Abstract
Purpose
After practice, augmented feedback is the most important factor that facilitates motor learning. We assess the potential effectiveness of two types of augmented auditory feedback on the re-learning of arm reaching in individuals with stroke: (a) real-time knowledge of performance (KP) feedback and (b) rhythmic cueing in the form of knowledge of results (KR) feedback.
Method
Five participants with stroke underwent short-term practice, reaching with their affected arm with KP, KR and no feedback, on separate days. We assessed range of motion of the upper extremity (shoulder, elbow) and trunk, mean error and variability of the performed trajectory, and movement time, before and after training.
Results
All participants benefitted from practice with feedback, though the effects varied across participants and feedback type. In three participants, KP feedback increased elbow extension and reduced compensatory trunk flexion. In four participants, KR feedback reduced movement time taken to perform the reach. Of note, one participant benefitted mostly from KP feedback, which increased shoulder flexion and elbow extension, and decreased compensatory trunk flexion and mean error.
Conclusions
Within day practice with augmented auditory feedback improves reaching in individuals with stroke. This warrants further investigation with longer practice periods in a larger sample size.
Keywords: Knowledge of performance, knowledge of results, motor learning
Introduction
Stroke is one of the leading causes of disability, with an estimated incidence of 795 000 cases per year in the USA (American Heart Association www.strokeassociation.org). At 6 months post-stroke, only about 11% of stroke survivors show complete functional recovery of the upper limb [1]. There are a variety of therapeutic techniques to treat the upper extremity post-stroke, though currently no regime is the gold standard [2–4]. However, the application of motor learning principles is one approach that may support and enhance therapeutic gains during rehabilitation [5,6]. Specifically, augmented feedback is one of the most potent factors that can influence motor learning [7,8]. It is sensory information provided to the individual from the environment that supplements naturally or internally available information, derived for example from a person’s own proprioception and sensation. When the central nervous system is impaired post-stroke, the ability to process internal feedback information may become compromised [9]. Thus, the use of augmented feedback may be highly important for the re-learning of previously acquired skills post-stroke.
The use of augmented feedback to improve motor re-learning in stroke survivors is an approach that has been less systematically studied but shows promise [3,10–13]. In particular, the use of music as a form of augmented feedback may be a fun and motivating approach to facilitate motor stroke recovery [14–16]. Furthermore, individuals are better able to execute timely motor responses with sounds as opposed to visual cues [17–20], with these sensorimotor interactions supported by a tight link between cortical auditory and motor systems [21–23]. Even the mere act of listening to musical rhythms activates motor regions of the brain [24,25], which suggests a potential for music to prime the motor system and hence movements.
Augmented feedback can be broadly categorized into two types: knowledge of results (KR) and knowledge of performance (KP). KR is feedback information about the outcome or goal of the movement [7]. In contrast, KP is feedback information about the movement pattern or kinematics used to achieve the goal [7], and can thus inform individuals about their quality of movements. Specifically, the provision of rhythmic auditory cues, whereby movements are executed in synchrony with a sound, have been applied as a form of KR feedback to improve gait [26,27] and upper extremity motor control [28–30] post-stroke. Rhythmic feedback may be conceptualized as a type of KR since a movement that occurs in time with a sound indicates to the participant they have successfully achieved the task. The rhythmic cues do not inform individuals about the quality of their movements, however, the cueing might improve them because it provides a temporal framework for motor actions to map onto [31]. Preliminary findings suggest rhythmic cueing can improve range of motion (ROM) in the arm and as well decrease compensatory trunk flexion [30].
In contrast, others have shown the provision of KR feedback improves the end-point accuracy of the reach to a target, whereas KP feedback improves the movement kinematics implemented by the upper extremity to reach the target [32,33]. Specifically, KP feedback facilitates the use of shoulder flexion and elbow extension while reducing compensatory strategies such as trunk flexion [32,33]. This work applied feedback in the verbal and visual modalities. The application of KP feedback in the context of music has to our knowledge, not been studied.
Taken together, there is evidence for use of augmented feedback to increase ROM of the upper extremity and to reduce compensatory trunk movements, during reaching. However, it is unclear what type of feedback may be more effective, especially if one wants to emphasize KP or KR in the context of giving musical stimuli for motor rehabilitation. Thus, the aim of this proof-of-concept case series in five individuals with stroke is to explore the effects of short-term practice using music-based KP, KR, and no feedback on hemiplegic arm reaching ability. Evidence from short-term practice about which auditory feedback cue would be most optimal would then allow us to design and conduct a longer-term music-based intervention using the most promising feedback cue.
