Abstract
The nucleoside antibiotic, 5′-O-[N-(salicyl)sulfamoyl]adenosine (1), possesses potent whole-cell activity against Mycobacterium tuberculosis (Mtb), the etiological agent of tuberculosis (TB). This compound is also active in vivo, but suffers from poor drug disposition properties that result in poor bioavailability and rapid clearance. The synthesis and evaluation of a systematic series of lipophilic ester prodrugs containing linear and α-branched alkanoyl groups from two to twelve carbons at the 3′-position of a 2′-fluorinated analogue of 1 is reported with the goal to improve oral bioavailability. The prodrugs were stable in simulated gastric fluid (pH 1.2) and under physiological conditions (pH 7.4). The prodrugs were also remarkably stable in mouse, rat, and human serum (relative serum stability: human~rat>>mouse) displaying a parabolic trend in the SAR with hydrolysis rates increasing with chain length up to eight carbons (t1/2 = 1.6 h for octanoyl prodrug 7 in mouse serum) and then decreasing again with higher chain lengths. The permeability of the prodrugs was also assessed in a Caco-2 cell transwell model. All of the prodrugs were found to have reduced permeation in the apical-to-basolateral direction and enhanced permeation in the basolateral-to-apical direction relative to the parent compound 2, resulting in efflux ratios 5–28 times greater than 2. Additionally, Caco-2 cells were found to hydrolyze the prodrugs with SAR mirroring the serum stability results and a preference for hydrolysis on the apical side. Taken together, these results suggest that the described prodrug strategy will lead to lower than expected oral bioavailability of 2 and highlight the contribution of intestinal esterases for prodrug hydrolysis.
Keywords: Prodrug, Nucleoside, Tuberculosis, Siderophore Biosynthesis Inhibitor
Graphical abstract
1. Introduction
Tuberculosis (TB), one of the oldest recorded diseases of humankind, is caused by the slowgrowing bacterium Mycobacterium tuberculosis (Mtb) as well as several closely related mycobacterial species. TB is a devastating disease clinically manifested as a persistent cough followed by hemoptysis, general malaise and fatigue, and severe weight loss as the disease progresses. TB is extremely difficult to treat relative to other bacterial infections due to a number of unique factors in the pathology and metabolism of Mtb.1–4 Thus, in the case of the simplest drug-sensitive TB, one must employ a four-drug regimen comprised of isoniazid, rifampicin, ethambutol, and pyrazinamide for the first two months followed by 4–7 months of isoniazid and rifampicin. Drug-resistant strains are even more challenging to treat with corresponding lower cure rates. As a result, TB has now overtaken malaria and HIV as the leading cause of infectious disease mortality.5 The development of new antitubercular agents that are effective against drug-resistant strains and reduce the duration of treatment will be necessary to bring TB back under control.
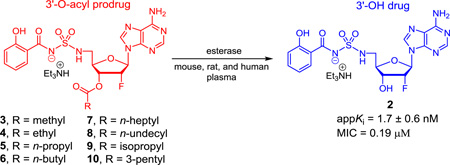
The nucleoside antibiotic 5′-O-[N-(salicyl)sulfamoyl]adenosine (Sal-AMS (1), Figure 1) originally described by Quadri, Tan and co-workers was rationally designed to inhibit siderophore biosynthesis in Mtb, an essential process under iron-deficient conditions found in the host.6–9 Sal-AMS (1) possesses nanomolar enzyme inhibition of MbtA, which catalyzes the first committed step of mycobactin biosynthesis, and potent on-target whole-cell activity. Proof-of-concept in vivo efficacy was also demonstrated; however, 1 suffers from poor physicochemical properties that result in high clearance and low oral bioavailability.10 To further advance this new class of antibiotics, we previously explored a large number of modifications to 1.8,11–16 The 2′-Fluoro analogue 2 emerged as a lead compound with improved in vitro antitubercular activity (2-fold more potent than 1) while displaying enhanced in vitro (Caco-2 permeability of 4.2 × 10−6 cm/s or 3.5-fold greater than 1, indicative of a medium-permeable compound) and in vivo drug disposition properties (3-fold reduced clearance resulting in a commensurate 3-fold improved oral exposure relative to 1), but no improvement in bioavailability.15
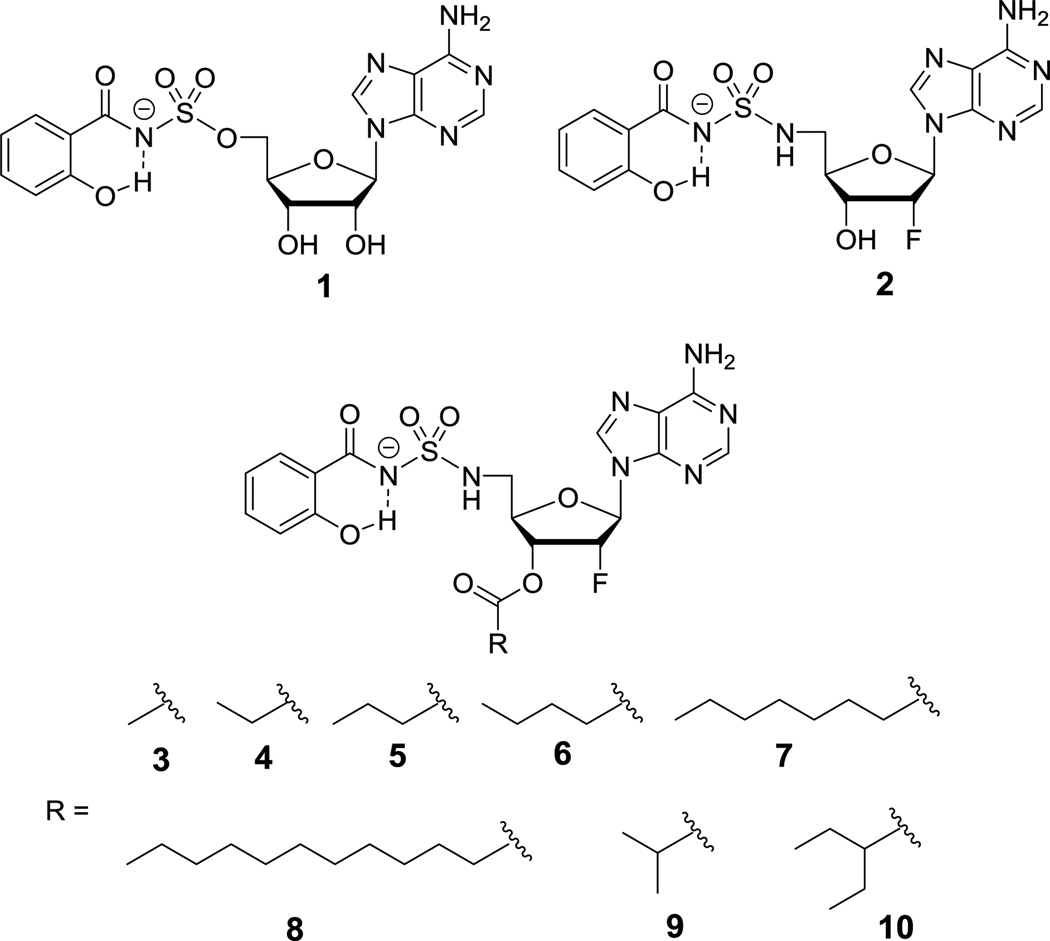
Figure 1.
Structure of Sal-AMS (1), 2′-deoxy-2′-fluoro analogue 2, and ester prodrugs 3–10.
A common strategy to improve oral bioavailability is to synthesize an ester prodrug that increases the lipophilicity and thereby enhances gastrointestinal absorption.17,18 The ester prodrug is then cleaved by serum or tissue esterases to release the parent drug. Herein we report the synthesis, chemical and enzymatic stability, as well as the in vitro membrane permeability of a systematic series of eight prodrugs (3–10) of the 2′-fluoro analogue 2 through esterification at the 3′-OH group (Figure 1). Prodrugs 3–8 contain a linear alkyl chain from two to twelve carbons while 9 and 10 are branched at the α-position to explore the importance of steric hindrance of the promoiety. The pivaloyl ester, which contains a tertiary carbon at the α-position, was not prepared due to the chronic toxicity of pivalate.19 The phenolic hydroxyl group was left unmasked because the formation of an intramolecular hydrogen bond with the charged acylsulfamide moiety was expected to shield the polarity through charge delocalization.14
2. Results and Discussion
2.1. Chemistry
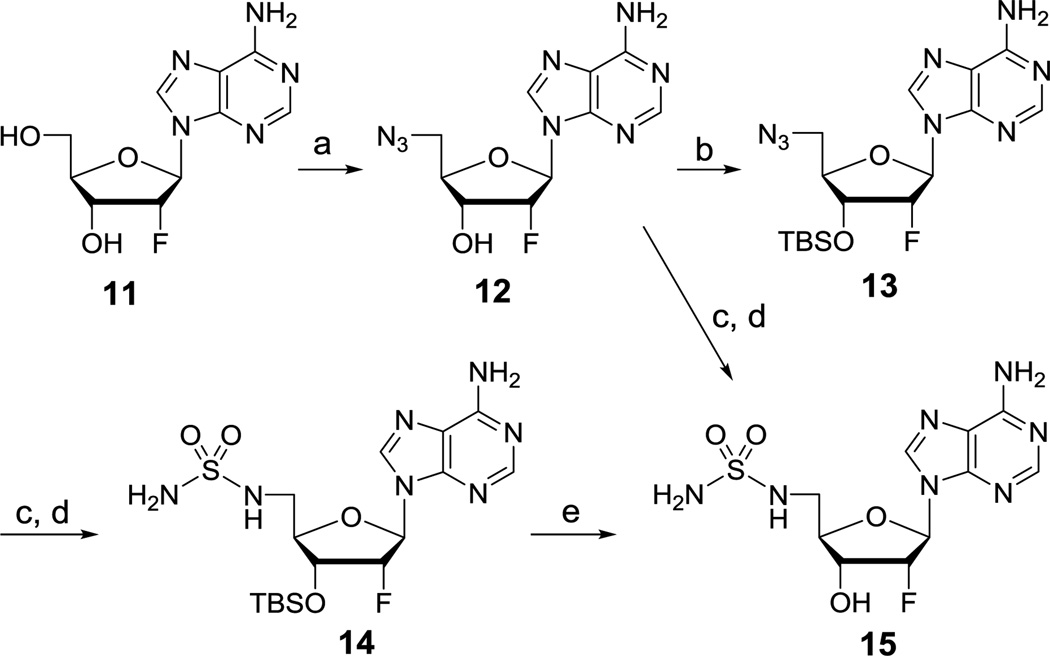
Synthesis of the key intermediate 15 for preparation of the prodrugs began with commercially available 2′-deoxy-2′-fluoroadenosine 11 (Scheme 1). Regioselective 5′-azidation of 11 with NaN3 using the classic Appel conditions (CBr4, PPh3, DMF) afforded 12 in 78% yield. The 3′-OH in 12 was then protected as the TBS ether 13 in 80% yield. Catalytic hydrogenation of 13 employing wet Pd/C led to reduction of the azide to the corresponding amine in quantitative yield. The crude aminonucleoside intermediate was refluxed with sulfamide (NH2SO2NH2) in 1,4-dioxane to provide 14 in 85% yield over two steps. Deprotection of the TBS group with HCl furnished the desired sulfamide 15 in quantitative yield. The conversion of azide 12 to sulfamide 15 was accomplished in four steps with a 68% overall yield by this optimized synthetic route. We also demonstrated that azide 12 could be directly converted to sulfamide 15 in 58% overall yield in two steps circumventing the TBS protection-deprotection sequence utilizing an analogous series of reactions for the conversion of 13 to 14. However, the former route was preferred due to its higher overall yield and avoidance of chromatographic purification of the polar nucleoside 15.
