Abstract
Objective
Provide a contemporary estimate of osteoarthritis (OA) by comparing accuracy and prevalence of alternative definitions of OA.
Methods
The Medical Expenditure Panel Survey (MEPS) household component (HC) records respondent-reported medical conditions as open-ended responses; professional coders translate these responses into ICD-9-CM codes for the medical conditions files. Using these codes and other data from the MEPS-HC medical conditions files, we constructed three case definitions of OA and assessed them against medical provider diagnoses of ICD-9-CM 715 [osteoarthrosis and allied disorders] in a MEPS subsample. The three definitions were: 1) strict = ICD-9-CM 715; 2) expanded = ICD-9-CM 715, 716 [other and unspecified arthropathies], OR 719 [other and unspecified disorders of joint]); and 3) probable = strict OR expanded + respondent-reported prior diagnosis of OA or other arthritis excluding rheumatoid arthritis (RA).
Results
Sensitivity and specificity of the three definitions were: strict – 34.6% and 97.5%; expanded – 73.8% and 90.5%; and probable – 62.9% and 93.5%.
Conclusion
The strict definition for OA (ICD-9-CM 715) excludes many individuals with OA. The probable definition of OA has the optimal combination of sensitivity and specificity relative to the two other MEPS-based definitions and yields a national annual estimate of 30.8 million adults with OA (13.4% of US adult population) for 2008 – 2011.
Osteoarthritis (OA) is the most common joint disorder in the US (1), affecting an estimated 12% of US adults aged 25 to 74 years. This prevalence estimate, based on clinical examinations conducted forty years ago (1971 to 1975) as part of the first National Health and Nutrition Survey (NHANES I) (2), may be an underestimate of current prevalence for at least two reasons. First, symptomatic knee OA prevalence tripled among men and doubled among women from the 1980s to the 2000s (3). Second, obesity, which is a risk factor for another common type of OA, hand OA (4, 5), has increased dramatically since NHANES I (6) (7).
We therefore generated an updated estimate of OA prevalence using data from the nationally representative Medical Expenditure Panel Survey (MEPS), which provides estimates of conditions with current adverse health impact (i.e., leading to healthcare utilization, disability, or that bothers the individual). Because it is healthcare utilization and disability that primarily drive the societal costs associated with OA, estimating the prevalence of OA with such adverse health impacts is important to public health practice.
Materials and Methods
Data Source
We analyzed data for adults ≥ 18 years in the combined 2008 – 2011 MEPS, an annual national survey representative of the civilian non-institutionalized US population. MEPS uses an overlapping panel design: each year, a new panel of sample households is selected from the previous year’s National Health Interview Survey (NHIS). Each panel is interviewed five times to collect data for a two-year period; each year therefore includes data from two panels. For the MEPS household component (HC), interviewers collect information from a household representative (the respondent) for all household members (subjects) on medical care utilization and associated expenditures (8). Final response rates for the 2008 – 2011 MEPS-HC ranged from 54% to 59% (9); sample sizes varied from 31,228 to 34,920 (10).
In the MEPS-HC, respondents report conditions that result in healthcare utilization (inpatient, ambulatory, or home health events, or prescribed medication purchases), disability days (days missed from work, at school, or spent in bed), or that otherwise bothered individuals. These conditions are translated by professional coders into ICD-9-CM codes before release in the MEPS-HC medical conditions file. The coder error rate (proportion of conditions found, in an audit, to be incorrectly coded) is ≤ 2.5% (11). MEPS also includes diagnostic data from medical providers in the Medical Provider Component (MPC), a non-representative sub-sample of MEPS-HC. The MPC sampling frame includes all visits from households with Medicaid enrollees or individuals with high-cost events and a smaller proportion of visits from other households (12). Furthermore, the MEPS-MPC includes encounter information from only those providers for whom respondents have authorized contact (13). After information from providers is collected, HC and MPC visits are linked probabilistically (i.e., encounters are matched based on the similarity of multiple characteristics such as visit date and services received) (13). Information from the MPC is used to validate items in the HC by determining whether respondent-reported diagnoses and procedures associated with medical utilization are also reported by a provider. We obtained access to 2008 – 2010 MEPS-MPC data via a National Center for Health Statistics (NCHS) Research Project Agreement to evaluate the performance of several OA definitions from the HC compared to the MPC; 2011 data were not available at the time of this analysis.
OA definitions
Criteria exist to define OA as radiographic (based on radiographs) (14), clinical (e.g., American College of Rheumatology criteria) (15), or symptomatic radiographic ( i.e., presence of both radiographic evidence [e.g., Kellgren-Lawrence grades (14)] and symptoms such as frequent pain in the same joint). From the clinical or public health perspective, symptomatic OA is the most relevant, as non-symptomatic OA is not likely to result in negative impacts such as medical utilization and/or disability. Based on findings from our previous work and review of MEPS documentation and literature on the accuracy of patient-reported OA, we developed three definitions of OA with adverse health impact in MEPS:
Strict: Our first definition was simply the presence of ICD-9-CM 715 (osteoarthrosis and allied disorders) in the MEPS-HC medical conditions file – the default definition of OA typically used for ICD-9-CM-coded data. Machlin et al. reported that OA based on ICD-9-CM 715 is substantially underreported (sensitivity=12.1%) when compared to MEPS-MPC data (16). Therefore, we expected that this “strict” definition would likely underestimate the number of people with OA.