Methods
Participants
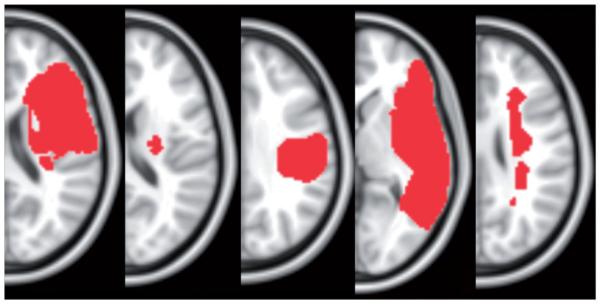
Five right-handed individuals (one female) with stroke gave written informed consent to participate in the study approved by the Institutional Review Board of Beth Israel Deaconess Medical Center, Boston. Inclusion criteria were as follows: first ischemic stroke in the middle cerebral artery territory, no previous or subsequent cerebral ischemia or hemorrhage, no hearing impairment according to subject’s report, and at least 10° of shoulder flexion and elbow extension. All individuals underwent the Upper Extremity Fugl Meyer (UE-FM) assessment of motor impairment. This consists of 30 voluntary UE motions observed by a rater and three tendon tap responses, with a maximal score of 66 [34]. Scores are also presented out of 56 since one participant had surgery to the wrist, preventing testing of this section. Thus, a new maximal UE-FM score of 56 was calculated that did not include any scores for wrist movements. See Table 1 for patient characteristics and Figure 1 for lesion location.
Table 1.
Participant demographics.
| ID | Age at assessment (years) |
Sex | Time since stroke (months – m, days – d) |
Affected side | Lesion volume (cc) | Upper extremity Fugl–Meyer |
|---|---|---|---|---|---|---|
| 01 | 55 | M | 108 m 24d | R | 169.24 | 14/66 (14/56) |
| 02 | 60 | F | 2m6d | L | 1.06 | 44/66 (39/56) |
| 03 | 52 | M | 57 m 3d | L | 39.19 | 53/66 (46/56) |
| 04 | 57 | M | 52 m 15d | L | 199.25 | 33/66 (33/56) |
| 05 | 64 | M | 14 m 10d | R | 10.26 | 30/66 (17/56) |
Figure 1.
Lesion location. Lesions are shown in the right hemisphere, superimposed on the standard MNI 152 template. The slice with the maximal lesion size is shown for each participant. Participant 1–5 shown from left to right.
Electrogonimeter sensor set up
Data were collected for the affected upper extremity using electrogoniometer sensors (Biometrics Ltd., UK). These sensors measured flexion and extension at the elbow, shoulder and trunk during reaching (Figure 2), from which we also derived real-time KP feedback. The distal endblock of the elbow goniometer was attached to the forearm with its center axis coincident with the center axis of the forearm; the proximal endblock was attached to the arm with its center axis coincident with the center axis of the arm. The distal endblock of the shoulder goniometer was attached to the arm, over the belly of the biceps muscle, while the proximal endblock was attached just lateral to the convex portion of the clavicle. The distal endblock of the trunk goniometer was attached to the trunk at the level of L5, while the proximal endblock was attached to the back of the chair. Subjects were instructed to sit upright such that the distal and proximal endblocks of the trunk goniometer were aligned at 180°.
Figure 2.
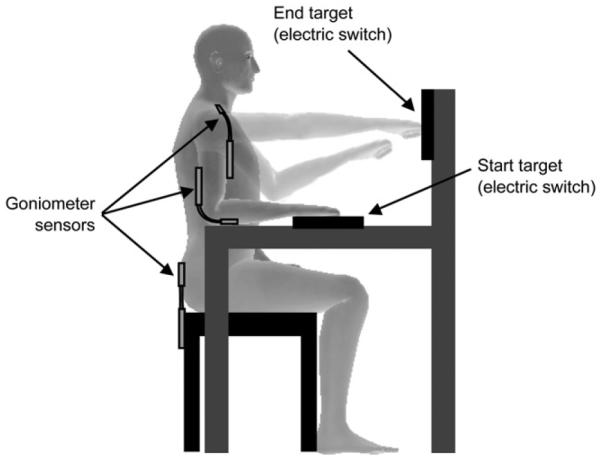
Experimental setup. Electrogoniometer sensors measure elbow, shoulder and trunk movements. The start and end targets incorporate electric switches that measured reaching times.
Reaching task
Participants were seated with their initial arm position in 90° elbow flexion, 0° shoulder flexion and abduction, with the hand resting on the start target, fist of the hand closed (Figure 2). The end target was placed in front of the participant, at arm’s length. This distance was measured at 90° shoulder flexion (no abduction/adduction), 0° elbow flexion. The start and end targets along with the upper extremity were aligned in the same plane. One trial of reaching encompassed the displacement of the fist from the start to end target and back. Hitting either targets produced a click sound, which was the sound of the electric switch embedded in the targets that was used to calculate the start and stop times for each trial. To become familiar with the general experimental setup, participants practiced three trials of reaching at their own pace with no feedback.
