Abstract
Early stress in the form of repetitive neonatal pain, in infants born very preterm, is associated with long-term dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and with poorer cognitive performance. Brain-derived neurotrophic factor (BDNF) which is important in synaptic plasticity and cognitive functions is reduced by stress. Therefore the BDNF Val66Met variant, which affects secretion of BDNF, may interact with early exposure to pain-related stress in children born very preterm, to differentially affect HPA regulation that in turn may be associated with altered cognitive performance.
The aims of this study were to investigate whether in children born very preterm, the BDNF val66met variant modulates the association between neonatal pain-related stress and cortisol levels at age 7 years, and if cortisol levels were related to cognitive function. Furthermore, we examined whether these relationships were sex-specific. Using a longitudinal cohort design, N=90 children born very preterm (24–32 weeks gestation) were followed from birth to age 7 years. Cortisol was assayed from hair as an index of cumulative stress and from saliva to measure reactivity to a cognitive challenge. BDNF Val66Met variant was genotyped at 7 years using real time PCR. Using generalized linear modeling, in boys with the Met allele, greater neonatal pain-related stress (adjusted for clinical risk factors) predicted lower hair cortisol (p=0·006) and higher reactivity salivary cortisol (p=0.002). In both boys and girls with the Met allele, higher salivary cortisol reactivity was correlated with lower IQ (r= −0.60; p=0.001) and poorer visual-motor integration (r= −0.48; p=0.008).
Our findings show associations between lower BDNF availability (presence of the Met allele) and vulnerability to neonatal pain/stress in boys, but not girls. This exploratory study suggests new directions for research into possible mechanisms underlying how neonatal pain/stress is related to cognitive performance in children born very preterm.
Keywords: BDNF rs6265, preterm, infant, cortisol, pain, sex
1. Introduction
Early adversity is known to affect behavioral and physiological systems in the long-term. Genetic variation can offer opportunities to unveil gene-environment interactions that may explain susceptibility and resiliency to early adverse environmental effects. Brain-derived neurotrophic factor (BDNF) is an important neurotrophin which is widely expressed in the brain especially in the prefrontal and hippocampal regions, and has long-term effects on neuronal survival, development and synaptic plasticity. Human postmortem (Chen et al., 2001) and animal studies have found that stress modifies BDNF expression: decreased expression in hippocampus and increased in amygdala (Rasmusson et al., 2002; Govindarajan et al., 2006; Lakshminarasimhan and Chattarji, 2012). BDNF is involved in cognitive functions (Hariri et al., 2003; Zhang et al., 2014; Zhang et al., 2013), with the pathophysiology of psychiatric disorders such as schizophrenia, major depression, and bipolar disorder (see (Notaras et al., 2015; Duman and Monteggia, 2006). The BDNF Val66Met (rs6265) variant affects intracellular processing and secretion of BDNF (Egan et al., 2001). The Met allele encodes a precursor protein with impaired function which results in lower BDNF availability, and hence is associated with alterations of human hippocampal function and episodic memory (Egan et al., 2003). Many studies have implicated the BDNF Val66Met polymorphism in psychiatric disorders such as schizophrenia, Alzheimer’s disease, and affective disorders (Ventriglia et al., 2002; Chen et al., 2006; Rybakowski, 2008; Verhagen et al., 2010). Sex differences have been found in BDNF levels (e.g. (Xiu et al., 2009; Zhang et al., 2014). However, the direction of interaction differs depending on the study population and outcome measures. Availability of BDNF is also affected by early environmental physical or psychological stress, which is reported to result in decreased neurotrophic support to certain BDNF-rich regions (Brown et al., 2003; Rasmusson et al., 2002). However, the relationships between early adversity, BDNF levels, and cognition in young children is still unknown.
Infants born very preterm are exposed to considerable procedural pain-related stress during weeks to months of hospitalization in the neonatal intensive care unit (NICU) (Grunau, 2007; Grunau, 2013). Since pain and stress cannot be distinguished in very preterm infants, we use the term “pain-related” stress (Grunau et al., 2013). Early life stress can permanently alter neural, hormonal, and behavioral systems and thereby contribute to increased vulnerability to cognitive problems later in life (Heim and Nemeroff, 2002; Richards and Wadsworth, 2004). Children born very prematurely perform more poorly in tasks that require attention and inhibition (Kulseng et al., 2006; Shum et al., 2008). Executive functions in children born very preterm have been identified as an area of difficulty in this population, even when intelligence is broadly normal (Mulder et al., 2009; Aarnoudse-Moens et al., 2012). One primary mechanism underling early programming effects of stress is re-setting of the hypothalamic-pituitary-adrenal (HPA) axis, reflected by long-term changes in cortisol the primary stress hormone in humans (Brummelte et al., 2012; Grunau, 2007; Grunau, 2013; Zouikr et al., 2014). Early exposure to stress has been shown to have prolonged effects on cognitive and affective functions (Richards and Wadsworth, 2004; Heim and Nemeroff, 2002; Heim et al., 2008); reviewed by (Lupien et al., 2009; Pechtel and Pizzagalli, 2011).