Expanded: Previous studies have found that many people with OA do not report a specific OA diagnosis but instead report their OA using more generic terms (e.g., simply arthritis, joint/cartilage wearing out) (17, 18). Our previous unpublished work using 1996 - 1999 MEPS data found that 55% of visits coded by medical providers with the specific OA code ICD-9-CM 715 in MEPS-MPC were coded in the respondent-reported MEPS-HC condition file with the less specific ICD-9-CM codes 716 (other and unspecified arthropathies) or 719 (other and unspecified disorders of joint). Based on these findings, we created an “expanded” definition that comprised the presence of any of the ICD-9-CM codes 715, 716, or 719.
Probable: In addition to conditions translated to ICD-9-CM codes in the MEPS-HC condition file, since 2001 MEPS respondents have been asked about the arthritis diagnosis history for all adult members of the household: [(Have/Has) (PERSON) ever been told by a doctor or other health professional that (PERSON) had arthritis?]. Starting in 2008, those answering yes are asked which type of arthritis they had been diagnosed with: osteoarthritis (OA), rheumatoid arthritis (RA), or some other kind of arthritis. We hypothesized that respondent-reported ICD-9-CM 716 or 719, combined with responses to the follow-up question of either “OA” or “some other kind” (i.e., those not reporting RA) probably had OA because, as prior studies indicate, many people who report non-specific arthritis actually have OA (17, 18). Our probable OA definition included all individuals who met the strict definition (715) plus the subset of subjects in the expanded definition (716 or 719) who also reported a prior, non-RA arthritis diagnosis. Because 93% of those meeting the strict definition also reported the prior non-RA diagnosis, we did not require it for that subset.
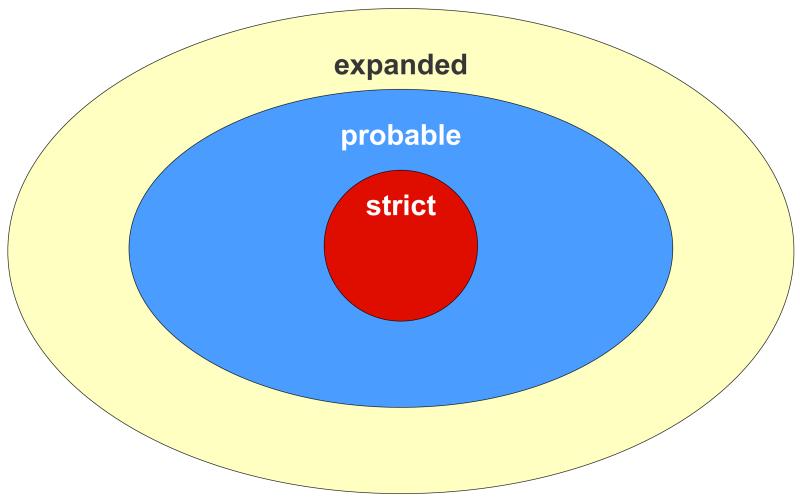
The conceptual relationship among the three definitions is displayed graphically in Figure 1.
Figure 1. Relationships of three case definitions for OA in MEPS-HC.
 strict: ICD-9-CM 715
strict: ICD-9-CM 715  expanded: ICD-9-CM 715 or 716 or 719
expanded: ICD-9-CM 715 or 716 or 719
 probable: strict (ICD-9-CM 715) plus those expanded definition ICD-9-CM 716 or 719 subjects who also reported a prior nonRA arthritis diagnosis
probable: strict (ICD-9-CM 715) plus those expanded definition ICD-9-CM 716 or 719 subjects who also reported a prior nonRA arthritis diagnosis
OA, osteoarthritis; MEPS, Medical Expenditure Panel Survey; HC, Household Component
Measuring Performance
Upon creating these definitions, we examined their performance with sensitivity, specificity, and positive predictive value in the MEPS-HC compared with the MEPS-MPC, using ICD-9-CM 715 (osteoarthrosis and allied disorders) coded by providers in the MPC as the gold standard.
Sensitivity
Assuming effective communication of diagnoses from provider to patient and accurate matching of MPC and HC encounters, we would expect provider-reported ICD-9-CM 715 to be captured by the three case definitions. Because of MPC sampling priorities and incomplete matching, many MEPS subjects do not have any MPC data matched to their medical encounters in the HC, while others have MPC data matched to some – but not all – of their encounters. We therefore assessed sensitivity in a dataset which included all HC adults who had at least one MPC encounter, regardless of whether it matched an encounter in the HC (Subset A). This dataset contained HC data on all of the subjects’ diagnoses and their responses to the arthritis and joint symptoms questions, which ensured that any provider diagnosis of ICD-9-CM 715 (our gold standard) could be evaluated against our three case definitions.
