Abstract
Purpose
Treatment of progression in high-risk neuroblastoma remains challenging despite improved survival. We retrospectively evaluated outcomes in children with a first progression that included soft-tissue masses.
Methods
We reviewed records of 903 consecutive children with high-risk neuroblastoma diagnosed between 2004 and 2014, and identified 42 whose first progression included soft-tissue masses. Data on demographics, disease characteristics, treatment, and survival were collected. Primary outcome was 5-year overall survival (OS) from time of first progression. Secondary outcomes were local disease-free progression (LDFR) and progression-free survival (PFS) post-progression. We evaluated the prognostic significance of concomitant bone/bone marrow involvement, MYCN status, and multifocality of soft tissue relapse. Data are given as median(range).
Results
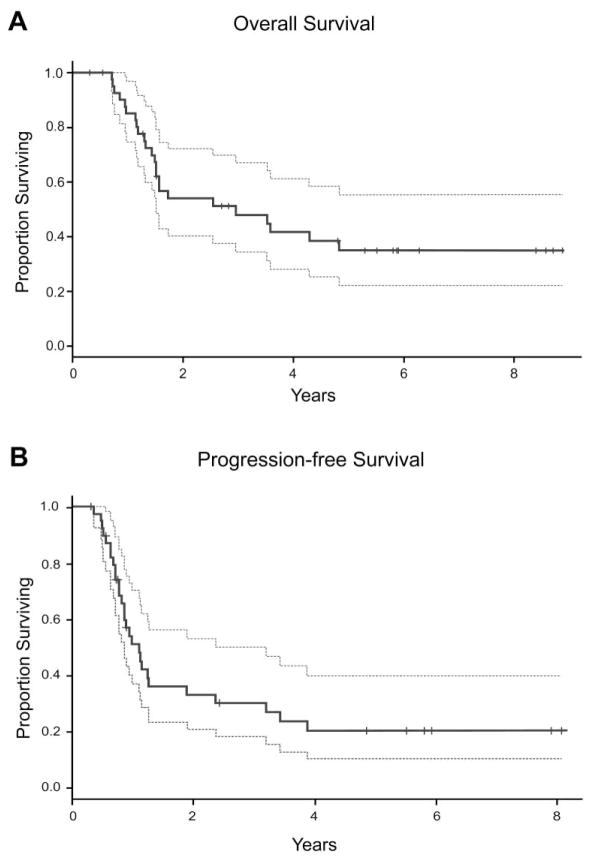
Median age at diagnosis was 3.0 (1 – 10.7) years. Median time to first relapse or progression was 1.2 (0.1 – 4.5) years after complete remission or minimal stable residual disease Twelve (29%) patients had concomitant bone or marrow involvement at progression/relapse. There were 11 (26%) patients with INSS stage 3 disease (all with MYCN amplification), and 31 (74%) with stage 4 disease (12 with MYCN amplification). Nine (21%) patients had multifocal soft tissue progression. R1 resection was achieved in 41 children (95%), 38 (95%) of whom also received salvage radiation therapy. Five-year OS post-progression was 35% (95%CI: 19–51%), 5-year LDFS was 52% (95%CI: 32–72%), and 5-year PFS post-progression was 20% (95%CI: 6–34%).
Conclusion
Among children with high-risk neuroblastoma who underwent aggressive treatment of a first soft-tissue recurrence, 5-year post-progression overall survival was 34%. Multifocality and MYCN amplification were the predominant prognostic correlates for worse survival.
Keywords: High-risk Neuroblastoma, Survival, Progression, MYCN, Multifocal Disease
Introduction
Neuroblastoma is the most prevalent non-CNS solid tumor in the pediatric population, and 30–50% of patients with neuroblastoma present with high-risk disease [1]. Despite aggressive multimodality treatment, overall mortality remains approximately 50% [1,2]. Although survival in high-risk neuroblastoma has improved, survival after progression remains poor. London et al. found that in a subset of patients with low or intermediate risk neuroblastoma (stage 1, 2, 3, or 4S with no MYCN amplification (MYCN-NA), cure was possible post-recurrence [3]. Patients with stage 4 disease or MYCN amplification (MYCN-A), however, had poor overall survival following relapse. The poor prognosis of patients with relapsed high-risk neuroblastoma can complicate decision making with respect to the use of aggressive multimodality treatment. In an effort to maximize quality of life, clinicians may opt to forego aggressive therapies in some patients. To identify those patients who might benefit from salvage therapy, we performed a retrospective analysis of data from patients with high-risk relapsed neuroblastoma with a soft tissue component to determine which clinical and biologic factors are associated with prolonged overall survival.