Neurodevelopmental and behavioral problems are highly prevalent among children born very preterm (Aarnoudse-Moens et al., 2009), however, little is known about the etiology of these difficulties, and particularly whether altered endogenous cortisol expression following early stress may play a role. In a longitudinal study, we found the trajectory of salivary cortisol expression was altered in very preterm infants while in the NICU (Grunau et al 2005), and compared to infants born full-term term long after NICU discharge (Grunau et al., 2007). Importantly, cumulative neonatal stress (higher number of skin-breaking procedures from birth to term adjusted for clinical confounders related to prematurity) was associated with these altered cortisol levels, Furthermore, altered hair and salivary cortisol levels were evident at school-age in children born very preterm (Grunau et al., 2013; Brummelte et al., 2015; Kajantie and Raikkonen, 2010; Buske-Kirschbaum et al., 2007). While endogenous cortisol levels play an important role in brain function, there is a dearth of knowledge of the relationship between cognitive performance and cortisol expression at school age in children born preterm.
In rodent studies, long-term effects of pain on the HPA axis appear to depend on the type of pain induced in the neonatal period. In a model of repetitive neonatal pin prick pain, while increased anxiety was evident in adulthood there was no effect on corticosterone level (Anand et al., 1999; Walker et al., 2003). Walker et al using the same pin-prick model, also found no effect on corticosterone, but concluded this might be due to increased maternal licking and grooming by dams of pups in the pain group, suggesting maternal behavior might buffer effects of pain thereby preventing HPA changes. On the other hand, inflammatory pain induced by carrageenan did show long-term alteration in HPA axis regulation (Victoria et al., 2013a), anxiety (Victoria et al., 2013b), and memory (Henderson et al., 2015). The conditions under which neonatal pain leads to changes in neuroendocrine function, the relationship with cognition, and underlying mechanisms are unclear.
Hair cortisol provides an integrated index of cumulative stress level during an extended period of time while salivary cortisol reflects short-term activation and reactivity of the HPA axis (Sauve et al., 2007; Steudte et al., 2011; van Holland et al., 2012). Hair and salivary cortisol serve as non-invasive complementary biomarkers of stress hormone regulation (van Holland et al., 2012).
Our previous studies have shown that cortisol levels in very preterm children at school age were associated with neonatal pain-related stress in a sex-dependent way (Brummelte et al., 2015; Grunau et al., 2013). In preterm boys with the minor allele in NFKBIA rs2233409 (NF-kappa-B inhibitor alpha) which regulates the NF-κB mediated inflammatory response (Hayden and Ghosh, 2008; Lynn et al., 2008)) greater procedural neonatal pain/stress predicted lower hair cortisol at age 7 years, (Grunau et al., 2013). Moreover, greater neonatal pain/stress also predicted lower salivary cortisol levels and altered pattern of diurnal cortisol (Brummelte et al., 2015). Sex-dependent effects of early life stress and adversity have been found in animal (e.g. (Darnaudery and Maccari, 2008), and human studies (Sandman et al., 2013; Ruttle et al., 2014). However, stress responses appear to be context-dependent and timing of the developmental stage influences whether male or female are vulnerable or resilent to early life adversity (Khashan et al 2008; Mueller and Bale 2008; Kundakovic et al 2013; Jacobson-Pick and Richter 2010; Weathington et al 2012).
Multiple biopsychosocial mechanisms may contribute to these sex differences in pain, including sex hormones, endogenous opioid function, and genetic factors (Zubieta et al., 2003; Mogil, 2012; Craft, 2007). Epidemiologic and clinical findings consistently show that women are at higher risk for chronic pain and may experience more severe clinical pain, and women show greater pain sensitivity than men (reviewed by (Ruau et al., 2012; Bartley and Fillingim, 2013). However, most studies examined post-puberty samples. Developmental differences in pain and stress response between boys and girls before puberty are still largely unknown.