Specificity and Positive Predictive Value (PPV)
We examined specificity and PPV using a subgroup of Subset A — only adults with matched HC and MPC encounters (Subset B). We did this because subjects without matched MPC encounters have little opportunity to be identified as having OA by providers. That information bias has the potential to decrease the PPV. Although Subset B represents the best scenario for using MPC data to calculate specificity and PPV, it is still less than optimally suited to evaluating specificity and PPV because 1) it is very likely that many provider encounters are not included and 2) respondents reporting OA due to disability or bothersomeness without medical utilization cannot be evaluated. The specificity and PPV for our respondent-reported case definitions against provider diagnosis of ICD-9-CM 715 (our gold standard) may therefore be underestimates.
Proportion symptomatic
MEPS respondents are also asked whether adult subjects in the household experienced pain, swelling, or stiffness around a joint in the last 12 months. Given the importance of symptoms to an OA definition with adverse health impact, we estimated the proportion of adults reporting joint symptoms for each case definition of OA.
Annual Prevalence Estimates
The annual number of US adults satisfying each of three definitions and the associated proportions were estimated using the 2008 – 2011 MEPS-HC; this was the only use of 2011 MEPS data in this analysis.
Statistical analysis
All analyses were conducted in SAS 9.3 using SURVEY procedures (19) to account for the MEPS complex sampling design. Sampling weights were applied when estimating prevalence to derive nationally representative estimates. Variance estimates were based on Taylor series linearization. Performance estimates were not statistically different at α = 0.05 unless explicitly noted.
Results
Compared to the entire adult HC sample for 2008 - 2010 (n=68,270), those in Subset A (n=24,022) and Subset B (n=20,372) were significantly older (≥65 years; 16.7% vs. 25.0% and 26.0% ), more likely to be the survey respondent (53.2% vs. 61.9% and 61.8% , women (51.6% vs. 60.8% and 60.0% ), white non-Hispanic (68.2% vs. 72.5% and 73.0%), report a usual place for care ( 75.9% vs. 87.3% and 87.8%), poor health (22.9% vs. 35.3% and 38.4%), limitation in work, housework, or school (12.2% vs. 22.3% and 24.6%) and public only insurance (specifically Medicaid; 9.8% vs. 17.4% and 18.3%). They were also less likely to have completed high school (15.9% vs. 18.1% and 18.5%), be uninsured, (15.6% vs. 8.7% and 8.5%) or have private insurance (65.9% vs. 61.7% and 60.6%) (Table 1). Only one characteristic was statistically significantly different between the two subsets: Subset A had a slightly higher proportion of excellent/very good perceived health status (29.0% vs. 25.8%) and a correspondingly lower proportion of fair/poor perceived health (35.3% vs. 38.4%) when compared to Subset B.
Table 1. Distribution of socio-demographic variables among subjects in MEPS-HC and MEPS-MPC, overall and by Subsets A & B, age ≥ 18 years, weighted, 2008 - 2010*.
| MEPS-HC* (n=68,270) | Subset A† (n=24,022) | Subset B‡ (n=20,372) | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| category | characteristic | Proportion (95% CI) | Proportion (95% CI) | Proportion (95% CI) | |||
| Age (in years) | 18 - 44 | 48.5 | (47.5 — 49.5) | 38.7 | (37.4 — 40.0) | 38.0 | (36.7 — 39.3) |
| 45 - 64 | 34.8 | (34.0 — 35.6) | 36.3 | (35.1 — 37.4) | 36.0 | (34.8 — 37.1) | |
| 65+ | 16.7 | (15.9 — 17.5) | 25.0 | (23.8 — 26.3) | 26.0 | (24.7 — 27.3) | |
| Subject was respondent in all rounds |
No | 46.8 | (46.2 — 47.5) | 38.1 | (37.3 — 39.0) | 38.2 | (37.2 — 39.1) |
| Yes | 53.2 | (52.5 — 53.8) | 61.9 | (61.0 — 62.7) | 61.8 | (60.9 — 62.8) | |
| Sex | Men | 48.4 | (48.0 — 48.9) | 39.2 | (38.4 — 39.9) | 40.0 | (39.2 — 40.9) |
| Women | 51.6 | (51.1 — 52.0) | 60.8 | (60.1 — 61.6) | 60.0 | (59.1 — 60.8) | |
