Abstract
The anterior eye is comprised of an avascular cornea surrounded by a dense periocular vascular network and therefore serves as an excellent model for angiogenesis. Although signaling through PlexinD1 underlies various vascular patterning events during embryonic development, its role during the formation of the periocular vascular network is yet to be determined. Our recent study showed that PlexinD1 mRNA is expressed by periocular angioblasts and blood vessels during ocular vasculogenesis in patterns that suggest its involvement with Sema3 ligands that are concurrently expressed in the anterior eye. In this study, we used in vivo knockdown experiments to determine the role of PlexinD1 during vascular patterning in the anterior eye of the developing avian embryos. Knockdown of PlexinD1 in the anterior eye caused mispatterning of the vascular network in the presumptive iris, which was accompanied by lose of vascular integrity and profuse hemorrhaging in the anterior chamber. We also observed ectopic vascularization of the cornea in PlexinD1 knockdown eyes, which coincided with the formation of the limbal vasculature in controls. Finally we show that Sema3E and Sema3C transcripts are expressed in ocular tissue that is devoid of vasculature. These results indicate that PlexinD1 plays a critical role during vascular patterning in the iris and limbus, and is essential for the establishment of corneal avascularity during development. We conclude that PlexinD1 is involved in vascular response to antiangiogenic Sema3 signaling that guides the formation of the iris and limbal blood vessels by inhibiting VEGF signaling.
Keywords: PlexinD1, Vasculogenesis, Angiogenesis, Ocular development, Cornea, Periocular vascular plexus
INTRODUCTION
Corneal avascularity is required to maintain transparency and vision clarity. Avascularity is maintained throughout adult life, but it is first established concomitantly with both the formative morphological events of the embryonic cornea and the vascularization of the periocular region. This process occurs when neural crest cells migrate between the lens and overlying ectoderm to form the corneal endothelium and stroma (Creuzet et al., 2005; Hay, 1980; Lwigale et al., 2005), while migratory angioblasts undergo vasculogenesis in the periocular region. The angioblasts and nascent blood vessels do not enter the cornea, but instead form a dense vascular network in the periocular region (Hiruma, 1996; Kwiatkowski et al., 2013), which distributes oxygen and nutrients to the tissues in the anterior eye including the cornea.
The mechanism by which angioblasts and ocular blood vessels form the dense vascular network in the anterior eye as well as their exclusion from the adjacent embryonic cornea remains unclear. A debated hypothesis speculates that the limbus epithelium functions as a physical barrier against vascularization of the cornea (Ellenberg et al., 2010; Lim et al., 2009; Ma et al., 2004). However, substantial evidence also suggests that the cornea provides a chemical barrier by secreting antiangiogenic factors that restrict vascular invasion, also known as neovascularization. Much of the evidence supporting this hypothesis originates from studying the role of antiangiogenic factors in adult corneas. For example, the mammalian adult corneal epithelium expresses the proangiogenic vascular endothelial growth factor (VEGF), but neovascularization is prevented by the presence of soluble VEGF receptor-1 (sVEGFR-1, also known as sFlt1). Knockdown of sFlt1 increases the bioavailability of VEGF and results in corneal neovascularization (Ambati et al., 2006; Ambati, et al., 2007). Other endogenously expressed antiangiogenic factors that promote corneal avascularity include thrombospondin, pigment epithelium-derived factor (PEDF), Slit2, and Netrin1 (Aiello et al., 1995; Ellenberg et al., 2010; Han and Zhang, 2010; Jin et al., 2010).
The role of antiangiogenic factors during corneal development is supported by our recent study showing that lens-derived Semaphorin3A (Sema3A) inhibits VEGF-induced angioblast migration and vascularization of the cornea by signaling through a common Neuropilin1 (Nrp1) receptor (McKenna et al., 2014). Loss of Sema3A (in chick) or Nrp1/Sema signaling (in mouse) resulted in spontaneous vascularization in the embryonic cornea. Besides Sema3A, other antiangiogenic factors are expressed in the anterior eye including Sema3E, Sema3F (Chilton and Guthrie, 2003), sFlt1, Netrin1, Netrin4 (Kwiatkowski et al., 2013), and CXCL14 (Ojeda et al., 2013), however their putative function during the formation of the periocular vascular network and in establishing corneal avascularity alongside Sema3A have not been demonstrated. Pertinent to this study is the vivid expression of Sema3E in the optic cup and iridocorneal angle, and the concomitant expression of PlexinD1 by angioblasts and the forming periocular vascular network (Kwiatkowski et al., 2013). Interestingly, PlexinD1 expression coincides with Nrp1 during the formation of the periocular vasculature, but it is not clear whether PlexinD1 functions independently or in conjunction with Nrp1 to regulate vascular patterning during ocular development.
PlexinD1 is a receptor for secreted Sema3 ligands. During embryonic development, it is expressed by endothelial cells, retinal blood vessels, and cardiac tissue (Fukushima, 2011; Gay, 2011; Oh and Gu, 2013a; Toyofuku, 2008). Endothelial PlexinD1 signaling inhibits angiogenesis by preventing endothelial migration, upregulating endothelial expression of sVEGFR-1, and antagonizing VEGF-induced relaxation of lateral inhibition (Kim, 2011, Torres-Vazquez et al., 2004; Zygmunt, 2011). This role has been exemplified in several contexts, including in the developing retina and in somites where knockout of PlexinD1 disrupts vascular patterning (Fukushima et al., 2011; Gay et al., 2011; Gu et al., 2005; Kim et al., 2011; Meadows et al., 2013; Oh and Gu, 2013b; Torres-Vázquez et al., 2004). In this study, we aim to identify a new role of PlexinD1 during the formation of the periocular vascular network and maintenance of corneal avascularity during ocular development. We utilized RCAS-mediated overexpression of PlexinD1-shRNA in chick and Tg(tie1:H2B:eYFP) quail embryos (Sato et al., 2010) to knockdown PlexinD1 in the anterior eye. Our results show that in the periocular region PlexinD1 is requisite for proper function of endothelial cells that is required for proper patterning and formation of the iridial and limbal vasculature, and for the maintenance of corneal avascularity during development. Thus, PlexinD1 plays a critical role in the vascular guidance cues that are essential for the formation and patterning of the intricate vasculature of the anterior eye.
METHODS
Embryos
Fertilized White Leghorn chicken eggs were obtained from the Texas A&M University Poultry Center (College Station, TX). Fertilized Tg(tie1:H2B:eYFP) transgenic quail eggs were obtained from Ozark Egg Company (Stover, MO). All experiments involving embryos were approved by the Institutional Animal Care and Use Committee (IACUC) at Rice University.
Immunohistochemistry
Tg(tie1:H2B:eYFP) quail and chick eyes were collected at embryonic day (E)12, fixed overnight at 4°C in 4% PFA, and washed in PBS containing 0.1% Triton. Some eyes were embedded in gelatin and cryosectioned at 12μm. Whole-mount and sectioned eyes were immunostained using standard protocols. Primary antibodies used were: mouse anti-GFP monoclonal antibody (1:2000, IgG1, Covance), rabbit anti-GFP polyclonal primary antibody (1:500, IgG, Invitrogen), rabbit anti-mCherry polyclonal antibody (1:500, IgG, Abcam), and mouse anti-Claudin-5 (1:250, IgG1, Fisher). The secondary antibodies (Alexa 488 sheep anti-mouse, Alexa 488 anti-rabbit antibody, and Alexa 594 sheep anti-rabbit, Invitrogen) were used at a concentration of 1:200.
Section in situ hybridization and immunostaining for GFP
Tg(tie1:H2B:eYFP) quail eggs were incubated at 37°C and embryos were collected at E7, E10, and E12. Eyes were enucleated and fixed overnight at 4°C in modified Carnoy's fixative (60% ethanol, 30% formaldehyde, and 10% glacial acetic acid). Fixed eye balls were dehydrated through ethanol series, embedded in paraffin, and sectioned at 12 μm. Riboprobes for PlexinD1, Sema3C, and Sema3E were generated as previously described (Kwiatkowski et al., 2013). RNA in situ hybridization was performed in sectioned tissue as described (Etchevers et al., 2001). After color reaction, sections were post-fixed in 4% paraformaldehyde (PFA) then immunostained for GFP as described above to fluorescently label the endothelial cells in Tg(tie1:H2B:eYFP) embryos.