Patients and Methods
Patient Selection
With Institutional Review Board approval, we reviewed the electronic medical records of 903 consecutive patients who were diagnosed with neuroblastoma between 2004 and 2014. Patients were included in our analysis if they met the following criteria: (1) high-risk disease by both International Neuroblastoma Risk Group (INRG) and International Neuroblastoma Staging System (INSS) criteria (i.e., stage 4 disease and age ≥ 18 months at diagnosis, stage 4 disease and age < 18 months at diagnosis with MYCN-A, or stage 3 disease with MYCN-A); (2) surgical treatment at our institution at any point during treatment; (3) soft tissue disease component during first relapse or progression; (4) disease-free or stable minimal residual disease period prior to progression/relapse; (5) age younger than 12 years at the time of diagnosis. Soft tissue disease was defined as connective tissue, muscle, or lymph node involvement.
Demographic and initial treatment
Characteristics of initial disease course were analyzed, including age at diagnosis, INSS stage, International Neuroblastoma Pathology Classification (INPC; unfavorable vs. favorable histology), DNA ploidy, and MYCN amplification status. Initial treatment data were also obtained, including whether patients underwent stem cell transplantation, chemotherapy, radiation therapy (external beam or intraoperative radiation therapy), immunotherapy, and extent of resection. Gross total resection was defined as removal of all visible and palpable tumor, as documented in operative reports.
Relapse/Progression
Relapse was defined as the presence of new tumor in a patient with previously documented complete remission. Progression was defined as an increase of >20% in tumor size, the presence of a new focus of disease, or new metaiodobenzylguanidine (MIBG)-active focus in a patient with documented minimal stable residual disease. Relapse/progression was defined as locoregional if confined to the primary tumor bed, and systemic if metastatic disease was present. Systemic disease was further characterized as skeletal bone, bone marrow, or distant soft tissue disease. Soft tissue disease was divided into focal and multifocal categories. Treatment data was collected including whether patients received chemotherapy, radiation therapy (RT), immunotherapy, and/or stem cell transplant. Type of operation and extent of resection were also noted.
The primary endpoint was overall survival following date of first relapse (OS post-progression). OS post-progression was defined as the interval between diagnosis of first progression/relapse and death or date of last follow-up. Time to first relapse (TTFR) was calculated from the first documented complete remission (CR), or when unavailable, treatment end date in patients with no residual disease. In patients who did not have a CR, TTFR was calculated from the date of minimal stable residual disease. Patients were divided into three categories based on TTFR: < 6 months, 6–18 months, or > 18 months after CR. Secondary endpoints were local disease-free survival (LDFS) and progression-free survival (PFS) post-progression. For LDFS, local disease was defined as the site of initial tumor progression, and LDFS was calculated as the interval between the operation for first progression and the time of second progression/relapse at the new local disease site. PFS post-progression was defined as the interval between diagnosis of first progression/relapse and the date of second progression/relapse. If patients did not relapse/progress (PFS post-progression, LDFS) or die (OS post-progression), the date of last follow-up was used.
Statistical Analyses
All statistical analyses were performed using R statistical software (version 3.1.1; The R Foundation/R Project for Statistical Computing; http://www.r-project.org/). Survival curves were generated using the Kaplan-Meier method and differences were assessed with log-rank tests, where appropriate. Data are given as median (ranges) and P <0.05 was considered statistically significant.
Results
Demographics, initial presentation, and treatment of initial disease
We identified 42 children who developed a first relapse/progression with a soft tissue component. There were 26 male (62%) and 16 (38%) female. The median age at diagnosis was 3.0 (1–10.7) years and median time to first relapse/progression was 1.2 (0.1 – 4.5) years after complete remission or minimal stable residual disease At diagnosis, 11 (26%) patients had INSS stage 3 disease (all MYCN-amplified), and 31 (74%) had stage 4 disease, with MYCN amplification in 12 (39%). A majority had an adrenal primary tumor (n=31, 74%). Ninety-seven percent of children had unfavorable INPC histology at diagnosis (n=33 of 34 with available data). Ploidy data were only available for 13 patients (diploid: n=7, 54%; hyperdiploid: n=6, 46%).