| Race/ethnicity | Hispanic | 13.8 | (12.3 — 15.3) | 10.8 | (09.4 — 12.2) | 10.6 | (09.2 — 12.1) |
| White NonHispanic | 68.2 | (66.4 — 70.0) | 72.5 | (70.7 — 74.4) | 73.0 | (71.2 — 74.9) | |
| Black NonHispanic | 11.4 | (10.2 — 12.6) | 11.9 | (10.6 — 13.2) | 11.7 | (10.4 — 13.1) | |
| Other NonHispanic | 6.6 | (05.7 — 07.6) | 4.8 | (03.8 — 05.8) | 4.6 | (03.6 — 05.6) | |
| MSA status | NonMSA | 15.9 | (13.3 — 18.5) | 19.4 | (16.1 — 22.7) | 19.7 | (16.3 — 23.1) |
| MSA | 84.1 | (81.5 — 86.7) | 80.6 | (77.3 — 83.9) | 80.3 | (76.9 — 83.7) | |
| Census region | Northeast | 18.4 | (17.1 — 19.8) | 18.5 | (16.9 — 20.1) | 18.2 | (16.6 — 19.9) |
| Midwest | 21.9 | (20.7 — 23.2) | 25.6 | (23.8 — 27.3) | 25.7 | (23.8 — 27.5) | |
| South | 36.5 | (34.9 — 38.2) | 35.4 | (33.4 — 37.3) | 35.4 | (33.4 — 37.5) | |
| West | 23.1 | (21.7 — 24.5) | 20.6 | (18.9 — 22.3) | 20.6 | (19.0 — 22.3) | |
| Education | Less than high school | 15.9 | (15.2 — 16.6) | 18.1 | (17.2 — 19.0) | 18.5 | (17.5 — 19.5) |
| High school | 30.8 | (29.9 — 31.6) | 31.5 | (30.5 — 32.6) | 31.7 | (30.6 — 32.8) | |
| Greater than High school |
53.3 | (52.2 — 54.4) | 50.4 | (49.1 — 51.6) | 49.8 | (48.4 — 51.1) | |
| Usual place for care | No (access issue) | 5.8 | (05.4 — 06.2) | 3.7 | (03.4 — 04.1) | 3.7 | (03.3 — 04.0) |
| No (other) | 18.3 | (17.5 — 19.1) | 9.0 | (08.3 — 09.6) | 8.6 | (07.9 — 09.2) | |
| Yes | 75.9 | (74.9 — 76.8) | 87.3 | (86.5 — 88.1) | 87.8 | (87.0 — 88.5) | |
| Worst perceived health status in any round |
Excellent/very good | 40.3 | (39.4 — 41.3) | 29.0 | (27.9 — 30.0) | 25.8 | (24.9 — 26.8) |
| Good | 36.7 | (36.0 — 37.4) | 35.7 | (34.8 — 36.6) | 35.7 | (34.7 — 36.7) | |
| Fair/poor | 22.9 | (22.2 — 23.6) | 35.3 | (34.3 — 36.4) | 38.4 | (37.3 — 39.6) | |
| Any limitation in work, housework, or school |
No limitation | 87.8 | (87.2 — 88.4) | 77.7 | (76.5 — 78.8) | 75.4 | (74.2 — 76.7) |
| Limitation | 12.2 | (11.6 — 12.8) | 22.3 | (21.2 — 23.5) | 24.6 | (23.3 — 25.8) | |
| Health insurance status |
Any private | 65.9 | (64.7 — 67.0) | 61.7 | (60.2 — 63.2) | 60.6 | (59.0 — 62.1) |
| Public only | 18.6 | (17.7 — 19.4) | 29.6 | (28.3 — 31.0) | 30.9 | (29.5 — 32.3) | |
| Uninsured | 15.6 | (14.7 — 16.4) | 8.7 | (08.0 — 09.3) | 8.5 | (07.9 — 09.2) | |
| Medicaid coverage during year |
No | 90.2 | (89.6 — 90.9) | 82.6 | (81.5 — 83.7) | 81.7 | (80.6 — 82.9) |
| Yes | 9.8 | (09.1 — 10.4) | 17.4 | (16.3 — 18.5) | 18.3 | (17.1 — 19.4) | |
All adults with positive weights in MEPS-HC.
Subjects with at least one MEPS-MPC medical encounter; used to compute sensitivity.
Subjects with at least one MEPS-HC encounter matched to a MEPS-MPC medical encounter diagnosis; used to compute specificity and PPV.
MEPS-HC, Medical Expenditure Panel Survey – household component; MEPS-MPC, Medical Expenditure Panel Survey – Medical Provider Component.
Performance measures for the three definitions against provider diagnoses in the MPC are presented in Table 2. As expected, the strict definition (n=4,838) had the lowest sensitivity (34.6%), highest specificity (97.5%) and highest PPV (33.5%). Also as expected, the expanded (n=19,035) definition had the highest sensitivity (73.8%), lowest specificity (90.5%) and lowest PPV (26.3%) of all three definitions. The expanded definition performance measures were statistically significantly different from those of the other two definitions. Performance measure estimates for the probable (n=11,230) definition fell between those of the strict and expanded definitions, at 62.9%, 93.5%, and 31.4% for sensitivity, specificity, and PPV, respectively. The probable definition showed statistically significant differences in sensitivity, specificity, and PPV compared with the expanded definition, and in sensitivity and specificity compared with the strict definition. In terms of detecting symptomatic OA, the three definitions performed similarly; pain, aching, stiffness, or swelling around a joint in the past twelve months was reported for 89.1% of expanded, 91.2% for probable, and 92.3% for the strict definition.