Generation of RCAS-shRNA constructs
Using free online tools (Invitrogen, Dharmacon, Genscript), PlexinD1-specific sequences having 21 base pairs, GC content ranging between 30-50%, and beginning with a guanine residue were selected as PlexinD1-shRNA sequences. A scrambled (SCR) sequence (Ferrario et al., 2012) was also selected to serve as a negative control shRNA. The PlexinD1-specific and scrambled sequences were incorporated into complementary pairs of oligomers (Sigma) comprising a stem-loop sequence and restriction endonuclease sites to facilitate future cloning steps (Fig. S1). pSLAX-mCherry-cU6-(PlexinD1-shRNA) and pSLAX-(mCherry)-cU6-(SCR-shRNA) were generated from a pSLAX-GFP-cU6-(Sox9-shRNA) shuttle vector (Deneen et al., 2006), a gift from David Anderson (Caltech), and pFlk1:myr-mCherry vector (Larina et al., 2009), a gift from Mary Dickinson (Baylor College of Medicine). mCherry-cU6-(PlexinD1-shRNA) and mCherry-cU6-(SCR-shRNA) fragments were cloned into the ClaI restriction site of RCASBP(A) or RCASBP(B), referred to herein as RCASA or RCASB, using PCR-based cloning kits (InFusion, Clontech or CloneEZ, Genscript).
Cell culture infection and production of RCAS-shRNA viral stocks
A DF-1 chicken fibroblast cell line (ATCC) was maintained in Dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Invitrogen). DF-1 cells were seeded into 12-well plates (MidSci) and incubated at 37°C and 5% CO2 for two days until ~50% confluent. Cultures were transfected using Lipofectamine (Invitrogen) according to manufacturer's protocol then reincubated until they reached 95% confluence. At least 90% of the cells were infected by RCAS virus, as determined by expression of mCherry. After screening for fluorescence, cell cultures were collected to isolate total RNA and generate cDNA using superscript III reverse transcriptase (Invitrogen). Transcription of PlexinD1 was analyzed by semi-quantitative reverse transcription PCR (RT-PCR) using MyTaq hotstart PCR mastermix (Bioline) and gel electrophoresis. Transcription of GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was used as a loading control.
For production of virus, DF-1 cells transfected with shRNA-expressing RCASA and RCASB vectors were expanded in T75 flasks (MidSci) for up to a week until the cultures reached 80% confluence. Media was replaced with fresh DMEM without FBS. Cultures were reincubated for four days and the medium was harvested, pooled, and filtered through 0.45μm syringe filters (Millipore) to remove cell debris. Medium was ultracentrifuged (Beckman-Coulter Optia L-80 XP) at 21,000 rpm for 1.5 hours at 4°C. Pellets containing the virus particles were resuspended in 500 μl DMEM (approximately 1-3 × 108 infectious units per ml) and stored in 10 μl aliquots at −80°C.
Viral infection of embryos in ovo
Fertilized chick and Tg(tie1:H2B:eYFP) quail eggs were incubated sideways at 37°C for 24 hours to obtain Hamburger-Hamilton stage (HH) 6-8 (Hamburger and Hamilton, 1951). Eggs were windowed as previously described (Spurlin and Lwigale, 2013). Concentrated RCAS virus was injected between the vitelline membrane and the cranial region of the embryos using a pressure-based microinjection system (Picospritzer III, Parker Hannifin). Injected eggs were sealed with packaging tape and reincubated at 37°C. Embryos were collected between E7 and E12 and screened for robust mCherry expression in the anterior ocular region before further analysis.
Imaging
Brightfield images of whole-mount eyes were captured using a Zeiss Axiocam mounted onto a dissecting microscope (Stemi 2000-CS, Zeiss). Fluorescent and differential interference contrast (DIC) images of whole-mount and sectioned tissue were captured using a Zeiss Axiocam mounted on an AxioImager2 fluorescent microscope (Zeiss).
Quantification of vascular patterning defects
The area of corneal vascularization was quantified in E12 Tg(tie1:H2B:eYFP) PlexinD1 knockdown eyes and controls using ImageJ. Blood vessels of the iris, including the arterial vessels in the temporal region of the eye, were not included. Statistical analysis was performed using the unpaired two-tailed student's t-test.
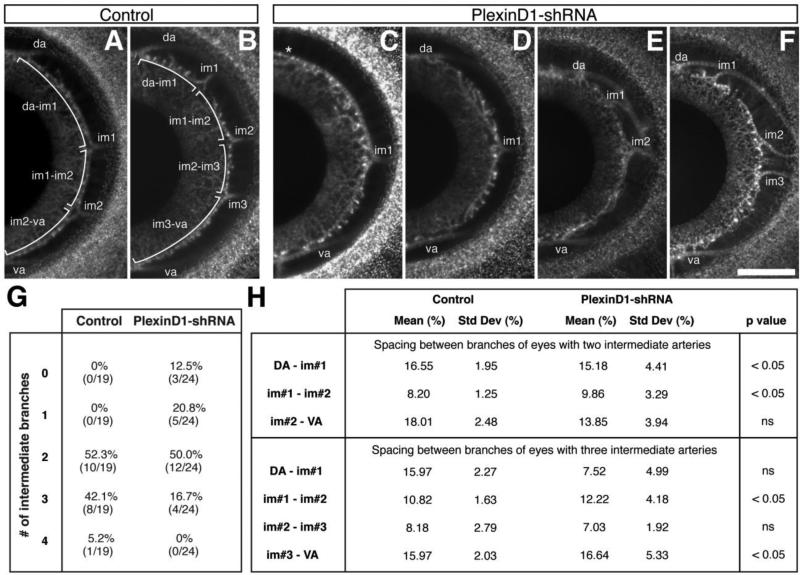
Low magnification (2.5x) images showing the contact points of the dorsal, ventral, and intermediate ciliary vessels with the iridial ring artery (Figure 5A-B, asterisks) were generated using anterior eyes collected from E12 Tg(tie1:H2B:eYFP) quail expressing PlexinD1-shRNA, SCR-shRNA, or uninfected controls. Images of eyes that retained two or three intermediate vessels were used to quantify patterning defects relative to the arterial vessels in the temporal region of each eye. The arc length between each marked location (brackets) was measured relative to the total circumference of the iridial ring artery using ImageJ. Statistical analysis was conducted using the two-tailed F-test.
Figure 5. Variation of the branching pattern of the intermediate branches from the temporal ciliary artery caused by PlexinD1 knockdown.
(A and B) Flat-mounts of anterior eyes from control embryos showing the number and spacing between the intermediate branches (brackets) that feed into the iridial vasculature. (C – F) Flat-mount of anterior eyes from PlexinD1 knockdown embryos showing variation in the number of intermediate arteries and the distances between them. Asterisk in (C) indicates missing dorsal intermediate artery. (G) Quantification of the intermediate branches. (H) Quantification of the separation between the intermediate branches. Spacing between branches was calculated as described in the methods section. Abbreviations: da, dorsal intermediate artery; va, ventral intermediate artery; im1, intermediate branch 1; im2, intermediate branch 2; im3, intermediate branch 3. Scale bar = 500 μm.