During the initial disease course, all children underwent aggressive multimodality treatment, including both neoadjuvant and adjuvant chemotherapy (93%, n=39), adjuvant radiation therapy (100%, n=42), and stem cell transplant (77%, n=27). All had resection of the primary tumor. Of the 32 with known resection margins, 66% (n=21) had a gross total resection and 34% (n=11) had a subtotal resection. Following initial treatment, 62% of children (n=26) received immunotherapy, and 60% (n=25) received cis-retinoic acid.
Seventy-four percent (n=31) achieved a complete remission and 20% (n=8) had stable minimal residual disease. In 3 (7%), children complete remission could not be documented but they were completely off treatment at the time of relapse.
Characteristics of relapse/progression
The median TTFR (as calculated from the end of treatment or CR/stable minimal residual disease) was 1.2 years (range 0.1–4.5 years), with 8 (19%) relapsing in the first 6 months, 21 (50%) between 6 and 18 months, and 13 (31%) in > 18 months. Locoregional relapse, with disease confined to the primary tumor bed, occurred in 43% (n=18) of patients, and metastatic disease, including distant soft tissue, bone marrow, or skeletal bone disease, developed in the remaining 57% (n=24). Concomitant skeletal bone and/or bone marrow involvement at progression was evident in 12 (29%) patients. Nine (21%) patients had multifocal soft tissue disease. Patients with soft tissue progression had additional skeletal bone metastases in 26% (n=11), bone marrow involvement in 7% (n=3), pulmonary metastases in 5% (n=2), liver metastases in 5% (n=2), and central nervous system disease in 2% (n=1).
Treatment of Relapse/Progression
All children underwent aggressive multimodality treatment for disease progression/relapse, with 95% (n=40) receiving salvage chemotherapy and 95% (n=38) receiving RT (external beam RT in 20 patients, and intraoperative RT in 18). All patients underwent surgical resection, with R1 resection achieved in 40 children (98%). Only 6.5% (n=2) of patients underwent stem cell transplantation for treatment of their first relapse/progression. Seventy-seven percent of patients (n=30) received immunotherapy following first relapse/progression.
Predictors of prolonged overall survival post-relapse
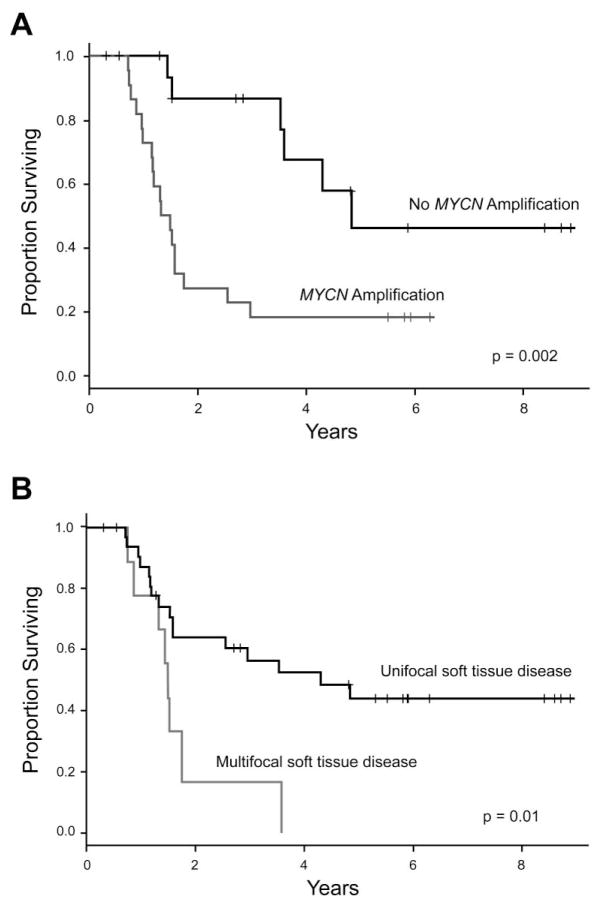
The 5-year OS from time of diagnosis for the entire cohort was 49% (95% confidence interval [CI]: 33–65%) and the 5-year OS post-progression was 35% (95% CI: 19–51%; Figure 1). There was no significant difference in 5-year OS post-progression in the TTFR < 6 months, 6–18 months, and ≥ 18 months groups. The only disease characteristics associated with worse 5-year OS post-progression were MYCN-A and multifocal soft tissue disease. In the 57.5% of patients with MYCN-A, 5-year OS post-progression was 18% (95% CI: 2–35%), compared to 46% (95% CI: 15–78%; p=0.002) in the 42.5% of patients with MYCN-NA (p=0.002, Figure 2A). Of the 9 patients with multifocal soft tissue disease, there were no survivors at 5 years as compared to a 44% (25–63%) 5-year OS post-progression in those with unifocal soft tissue disease (p=0.01, Figure 2B). The presence of either skeletal bone or bone marrow disease at time of progression did not have an impact on 5-year OS post-progression (44%, 95% CI: 13–76%) compared to patients without skeletal bone or bone marrow disease (32%, 95% CI: 13–50%).