Table 2. Comparison of Three definitions of OA using MEPS data.
| Definition‡ | Performance Measures (2008 - 2010)* | Annual Prevalence 2008 - 2011* |
Symptomatic† 2008 - 2011* |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Sensitivity‡ (95% CI) |
Specificity‡ (95% CI) |
PPV‡ (95% CI) | sample n |
estimate |
||||||
| millions | (%) | % | ||||||||
| Strict | 34.6 | (31.5 – 37.8) | 97.5 | (97.2 – 97.9) | 33.5 | (29.3 – 37.6) | 4,838 | 14.4 | (6.3) | 92.3% |
| Expanded | 73.8 | (71.1 – 76.5) | 90.5 | (89.8 – 91.1) | 26.3 | (24.1 – 28.5) | 19,035 | 51.4 | (22.4) | 89.1% |
| Probable | 62.9 | (59.8 – 65.9) | 93.5 | (93.0 – 94.0) | 31.4 | (28.8 – 34.1) | 11,230 | 30.8 | (13.4) | 91.8% |
Calculation of performance measures requires the use of restricted MEPS-MPC data; 2011 MPC data was not available when our access to these data was granted.
Assessed with the following question asked at the first and last assessment at each year “[Have/Has] [PERSON] had pain, aching, stiffness or swelling around a joint in the past 12 months?”.
Strict, ICD-9-CM diagnosis 715; Expanded, ICD-9-CM diagnosis 715 , 716, or 719; Probable, ICD-9-CM 715 OR [716 or 719 AND prior non-RA arthritis diagnosis – assessed with a question asked of all adults “(Have/Has) (PERSON) ever been told by a doctor or other health professional that (PERSON) had arthritis?” and a follow-up question prompting the individual for the type of arthritis].
Sensitivity estimated on Subset A – participants with at least one MEPS-MPC medical counter diagnosis (n=24,022). Specificity and PPV estimated on Subset B – participants with MEPS-HC condition associated with at least one MEPS-MPC medical encounter diagnosis matched to a HC diagnosis (n=20,372).
OA, osteoarthritis; MEPS, Medical Expenditure Panel Survey; CI, Confidence Interval; PPV, Positive Predictive Value; ICD-9-CM, International Classification of Diseases Version 9 Clinical Modification; MPC, Medical Provider Component; HC, Household Component.
The annual number of estimated cases and prevalence proportion for each of the definitions are also provided in Table 2. The strict definition yielded the lowest estimates (14.4 million, 6.3% of the adult population) while the expanded definition resulted in the highest estimates (51.4 million or 22.4% of the adult population). The probable prevalence estimate was between those of the other two definitions, at 30.8 million or 13.4% of adults.
Discussion
Performance estimates for the three OA case-finding definitions in MEPS varied substantially. Sensitivity estimates ranged from 34.6% (strict) to 73.8% (expanded), specificity from 90.5% (expanded) to 97.5% (strict), and PPV from 26.3% (expanded) to 33.5% (strict). Probable almost doubled the sensitivity of the strict definition (from 34.6% to 62.9%, a statistically significant increase) while only modestly decreasing specificity (97.5% to 93.5%, a statistically significant difference) and PPV (33.5% to 31.4%).
There are reasons to believe that probable more accurately captures adults in MEPS with OA-associated adverse health impact than the other two definitions evaluated. The strict definition (ICD-9-CM 715 in the HC condition file), yields a sensitivity (34.6%) that is statistically significantly lower than all other definitions evaluated, and its prevalence proportion (6.3%) is substantially below the 2005 census/NHANES I estimate for adults age 25 and over (12%) (2). Although the expanded definition results in a significantly higher sensitivity than probable, its specificity and PPV are significantly lower, most likely because other conditions are being reported using these ICD-9-CM codes 716 and 719. The expanded specificity of 90.5% equates to a false positive rate of 9.5%. For these reasons it may be an overestimate.
The probable definition restricts the expanded definition by requiring individuals reporting condition records coded as ICD-9-CM 716 or 719 (but not 715) to also have been told by a provider that they had non-RA arthritis. This definition represents a reasonable middle ground between the underestimate of the strict definition and the overestimate of the expanded definition. The overwhelming majority of these individuals (92%) also report joint symptoms; the symptoms of the remaining 8% may be alleviated due to the effective use of medication. Furthermore, the similarity of the prevalences from the probable estimate (13.4%) to the NHANES I estimate (12.0%) gives further credence to it being the definition of choice for estimating prevalence of OA from MEPS. The probable definition yielded an estimated 30.8 million or 12.3% of the adult civilian non-institutionalized population.