In the present study we investigated whether the BDNF Val66Met variant contributed to susceptibility or resiliency to long-term effects of early physical pain/stress. We examined whether the BDNF Val66Met genotype modulates the association between neonatal procedural pain-related stress and cortisol levels (hair to index cumulative stress and saliva to measure reactivity), in a longitudinal cohort study of children born very preterm. We also examined whether sex affected the relationships between cortisol levels, BDNF Val66Met polymorphism, and cognitive outcomes. We hypothesized that in children born very preterm, BDNF Val66Met polymorphism would modify the effect of neonatal exposure to pain/stress on hair and salivary cortisol levels, and that the relationship between the BDNF variant, HPA axis and cognitive outcomes would be sex-dependent. This is the first study in very preterm children to address cortisol levels in relation to the BDNF Val66Met variant and the relationship with cognition.
2. Methods
2.1. Subjects
A total of 106 school age children (53 boys, 53 girls) born preterm at 25 to 32 weeks gestational age (GA) in 2000 to 2004, admitted to the NICU at the Children’s & Women’s Health Centre of BC, were seen at mean age 7.69 years (SD 0.31) as part of a longitudinal study on the long-term effects of pain-related stress in children born very preterm (e.g. (Grunau et al., 2007; Grunau et al., 2009; Brummelte et al., 2015; Grunau et al., 2013). Parent informed consent and child assent were obtained. None of the children had a major sensory impairment (blind or sensory-neural hearing loss requiring amplification), motor impairment (non-ambulatory cerebral palsy) or intellectual impairment (Wechsler Intelligence Scale for Children 4th edition full scale IQ < 70). One boy with a diagnosis of autism, 4 children (2 boys, 2 girls) with severe brain injury on neonatal ultrasound (periventricular leukomalacia or grade III–IV intraventricular hemorrhage) and 11 (8 boys, 3 girls) receiving medications that affect cortisol (e.g. to treat Attention Deficit Hyperactivity Disorder or asthma) were excluded. Characteristics of the 90 (42 boys, 48 girls) children included in this study are provided in Table 1. This study was approved by the Clinical Research Ethics Board of the University of British Columbia and the Children’s & Women’s Health Centre of BC, and conforms to the conventions set out in the Declaration of Helsinki.
Table 1.
Child and Mother Characteristics by sex
| Characteristic (N=95) | Boys n=42 | Girls n=48 | p-value |
|---|---|---|---|
| Child | |||
| Gestational age at birth (weeks) mean (SD) | 29.50 (2.34) | 29.69 (2.45) | 0.712 |
| Birth weight (g) mean (SD) | 1,377 (474) | 1,289 (380) | 0.322 |
| Child age at testing (y) (SD) | 7.7 (0.31) | 7.7 (0.27) | 0.349 |
| Skin-breaking procedures (SD) | 107 (85) | 90 (78) | 0.305 |
| Cumulative dose of Morphine (SD) | 1.2 (3.8) | 1.3 (4.1) | 0.934 |
| Days of mechanical ventilation (SD) | 7.8 (11.5) | 9.3 (18.3) | 0.636 |
| Illness severity day 1 SNAP-II (SD) | 11.5 (10.1) | 9.6 (11.6) | 0.397 |
| Number of surgeries (n) mean (SD) | 0.3 (0.7) | 0.2 (0.6) | 0.263 |
| Number of postnatal infection (n) mean (SD) | 0.3 (0.4) | 0.2 (0.4) | 0.811 |
|
| |||
| Mother | |||
| Education (years) mean (SD) | 16.2 (3.0) | 15.5 (2.6) | 0.265 |
| Beck Depression Index-II mean (SD) | 7.22 (8.26) | 7.14 (6.13) | 0.956 |
| State anxiety mean (SD) | 29.36 (10.17) | 30.76 (8.37) | 0.463 |
| Trait anxiety mean (SD) | 34.29 (9.69) | 37.78 (8.34) | 0.062 |
2.2. Neonatal data
Medical and nursing chart review was carried out by an experienced research neonatal nurse. Variables included, but were not limited to gestational age, birth weight, early illness severity (SNAP-II) on day 1, number of skin-breaking procedures (e.g. heel lance, intramuscular injection, intravenous or central line insertion), days of mechanical ventilation, postnatal infection, number of surgeries, and daily dose of morphine (Table 1). Neonatal pain-related stress was operationalized as the sum of all skin-breaking procedures from birth to term equivalent age or hospital discharge, whichever came first, adjusted statistically for clinical confounders associated with prematurity. Cumulative exposure to morphine was calculated as the average daily dose (i.e. intravenous dose plus intravenous-equivalent oral dose) adjusted for daily body weight, multiplied by the number of days morphine was given. Morphine was standard analgesia in our NICU for preterm infants on mechanical ventilation; in addition 7 children were exposed to the sedative midazolam (2 boys, 5 girls). Fentanyl was only used during surgery. During the period 2000–2004 when these infants were in the NICU little non-pharmacological pain management was used in our centre.
