Abstract
Background
Fatigue is a multidimensional condition that is difficult to treat with standard monoaminergic antidepressants. Ketamine, an N-methyl-D-aspartate receptor (NMDAR) antagonist produces rapid and robust improvements in depressive symptoms in treatment-resistant depression. However, there is a dearth of literature examining the anti-fatigue effects of ketamine. We hypothesize that ketamine will rapidly improve fatigue symptoms in treatment-resistant depressed patients.
Methods
This is an exploratory analysis of data obtained from two double-blind, randomized, placebo-controlled, crossover trials. A total of 36 participants with treatment-resistant bipolar I or II disorder in a depressive episode (maintained on therapeutic levels of lithium or valproate) received a single infusion of ketamine hydrochloride intravenously (0.5mg/kg over 40 minutes) or placebo. A post-hoc analysis compared fatigue scores on ketamine vs. placebo at 10 time points from baseline through 14 days post-treatment using the National Institute of Health-Brief Fatigue Inventory.
Results
A linear mixed model showed that ketamine significantly lowered fatigue scores compared to placebo from 40 minutes post-treatment to Day 14 with the exception of Day 7. The largest difference in anti-fatigue effects between placebo and ketamine was at day 2 (d=.58, p < .05). The effect remained significant after controlling for changes in non-fatigue depressive symptoms.
Limitation
The retrospective nature and a small sample size are study limitations.
Conclusions
Ketamine rapidly improved fatigue relative to placebo in a group of individuals with treatment-resistant bipolar depression. NMDAR is a glutamate receptor; hence, glutamate may represent a valuable target to study the clinical efficacy of new anti-fatigue approaches in multiple disorders.
Fatigue is a common, distressing condition that is often associated with several medical disorders (e.g. anemia, thyroid dysfunction, cancer) and psychosocial factors (Ryan et al., 2007; Portenoy and Itri, 1999; Horneber et al., 2012). The causes and mechanisms of fatigue are unknown; however, it is believed to be a complex and multifactorial condition that is influenced by somatic, affective and cognitive factors (Berger & Mitchell, 2008). Patients describe fatigue as diminishing vitality, work and activities because of muscular weakness, and/or impairment in their cognitive functioning (Vogelzang et al., 1997; Portenoy and Itri, 1999). In fact, physical impairment and disability from fatigue are common and have negative economic consequences at the individual and societal levels. For example, the direct and indirect economic costs of chronic fatigue is estimated to be between $17-24 billion annually (Jason et al., 2008), where $9.1 billion of which can be attributed to lost household and labor force productivity (Reynolds et al., 2004).
Patients experiencing fatigue also feel a sense of hopelessness, worthlessness, guilt and suicidal ideation (Ahlberg et al., 2003). Fatigue has long been identified as a core depressive symptom (Swindle et al., 2001; Buchwald & Rudick-Davis, 1993). The relationship between fatigue and depression is poorly understood, but it is postulated that both conditions share common mechanisms related to disrupted rest-activity rhythms (Roscoe et al., 2002), 5-hydroxytryptamine (5-HT) dysfunction (Andrews et al., 2004), and altered hypothalamic-pituitary-adrenal (HPA)-axis activity (Vgontzas and Chrousos, 2002).
The relationship of fatigue with depression came into focus when clinicians observed the failure of antidepressants to restore energy after documented efficacy in relieving affective symptoms of depression (Ferguson et al., 2014; Reuter et al., 2006). In addition, up to one-third of individuals with major depressive disorder (MDD) who achieved remission or response continued to experience fatigue (Fava, 2003: Nierenberg et al., 1999). One recent study noted that more than 90% of patients with MDD complain of severe fatigue, even if >80% of these patients were already on antidepressants (Ferrentinos et al., 2010). Without adequate empirical support, several pharmacological agents that are known to increase norepinephrine and dopamine levels including venlafaxine, bupropion, fluoxetine, and sertraline have been proposed to be the first-line treatment for depressed patients with prominent fatigue (Demyttenaere et al., 2005). Augmentation of the proposed first-line agents with stimulants such as modafinil, also provides relief from fatigue (DeBattista et al., 2004), by releasing histamine in the hypothalamus (Ishizuka et al., 2003), as well as dopamine and norepinephrine in the cortex (Bymaster et al., 2002). Central nervous system (CNS) stimulants, such as amphetamines and methylphenidate have also been observed to improve fatigue in patients with major depressive disorders by blocking the reuptake of norepinephrine and dopamine (Xu et al., 2000). However, these agents abate fatigue less frequently and slowly (Demyttenaere et al., 2005). So, rapid relief from fatigue is essential to attain full remission from depression.
