Abstract
The vitamin D receptor (VDR) is a critical mediator of the biological actions of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3). As a nuclear receptor, ligand activation of the VDR leads to the protein’s binding to specific sites on the genome that results in the modulation of target gene expression. The VDR is also known to play a role in the hair cycle, an action that appears to be 1,25(OH)2D3-independent. Indeed, in the absence of VDR as in hereditary 1,25-dihydroxyvitamin D resistant rickets (HVDRR) both skin defects and alopecia emerge. Recently, we generated a mouse model of HVDRR without alopecia wherein a mutant human VDR lacking 1,25(OH)2D3-binding activity was expressed in the absence of endogenous mouse VDR. While 1,25(OH)2D3 failed to induce gene expression in these mice, resulting in an extensive skeletal phenotype, the receptor was capable of restoring normal hair cycling. We also noted a level of secondary hyperparathyroidism that was much higher than that seen in the VDR null mouse and was associated with an exaggerated bone phenotype as well. This suggested that the VDR might play a role in parathyroid hormone (PTH) regulation independent of 1,25(OH)2D3. To evaluate this hypothesis further, we contrasted PTH levels in the HVDRR mouse model with those seen in Cyp27b1 null mice where the VDR was present but the hormone was absent. The data revealed that PTH was indeed higher in Cyp27b1 null mice compared to VDR null mice. To evaluate the mechanism of action underlying such a hypothesis, we measured the expression levels of a number of VDR target genes in the duodena of wildtype mice and in transgenic mice expressing either a normal or a hormone-binding deficient mutant VDRs. We also compared expression levels of these genes between VDR null mice and Cyp27b1 null mice. In a subset of cases, the expression of VDR target genes was lower in mice containing the VDR as opposed to mice that did not. We suggest that the VDR may function as a selective suppressor/de-repressor of gene expression in the absence of 1,25(OH)2D3.
Keywords: 1,25-dihydroxyvitamin D3-independent action; transcription repressor; transcriptional de-repressor; VDR null mice; Cyp27b1 null mice
1. Introduction
The vitamin D receptor (VDR) is a nuclear factor which mediates the biological actions of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3). One of the prominent activities of this hormone is to maintain mineral homeostasis in higher vertebrates via the regulation of gene expression by the VDR in intestine, kidney, bone and parathyroid gland (PTG) [1]. Parathyroid hormone (PTH), a calciotropic regulator secreted from the PTG, directly influences 1,25(OH)2D3 target tissues such as kidney and bone but not the intestine. Indeed, PTH profoundly upregulates the expression of renal Cyp27b1 to increase the production of 1,25(OH)2D3 which in turn activates the VDR [2]. 1,25(OH)2D3-bound VDR modulates the expression of a network of genes to raise calcium absorption in intestine [3] while influencing bone cell differentiation in conjunction with PTH to affect bone remodeling [4]. Increased levels of 1,25(OH)2D3 also exert a suppressive negative feedback loop in the PTG to reduce the production of PTH, although the molecular mechanism of such action remains unclear [5]. In kidney, ligand-activated VDR regulates target genes not only to repress Cyp27b1 gene expression to control 1,25(OH)2D3 production but also to facilitate calcium reabsorption [2].
The action of the VDR is initiated through its interaction with 1,25(OH)2D3, which results in the formation of a heterodimer with retinoid X receptor (RXR), the binding of this heterodimer to vitamin D response element (VDRE) at target genes, and the recruitment of various molecular machines that are capable of altering chromatin structure [6–10]. Our recent unbiased genome-wide studies using ChIP-sequencing (ChIP-seq) analysis coupled with to RNA-sequencing (RNA-seq) analysis in bone cells [11] and mouse small intestine [3] suggest that VDR binding sites are enriched for VDREs but also contain adjacent sequences capable of interacting with additional transcription factors that may be involved. These studies have also revealed that 1,25(OH)2D3/VDR target genes are regulated through multiple enhancers located predominantly within introns and intergenic regions but less frequently in regions near target gene promoters.