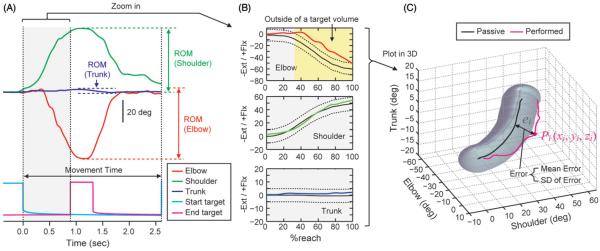
The signals of the goniometer sensors were amplified (K800 Amplifier, Biometrics Ltd.), synchronized with the electric switches (two 1.5 V batteries), converted from analog to digital at a frequency of 200 Hz (ADA 16-32/ 2(CB)F, Contec Co., Ltd.), and recorded on a personal computer with a custom written program in C++. A typical example of collected data during a forward and backward reach is shown in Figure 3(A).
Figure 3.
Data analysis. (A) A typical example of data recorded from electrogoniometers (red: elbow, green: shoulder, blue: trunk) and electric switches (cyan: start target, magenta: end target). Movement time was calculated from the start target signal. ROM was calculated from the electrogoniometer sensor. (B) Data are shown for the reach, from time of hand lift-off from the start switch to time when the hand hits the target switch. The time axis (i.e. x-axis) is denoted as percentage of reach; the y-axis is joint angle in degrees. The positive and negative values on the y-axis denote joint flexion (Flx) and extension (Ext), respectively. The black solid and dashed lines represent the target trajectory and its volume, respectively. The yellow area represents the portion of the reach performed outside the target volume. (C) Three-dimensional plot in elbow-, shoulder-, and trunk-joint angle space. Black line and gray area indicate the target trajectory and its volume, respectively. Magenta line indicates a performed movement. The mean error and SD was calculated at each time frame of a performed movement trajectory to assess the degree to which the movement deviates from the target.
Study conditions
We explored the effects of two types of augmented auditory feedback, continuous KP and rhythmic KR, and no feedback, on arm reaching ability. In the KP condition, participants performed arm reaches between targets while receiving real-time continuous auditory feedback throughout the trajectory of the reach. In this condition, real-time information (data sampled at 100 Hz, i.e. every 10 ms) about how the elbow, shoulder and trunk moved during the reach was provided. Participants heard a consonant (e.g. pleasant) sound played continuously (details in section: ‘‘Consonant and dissonant stimuli’’) if movements were performed accurately. That is, data from the participant’s elbow, shoulder and trunk were within a predetermined target trajectory volume (details in section: ‘‘Generation of target trajectory volume’’). A dissonant (e.g. unpleasant) sound was heard in real-time if data from any joint was outside the target trajectory volume. When this occurred, participants were instructed to adjust their elbow, shoulder and/or back since one or more of these joints were moving incorrectly. For example, the yellow area in Figure 3(B) illustrates data from the elbow that was outside the target volume and a dissonant sound played. Participants did not know which specific joint produced the error. We choose this approach to minimize the cognitive processing load; the task could be too difficult if an individual with stroke has to learn to associate different sounds with different joint movements. Furthermore, it is well-known that too much feedback information may slow down learning, in healthy individuals [7]. Thus, we did not encode every possible kinematic variable as a feedback signal.
In the rhythmic auditory KR condition, participants performed arm reaches between start and end targets repeatedly, synchronizing the contact of fist-to-target in time with a metronome, a device that produces a cue at a set time interval, e.g. 1 Hz. If movements are synchronized with the metronome, this indicates correct performance. If the reach lags behind or occurs ahead of the metronome, this indicates an error. Prior to testing, a comfortable metronome pace was selected for each subject.
Thus, the KP and KR conditions differed in that one provides continuous real-time feedback throughout the movement trajectory, and the other provides rhythmical feedback at the end points of movement. In both conditions, auditory feedback was provided on every trial. In the no-feedback condition participants performed reaching with no auditory feedback. Sounds were played binaurally through headphones and loudness was set at a comfortable level for each participant. Participants closed their eyes to ensure only feedback from the auditory domain could be used.
Study design
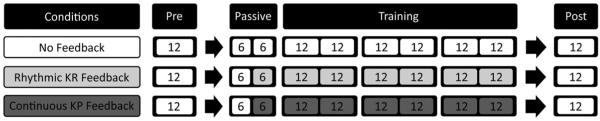
Three conditions (rhythmic KR, continuous KP, no feedback) were tested on separate days, randomized for presentation order across participants. For each condition, we first obtained baseline pre-training data whereby participants performed 12 trials of reaching without feedback. Second, the experimenter passively moved the arm between the start and end targets for six trials. This data was used to create a target trajectory volume (details in section: ‘‘Generation of the target trajectory volume’’) from which the real-time continuous auditory feedback signal was generated and used for the training period. Third, the experimenter then performed an additional six trials of passive reaching to demonstrate each of the feedback conditions. Fourth, participants practiced reaching with one of the three conditions. This training comprised 72 trials of reaching broken into six blocks of 12 trials with rest provided between blocks. Lastly, participants performed another 12 trials of post-training reaching with no feedback. Thus, the pre- and post-training (no feedback) trials allowed us to evaluate short-term retention effects for each of the conditions (Figure 4).