The rapid antidepressant effect of a noncompetitive glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, such as a low dose ketamine, is well documented in individuals with MDD, even those with treatment-resistant depressive conditions (Berman et al., 2000; Price et al., 2009; Zarate et al., 2006). Ketamine’s rapid anti-depressant effects are believed to be caused by disinhibiting gamma aminobutyric acid (GABA) inputs thus enhancing the firing rate of glutamatergic neurons, increasing presynaptic release of glutamate, and consequently increasing extracellular levels of glutamate which favors α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR) over NMDA receptors (Diazgranados et al., 2010; Machado-Vieira et al., 2009; Zarate et al., 2006). However, its effect on fatigue especially in individuals with MDD has not been systematically investigated. There are very few trials that explored the effects of NMDA receptor antagonists on fatigue. One study showed a reduction in perceived fatigue in individuals with multiple sclerosis after a month of amantadine treatment (Shaygannejad et al, 2012; Ledinek et al., 2013).
In this analysis we investigated the rapid anti-fatigue effects of a low dose ketamine in individuals with treatment-resistant depression. We hypothesized that a ketamine infusion would produce a rapid reduction in fatigue symptoms in patients with treatment-resistant depression compared to placebo. Considering the strong correlation between fatigue and depression (Passik et al., 1998, Roscoe et al., 2002), information from this study would provide initial evidence of the role of NMDA receptor in fatigue.
Methods
Design and subjects
This is an exploratory analysis of data collected from the original studies by DiazGranados et al. (2010) and Zarate et al. (2012), as well as three additional patients who participated in the same protocol and followed identical procedures. This study focuses on the anti-fatigue effects of ketamine. The original studies were double-blind, randomized, placebo-controlled, crossover clinical studies exploring the efficacy of ketamine as an intervention in reducing depressive symptoms in bipolar depression. Informed consent was obtained for all study participants. The study was conducted at the National Institutes of Health (NIH) Clinical Center, Bethesda, Maryland. Participants with treatment-resistant bipolar I or II depression by DSM-IV criteria (maintained on therapeutic levels of lithium or valproate) received a single infusion of ketamine hydrochloride intravenously (IV) at a dose of 0.5mg/kg over 40 minutes or normal saline delivered IV over 40 minutes as placebo. The clinical trials had two experimental periods, which were separated by two weeks.
Measure
Fatigue was assessed using the 7-item, clinician-administered NIH-Brief Fatigue Inventory (NIH-BFI) (Saligan et al., 2015). The NIH-BFI was developed from items of existing clinician-administered psychiatric scales administered in the NIMH clinical trial (i.e., Hamilton Depression Rating Scale (17 Item) [HDRS], Montgomery-Asberg Depression Rating Scale [MADRS], Young Mania Rating Scale [YMRS], and Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression [SIGH-SAD]). The range of the scale is from 0 to 34, where a higher score suggests worse fatigue symptoms.
Data analyses
A linear mixed model with restricted maximum likelihood estimates and a compound symmetry covariance structure were used to assess the course of fatigue scores on ketamine versus placebo from 40 minutes through day 14 post-infusion with baseline as a covariate. Both drug and time were within-subjects factors and the interaction between them was included in the model. Post hoc simple effects tests were used to evaluate the difference between ketamine and placebo at each time point. Significance was evaluated at p≤.05, two-tailed, and unadjusted values are reported unless indicated otherwise. Cohen’s d was used to present the size of effects of the study drug versus placebo. Pearson correlations were used to examine the relationship between percent change in fatigue from baseline on ketamine with factors (e.g. body mass index (BMI), family history of alcohol abuse, prior suicide attempt) previously found related to percent change in depression at 230 minutes, day 1, and day 7 post treatment. Statistics were calculated using IBM SPSS 21.
Results
Sample
Data from 36 randomized study participants were included. Twenty-six patients completed both phases of the crossover and eight patients dropped out prior to the second phase. All available data was used for analysis. Further information on the study enrollment can be found in the original manuscripts (DiazGranados et al., 2010; Zarate et al., 2012). The sample included 58% females at an average age of 46.7. All participants began the study with an NIHBFI score of at least 12 points with an average of 18.9 at the study baseline. Patients were moderately depressed on average (Table 1).
Table 1.