Since the syndrome of hereditary 1,25-dihydroxyvitamin D resistant rickets (HVDRR) was first identified [12], the underlying involvement of the VDR was suspected. Further biochemical and genetic studies of patient samples and cells not only supported but eventually confirmed this hypothesis [13], prompting the development of a mouse model in which the VDR was similarly mutated [14–17]. Further understanding of the roles of the 1,25(OH)2D3/VDR system were extended through studies of the syndrome of vitamin D dependency rickets type 1 (VDDR-1), a disease caused by mutations in the CYP27B1 gene that resulted in a failure of 1,25(OH)2D3 production [18], and the development of cognate Cyp27b1 null mouse models [19–21]. These human syndromes and mouse models in which 1,25(OH)2D3-mediated transcriptional activity of the VDR was compromised share a similar biological phenotype, such as abnormal mineral homeostasis and skeletal defects that include rickets and growth retardation, indicating the importance of 1,25(OH)2D3-dependent activation of the VDR. This transcriptional activity was also suggested to modulate proliferation and differentiation of epidermal keratinocytes in skin as well [22]. However, prompted by selective differences in the presence or absence of alopecia in humans as well as the observation that Vdr null but not Cyp27b1 null mice are alopecic, the concept emerged that the VDR might function in keratinocytes in the absence of 1,25(OH)2D3. This concept was further supported by studies of VDR null mice in which a mutant human VDR lacking 1,25(OH)2D3 binding activity due to a leucine to serine replacement at amino acid 233 (L233S) was expressed specifically in keratinocytes and led to normal hair growth [23].
We recently generated a humanized VDR mouse model using a bacterial artificial chromosome (BAC) that contained either the entire wildtype human VDR gene [24] or a mutant version (L233S) as just documented [25]. Although the wildtype VDR fully rescued transcription, mineral homeostasis and skin abnormalities, the mutant VDR rescued only the alopecia, supporting the concept that the control of hair follicle cycling by the VDR is 1,25(OH)2D3 ligand-independent. Interestingly, we found that the presence of this mutant form of the VDR also led to an exaggerated phenotype relative to PTH secretion and lower bone mineral density (BMD) than that seen in the VDR null mouse, suggesting the possibility that 1,25(OH)2D3-independent actions of the VDR might extend beyond the skin [25]. In the present report, we present data suggesting that the VDR expressed both in the mutant humanized mouse models we generated and in the Cyp27b1 null mouse model may function to suppress/de-repress specific target genes in the absence of 1,25(OH)2D3 not only in skin but in other mouse tissues as well.
2. Materials and Methods
2.1. Animal Study
VDR null mice [15] and Cyp27b1 null mice [21] used in this study were obtained from the Jackson Laboratories. Generation of humanized VDR mice (hVDRWT/VDR−/−) and mutant humanized VDR mice (T805/VDR−/−, T806/VDR−/− and T807/VDR−/−) were previously described [24, 25]. Wildtype mice were littermates from C57BL/6 mice (Harlan). All mice were fed standard rodent chow diet (5008; Harlan Teklad) after weaning until sacrifice at 8–10 weeks of age. Mice were exposed to a 12-hour light-dark cycle and all animal studies were reviewed and approved by the Research Animal Care and Use Committee of University of Wisconsin-Madison.
2.2. Serum PTH measurement
Blood collection and measurement of serum PTH levels were previously described [24].
2.3. Reverse transcription-polymerase chain reaction (RT-PCR)
Preparation of RNAs and cDNAs from tissues were previously described [24]. Quantitative polymerase chain reaction (qPCR) was performed as previously described [25]. TaqMan primers (Applied Biosystems) for qPCR are available upon request.
2.4. Statistical Analysis
Student’s unpaired t test was used to identify significant differences between groups (p < 0.05).
