Abstract
The study of cancer initiation, growth and metastasis has traditionally been focused on cancer cells, and the view that they proliferate due to uncontrolled growth signaling owing to genetic derangements. However, uncontrolled growth in tumors cannot be explained solely by aberrations in cancer cells themselves. To fully understand the biological behavior of tumors, it is essential to understand the microenvironment in which cancer cells exist, and how they manipulate the surrounding stroma to promote the malignant phenotype.
Ovarian cancer is the leading cause of death from gynecologic cancer worldwide. The majority of patients will have objective responses to standard tumor debulking surgery and platinum-taxane doublet chemotherapy, but most will experience disease recurrence and chemotherapy resistance. As such, a great deal of effort has been put forth to develop therapies that target the tumor microenvironment in ovarian cancer. Herein, we review the key components of the tumor microenvironment as they pertain to this disease, outline targeting opportunities and supporting evidence thus far, and discuss resistance to therapy.
Keywords: Ovarian cancer, tumor microenvironment
Introduction
Background
The study of cancer initiation, growth, and metastasis has traditionally been focused on cancer cells. This view postulates that cancer cells proliferate due to uncontrolled growth signaling pathways owing to derangements in both oncogenes and tumor suppressor genes[1]. However, despite the significant contributions of these pathways in the metastatic transformation of cells, the uncontrolled growth that occurs in tumors cannot be explained solely by aberrations in the cancer cells themselves. Tumors are complex tissues composed of tumor cells, as well as stroma consisting of blood and lymphoid vessels, nerves, fibroblasts and extracellular matrix proteins, endothelial cells, pericytes, and immune cells[1]. These collectively comprise the tumor microenvironment. To fully understand the biological behavior of tumors, it is essential to consider the context in which cancer cells exist, and how they manipulate and are manipulated by the surrounding stroma to promote the malignant phenotype[2].
Epidemiology
Ovarian cancer is the second most common gynecologic malignancy but is the most common cause of death from gynecologic cancer worldwide[3, 4]. Epidemiology, treatment and prognosis vary greatly by histopathologic subtype. Epithelial ovarian carcinoma (EOC) comprises approximately 85 percent of ovarian malignancies [5, 6], with high-grade serous (HGSC) being the most common histology.
While HGSC was historically thought to arise from the ovarian surface epithelium (OSE), contemporary paradigms suggest that other sources are more likely. Studies examining the distal, fimbriated end of the fallopian tubes in patients with serous carcinoma classified as either ovarian, fallopian tube or primary peritoneal in origin demonstrated that approximately 50% of patients had tubal intraepithelial carcinoma (TIC) present[7]. This suggests that TIC may be the precursor lesion and an important initiating factor in pelvic serous carcinoma[8]. Cells in the hilum of the ovary may be an alternative source of stem cells [9] and may have increased susceptibility to malignant transformation [9]. The primary mode of spread of HGSC was traditionally thought to be continuous exposure of the peritoneal surfaces to exfoliated tumor cells, however, there is evidence pointing to hematogenous mode of spread being an important component of the metastatic process [10] [11]. Ovarian cancer cells have tropism for the omentum, which is likely mediated by a variety of factors produced by omental adipocytes [12].
Herein, we review the key components of the tumor microenvironment as they pertain to ovarian cancer, discuss targeting opportunities for individual stromal cell types as well as their prognostic potential, and outline emerging areas of research. Emphasis will be placed on fibroblasts, endothelial cells, and the immune components of the tumor microenvironment.
Cancer-Associated Fibroblasts
Background
Fibroblasts are the principal cellular component of connective tissue and are largely responsible for its maintenance and regeneration. The functions of fibroblasts include production and deposition of types I, III and V collagen and fibronectin, which are key components the fibrillar extracellular matrix[13], as well as synthesis of basement membrane proteins laminin and type IV collagen[14]. In addition, fibroblasts have an important role in the turnover and maintenance of the extracellular matrix by producing proteases such as matrix metalloproteinases [14]. Importantly, fibroblasts are crucial components in the process of wound healing, whereby they localize to wounds, generate extracellular matrix proteins, and aid in the contracture of the lesions that they occupy[13, 15]. Additionally, these fibroblasts gain contractile strength[16] by expressing characteristically increased levels of α-smooth muscle actin (α-SMA)[13]. This phenomenon is mediated by growth factors such as TGF-β[17, 18]. Once the wound has completed healing, activated fibroblasts undergo apoptosis [19, 20].
The importance of fibroblasts in tumor development is well established. Initial studies showed that injection of carcinogenic Rous sarcoma virus in chickens led to development of tumors [21]. Tumors have been described as “wounds that do not heal.”[22] Similarly, cancer cells have the ability to induce a reactive fibroblast phenotype, termed cancer-associated fibroblasts (CAF). CAFs are similar to activated fibroblasts in that they express α-SMA, but do not undergo apoptosis and do not lose their activated phenotype[23]. In addition, they express fibroblast activation protein (FAP)[15]. The interaction between cancer cells and fibroblasts in the tumor microenvironment is complex. CAFs can initially restrict tumor progression, similar to the relationship between cancer cells and immune components of the microenvironment[24]. However, CAFs eventually become activated by growth factors such as TGF-β1, platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), and interleukin-6 (IL-6). Vascular endothelial growth factor (VEGF), described in detail in the following section, is released by cancer cells and induces an influx of fibroblasts and thus, an increase in both the volume of tumor stroma[25]. CAFs contribute to vascular stabilization in ovarian and other cancers[26]. Lysophosphatidic acid produced by ovarian cancer cells has been shown to promote differentiation of adipose tissue-derived mesenchymal stem cells to CAFs[27], demonstrating not only the close interactions between CAFs and tumor cells, but also the ability of tumor cells to modify the surrounding microenvironment. Finally, the Hedgehog pathway, whose role is primarily developmental, has been implicated in the carcinogenesis of many cancer types [28, 29]. Hedgehog ligands produced by stromal cells provide essential growth signaling for tumor cells, emphasizing the interaction between tumor cells and their microenvironment. [30]. Overall, the result of the interaction between tumor cells and CAFs is a reciprocal, positive feedback mechanism in which cancer cells produce factors that activate and maintain CAFs, which in turn promote tumor progression by increasing cancer cell proliferation, angiogenesis, and remodeling of the extracellular matrix[23].