2.3. Cortisol Measurement
Cortisol levels were assayed from saliva and hair samples collected by research assistants at the same study visit at 7 years old. The baseline samples of most study visits were collected in the morning (average time at 10 am, ranged between 9 – 11 am), except 3 children were seen at noon.
2.3.1. Longer-term Integrated Hair Cortisol Level
Cortisol was assayed from hair samples to reflect stress in the prior two months, as described in our previous published studies (Grunau et al., 2013; Chau et al., 2014). Hair samples were collected from the vertex posterior of the head, as it has been shown to have the lowest coefficient of variation in hair cortisol concentrations (Sauve et al., 2007). A cluster of hair strands approximately half the diameter of a pencil, were cut from five small spots at the base of the hair shaft, at the level of the scalp. The hair samples were secured on a card with tape, labeled, and stored in individual sealable plastic bags at room temperature until analysis. The most proximal 2 cm from each hair sample were used for the assay. Selected hair was weighed, and 10–15 mg from each sample was placed in scintillation vials. To remove external contaminants, hair samples were washed twice by immersing each sample in 3 mL of isopropanol and incubating it at room temperature at 100 RPM for 3 minutes. After decanting the isopropanol, samples were allowed to dry for at least 12 hours. After drying, 1 mL of methanol was added to each scintillation vial. Hair was then finely minced with surgical scissors, cleaning the scissors between samples. The vials were sealed with paraffin film and incubated for 16 hours at 50°C at 100 RPM. Following methanol extraction each cortisol-containing methanol solution was pipetted into 5 mL test tubes and evaporated on a test tube hot plate under a steady stream of nitrogen gas. The residue was then reconstituted with 250 μL of phosphate buffered saline. These reconstituted samples were analyzed using the salivary enzyme linked immunoassay kit (Alpco Diagnostics, Salem, NH, USA). The intra-assay and inter-day coefficients of variation were 8·867% and 5·124%, respectively. The kit reported a sensitivity of 1.0 ng/mL.
2.3.2. Short-term Salivary Cortisol Reactivity
Two baseline samples were collected ~30 minutes after arrival to the study to allow children to adapt to the study location. These samples were averaged to get a baseline cortisol level. A “cognitive stressor” response sample was taken 20 minutes after the end of computerized executive functions tasks that required inhibitory control, working memory and visual spatial memory skills, that preterm children find challenging (Kulseng et al., 2006; Shum et al., 2008; Mulder et al., 2009; Aarnoudse-Moens et al., 2012). Saliva samples were stored at −20°C until assayed using the Salimetrics High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit for quantitative determination of salivary cortisol (Salimetrics LLC, State College, PA). Cortisol values were examined for outliers, then winsorized (Tukey, 1977) and log transformed prior to analysis (Gunnar et al., 2009). Reactivity salivary cortisol was calculated using the following formula: [log (post-cognitive stressor cortisol) – log(baseline cortisol)]/[log (baseline cortisol)].
2.4. Genetic analysis
Genomic DNA was collected and extracted from saliva using Oragene OG-500 and prepIT-L2P collection kit (DNA Genotek/OraSure Technologies Inc., Bethlehem, Pennsylvania). Final purified genomic DNA concentration was ranged from 24 ng/μL to 437 ng/μL. The BDNF Val66Met variant was genotyped using TaqMan SNP Genotyping Assay reagents and primers specific for BDNF rs6265 (C_11592758_10), and an Applied Biosystems 7500 Real Time PCR System (Applied Biosystems, Carlsbad, California). Call rate was 100%. The Hardy-Weinberg equilibrium of allelic distribution of the cohort was examined and compared to the population distribution by chi-square test.
2.5. Child Cognitive function assessment
To assess cognitive functions, the Wechsler Intelligence Scale for children (WISC-IV; (Wechsler, 1989)) was administered to preterm children at age 7 years during their visit to the Centre. Children completed computerized executive function tasks including the Dots task and a Flanker task (Diamond et al., 2007; Weikum et al., 2013). The computerized tasks were time constrained and novel to the children, and had been shown to be challenging for healthy children attending regular school (Diamond et al., 2007). Therefore we hypothesized the tasks were also be challenging for children born very preterm. The Beery-Buktenica Developmental Test of Visual-Motor Integration, 6th Edition (Beery VMI; (Beery and Beery, 2004) was used to measure eye-hand co-ordination.