Sociodemographic and clinical characteristics of the sample (N=36).
| Characteristics | Mean | SD |
|---|---|---|
| Age | 46.7 | 11.1 |
| Age of Onset (Years) | 17.9 | 7.3 |
| Length of Illness (Years) | 28.8 | 10.6 |
| Length of Current Episode (Months) | 17.1 | 20.1 |
| Body Mass Index | 30.1 | 6.0 |
| NIH-BFI | 18.9 | 3.4 |
| MADRS | 33.9 | 5.0 |
| HDRS | 22.1 | 4.0 |
| YMRS | 5.0 | 2.8 |
| Characteristics | n | % |
| Sex (Female) | 21 | 58 |
| Depression Subtype | ||
| Atypical | 7 | 19 |
| Melancholic | 13 | 36 |
| Other (i.e. Not Atypical or Melancholic) | 16 | 44 |
| Illness History | ||
| Psychiatric Hospitalization | 30 | 91 |
| Suicide Attempt | 18 | 51 |
| ECT Exposure | 17 | 19 |
| Comorbidity | ||
| Anxiety Disorder | 19 | 53 |
| PTSD | 6 | 17 |
| Psychosis | 9 | 26 |
| Alcohol Abuse | 19 | 53 |
| Substance Abuse | 17 | 47 |
| Family History | ||
| Alcohol Dependence (First Degree) | 13 | 36 |
| Mood Disorder | 32 | 89 |
| Anxiety Disorder | 9 | 26 |
| Suicide | 10 | 36 |
ECT = Electroconvulsive Therapy; PTSD = Post Traumatic Stress Disorder; NIH-BFI = National Institutes of Health, Brief Fatigue Inventory; HDRS = Hamilton Depression Rating Scale; MADRS = Montgomery-Asberg Depression Rating Scale; YMRS = Young Mania Rating Scale; PTSD = post-traumatic stress disorder
Analysis
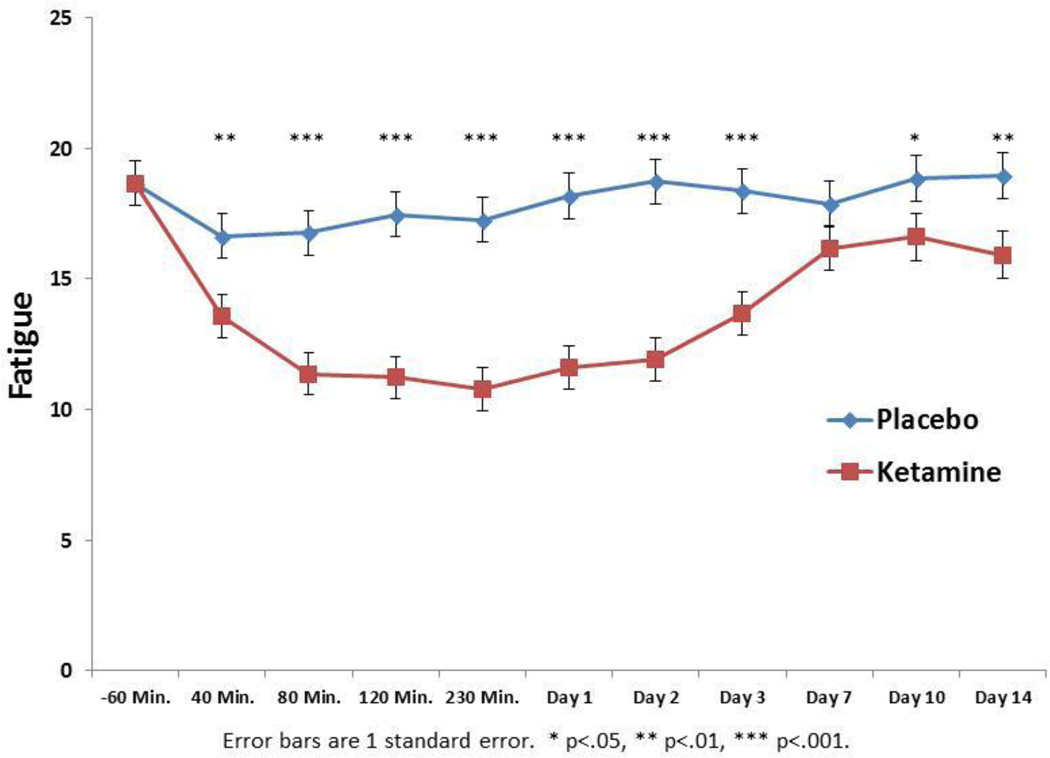
The linear mixed model showed a significant drug by time interaction (F9,549=3.76, p < .001). Bonferroni post hoc tests indicated significantly lower scores from 40 minutes post treatment through the end of the study (Day 14) with the exception of day 7 (p=.10). The effect size of the ketamine-placebo difference was greatest at day 2 (d=0.59) and smallest at day 7 (d=0.14). Thus, ketamine appeared to improve fatigue symptoms compared to placebo very rapidly, achieving its greatest effect over placebo at day 2 (Figure 1). The drug by time interaction remained significant when controlling for depression (MADRS without fatigue items) at each time point (F9,553=4.19, p < .001), suggesting that the anti-fatigue effect of ketamine cannot be explained solely by its anti-depressant effect.