3. Results and Discussion
3.1. Potential role of the VDR as a de-repressor of PTH production in the absence of 1,25(OH)2D3
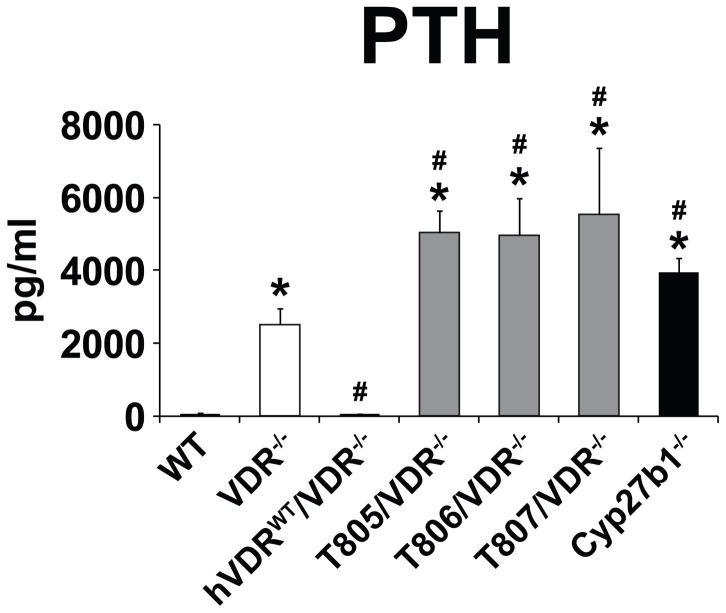
Our previous studies describe the introduction of BAC clone transgenes containing the human VDR gene and its regulatory features into the VDR null background [24, 25]. In addition to several wildtype hVDR strains, three transgenic strains designated T805/VDR−/−, T806/VDR−/−, and T807/VDR−/− were also created that produced a human L233S mutant VDR protein incapable of binding 1,25(OH)2D3 [25]. VDR protein expression in the tissues of these mice ranged from that seen in wildtype mice (T807/VDR−/−) to levels approximately 10-fold lower (T805/VDR−/−) [25]. None of these mice exhibited alopecia; however, all were hypocalcemic and phosphatemic, exhibited high levels of PTH and displayed skeletal defect analogous to those seen in VDR null mice due to a failure of the VDR to direct 1,25(OH)2D3-dependent transcription [25]. Interestingly, while serum PTH levels as documented in Fig. 1 were normalized in VDR null mice rescued through expression of the wildtype human VDR, levels of this hormone were substantially higher in VDR null mice expressing the mutant L233S VDR form [25]. As this finding suggested a potential ligand-independent de-repressing action of the VDR in the PTG, we compared the levels of PTH in these mice with those in Cyp27b1 null mice where wildtype mouse receptor was expressed normally in the absence of 1,25(OH)2D3. As can be seen in Fig. 1, PTH levels in these mice were much higher than in VDR null mice as well. Importantly, these data are consistent with an earlier report in a similar strain of hypocalcemic mice [26]. The authors at that time also concluded that the increased levels of PTH seen in Cyp27b1 null mice correlated directly with PTG size that exceeded that seen in VDR null mice and that this effect was independent of calcemic conditions [26]. Importantly, their further studies revealed that PTG size was also greater in Cyp27b1 null mice even in the absence of the VDR. This study also suggested an alternative possibility that PTG cell proliferation was regulated by serum calcium and 1,25(OH)2D3 and not by the VDR, although the idea that VDR protein might exert a direct impact on this tissue to accelerate PTH production separate from the hormone cannot be ruled out.
Fig. 1.
Analysis of serum PTH levels. PTH levels were assessed in EDTA-plasma obtained from wildtype (WT), VDR null mice (VDR−/−; white bar), wildtype humanized VDR mice (hVDRWT/VDR−/−), 3 mutant humanized VDR mice (T805/VDR−/−, T806/VDR−/− and T807/VDR−/−; gray bars) and Cyp27b1 null mice (Cyp27b1−/−; black bar) as indicated. Data are presented as the mean ± SEM (n=5–7 per strain). *p < 0.05 compared with wildtype mice, #p < 0.05 compared with VDR null mice.