2.6. Parental factors
Questionnaires were completed by the child’s primary caregiver (88 mothers and 2 fathers). The Beck Depression Inventory – 2nd Edition (BDI-II) (Beck et al., 1996), a 21-item self-report multiple choice test, was used to assess the presence and severity of symptoms of depression; the BDI-II has an alpha coefficient of 0.80. The State-Trait Anxiety Inventory (Spielberger, C.D., Gorsuch, R.L., Lushene, R., Vagg, P.R., Jacobs, G.A., 1983) that comprises two 20-statement self-report scales, was used to measure state and trait anxiety. The S-Anxiety scale (STAI Form Y-1) evaluates how respondents feel “right now, at this moment” and the T-Anxiety scale (STAI Form Y-2) how respondents generally feel.
2.7. Statistical analysis
T-test was used to compare the neonatal characteristics between preterm girls and boys, and cognitive performance between BDNF Val/Val and Met allele carriers (Val/Met and Met/Met genotypes). Pearson chi-square test was used to compare the genotype distribution between preterm girls and boys. Due to the low frequencies of the BDNF Met allele (Table 2), subjects with the Met/Met and the Val/Met genotypes were grouped together in all analyses. ANCOVA was used to examine the sex differences in salivary reactivity cortisol levels adjusted for concurrent maternal factors (depressive and anxiety symptoms). To examine the relationship between genotypes, cumulative neonatal pain/stress (adjusted for clinical confounders) and hair cortisol level, Generalized Linear Modeling (GZLM) was used. GZLM, which provides an extension of general linear models (GLM) to permit examination of correlated clinical confounders, relaxes the requirement of equality or constancy of variances that is required in traditional linear models, and allows correlated confounding variables to be entered as covariates. SPSS version 22 was used for GZLM and all other statistics.
Table 2.
BDNF Val66Met allelic distribution between preterm boys and girls
| BDNF Val66Met rs6265 | Boys (n=45) | Girls (n=50) | Chi-square p-value a | HWE p-value b |
|---|---|---|---|---|
| Val/Val | 29 | 35 | 0.564 | 0.143 |
| Val/Met + Met/Met | 16 | 15 |
p-values from Pearson Chi-Square tests between boys and girls.
p-values from Hardy-Weinberg Equilibrium between allelic distributions.
3. Results
3.1. Subjects characteristics
Child and parent characteristics were compared by sex, and neonatal clinical information by sex (see Table 1). The BDNF Val66Met genotype distribution by sex is shown in Table 2. There was no significant difference in genotype frequency between boys and girls (p = 0.56), and the distribution of genotypes had no significant discrepancy from the Hardy-Weinberg equilibrium (p = 0.143).
3.2. BDNF Val66Met genotype interacts with neonatal pain and predicts hair cortisol levels in preterm boys
Due to the sex differences in hair (Grunau et al, 2013) and salivary cortisol levels (Brummelte et al, 2015) in this cohort, modeling of the neonatal predictors was carried out separately for boys and girls. A GZLM model was constructed with the BDNF Val66Met genotype and postnatal infection as factors, and number of skin-breaking procedures (neonatal pain-related stress), number of surgeries, cumulative morphine, early illness severity (SNAP II on day 1), and days of mechanical ventilation, as covariates, to predict hair cortisol level.
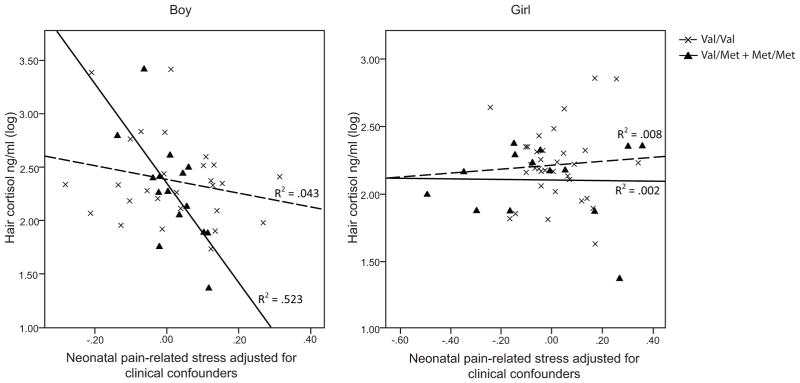
We found that, in preterm boys, but not girls, an interaction between the BDNF Val66Met genotype and extent of neonatal pain-related stress exposure on hair cortisol levels. Lower hair cortisol was observed in boys with the BDNF 66 Val/Met and Met/Met genotypes that had greater exposure to neonatal pain-related stress (B = −1.36, p = 0.006, r2 = 0.523), independent of morphine dosage, early illness severity, days on mechanical ventilation, gestational age, and postnatal infection. This relationship was not observed in boys with the BDNF 66Val/Val genotype. Regression parameters are shown in Table 3. The interactions between BDNF Val66Met genotypes and neonatal pain-related stress and their relationships with hair cortisol are shown in Figure 1.