Cancer-associated Fibroblasts as a Therapeutic Target
There are several factors that make CAFs an attractive target for therapy. They comprise a large portion of tumor mass of solid tumors, and there is constant two-way crosstalk between them and cancer cells. Furthermore, fibroblasts are relatively genetically stable compared to cancer cells, and conventional challenges of acquired resistance could potentially be avoided[23]. Recent studies have demonstrated that CAFs contribute to tumor growth and metastasis in ovarian cancer, and may be a clinically important target for diagnosis, treatment and surveillance [31, 32]. Infiltration of CAFs into ovarian carcinoma spheroids leads to vascular stabilization of the tumors via expression of angiopoietin-1 and angiopoietin-2[33]. Immunohistochemical analysis of benign, borderline and malignant ovarian specimens demonstrated lack of CAFs in benign ovarian tissue and abundant CAFs in ovarian carcinoma [31]. The quantity of CAFs was increased in ovarian carcinoma specimens with disease stage, and patients with lymph node and omental metastases had significantly higher α-SMA expression in their tumors as compared to those without metastatic disease. This suggests not only that CAFs may be necessary for metastases to occur, but that metastatic sites recruit stromal components to optimize cell survival and further metastasis. In addition, FAP expression level within tumors was found to correlate with platinum resistance and shortened interval to recurrence[34]. Furthermore, FAP silencing led to a decrease in ovarian cancer cell growth in vivo. Similarly, fibroblast growth factor receptor-3 (FGFR-3), whose ligand is FGF, has been shown to have significantly higher expression in clear cell ovarian cancer samples, as compared to normal ovarian tissue, and knockdown of FGFR-3 slowed cell migration and proliferation[35]. The reciprocal relationship between tumor cells and CAFs was further demonstrated when conditioned media from SKOV3 ovarian cancer cells led to differentiation of fibroblasts into CAFs, as evidenced by increased expression of α-SMA[36]. Although the latter two studies demonstrate the importance of fibroblasts in ovarian cancer progression, it is important to note that clear cell ovarian cancer is biologically and histologically distinct from high grade serous cancer. Finally, there is some evidence indicating that mutations in tumor suppressor genes in CAFs may contribute to the interaction between CAFs and cancer cells, leading to tumor progression. Of particular interest is p53, which is mutated in nearly all high grade serous tumors[37], but not in other histological subtypes. In an in vivo breast cancer model, there was significantly increased tumor size when tumor cells were injected into p53-null mice, as compared to tumor size in wild-type p53 mice[38]. This suggests a potential role for p53 in the surrounding tumor stroma, irrespective of p53 status of the tumor cells. Further, CAFs isolated from breast and colon cancer specimens have been shown to have aberrations in p53, including inactivation mutations[39] and intact, but non-functional protein[40]. Despite the frequency of p53 mutations in epithelial ovarian cancer, p53 in CAFs in ovarian cancer tumor stroma, and any potential role in tumorigenesis, has yet to be elucidated.
Fibroblast growth factor receptor (FGFR) isoforms have proven to be important targets in the treatment of solid tumors, based on preclinical and early clinical work. Lucitanib is a receptor tyrosine kinase inhibitor that acts on FGFR isoforms 1 through 3, vascular endothelial growth factor receptor (VEGFR) isoforms 1 through 3, and PDGF receptors (PDGFR) α and β, and is currently in phase II trials in patients with metastatic breast cancer and FGF amplifications (NCT02202746, NCT02053636). Dovitinib is a non-specific receptor tyrosine kinase inhibitor whose targets include FGFR3, and is currently undergoing phase I trials in solid tumors (NCT01497392) and phase II trials in urothelial cancer (NCT01732107) and prostate cancer (NCT01741116). Several other, similar receptor tyrosine kinase inhibitors targeting the FGF receptor are undergoing phase I evaluation. Finally, nintedanib is a non-specific receptor tyrosine kinase inhibitor of VEGFR 1-3, FGFR 1-3, and PDGFR α and β that is currently undergoing phase I, II, and III clinical testing as monotherapy and in combination with chemotherapy for first line and recurrent ovarian cancer (e.g., NCT01610869, NCT01669798, EudraCT 2013-002109-73). In an initial randomized, phase II, placebo-controlled trial, patients with ovarian cancer who had completed chemotherapy for recurrent disease were treated with nintedanib as maintenance therapy. Progression-free rates were 16.3% in the nintedanib and 5.0% in the placebo groups (HR=0.65, p=0.06)[41]. This prompted a phase III trial which demonstrated an improvement in progression-free survival (PFS) when nintedanib was used in the up-front setting in combination with carboplatin and paclitaxel, as compared to carboplatin and paclitaxel alone (27.1 versus 20.8 months, respectively, hazard ratio=0.84, 95% confidence interval=0.72–0.98, p=0.024)[42].
As evidenced above, the majority of fibroblast-directed therapies currently being tested in clinical trials are non-specific to FGFR. A notable exception is an FGFR-selective antibody drug conjugate currently in phase I trials (NCT02368951). Although results have been modest thus far, therapies that target multiple receptor types may prove to be helpful in circumventing common resistance mechanisms [43, 44].