Figure 4.
Experimental protocol. The number of trials in each block is shown. Light gray boxes denote trials where rhythmic KR feedback is provided while dark gray boxes denote trials where continuous KP feedback is provided.
Consonant and dissonant stimuli
Two types of distinguishable sounds, one signaling error and the other correct performance, were created for the continuous KP condition. Consonant sounds are pleasant and more preferred than dissonant sounds [35]; the latter is perceptually harsh and unpleasant since they create a sensation of beating [36]. Two pairs of consonant/dissonant stimuli were created, pair 1: C-G/C-C#; pair 2: C#-G#/G-G# (frequency C ¼ 261.626 Hz; C# ¼ 277.183 Hz; G ¼ 391.995 Hz; G# ¼ 415.305 Hz). Each stimulus in a pair comprised of two pure tones; a consonant stimulus forms the interval of a perfect fifth (i.e. seven semitones apart), and a dissonant stimulus forms the interval of a minor second (i.e. one semitone apart). The peak amplitude of the stimuli was normalized such that there were no differences in sound intensity, only changes in sound consonance and dissonance.
Participants underwent a hearing test to ensure they could discriminate between consonant and dissonant sound pairs, and thus perform the reaching task using continuous KP feedback. In this hearing test, participants pressed a button when they heard a change in sound. In half the trials, the consonant stimulus was presented first and the dissonant stimulus presented at 1, 2, 3 or 4 s following (total trial duration is 5 s). This prevented participants from anticipating and thus predicting when the stimulus would occur. There were four orders of stimuli presentation (consonant to dissonant, dissonant to consonant, for each of stimuli pair 1 and pair 2), four time points at which the change occurred, and two trials per configuration, rendering 32 trials. An additional 32 trials were also included where by there was no change in the sound stimulus (participants were instructed to not press the button). Here, stimuli (C-G, C#-G#, C-C# and G-G#) were played throughout the 5-s period for eight trials each. All trials were randomly presented with sounds presented at a comfortable level, binaurally through a headphone (HD 201, Sennheiser). To determine whether participants could discriminate between consonant and dissonant sound pairs, we calculated the percentage of correct responses and reaction time to changes in the stimuli.
Generation of the target trajectory volume
A target trajectory volume was created to define what would be considered correct and incorrect reaching performance for the continuous KP feedback condition. This volume was derived from the six trials of passive movement so that it would be individualized for each participant. This enabled us to take into consideration each person’s impairment level and thus the amount of improvement that could be realistically seen. First, we normalized the time taken between lift-off of the hand from the start target and contact of the hand with the end target (an example of the time normalization is shown as the gray area in Figure 3A and percent (%) reach in Figure 3B). Second, we averaged the time-series data across the six trials of passive movement to create a target trajectory. Third, the target trajectory volume was defined as ±10° of the target trajectory for the elbow and shoulder, and ±5° of the target trajectory for the trunk (Figure 3C). For example, the black solid and dashed lines in Figure 3(B) denote the target trajectory and its volume, respectively. These ranges take into consideration the natural variability of movements during arm reaching. Electrogoniometer data from healthy participants in our pilot testing showed that inter-trial variability (2 SD) across 100 trials of reaching were 7.63°, 6.41° and 1.83° at elbow, shoulder and trunk, respectively. This pilot work shows ±10° range for the elbow and shoulder, and ±5° range for the trunk, accommodated the natural variability of movements.
Data analysis
The pre- and post-no-feedback trials for each condition were analyzed to determine the short-term effects of practice. For each reach, we calculated ROM at each joint (elbow, shoulder, trunk) and movement time. ROM is defined as the maximal change in joint angle during the reach (see Figure 3A). Movement time is defined as the time when the hand lifts off from the start switch and returns to it (see Figure 3A). As a secondary outcome measure, we assessed how a performed movement deviates from a target trajectory. We define an error at ith sampled time frame (ei) as,
| (1) |
where xi, yi and zi, are ith sampled time frame of the performed joint angle at the elbow, shoulder and trunk, respectively, while xj, yj and zj, are jth sampled time frame of the target joint angles. Namely, the error (ei) was calculated as the minimum distance from a point (Pi) to the target trajectory in the three-dimensional plot in elbow-, shoulder, and trunk-joint angle space (Figure 3C). We calculated mean and SD of the errors across the sampled time frames. Mean error and its SD were calculated only for the forward portion of the reach (i.e. the time when the hand lifts off from the start switch and hits the target switch). This is because creation of the continuous feedback is based on the forward portion of the reach toward the end target.