Scheme 1.
Reagents and conditions: (a) CBr4, PPh3, NaN3, DMF, 0 °C to rt, 24 h, 78%; (b) TBSCl, imidazole, DMAP, DMF, rt, 7 h, 80%; (c) Pd/C, H2, MeOH, rt, 4–6 h; (d) sulfamide, 1,4-dioxane, reflux, 13–17 h, 85% (13 to 14), 58% (12 to 15); (e) 4 M HCl in 1,4-dioxane, MeOH, rt, 3 h, quant.
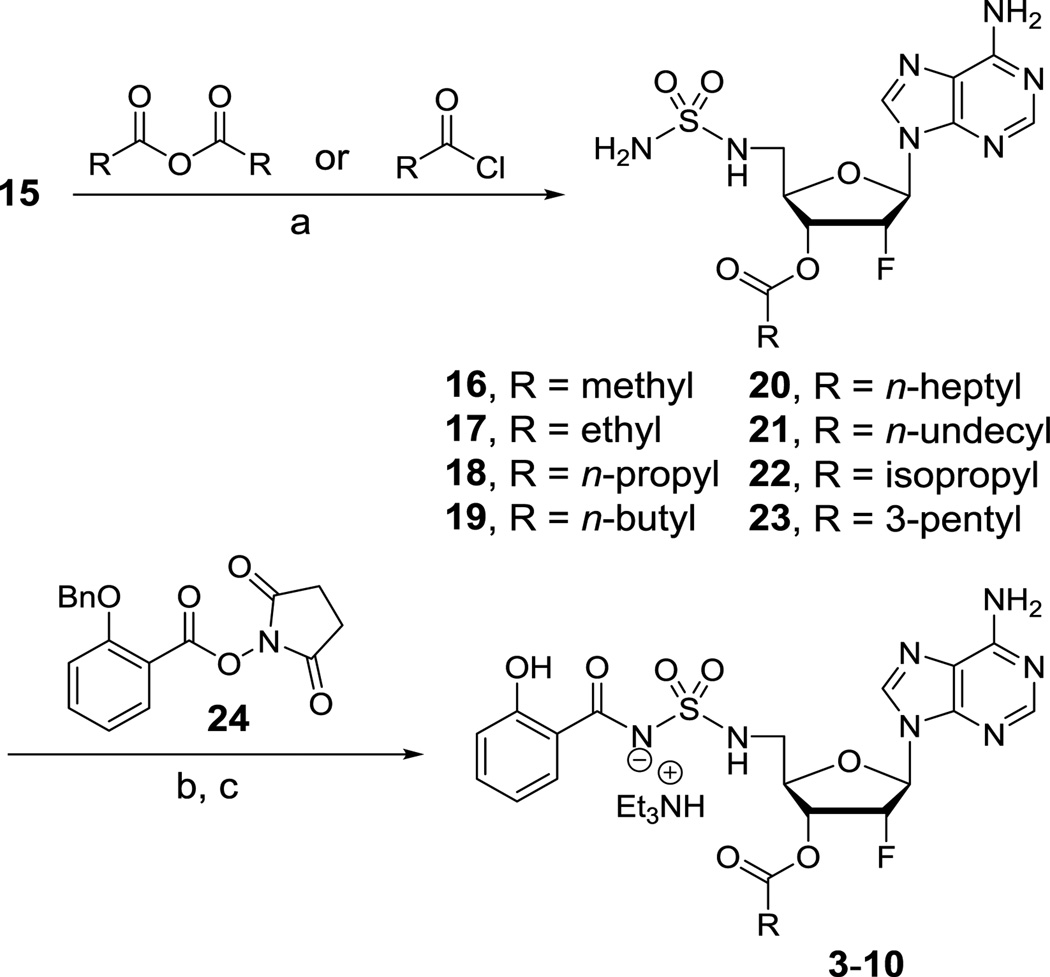
With an efficient route to the common intermediate 15 in place, we then developed a reliable procedure for introduction of the promoieties onto the 3′-OH of the nucleoside. Although selective acylation of the 3′-OH over the sulfamide could be accomplished at −5 °C; in practice the extended reaction times made this experimentally impractical. Thus, the reactions were conducted at 0 °C leading to dual acylation. The acyl group on the sulfamide moiety was subsequently cleaved in situ by treatment with formic acid in methanol. By this method, the 3′-O-acylated sulfamides 16–23 were obtained in 61–88% yield. The salicyl group was introduced by Cs2CO3 mediated coupling with N-hydroxysuccinimdyl ester 24 followed by hydrogenolysis of the benzyl protected phenol and purification by column chromatography (coelution with 2% Et3N) afforded prodrugs 3–10 as triethylammonium salts in 37–57% yield over these final two steps.
2.2. Aqueous and plasma stability
The ideal prodrug for our application should be stable in aqueous solutions (for 1–2 hours to enable oral absorption), but rapidly hydrolyzed to the parent drug in plasma. The aqueous stability of the prodrugs 3–10 was thus studied in simulated gastric fluid (SGF, pH 1.2) and HEPES buffer (pH 7.4), which mimic gastric and physiological pH, respectively. The prodrugs 3–10 (100 µM) were incubated at 37 °C in the indicated buffers containing 2% DMSO and the amount of both hydrolyzed product (i.e. parent drug 2) and the prodrug remaining in the solution were monitored by HPLC. Most of the prodrugs were extremely stable and showed little degradation or hydrolysis at 2 hours in both buffers (Table 1). Surprisingly, in the case of most lipophilic dodecanoyl prodrug 8 at pH 1.2, only 76% of the prodrug remained in solution after 2 hours at 37 °C. Chemical hydrolysis to release the parent drug 2 was not observed. Instead we noticed that the prodrug began to slowly precipitate over time, due to the low solubility at pH 1.2. This was also observed with octanoyl prodrug 7 to a minor extent (93% remaining in solution at 2 hours). Only the acetate prodrug 3 was slightly hydrolyzed to parent drug 2 (8% hydrolyzed at pH 7.4 at 2 h). These results confirm that the prodrugs are stable under aqueous conditions.
Table 1.
Aqueous and plasma stability of drug 2 and prodrugs 3–10
| Compound | cLogPa | Aqueous stability % remaining at 2 h |
Plasma stability % remaining at 6 h OR (t1/2, h) |
|||
|---|---|---|---|---|---|---|
| SGFb (pH 1.2) |
HEPES (pH 7.4) |
Mouse | Rat | Human | ||
| 2 | −0.13 | 100 | 100 | 100 | 100 | 100 |
| 3 | 0.08 | 97 ± 1 | 92 ± 4 | (4.6 ± 0.1) | (5.7 ± 0.2) | 61 ± 9 |
| 4 | 0.61 | 97 ± 1 | 100 | (3.3 ± 0.1) | 58 ± 1 | 70 ± 1 |
| 5 | 1.14 | 97 ± 2 | 100 | (2.2 ± 0.1) | 61 ± 0.2 | 64 ± 3 |
| 6 | 1.67 | 98 ± 2 | 100 | (1.6 ± 0.04) | 53 ± 0.8 | 66 ± 2 |
| 7 | 3.26 | 93 ± 2 | 98 ± 2 | (0.7 ± 0.1) | 86 ± 0.1 | 93 ± 0.3 |
| 8 | 5.37 | 76 ± 5 | 100 | (1.6 ± 0.5) | 95 ± 0.1 | 98 ± 0.2 |
| 9 | 0.92 | 98 ± 1 | 100 | (3.7 ± 0.2) | 72 ± 3 | 94 ± 1 |
| 10 | 1.98 | 100 | 100 | 83 ± 5 | 93 ± 2 | 94 ± 2 |
calculated using ChemBioDraw Ultra 14.0;
simulated gastric fluid.