Specifying a case definition for OA, whether clinical or epidemiological, is challenging for multiple reasons. For clinical OA, ACR criteria include symptoms plus findings from physical examination, radiography, and/or laboratory tests. Early OA can be missed by relying entirely on symptom history and physical exam signs; the ACR criteria seem to reflect more advanced disease (20). Additionally, some individuals with extensive radiographic damage from OA have no symptoms, whereas others have symptoms but no evidence of radiographic damage (17). Defining an epidemiologic case for the purpose of estimating prevalence is no less problematic. According to Chubak et al. (21), sensitivity should be prioritized over other performance measures when the benefit of identifying all persons with the condition of interest outweighs the negative consequence of including more individuals without the condition. One scenario in which sensitivity is routinely prioritized is when the study generalizability is important, especially if less sensitive definitions are differentially sensitive based on disease severity; an example of this is analysis of treatment effectiveness of a specific disease, where selecting more severe patients may fail to detect treatments that work for mild, but not severe disease (21). This scenario aligns with our objective for the selected case definition: to quantify the proportion of the population US adults with OA that impacts their health in some way. Therefore maximizing sensitivity was our first priority. However, as sensitivity increases the specificity decreases, leading to more false positives. Because specificity calculations are based on a large numbers of people without the disease in question, a difference of just a few percentage points may erroneously classify millions of people as cases. Thus, an epidemiologic case definition must strike a balance between maximizing sensitivity while minimizing false positives, i.e., preserving specificity and PPV. As mentioned previously, probable best strikes this balance.
Our reference standard for calculating the sensitivity, specificity, and PPV was an ICD-9-CM OA diagnosis (715) in the MPC, which is likely an incomplete and potentially problematic gold standard. Office-based MPC records (which provide the majority of HC and MPC events) are derived primarily from billing records (12). Katz et al. found sensitivity of 100% and 90% for hip and knee OA respectively, and a PPV of 83% for each, when OA-coded Medicare claims from eight rheumatology practices were compared to the authors’ classification of visits from medical record review as the gold standard (22). Harrold and colleagues reported much higher levels of misclassification of OA from HMO claims when compared to chart review (PPV = 62%) (23). The higher PPV estimate reported by Katz et al. may be explained in part by their data source being restricted to rheumatology practices, which have both a higher prevalence of OA (leading to higher PPV estimates) and personnel more skilled in the diagnosis (and presumably ICD-9-CM coding) for OA than the less specialized providers in the Harrold et al. study. The MPC is based on information from a variety of providers making it more similar to the Harrold et al. study sample. Therefore validation of the MPC against a chart-review as the gold standard would probably yield results (i.e., a lower PPV) more similar to Harrold et al. than to Katz et al.
One method to ascertain OA is to ask respondents whether they have ever been medically diagnosed with OA, as has been done in some other studies. Sensitivity, specificity, and PPV for such respondent-reported OA against clinical criteria or radiographic evidence plus symptoms were 73%, 96%, and 86% for knee OA and 81%, 94%, and 61% for hip OA using an internet-based questionnaire (24), and 69%, 66%, 78% for overall OA in a population-based study of women (17). A small clinic-based sample estimated PPV of 89% (25). The PPV for a case definition requiring patient-report of joint pain or swelling in the past six months along with one of three terms (OA, degenerative arthritis, or “joint/cartilage wearing out”) was 81%, with a sensitivity of 42% and a specificity of 96% (26). Although all of our definitions require adverse health impact from utilization, disability, or bothersomeness, one could assume that respondent-report of pain or swelling in the past six months would be synonymous with bothersomeness; all three definitions had high reported proportions of symptomatology. It is interesting that the PPVs in all of these studies are substantially higher than those calculated for any of our definitions. These studies were conducted on populations that were relatively affluent and well-educated, and one noted a statistically significant difference between the sensitivity of the college educated subpopulation vs. those with less education (24). Because no study has compared accuracy of OA self-report to administrative data, accuracy of these measures compared to a clinical reference standard is unknown.
This study has several limitations. First, it was focused on those having OA with adverse health impact and does not capture those with early or non-symptomatic radiographic OA—a population that might be targeted with preventive measures to slow disease progression. Second, the MPC subsample used to calculate performance measures is not representative of the entire US population. The MPC sample was enriched with individuals with Medicaid or high-cost events, who often are not aware of the healthcare services they have received or the associated charges and payments (27). As we explored, the MPC subsets used for analysis were significantly older, more likely to be women, white non-Hispanic, physically limited, report poor health, and to have Medicaid than the MEPS-HC overall. This may have biased our results. If those with OA in the MPC represent those who were less likely to report OA in the HC file, then our sensitivity estimates could be too low; if those with OA in the MPC were more likely to report OA, then our sensitivity estimates could be too high. Third, the prevalence proportion of our recommended estimate (probable ) at 13.4% is only slightly higher than the 2005 NHANES/census-based 12% estimate even though prevalence of obesity, a risk factor for hand and knee OA (5), has increased since the 1971-75 NHANES (7). One possible reason for our lower than expected prevalence is the sensitivity of probable — at 62.9% it does not capture 37% of adults with OA. Another reason for our lower than anticipated estimate may be our more restrictive definition, requiring OA with adverse health impact, while the 1971-75 NHANES definition was based on clinical examination only. Finally, the PPV of probable, although most likely an underestimate of the PPV compared to clinical criteria, is quite low (31.4%). This may be because some individuals coded as 716 or 719 and non-RA arthritis might not actually have OA, but rather, non-specific symptoms, or perhaps another rheumatic condition such as gout or fibromyalgia. Future research addressing some of these study limitations may result in even more accurate OA prevalence estimates. For example, validation of a definition for general OA with adverse health impact in a population with complete medical records such as the Rochester Epidemiology Project (28) could be especially helpful.