Table 3.
GENLIN parameter tables BDNF Val66Met in preterm boys – Hair Cortisol
| Ba | Std. Error | t value | p-value | |
|---|---|---|---|---|
| BDNF 66Val/Val | 0b | - | - | - |
| BDNF 66 Val/Met + Met/Met | 2.53 | 0.91 | 2.797 | 0.009 |
| Postnatal infection present | 0.27 | 0.20 | 1.361 | 0.183 |
| Postnatal infection absent | 0b | - | - | - |
| Neonatal pain-related stress | −0.72 | 0.51 | 1.404 | 0.170 |
| Cumulative morphine dose | 0.05 | 0.33 | 0.137 | 0.892 |
| Days of mechanical ventilation | 0.25 | 0.27 | 0.92 | 0.364 |
| Gestational age | −0.10 | 0.05 | 1.904 | 0.066 |
| Severity of Illness | −0.006 | 0.009 | ||
| Number of surgeries | 0.19 | 0.13 | 1.456 | 0.155 |
| BDNF Val/Val x Neonatal pain-related stress | 0b | - | - | - |
| BDNF Val/Met and Met/Met x Neonatal pain-related stress | −1.36 | 0.47 | 2.922 | 0.006 |
B is the unstandardized regression coefficient showing the effect on the outcome variable per unit change of each individual predictor
Denotes parameter is redundant
Figure 1.
Hair cortisol predicted by neonatal pain-related stress and BDNF Val66Met polymorphism in very preterm children.
GZLM results of 45 boys (29 Val/Val; 16 Val/Met or Met/Met) and 50 girls (35 Val/Val; 15 Val/Met or Met/Met) were shown in the figure.
Solid lines represented children with Val/Met or Met/Met genotypes; dashed lines represented children with Val/Val genotype.
Analyses were done with or without SNAP-II and number of surgeries without significant effect on the model. We re-ran the model adding concurrent stressors (mother’s depressive and anxiety symptoms). Results of the models remained the same (p = 0.016), and there was no significant effect for the concurrent stressors. Results also remained unchanged (p = 0.035) after we removed children who had neonatal exposure to midazolam and/or fentanyl.
3.3. BDNF Val66Met genotype interacts with neonatal pain to predict salivary reactivity cortisol in boys at 7 yrs
An independent t-test revealed no sex difference in salivary reactivity cortisol levels (p = 0.514). The main effect for sex remained non-significant (p = 0.452) after ANCOVA was used to control for concurrent maternal factors (self-reported anxiety (p = 0.706), depressive symptoms (p = 0.982), parental stress (p = 0.724), and mother’s years of education (p = 0.157).
Similar to the analyses of hair cortisol, GZLM models were constructed separately for boys and girls with the BDNF Val66Met genotype and postnatal infection as factors, and number of skin-breaking procedures (neonatal pain-related stress), number of surgeries, cumulative morphine, early illness severity (SNAP II on day 1), and days of mechanical ventilation, as covariates, to predict reactivity cortisol level.
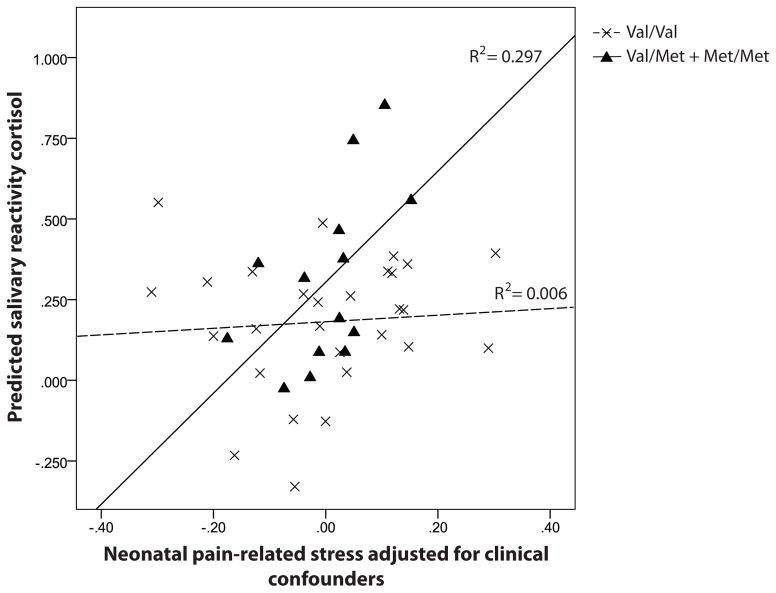
We found that, in preterm boys, but not girls, there was a significant interaction between the BDNF Val/Met + Met/Met genotypes and neonatal pain-related stress (B = 0.162, p = 0.002, r2 = 0.297). Higher salivary reactivity cortisol was in boys with the Val/Met and Met/Met genotypes who had the greatest neonatal procedural pain-related stress, independent of morphine dosage, early illness severity (SNAP-II day 1), days on mechanical ventilation, gestational age, and postnatal infection. Detailed regression parameters are shown in Table 4. The interactions between BDNF Val66Met genotypes and neonatal pain-related stress and their relationships with salivary reactivity cortisol in very preterm boys are shown in Figure 2.