Figure 1.
Mean fatigue scores with ketamine and placebo. Fatigue was measured by the National Institutes of Health – Brief Fatigue Inventory. Fatigue significantly decreased 40 minutes post treatment until the end of the study (Day 14) with the exception of day 7.
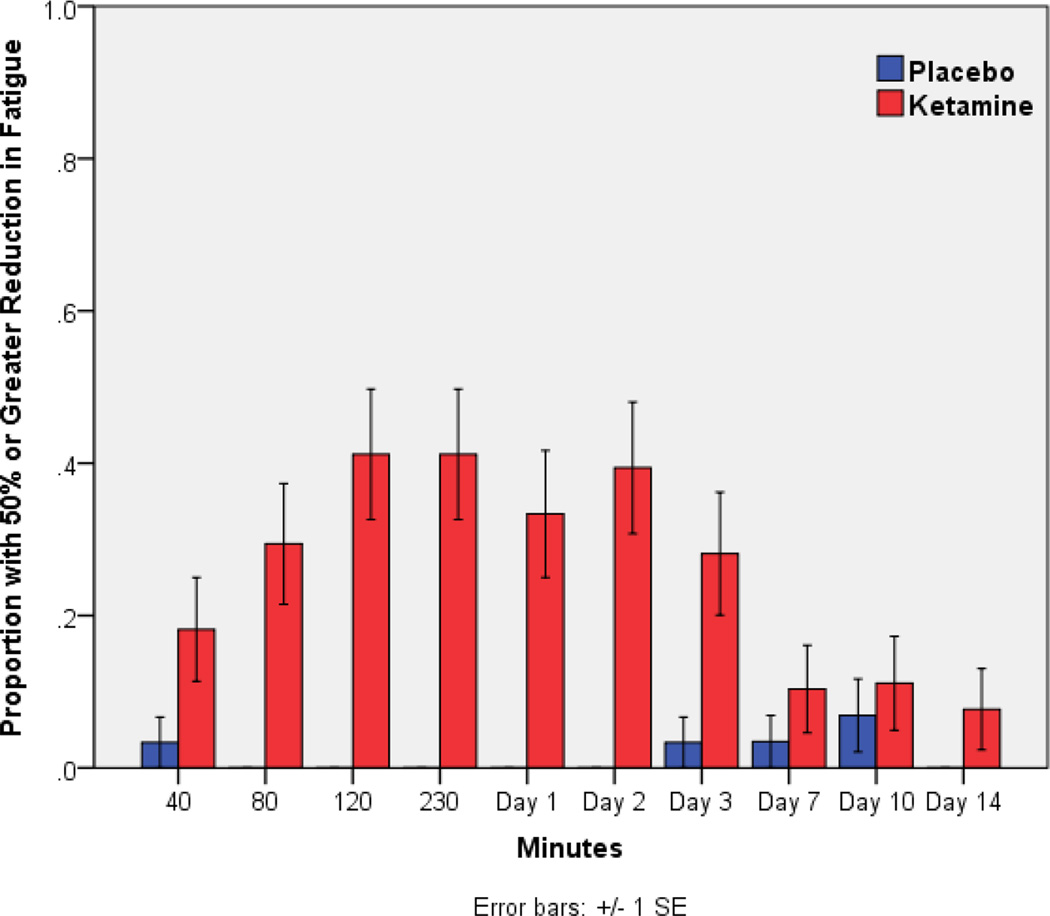
Taking a more clinical view of the changes in fatigue, 65% of patients had a response (> 50% improvement from baseline) on ketamine when considering the maximum change during the trial; only 10% had comparable changes on placebo. Looking at specific time points, the highest percentage of patients with substantial improvement on ketamine occurred at both 120 and 230 minutes (41%). This suggests rapid and clinically meaningful improvement in fatigue symptoms on ketamine in a matter of hours (Figure 2).
Figure 2.
Study responders. Participants who had >50% reduction in fatigue score compared to baseline with placebo or ketamine infusion. Fatigue measured by the National Institutes of Health – Brief Fatigue Inventory.