This study is important in several respects. First, it is the most contemporary national prevalence estimate of OA. Second, it is based on a large national population sample rather than a regional or clinic-based sample. Third, it clearly demonstrates how the use of ICD-9-CM code 715 alone to estimate treated prevalence in MEPS provides results that are likely substantially underestimated. Fourth, it provides a definition with superior performance to only ICD-9-CM code 715 for estimating prevalence of OA with adverse health impact.
The strict definition (ICD-9-CM 715) excludes many individuals with OA. We recommend that analysts use probable instead, which results in an annual prevalence of OA with adverse health impact in 2008 – 2011 of 30.8 million, or 13.4% of the US adult civilian non-institutionalized population.
Significance and Innovations.
This study provides the most recent estimate of OA prevalence and is based on a national population sample (the Medical Expenditure Panel Survey) rather than a regional or clinic-based sample. The definition of OA prevalence is restricted to cases resulting in healthcare utilization, disability, or other adverse impacts among US adults.
Using medical provider-reported diagnoses of ICD-9-CM 715 (osteoarthrosis and allied disorders) as a gold standard to evaluate possible case definitions of respondent-reported OA in MEPS, we provide a definition that more accurately captures adults with OA that impacts their health, utilization, or activities in a meaningful way, than would the use of ICD-9-CM 715 alone. This recommended definition results in an estimate of 30.8 million adults (13.4% of the civilian non-institutionalized adult U.S. population) and can be used for other analytic applications in MEPS, such as cost-of-illness estimates.
Acknowledgements
We thank Ray Kunz and Doris Lefkowitz, both of the Agency for Healthcare Research and Quality (AHRQ), as well as Ajay Yesupriya, Peter Meyer, Carolyn Neal, and Karon Lewis of the National Center for Health Statistics Atlanta Research Data Center for making our proposed analysis of MEPS-MPC data a reality. Steve Machlin and Joel Cohen of AHRQ provided advice in the design phase of this analysis, for which we are extremely grateful.
Financial support: Ms. Cisternas’s work on this project was supported by contracts from the Centers for Disease control and prevention (contract numbers 200-2009-M-32656, 200-2010-M-3603, and 200-2011-M-42091). Dr. Sacks’ work was made possible by a contract with the National Association of Chronic Disease Directors (contract nos. 0612011 and 095213), as was Mr. Pasta’s work (contract no. 2212013).
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
None of the authors have financial interests that could create a conflict of interest or an appearance of such a conflict.
References
- 1.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clinics in geriatric medicine. 2010;26(3):355–69. doi: 10.1016/j.cger.2010.03.001. Epub 2010/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and rheumatism. 2008;58(1):26–35. doi: 10.1002/art.23176. Epub 2008/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen US, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Annals of internal medicine. 2011;155(11):725–32. doi: 10.1059/0003-4819-155-11-201112060-00004. Epub 2011/12/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheumatic diseases clinics of North America. 2013;39(1):1–19. doi: 10.1016/j.rdc.2012.10.004. Epub 2013/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC musculoskeletal disorders. 2008;9:132. doi: 10.1186/1471-2474-9-132. Epub 2008/10/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis and rheumatism. 2008;59(9):1207–13. doi: 10.1002/art.24021. Epub 2008/09/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shields M, Carroll MD, Ogden CL. Adult obesity prevalence in Canada and the United States. NCHS data brief. 2011;(56):1–8. Epub 2011/05/20. [PubMed] [Google Scholar]
- 8.Agency for Healthcare Research and Quality . MEPS-HC Sample Design and Collection Process. Rockville, MD: [cited 2014 15 April 2014]; Available from: http://www.meps.ahrq.gov/mepsweb/survey_comp/hc_data_collection.jsp. [Google Scholar]
- 9.Agency for Healthcare Research and Quality . MEPS-HC Response Rates by Panel. Rockville, MD: Apr 15, 2014. Available from: http://meps.ahrq.gov/survey_comp/hc_response_rate.jsp. [Google Scholar]
- 10.Agency for Healthcare Research and Quality . MEPS-HC Sample Sizes. Rockville, MD: Feb 13, 2015. Available from: http://meps.ahrq.gov/mepsweb/survey_comp/hc_sample_size.jsp. [Google Scholar]