Next, the stability of the prodrugs in mouse, rat, and human plasma at 37 °C was investigated. The prodrugs were hydrolyzed very slowly in rat and human plasma. On the other hand, most of the prodrugs were hydrolyzed more rapidly by mouse plasma and followed first order kinetics allowing determination of their half-lives. For prodrugs that showed less than 50% hydrolysis at 6 hours, the percentage of prodrug remaining at this terminal time point was measured. The half-lives and percent remaining at 6 hours are listed in Table 1. In mouse plasma, the fastest rate of hydrolysis was observed with octanoyl prodrug 7 with a half-life (t1/2) of 0.7 hours. The hydrolysis rate was slightly slower for prodrugs with larger alkyl groups such as dodecanoyl 8 (t1/2 = 1.6 h) and much slower for branched alkyl chains such as 9 and 10. It was also slower for prodrugs 3, 4, 5, and 6 containing shorter ester promoieties with a clear trend paralleling chain length. In rat and human plasma, the hydrolysis was slow for all prodrugs with a trend of slower hydrolysis for longer and branched promoiet ies. Nonetheless, the prodrugs studied were far more susceptible to enzymatic hydrolysis than chemical hydrolysis in aqueous buffers. We also confirmed that the parent compound 2 was 100% stable at the last incubation time in both the aqueous and plasma stability experiments.
2.3 Caco-2 permeability
A representative set of prodrugs (acetyl 3, butanoyl 5, octanoyl 7, isobutyryl 9, and 2-ethylbutyryl 10) along with the parent drug 2 were then evaluated in the bidirectional Caco-2 cell transwell model to measure their permeability and potential for improved oral absorption. Because ester prodrugs can be hydrolyzed by esterases produced by Caco-2 cells, the concentration of both the parent compound and the prodrug were monitored. The permeability coefficients Papp of each prodrug for transport from the apical-to-basolateral (AP-BL) and the converse direction (BL-AP) were determined under initial velocity conditions (<10% transport of prodrug at the last time point) by measuring the transport of compound across the Caco-2 monolayer as a function of time. The permeability coefficients reported herein represent the sum of each prodrug and the parent drug 2 (produced by prodrug hydrolysis before, during, or after transport) detected in the acceptor compartment. The efflux ratio is the ratio of Papp values in the BL-AP and AP-BL direction. The results show the all of the prodrugs evaluated, except 7, had substantially lower permeation in AP-BL direction compared to that of parent drug 2 (Table 2). The prodrugs all had higher permeation in BL-AP direction resulting in high efflux ratios ranging from 1.5 to 8.5 compared to a value of just 0.3 for 2 (Table 2). These high efflux ratios suggest that these prodrugs will almost certainly have poor oral bioavailability.
Table 2.
Caco-2 permeability coefficients (Papp) of drug 2 and selected prodrugs, and relative amount of prodrug remaining intact at 2 h in apical side (RAAP) and in basolateral side (RABL). AP-BL and BL-AP signs signify the donor solution is in apical and basolateral side, respectively.
| Compound | Papp (×10−6 cm/s) | Efflux ratio |
RAAP (%) | RABL (%) | |||
|---|---|---|---|---|---|---|---|
| AP-BLa | BL-APa | AP-BL | BL-AP | AP-BL | BL-AP | ||
| 216 | 4.2 | 1.3 | 0.3 | ||||
| 3 | 1.1 | 1.6 | 1.5 | 93 | 88 | 81 | 86 |
| 5 | 0.5 | 3.5 | 7.0 | 74 | 15 | 63 | 93 |
| 7 | 3.9 | 6.9 | 1.8 | 29 | 1 | 3 | 90 |
| 9 | 0.4 | 3.4 | 8.5 | 88 | 53 | 73 | 95 |
| 10 | 0.5 | 3.8 | 7.6 | 97 | 78 | 90 | 99 |
donor concentration = 100 µM
Since Caco-2 cells contain esterases, we measured the residual amount of each prodrug remaining intact at 2 hours in both the apical and basolateral side. The amount of prodrug remaining in the apical side is represented by RAAP. Similarly, the amount of prodrug remaining in the basolateral side is represented as RABL (Table 2). The RAAP and RABL values also depend on which side of the monolayer, the prodrug is introduced. Thus, RAAP for AP-BL signifies the relative residual amount of prodrug in the apical solution when the donor solution is in the apical side. This value correlates the amount of hydrolyzed prodrug released by Caco-2 cells into the apical side. On the other hand, RAAP for BL-AP is the relative amount of prodrug in the apical side when the donor solution is in the basolateral side and corresponds to the amount of hydrolysis occurring while the prodrug is passing through the cell monolayer as well as after permeation in the apical side. Similarly, the relative amount of prodrug in the basolateral solution (RABL) was calculated for both conditions where the donor solution is in either the apical (AP-BL) or basolateral (BL-AP) side. As an example, octanoyl prodrug 7 was almost completely hydrolyzed during transport across the Caco-2 cells, with only 1 and 3% remaining at 2 hours in the apical and basolateral sides, respectively, after BL-AP and AP-BL permeation. The result also showed that the prodrugs are hydrolyzed not only during the permeation across the monolayer by cytosolic esterases, but also on both sides of the monolayer, presumably by secreted esterases.20,21 Additionally, Caco-2 cells contain more esterases on the apical side than on the basolateral side because the prodrugs were hydrolyzed to a greater extent on the apical side than on the basolateral side. Prodrugs with short promieties such as acetyl 3 or branched chain promoieties such as isobutyryl 9 and 2-ethylbutyryl 10 were hydrolyzed to a lesser extent in these experiments. The higher stability of these prodrugs was also observed in aqueous and plasma stability studies.
3. Conclusion
A systematic series of prodrugs of the 2′-fluoro nucleoside 2 with linear and α-branched alkanoyl groups from two to twelve carbons were synthesized through esterification at the 3′-OH position of the nucleoside in order to improve membrane permeability and oral bioavailability. We developed a scalable synthesis of the key intermediate 9-[2,5-dideoxy-2-fluoro-5-(N-sulfamoyl) amino-β-d-arabinofuranosyl]adenine 15 and reliable conditions for direct installation of the ester promoieties on the unprotected nucleoside. The ester prodrugs 3–10 were stable in aqueous conditions at pH 1.2 and 7.4 and surprisingly showed high serum stability in rat and human serum with most prodrugs showing less than 50% hydrolysis at 6 hours. By contrast, the prodrugs were hydrolyzed more rapidly in mouse serum with half-lives ranging from 0.7 to 4.6 hours for 3–9 showing a parabolic relationship with maximal cleavage rates for the octanoyl prodrug 7 (t1/2 = 0.7 hours). The cleavage rates decreased proportionately with alkanoyl chain-length from eight to two carbons and also decreased for longer and α-branched alkanoyl promoieties. The relative hydrolysis in rat and human serum, although substantially lower in magnitude, also paralleled this trend. Selected prodrugs were then evaluated in the Caco-2 transwell permeability assay to evaluate membrane permeability and potential for enhanced oral bioavailability relative to 2. Surprisingly, all of the prodrugs were found to have reduced permeation in the apical-to-basolateral direction with permeability coefficients (Papp) ranging from 0.4 to 3.9 × 10−6 cm/s, compared to 2, whose Papp was 4.2 × 10−6 cm/s. More significantly, all of the prodrugs were found to have enhanced permeation in the basolateral-to-apical direction with Papp’s ranging from 1.6 to 6.9 × 10−6 cm/s, compared to 2, whose Papp was 1.2 × 10−6 cm/s. As a result the efflux ratios of 3–10 were found to be 5 to 28-fold greater than 2. These data indicate that despite greater cLogP values (Table 1), the prodrugs are substrates for efflux pumps and are also hydrolyzed intracellularly. As expected, the more sterically hindered isobutyryl prodrug 9 was hydrolyzed to a lesser extent than the butyryl prodrug 5, but the overall permeability was lower likely due to efflux pumps on the apical membrane. Further examination showed that the Caco-2 cells were able to hydrolyze the prodrugs with the same trend observed with the serum stability studies. Thus, octanoyl prodrug 7 was largely hydrolyzed by esterases on the apical side and only 29% percent remained in the apical compartment at 2 hours. The amount of intact prodrug 7 that was successfully transported across the Caco-2 monolayer during AP-BL experiment was only 1% of the total amount (2 + 7) indicating rapid hydrolysis by cellular esterases or efflux of 7 back into the apical compartment. These results collectively suggest that the described prodrug strategy using simple alkanoyl esters will not be successful to improve the oral bioavailability of 2. While this approach was ultimately unsuccessful, it has provided a useful benchmark to guide future studies and also highlights the potential contribution of intestinal epithelial cells for release of ester prodrugs, which in some cases may be faster than serum esterases.
4. Experimental section
4.1. Chemistry
4.1.1 General Chemistry Methods
All commercial reagents were used as provided unless otherwise indicated. 2′-Deoxy-2′-fluoroadenosine was purchased from Metkinen Chemistry, Finland. ACS and HPLC grade solvents were purchased from Fischer Scientific. An anhydrous solvent dispensing system using packed columns of neutral alumina was used for drying THF and CH2Cl2, while packed columns of 4 Å molecular sieves were used to dry DMF, and the solvents were dispensed under nitrogen. All reactions were performed under an inert atmosphere of argon in oven dried (130–150 °C) glassware. Thin-layer chromatography (TLC plate, Merck) was performed on a pre-coated silica gel 60 F254 plates. The detection of compounds was carried out with UV light. Flash chromatography was performed with silica gel P60 (Silicycle) with the indicated solvent system. All NMR spectra were recorded on Varian 400 or 600 MHz spectrometers. 1H NMR spectra were referenced to residual CDCl3 (7.27 ppm), DMSO-d6 (2.50 ppm), or CD3OD (3.31 ppm); 13C NMR spectra were referenced to CDCl3 (77.23 ppm) DMSO-d6 (39.51 ppm), or CD3OD (49.15 ppm). NMR chemical shift data are reported as follows: chemical shift, multiplicity (br = broad, s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), coupling constant, integration. Coupling constants are given in Hertz (Hz). High-resolution mass spectra (HRMS) were obtained on a Bruker BioTOF II instrument.
