Neurological Sciences. 1997;150:S316. [Google Scholar]
- 45.Pitts T. Using Voluntary Cough To Detect Penetration and Aspiration During Oropharyngeal Swallowing in Patients with Parkinson Disease. Chest. 2010;138(6):1426. doi: 10.1378/chest.10-0342. [DOI] [PubMed] [Google Scholar]
- 46.Rosenbek J, Robbins J, Roecker E, Coyle J, Wood J. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 47.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 48.Mann G. MASA, The Mann Assessment of Swallowing Ability. Clifton Park, NY: Singular Thomson Learning; 2002. [Google Scholar]