RESULTS
Validation of shRNA-mediated knockdown of PlexinD1
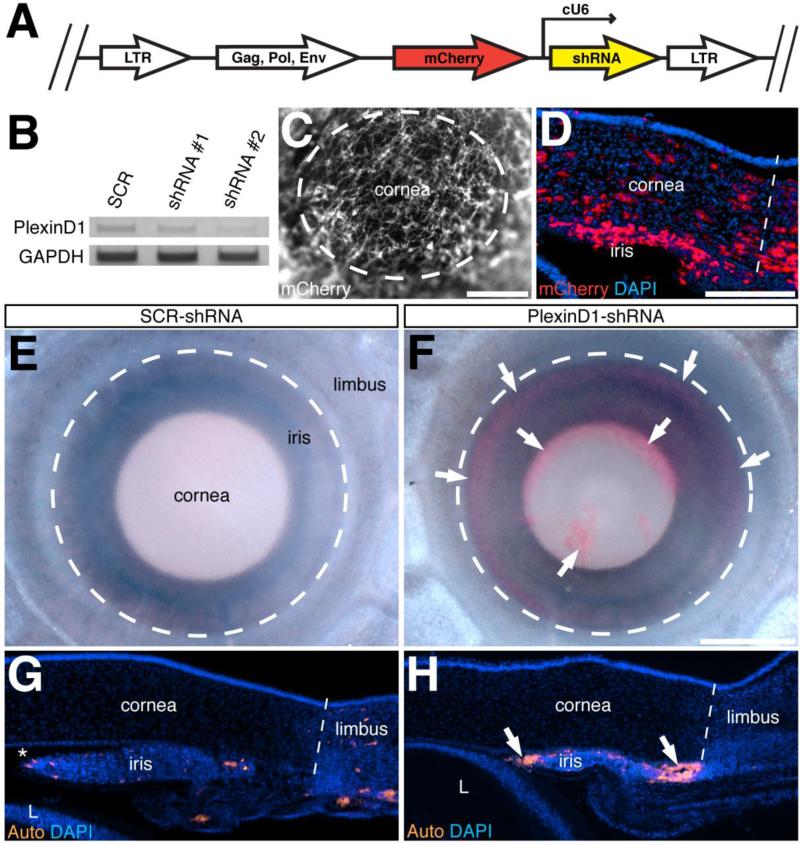
Previously, we showed that PlexinD1 and other angiogenic receptors are expressed by angioblasts and forming vasculature in the anterior eye (Kwiatkowski et al., 2013). To determine the function of PlexinD1 during anterior eye development in avian embryos, we generated RCASBP (replication competent avian sarcoma-leukosis virus with a spice acceptor and encoding Bryan-strain polymerase) vectors (Hughes, 2004; Hughes et al., 1987) to promote stable transfection of custom-designed PlexinD1-specific small-hairpin RNA (shRNA). The constructs comprised of an mCherry reporter sequence and a chicken U6 promoter driving PlexinD1 or scrambled (SCR) shRNA were cloned into RCASBP(A or B) (Fig. 1A). To test the knockdown efficiency of the PlexinD1-shRNA, we took advantage of the endogenous expression of PlexinD1 by DF-1 cells. Semi-quantitative RT-PCR analysis revealed no effect on PlexinD1 expression in DF-1 cells transfected with SCR-shRNA. However, there was a considerable reduction of PlexinD1 expression in cells transfected with PlexinD1-shRNA#1 and PlexinD1-shRNA#2 (Fig. 1B). The level of GAPDH was unaffected demonstrating specificity of the shRNA-mediated knockdown of PlexinD1. Given the high degree of PlexinD1 knockdown by PlexinD1-shRNA#2, subsequent experiments use this virus, here after designated as PlexinD1-shRNA. Only embryos showing vivid expression of mCherry in the anterior eye (Fig. 1C and 1D) were used for this study.
Figure 1. Generation and validation of RCAS-shRNA constructs.
(A) Schematic representation of the RCASBP vector encoding mCherry reporter and either PlexinD1-specific or scrambled-shRNA. (B) shRNA-mediated knockdown of PlexinD1 was tested in vitro using DF-1 cells. Endogenous expression of PlexinD1 in DF-1 cells was not affected by the scrambled-shRNA, but PlexinD1-knockdown was strongest in cells expressing PlexinD1-shRNA #2. (C - D) Whole-mount (C) and cross-section (D) of the anterior eye of an E7 embryo showing robust expression of mCherry indicating targeting of shRNA to the cornea and limbus region. (E - F) Bright field images of E12 eyes from embryos injected with either scrambled-shRNA (E) or PlexinD1-shRNA (F). Only the embryos injected with PlexinD1-shRNA showed hemorrhaging in the anterior eye (F; arrows). (G - H) Cross-sections through the anterior eyes counterstained with DAPI and analyzed for autofluorescence of blood cells showing location of blood cells in the vasculature of embryos injected with scrambled-shRNA (G), and hemorrhage in the anterior chamber of embryos injected with PlexinD1-shRNA (H; arrows). Dotted lines (G – H) represent the boundary between the cornea and limbus. Asterisk in (G) indicates the anterior chamber. Abbreviation: L, lens. Scale bars: 400 μm (C); 200 μm (D, G, H); 250 μm (E, F).
PlexinD1 knockdown causes hemorrhaging in the anterior eye during development
Formation of the vascular network in the anterior eye begins with the arrival of angioblasts in the periocular region by E3, which undergo vasculogenesis to form a primitive vascular network. Angiogenesis and vascular remodeling events then adapt the network as directed by developing ocular tissues (Hughes, 1934; Hiruma, 1996; Kwiatkowski et al., 2013). To determine the function of PlexinD1 during this process, we initially evaluated embryos expressing PlexinD1-shRNA for obvious vascular defects. Gross analysis of the anterior eye of PlexinD1 knockdown embryos did not reveal obvious vascular defects until E7 when minor hemorrhage was first observed (data not shown). However, substantial hemorrhage into the anterior chamber was observed in E12 chick and quail embryos following PlexinD1 knockdown in the anterior chamber (Fig. 1F and 1H, arrows; n = 15/29). In contrast, none of the control embryos expressing SCR-shRNA showed hemorrhaging in the anterior chamber (Fig. 1E and 1G; n = 25). These results suggest that PlexinD1 plays an important role during the formation of the vascular network in the anterior eye.
PlexinD1 is expressed in the periocular vasculature of the iris and limbus
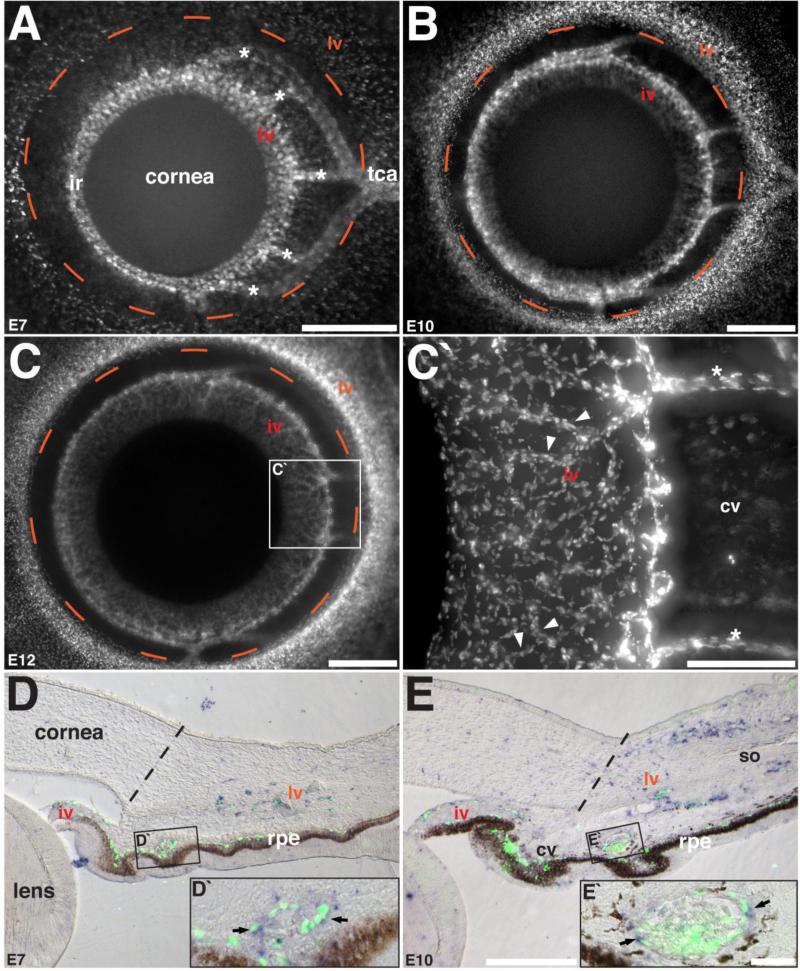
Given that the vascular phenotype in PlexinD1 knockdown eyes occurred at a much later stage of ocular development (E12) than previously examined (Kwiatkowski et al., 2013), we further characterized the vasculogenesis process and the expression of PlexinD1 at later stages of development to identify the defects that caused the hemorrhaging. At E7, the iridial ring artery is prominent in the nasal region of the eye, while the formation of the iridial vasculature commences in the temporal half (Fig. 2A). Also at this time the endothelial cells coalesce to form the intermediate branches that connect the iridial ring and temporal ciliary arteries. In the images of the anterior eye shown (Fig. 2A-2C’), the avascular cornea overlays the developing iris and its boundaries approximate the dotted circle that delineates the nascent limbal vasculature. By E10, the iridial vasculature continues to form in a radial pattern originating from the iridial ring artery (Fig. 2B). At E12, the iridial vasculature is comprised of several radial vessels that bifurcate into smaller vessels to form a dense micro-vascular network, which covers most of the area of the iris (Fig. 2C and 2C’). By this time the vascular network in the limbus region is dense but the adjacent cornea remains avascular.