3.2. Role of the VDR in bone in the absence of 1,25(OH)2D3
L233S VDR mutant mice also display reduced skeletal BMD relative to VDR null mice [25]. This contrast with VDR null mice was also observed in a recent study in mice in which a mutation was introduced into the endogenous mouse Vdr gene different from ours [27]; this created a unique form of the mouse VDR that was unable to bind 1,25(OH)2D3 as well, but could be activated by a synthetic ligand. These data suggest that the presence of the VDR protein could exert an effect on not only the PTG but on the skeleton as well, although this phenotype could also be derived from higher levels of PTH. Interestingly, mice with the knock-in mutation exhibited a more severe bone defect than seen in VDR null mice, as manifested by shorter femur length [27]. This defect was not observed in our own studies despite similarities in both experimental age and dietary content. Although a reduced femur shaft length was not seen in earlier studies of the Cyp27b1 mouse, this investigation was conducted on animals that were considerably older and maintained under different dietary conditions [26]. We conclude that while the skeletal defects seen in each of these three studies appear to be inconsistent, and sensitivity of skeletal elements to high levels of PTH could account for the results observed, the idea that a ligand binding-deficient receptor could be active in the skeleton remains a viable possibility.
3.3. Role of the VDR as a transcription repressor in the absence of 1,25(OH)2D3
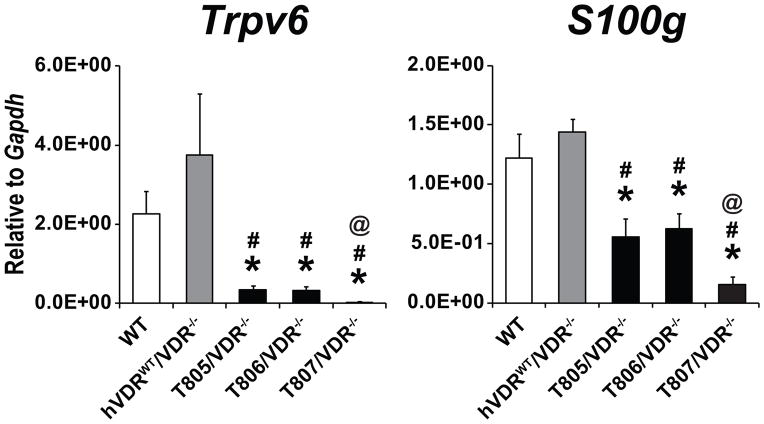
As with 1,25(OH)2D3-dependent activity, ligand-independent actions of the VDR are likely to occur at the level of transcription. Given the complexity associated with mechanisms related to PTH upregulation and the potential for this hormone to impact the skeleton separate from the VDR, we focused our search for additional 1,25(OH)2D3-independent actions at genes that were expressed in the intestine in our mouse strains and not influenced by PTH. Accordingly, we chose genes such as Trpv6 and S100g (calbindin D9k) that represent direct targets of vitamin D action and that are involved in calcium absorption. As can be seen in Fig. 2, residual expression of both genes was highest in normal wildtype mice and in mice that expressed the wildtype hVDR transgene in a mouse VDR null background. In contrast, the expression of these genes in mice producing various levels of the L233S mutant VDR were significantly depressed. It is interesting that the highest level of expression of the L233S mutant in the T807/VDR−/− strain was capable of the greatest suppression of basal Trpv6 and S100g expression and while the level of suppression in the two strains with lower level of VDR expression (T806/VDR−/− and T805/VDR−/−) was less relative to the two control mouse strains, it was still highly significant. This observation is consistent with our earlier finding that while lower levels of VDR protein were also able rescue the alopecia seen in this VDR null rescue strain (T805/VDR−/−), it was clear that the skin phenotype of the T805/VDR−/− strain was not entirely normal [25]. This finding directly at the level of gene expression in the intestine, a tissue unaffected by high circulating levels of PTH, suggests that in the absence of 1,25(OH)2D3-activation, the VDR is indeed capable of suppressing the expression of genes which are in turn induced in the presence of the hormone. Importantly, the recent study of VDR knock-in mutant mice also revealed a reduction in basal expression of Trpv6, Cyp24a1 and Slc30a10 in the intestine [27].
Fig. 2.