For each condition, data for each dependent measure were averaged across the 12 pre- and post-reaching trials separately, and a ratio of post/pre was calculated. A value of 1 indicates no change, values >1 indicate measures post-training were greater than those pre-training (i.e. increase ROM at elbow and shoulder) and values <1 indicate that measures post-training were reduced relative to pre-training (i.e. decreased ROM at trunk, movement time, mean error and its SD). Given five participants were tested, data are presented and discussed as a case series.
Magnetic resonance imaging
We obtained magnetic resonance imaging (MRI) images on a 3-T GE scanner to delineate the stroke lesion. For each participant, we obtained a T1-weighted high-resolution scan (0.93 × 0.93 × 1.5 mm3) and a set of axial fluid-attenuated inversion recovery (FLAIR) images (0.5 × 0.5 × 5 mm3). Lesions were drawn on each participant’s normalized T1-weighted image, using the FLAIR for additional guidance on lesion delineation (Figure 1).
Results
Perceptual hearing test
Mean percentage of correct response to the stimulus was 96.9 ± 2.4%. Mean reaction time to a change in the stimulus was 620.08 ± 80.52 ms.
Effect of feedback on reaching performance
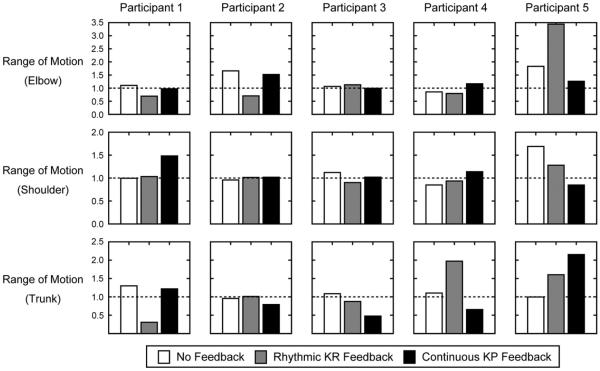
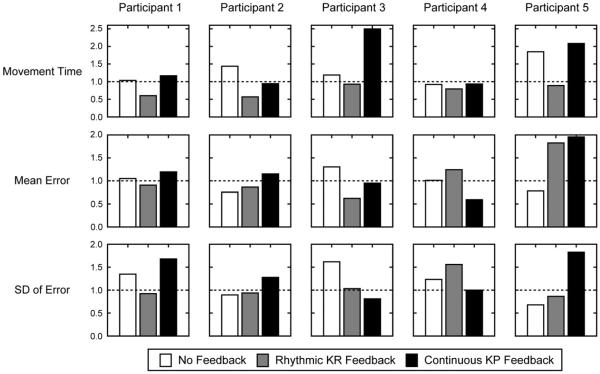
All five participants demonstrated some benefit to practice with feedback. Responses varied across feedback type and participants (Figures 5 and 6, Table 2), however, some general trends include: (1) continuous KP feedback increased elbow extension and reduced compensatory trunk flexion in three individuals; (2) rhythmic KR feedback reduced movement time for reaching in four individuals. Below, we describe the effects in each participant.
Figure 5.
Change in range of motion at elbow, shoulder and trunk after training. A value of 1 (dashed horizontal lines) indicates no change, values >1 indicate that measures post-training were greater than those pre-training, and values <1 indicate that measures post-training were reduced relative to pre-training.
Figure 6.
Change in movement time, mean error and variability of error after training. A value of 1 (dashed horizontal lines) indicates no change, values >1 indicate that measures post-training were greater than those pre-training and values <1 indicate that measures post-training were reduced relative to pre-training.
Table 2.
Summary of results.
| Measure | Participant 1 |
Participant 2 |
Participant 3 |
Participant 4 |
Participant 5 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NF | KR | KP | NF | KR | KP | NF | KR | KP | NF | KR | KP | NF | KR | KP | |
| ROM Elbow | ** | ** | * | * | ** | ** | ** | ||||||||
| ROM Shoulder | ** | * | ** | ** | |||||||||||
| ROM Trunk | ** | ** | * | ** | ** | ||||||||||
| Movement Time | ** | ** | ** | * | |||||||||||
| Mean Error | ** | * | ** | ** | ** | ||||||||||
| SD of Error | * | * | ** | * | |||||||||||
ROM, range of motion; SD: standard deviation; NF: no feedback; KR: rhythmic knowledge of results feedback; KP: continuous knowledge of performance feedback.