Angiogenesis & Endothelial Cells
Background
The formation of new blood vessels is essential for tumor growth and metastasis [45]. Angiogenesis is a central hallmark of cancer and is crucial for solid tumor growth and metastasis[1]. Early studies demonstrated that tumor growth in isolated perfused organs was significantly decreased in the absence of tumor vascularization[46, 47], and that without adequate vascularization, tumor cells undergo necrosis or apoptosis[48, 49]. An “angiogenic switch” becomes activated during the early stages of tumor development and is a key step in tumorigenesis[45]. This can be activated by conditions that require increased oxygen and nutrient delivery to the tumor, including hypoxia, hypoglycemia, mechanical stress, and inflammation[50]. There are other mechanisms by which tumors produce a microcirculation to acquire oxygen and nutrients. In contrast to angiogenesis, several tumor types display vascular cooption, a process by which a tumor mass coopts already-established host vessels, allowing the tumor to be initially well-vascularized [51]. Moreover, aggressive tumors are capable of directly contributing to vasculature, a process described as vasculogenic mimicry [52, 53]. In addition, mutations in tumor suppressor genes and oncogenes can alter the balance between pro-angiogenic and anti-angiogenic factors to promote tumor growth [1, 54]. Well-recognized promoters of angiogenesis include VEGF [55, 56], FGF1 and 2, and their associated receptors. In addition to these pathways, PDGF, epidermal growth factor (EGF), angiopoietins, and hepatocyte growth factor (HGF) are growth factors known to contribute to tumor angiogenesis [57]. These growth factors bind to receptor tyrosine kinases, leading to the initiation of intracellular signaling. While the mechanisms regulating angiogenesis in tumors are complex and multi-factorial, VEGF has emerged as a dominant pathway. The VEGF family of molecules includes VEGF-A, -B, -C, -D and placental growth factor (PIGF) [58]. VEGF is constitutively expressed in most human cancers[58], and is mediated via hypoxia-inducible transcription factors 1α and 2α [59]. Additionally, VEGFR-3 plays an important role in sustaining angiogenesis, even in the presence of VEGFR-2 inhibitors [60]. Traditionally, the relationship between the VEGF ligands and their receptors has been described as paracrine; however, there is evidence that VEGF can be produced by stromal cells in the tumor microenvironment[25] as well as by hematopoietic stem cells[61] and VEGFRs can be expressed directly on cancer cells[62].
Targeting the VEGF Pathway
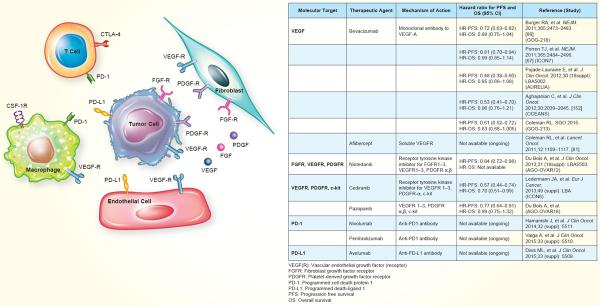
Despite a modest increase in survival in women with ovarian cancer over the past several decades, a significant proportion of women will experience disease recurrence[5], as the median progression free survival in these patients is approximately 18 months[4]. While patients with platinum-sensitive relapsed disease can be re-treated with platinum-based therapy, the options for those with platinum-resistant or -refractory disease are limited [63, 64]. Among the various options, anti-angiogenesis strategies are attractive. Bevacizumab (a monoclonal antibody to VEGF-A) is the only anti-angiogenic therapy that is approved by the Food and Drug Administration (FDA) in combination with chemotherapy in patients with platinum-resistant recurrent ovarian cancer treated with 1 or 2 prior regimens. Approval was based on the results of the AURELIA trial, which compared bevacizumab plus conventional chemotherapy (paclitaxel, pegylated liposomal doxorubicin, or topotecan), to chemotherapy alone in patients with platinum-resistant recurrent epithelial ovarian cancer [65]. Adding bevacizumab to chemotherapy resulted in statistically improved progression free survival (median PFS 3.4 months with chemotherapy alone versus 6.7 months when bevacizumab was added) and overall response rate, however, did not improve overall survival[65]. In addition, bevacizumab is approved for frontline therapy for ovarian cancer in many countries outside the United States, based on findings from the GOG-218 and ICON7 trials [66, 67]. To date, there have been five positive phase III trials with bevacizumab in combination with chemotherapy for patients with newly diagnosed or relapsed ovarian cancer (Table 1).
Table 1.
Summary of therapies targeting the tumor microenvironment in ovarian cancer. The agents listed below have demonstrated safety/efficacy in phase I/II trials, and/or improved survival in phase III trials.
Other Anti-Angiogenic Therapeutic Targets
Despite FDA approval, improvements in overall survival are modest in patients using bevacizumab, and resistance is common, emphasizing the need for development of alternative anti-angiogenic therapies [43].
PDGF has four isoforms (A-D) that bind to specific receptors, PDGFR-α and –β. PDGF is secreted by endothelial cells at the site of angiogenesis and attracts pericytes to the region in order to stabilize newly formed blood vessels[68]. Inhibition of PDGFR prevents pericyte coverage of new blood vessels, leading to vessel destabilization and subsequently preventing oxygen and nutrient flow to tumor cells[69]. As detailed above in the discussion about fibroblasts, therapies targeting PDGF also target VEGFR and FGFR isoforms, as well as other receptor types. Blockade of the PDGF pathway may enhance the effectiveness of VEGF pathway blockade [70, 71]. Targeted agents with completed trials which have shown effectiveness in ovarian cancer are highlighted below.
Cediranib is a receptor tyrosine kinase inhibitor that inhibits VEGFR 1-3, PDGFR-α, and c-kit. In an initial phase II trial, cediranib yielded a PFS of 5.2 months, with partial responses in 17% of enrolled patients with recurrent ovarian cancer [72]. The follow-up phase III trial (ICON6) demonstrated a prolonged PFS and OS in the group of patients who received chemotherapy plus cediranib followed by 18 months of cediranib for maintenance, as compared to chemotherapy plus cediranib with placebo maintenance or chemotherapy plus placebo with placebo maintenance (median PFS 11.1 versus 8.7 months, hazard ratio=0.57, 95% confidence interval=0.45–0.74)[73, 74]. There are additional ongoing trials with cediranib alone (NCT00278343) and in combination with other targeted therapies for ovarian cancer (e.g., temsirolimus; NCT01065662). A phase II trial with olaparib plus cediranib demonstrated an improvement in PFS when the two agents were used in combination as compared to the use of olaparib alone (median PFS 17.7 versus 9 months, hazard ratio=0.42, 95% confidence interval=0.23–0.76, p=0.005)[75].