Figure 1.
Overall survival (A) and progression-free survival (B) following first soft tissue progression/relapse. Dotted lines represent 95% confidence intervals. Overall survival was calculated from date of diagnosed first progression to the date of last follow-up. Progression-free survival was calculated from date of diagnosis of the first progression.
Figure 2.
Effect of MYCN amplification (A) and focality of soft tissue relapse (B) on overall survival following first soft-tissue progression.
Local disease-free survival and progression-free survival
Following initial progression, 28 children had a second progression, 29% (n=8) of whom were alive at the time of follow-up. The 5-year PFS from first progression was 20% (95% CI: 6–34%). There was no difference in children with or without MYCN-A. Only 13 (34%) had a second progression/recurrence at the initial progression site. The 5-year LDFS was 52% (95% CI: 32–72%). In children with MYCN-A, the 5-year LDFS was 33% (95% CI: 6–59%), and in those with MYCN-NA it was 65% (95% CI: 37–94%), although this was not statistically significant.
Discussion
London et al. found that in that subset with low or intermediate risk neuroblastoma, post-progression cure was possible [3]. However, in those with stage 4 disease or MYCN amplification, the 5-year OS post-progression was only 7% to 8%. Other studies have found similarly dismal results in high-risk relapsed patients [4,5]. We found that those with high-risk neuroblastoma with a soft tissue relapse/progression comprise a unique subset of patients with a much higher 5-year OS post-progression of 35% (95% CI: 19–51%). We found that the worst predictors for OS post-progression were MYCN amplification and multifocal soft tissue progression. Of note, although those with MYCN amplification did significantly worse than those without MYCN amplification, their 5-year OS post-progression of 18% (95% CI: 2–35%) was still better than that reported in previous studies [3–5]. Chilren with multifocal soft tissue disease, however, had a much worse prognosis, with no survivors at 5 years. Our data suggest that those with high-risk relapsed soft tissue disease fare much better than previously thought, even when MYCN amplification is present.
Following surgical resection, we observed a 5-year LDFS of 52% (95% CI: 32–72%), suggesting that surgery might play an important role in the management of this population. Unlike other studies [3–5], we did not find an association between OS post-progression and time to first progression.
The risks and benefits of aggressive multimodality salvage therapy must be carefully weighed In those with high-risk relapsed neuroblastoma. We have found that those with unifocal soft tissue disease represent a unique population that may be salvageable with multimodality treatment, including surgical resection when feasible. Even in those children with bone and/or bone marrow disease, surgical resection might play an important role in multimodality salvage therapy, particularly when soft tissue disease is limited to a single focus. In these patients, achieving a second remission is critical. However, in those with multifocal soft tissue recurrence, the limited benefit of aggressive treatment should be weighed against the poor likelihood for a prolonged survival post-progression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a Children’s Oncology Group study. J Clin Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.London WB, Castel V, Monclair T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the International Neuroblastoma Risk Group project. J Clin Oncol. 2011;29:3286–3292. doi: 10.1200/JCO.2010.34.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santana VM, Furman WL, McGregor LM, et al. Disease control intervals in high-risk neuroblastoma. Cancer. 2008;112:2796–2801. doi: 10.1002/cncr.23507. [DOI] [PubMed] [Google Scholar]
- 5.Lau L, Tai D, Weitzman S, et al. Factors influencing survival in children with recurrent neuroblastoma. J Pediatr Hematol Oncol. 2004;26:227–232. doi: 10.1097/00043426-200404000-00003. [DOI] [PubMed] [Google Scholar]