Post/pre is greater than 1.1 for ROM Elbow and ROM Shoulder or less than 0.9 for ROM Trunk, Movement Time, Mean Error, and SD of Error.
Post/pre is greater than 1.2 for ROM Elbow and ROM Shoulder or less than 0.8 for ROM Trunk, Movement Time, Mean Error, and SD of Error.
Participant 1
Short-term performance was improved when practice occurred with, as opposed to without, feedback. Specifically, continuous KP feedback enhanced shoulder flexion, while rhythmic KR feedback decreased compensatory trunk flexion and movement time for reaching.
Participant 2
Short-term performance was improved when practice occurred both with and without feedback. Specifically, practice without feedback improved elbow extension, and reduced mean error and variability of error. Practice with rhythmic KR feedback reduced movement time for reaching and mean error. Practice with continuous KP feedback increased shoulder flexion and decreased compensatory trunk flexion.
Participant 3
Short-term performance was improved when practice occurred both with and without feedback. Specifically, practice without feedback improved shoulder flexion. Practice with rhythmic KR feedback increased elbow extension, and decreased compensatory trunk flexion and mean error. Practice with continuous KP feedback also reduced compensatory trunk flexion and variability of error.
Participant 4
Short-term performance was improved when practice occurred with, as opposed to without, feedback. Specifically, this individual appeared to benefit mostly from practice with continuous KP: increased shoulder flexion and elbow extension, and decreased compensatory trunk flexion and mean error. Practice with rhythmic KR feedback only reduced movement time for reaching.
Participant 5
Short-term performance was improved when practice occurred both with and without feedback. Specifically practice without feedback improved shoulder flexion and elbow extension, and decreased mean error and variability of error. Practice with rhythmic KR feedback also increased shoulder flexion and elbow extension, and reduced movement time for reaching and variability of error. Practice with continuous KP feedback only improved shoulder flexion.
Discussion
The aim of this proof of concept case series was to determine what type of music-based augmented feedback could improve reaching movements in the affected extremity in individuals with stroke. The optimal outcome of this training would be that participants could use feedback to reduce compensatory trunk movements and increase ROM in the shoulder and elbow, thus improving their reaching ability. If practice would occur over a longer period of training, changes in ROM would likely manifest in reductions in motor impairment [33]. Our preliminary pilot findings in five individuals suggest that there may be some benefit of providing music-based augmented feedback, however, effects differed across participants and feedback type. This motivates the need to test more individuals across a longer period of time to determine whether and which of these effects are long lasting.
Our findings show that in four participants, practice with rhythmic KR feedback reduced movement time for reaching. The fifth participant (#3) also showed some reduction in movement time, but smaller in magnitude. These results concur with prior research that suggests 2 weeks training on a reaching task with rhythmic auditory cues also reduces movement time in individuals with chronic stroke [30]. This prior research also found rhythmic training to reduce compensatory trunk flexion, and increase shoulder flexion and elbow extension. However, our findings from short-term practice suggest only some participants’ benefit from rhythmic KR to enhance shoulder flexion (participant #5) and elbow extension (participants #3, #5), and reduce compensatory trunk flexion (participants #1, #3).
Similarly, our findings provide modest evidence that practice with continuous KP feedback can also increase shoulder flexion (participants #1, #4) and elbow extension (participants #2, #4, #5), and reduce compensatory trunk flexion (participants #2, #3, #4). These findings concur with prior work showing KP relative to KR feedback improves elbow and shoulder ROM along with their joint inter-coordination, and decreases compensatory trunk flexion [32,33]. In particular, participant #4 seemed to ideally benefit from continuous KP feedback. This individual was able to increase shoulder flexion and elbow extension, and decrease compensatory trunk flexion, after practice, relative to practice with no feedback or rhythmic KR feedback.
As a secondary outcome measure, we assessed whether mean error and its variability decreased after practice. Some participants were able to reduce the mean error and variability of their elbow, shoulder and trunk movements. However, there was no clear pattern of findings across participants. Mean error was reduced in participants #2 and #3 with rhythmic KR feedback, and in participant #4 with continuous KP feedback. Variability was reduced in participant #5 with rhythmic KR feedback, and in participant #3 with continuous KP feedback. These findings suggest that mean error and variability may not be sensitive nor relevant measures of improvement for our tasks. Improvements in ROM can occur independent of changes in mean error.
Together, the findings of this case series indicate that the use of music-based augmented auditory feedback may have some modest short-term practice effects in improving aspects of reaching in stroke patients. However, it is unclear at this point, which feedback type, rhythmic KR or continuous KP, is more optimal. Participants showed short-term improvements in performance after practice with both types of feedback. Thus, it may be that an intervention comprising both rhythmic KR and continuous KP feedback would be most effective.