Basal expression of VDR target genes in duodenum of humanized VDR null mice. Expression of the indicated VDR target genes was measured in duodenum of wildtype (WT; white bars), wildtype humanized VDR mice (hVDRWT/VDR−/−; gray bars) and 3 mutant humanized VDR mice (T805/VDR−/−, T806/VDR−/− and T807/VDR−/−; black bars) by qPCR. Expression levels of the gene transcripts were normalized to Gapdh and presented as the mean ± SEM (5–7 mice per strain). *p < 0.05 compared with wildtype mice, #p < 0.05 compared with wildtype humanized VDR mice, @p < 0.05 compared with T805/VDR−/− and T806/VDR−/− mice.
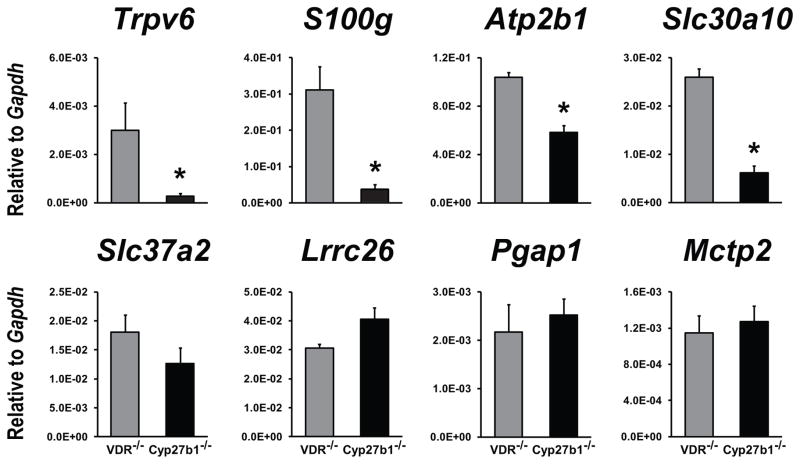
We have recently identified a gene network in the intestine of Cyp27b1 null mice that is regulated by 1,25(OH)2D3 and plays a role in the active uptake of duodenal calcium [3]. To confirm and extend the results obtained in the VDR null mouse strain expressing the human L233S VDR mutant, we contrasted in a final study the basal expression of not only Trpv6 and S100g but other newly discovered target genes as well in Cyp27b1 null mice with that of VDR null mice maintained on a normal calcium and phosphate diet. As documented in Fig. 3, although Trpv6, S100g, Atp2b1 and Slc30a10 were all significantly lower in Cyp27b1 null mice vs VDR null mice, other genes such as Slc37a1, Lrrc26, Pgap1 and Mctp2 were not repressed. These data combined with VDR protein-dependent suppression seen in the L233S mutant strains suggest that the VDR is indeed capable of the selective repression of target genes in the absence of ligand. This effect is consistent with the 1,25(OH)2D3-independent effects of the VDR in hair follicle cycling and could highlight a role for the VDR in the regulation of gene expression that is more extensive that currently appreciated. These data also suggest that the VDR may be similar to other nuclear receptors such as the thyroid and retinoid receptors that are known to bind to DNA and to act as repressors of gene expression in the absence of their cognate ligands [28–32].
Fig. 3.
Basal expression of VDR target genes in duodenum of Cyp27b1 null mice. Expression of the indicated VDR target genes was measured in duodenum of VDR null mice (VDR−/−; gray bars) and Cyp27b1 null mice (Cyp27b1−/−; black bars) by qPCR. Expression levels of the gene transcripts were normalized to Gapdh and presented as the mean ± SEM (5–7 mice per strain). *p < 0.05 compared with VDR null mice.