4.1.2. 9-[5-Azido-2,5-dideoxy-2-fluoro-β-d-ribofuranosyl]adenine (12)
To a solution of 2′-deoxy-2′-fluoroadenosine 11 (1.70 g, 6.37 mmol) in DMF (60 mL) was added triphenylphosphine (4.96 g, 18.91 mmol, 3.0 equiv) and NaN3 (2.05 g, 31.54 mmol, 5.0 equiv). After 5 min at rt, the reaction mixture was cooled to 0 °C, CBr4 (6.27 g, 18.91 mmol, 3.0 equiv) was added, and the reaction mixture was gradually warmed to rt. After 24 h, the reaction was quenched with methanol and the resulting mixture was concentrated under reduced pressure. The crude product was purified by flash column chromatography (SiO2, hexanes–EtOAc–EtOH, 1:3:0–0:100:5) to yield the title compound (1.44 g, 78%) as a white amorphous solid: Rf = 0.35 (5:95 EtOH/EtOAc); 1H NMR (400 MHz, CD3OD) δ 8.25 (s, 1H), 8.21 (s, 1H), 6.29 (dd, J = 19.4, 1.8 Hz, 1H), 5.53 (ddd, J = 52.8, 4.5, 1.8 Hz, 1H), 4.81 (ddd, J = 20.7, 12.5, 4.5 Hz, 1H), 4.21–4.14 (m, 1H), 3.74 (dd, J = 13.5, 2.8 Hz, 1H), 3.56 (dd, J = 13.5, 2.8 Hz, 1H); 13C NMR (100 MHz, CD3OD) δ 157.5, 154.2, 150.4, 141.5, 120.7, 94.8 (d, J = 185 Hz), 88.8 (d, J = 31 Hz), 83.0, 71.2 (d, J = 17 Hz), 52.4; HRMS (ESI+) m/z calcd for C10H11FN8O2Na+ [M + Na]+ 317.0881, found 317.0880 (Δ 0.3 ppm).
4.1.3. 9-[5-Azido-3-O-(tert-butyldimethylsilyl)-2,5-dideoxy-2-fluoro-β-d-rib` ofuranosyl]adenine (13)
To a solution of azide 12 (3.17 g, 10.8 mmol, 1.0 equiv) in DMF (110 mL) was added imidazole (4.40 g, 64.6 mmol, 6.0 equiv), DMAP (1.32 g, 10.8 mmol, 1.0 equiv) and TBSCl (4.87 g, 32.3 mmol, 3.0 equiv) and the reaction mixture was stirred for 6 h at rt. At 6 h, more TBSCl (1.62 g, 10.8 mmol, 1.0 equiv) was added. After an additional 1 h at rt, the reaction was quenched with methanol and the resulting mixture was concentrated under reduced pressure. The crude product was purified by flash column chromatography (SiO2, hexanes–EtOAc, 1:1–1:3) to yield the title compound (3.51 g, 80%) as a white amorphous solid: Rf = 0.40 (1:2 EtOAc/hexanes); 1H NMR (400 MHz, CD3OD) δ 8.25 (s, 1H), 8.21 (s, 1H), 6.26 (dd, J = 17.4, 1.6 Hz, 1H), 5.64 (ddd, J = 53.1, 4.6, 1.6 Hz, 1H), 5.11 (ddd, J = 18.9, 7.5, 4.6 Hz, 1H), 4.20–4.13 (m, 1H), 3.71 (dd, J = 13.7, 2.9 Hz, 1H), 3.44 (dd, J = 13.7, 4.2 Hz, 1H), 0.96 (s, 9H), 0.21 (s, 6H); 13C NMR (100 MHz, CD3OD) δ 157.6, 154.1, 150.4, 142.2, 120.8, 94.0 (d, J = 189 Hz), 89.0 (d, J = 35 Hz), 83.6, 72.2 (d, J = 16 Hz), 51.8, 26.3, 19.1, −4.5, −4.8; HRMS (ESI+) m/z calcd for C16H25FN8O2SiNa+ [M + Na]+ 481.1746, found 431.1758 (Δ 2.8 ppm).
4.1.4. 9-[3-O-(tert-Butyldimethylsilyl)-2,5-dideoxy-2-fluoro-5-(N-sulfamoyl)amino-β-d-arabinofuranosyl] adenine (14)
To a solution of compound 13 (2.97 g, 7.28 mmol, 1.0 equiv) in methanol (70 mL) was added 10% Pd/C wetted with 55% water (2.00 g, TCI America). The flask was evacuated, purged with hydrogen (balloon pressure) and the reaction was stirred under an atmosphere of hydrogen for 4 h at rt. The resulting mixture was filtered through a short pad of Celite and the filtrate was concentrated under reduced pressure to give the corresponding amine as a white solid. To a solution of the solid in 1,4-dioxane (70 mL) was added sulfamide (2.80 g, 29.1 mmol, 4.0 equiv) and the resulting mixture was refluxed for 13 h. The reaction mixture was concentrated under reduced pressure and the crude product was purified by flash column chromatography (SiO2, hexanes–EtOAc–EtOH, 1:3:0–0:100:15) to yield the title compound (2.73 g, 81% over 2 steps) as a white amorphous solid: Rf = 0.58 (5:95 EtOH/EtOAc); 1H NMR (400 MHz, CD3OD) δ 8.26 (s, 1H), 8.25 (s, 1H), 6.22 (dd, J = 14.3, 4.9 Hz, 1H), 5.63 (dt, J = 52.2, 4.9 Hz, 1H), 4.83 (ddd, J = 17.3, 8.6, 4.9 Hz, 1H), 4.32–4.25 (m, 1H), 3.44 (dd, J = 13.7, 2.8 Hz, 1H), 3.35 (dd, J = 13.7, 3.5 Hz, 1H), 0.96 (s, 9H), 0.19 (s, 3H), 0.17 (s, 3H); 13C NMR (100 MHz, CD3OD) δ 157.7, 154.2, 150.1, 142.4, 121.1, 92.6 (d, J = 194 Hz), 89.1 (d, J = 32 Hz), 85.9, 72.9 (d, J = 15 Hz), 45.4, 26.4, 19.2, −4.48, −4.71; HRMS (ESI+) m/z calcd for C16H28FN7O4SSiNa+ [M + Na]+ 484.1569, found 484.1566 (Δ 0.6 ppm).
4.1.5. 9-[2,5-Dideoxy-2-fluoro-5-(N-sulfamoyl)amino-β-d-ribofuranosyl]adenine (15)
This was prepared using two different methods.
Method A
To a solution of sulfamide 14 (210 mg, 0.454 mmol) in methanol (12 mL) was added 4 M HCl in 1,4-dioxane (4.0 mL). After 3 h, the reaction mixture was concentrated under reduced pressure to yield the title compound as the hydrochloride salt (182 mg, quant.) as a white amorphous solid, which was used in the next step without further purification. Characterization data is shown below in Method B.
Method B
To a solution of azide 12 (21.2 mg, 0.0721 mmol) in methanol (1.0 mL) was added 10% Pd/C wetted with 55% water (20 mg). The flask was evacuated, back-filled with hydrogen and the reaction mixture was stirred under an atmosphere of hydrogen for 6 h at rt. The reaction mixture was filtered through a short pad of Celite and the filtrate was concentrated under reduced pressure to give the corresponding amine as a white solid. To a solution of the solid aminonucleoside in 1,4-dioxane (1.0 mL) was added sulfamide (27.7 mg, 0.288 mmol, 4.0 equiv) and the resulting mixture was refluxed for 17 h. The reaction mixture was concentrated under reduced pressure and the crude product was purified by flash column chromatography (SiO2, EtOAc–MeOH, 100:0–100:5–75:25) to yield the title compound (14.5 mg, 58% over 2 steps) as a white amorphous solid: Rf = 0.23 (5:95 MeOH/EtOAc); 1H NMR (400 MHz, CD3OD) δ 8.54 (s, 1H), 8.43 (s, 1H), 6.36 (dd, J = 16.9, 2.6 Hz, 1H), 5.49 (ddd, J = 52.4, 4.4, 2.6 Hz, 1H), 4.67 (ddd, J = 16.9, 6.7, 4.4 Hz, 1H), 4.28–4.21 (m, 1H), 3.49 (dd, J = 14.1, 3.2 Hz, 1H), 3.39 (dd, J = 14.1, 4.7 Hz, 1H); 1H NMR (400 MHz, DMSO-d6) δ 8.35 (s, 1H), 8.16 (s, 1H), 7.41 (s, 2H), 7.22 (dd, J = 7.1, 4.9 Hz, 1H), 6.60 (s, 2H), 6.24 (dd, J = 17.1, 3.7 Hz, 1H), 5.82 (d, J = 5.6 Hz, 1H), 5.54 (ddd, J = 52.8, 5.7, 3.7 Hz, 1H), 4.52 (ddd, J = 14.3, 11.0, 5.7 Hz, 1H), 4.17–4.07 (m, 1H), 3.36–3.26 (m, 1H), 3.21–3.09 (m, 1H); 13C NMR (100 MHz, CD3OD) δ 151.9, 149.6, 145.6, 144.7, 121.1, 94.6 (d, J = 189 Hz), 89.3 (d, J = 34 Hz), 84.1, 71.3 (d, J = 16 Hz), 45.0; 13C NMR (100 MHz, DMSO-d6) δ 156.3, 152.7, 148.6, 140.1, 119.4, 92.4 (d, J = 189 Hz), 86.2 (d, J = 32 Hz), 82.2, 69.6 (d, J = 16 Hz), 44.3; HRMS (ESI+) m/z calcd for C10H14FN7O4SNa+ [M + Na]+ 370.0704, found 370.0691 (Δ 3.5 ppm).