Limitations to our study include the short-term nature of the training (1 day, 72 trials) with no evaluation of retention beyond the immediate post-training period. The reason for the short-term nature of our study was to determine if one could quickly establish which feedback cue is most optimal. This information would then allow us to design an intervention over a longer period of practice where one would also expect retention effects over a longer period. Feedback was also provided on every trial (though required for the rhythmic condition). This may not be as effective for motor learning as it is thought that the provision of less feedback is better [7]. We also did not perform statistics due to the case series nature of our study.
Findings from our case series support the notion that enhancing sensory feedback can improve motor performance, which ultimately may allow brain-injured patients to relearn motor skills. We encourage future research to train participants over a longer period so that the long-term effectiveness of these different types of music-based augmented feedback can be fully evaluated.
IMPLICATIONS FOR REHABILITATION.
After practice, augmented feedback is the second most important factor that facilitates motor learning.
Music-based augmented auditory feedback has potential to enhance reaching abilities in individuals with stroke.
Future studies are warranted to evaluate the long-term effectiveness of this feedback over a longer training period in a larger sample size.
Acknowledgements
We thank the individuals with stroke for their time in participating in the research. We thank the Grammy Foundation to the first author for financial support of this research. We thank Dr. Masaya Hirashima for his technical help with the development of continuous auditory feedback.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
References
- 1.Kwakkel G, Kollen BJ, van der Grond J, Prevo AJH. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–6. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- 2.Hömberg V. Neurorehabilitation approaches to facilitate motor recovery. Handbook Clin Neurol. 2013;110:161–73. doi: 10.1016/B978-0-444-52901-5.00014-9. [DOI] [PubMed] [Google Scholar]
- 3.Liepert J. Evidence-based therapies for upper extremity dysfunction. Curr Opin Neurol. 2010;23:678–82. doi: 10.1097/WCO.0b013e32833ff4c4. [DOI] [PubMed] [Google Scholar]
- 4.Carter AR, Connor LT, Dromerick AW. Rehabilitation after stroke: current state of the science. Curr Neurol Neurosci Rep. 2010;10:158–66. doi: 10.1007/s11910-010-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winstein CJ, Wolf SL. Task-oriented training to promote upper extremity recovery. In: Stein J, Harvey R, Macko R, et al., editors. Stroke recovery and rehabilitation. Demos Medical Publishing; 2009. pp. 267–90. [Google Scholar]
- 6.Winstein C, Lewthwaite R, Blanton SR, et al. Infusing motor learning research into neurorehabilitation practice. J Neurologic Phys Ther. 2014;38:190–200. doi: 10.1097/NPT.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt R, Lee T. Augmented feedback. In: Schmidt R, Lee T, editors. Motor control and learning. 5th Human Kinetics; 2011. pp. 393–428. [Google Scholar]
- 8.Wulf G, Shea C. Understanding the role of augmented feedback: the good, the bad, and the ugly. Skill acquisition in sport: research, theory and practice. 2004:121–44. [Google Scholar]
- 9.Buxbaum LJ, Johnson-Frey SH, Bartlett-Williams M. Deficient internal models for planning hand-object interactions in apraxia. Neuropsychologia. 2005;43:917–29. doi: 10.1016/j.neuropsychologia.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Molier BI, Van Asseldonk EHF, Hermens HJ, Jannink MJA. Nature, timing, frequency and type of augmented feedback; does it influence motor relearning of the hemiparetic arm after stroke? A systematic review. Disabil Rehabil. 2010;32:1799–809. doi: 10.3109/09638281003734359. [DOI] [PubMed] [Google Scholar]
- 11.Subramanian SK, Massie CL, Malcolm MP, Levin MF. Does provision of extrinsic feedback result in improved motor learning in the upper limb poststroke? A systematic review of the evidence. Neurorehabil Neural Repair. 2010;24:113–24. doi: 10.1177/1545968309349941. [DOI] [PubMed] [Google Scholar]
- 12.Van Dijk H, Jannink MJA, Hermens HJ. Effect of augmented feedback on motor function of the affected upper extremity in rehabilitation patients: a systematic review of randomized controlled trials. J Rehabil Med. 2005;37:202–11. doi: 10.1080/16501970510030165. [DOI] [PubMed] [Google Scholar]
- 13.Van Vliet PM, Wulf G. Extrinsic feedback for motor learning after stroke: what is the evidence? Disabil Rehabil. 2006;28:831–40. doi: 10.1080/09638280500534937. [DOI] [PubMed] [Google Scholar]