Figure 2. Patterning of the iridial and limbal vasculature during avian ocular development and the expression of PlexinD1.
(A – C) Whole-mount anterior eyes of Tg(tie1:H2B:eYFP) quail embryos showing vascular patterning at E7, E10, and E12. (C’) High magnification of selected region in C showing the microvessels of the iridial network (arrowheads). (D – E) Section in situ hybridization and immunostaining showing co-localization of PlexinD1 with GFP in the forming ocular vasculature (arrows). Dashed orange line represents the boundary between the limbal vasculature and the corneal periphery and the dashed black lines represent the corneal/limbus boundary. Asterisks indicate the intermediate branches from the temporal ciliary artery. Abbreviations: iv, vascular network of the iris; lv, vascular network of the limbus; cv, vascular network of the ciliary body; tca, temporal ciliary artery; rpe, retinal pigment epithelium; so, scleral ossicle. Scale bars = 500 μm (A – C), 200 μm (C‘), 200 μm (D, E), 25 μm (D‘, E‘).
Section in situ hybridization of eyes analyzed at E7 and E10 showed that robust expression of PlexinD1 is maintained during the formation of the vascular network of the iris and limbus (Fig. 2D and 2E). We confirmed that PlexinD1 is expressed in the vascular networks of the anterior eye by immunostaining the sections with anti-GFP antibody to label Tg(tie1:H2B:eYFP) endothelial cells and vessels, which co-localized (Fig. 2D, 2D’ and 2E, 2E’). In addition, the histology shows that the iridial and ciliary networks are continuous with the choroidal blood vessels located above the retinal pigment epithelium. The limbal network is separate from the iridial and ciliary networks. At E10, the limbal network is invaginated by the condensing mesenchyme of the avascular scleral ossicle (Jourdeuil and Franz-Odendaal, 2012), resulting in the formation of a “C”-shaped network by E10 (Fig. 2E).
Knockdown of PlexinD1 induces ectopic vascularization of the cornea
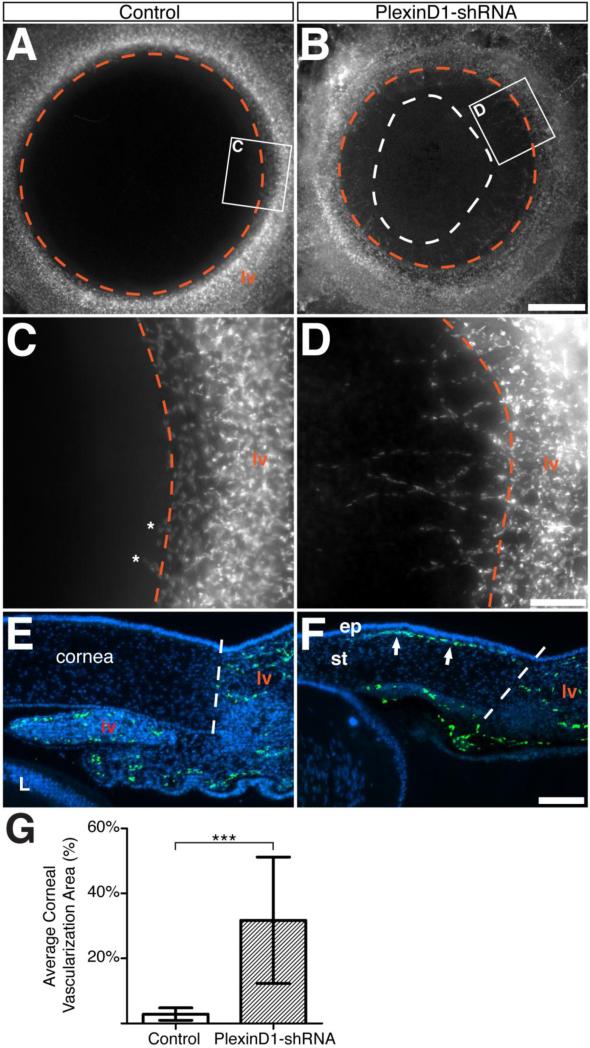
Since PlexinD1 is expressed by the periocular angioblasts and forming vasculature, (Fig. 2D and 2E; Kwiatkowski et al., 2013), we sought to determine whether it is involved in the inhibition of corneal vasculogenesis during development. Although PlexinD1 is expressed in the forming periocular vasculature early during corneal development (Kwiatkowski et al., 2013; Fig. S2), we did not observe ectopic vascularization of the cornea between E3-E7 despite robust expression of PlexinD1-shRNA in the anterior eye (data not shown), indicating that the vasculogenesis process in the anterior eye was unaffected during this period of ocular development. However, several PlexinD1 knockdown eyes examined at advanced stages of development (E10-E12) showed ingression of endothelial cells from the limbal vasculature into several regions of the corneal perimeter (Fig. 3B, 3D, and 3F arrows; n = 8/24). In contrast, very few endothelial cells were localized at the corneal margin of uninjected and SCR-shRNA injected embryos (Fig. 3A and 3C, n = 19). Cross-sections through the corneas of PlexinD1 knockdown embryos revealed ectopic GFP-positive endothelial cell nuclei in the anterior region, subjacent to the corneal epithelium (Fig. 3F, arrows), which were absent in control corneas (Fig. 3E). Comparison of the area covered by GFP-positive endothelial nuclei in corneas revealed significant vascularization of PlexinD1 knockdown corneas (Fig. 3G; P = 0.0009). Given that ectopic endothelial cells were only observed in the cornea after the formation of the limbal vasculature, our results suggest that knockdown of PlexinD1 disrupts normal endothelial cell function, which leads to abnormal sprouting and ectopic vascularization of the developing cornea.
Figure 3. Neovascularization of the cornea caused by knockdown of PlexinD1.
(A – D) Whole-mount images of flat-mounted E12 anterior eyes after removal of the iris to show the extent of corneal vascularization. (A and C) Cornea of control embryo injected with scrambled-shRNA show the characteristic avascularity with only a few endothelial cells in the periphery (C, asterisks). (B and D) Ectopic ingression of endothelial cells into the cornea of an embryo injected with PlexinD1-shRNA. The white dotted line in (B) indicates the extent of corneal vascularization. (E – F) Cross-sections of E12 anterior eyes showing absence of endothelial cells in a control cornea (E), and ingression of endothelial cells into the anterior stroma (F, arrowheads). The dashed lines represent the corneal/limbus boundary. (G) Quantification of the area covered by endothelial cells between the limbal vascular boundary and cornea (P = 0.0009). Abbreviations: lv, limbal vasculature; iv, iridial vasculature; L, lens; ep, corneal epithelium; st, corneal stroma. Scale bars: 500 μm (A, B), 100 μm (C - F).