3.4. Advancing insights into 1,25(OH)2D3-independent actions of the VDR
Further studies to define the molecular mechanisms that enable the VDR to modulated genes in the absence of 1,25(OH)2D3 will be necessary. The first requirement will be to identify fully the sites of action of the VDR at genes whose expression can be suppressed by the VDR alone. Accordingly, we have recently performed genome-wide in vivo ChIP-seq analysis using intestine from vehicle- or 1,25(OH)2D3-treated wildtype mice [3]. This analysis has identified the presence of VDR binding sites at target genes such as Trpv6 and Cyp24a1 reported earlier [33–35] as well as near genes such S100g, Atp2b1, Cldn2 and Pdlim2, for which sites were previously unknown. Regulatory sites were also identified for a broader arrays of genes that were regulated by 1,25(OH)2D3 as well. It is at these sites that the actions of the VDR will need to be examined. An important complexity of this regulation is the finding that most genes are controlled by multiple regulatory regions often located at sites that are highly remote relative to the genes’ transcriptional start sites. Thus, an understanding of the interacting roles of these regulatory regions will also be necessary. Assuming that unliganded VDR functions at the level of DNA, a second requirement will be to identify sites on the genome to which the VDR is prebound prior to ligand activation. In this regard, recent genome-wide studies in both cells in culture and in mice in vivo suggest that while the majority of VDR DNA binding occurs in response to 1,25(OH)2D3, a smaller subset of binding sites on DNA are indeed pre-occupied by the VDR even in the absence of the hormone [9, 36–38]. The presence of the VDR at sites for which gene suppression has been noted will therefore be a likely prerequisite. Finally, we would anticipate that VDR-mediated suppression of gene expression will likely require the recruitment of coregulatory factors such as a histone deacetylase (HDAC) by the unliganded VDR that would serve to condense chromatin surrounding these gene loci thus restricting the expression of the gene of interest. Fully defining these principles will not be an easy task.
4. Conclusions
Although the VDR is a well-established critical mediator of the biological actions of 1,25(OH)2D3, the protein’s actions in tissues in the absence of this ligand are not fully understood. Nevertheless, 1,25(OH)2D3-independent action of the VDR within the hair follicle to facilitate cycling is now fully accepted, although the molecular mechanisms integral to this process remain elusive. In this report, we present several distinct features of the mouse models in which the ability of VDR to regulate gene expression in response to 1,25(OH)2D3 is abrogated yet support a potential role of the VDR as a transcription regulator in the absence of the hormone in vivo.
Acknowledgments
We thank members of the Pike laboratory for their contributions to this work. We also acknowledge David Nehls, Regina Berget and Douglas Jacobson for the animal husbandry associated with this study. This study was supported by the National Institutes of Health grants DK-072281, DK-073995 and AR-045173 to JWP.
Abbreviations
- VDR
vitamin D receptor
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- PTG
parathyroid gland
- PTH
parathyroid hormone
- RXR
retinoid X receptor
- VDRE
vitamin D response element
- ChIP-seq
ChIP-sequencing
- RNA-seq
RNA-sequencing
- HVDRR
hereditary 1,25-dihydroxyvitamin D resistant rickets
- VDDR-1
vitamin D dependency rickets type 1
- BAC
bacterial artificial chromosome
- qPCR
quantitative polymerase chain reaction
References
- 1.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78(4):1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 2.Murayama A, Takeyama K, Kitanaka S, Kodera Y, Kawaguchi Y, Hosoya T, Kato S. Positive and negative regulations of the renal 25-hydroxyvitamin D3 1alpha-hydroxylase gene by parathyroid hormone, calcitonin, and 1alpha,25(OH)2D3 in intact animals. Endocrinology. 1999;140(5):2224–2231. doi: 10.1210/endo.140.5.6691. [DOI] [PubMed] [Google Scholar]