Sorafenib acts on VEGFR 1-3, PDGFR-β, and Raf-1, and is FDA approved for use in advanced renal cell and hepatocellular carcinoma. In an initial phase II study, 24% of patients had stable disease for 6 months, and 3.4% of patients had partial responses when patients with recurrent ovarian cancer were given sorafenib alone[76]. However, completed trials have demonstrated a high rate of toxicity leading to increased frequency of dose reductions and treatment discontinuation [76, 77]. Trials testing sorafenib in combination with bevacizumab (NCT00436215) and carboplatin and paclitaxel (NCT003900611) are ongoing. Pazopanib, an inhibitor of VEGFR 1-3, PDGFR α and β, and c-kit, is FDA approved for use in advanced sarcoma and renal cell carcinoma. A phase II of patients with recurrent ovarian cancer demonstrated 3 partial responses and a CA-125 response rate of 31%[78]. A phase III trial of pazopanib maintenance therapy after first-line chemotherapy showed prolonged PFS with pazopanib as compared to placebo (17.9 versus 12.3 months, hazard ratio=0.77, 95% confidence interval =0.64–0.91, p=0.002), but substantially more toxicity, particularly among the Asian cohort[79]. None of the receptor tyrosine kinase inhibitors have been FDA approved for use in ovarian cancer so far.
Aflibercept is a soluble decoy VEGF receptor that binds to circulating VEGF-A and –B molecules. This acts as a “VEGF trap,” binding VEGF at very high affinity, thereby decreasing the amount of circulating VEGF available to act on its receptors [80]. It is FDA approved for the treatment of neovascular (wet) age-related macular degeneration, however, it has shown promise in recurrent ovarian cancer. In a phase II trial in which aflibercept was given in combination with docetaxel, there was an overall response rate of 54% (25 of 46 patients), with 11 patients with a complete response and 14 with a partial response [81]. A subsequent phase II study showed that aflibercept was effective at reducing symptomatic malignant ascites in this patient population, however, frequency of fatal intestinal perforations was higher in the aflibercept group than placebo (three events versus one)[82].
Resistance to Anti-VEGF therapy
Despite the success of anti-VEGF therapies, the duration of effect is often short as tumors quickly become resistant to therapy[43] via a variety of mechanisms. While the modes of resistance are varied, a common contributing factor is that patients are treated with anti-VEGF therapies irrespective of the characteristics of their tumors. The stromal components of the tumor microenvironment may offer important means of resistance to VEGF blockade. The Notch signaling pathway has been shown to have a central role in anti-VEGF resistance and is closely related to the VEGF pathway[83]. Tumors with increased activity of the Notch pathway via the DLL4 ligand, produced by endothelial cells, had increased formation of large vessels, thereby decreasing sensitivity to anti-VEGF therapy [84]. This finding emphasizes the need for molecular testing and tumor evaluation prior to initiating targeted therapy. Tumor-associated macrophages (TAMs), which are discussed in the next section in the context of their immune properties, have also been implicated in resistance to anti-VEGF therapies. As a major component of the tumor microenvironment, TAMs contribute to tumor growth and metastasis via several mechanisms, including promoting angiogenesis[85]. Sub-populations of TAMs that produce Tie2 lead to vasculogenic mimicry in the tumor via the production of primitive capillary-like structures[86]. Given their ability to induce pro-angiogenic pathways, TAMs in the tumor microenvironment likely play an important role in resistance to anti-VEGF therapy.
Immune Components of the Tumor Microenvironment
Background
Immune cells are present not only in the tumor microenvironment, where they interact closely with fibroblasts and endothelial cells, but also in areas of the tumor predominated by cancer cells [87]. The importance of the interaction between cancer cells and immune cells was first described in 1863 by Virchow, who observed that cell proliferation was enhanced at sites of tissue injury and resultant inflammation[88, 89]. This concept is demonstrated by the fact that approximately 15% of cancers globally can be attributed to infectious etiology [90]. For example, infection with human papillomavirus is instrumental in the pathogenesis of cervical dysplasia and progression to squamous cell carcinoma of the cervix. Chronic inflammatory conditions not related to infections are also known to predispose to cancer, exemplified by the role of ulcerative colitis in the development of colorectal cancer[91]. The development of colitis-associated colorectal cancer is driven by IL-6 produced by immune cells in the intestinal microenvironment, which protects premalignant cells from apoptosis[92]. Tumors have hence been described as a “Darwinian microenvironment,” which adapt and select for the level of inflammation that maximally promotes their growth and metastasis [93].
When tissue is injured, platelets accumulate at the site of injury. In this setting, platelets serve a dual purpose: initiating both coagulation and the host inflammatory response. Platelets secrete plasma proteins, coagulation factors, and cellular growth factors including platelet-derived growth factor (PDGF), transforming growth factor-α and –β (TGF-α and TGF–β), and basic fibroblast growth factor (bFGF) [88], all of which potentiate the inflammatory response. In addition to promoting formation of the extracellular matrix and new vasculature, platelets are instrumental in neutrophil chemotaxis. Not all tumors are characterized by a classical inflammatory response, but tumor infiltrating immune cells can be present in smaller quantities and still have influential effects on tumor growth and metastasis[94]. Given the depth and breadth of the involvement of the immune system in cancer, emphasis will be placed on T-lymphocytes and macrophages, and their role in the treatment of ovarian cancer.
Lymphocytes and Associated Therapies
In 1984, Rosenberg and colleagues used an infusion of interleukin-2 (IL-2), a potent cytokine that induces proliferation of lymphocytes, to treat a patient with progressive metastatic melanoma. This patient had a complete response to treatment [95]. IL-2 is primarily secreted by antigen-stimulated CD4+ T cells, but can also be secreted by CD8+ T cells, natural killer cells, and activated dendritic cells [95]. Since its initial use, recombinant IL-2 has been FDA approved for the treatment of metastatic melanoma and renal cell carcinoma. Administration of tumor-infiltrating lymphocytes isolated from tumors, and propagated in IL-2, has also shown to be effective in metastatic melanoma [96–98].