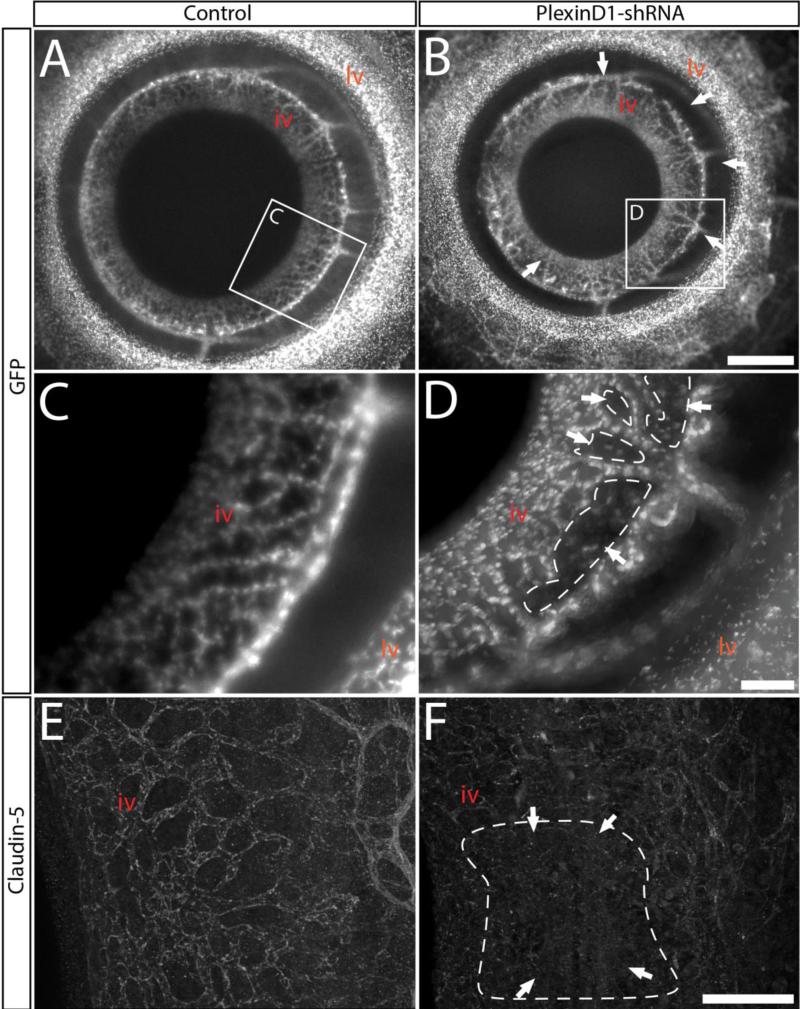
Disruption of the Iridial vasculature due to PlexinD1 knockdown is associated with hemorrhaging into the anterior chamber
Hemorrhage into the anterior chamber observed in the eyes of PlexinD1 knockdown embryos (Fig. 1F and 1H) suggest that a defect in adjacent vasculature may cause the leakage of blood into this region. Because the iridial vascular network is located on the surface of the iris, which extends into the anterior chamber (Fig. 2D and 2E), we examined PlexinD1 knockdown eyes for defects in the formation of these vessels. The vascular defects associated with PlexinD1 knockdown was reflected in the large gaps observed in the radial iridial microvessels (Fig. 4B and 4D; Fig. S3; n = 13/24). These gaps were absent in the iridial vascular network of control eyes (Fig. 4A and 4C; n = 19). Further analysis revealed that the presence of the gaps in the iridial radial vessels corresponded with the hemorrhaging in the anterior chamber (n = 9/13). To determine whether the hemorrhage in PlexinD1 knockdown eyes is associated with defects in vascular permeability, we immunostained E12 anterior eyes for Claudin-5, a major constituent of endothelial cell tight junctions (Tsukita et al., 2002; Ohtsuki et al., 2007). In control eyes Claudin-5 staining matched the microvessels of the iridial vasculature (Fig. 4E). In contrast, PlexinD1 knockdown eyes showed patchy staining for Claudin-5 (Fig. 4F; n = 4/6). Together with the gaps seen in PlexinD1 knockdown eyes, our observations suggest a disruption of vascular tight junctions, which may facilitate leakage of the iridial microvessels and cause hemorrhaging in the anterior chamber.
Figure 4. Knockdown of PlexinD1 causes defects in patterning of the microvessels of the iridial network.
(A - D) Whole-mount images of flat-mounted E12 anterior eyes showing: (A and C) Organized radial vascularization of the iris in control eyes. (B and D) Gaps in the iridial vasculature (arrows) following PlexinD1 knockdown. (E and F) Iridial microvessels immunostained for Claudin-5. Note the consistent staining in control that labels the network (E), and the patchy staining in PlexinD1 knockdown eye (F, arrows). Abbreviations: lv, limbal vasculature; iv, iridial vasculature. Scale bars: 500 μm (A, B), 200 μm (C - D), 100 μm (E-F).
Knockdown of PlexinD1 disrupts the branching pattern of intermediate vessels from the temporal ciliary artery
To determine whether knockdown of PlexinD1 affected the patterning of large vessels in the anterior eye, we examined the temporal ciliary artery and intermediate vessels that feed into the iridial network (Fig. 2A-2F). Analysis of whole-mount control eyes at E12 (n = 19) revealed regular patterning of the intermediate branches from the temporal ciliary artery, which formed dorsal and ventral branches, and either two or three intermediate branches (Fig. 5A and 5B) at approximately the same frequency (Fig. 5G). The PlexinD1 knockdown eyes (n = 24) showed variations in the patterning of the intermediate vessels (Fig. 5C-5F). In some instances, either the dorsal or ventral branch was absent (n = 3/24; Fig. 5C). In cases where the PlexinD1 knockdown eyes showed proper formation of the dorsal and ventral branches, the number of intermediate branches varied between one and three (Fig. 5D-5F and Fig. 5G). In addition, there was considerable variation in the spacing between the intermediate vessels (compare Fig. 5A with Fig. 5E and Fig. 5B with Fig. 5F). For PlexinD1 knockdown eyes with two intermediate branches, significant differences in spacing were observed between the dorsal and first intermediate arteries, and between the first and second intermediate arteries (Fig. 5H; P < 0.05). For eyes with three intermediate arteries, significant spacing was observed between first and second branches and also between the third and ventral branches (Fig. 5H; P < 0.05). Altogether, these results suggest that PlexinD1 plays a role during the branching and formation of the intermediate vessels from the temporal ciliary artery.
Expression of PlexinD1 ligands in the anterior eye during development
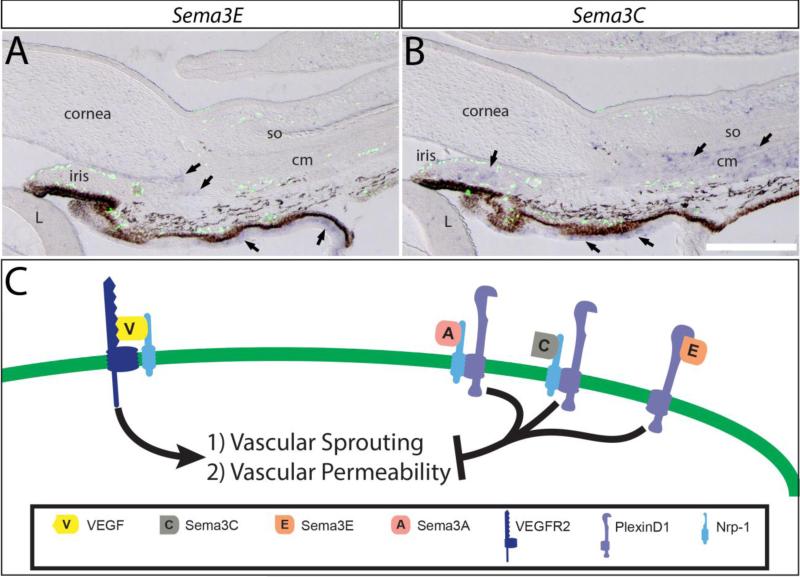
Endothelial cell guidance associated with PlexinD1 has been shown to act in response to either direct binding of Sema3E (Gu et al., 2005; Kim et al., 2011), or indirect transduction of Sema3C signaling via interaction with Nrp1 co-receptors (Gitler et al., 2004; Torres-Vázquez et al., 2004; Banu et al., 2006; Salikhova et al., 2008). To identify the putative extracellular signals that guide vascular patterning in the anterior eye via PlexinD1, we examined the expression patterns of Sema3E and Sema3C. We performed section in situ hybridization on E12 Tg(tie1:H2B:eYFP) quail eyes followed by immunostaining for GFP to identify the relative location of endothelial cells and regions of Sema3E and Sema3C expression. As previously shown in chick (Kwiatkowski et al., 2013), Sema3E was expressed in the optic cup region of the presumptive iris and ciliary body, and in the iridocorneal angle (Fig. 6A, arrows). Both regions of Sema3E expression are devoid of GFP-positive endothelial cells. Analysis of Sema3C revealed an overlap with Sema3E expression in the optic cup region of the presumptive iris and ciliary body, as well as vivid expression in the stroma of the iris and ciliary muscle (Fig. 6B and Fig. S5). Similarly, the GFP-positive endothelial cells avoid the stroma of the iris and the ciliary muscle where Sema3C is expressed. Since the GFP-positive endothelial cells express PlexinD1 (Fig. 3D and 2E) and they are located in complementary regions to the expression of Sema3E and Sema3C, it is possible that the interaction between these two secreted ligands and endothelial cells is essential for the proper patterning of the periocular vasculature.