Using factors found to be associated with depression response to ketamine in a prior analysis (Niciu, et al., 2014), demographic characteristics such as BMI, family history of alcohol disorder, and prior suicide attempt were evaluated to determine whether they predicted the antifatigue effect of ketamine. For BMI, only the correlation at day 1 was significant (230 minutes: r=−.26, p=.14; Day 1: r=−.38, p=.03; Day 7: r=−.003, p=.99), which may be related to the rapid absorption and storage of ketamine in fat cells (Edwards et al., 2002). The correlations with family history of alcohol (230 minutes: r=−.02, p=.91; Day 1: r=−.21, p=.25; Day 7: r=−.14, p=.46) and prior suicide attempt (230 minutes: r=.12, p=.49; Day 1: r=.06, p=.76; Day 7: r=.21, p=.28) were not significant. Previous papers listed the time-limited, ketamine-specific adverse events that were reported by participants of the original clinical trials, which included dissociation, dry mouth, tachycardia, and increased blood pressure in >10% of subjects (DiazGranados et al., 2010; Zarate et al., 2012). No adverse event was significantly different between ketamine and placebo >80 minutes post infusion.
Discussion
The present finding is the first to describe a potential key role for ketamine as an anti-fatigue agent. In this study, ketamine significantly improved fatigue over placebo within 40 minutes, achieving its greatest efficacy at day 2. Further, the anti-fatigue effect of ketamine was not fully accounted for by its anti-depressant effect. The study finding provides a critical initial evidence of NMDA receptor inhibition as a potential therapeutic option for fatigue, although this finding needs to be replicated in a study solely designed to investigate the effects of NMDA receptor inhibition on fatigue.
Our study finding is novel and has great public health implications. The rapid anti-fatigue effects of ketamine and the consequential development of an effective long-term anti-fatigue treatment would greatly reduce or eliminate the daily physical and social interferences of fatigue at home and in the workplace, which are often reported by patients with cancer-related fatigue or with chronic fatigue syndrome (Bennet et al., 2007). On a broader scale, these fatigue interventions could address related economic issues, allowing individuals to return to work and maintain productivity, enhancing overall quality of life (Sabes-Figuera et al., 2010; Göksel Karatepe et al., 2011).
The etiology of fatigue remains elusive. Persistent immune (Stringer et al., 2013) or mitochondrial (Vernon et al., 2006) abnormalities have previously been proposed as potential causes of fatigue. Therapies to address these physiological abnormalities often provide temporary, but not long-lasting improvements in fatigue symptoms. The rapid anti-fatigue effects of ketamine are both an advantage and a limitation. It is an advantage because it shows that patients with severely debilitating fatigue may be able to find temporary and immediate relief. It can also be beneficial in specific physiologic conditions, such as reducing post-operative fatigue, since it has been found to have rapid antidepressant effects in post-operative states (Kudoh et al., 2002), as well as in depressed patients with comorbid pain syndrome or those with alcohol dependence (Correll & Futter, 2006; Liebrenz et al., 2009). However, its sedative and psychotomimetic side effects, and potential for addiction may limit its clinical use (Perry et al., 2007). Its short-acting, anti-fatigue effects also require that other therapeutic options must be considered to provide a more lasting relief from this distressing condition. For that reason, other drugs with similar mechanism of therapeutic action of ketamine will need to be developed for clinical use.
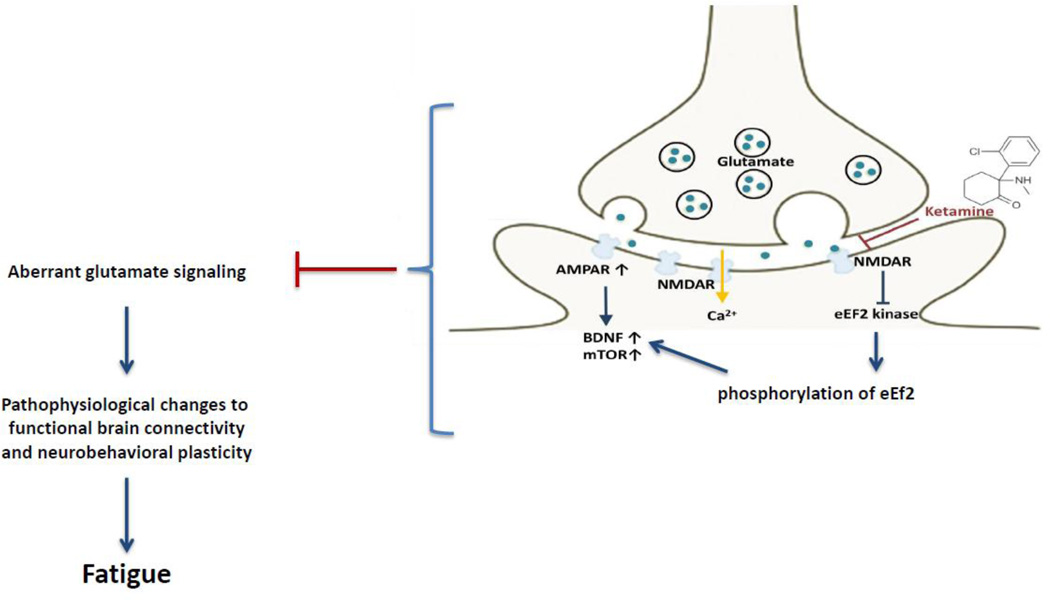