Table 4.
GENLIN parameter tables BDNF Val66Met in preterm boys – Reactivity Cortisol
| Ba | Std. Error | t value | p-value | |
|---|---|---|---|---|
| BDNF Val/Val | 0b | - | - | - |
| BDNF Val/Met + Met/Met | −2.95 | 0.90 | 10.70 | 0.003 |
| Postnatal infection present | 0.23 | 0.18 | 1.51 | 0.228 |
| Postnatal infection absent | 0b | - | - | - |
| Neonatal pain-related stress | 1.49 | 0.53 | 2.70 | 0.111 |
| Cumulative morphine dose | −0.42 | 0.33 | 1.62 | 0.213 |
| Days of mechanical ventilation | 0.13 | 0.23 | 0.34 | 0.562 |
| Gestational age | 0.09 | 0.04 | 4.20 | 0.049 |
| Severity of Illness | 0.01 | 0.01 | 2.58 | 0.119 |
| Number of surgeries | 0.01 | 0.13 | 0.01 | 0.939 |
| BDNF Val/Val x Neonatal pain-related stress | 0b | - | - | - |
| BDNF Val/Met + Met/Met x Neonatal pain-related stress | 1.62 | 0.48 | 11.28 | 0.002 |
B is the unstandardized regression coefficient showing the effect on the outcome variable per unit change of each individual predictor
Denotes parameter is redundant
Figure 2.
Salivary reactivity cortisol predicted by neonatal pain and BDNF Val66Met polymorphism in very preterm boys.
GZLM results of 45 boys (29 Val/Val; 16 Val/Met or Met/Met) were shown in the figure.
Solid lines represented children with Val/Met or Met/Met genotypes; dashed lines represented children with Val/Val genotype.
Analyses were done with or without SNAP-II and number of surgeries without significant effect on the model. We also re-ran the model adding in concurrent stressors (mother’s depressive and anxiety symptoms). Results of the model remained the same (p = 0.002), and there was no significant effect for the concurrent stressors (p = 0.935 and p = 0.270 respectively). Since 3 children were seen in the afternoon, we tried to exclude them and the results of the t-test, ANCOVA and GZLM remained unchanged. Results also remained unchanged (p = 0.008) after we removed children who had neonatal exposure to midazolam and/or fentanyl.
3.4. Correlation between cortisol (hair and salivary) and cognition
To understand the relationship between cortisol reactivity and cognitive outcomes, Pearson correlations were computed between hair and salivary reactivity cortisol levels, WISC-IV FSIQ scores, and Beery VMI scores. There was no significant correlation between both cortisol levels (hair and saliva) and WISC-IV FSIQ and Beery VMI scores, in boys and girls. Hair and salivary reactivity cortisol levels were significantly correlated in girls (r = 0.54, p < 0.001) but not in boys (r = −0.30, p = 0.069).