Figure 6. The limbal and iridial vasculature avoid ocular regions with strong expression of Sema3E and Sema3C.
Section in situ hybridization of anterior eyes from E12 Tg(tie1:H2B:eYFP) quail embryos showing expression of Sema3E, Sema3C, and GFP-positive endothelial cells. (A) Expression of Sema3E is strong in the optic cup region of the iris and ciliary body, and in the iridocorneal angle (arrows). (B) Sema3C expression is strong in the stroma of the iris, ciliary muscle, and in the optic cup region of the iris and ciliary body (arrows). (C) Schematic of the endothelial cell surface summarizing the involvement of PlexinD1/Nrp1 receptors and cues from Sema3 ligands in inhibition of VEGF signaling. The PlexinD1/Nrp1/Sema3 signaling promotes vascular stability by maintaining tight junctions between endothelial cells and preventing vascular sprouting. Absence of PlexinD1 decreases Sema3 signaling and permits endothelial response to VEGF signaling, which causes aberrant vascularization and hemorrhage. Abbreviations: L, lens; so, scleral ossicle; cm, ciliary muscle; Scale bar = 200 μm.
DISCUSSION
Although the function of PlexinD1 has been well established in the patterning and development of the intersomitic, cardiac, and retinal vasculature, very little is known about its function in the formation of the intricate vasculature of the anterior eye. In this study we demonstrated that endothelial cell expression of PlexinD1 is maintained throughout the formation of the vascular network in the anterior eye. Knockdown experiments using RCAS-mediated PlexinD1-shRNA indicate that PlexinD1 is required for maintaining corneal avascularity, and for the proper formation of the iridial and limbal vascular networks. Furthermore, we show that Sema3E and Sema3C are expressed in ocular regions that are avoided by the forming vasculature, suggesting that signaling of Sema3 ligands through PlexinD1 plays a critical role during the formation and patterning of the vascular network of the anterior eye.
Previously, we showed that the vascular network in the anterior eye of avian embryos forms via vasculogenesis (Kwiatkowski et al., 2013). This process begins as early as E2.5 with the migration of angioblasts into the periocular mesenchyme, and their subsequent clustering into vascular tubes that define the dense periocular network that never invades the developing cornea. Based on mRNA expression profiles, we inferred from this study that the presence of both pro- and anti-angiogenic factors, combined with the expression of their receptors by angioblasts and nascent vasculature, was responsible for the intricate patterning of the periocular network. Among the receptors that are expressed by the forming vascular network in the anterior eye is PlexinD1. Here we extended our analysis of PlexinD1 until advanced stages of eye development, and show that its expression is maintained in the periocular vasculature during the angiogenic processes that partition the iridial and limbal vascular networks. Therefore, it is plausible that signaling through PlexinD1 is involved in the guidance events that are responsible for the patterning of the iridial and limbal vasculature, and their stabilization.
PlexinD1 knockout mice exhibit patterning defects in the intersomitic (Gu et al., 2005) and retinal vasculature (Fukushima et al., 2011; Kim et al., 2011), and they die prenatally due to cardiovascular defects (Gitler et al., 2004; Zhang et al., 2009). In zebrafish embryos, PlexinD1 plays a role in the formation of the trunk segmental arteries and it is responsible for the defects in intersegmental vessels observed in out of bounds (obd) mutants (Torres-Vazquez et al., 2004, Childs et al., 2002; Lamont et al., 2009). In the present study, we showed for the first time that PlexinD1 is required during the formation of the vascular network in the anterior eye. We show that RCAS-shRNA mediated knockdown of PlexinD1 in avian embryos caused hemorrhage into the anterior chamber of the eye. In adults, breakdown of the endothelial barrier due to trauma or pathological neovascularization of the iris causes similar hemorrhage (hyphema) into the anterior chamber (Walton et al., 2002). Hemorrhage was also observed in the ventricular walls of mouse mutants lacking endothelial-specific expression of plexinD1 (Zhang et al., 2009). Our results show gaps in the iridial network following PlexinD1 knockdown, which may be an indication of abnormal vascular growth due to loss of endothelial tight junctions. This is consistent with the patchy staining of Claudin-5 in the iridial microvessels, which comprise the blood-aqueous-barrier (Cunha-Vaz, 1979; Freddo, 2013). Loss of Claudin-5 indicates disruption of the endothelial barrier of the iridial microvessels and accounts for the hemorrhage observed in the anterior chamber. VEGF has been shown to disrupt Claudin-5 expression and cause breakdown of the endothelial microvessels associated with the blood-brain barrier (Argaw et al., 2008). It is likely that in the absence of PlexinD1, the inhibitory Sema3 signals are substantially reduced, which permits increased VEGF signaling in the iridial microvessels.
We showed that expression of PlexinD1 is maintained in the limbal vascular network during its formation adjacent to the avascular cornea, and that knockdown of PlexinD1 caused significant ectopic vascularization of the corneal periphery. Interestingly, the neovascularization defects were only detected at advanced stages of corneal development despite the early expression of PlexinD1 by angioblasts in the periocular region (Kwiatkowski et al., 2013). It is likely that there is a minimal requirement for PlexinD1 during early guidance of angioblasts associated with initial vasculogenesis events of the periocular network. Therefore its absence does not cause ectopic migration of angioblasts during the formation of the corneal endothelium and stroma. Recently, we showed that the vasculogenesis process is under the guidance of Nrp1, which is also expressed by angioblasts during the formation of the periocular vasculature. Thus the relatively higher levels of Sema3A compared to VEGF in the cornea and adjacent lens prevent ectopic angioblast migration and vascularization of the cornea during the early phase of development (McKenna et al., 2014). The abundance of Sema3A may be sufficient to inhibit VEGF-induced vasculogenesis through competitive inhibition for Nrp1, thus functioning despite knockdown of Plexin receptors (Bagnard et al., 2001; Miao et al., 1999; Soker et al., 1998). Another interesting observation of this study was the specific projection of the ectopic vasculature towards the corneal epithelium (Fig. 3F). At this phase of corneal development and in adult corneas, the corneal epithelium expresses both VEGF and Sema3A (Morishige et al., 2010; Philipp et al., 2000; McKenna et al., 2014). Therefore absence of PlexinD1 may disrupt endothelial cell function causing their sprouting and projection towards the VEGF signals from the corneal epithelium via VEGFR2, and due to reduced Nrp1 response to Sema3A (Gitler et al., 2004; Acevedo et al., 2008; Zhang et al., 2009). PlexinD1 function in the anterior eye is different from what was previously reported in the mouse retina, where PlexinD1 expression was only expressed in the sprouting vessels (Kim et al., 2011). Since PlexinD1 is maintained in the anterior eye vasculature, the Sema3 signals that guide its formation may appear as gradients due to changes caused by the morphogenesis of the anterior chamber.
The microvessels of the iridial vascular network anastomose with the iridial ring artery and together with the intermediate arteries, are fed from the temporal ciliary artery (Fig.2; Hiruma, 1996). The branching pattern of the intermediate arteries from the temporal ciliary artery is consistent in control embryos, where the dorsal and ventral branches form the boundaries, and either two or three other branches form between them. However, in PlexinD1 knockdown eyes, the branching pattern of intermediate vessels from the temporal ciliary artery is random or absent in some cases. These defects may reflect inconsistent formation of the intermediate branches in the absence of PlexinD1, which inappropriately follow the existing guidance cues to the ectopic locations in the anterior eye. An analogous defect was observed in PlexinD1 knockout mice, which exhibit abnormal patterning of the coronary artery (Gitler et al., 2004; Gay et al., 2011).