4.1.6. General procedure for the preparation of compounds 16–23
To a solution of alcohol 15 (1.0 mmol, 1.0 equiv) in pyridine (6.0 mL) was added acid chloride (3.0 equiv) or anhydride (6.0 equiv) at 0 °C and the reaction mixture was stirred until the starting material disappeared as monitored by TLC. The reaction was quenched with methanol and the resulting mixture was concentrated under reduced pressure. To a solution of the residue in methanol (15 mL) was added formic acid (1.5 mL). After 16 h at rt, the reaction mixture was concentrated under reduced pressure. The crude product was purified by flash column chromatography (SiO2, hexanes–EtOAc–MeOH, 1:1:0–0:100:0–0:100:10) to yield the corresponding compound.
4.1.6.1. 9-[3-O-(Acetyl)-2,5-dideoxy-2-fluoro-5-(N-sulfamoyl)amino-β-d-ribofuranosyl]adenine (16)
Ester 16 (318 mg, 0.816 mmol, 88% in 2 steps) was obtained from alcohol 15 (356 mg, 0.928 mmol) and acetic anhydride as a pale yellow amorphous solid: Rf = 0.33 (5:95 MeOH/EtOAc); 1H NMR (400 MHz, CD3OD) δ 8.28 (s, 1H), 8.28 (s, 1H), 6.29 (dd, J = 15.9, 4.0 Hz, 1H), 5.94–5.71 (m, 1H), 5.70–5.58 (m, 1H), 4.48 (br s, 1H), 3.45 (br s, 2H), 2.17 (s, 3H); 13C NMR (100 MHz, CD3OD) δ 171.6, 157.5, 154.0, 150.1, 142.2, 121.1, 91.5 (d, J = 194 Hz), 89.2 (d, J = 33 Hz), 82.7, 72.8 (d, J = 14 Hz), 45.2, 20.6; HRMS (ESI+) m/z calcd for C12H16FN7O5SNa+ [M + Na]+ 412.0810, found 412.0806 (Δ 1.0 ppm).
4.1.6.2. 9-[2,5-Dideoxy-2-fluoro-3-O-(propionyl)-5-(N-sulfamoyl)amino-β-d-ribofuranosyl]adenine (17)
Ester 17 (274 mg, 0.679 mmol, 78% in 2 steps) was obtained from alcohol 15 (336 mg, 0.874 mmol) and propionic anhydride as a white amorphous solid: Rf = 0.30 (5:95 MeOH/EtOAc); 1H NMR (400 MHz, CD3OD) δ 8.28 (s, 1H), 8.27 (s, 1H), 6.29 (dd, J = 15.6, 4.7 Hz, 1H), 5.82 (ddd, J = 51.4, 4.9, 4.7 Hz, 1H), 5.65 (ddd, J = 10.1, 4.9, 4.3 Hz, 1H), 4.52–4.43 (m, 1H), 3.51–3.39 (m, 2H), 2.49 (q, J = 7.4 Hz, 2H), 1.18 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CD3OD) δ 175.0, 157.6, 154.1, 150.1, 142.2, 121.1, 91.6 (d, J = 195 Hz), 89.2 (d, J = 32 Hz), 82.7, 72.7 (d, J = 14 Hz), 45.3, 28.1, 9.5; HRMS (ESI+) m/z calcd for C13H18FN7O5SNa+ [M + Na]+ 426.0966, found 426.0964 (Δ 0.5 ppm).
4.1.6.3. 9-[3-O-(Butyryl)-2,5-dideoxy-2-fluoro-5-(N-sulfamoyl)amino-β-d-ribofuranosyl]adenine (18)
Ester 18 (317 mg, 0.759 mmol, 83% in 2 steps) was obtained from alcohol 15 (350 mg, 0.913 mmol) and butyric anhydride as a pale yellow amorphous solid: Rf = 0.35 (5:95 MeOH/EtOAc); 1H NMR (400 MHz, CD3OD) δ 8.28 (s, 1H), 8.27 (s, 1H), 6.29 (dd, J = 15.6, 4.7 Hz, 1H), 5.83 (ddd, J = 51.6, 5.1, 4.7 Hz, 1H), 5.66 (ddd, J = 10.1, 5.1, 4.7, 1H), 4.51–4.44 (m, 1H), 3.52–3.39 (m, 2H), 2.45 (t, J = 7.3 Hz, 2H), 1.76–1.64 (m, 2H), 0.99 (t, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CD3OD) δ 174.1, 157.6, 154.2, 150.1, 142.2, 121.1, 91.6 (d, J = 194 Hz), 91.1 (d, J = 33 Hz), 82.7, 72.6 (d, J = 14 Hz), 42.3, 36.7, 19.5, 14.0; HRMS (ESI+) m/z calcd for C14H20FN7O5SNa+ [M + Na]+ 440.1123, found 440.1151 (Δ 6.3 ppm).
4.1.6.4. 9-[2,5-Dideoxy-2-fluoro-3-O-(pentanoyl)-5-(N-sulfamoyl)amino-β-d-ribofuranosyl]adenine (19)
Ester 19 (322 mg, 0.746 mmol, 81% in 2 steps) was obtained from alcohol 15 (354 mg, 0.922 mmol) and pentanoic anhydride as a pale yellow solid: Rf = 0.40 (5:95 MeOH/EtOAc); 1H NMR (400 MHz, CD3OD) δ 8.28 (s, 1H), 8.28 (s, 1H), 6.38–6.21 (m, 1H), 5.95–5.70 (m, 1H), 5.72–5.58 (m, 1H), 4.54–4.39 (m, 1H), 3.57–3.37 (m, 2H), 2.58–2.35 (m, 2H), 1.78–1.52 (m, 2H), 1.52–1.31 (m, 2H), 1.06–0.82 (m, 3H); 13C NMR (100 MHz, CD3OD) δ 174.3, 157.3, 153.7, 150.1, 142.3, 121.1, 91.6 (d, J = 195 Hz), 89.3 (d, J = 33 Hz), 82.7, 72.6 (d, J = 14 Hz), 45.2, 34.5, 28.2, 23.3, 14.2; HRMS (ESI+) m/z calcd for C15H22FN7O5SNa+ [M + Na]+ 454.1279, found 454.1275 (Δ 0.9 ppm).
4.1.6.5. 9-[2,5-Dideoxy-2-fluoro-3-O-(octanoyl)-5-(N-sulfamoyl)amino-β-d-ribofuranosyl]adenine (20)
Ester 20 (340 mg, 0.719 mmol, 79% in 2 steps) was obtained from alcohol 15 (351 mg, 0.915 mmol) and octanoic anhydride as a white amorphous solid: Rf = 0.43 (5:95 MeOH/EtOAc); 1H NMR (400 MHz, CD3OD) δ 8.28 (s, 1H), 8.27 (s, 1H), 6.29 (dd, J = 15.4, 4.6 Hz, 1H), 5.83 (ddd, J = 51.5, 5.3, 4.6 Hz, 1H), 5.66 (ddd, J = 10.1, 5.3, 4.8 Hz, 1H), 4.52–4.45 (m, 1H), 3.52–3.41 (m, 2H), 2.47 (t, J = 7.3 Hz, 2H), 1.73–1.62 (m, 2H), 1.43–1.26 (m, 8H), 0.91 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CD3OD) δ 174.3, 157.7, 154.4, 150.1, 142.1, 121.1, 91.6 (d, J = 196 Hz), 89.2 (d, J = 32 Hz), 82.7, 72.6 (d, J = 14 Hz), 45.3, 34.8, 33.0, 30.2, 30.2, 26.1, 23.8, 14.5; HRMS (ESI+) m/z calcd for C18H28FN7O5SNa+ [M + Na]+ 496.1749, found 496.1736 (Δ 2.6 ppm).
4.1.6.6. 9-[2,5-Dideoxy-3-O-(dodecanoyl)-2-fluoro-5-(N-sulfamoyl)amino-β-d-ribofuranosyl]adenine (21)
Ester 21 (328 mg, 0.619 mmol, 68% in 2 steps) was obtained from alcohol 15 (350 mg, 0.913 mmol) and dodecanoic anhydride as a white amorphous solid: Rf = 0.48 (5:95 MeOH/EtOAc); 1H NMR (400 MHz, CD3OD) δ 8.28 (s, 1H), 8.27 (s, 1H), 6.29 (dd, J = 15.5, 4.7 Hz, 1H), 5.83 (ddd, J = 51.4, 5.1, 4.7 Hz, 1H), 5.66 (ddd, J = 10.0, 5.1, 4.7 Hz), 4.51–4.44 (m, 1H), 3.52–3.40 (m, 2H), 2.46 (t, J = 7.1 Hz, 2H), 1.72–1.62 (m, 2H), 1.42–1.23 (m, 16H), 0.89 (t, J = 6.5 Hz, 3H); 13C NMR (100 MHz, CD3OD) δ 174.3, 157.7, 154.4, 150.1, 142.1, 121.1, 91.5 (d, J = 196 Hz), 89.2 (d, J = 33 Hz), 82.7, 72.6 (d, J = 14 Hz), 45.3, 34.8, 33.2, 30.9, 30.9, 30.7, 30.6, 30.5, 30.3, 26.1, 23.9, 14.6; HRMS (ESI+) m/z calcd for C22H36FN7O5SNa+ [M + Na]+ 552.2375, found 552.2347 (Δ 5.0 ppm).
4.1.6.7. 9-[2,5-Dideoxy-2-fluoro-3-O-(isobutyryl)-5-(N-sulfamoyl)amino-β-d-ribofuranosyl]adenine (22)
Ester 22 (324 mg, 0.777 mmol, 85% in 2 steps) was obtained from alcohol 15 (350 mg, 0.913 mmol) and isobutyric anhydride as a pale yellow solid: Rf = 0.38 (5:95 MeOH/EtOAc); 1H NMR (400 MHz, CD3OD) δ 8.29 (s, 1H), 8.29 (s, 1H), 6.40–6.19 (m, 1H), 5.97–5.67 (m, 1H), 5.73–5.55 (m, 1H), 4.55–4.37 (m, 1H), 3.59–3.36 (m, 2H), 2.81–2.59 (m, 1H), 1.23 (br s, 6H); 13C NMR (100 MHz, CD3OD) δ 177.5, 157.0, 153.3, 150.0, 142.5, 121.1, 91.7 (d, J = 194 Hz), 89.3 (d, J = 34 Hz), 82.6, 72.5 (d, J = 14 Hz), 45.2, 35.1, 19.5, 19.3; HRMS (ESI+) m/z calcd for C14H20FN7O5SNa+ [M + Na]+ 440.1123, found 440.1129 (Δ 1.4 ppm).