Ovarian cancer was traditionally not believed to be an immunogenic tumor type, but there is now ample evidence suggesting the opposite[99]. The presence of intratumoral T cells was found to correlate with improved clinical outcome in advanced ovarian cancer[100], as had been previously demonstrated in patients with melanoma[101], colorectal[102], breast[103], prostate[104], renal cell[105], and esophageal cancers[106]. This finding has been confirmed by other investigators, who have also shown that intratumoral T-cells, despite predicting improved survival, were more prevalent in tumors with increased proliferation[107]. The evidence has led to an increase in the use of immune therapies in ovarian cancer. A phase II trial in which patients with platinum-resistant or –refractory ovarian cancer were administered weekly intraperitoneal recombinant IL-2 had a 17% complete response rate. This study also found a significant association between changes in peripheral lymphocytes and overall survival [108]. In addition to the use of IL-2, improved survival rates have been observed in patients who underwent adoptive transfer of tumor-infiltrating lymphocytes [109], as well as after treatment with CTLA-4 antibody [110, 111].
Programmed death 1 (PD-1) is an inhibitory immune checkpoint receptor expressed by activated T cells. PD-1 interacts with its ligands, programmed death-ligand 1 and 2 (PD-L1 and 2), present on tumor and stromal cells [112–114]. Blocking the interaction of PD-1 with its ligands has been shown to mediate antitumor activity in preclinical models [115–117]. PD-L1 is highly expressed in ovarian cancer cell lines and high expression is associated with poorer survival in patients [118]. Silencing PD-L1 in animal models has been shown to decrease peritoneal dissemination of ovarian tumors [119]. Pembrolizumab and nivolumab, humanized antibodies against PD-1, and avelumab, a humanized antibody against PD-L1 have shown response rates of 10–20% in patients with recurrent or refractory ovarian cancer [120–122]. In ovarian carcinosarcoma, PD-L1, PD-L2 and CD8+ tumor infiltrating lymphocytes are highly expressed, suggesting that PD-1/PD-L1 targeting may also be beneficial in this disease.[123]
Macrophages
Macrophages are the most abundant immune cell population in the tumor microenvironment [88, 124], and are termed tumor-associated macrophages (TAM) when present in association with tumors. TAM are derived from monocyte precursors[125] and are recruited to the tumor microenvironment by chemokines CCL2, CCL5, CXCL1, and others[126]. Once monocytes are recruited to tumor areas, the chemokines TGF-β, IL-10 and IL-4 promote their differentiation into the M2 macrophage phenotype [127, 128]. This phenotype has poor antigen presenting capacity, and promotes wound healing, tissue remodeling and angiogenesis[129]. As such, TAM predominantly accumulate in hypoxic areas of tumor due to HIF-1 dependent upregulation of CXCR4[130]. TAM survival is promoted in the tumor microenvironment by macrophage colony-stimulating factor (M-CSF) and VEGF, both produced by tumor cells[93]. TAM generally have pro-tumorigenic functions and as a result, high levels of TAMs in tumors are associated with poor prognosis[85, 93, 131]. Macrophages produce VEGF[132], PDGF[128] and other pro-angiogenic factors. There is also concurrent dissolution and remodeling of the extracellular matrix by MMPs, urokinase-type plasminogen activator (uPA) and its receptor, and plasmin produced by TAMs can enable tumor cell migration[133, 134]. TAMs also act as suppressors of anti-tumor immune responses by producing immunosuppressive chemokines including IL-10, TGF-β, and prostaglandin E2 (prostaglandin E2)[93, 127, 128], and producing chemokines such as CCL17, CCL18 and CCL22 that recruit only immune cell populations that lack cytotoxic activity. Finally, TAMs can directly stimulate growth of cancer cells via production of EGF, IL-6 and tumor necrosis factor[93, 135]. The multi-factorial nature by which TAMs promote tumor progression make them an appealing therapeutic target. Zoledronic acid, a bisphosphonate, has been shown to suppress MMP-9 production by TAMs, and could be a potential therapeutic approach [136].
Myeloid-Derived Suppressor Cells and Associated Therapies
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous group of myeloid-derived cell types including myeloid progenitor cells, and immature macrophages, granulocytes and dendritic cells[137]. They differ from TAMs in that they have granulocytic morphology and upregulation of both arginase 1 and inducible nitric oxide synthase, resulting in increased production of immunosuppressive nitric oxide and reactive oxygen species [138]. Thus, the presence of MDSCs in tumors leads to suppression of the tumor-directed immune response. This suppression must be abrogated in order for immune therapies to be successful. One such strategy is to promote the differentiation of MDSCs into immunocompetent mature myeloid cells. This has been accomplished by the use of all-trans retinoic acid, which has been shown to decrease MDSCs, increase antigen-specific T cell response, and prolong vaccine effect when used in combination with anti-tumor vaccines [139, 140]. While a variety of other approaches are under investigation, the role for these therapies in ovarian cancer has yet to be determined.
Resistance to Immune Therapy
While immune therapy has shown considerable promise in the treatment of ovarian and other cancers, immune suppression is an important mechanism of resistance. This is accomplished in a variety of ways, which often operate simultaneously. Tumor cells downregulate MHC class I molecules in order to avoid detection by T cells, and upregulate factors that are inhibitory to T cell signaling, such as PD-L1[141]. Ovarian cancer cells and TAMs produce CCL22, a chemokine that recruits T regulatory cells to the tumor. This particular T cell population inhibits tumor-specific T cell immunity and is associated with reduced patient survival [142]. This represents a mechanism by which tumors actively promote their own immune privilege. MDSCs appear to have multiple roles in decreasing the immune response to tumors. First, they are recruited to areas of hypoxia in tumors to stimulate angiogenesis [143, 144]. They also inhibit the activity of T cells and natural killer cells via TGF-β, IL-10, and reactive oxygen species, thereby dampening the tumor-directed immune response [138]. Finally, CAFs can inhibit recruitment of effector T cells to the tumor by overexpression of TGF-β, yielding an immunosuppressive effect[141]. These mechanisms of resistance highlight the importance of the interaction between immune cells and other components of the microenvironment, and provide important therapeutic opportunities. Indeed, initial reports of PD-1/PD-L1 blockade have yielded objective responses in patients with measurable recurrent ovarian cancer. While the range of response is in line with salvage chemotherapy, some of the responses were complete and occurred in chemotherapy refractory settings[120–122].