- 11.Agency for Healthcare Research and Quality . MEPS HC-146: 2011 Medical Conditions. Rockville, MD: Apr 30, 2015. Available from: http://meps.ahrq.gov/mepsweb/data_stats/download_data/pufs/h146/h146doc.shtml. [Google Scholar]
- 12.Westat . MEPS Medical Provider Component Annual Methodology Report. Rockville, MD: Jun 15, 2010. 2010. Report No. [Google Scholar]
- 13.Stagnitti MN, B K, S. Amy. Design, Methods, and Field Results of the Medical Expenditure Panel Survey Medical Provider Component (MEPS MPC)—2006 Calendar Year Data. Agency for Healthcare Research and Quality; Rockville, MD: 2008. [Google Scholar]
- 14.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Annals of the rheumatic diseases. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. Epub 1957/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altman RD. Criteria for classification of clinical osteoarthritis. The Journal of rheumatology Supplement. 1991;27:10–2. Epub 1991/02/01. [PubMed] [Google Scholar]
- 16.Machlin S, Cohen J, Elixhauser A, Beauregard K, Steiner C. Sensitivity of household reported medical conditions in the medical expenditure panel survey. Med Care. 2009;47(6):618–25. doi: 10.1097/MLR.0b013e318195fa79. Epub 2009/05/13. [DOI] [PubMed] [Google Scholar]
- 17.Szoeke CE, Dennerstein L, Wluka AE, Guthrie JR, Taffe J, Clark MS, et al. Physician diagnosed arthritis, reported arthritis and radiological non-axial osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16(7):846–50. doi: 10.1016/j.joca.2007.12.001. Epub 2008/01/30. [DOI] [PubMed] [Google Scholar]
- 18.Roux CH, Saraux A, Mazieres B, Pouchot J, Morvan J, Fautrel B, et al. Screening for hip and knee osteoarthritis in the general population: predictive value of a questionnaire and prevalence estimates. Annals of the rheumatic diseases. 2008;67(10):1406–11. doi: 10.1136/ard.2007.075952. Epub 2007/12/14. [DOI] [PubMed] [Google Scholar]
- 19.SAS Institute . SAS Online Doc 9.2. United States SAS Institute; Cary, North Carolina: 2002-2008. [Google Scholar]
- 20.Peat G, Thomas E, Duncan R, Wood L, Hay E, Croft P. Clinical classification criteria for knee osteoarthritis: performance in the general population and primary care. Annals of the rheumatic diseases. 2006;65(10):1363–7. doi: 10.1136/ard.2006.051482. Epub 2006/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chubak J, Pocobelli G, Weiss NS. Tradeoffs between accuracy measures for electronic health care data algorithms. Journal of Clinical Epidemiology. 2012;65(3):343–9. doi: 10.1016/j.jclinepi.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz JN, Barrett J, Liang MH, Bacon AM, Kaplan H, Kieval RI, et al. Sensitivity and positive predictive value of Medicare Part B physician claims for rheumatologic diagnoses and procedures. Arthritis and rheumatism. 1997;40(9):1594–600. doi: 10.1002/art.1780400908. Epub 1997/10/27. [DOI] [PubMed] [Google Scholar]
- 23.Harrold LR, Yood RA, Andrade SE, Reed JI, Cernieux J, Straus W, et al. Evaluating the predictive value of osteoarthritis diagnoses in an administrative database. Arthritis and rheumatism. 2000;43(8):1881–5. doi: 10.1002/1529-0131(200008)43:8<1881::AID-ANR26>3.0.CO;2-#. Epub 2000/08/16. [DOI] [PubMed] [Google Scholar]
- 24.Ratzlaff C, Koehoorn M, Cibere J, Kopec J. Clinical validation of an Internet-based questionnaire for ascertaining hip and knee osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20(12):1568–73. doi: 10.1016/j.joca.2012.08.017. Epub 2012/09/15. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Y, Macera CA, Davis DR, Ainsworth BE, Troped PJ, Blair SN. Physical activity and self-reported, physician-diagnosed osteoarthritis: is physical activity a risk factor? J Clin Epidemiol. 2000;53(3):315–22. doi: 10.1016/s0895-4356(99)00168-7. Epub 2000/04/13. [DOI] [PubMed] [Google Scholar]
- 26.March LM, Schwarz JM, Carfrae BH, Bagge E. Clinical validation of self-reported osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 1998;6(2):87–93. doi: 10.1053/joca.1997.0098. Epub 1998/08/06. [DOI] [PubMed] [Google Scholar]
- 27.Machlin SR, Taylor AK. Design, Methods, and Field Results of the 1996 Medical Expenditure Panel Survey Medical Provider Component. Agency for Healthcare Research and Quality; Rockville, MD: 2000. [Google Scholar]
- 28.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Pankratz JJ, Brue SM, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. International journal of epidemiology. 2012;41(6):1614–24. doi: 10.1093/ije/dys195. Epub 2012/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]