4.1.6.8. 9-[2,5-Dideoxy-3-O-(2-ethylbutyryl)-2-fluoro-5-(N-sulfamoyl)amino-β-d-ribofuranosyl]adenine (23)
Ester 23 (251 mg, 0.563 mmol, 61% in 2 steps) was obtained from alcohol 15 (354 mg, 0.923 mmol) and 2-ethylbutyryl chloride as a pale yellow solid: Rf = 0.43 (5:95 MeOH/EtOAc); 1H NMR (400 MHz, CD3OD) δ 8.33 (s, 1H), 8.31 (s, 1H), 6.37–6.24 (m, 1H), 5.94–5.72 (m, 1H), 5.73–5.60 (m, 1H), 4.54–4.40 (m, 1H), 3.55–3.36 (m, 2H), 2.42–2.28 (m, 1H), 1.87–1.47 (m, 4H), 1.05–0.81 (m, 6H); 13C NMR (100 MHz, CD3OD) δ 176.6, 156.2, 152.2, 150.0, 142.8, 121.1, 91.9 (d, J = 194 Hz), 89.4 (d, J = 32 Hz), 82.5, 72.4 (d, J = 15 Hz), 50.2, 45.0, 26.2, 26.2, 12.3, 12.1; HRMS (ESI+) m/z calcd for C16H24FN7O5SNa+ [M + Na]+ 468.1436, found 468.1424 (Δ 2.6 ppm).
4.1.7. General procedure for the preparation of prodrugs 3–10
To a solution of the 3′-esterified nucleoside (1.0 equiv) in DMF (5.0 mL, 0.2 M in limiting reagent) was added 248 (1.0 equiv) and Cs2CO3 (2.5 equiv) at 0 °C and the reaction mixture was gradually warmed to rt. After 16 h, the reaction mixture was concentrated under reduced pressure. The residue was dissolved in CH2Cl2 and washed with 1 M HCl and brine. The organic layer was dried over MgSO4, filtered, and the solvent was removed under reduced pressure to give the corresponding coupled product. To a solution of the coupled product in CH2Cl2 (10 mL) was added methanol (5.0 mL) and 10% Pd/C wetted with 55% water (450 mg). The flask was evacuated, back-filled with hydrogen gas and the reaction mixture was stirred under an atmosphere of hydrogen for 16 h. The reaction mixture was filtered through a short pad of Celite and the filtrate was concentrated under reduced pressure. The residue was purified by flash column chromatography (CH2Cl2–MeOH–Et3N, 100:0:2–100:10:2) to yield the corresponding title compound as the triethylammonium salt.
4.1.7.1. 9-[3-O-(Acetyl)-2,5-dideoxy-2-fluoro-5-{N-(N-2-hydroxybenzoyl)sulfamoyl}amino-β-d-ribofuranosyl]adenine Triethylammonium Salt (3)
The title compound 3 (195 mg, 41% in 2 steps) was obtained from 16 (307 mg, 0.788 mmol) as a white amorphous solid: Rf = 0.20 (10:90 MeOH/CH2Cl2); 1H NMR (600 MHz, 2:1 CDCl3–CD3OD) δ 8.33 (s, 1H), 8.22 (s, 1H), 7.94–7.91 (m, 1H), 7.32–7.27 (m, 1H), 6.86–6.82 (m, 1H), 6.82–6.78 (m, 1H), 6.21 (dd, J = 15.3, 4.5 Hz, 1H), 5.80 (dt, J = 51.6, 4.5 Hz, 1H), 5.61 (ddd, J = 10.5, 5.4, 4.5 Hz, 1H), 4.48–4.44 (m, 1H), 3.47 (dd, J = 13.6, 2.8 Hz, 1H), 3.42 (dd, J = 13.6, 4.0 Hz, 1H), 2.92 (q, J = 7.5 Hz, 6H), 2.17 (s, 3H), 1.20 (t, J = 7.4 Hz, 9H); 13C NMR (150 MHz, 2:1 CDCl3–CD3OD) δ 172.9, 169.8, 159.9, 155.4, 152.7, 148.5, 139.4, 132.6, 129.4, 119.1, 119.1, 117.8, 116.3, 90.0 (d, J = 195 Hz), 86.7 (d, J = 32 Hz), 80.6, 71.0 (d, J = 15 Hz), 45.9, 43.8, 19.6, 8.81; HRMS (ESI−) m/z calcd for C19H19FN7O7S [M − H]− 508.1056, found 508.1079 (Δ 4.5 ppm).
4.1.7.2. 9-[2,5-Dideoxy-2-fluoro-5-{N-(N-2-hydroxybenzoyl)sulfamoyl}amino-3-O-(propionyl)-β-d-ribofuranosyl]adenine Triethylammonium Salt (4)
The title compound 4 (194 mg, 47% over 2 steps) was obtained from 17 (270 mg, 0.668 mmol) as a pale yellow amorphous solid: Rf = 0.23 (10:90 MeOH/CH2Cl2); 1H NMR (600 MHz, 2:1 CDCl3–CD3OD) δ 8.33 (s, 1H), 8.22 (s, 1H), 7.94–7.90 (m, 1H), 7.32–7.26 (m, 1H), 6.86–6.83 (m, 1H), 6.83–6.78 (m, 1H), 6.21 (dd, J = 15.5, 4.3 Hz, 1H), 5.81 (ddd, J = 51.5, 4.6, 4.3 Hz, 1H), 5.61 (ddd, J = 10.1, 4.9, 4.6 Hz, 1H), 4.49–4.44 (m, 1H), 3.47 (dd, J = 13.8, 2.9 Hz, 1H), 3.43 (dd, J = 13.8, 4.0 Hz, 1H), 3.14 (q, J = 7.4 Hz, 6H), 2.46 (q, J = 7.2 Hz, 2H), 1.29 (t, J = 7.4 Hz, 9H), 1.18 (t, J = 7.2 Hz, 3H); 13C NMR (150 MHz, 2:1 CDCl3–CD3OD) δ 173.2, 172.8, 159.9, 155.4, 152.7, 148.5, 139.4, 132.6, 129.4, 119.1, 119.1, 117.8, 116.3, 90.0 (d, J = 196 Hz), 86.8 (d, J = 33 Hz), 80.6, 70.9 (d, J = 14 Hz), 46.2, 43.9, 26.6, 8.2, 8.1; HRMS (ESI−) m/z calcd for C20H21FN7O7S [M − H]− 522.1213, found 522.1302 (Δ 17.0 ppm, slightly displaced by nearby internal standard peak).
4.1.7.3. 9-[3-O-(Butyryl)-2,5-dideoxy-2-fluoro-5-{N-(N-2-hydroxybenzoyl)sulfamoyl}amino-β-d-ribofuranosyl]adenine Triethylammonium Salt (5)
The title compound 5 (260 mg, 57% over 2 steps) was obtained from 18 (300 mg, 0.719 mmol) as a white amorphous solid: Rf = 0.24 (10:90 MeOH/CH2Cl2); 1H NMR (600 MHz, 2:1 CDCl3–CD3OD) δ 8.33 (s, 1H), 8.21 (s, 1H), 7.94–7.90 (m, 1H), 7.32–7.26 (m, 1H), 6.87–6.83 (m, 1H), 6.82–6.78 (m, 1H), 6.20 (dd, J = 15.6, 4.3 Hz, 1H), 5.81 (ddd, J = 51.4, 5.3, 4.3 Hz, 1H), 5.61 (dt, J = 10.6, 5.3 Hz, 1H), 4.49–4.44 (m, 1H), 3.47 (dd, J = 13.6, 3.0 Hz, 1H), 3.43 (dd, J = 13.6, 4.3 Hz, 1H), 3.09 (q, J = 7.3 Hz, 6H), 2.44–2.38 (m, 2H), 1.74–1.65 (m, 2H), 1.27 (t, J = 7.6 Hz, 9H), 0.98 (t, J = 7.6 Hz, 3H); 13C NMR (150 MHz, 2:1 CDCl3–CD3OD) δ 172.8, 172.4, 159.9, 155.4, 152.7, 148.5, 139.4, 132.6, 129.4, 129.4, 119.1, 117.8, 116.3, 90.0 (d, J = 196 Hz), 86.8 (d, J = 33 Hz), 80.7, 70.8 (d, J = 16 Hz), 46.2, 43.9, 35.2, 17.8, 12.8, 8.3; HRMS (ESI−) m/z calcd for C21H23FN7O7S [M − H]− 536.1369, found 536.1344 (Δ 4.7 ppm).