- 3.Lee SM, Riley EM, Meyer MB, Benkusky NA, Plum LA, DeLuca HF, Pike JW. 1,25-Dihydroxyvitamin D3 Controls a Cohort of Vitamin D Receptor Target Genes in the Proximal Intestine that is Enriched for Calcium Regulating Components. J Biol Chem. 2015 doi: 10.1074/jbc.M115.665794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.St John HC, Meyer MB, Benkusky NA, Carlson AH, Prideaux M, Bonewald LF, Wesley Pike J. The parathyroid hormone-regulated transcriptome in osteocytes: Parallel actions with 1,25-dihydroxyvitamin D3 to oppose gene expression changes during differentiation and to promote mature cell function. Bone. 2014;72C:81–91. doi: 10.1016/j.bone.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nygren P, Larsson R, Johansson H, Ljunghall S, Rastad J, Akerström G. 1,25(OH)2D3 inhibits hormone secretion and proliferation but not functional dedifferentiation of cultured bovine parathyroid cells. Calcif Tissue Int. 1988;43(4):213–218. doi: 10.1007/BF02555137. [DOI] [PubMed] [Google Scholar]
- 6.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 8.Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25(1):45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 9.Pike JW, Lee SM, Meyer MB. Regulation of gene expression by 1,25-dihydroxyvitamin D3 in bone cells: exploiting new approaches and defining new mechanisms. Bonekey Rep. 2014;3:482. doi: 10.1038/bonekey.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer MB, Benkusky NA, Pike JW. 1,25-Dihydroxyvitamin D3 induced histone profiles guide discovery of VDR action sites. J Steroid Biochem Mol Biol. 2014;144(Pt A):19–21. doi: 10.1016/j.jsbmb.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St John HC, Bishop KA, Meyer MB, Benkusky NA, Leng N, Kendziorski C, Bonewald LF, Pike JW. The osteoblast to osteocyte transition: epigenetic changes and response to the vitamin D3 hormone. Mol Endocrinol. 2014;28(7):1150–1165. doi: 10.1210/me.2014-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks MH, Bell NH, Love L, Stern PH, Orfei E, Queener SF, Hamstra AJ, DeLuca HF. Vitamin-D-dependent rickets type II. Resistance of target organs to 1,25-dihydroxyvitamin D. N Engl J Med. 1978;298(18):996–999. doi: 10.1056/NEJM197805042981804. [DOI] [PubMed] [Google Scholar]
- 13.Feldman D, Malloy PJ. Mutations in the vitamin D receptor and hereditary vitamin D-resistant rickets. Bonekey Rep. 2014;3:510. doi: 10.1038/bonekey.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16(4):391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 15.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94(18):9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci U S A. 2001;98(23):13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erben RG, Soegiarto DW, Weber K, Zeitz U, Lieberherr M, Gniadecki R, Möller G, Adamski J, Balling R. Deletion of deoxyribonucleic acid binding domain of the vitamin D receptor abrogates genomic and nongenomic functions of vitamin D. Mol Endocrinol. 2002;16(7):1524–1537. doi: 10.1210/mend.16.7.0866. [DOI] [PubMed] [Google Scholar]
- 18.Feldman D, Malloy PJ, Miller WL. Genetic disorders of vitamin D synthesis and action. In: Thakker RV, Whyte MP, Eisman J, Igarashi T, editors. Genetics of Bone Biology and Skeletal Disease. Elsevier; San Diego, CA, USA: 2013. pp. 537–552. [Google Scholar]
- 19.Dardenne O, Prud’homme J, Arabian A, Glorieux FH, St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 2001;142(7):3135–3141. doi: 10.1210/endo.142.7.8281. [DOI] [PubMed] [Google Scholar]
- 20.Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98(13):7498–7503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanhooke JL, Prahl JM, Kimmel-Jehan C, Mendelsohn M, Danielson EW, Healy KD, DeLuca HF. CYP27B1 null mice with LacZreporter gene display no 25-hydroxyvitamin D3-1alpha-hydroxylase promoter activity in the skin. Proc Natl Acad Sci U S A. 2006;103(1):75–80. doi: 10.1073/pnas.0509734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bikle DD. Vitamin D and the skin: Physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13(1):3–19. doi: 10.1007/s11154-011-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skorija K, Cox M, Sisk JM, Dowd DR, MacDonald PN, Thompson CC, Demay MB. Ligand-independent actions of the vitamin D receptor maintain hair follicle homeostasis. Mol Endocrinol. 2005;19(4):855–862. doi: 10.1210/me.2004-0415. [DOI] [PubMed] [Google Scholar]