Ketamine has high lipid solubility and low plasma protein binding, which facilitates its rapid transfer across the blood-brain-barrier (Prommer, 2012). Although the neurobiological mechanisms of ketamine’s antidepressant effects are not fully clear, it is believed that ketamine’s rapid antidepressant effects are mostly mediated by activation of AMPAR which induces a rapid dissociation of glutamate and less driven by NMDA receptor antagonism (Zarate et al., 2006). Ketamine initially serves as a noncompetitive NMDA receptor antagonist and subsequently enhances AMPA throughput, by increasing the presynaptic release of glutamate thus enhancing the rate of glutamate favoring AMPA receptors over NMDA receptors (Aan Het Rot, 2012; Prommer, 2012). Antagonizing AMPA receptors prior to ketamine infusion selectively abolished its antidepressant effects, confirming that ketamine’s antidepressant effect was driven mostly by AMPA throughput (Maeng et al., 2008). Although, we observed that the anti-fatigue effect of ketamine is not contributed solely by its anti-depressant effect, we hypothesize that the AMPAR activation may be a biologic mechanism that is shared by fatigue and depression. Figure 3 illustrates the biologic correlates of ketamine’s potential anti-fatigue effects.
Figure 3.
Potential Anti-fatigue effects of ketamine. Ketamine increases extracellular glutamate release favoring AMPA receptors over NMDA receptors. Post-synaptically, administration of ketamine leads to upregulation of BDNF levels by inhibiting eEF2 kinase. In addition, activation of the mTOR-dependent protein synthesis leads to enhancement of synaptogenesis. We hypothesize that aberrant glutamate signaling plays a role in fatigue pathogenesis by affecting functional brain connectivity and altering neurobehavioral plasticity. Ketamine alleviate fatigue symptoms by targeting these aberrant glutamate signaling pathways.
Although the study revealed novel findings, but the retrospective nature of this investigation is a limitation. Since the study findings was based on an exploratory analysis, we were unable to determine the effect of ketamine on other symptoms that cluster with fatigue, such as sleep, since sleep was not measured as a study outcome in the original study. However, we were able to determine that the anti-fatigue effects of ketamine remained significant even after controlling for non-fatigue depressive symptoms. In addition, multiple comparisons with a small sample size limited the generalizability of our findings. The use of active placebo has been used in other ketamine studies to optimize randomization of ketamine trials because active placebo such as midazolam mimics the psychotomimetic effects of ketamine (Murrough et al., 2013). However, prior studies showed that the anti-depressant effects of ketamine remained distinct when compared with active (Murrough et al., 2013) or an inactive placebo (Zarate et al., 2006).
Further, this study has shown the ability of NIH-BFI to measure changes in fatigue symptoms after ketamine infusion. The continued use of NIH-BFI in succeeding fatigue studies may validate its utility in clinical trials. This study was limited because of a small sample size. Further investigation is warranted to confirm the anti-fatigue effects of ketamine, especially in different fatiguing conditions, such as depressed and non-depressed populations, individuals with inflammatory versus non-inflammatory conditions, or those with neurovegetative disorders. The addition of neuroimaging during the rapid change in fatigue symptoms would be ideal to identify brain areas that may be involved in fatigue.
Conclusion
There is no current Food and Drug Administration-approved treatment for fatigue. The findings from this study suggest a novel mechanism supporting the role of glutamatergic system in the pathophysiology and therapeutics of fatigue. Further understanding of the role of the glutamatergic system in fatigue conditions will move us closer to understanding the neurobiology of fatigue and identifying potential therapeutic targets. Currently, patients with fatigue remain vulnerable to impaired global functioning and depression. Understanding its etiology and developing effective agents for treatment would have a significant public health impact to help those who are affected by this debilitating condition. The NIH recognizes the need to advance understanding of fatigue as a key priority area. There are currently trans-NIH activities that promote research to shed light on the causes of fatigue.
Highlights.
NMDA receptor inhibition as a potential therapeutic option for fatigue.
The potential role of glutamatergic system in the pathophysiology and therapeutics of fatigue.
The utility of NIH-BFI to measure changes in fatigue symptoms in a clinical trial.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Leorey N. Saligan, Email: saliganl@mail.nih.gov.
David A. Luckenbaugh, Email: luckenbd@mail.nih.gov.
Elizabeth E. Slonena, Email: elizabeth.slonena@nih.gov.