In the developing avian eye, PlexinD1 expression is specific to the forming vascular network (Fig. 2D and 2E; Kwiatkowski et al., 2013). Since vascular defects are observed in the absence of direct PlexinD1 signaling mediated by Sema3E (Gu et al., 2005; Kim et al., 2011), and Sema3C (Gitler et al., 2004; Torres-Vázquez et al., 2004; Banu et al., 2006), we examined the expression of these two potential ligands during ocular development. Our results show that Sema3E is expressed in the mesenchyme of the iridocorneal angle, which is avoided by the ocular blood vessels. This indicates that Sema3E signaling from the iridocorneal angle separates the limbal vasculature from the iridial and choroidal networks. Surprisingly, we did not observe coalescence of these two networks following PlexinD1 knockdown, which implies that other anti-angiogenic signals or receptors may be responsible for separating these networks. We also show that Sema3C is expressed in the ciliary muscle, stroma of the iris, and it overlaps with Sema3E in the optic cup region of the presumptive iris. Sema3C interacts with PlexinD1 in the presence of Nrp1 (Gitler et al., 2004), and Sema3C knockout mice have cardiac defects that are similar to PlexinD1 mutants (Feiner et al., 2001). Interestingly, Sema3C/PlexinD1 signaling elicits a proangiogenic response from endothelial cells (Banu 2006; Joza et al., 2013; Miyato et al., 2012) or antiangiogenic response in retinal microvessels (Yang et al., 2015). Therefore, further studies might be necessary to identify the exact function of Sema3C during ocular vasculogenesis.
The vascular defects observed in the limbal and iridial networks during advanced eye development when PlexinD1 signaling is perturbed indicate that it is involved in signal transductions that refine the network of blood vessels in the limbus and iris. VEGF signaling in the anterior eye is critical for angioblast migration and establishment of the limbal vasculature by signaling through the VEGFR2 and Nrp1 receptors that are expressed by endothelial cells (Kwiatkowski et al., 2013). We propose that PlexinD1 functions both independently and conjunction with Nrp1 to elevate endothelial response to Sema3 ligands and attenuate VEGF signaling (Fig. 6C). The absence of PlexinD1 lowers Sema3 and favors VEGF signaling in endothelial cells, which leads to vascular sprouting in the limbus and loss of tight junctions in the iridial microvessels. Thus, PlexinD1 and Nrp1 receptors function interdependently to convey Sema3 signals required to inhibit VEGF signaling, a critical step for the establishment of the intricate vascular network of the anterior eye and maintenance of corneal avascularity during development.
Supplementary Material
Highlights.
PlexinD1 is expressed in the periocular vascular network during eye development.
Knockdown of PlexinD1 permits ectopic vascularization of the cornea.
Knockdown of PlexinD1 disrupts vascular patterning in the iris and causes hemorrhaging into the anterior chamber.
Vascular defects following PlexinD1 knockdown in the anterior eye suggest its role in ocular vasculogenesis in response to Sema3 signaling.
ACKNOWLEDGMENTS
We are grateful to Drs. Benjamine Deneen, Mary Dickenson, and Stephen Hughes for the expression plasmids and RCAS vectors. We thank Ruda Cui and Dr. Sinu Jasrapuria-Agrawal for reading the manuscript. This work was supported by NIH grant EY022158 to P.Y.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Acevedo LM, Barillas S, Weis SM, Göthert JR, Cheresh DA. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood. 2008;111:2674–2680. doi: 10.1182/blood-2007-08-110205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl. Acad. Sci. U. S. A. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJC, Richter E, Sakurai E, Newcomb MT, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambati BK, Patterson E, Jani P, Jenkins C, Higgins E, Singh N, Suthar T, Vira N, Smith K, Caldwell R. Soluble vascular endothelial growth factor receptor-1 contributes to the corneal antiangiogenic barrier. Br. J. Ophthalmol. 2007;91:505–508. doi: 10.1136/bjo.2006.107417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci USA. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagnard D, Vaillant C, Khuth S-T, Dufay N, Lohrum M, Puschel AW, Belin M-F, Bolz J, Thomasset N. Semaphorin3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J. Neurosci. 2001;21:3332–3341. doi: 10.1523/JNEUROSCI.21-10-03332.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banu N, Teichman J, Dunlap-Brown M, Villegas G, Tufro A. Semaphorin 3C regulates endothelial cell function by increasing integrin activity. FASEB J. 2006;20:2150–2152. doi: 10.1096/fj.05-5698fje. [DOI] [PubMed] [Google Scholar]
- 8.Childs S, Chen J-N, Garrity DM, Fishman MC. Patterning of angiogenesis in the zebrafish embryo. Dev. Camb. Engl. 2002;129:973–982. doi: 10.1242/dev.129.4.973. [DOI] [PubMed] [Google Scholar]
- 9.Chilton JK, Guthrie S. Cranial expression of class 3 secreted semaphorins and their neuropilin receptors. Dev. Dyn. 2003;228:726–733. doi: 10.1002/dvdy.10396. [DOI] [PubMed] [Google Scholar]
- 10.Creuzet S, Vincent C, Couly G. Neural crest derivatives in ocular and periocular structures. Int. J. Dev. Biol. 2005;49:161–171. doi: 10.1387/ijdb.041937sc. [DOI] [PubMed] [Google Scholar]
- 11.Cunha-Vaz J. The blood-ocular barriers. Surv. Ophthalmol. 1979;23:279–296. doi: 10.1016/0039-6257(79)90158-9. [DOI] [PubMed] [Google Scholar]
- 12.Deneen B, Ho R, Lukaszewicz A, Hochstim CJ, Gronostajski RM, Anderson DJ. The Transcription Factor NFIA Controls the Onset of Gliogenesis in the Developing Spinal Cord. Neuron. 2006;52:953–968. doi: 10.1016/j.neuron.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Ellenberg D, Azar DT, Hallak JA, Tobaigy F, Han KY, Jain S, Zhou Z, Chang J-H. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog. Retin. Eye Res. 2010;29:208–248. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etchevers HC, Vincent C, Douarin NML, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- 15.Feiner L, Webber AL, Brown CB, Lu MM, Jia L, Feinstein P, Mombaerts P, Epstein JA, Raper JA. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development. 2001;128:3061–3070. doi: 10.1242/dev.128.16.3061. [DOI] [PubMed] [Google Scholar]
- 16.Ferrario JE, Baskaran P, Clark C, Hendry A, Lerner O, Hintze M, Allen J, Chilton JK, Guthrie S. Axon guidance in the developing ocular motor system and Duane retraction syndrome depends on Semaphorin signaling via alpha2-chimaerin. Proc. Natl. Acad. Sci. U. S. A. 2012;109:14669–14674. doi: 10.1073/pnas.1116481109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freddo TF. A contemporary concept of the blood–aqueous barrier. Prog. Retin. Eye Res. 2013;32:181–195. doi: 10.1016/j.preteyeres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S-I, Uemura A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J. Clin. Invest. 2011;121:1974–1985. doi: 10.1172/JCI44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gay CM, Zygmunt T, Torres-Vázquez J. Diverse functions for the semaphorin receptor PlexinD1 in development and disease. Dev. Biol. 2011;349:1–19. doi: 10.1016/j.ydbio.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gitler AD, Lu MM, Epstein JA. PlexinD1 and Semaphorin Signaling Are Required in Endothelial Cells for Cardiovascular Development. Dev. Cell. 2004;7:107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and Plexin-D1 Control Vascular Pattern Independently of Neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 22.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 23.Han X, Zhang M-C. Potential anti-angiogenic role of Slit2 in corneal neovascularization. Exp. Eye Res. 2010;90:742–749. doi: 10.1016/j.exer.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Hay ED. Development of the vertebrate cornea. Int. Rev. Cytol. 1980;63:263–322. doi: 10.1016/s0074-7696(08)61760-x. [DOI] [PubMed] [Google Scholar]