- 24.Lee SM, Bishop KA, Goellner JJ, O’Brien CA, Pike JW. Mouse and Human BAC Transgenes Recapitulate Tissue-Specific Expression of the Vitamin D Receptor in Mice and Rescue the VDR-Null Phenotype. Endocrinology. 2014;155(6):2064–2076. doi: 10.1210/en.2014-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SM, Goellner JJ, O’Brien CA, Pike JW. A humanized mouse model of hereditary 1,25-dihydroxyvitamin D-resistant rickets without alopecia. Endocrinology. 2014;155(11):4137–4148. doi: 10.1210/en.2014-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, Goltzman D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem. 2004;279(16):16754–16766. doi: 10.1074/jbc.M310271200. [DOI] [PubMed] [Google Scholar]
- 27.Huet T, Laverny G, Ciesielski F, Molnár F, Ramamoorthy TG, Belorusova AY, Antony P, Potier N, Metzger D, Moras D, Rochel N. A vitamin D receptor selectively activated by gemini analogs reveals ligand dependent and independent effects. Cell Rep. 2015;10(4):516–526. doi: 10.1016/j.celrep.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 28.Ignar-Trowbridge D, Nelson K, Bidwell M, Curtis S, Washburn T, McLachlan J, Korach K. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci U S A. 1992;89(10):4658–4662. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma ZQ, Santagati S, Patrone C, Pollio G, Vegeto E, Maggi A. Insulin-like growth factors activate estrogen receptor to control the growth and differentiation of the human neuroblastoma cell line SK-ER3. Mol Endocrinol. 1994;8(7):910–918. doi: 10.1210/mend.8.7.7984152. [DOI] [PubMed] [Google Scholar]
- 30.Arnold SF, Obourn JD, Jaffe H, Notides AC. Phosphorylation of the human estrogen receptor by mitogen-activated protein kinase and casein kinase II: consequence on DNA binding. J Steroid Biochem Mol Biol. 1995;55(2):163–172. doi: 10.1016/0960-0760(95)00177-2. [DOI] [PubMed] [Google Scholar]
- 31.Patrone C, Gianazza E, Santagati S, Agrati P, Maggi A. Divergent pathways regulate ligand-independent activation of ER alpha in SK-N-BE neuroblastoma and COS-1 renal carcinoma cells. Mol Endocrinol. 1998;12(6):835–841. doi: 10.1210/mend.12.6.0114. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, Chen Z, Beroukhim R, Wang H, Lupien M, Wu T, Regan MM, Meyer CA, Carroll JS, Manrai AK, Jänne OA, Balk SP, Mehra R, Han B, Chinnaiyan AM, Rubin MA, True L, Fiorentino M, Fiore C, Loda M, Kantoff PW, Liu XS, Brown M. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138(2):245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer MB, Zella LA, Nerenz RD, Pike JW. Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J Biol Chem. 2007;282(31):22344–22352. doi: 10.1074/jbc.M703475200. [DOI] [PubMed] [Google Scholar]
- 34.Meyer MB, Goetsch PD, Pike JW. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1alpha,25-dihydroxyvitamin D3. J Biol Chem. 2010;285(20):15599–15610. doi: 10.1074/jbc.M110.119958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pike JW, Meyer MB. Regulation of mouse Cyp24a1 expression via promoter-proximal and downstream-distal enhancers highlights new concepts of 1,25-dihydroxyvitamin D(3) action. Arch Biochem Biophys. 2012;523(1):2–8. doi: 10.1016/j.abb.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer MB, Goetsch PD, Pike JW. Genome-wide analysis of the VDR/RXR cistrome in osteoblast cells provides new mechanistic insight into the actions of the vitamin D hormone. J Steroid Biochem Mol Biol. 2010;121(1–2):136–141. doi: 10.1016/j.jsbmb.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer MB, Goetsch PD, Pike JW. VDR/RXR and TCF4/β-catenin cistromes in colonic cells of colorectal tumor origin: impact on c-FOS and c-MYC gene expression. Mol Endocrinol. 2012;26(1):37–51. doi: 10.1210/me.2011-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pike JW, Meyer MB. Fundamentals of vitamin D hormone-regulated gene expression. J Steroid Biochem Mol Biol. 2013 doi: 10.1016/j.jsbmb.2013.1011.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]