Abstract
Objective
To test the hypothesis that participants with stroke will exhibit appropriate increases in muscle activation of the paretic leg when taking a non-paretic long step compared to steady state walking, with a consequent increase in biomechanical output and symmetry during the stance phase of the modified gait cycle.
Design
Single-session observational study
Setting
Clinical research center in an outpatient hospital setting.
Participants
Fifteen adults with chronic post-stroke hemiparesis.
Interventions
Participants walked on an instrumented treadmill while kinetic, kinematic and electromyographical data were recorded. Participants performed steady state walking and a separate trial of the long step adaptability task in which they were instructed to intermittently take a longer step with the non-paretic leg.
Main Outcome Measure(s)
Forward progression, propulsive force, and neuromuscular activation during walking.
Results
Participants performed the adaptability task successfully and demonstrated greater neuromuscular activation in appropriate paretic leg muscles, particularly heightened activity in paretic plantarflexor muscles. Propulsion and forward progression by the paretic leg were also increased.
Conclusions
These findings support the assertion that the non-paretic long step task may be effective for use in post-stroke locomotor rehabilitation in order to engage the paretic leg and promote recovery of walking.
Keywords: walking, stroke, electromyography, biomechanics, rehabilitation
Introduction
A major goal of locomotor rehabilitation after stroke is to induce recovery of coordinated paretic limb control through experience-dependent neuroplasticity. This requires an intense, repetitive and task-specific dosage of walking therapy. However, engaging the paretic leg during locomotor rehabilitation is challenging due to the common use of compensatory biomechanical strategies. As a result, practice of steady state walking may fail to deliver the appropriate training stimulus needed to optimize recovery. Indeed, some prior studies have reported that steady state walking or pedaling does not improve paretic limb coordination and may reinforce compensatory patterns1–3.
A potentially beneficial approach for achieving an intense and targeted training stimulus in the paretic leg is through the use of locomotor adaptability tasks. Locomotor adaptability involves modifying typical walking to meet task objectives and environmental demands4. Adaptability tasks often require increased biomechanical output during specific subtasks of gait. For example, it has been shown in healthy adults that taking an isolated longer step requires the stance leg to increase its support and propulsion of the body, which requires an increase in plantarflexor output5. This particular subtask of gait is important after stroke because impaired support and propulsion contribute to slow and asymmetrical walking6,7.
This study seeks to determine if a long step adaptability task is effective for increasing neuromuscular output of the paretic leg plantarflexors during walking after stroke. We hypothesize that participants will increase activation of paretic plantarflexor muscles when taking a non-paretic long step compared to during steady state walking, with a consequent increase of paretic leg biomechanical output and symmetry during the stance phase of the modified gait cycle. If confirmed, this finding will support the use of a long step adaptability task to train the generation of propulsion in future rehabilitation studies and will warrant investigation of other adaptability tasks that may similarly enhance neuromuscular output for other subtasks of gait.
Methods
Participants
Participants with chronic post-stroke hemiparesis due to unilateral stroke were recruited for this study. Participants were required to be capable of walking 10m in the laboratory without the use of an assistive device. Exclusion criteria included significant pain, contractures, major sensory deficits or cardiorespiratory symptoms that would prevent compliance with the study protocol. Study procedures were approved by the University of Florida’s IRB.
Procedures
Participants walked on a treadmill with independent force plates embedded on the left and right sides (Techmachine, Andrezieux Boutheon, France). Reflective markers were attached to anatomical landmarks using a modified Helen Hayes marker set and recorded using a 12 camera motion capture system (VICON, Colorado, USA). Surface electromyography (EMG) was recorded bilaterally with bipolar Ag-AgCl electrodes from tibialis anterior (TA), soleus (SO), medial gastrocnemius (MG), vastus medialis (VM), rectus femoris (RF), lateral hamstrings (LH), medial hamstrings (MH) and gluteus medius (GM) (Konigsberg Instruments, Pasedana, CA). Kinetic and kinematic data were acquired at 200Hz and EMG data were acquired at 2000 Hz using VICON Workstation v4.5. Data were analyzed using Matlab 7.0 (The Mathworks, Natick, MA) and JMP statistical software (v8.0, SAS Institute, Cary, NC).
Participants performed two 30-second walking trials. The treadmill speed was chosen by each participant as being comfortable and typical of his/her regular walking speed. They then performed a one minute trial at the same speed but were instructed to take a long step with the non-paretic leg approximately every 5th gait cycle. This allowed participants to regain a steady state walking pattern before taking the next long step. Participants wore a safety harness secured to an overhead support and some individuals used a semi-rigid ankle brace to provide medial/lateral joint stability without restricting plantarflexion or dorsiflexion. Participants did not hold onto a railing or use any other assistive device.