- 25.Hiruma T. Formation of the ocular arteries in the chick embryo: observations of corrosion casts by scanning electron microscopy. Anat. Embryol. (Berl.) 1996;193:585–592. doi: 10.1007/BF00187930. [DOI] [PubMed] [Google Scholar]
- 26.Hughes AFW. On the Development of the Blood Vessels in the Head of the Chick. Philos. Trans. R. Soc. Lond. 1934;224:75–130. [Google Scholar]
- 27.Hughes SH. The RCAS vector system. Folia Biol. (Praha) 2004;50:107–119. [PubMed] [Google Scholar]
- 28.Hughes SH, Greenhouse JJ, Petropoulos CJ, Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J. Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin J, Ma J-X, Guan M, Yao K. Inhibition of chemical cautery-induced corneal neovascularization by topical pigment epithelium-derived factor eyedrops. Cornea. 2010;29:1055–1061. doi: 10.1097/ICO.0b013e3181cc7987. [DOI] [PubMed] [Google Scholar]
- 30.Jourdeuil K, Franz-Odendaal TA. Vasculogenesis and the Induction of Skeletogenic Condensations in the Avian Eye. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2012;295:691–698. doi: 10.1002/ar.22429. [DOI] [PubMed] [Google Scholar]
- 31.Joza S, Wang J, Tseu I, Ackerley C, Post M. Fetal, but Not Postnatal, Deletion of Semaphorin-Neuropilin-1 Signaling Affects Murine Alveolar Development. Am. J. Respir. Cell Mol. Biol. 2013;49:627–636. doi: 10.1165/rcmb.2012-0407OC. [DOI] [PubMed] [Google Scholar]
- 32.Kim J, Oh W-J, Gaiano N, Yoshida Y, Gu C. Semaphorin 3E–Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev. 2011;25:1399–1411. doi: 10.1101/gad.2042011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwiatkowski S, Munjaal RP, Lee T, Lwigale PY. Expression of pro- and anti-angiogenic factors during the formation of the periocular vasculature and development of the avian cornea. Dev. Dyn. 2013;242:738–751. doi: 10.1002/dvdy.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamont RE, Lamont EJ, Childs SJ. Antagonistic interactions among Plexins regulate the timing of intersegmental vessel formation. Dev. Biol. 2009;331:199–209. doi: 10.1016/j.ydbio.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 35.Larina IV, Shen W, Kelly OG, Hadjantonakis A-K, Baron MH, Dickinson ME. A Membrane Associated mCherry Fluorescent Reporter Line for Studying Vascular Remodeling and Cardiac Function During Murine Embryonic Development. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2009;292:333–341. doi: 10.1002/ar.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim P, Fuchsluger TA, Jurkunas UV. Limbal stem cell deficiency and corneal neovascularization. Semin. Ophthalmol. 2009;24:139–148. doi: 10.1080/08820530902801478. [DOI] [PubMed] [Google Scholar]
- 37.Lwigale PY, Cressy PA, Bronner-Fraser M. Corneal keratocytes retain neural crest progenitor cell properties. Dev. Biol. 2005;288:284–293. doi: 10.1016/j.ydbio.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 38.Ma DH-K, Yao J-Y, Yeh L-K, Liang S-T, See L-C, Chen H-T, Lin K-Y, Liang C-C, Lin K-K, Chen J-K. In vitro antiangiogenic activity in ex vivo expanded human limbocorneal epithelial cells cultivated on human amniotic membrane. Invest. Ophthalmol. Vis. Sci. 2004;45:2586–2595. doi: 10.1167/iovs.03-1338. [DOI] [PubMed] [Google Scholar]
- 39.McKenna CC, Ojeda AF, Spurlin J, III, Kwiatkowski S, Lwigale PY. Sema3A maintains corneal avascularity during development by inhibiting VEGF induced angioblast migration. Dev. Biol. 2014 doi: 10.1016/j.ydbio.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meadows SM, Ratliff LA, Singh MK, Epstein JA, Cleaver O. Resolution of defective dorsal aortae patterning in Sema3E-deficient mice occurs via angiogenic remodeling. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2013;242:580–590. doi: 10.1002/dvdy.23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miao H-Q, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 Mediates Collapsin-1/Semaphorin III Inhibition of Endothelial Cell Motility: Functional Competition of Collapsin-1 and Vascular Endothelial Growth Factor-165. J. Cell Biol. 1999;146:233–241. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyato H, Tsuno NH, Kitayama J. Semaphorin 3C is involved in the progression of gastric cancer. Cancer Sci. 2012;103:1961–1966. doi: 10.1111/cas.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morishige N, Teranishi S, Kondo T, Nishida T. Abnormal cytokeratin expression in low-grade conjunctival intraepithelial neoplasia. Clin. Experiment. Ophthalmol. 2010;38:899–900. doi: 10.1111/j.1442-9071.2010.02351.x. [DOI] [PubMed] [Google Scholar]
- 44.Oh WJ, Gu C. The role and mechanism-of-action of Sema3E and Plexin-D1 in vascular and neural development. Semin. Cell Dev. Biol. 2013a;24:156–162. doi: 10.1016/j.semcdb.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh WJ, Gu C. Establishment of neurovascular congruency in complex tissue by a novel independent patterning mechanism. Neuron. 2013b;80:458–469. doi: 10.1016/j.neuron.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ojeda AF, Munjaal RP, Lwigale PY. Expression of CXCL12 and CXCL14 during eye development in Chick and Mouse. Gene Expr. Patterns GEP. 2013;13 doi: 10.1016/j.gep.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohtsuki S, Sato S, Yamaguchi H, Kamoi M, Asashima T, Terasaki T. Exogenous expression of claudin-5 induces barrier properties in cultured rat brain capillary endothelial cells. J Cell Physiol. 2007;210:81–86. doi: 10.1002/jcp.20823. [DOI] [PubMed] [Google Scholar]
- 48.Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest. Ophthalmol. Vis. Sci. 2000;41:2514–2522. [PubMed] [Google Scholar]
- 49.Salikhova A, Wang L, Lanahan AA, Liu M, Simons M, Leenders WPJ, Mukhopadhyay D, Horowitz A. Vascular Endothelial Growth Factor and Semaphorin Induce Neuropilin-1 Endocytosis via Separate Pathways. Circ. Res. 2008;103:e71–e79. doi: 10.1161/CIRCRESAHA.108.183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato Y, Poynter G, Huss D, Filla MB, Czirok A, Rongish BJ, Little CD, Fraser SE, Lansford R. Dynamic Analysis of Vascular Morphogenesis Using Transgenic Quail Embryos. PLoS ONE. 2010;5:e12674. doi: 10.1371/journal.pone.0012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 52.Torres-Vázquez J, Gitler AD, Fraser SD, Berk JD, Van N. Pham, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-Plexin Signaling Guides Patterning of the Developing Vasculature. Dev. Cell. 2004;7:117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Toyofuku T, Yoshida J, Sugimoto T, Yamamoto M, Makino N, Takamatsu H, Takegahara N, Suto F, Hori M, Fujisawa H, et al. Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev. Biol. 2008;321:251–262. doi: 10.1016/j.ydbio.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 54.Tsukita S, Furuse M. Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol. 2002;14:531–536. doi: 10.1016/s0955-0674(02)00362-9. [DOI] [PubMed] [Google Scholar]
- 55.Walton W, Von Hagen S, Grigorian R, Zarbin M. Management of Traumatic Hyphema. Surv. Ophthalmol. 2002;47:297–334. doi: 10.1016/s0039-6257(02)00317-x. [DOI] [PubMed] [Google Scholar]
- 56.Yang W-J, Hu J, Uemura A, Tetzlaff F, Augustin HG, Fischer A. Semaphorin-3C signals through Neuropilin-1 and PlexinD1 receptors to inhibit pathological angiogenesis. EMBO Mol. Med. 2015 doi: 10.15252/emmm.201404922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Singh MK, Degenhardt KR, Lu MM, Bennett J, Yoshida Y, Epstein JA. Tie2Cre-mediated inactivation of plexinD1 results in congenital heart, vascular and skeletal defects. Dev. Biol. 2009;325:82–93. doi: 10.1016/j.ydbio.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zygmunt T, Gay CM, Blondelle J, Singh MK, Flaherty KM, Means PC, Herwig L, Krudewig A, Belting H-G, Affolter M, et al. Semaphorin-PlexinD1 Signaling Limits Angiogenic Potential via the VEGF Decoy Receptor sFlt1. Dev. Cell. 2011;21:301–314. doi: 10.1016/j.devcel.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.