- 14.Schneider S, Münte T, Rodriguez-Fornells A, et al. Music-supported training is more efficient than functional motor training for recovery of fine motor skills in stroke patients. Music Percept. 2010;27:271–80. [Google Scholar]
- 15.Schneider S, Schönle PW, Altenmüller E, Münte TF. Using musical instruments to improve motor skill recovery following a stroke. J Neurol. 2007;254:1339–46. doi: 10.1007/s00415-006-0523-2. [DOI] [PubMed] [Google Scholar]
- 16.Thompson W, Schlaug G. The healing power of music. Sci Am. 2015:33–41. [Google Scholar]
- 17.Hove MJ, Spivey MJ, Krumhansl CL. Compatibility of motion facilitates visuomotor synchronization. J Exp Psychol Hum Percept Perform. 2010;36:1525–34. doi: 10.1037/a0019059. [DOI] [PubMed] [Google Scholar]
- 18.Patel AD, Iversen JR, Chen Y, Repp BH. The influence of metricality and modality on synchronization with a beat. Exp Brain Res. 2005;163:226–38. doi: 10.1007/s00221-004-2159-8. [DOI] [PubMed] [Google Scholar]
- 19.Repp B, Penel A. Auditory dominance in temporal processing: new evidence from synchronization with simultaneous visual and auditory sequences. J Exp Psychol Hum Percept Perform. 2002;28:1085–99. [PubMed] [Google Scholar]
- 20.Repp BH, Penel A. Rhythmic movement is attracted more strongly to auditory than to visual rhythms. Psychol Res. 2004;68:252–70. doi: 10.1007/s00426-003-0143-8. [DOI] [PubMed] [Google Scholar]
- 21.Chen JL, Zatorre RJ, Penhune VB. Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. NeuroImage. 2006;32:1771–81. doi: 10.1016/j.neuroimage.2006.04.207. [DOI] [PubMed] [Google Scholar]
- 22.Zatorre RJ, Chen JL, Penhune VB. When the brain plays music: auditory-motor interactions in music perception and production. Nature reviews. Neuroscience. 2007;8:547–58. doi: 10.1038/nrn2152. [DOI] [PubMed] [Google Scholar]
- 23.Bangert M, Altenmüller EO. Mapping perception to action in piano practice: a longitudinal DC-EEG study. BMC Neurosci. 2003;4:26. doi: 10.1186/1471-2202-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JL, Penhune VB, Zatorre RJ. Listening to musical rhythms recruits motor regions of the brain. Cerebral Cortex. 2008;18:2844–54. doi: 10.1093/cercor/bhn042. [DOI] [PubMed] [Google Scholar]
- 25.Grahn JA, Brett M. Rhythm and beat perception in motor areas of the brain. J Cogn Neurosci. 2007;19:893–906. doi: 10.1162/jocn.2007.19.5.893. [DOI] [PubMed] [Google Scholar]
- 26.Thaut MH, Leins AK, Rice RR, et al. Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke: a single-blind, randomized trial. Neurorehabil Neural Repair. 2007;21:455–9. doi: 10.1177/1545968307300523. [DOI] [PubMed] [Google Scholar]
- 27.Thaut MH, McIntosh GC, Rice RR. Rhythmic facilitation of gait training in hemiparetic stroke rehabilitation. J Neurol Sci. 1997;151:207–12. doi: 10.1016/s0022-510x(97)00146-9. [DOI] [PubMed] [Google Scholar]
- 28.Luft AR, McCombe-Waller S, Whitall J, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2004;292:1853–61. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitall J, McCombe Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000;31:2390–5. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- 30.Malcolm MP, Massie C, Thaut M. Rhythmic auditory-motor entrainment improves hemiparetic arm kinematics during reaching movements: a pilot study. Top Stroke Rehabil. 2009;16:69–79. doi: 10.1310/tsr1601-69. [DOI] [PubMed] [Google Scholar]
- 31.Thaut MH, Kenyon GP, Schauer ML, McIntosh GC. The connection between rhythmicity and brain function. IEEE Eng Med Biol Mag. 1999;18:101–8. doi: 10.1109/51.752991. [DOI] [PubMed] [Google Scholar]
- 32.Cirstea CM, Ptito A, Levin MF. Feedback and cognition in arm motor skill reacquisition after stroke. Stroke. 2006;37:1237–42. doi: 10.1161/01.STR.0000217417.89347.63. [DOI] [PubMed] [Google Scholar]
- 33.Cirstea MC, Levin MF. Improvement of arm movement patterns and endpoint control depends on type of feedback during practice in stroke survivors. Neurorehabil Neural Repair. 2007;21:398–411. doi: 10.1177/1545968306298414. [DOI] [PubMed] [Google Scholar]
- 34.Gladstone DJ, Danells CJ, Black SE. The Fugl–Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–40. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 35.McDermott JH, Lehr AJ, Oxenham AJ. Individual differences reveal the basis of consonance. Curr Biol. 2010;20:1035–41. doi: 10.1016/j.cub.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDermott JH, Oxenham AJ. Music perception, pitch, and the auditory system. Curr Opin Neurobiol. 2008;18:452–63. doi: 10.1016/j.conb.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]