Finally, immune cell recruitment in the presence of induced hypoxia (e.g. anti-angiogenic therapy) may define angiogenic escape. Therapeutic targets, for example CSF-1R, are now entering clinical investigation as an opportunity to reverse this phenotype. VEGF produced by tumor and endothelial cells can also contribute to resistance to immune therapies, highlighting the interplay between tumor microenvironment components. VEGF can serve as a chemoattractant for immature myeloid cells from the bone marrow [145] to tumor sites. Exogenously administered VEGF was shown to decrease the number of mature CD4+/CD8+ thymocytes in animal models and inhibited dendritic cell maturation[146]. VEGF can also induce expression of Fas ligand, a known regulator of T cell apoptosis[147, 148], on human tumor endothelial cells [149, 150], resulting in the preferential apoptosis of tumor-infiltrating CD8+ T cells[149]. Overall, VEGF appears to promote tumor growth via diminishing the microenvironment immune cell population.
Conclusions
The treatment of epithelial ovarian cancer, particularly in the setting of platinum-resistant or - refractory disease, remains a challenge. Theoretically, targeting the tumor microenvironment is advantageous because stromal components do not develop mutations or genetic aberrations as frequently as do tumor cells. However, the intricate signals between components of the tumor microenvironment can ultimately lead to adaptive resistance and treatment failure. Many of the strategies outlined in this article hold hope for improving the efficacy of microenvironment-targeted therapies and enhancing patient outcomes.
Acknowledgments
Role of Funding Source JMH is supported by a NIH T32 Training Grant CA101642. This work was also supported in part by NIH grants (P50CA083639, CA109298, P50CA098258, U54CA151668, UH2TR000943, CA177909, CA016672, U54CA96300 and U54CA96297), CPRIT RP 110595, an Ovarian Cancer Research Fund Program Project Development Grant, Department of Defense Grants (OC120547 and OC093416), The Betty Ann Asche Murray Distinguished Professorship, the RGK Foundation, the Gilder Foundation and the Blanton-Davis Ovarian Cancer Research program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement JMH and AKS report no conflicts of interest. RLC is a consultant for Clovis Oncology, Bayer, AstraZeneca and GlaxoSmithKline. He is a speaker on behalf of AstraZeneca and GlaxoSmithKline. He serves on the scientific advisory board for Amgen, Clovis Oncology, Bayer, GlaxoSmithKline and the National Comprehensive Cancer Network. He participates in research funded by Clovis Oncology and EMD Serono.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316(8):1324–31. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 4.Torre LA, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015 doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Morgan RJ, Jr., et al. Epithelial ovarian cancer. J Natl Compr Canc Netw. 2011;9(1):82–113. doi: 10.6004/jnccn.2011.0008. [DOI] [PubMed] [Google Scholar]
- 6.Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10(11):803–8. doi: 10.1038/nrc2946. [DOI] [PubMed] [Google Scholar]
- 7.Kindelberger DW, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31(2):161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 8.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26(32):5284–93. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flesken-Nikitin A, et al. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495(7440):241–5. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pradeep S, et al. Hematogenous metastasis of ovarian cancer: rethinking mode of spread. Cancer Cell. 2014;26(1):77–91. doi: 10.1016/j.ccr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips KG, et al. Optical quantification of cellular mass, volume, and density of circulating tumor cells identified in an ovarian cancer patient. Front Oncol. 2012;2:72. doi: 10.3389/fonc.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomasek JJ, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 14.Chang HY, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99(20):12877–82. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 16.Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27(5):549–50. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 17.Sieweke MH, et al. Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-beta. Science. 1990;248(4963):1656–60. doi: 10.1126/science.2163544. [DOI] [PubMed] [Google Scholar]
- 18.Cai J, et al. Fibroblasts in omentum activated by tumor cells promote ovarian cancer growth, adhesion and invasiveness. Carcinogenesis. 2012;33(1):20–9. doi: 10.1093/carcin/bgr230. [DOI] [PubMed] [Google Scholar]
- 19.Desmouliere A, et al. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146(1):56–66. [PMC free article] [PubMed] [Google Scholar]
- 20.Rasanen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010;316(17):2713–22. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Dolberg DS, et al. Wounding and its role in RSV-mediated tumor formation. Science. 1985;230(4726):676–8. doi: 10.1126/science.2996144. [DOI] [PubMed] [Google Scholar]
- 22.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 23.Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011;1(4):482–97. [PMC free article] [PubMed] [Google Scholar]
- 24.McAnulty RJ. Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cell Biol. 2007;39(4):666–71. doi: 10.1016/j.biocel.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Fukumura D, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94(6):715–25. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 26.Granot D, et al. In vivo imaging of the systemic recruitment of fibroblasts to the angiogenic rim of ovarian carcinoma tumors. Cancer Res. 2007;67(19):9180–9. doi: 10.1158/0008-5472.CAN-07-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon ES, et al. Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblast-like cells. Stem Cells. 2008;26(3):789–97. doi: 10.1634/stemcells.2007-0742. [DOI] [PubMed] [Google Scholar]
- 28.Heller E, et al. Hedgehog signaling inhibition blocks growth of resistant tumors through effects on tumor microenvironment. Cancer Res. 2012;72(4):897–907. doi: 10.1158/0008-5472.CAN-11-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris LG, Samant RS, Shevde LA. Hedgehog signaling: networking to nurture a promalignant tumor microenvironment. Mol Cancer Res. 2011;9(9):1165–74. doi: 10.1158/1541-7786.MCR-11-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dierks C, et al. Essential role of stromally induced hedgehog signaling in B-cell malignancies. Nat Med. 2007;13(8):944–51. doi: 10.1038/nm1614. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, et al. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011;303(1):47–55. doi: 10.1016/j.canlet.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Schauer IG, et al. Cancer-associated fibroblasts and their putative role in potentiating the initiation and development of epithelial ovarian cancer. Neoplasia. 2011;13(5):393–405. doi: 10.1593/neo.101720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilead A, Meir G, Neeman M. The role of angiogenesis, vascular maturation, regression and stroma infiltration in dormancy and growth of implanted MLS ovarian carcinoma spheroids. Int J Cancer. 2004;108(4):524–31. doi: 10.1002/ijc.11583. [DOI] [PubMed] [Google Scholar]