Data analysis
Ground reaction force (GRF) and kinematic data were low-pass filtered (20 Hz and 10 Hz, respectively) with a fourth-order Butterworth filter with zero phase lag. Pelvis and foot center of mass (COM) locations were obtained by fitting the marker trajectories to an eight-segment musculoskeletal model using Visual3D (C-Motion, Inc., Germantown MD).
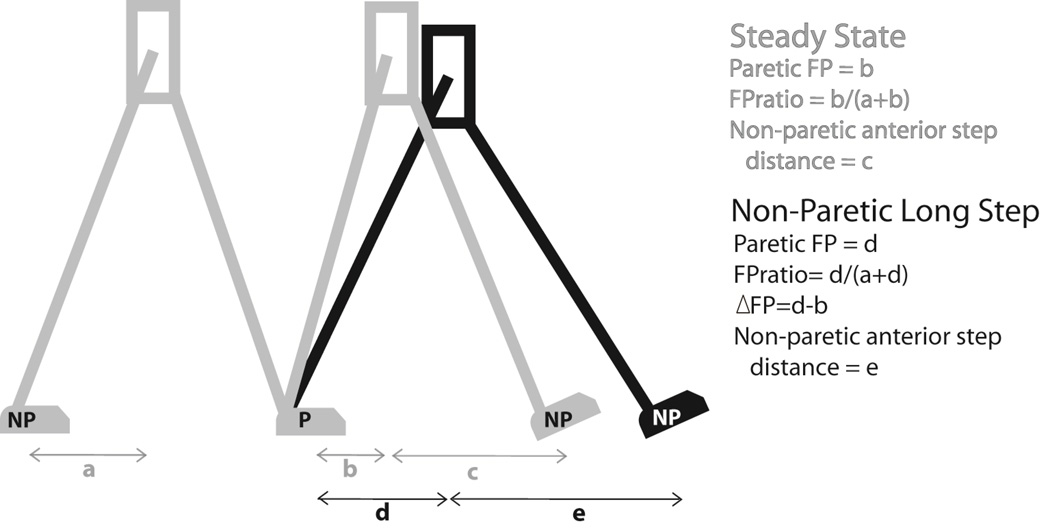
Forward progression (FP) of the body during the single limb support phase was expressed by calculating the horizontal distance between the pelvis COM and trailing foot COM at the instant of leading foot heel strike (see “a”, “b” and “c” in Figure 1). An FP ratio was calculated as [paretic/(paretic+nonparetic)] to quantify the symmetry of FP for each walking condition. Anterior step distance was calculated as the difference between the pelvis COM and leading foot center of mass at the instance of leading foot heel strike (see “c” and “d” in Figure 1). Step length is equivalent to the sum of the forward progression and anterior step distance measurements. Propulsion was calculated as the time integral of the anteriorly-directed horizontal component of the ground reaction force.
Figure 1.
Illustrative comparison of forward progression and anterior foot placement during steady state walking (in gray) and non-paretic long step (in black).
EMG signals were high-pass filtered (40 Hz) with a fourth-order Butterworth filter, de-biased and rectified. Paretic leg EMG and propulsion were averaged over the second half of paretic leg single limb support. This phase of the gait cycle was selected because it is a key period of forward progression in which the paretic leg alone is supporting the body. Therefore, non-paretic compensatory contributions to support and propulsion will not influence the results. EMG amplitude was normalized for each participant by calculating a percent change between steady state walking and the long step task.
Statistics
Comparisons between steady state walking and the long step task for each variable were conducted using repeated measures t-tests (one tailed, consistent with directional hypotheses). Associations between continuous variables were evaluated with Pearson’s correlation test. Statistical significant was accepted at p<0.05.
Results
Fifteen adults (8 male and 7 female) participated in this study. The mean age was 59.9±11.9 years and time since stroke was 39.8±34.3 months (range of 9 – 117 months). Preferred overground walking speed was 0.54 ± 0.25 m/s and the lower extremity Fugl-Meyer score was 23.7 ± 5.7 (out of 34 possible points).
During steady state walking, 13 out of 15 participants exhibited lower paretic FP (Figure 1 “a”) than non-paretic FP (Figure 1 “b”). The remaining two participants were approximately symmetric. On average, the FP distances for non-paretic FP and paretic FP during steady state walking were 15.10 ± 4.22 cm and 8.73 ± 5.07 cm, p<0.001. The group average FP ratio was 0.35 ± 0.11 (where 0.50 indicates symmetry). Paretic FP had a significant positive association with self-selected overground walking speed (r=0.71, p=0.003). Paretic FP was not associated with age (p = 0.33) or time since stroke (p=0.62).