4.1.7.4. 9-[2,5-Dideoxy-2-fluoro-5-{N-(N-2-hydroxybenzoyl)sulfamoyl}amino-3-O-(pentanoyl)-β-d-ribofuranosyl]adenine Triethylammonium Salt (6)
The title compound 6 (173 mg, 37% over 2 steps) was obtained from 19 (310 mg, 0.718 mmol) as a white amorphous solid: Rf = 0.26 (10:90 MeOH/CH2Cl2); 1H NMR (600 MHz, 2:1 CDCl3–CD3OD) δ 8.33 (s, 1H), 8.20 (s, 1H), 7.94–7.90 (m, 1H), 7.32–7.27 (m, 1H), 6.86–6.83 (m, 1H), 6.83–6.78 (m, 1H), 6.20 (dd, J = 15.6, 4.4 Hz, 1H), 5.81 (ddd, J = 51.7, 4.9, 4.4 Hz, 1H), 5.60 (dt, J = 10.3, 4.9 Hz, 1H), 4.49–4.41 (m, 1H), 3.48 (dd, J = 13.8, 3.1 Hz, 1H), 3.43 (dd, J = 13.8, 4.1 Hz, 1H), 3.10 (q, J = 7.2 Hz, 6H), 2.46–2.41 (m, 2H), 1.68–1.61 (m, 2H), 1.43–1.35 (m, 2H), 1.27 (t, J = 7.2 Hz, 9H), 0.94 (t, J = 7.1 Hz, 3H); 13C NMR (150 MHz, 2:1 CDCl3–CD3OD) δ 172.9, 172.6, 160.0, 155.4, 152.8, 148.6, 139.4, 132.7, 129.4, 119.2, 119.2, 117.9, 116.4, 90.0 (d, J = 196 Hz), 86.8 (d, J = 32 Hz), 80.7, 70.9 (d, J = 15 Hz), 46.2, 43.9, 33.1, 26.4, 21.7, 13.0, 8.3; HRMS (ESI−) m/z calcd for C22H25FN7O7S [M − H]− 550.1526, found 550.1543 (Δ 3.1 ppm).
4.1.7.5. 9-[2,5-Dideoxy-2-fluoro-5-{N-(N-2-hydroxybenzoyl)sulfamoyl}amino-3-O-(octanoyl)-β-d-ribofuranosyl]adenine Triethylammonium Salt (7)
The title compound 7 (250 mg, 52% over 2 steps) was obtained from 20 (329 mg, 0.695 mmol) as a white amorphous solid: Rf = 0.30 (10:90 MeOH/CH2Cl2); 1H NMR (400 MHz, CD3OD) δ 8.31 (s, 1H), 8.30 (s, 1H), 7.90–7.83 (m, 1H), 7.30–7.22 (m, 1H), 6.82–6.72 (m, 2H), 6.26 (dd, J = 15.7, 4.2 Hz, 1H), 5.85 (ddd, J = 51.7, 5.1, 4.2 Hz, 1H), 5.67 (dt, J = 10.6, 5.1 Hz, 1H), 4.47–4.39 (m, 1H), 3.43–3.35 (m, 2H), 3.16 (q, J = 7.4 Hz, 6H), 2.45–2.35 (m, 2H), 1.69–1.57 (m, 2H), 1.39–1.23 (m, 8H), 1.26 (t, J = 7.4 Hz, 9H), 0.90 (t, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CD3OD) δ 174.4, 174.2, 162.0, 157.6, 154.5, 150.4, 141.8, 134.1, 131.1, 121.1, 120.9, 119.3, 118.0, 92.0 (d, J = 195 Hz), 88.8 (d, J = 32 Hz), 82.5, 72.8 (d, J = 15 Hz), 48.0, 45.7, 34.7, 33.0, 30.2 (×2), 26.1, 23.8, 14.6, 9.5; HRMS (ESI−) m/z calcd for C25H31FN7O7S [M − H]− 592.1995, found 592.1967 (Δ 4.7 ppm).
4.1.7.6. 9-[2,5-Dideoxy-3-O-(dodecanoyl)-2-fluoro-5-{N-(N-2-hydroxybenzoyl)sulfamoyl}amino-β-d-ribofuranosyl]adenine Triethylammonium Salt (8)
The title compound 8 (175 mg, 39% over 2 steps) was obtained from 21 (317 mg, 0.598 mmol) as a white amorphous solid: Rf = 0.36 (10:90 MeOH/CH2Cl2); 1H NMR (600 MHz, 2:1 CDCl3–CD3OD) δ 8.34 (s, 1H), 8.20 (s, 1H), 7.94–7.90 (m, 1H), 7.33–7.27 (m, 1H), 6.87–6.83 (m, 1H), 6.83–6.78 (m, 1H), 6.20 (dd, J = 15.5, 4.1 Hz, 1H), 5.81 (ddd, J = 51.6, 5.1, 4.1 Hz, 1H), 5.60 (dt, J = 10.1, 5.1 Hz, 1H), 4.50–4.42 (m, 1H), 3.47 (dd, J = 13.9, 3.2 Hz, 1H), 3.43 (dd, J = 13.9, 4.1 Hz, 1H), 3.16 (q, J = 7.6 Hz, 6H), 2.48–2.38 (m, 2H), 1.70–1.62 (m, 2H), 1.38–1.22 (m, 16H), 1.30 (t, J = 7.6 Hz, 9H), 0.88 (t, J = 7.2 Hz, 3H); 13C NMR (150 MHz, 2:1 CDCl3–CD3OD) δ 172.6 (2C), 172.6, 159.9, 155.4, 152.8, 148.6, 139.4, 132.7, 129.4, 119.2, 119.0, 117.9, 116.4, 90.0 (d, J = 197 Hz), 86.8 (d, J = 32 Hz), 80.7, 70.9 (d, J = 14 Hz), 46.3, 43.9, 33.3, 31.4, 29.1 (2C), 29.0, 28.9, 28.8, 28.6, 24.3, 22.2, 13.4, 8.1; HRMS (ESI−) m/z calcd for C29H39FN7O7S [M − H]− 648.2621, found 648.2592 (Δ 4.5 ppm).
4.1.7.7. 9-[2,5-Dideoxy-2-fluoro-5-{N-(N-2-hydroxybenzoyl)sulfamoyl}amino-3-O-(isobutyryl)-β-d-ribofuranosyl]adenine Triethylammonium Salt (9)
The title compound 9 (193 mg, 41% over 2 steps) was obtained from 22 (309 mg, 0.741 mmol) as a pale yellow amorphous solid: Rf = 0.24 (10:90 MeOH/CH2Cl2); 1H NMR (600 MHz, 2:1 CDCl3–CD3OD) δ 8.33 (s, 1H), 8.21 (s, 1H), 7.95–7.90 (m, 1H), 7.32–7.27 (m, 1H), 6.86–6.83 (m, 1H), 6.83–6.78 (m, 1H), 6.20 (dd, J = 15.7, 4.6 Hz, 1H), 5.81 (dt, J = 51.2, 4.6 Hz, 1H), 5.60 (ddd, J = 10.0, 5.2, 4.6 Hz, 1H), 4.50–4.42 (m, 1H), 3.47 (dd, J = 13.7, 3.2 Hz, 1H), 3.43 (dd, J = 13.7, 4.3 Hz, 1H), 3.15 (q, J = 7.2 Hz, 6H), 2.73–2.65 (m, 1H), 1.29 (t, J = 7.2 Hz, 9H), 1.24 (d, J = 5.7 Hz, 3H), 1.22 (d, J = 5.7 Hz, 3H); 13C NMR (150 MHz, 2:1 CDCl3–CD3OD) δ 175.9, 172.8, 159.9, 155.4, 152.8, 148.6, 139.5, 132.7, 129.4, 119.2, 119.1, 117.9, 116.4, 90.0 (d, J = 196 Hz), 86.9 (d, J = 33 Hz), 80.7, 70.8 (d, J = 16 Hz), 46.3, 44.0, 33.4, 18.3, 18.1, 8.2; HRMS (ESI−) m/z calcd for C21H23FN7O7S [M − H]− 536.1369, found 536.1373 (Δ 0.7 ppm).
4.1.7.8. 9-[2,5-Dideoxy-3-O-(2-ethylbutyryl)-2-fluoro-5-{N-(N-2-hydroxybenzoyl)sulfamoyl}amino-β-d-ribofuranosyl]adenine Triethylammonium Salt (10)
The title compound 10 (185 mg, 51% over 2 steps) was obtained from 23 (241 mg, 0.542 mmol) as a white amorphous solid: Rf = 0.33 (10:90 MeOH/CH2Cl2); 1H NMR (400 MHz, CD3OD) δ 8.32 (s, 1H), 8.31 (s, 1H), 7.89–7.83 (m, 1H), 7.30–7.22 (m, 1H), 6.82–6.72 (m, 2H), 6.26 (dd, J = 16.1, 4.0 Hz, 1H), 5.88 (ddd, J = 51.6, 5.1, 4.0 Hz, 1H), 5.69 (dt, J = 10.7, 5.1 Hz, 1H), 4.47–4.40 (m, 1H), 3.41–3.35 (m, 2H), 3.11 (q, J = 7.4 Hz, 6H), 2.38–2.27 (m, 1H), 1.73–1.50 (m, 4H), 1.24 (t, J = 7.4 Hz, 9H), 0.97–0.88 (m, 6H); 13C NMR (100 MHz, CD3OD) δ 176.5, 174.5, 162.0, 157.6, 154.5, 150.4, 141.9, 134.1, 131.0, 121.1, 120.9, 119.3, 118.0, 92.0 (d, J = 195 Hz), 88.9 (d, J = 32 Hz), 82.6, 72.8 (d, J = 15 Hz), 50.2, 48.0, 45.8, 26.2 (2C), 12.3, 12.1, 9.6; HRMS (ESI−) m/z calcd for C23H27FN7O7S [M − H]− 564.1682, found 564.1703 (Δ 3.7 ppm).
4.2. HPLC method
Reversed-phase HPLC analysis was performed on an Agilent 1260 instrument (Agilent Technologies, Santa Clara, CA) equipped with a Phenomenex Gemini C18, 5 micron, 4.6 × 250 mm column (Phenomenex, Torrence, CA). The mobile phase consisted of 20 mM triethylammonium bicarbonate (TEAB) buffer, pH 7.5 as aqueous (A) and acetonitrile as organic (B) components. The 20 mM TEAB buffer was prepared by freshly diluting a 1 M stock which, in turn, was prepared by bubbling CO2 into 1 M aqueous trimethylamine at 4 °C until the pH reached 7.5. Elution was performed with the following gradient: 5 to 70% B over 10 min, 90% B from 10 to 12 min, isocratic at 90% B from 12 to 16 min, and re-equilibration at 5% B for 3 min as a postrun before the next injection. The retention times and purity of the parent drug 2 and prodrugs 3–10 are listed in the supporting information (Table S1) along with HPLC traces. The detection wavelength was set at 254 nm and the flow rate was 1.0 mL/min. The precision and accuracy of the analytical methods was ≤ 10% at all concentrations. Standard curves were processed in duplicate with the samples.