- 34.Mhawech-Fauceglia P, et al. Cancer Microenviron. 2014. Stromal Expression of Fibroblast Activation Protein Alpha (FAP) Predicts Platinum Resistance and Shorter Recurrence in patients with Epithelial Ovarian Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itamochi H, et al. Fibroblast growth factor receptor 2 is associated with poor overall survival in clear cell carcinoma of the ovary and may be a novel therapeutic approach. Int J Gynecol Cancer. 2015;25(4):570–6. doi: 10.1097/IGC.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 36.Yao Q, et al. CLIC4 mediates TGF-beta1-induced fibroblast-to-myofibroblast transdifferentiation in ovarian cancer. Oncol Rep. 2009;22(3):541–8. doi: 10.3892/or_00000469. [DOI] [PubMed] [Google Scholar]
- 37.Cancer Genome Atlas Research, N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiaris H, et al. Evidence for nonautonomous effect of p53 tumor suppressor in carcinogenesis. Cancer Res. 2005;65(5):1627–30. doi: 10.1158/0008-5472.CAN-04-3791. [DOI] [PubMed] [Google Scholar]
- 39.Wernert N, et al. Presence of genetic alterations in microdissected stroma of human colon and breast cancers. Anticancer Res. 2001;21(4A):2259–64. [PubMed] [Google Scholar]
- 40.Hawsawi NM, et al. Breast carcinoma-associated fibroblasts and their counterparts display neoplastic-specific changes. Cancer Res. 2008;68(8):2717–25. doi: 10.1158/0008-5472.CAN-08-0192. [DOI] [PubMed] [Google Scholar]
- 41.Ledermann JA, et al. Randomized phase II placebo-controlled trial of maintenance therapy using the oral triple angiokinase inhibitor BIBF 1120 after chemotherapy for relapsed ovarian cancer. J Clin Oncol. 2011;29(28):3798–804. doi: 10.1200/JCO.2010.33.5208. [DOI] [PubMed] [Google Scholar]
- 42.Du Bois A, et al. “AGO-OVAR 12: A randomized placebo-controlled GCIG/ENGOT-Intergroup phase III trial of standard frontline chemotherapy+/−nintedanib for advanced ovarian cancer.”. Int J Gynecol Cancer. 2013;23.8(Supplement 1) [Google Scholar]
- 43.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi HJ, et al. Anti-vascular therapies in ovarian cancer: moving beyond anti-VEGF approaches. Cancer Metastasis Rev. 2015;34(1):19–40. doi: 10.1007/s10555-014-9538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 46.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175(3):409–16. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 48.Brem S, et al. Prolonged tumor dormancy by prevention of neovascularization in the vitreous. Cancer Res. 1976;36(8):2807–12. [PubMed] [Google Scholar]
- 49.Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1(2):149–53. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 50.Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21(3):505–15. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 51.Holash J, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–8. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 52.Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156(2):361–81. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maniotis AJ, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155(3):739–52. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 55.Kim KJ, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–4. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 56.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69(Suppl 3):11–6. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 57.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 58.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–49. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 60.Tammela T, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454(7204):656–60. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 61.Gerber HP, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417(6892):954–8. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 62.Spannuth WA, et al. Functional significance of VEGFR-2 on ovarian cancer cells. Int J Cancer. 2009;124(5):1045–53. doi: 10.1002/ijc.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374(9698):1371–82. doi: 10.1016/S0140-6736(09)61338-6. [DOI] [PubMed] [Google Scholar]
- 64.Stockler MR, et al. Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J Clin Oncol. 2014;32(13):1309–16. doi: 10.1200/JCO.2013.51.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pujade-Lauraine E, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–8. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 66.Burger RA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 67.Perren TJ, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 68.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 69.Bergers G, et al. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111(9):1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Erber R, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18(2):338–40. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 71.Jo N, et al. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol. 2006;168(6):2036–53. doi: 10.2353/ajpath.2006.050588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matulonis UA, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol. 2009;27(33):5601–6. doi: 10.1200/JCO.2009.23.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raja FA, et al. Initial toxicity assessment of ICON6: a randomised trial of cediranib plus chemotherapy in platinum-sensitive relapsed ovarian cancer. Br J Cancer. 2011;105(7):884–9. doi: 10.1038/bjc.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ledermann JA, Perren TJ, Raja FA. Randomised double-blind phase III trial of cediranib (AZD 2171) in relapsed platinum sensitive ovarian cancer: Results of the ICON6 trial. European Cancer Congress 2013 (ECCO-ESMO-ESTRO); Sep 30, 2013. Abstract, 2013. [Google Scholar]
- 75.Liu JF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15(11):1207–14. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matei D, et al. Activity of sorafenib in recurrent ovarian cancer and primary peritoneal carcinomatosis: a gynecologic oncology group trial. J Clin Oncol. 2011;29(1):69–75. doi: 10.1200/JCO.2009.26.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herzog TJ, et al. A randomized phase II trial of maintenance therapy with Sorafenib in front-line ovarian carcinoma. Gynecol Oncol. 2013;130(1):25–30. doi: 10.1016/j.ygyno.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 78.Friedlander M, et al. A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol Oncol. 2010;119(1):32–7. doi: 10.1016/j.ygyno.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 79.du Bois A, et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol. 2014;32(30):3374–82. doi: 10.1200/JCO.2014.55.7348. [DOI] [PubMed] [Google Scholar]
- 80.Holash J, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–8. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coleman RL, et al. Phase 1–2 study of docetaxel plus aflibercept in patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer. Lancet Oncol. 2011;12(12):1109–17. doi: 10.1016/S1470-2045(11)70244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gotlieb WH, et al. Intravenous aflibercept for treatment of recurrent symptomatic malignant ascites in patients with advanced ovarian cancer: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Oncol. 2012;13(2):154–62. doi: 10.1016/S1470-2045(11)70338-2. [DOI] [PubMed] [Google Scholar]
- 83.Li JL, Harris AL. Crosstalk of VEGF and Notch pathways in tumour angiogenesis: therapeutic implications. Front Biosci (Landmark Ed) 2009;14:3094–110. doi: 10.2741/3438. [DOI] [PubMed] [Google Scholar]
- 84.Li JL, et al. DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res. 2011;71(18):6073–83. doi: 10.1158/0008-5472.CAN-11-1704. [DOI] [PubMed] [Google Scholar]
- 85.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 86.Scavelli C, et al. Vasculogenic mimicry by bone marrow macrophages in patients with multiple myeloma. Oncogene. 2008;27(5):663–74. doi: 10.1038/sj.onc.1210691. [DOI] [PubMed] [Google Scholar]