All study participants successfully performed the long step task with at least a small increase (i.e., ≥10%) of paretic FP relative to steady state walking. On average, paretic FP increased 113% over steady state walking (p<0.001). This change in FP was positively associated with an increase in anterior step distance by the non-paretic leg (Figure 1 “c” and “e”) (r=0.70, p=0.003). The average FPratio during the non-paretic long step task increased to 0.54 ± 0.11, indicating improved symmetry compared to the ratio of 0.35 observed during steady state walking.
Propulsion from the paretic leg increased 319% between steady state walking and the non-paretic long step (2.08 ± 2.42 N·s vs. 8.71 ± 5.12 N·s, p<0.0001). EMG amplitude from the paretic leg also increased significantly for most muscles between steady state walking and the non-paretic long step. These included TA (46.9±62.4%,p=0.04), SO (54.7±66.0%, p=0.01), MG (35.4±50.4%, p=0.04), VM (49.6±50.2%, p=0.01) and RF (33.6±30.1%, p=0.02). Of these muscles, the correlation between the change in EMG and change in propulsion was not significant, but a trend was evident for SO (r=0.53, p=0.08), MG (r=0.62, p=0.05) and VM (r=0.52, p=0.08). Only the changes for SO and MG were positively correlated with the change in paretic FP (r=0.76, p=0.02 for each muscle).
Discussion
The primary finding of this study is that a long step task with the non-paretic leg is effective for inducing an appropriate increase in paretic leg neuromuscular activation and biomechanical output during the stance phase of walking. Consistent with the strategy for taking a longer step in healthy adults, the post-stroke participants were able to use the paretic leg to increase propulsion during stance5. The EMG results are consistent with the known role of each respective muscle for supporting body weight and generating propulsion during the stance phase of walking8. There was considerable variability in the magnitude of task-dependent changes in EMG among participants, but the directionality of change (i.e., increase activity during long step) was largely consistent. This finding supports the assertion that participants used a strategy of more intense activation of the paretic leg, and did not need to rely solely on a strategy of limb flexion by the leading non-paretic leg to increase step length5.
Study Limitations
This study examined only a long step adaptability task. Other adaptability tasks (e.g., high step, turning, etc.) may also be effective for engaging the paretic limb during walking rehabilitation, but were not addressed here.
Conclusion
The non-paretic long step task may be effective for use in post-stroke locomotor rehabilitation in order to more effectively engage the paretic leg and induce neuroplastic recovery. These findings add to other potential benefits of using adaptability tasks in rehabilitation, including more effective engagement of cerebral circuits of locomotor control9,10 and high functional importance of adaptability tasks4.
Acknowledgement
Funding
This work was supported by the National Institutes of Health (HD-46820 and GM-109040) and by the US Department of Veterans Affairs Rehabilitation Research and Development Service (Merit Review B3983R and Career Development Awards B4888M and B7176W). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kautz SA, et al. Coordination of hemiparetic locomotion after stroke rehabilitation. Neurorehabil Neural Repair. 2005;19(3):250–258. doi: 10.1177/1545968305279279. [DOI] [PubMed] [Google Scholar]
- 2.Combs SA, Dugan EL, Ozimek EN, Curtis AB. Effects of body-weight supported treadmill training on kinetic symmetry in persons with chronic stroke. Clinical Biomechanics. 2012;27(9):887–892. doi: 10.1016/j.clinbiomech.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Den Otter AR, Geurts AC, Mulder T, Duysens J. Gait recovery is not associated with changes in the temporal patterning of muscle activity during treadmill walking in patients with post-stroke hemiparesis. Clin Neurophysiol. 2006;117(1):4–15. doi: 10.1016/j.clinph.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanian CK, Clark DJ, Fox EJ. Walking adaptability after a stroke and its assessment in clinical settings. Stroke Res Treat. 2014;2014:591013. doi: 10.1155/2014/591013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varraine E, Bonnard M, Pailhous J. Intentional on-line adaptation of stride length in human walking. Exp Brain Res. 2000;130(2):248–257. doi: 10.1007/s002219900234. [DOI] [PubMed] [Google Scholar]
- 6.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88(1):43–49. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Bowden M, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006;37(3):872–876. doi: 10.1161/01.STR.0000204063.75779.8d. [DOI] [PubMed] [Google Scholar]
- 8.Clark DJ, et al. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol. 2010;103(2):844–857. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark DJ, et al. Synchronous EMG activity in the piper frequency band reveals the corticospinal demand of walking tasks. Annals of Biomedical Engineering. 2013;41(8):1778–1786. doi: 10.1007/s10439-013-0832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark DJ, Rose DK, Ring SA, Porges EC. Utilization of central nervous system resources for preparation and performance of complex walking tasks in older adults. Front Aging Neurosci. 2014;6:217. doi: 10.3389/fnagi.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]