Rodrigo Machado-Vieira, Email: rodrigo.machadovieira@nih.gov.
Carlos A. Zarate, Jr., Email: zaratec@mail.nih.gov.
References
- Aan Het Rot M, Zarate CA, Jr, Charney DS, Mathew SJ. Ketamine for depression: Where do we go from here? Biol. Psychiatry. 2012;72:537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362:640–650. doi: 10.1016/S0140-6736(03)14186-4. [DOI] [PubMed] [Google Scholar]
- Berger AM, Mitchell SA. Modifying cancer-related fatigue by optimizing sleep quality. J. Natl. Compr. Canc. Netw. 2008;6:3–13. doi: 10.6004/jnccn.2008.0002. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Buchwald AM, Rudick-Davis D. The symptoms of major depression. J. Abnorm. Psychol. 1993;102:197–205. doi: 10.1037//0021-843x.102.2.197. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Lueke SK, Threlkeld PJ, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Correll GE, Futter GE. Two case studies of patients with major depressive disorder given lowdose (subanesthetic) ketamine infusions. Pain Med. 2006;7:92–95. doi: 10.1111/j.1526-4637.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- DeBattista C, Lembke A, Solvason HB, Ghebremichael R, Poirier J. A prospective trial of modafinil as an adjunctive treatment of major depression. J. Clin. Psychopharmacol. 2004;24:87–90. doi: 10.1097/01.jcp.0000104910.75206.b9. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, De Fruyt J, Stahl SM. The many faces of fatigue in major depressive disorder. Int. J. Neuropsychopharm. 2005;8:93–105. doi: 10.1017/S1461145704004729. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SR, Minto CF, Mather LE. Concurrent ketamine and alfentanil administration: pharmacokinetic consideration. Br. J. Anaesth. 2002;88:94–100. doi: 10.1093/bja/88.1.94. [DOI] [PubMed] [Google Scholar]
- Fava M. Symptoms of fatigue and cognitive/executive dysfunction in major depressive disorder before and after antidepressant treatment. J. Clin. Psychiatry. 2003;64(suppl 14):30–34. [PubMed] [Google Scholar]
- Ferguson M, Dennehy EB, Marangell LB, Martinez J, Wisniewski SR. Impact of fatigue on outcome of selective serotonin reuptake inhibitor treatment: Secondary analysis of STAR*D. Curr. Med. Res. Opin. 2014;30:2109–2118. doi: 10.1185/03007995.2014.936553. [DOI] [PubMed] [Google Scholar]
- Ferrentinos P, Kontaxakis V, Havaki-Kontaxaki B, et al. The Fatigue Questionnaire: Standardization in patients with major depression. Psychiatry Res. 2010;177:114–119. doi: 10.1016/j.psychres.2009.01.029. [DOI] [PubMed] [Google Scholar]
- Göksel Karatepe A, Kaya T, Günaydn R, Demirhan A, Ce P, Gedizlioğlu M. Quality of life in patients with multiple sclerosis: the impact of depression, fatigue, and disability. Int. J. Rehabil. Res. 2011;34:290–298. doi: 10.1097/MRR.0b013e32834ad479. [DOI] [PubMed] [Google Scholar]
- Horneber M, Fischer I, Dimeo F, Ruffer JU, Weis J. Cancer-related fatigue: Epidemiology, pathogenesis, diagnosis, and treatment. Dtsch. Arztebl. Int. 2012;109:161–171. doi: 10.3238/arztebl.2012.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T, Sakamoto Y, Sakurai T, Yamatodani A. Modafinil increases histamine release in the anterior hypothalamus of rats. Neuroscience Letters. 2003;339:143–146. doi: 10.1016/s0304-3940(03)00006-5. [DOI] [PubMed] [Google Scholar]
- Jason LA, Benton MC, Valentine L, Johnson A, Torres-Harding S. The economic impact of ME/CFS: individual and societal costs. Dyn. Med. 2008;7:6. doi: 10.1186/1476-5918-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh A, Takahira Y, Katagai H, Takazawa T. Small-dose ketamine improves the postoperative state of depressed patients. Anesth. Analg. 2002;95:114–118. doi: 10.1097/00000539-200207000-00020. [DOI] [PubMed] [Google Scholar]
- Ledinek AH, Sajko MC, Rot U. Evaluating the effects of amantadin, modafinil and acetyl-L-carnitine on fatigue in multiple sclerosis--result of a pilot randomized, blind study. Clin. Neurol. Neurosurg. 2013;115(Suppl 1):S86–S89. doi: 10.1016/j.clineuro.2013.09.029. [DOI] [PubMed] [Google Scholar]
- Liebrenz M, Stohler R, Borgeat A. Repeated intravenous ketamine therapy in a patient with treatment-resistant major depression. World. J. Biol. Psychiatry. 2009;10:640–643. doi: 10.1080/15622970701420481. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA., Jr Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol. Ther. 2009;123:143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CM, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am. J. Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Luckenbaugh DA, Ionescu DF, et al. Riluzole likely lacks efficacy in ketamine non-responders. J. Psych. Res. 2014;58:197–199. doi: 10.1016/j.jpsychires.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J. Clin. Psychiatry. 1999;60:221–225. doi: 10.4088/jcp.v60n0403. [DOI] [PubMed] [Google Scholar]