- 87.Negus RP, et al. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol. 1997;150(5):1723–34. [PMC free article] [PubMed] [Google Scholar]
- 88.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 90.Parkin DM, P.P., Muñoz N, Ferlay J. In: Infections and Human Cancer. R Newton VB, Weiss RA, editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1999. [Google Scholar]
- 91.Ekbom A, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323(18):1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 92.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 94.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 95.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–8. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233(4770):1318–21. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 97.Dillman RO, et al. Continuous interleukin-2 and tumor-infiltrating lymphocytes as treatment of advanced melanoma. A national biotherapy study group trial. Cancer. 1991;68(1):1–8. doi: 10.1002/1097-0142(19910701)68:1<1::aid-cncr2820680102>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 98.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21(2):233–40. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kandalaft LE, et al. Immunotherapy for ovarian cancer: what's next? J Clin Oncol. 2011;29(7):925–33. doi: 10.1200/JCO.2009.27.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang L, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 101.Halpern AC, Schuchter LM. Prognostic models in melanoma. Semin Oncol. 1997;24(1 Suppl 4):S2–7. [PubMed] [Google Scholar]
- 102.Naito Y, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58(16):3491–4. [PubMed] [Google Scholar]
- 103.Marrogi AJ, et al. Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int J Cancer. 1997;74(5):492–501. doi: 10.1002/(sici)1097-0215(19971021)74:5<492::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 104.Vesalainen S, et al. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer. 1994;30A(12):1797–803. doi: 10.1016/0959-8049(94)e0159-2. [DOI] [PubMed] [Google Scholar]
- 105.Nakano O, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61(13):5132–6. [PubMed] [Google Scholar]
- 106.Schumacher K, et al. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61(10):3932–6. [PubMed] [Google Scholar]
- 107.Adams SF, et al. Intraepithelial T cells and tumor proliferation: impact on the benefit from surgical cytoreduction in advanced serous ovarian cancer. Cancer. 2009;115(13):2891–902. doi: 10.1002/cncr.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vlad AM, et al. A phase II trial of intraperitoneal interleukin-2 in patients with platinum-resistant or platinum-refractory ovarian cancer. Cancer Immunol Immunother. 2010;59(2):293–301. doi: 10.1007/s00262-009-0750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fujita K, et al. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin Cancer Res. 1995;1(5):501–7. [PubMed] [Google Scholar]
- 110.Hodi FS, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100(8):4712–7. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hodi FS, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105(8):3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dong H, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 113.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 116.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hamanishi J, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abiko K, et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res. 2013;19(6):1363–74. doi: 10.1158/1078-0432.CCR-12-2199. [DOI] [PubMed] [Google Scholar]
- 120.Hamanishi J. Efficacy and safety of anti-PD-1 antibody (Nivolumab: BMS-936558, ONO-4538) in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2014;32(5s)(suppl) doi: 10.1200/JCO.2015.62.3397. abstr 5511. [DOI] [PubMed] [Google Scholar]
- 121.Disis ML. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with previously treated, recurrent or refractory ovarian cancer: A phase Ib, open-label expansion trial. J Clin Oncol. 2015;33(suppl) abstr 5509. [Google Scholar]
- 122.Varga A. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: Interim results from a phase Ib study. J Clin Oncol. 2015;33(suppl) abstr 5510. [Google Scholar]
- 123.Peng W, et al. PDL1, PDL2, and CD8+ TIL expression in ovarian carcinosarcoma. J Clin Oncol. 2015;33(15 supplement) [Google Scholar]
- 124.Allavena P, et al. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66(1):1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 125.Mantovani A, et al. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13(7):265–70. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 126.Mantovani A, et al. Chemokines in the recruitment and shaping of the leukocyte infiltrate of tumors. Semin Cancer Biol. 2004;14(3):155–60. doi: 10.1016/j.semcancer.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 127.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 128.Mantovani A, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 129.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23(4):344–6. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 130.Schioppa T, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198(9):1391–402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dave SS, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351(21):2159–69. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 132.Cursiefen C, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113(7):1040–50. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang S, et al. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94(15):1134–42. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- 134.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 135.Sica A, et al. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42(6):717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 136.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114(5):623–33. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Youn JI, et al. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mirza N, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66(18):9299–307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kusmartsev S, et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63(15):4441–9. [PubMed] [Google Scholar]
- 141.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 143.Yang L, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6(4):409–21. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 144.Murdoch C, et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8(8):618–31. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 145.Kim R, et al. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66(11):5527–36. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 146.Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res. 2001;23(2–3):263–72. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]
- 147.Ju ST, et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373(6513):444–8. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 148.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30(2):180–92. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Motz GT, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20(6):607–15. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yu JS, et al. Intratumoral T cell subset ratios and Fas ligand expression on brain tumor endothelium. J Neurooncol. 2003;64(1–2):55–61. doi: 10.1007/BF02700020. [DOI] [PubMed] [Google Scholar]
- 151.Aghajanian C, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17):2039–45. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]