4.3. Aqueous stability
A previously described method was followed to determine the stability of prodrugs in aqueous buffers.22 The parent drug 2 and prodrugs 3–10 (100 µM) were incubated at 37 °C in simulated gastric fluid (pH 1.2) and 100 mM HEPES (pH 7.4). In triplicate, buffers (980 µL) were pre-incubated for 5 min at 37 °C, 20 µL of a 20.0 mM DMSO stock solution of parent drug 2 or prodrugs 3–10 were added, the mixtures were briefly vortexed (3 sec), and incubated at 37 °C. Aliquots (100 µL) were removed from the incubation solution at 0, 5, 10, 15, 30, 45, 60, and 120 min, and immediately placed on ice and injected (50 µL) onto the HPLC. Once we realized that the prodrugs are quite stable in these buffers, only 0 and 120 min time points were used for subsequent HPLC analysis. Stability was determined by calculating the percentage of prodrug remaining after the 120 min incubation time.
4.4. Plasma stability
The plasma stability of prodrugs was investigated with CD-1 female pooled mouse plasma, Sprague-Dawley female pooled rat plasma, and female pooled human plasma (BioChemed, Winchester, VA). In triplicate, plasma (980 µL) was pre-incubated for 5 min at 37 °C followed by the addition of 20 µL of a 10.0 mM DMSO stock solution of 2 and prodrugs 3–10. Aliquots (100 µL) were withdrawn at 0, 10, 30, 60, 120, 240, and 360 min and immediately quenched with an equal volume of 10% TCA to precipitate the proteins. The samples were vortexed, centrifuged (30 sec at 15,000 × g), and 140 µL of the supernatant were collected in HPLC vials and kept in ice. Samples were analyzed by injecting 100 µL of samples onto the HPLC. The stability of the prodrugs was determined by calculating the half-lives of the parent compounds.
4.5. Caco-2 permeability
Studies to determine permeability of prodrugs in Caco-2 monolayers followed an adapted protocol described by Hubatsch and co-workers23 and a method previously published from our group.16 Caco-2 cells (ATCC® HTB-37™) from the stock were thawed and cultivated in Dulbecco’s modified Eagle’s medium (DMEM) media containing 17% fetal calf serum and 1% PEST (penicillin 10,000 U mL−1, streptomycin 10,000 µg mL−1). Cells of desired passage number (50–70) were seeded on transwell membrane plate (12-well, 12 mm insert, 0.4 µm pore, Corning No. 3401) and grown for 21 days (37 °C, CO2 incubator, 5% CO2, water saturated atmosphere). The medium was changed every other day to support the required amount of growth. On the day of experiment, the media in the plates was aspirated and the apical side washed twice with cell assay buffer (CAB) pH 6.0 (3.56 g NaCl, 1.05 g NaHCO3, 0.9 g glucose, 0.975 g MES, 0.112 g KCl, 0.147 g MgSO4, 0.10 g CaCl2, 0.035 g K2HPO4 in 500 mL of water). The basolateral side was washed with CAB pH 7.4 (Same composition as CAB 6.0, except 1.19 g HEPES instead of MES). The apical side was filled with 0.5 mL of CAB, pH 6.0, and the basolateral side was filled with 1.5 mL of CAB, pH 7.4. With an EVOM epithelial voltammeter, electrical resistance across the differentiated Caco-2 cells were measured in triplicate. Only the wells with transepithelial electrical resistance (TER) of more than 300 Ωcm2 were used for the studies. The plate with the CAB was preincubated in an incubator shaker for 60 min, 65 rpm. Donor solution (2.5 mL) for the apical side was made with the test compound (12.5 µL, 20 mM DMSO stock), luciferin yellow (12.5 µL, 20 mM in water) and CAB pH 6 (2.475 mL). Donor solution (6 mL) for the basolateral side was made with the test compound (30 µL, 20 mM in DMSO), luciferin yellow (30 µL, 20 mM in water) and CAB pH 7.4 (5.97 mL). The concentration of test compound in both donor solutions was 100 µM. Luciferin yellow was added to assess the intactness of tight junctions in the donor solutions. The CAB solutions were aspirated and the experiment was initiated by filling the apical side with 0.5 mL of donor solution and the basolateral side with 1.5 mL of donor solution. Samples (100 µL) were taken out at 0, 30, 60 and 120 minutes in triplicates from both the apical and basolateral sides and were analyzed by HPLC. Papp AP-BL and Papp BL-AP, and efflux ratio were calculated based on the cumulative sampling, correcting for the successive dilutions. The Papp values were calculated using equation (1):
| (eq 1) |
where is the steady state flux, A is the surface area of the filter (cm2) and C0 is the initial concentration in the donor chamber.
Supplementary Material
Scheme 2.
Reagents and conditions: (a) 1. Anhydride or acid chloride, pyridine, 0 °C; 2 h 2. Formic acid, MeOH, rt, 16 h, 61–88%; (b) 24, Cs2CO3, DMF, 0 °C to rt, 16 h; (c) Pd/C, H2, CH2Cl2/MeOH (2:1), rt, 16 h, 37–57% over two steps.
Acknowledgments
This work was supported by a grant from the NIH (AI070219 to C.C.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmc.xxxx.xx.xxx.
References and notes
- 1.Lenaerts A, Barry CE, 3rd, Dartois V. Immunol. Rev. 2015;264:288–307. doi: 10.1111/imr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dartois V. Nat. Rev. Microbiol. 2014;12:159–167. doi: 10.1038/nrmicro3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dartois V, Barry CE., 3rd Bioorg. Med. Chem. Lett. 2013;23:4741–4750. doi: 10.1016/j.bmcl.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warner DF. Cold Spring Harb. Perspect. Med. 2014;5 doi: 10.1101/cshperspect.a021121. pii: a021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. http://www.who.int/mediacentre/news/releases/2015/tuberculosis-mortality/en/
- 6.Ferreras JA, Ryu JS, Lello FD, Tan DS, Quadri LEN. Nat. Chem. Biol. 2005;1:29–32. doi: 10.1038/nchembio706. [DOI] [PubMed] [Google Scholar]
- 7.Miethke M, Bisseret P, Beckering CL, Vignard D, Eustache J, Marahiel MA. FEBS J. 2006;273:409–419. doi: 10.1111/j.1742-4658.2005.05077.x. [DOI] [PubMed] [Google Scholar]
- 8.Somu RV, Boshoff H, Qiao C, Bennett EM, Barry CE, 3rd, Aldrich CC. J. Med. Chem. 2006;49:31–34. doi: 10.1021/jm051060o. [DOI] [PubMed] [Google Scholar]
- 9.Duckworth BP, Wilson DJ, Nelson KM, Boshoff H, Barry CE, 3rd, Aldrich CC. ACS Chem. Biol. 2012;7:1653–1658. doi: 10.1021/cb300112x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lun S, Guo H, Adamson J, Cisar JS, Davis TD, Chavadi SS, Warren JD, Quadri LEN, Tan DS, Bishai WR. Antimicrob. Agents Chemother. 2013;57:5138–5140. doi: 10.1128/AAC.00918-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somu RV, Wilson DJ, Bennett EM, Boshoff HI, Celia L, Beck BJ, Barry CE, 3rd, Aldrich CC. J. Med. Chem. 2006;49:7623–7635. doi: 10.1021/jm061068d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiao CH, Gupte A, Boshoff HI, Wilson DJ, Bennett EM, Somu RV, Barry CE, 3rd, Aldrich CC. J. Med. Chem. 2007;50:6080–6094. doi: 10.1021/jm070905o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neres J, Labello NP, Somu RV, Boshoff HI, Wilson DJ, Vannada J, Chen L, Barry CE, 3rd, Bennett EM, Aldrich CC. J. Med. Chem. 2008;51:5349–5370. doi: 10.1021/jm800567v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelhart CA, Aldrich CC. J. Org. Chem. 2013;78:7470–7481. doi: 10.1021/jo400976f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawadi S, Viswanathan K, Boshoff HI, Barry CE, 3rd, Aldrich CC. J. Org. Chem. 2015;80:4835–4850. doi: 10.1021/acs.joc.5b00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson KM, Viswanathan K, Dawadi S, Duckworth BP, Boshoff HI, Barry CE, 3rd, Aldrich CC. J. Med. Chem. 2015;58:5459–5475. doi: 10.1021/acs.jmedchem.5b00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F, Maag H, Alfredson T. J. Pharm. Sci. 2008;97:1109–1134. doi: 10.1002/jps.21047. [DOI] [PubMed] [Google Scholar]
- 18.Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Nat. Rev. Drug Discovery. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 19.Brass EP. Pharmacol. Rev. 2002;54:589–598. doi: 10.1124/pr.54.4.589. [DOI] [PubMed] [Google Scholar]
- 20.Imai T, Imoto M, Sakamoto H, Hashimoto M. Drug Metab. Dispos. 2005;33:1185–1190. doi: 10.1124/dmd.105.004226. [DOI] [PubMed] [Google Scholar]
- 21.Ohura K, Nozawa T, Murakami K, Imai T. J. Pharm. Sci. 2011;100:3985–3994. doi: 10.1002/jps.22628. [DOI] [PubMed] [Google Scholar]
- 22.Teitelbaum AM, Meissner A, Harding RA, Wong CA, Aldrich CC, Remmel RP. Bioorg. Med. Chem. 2013;21:5605–5617. doi: 10.1016/j.bmc.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubatsch I, Ragnarsson EG, Artursson P. Nat. Protoc. 2007;2:2111–2119. doi: 10.1038/nprot.2007.303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.