- Passik SD, Dugan W, McDonald MV, Rosenfeld B, Theobald DE, Edgerton S. Oncologists’ recognition of depression in their patients with cancer. J. Clin. Oncol. 1998;16:1594–1600. doi: 10.1200/JCO.1998.16.4.1594. [DOI] [PubMed] [Google Scholar]
- Perry EB, Jr., Cramer JA, Cho HS, et al. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology (Berl) 2007;192:253–260. doi: 10.1007/s00213-007-0706-2. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Itri LM. Cancer-related fatigue: Guidelines for evaluation and management. Oncologist. 1999;4:1–10. [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prommer EE. Ketamine for pain: An update for uses in palliative care. J. Palliat. Med. 2012;15:474–483. doi: 10.1089/jpm.2011.0244. [DOI] [PubMed] [Google Scholar]
- Reynolds KJ, Vernon SD, Bouchery E, Reeves WC. The economic impact of chronic fatigue syndrome. Cost Eff. Resour. Alloc. 2004;2:4. doi: 10.1186/1478-7547-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter K, Classen CC, Roscoe JA, Morrow GR, Kirshner JJ, Rosenbluth R, Flynn PJ, Shedlock K, Spiegel D. Association of coping style, pain, age and depression with fatigue in women with primary breast cancer. Psychooncology. 2006;15:772–779. doi: 10.1002/pon.1012. [DOI] [PubMed] [Google Scholar]
- Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Matteson S, Rakita D, Andrews PL. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support. Care Cancer. 2002;10:329–336. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(Suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- Sabes-Figuera R, McCrone P, Hurley M, King M, Donaldson AN, Ridsdale L. The hidden cost of chronic fatigue to patients and their familiesB.MC. Health Serv. Res. 2010;10:56. doi: 10.1186/1472-6963-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saligan LN, Luckenbaugh DA, Slonena EE, Machado-Vieira R, Zarate CA., Jr Development of a clinician-administered National Institutes of Health-Brief Fatigue Inventory: a measure of fatigue in the context of depressive disorders. J. Psychiatr. Res. 2015;68:99–105. doi: 10.1016/j.jpsychires.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaygannejad V, Janghorbani M, Ashtari F, Zakeri H. Comparison of the effect of aspirin and amantadine for the treatment of fatigue in multiple sclerosis: a randomized, blinded, crossover study. Neurol. Res. 2012;34:854–858. doi: 10.1179/1743132812Y.0000000081. [DOI] [PubMed] [Google Scholar]
- Stringer EA, Baker KS, Carroll IR, et al. Daily cytokine fluctuations, driven by leptin, are associated with fatigue severity in chronic fatigue syndrome: evidence of inflammatory pathology. J. Transl. Med. 2013;11:93. doi: 10.1186/1479-5876-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindle R, Kroenke K, Braun LA. Energy and improved workplace productivity in depression. In: Sorkin A, Summers K, Farquhar I, editors. Investing in Health: The Social and Economic Benefits of Health Care Innovation. Vol. 14. Greenwich, CT, USA: Elsevier Science Ltd.; 2001. pp. 323–341. [Google Scholar]
- Vernon SD, Whistler T, Cameron B, Hickie IB, Reeves WC, Lloyd A. Preliminary evidence of mitochondrial dysfunction associated with post-infective fatigue after acute infection with Epstein Barr virus. B.M. C. Infect. Dis. 2006;6:15. doi: 10.1186/1471-2334-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: Multiple interactions and disturbances in sleep disorders. Endocrinol. Metab. Clin. North Am. 2002;31:15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- Vogelzang NJ, Breitbart W, Cella D, Curt GA, Groopman JE, Horning SJ, Itri LM, Johnson DH, Scherr SL, Portenoy RK. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: Results of a tripart assessment survey. Semin. Hematol. 1997;34(3 Suppl 2):4–12. [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat. Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SLV, Ramamoorthy A, Moaddel R, Wainer I. Relationship of ketamine’s plasma metabolites with response and diagnosis, and side effects in Major Depression. Biol. Psychiatry. 2012;72:331–338. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]