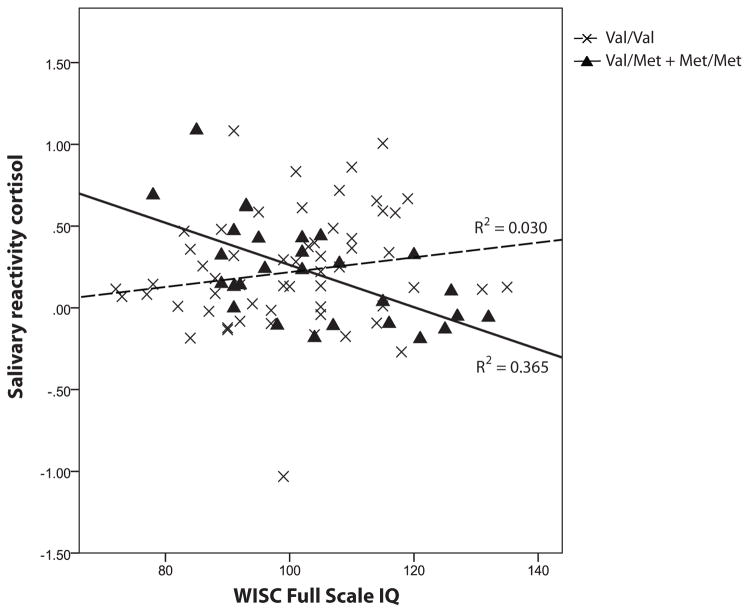
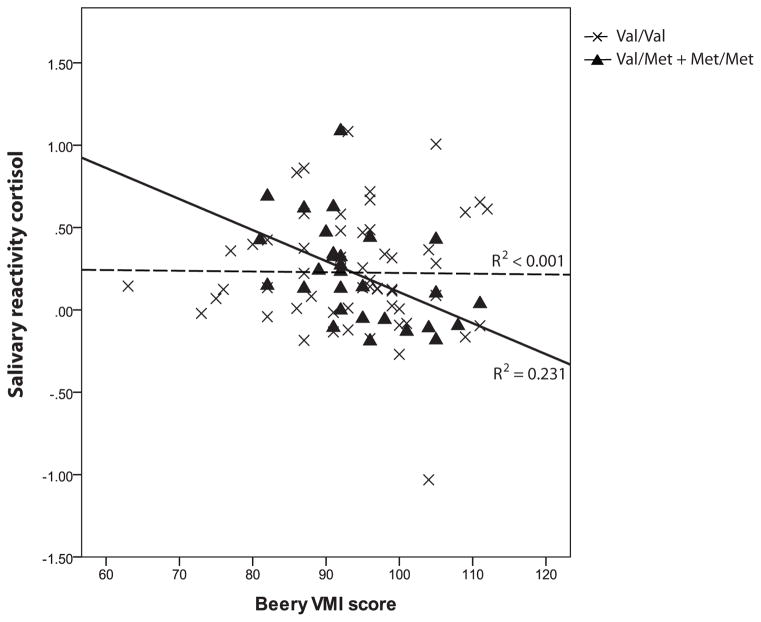
In children with BDNF 66Val/Met + Met/Met genotypes, greater salivary reactivity cortisol was correlated with lower WISC-IV total scores and Beery VMI scores (r = −0.60, p = 0.001 and r = −0.48, p = 0.008 respectively). In contrast, among children with the BDNF 66Val/Val genotype there was no significant correlation (r = 0.21, p = 0.117 and r = 0.104, p = 0.436 respectively). The correlations were shown in Figure 3a and b. The results did not differ by sex. Partial correlations remained significant after adjusting for parents’ years of education, parental depression and trait anxiety (p = 0.028 and 0.018 respectively). There was no significant correlation between hair cortisol and cognitive outcomes in either BDNF genotypes. T-tests showed no significant differences of WISC-IV total IQ score and Beery VMI score between BDNF Val66Met genotypes (p = 0.245 and p = 0.600 respectively).
Figure 3.
Figure 3a. Correlation between salivary reactivity cortisol and WISC Full Scale IQ for BDNF Val66Met polymorphism in very preterm children
Figure 3b. Correlation between salivary reactivity cortisol and Beery VMI for BDNF Val66Met polymorphism in very preterm children
4. Discussion
We found that the association between neonatal pain-related stress (adjusted for clinical confounders of prematurity) and cortisol levels (hair and saliva) at age 7 years differed depending on the BDNF Val66Met genotype in very preterm boys but not girls. Greater neonatal pain/stress was associated with higher salivary reactivity cortisol to stress of cognitive testing, and to lower hair cortisol, but only in boys with the BDNF 66Met allele (Val/Met and Met/Met genotypes). In contrast to the sex-dependent relationship between neonatal physical stress and cortisol levels at age 7 years, the relationship of higher salivary cortisol reactivity and poorer cognitive performance was evident in both boys and girls with BDNF 66Met allele. Hair cortisol was not related to cognitive ability for either sex. This is the first study, to our knowledge, to address cortisol levels in relation to the BDNF Val66Met variant and the relationship with cognition in children born very preterm. Our findings further highlight the importance of sex-specific effects of early stress exposure on the HPA axis.
BDNF plays an important role in promoting and modifying growth, development, survival of neuronal populations and neuronal plasticity (Huang and Reichardt, 2001). Studies indicated that the BDNF Val66Met variant is related to hippocampus-mediated memory performance in adults with specific neuropsychiatric disorders (Egan et al., 2003; Hariri et al., 2003; Zhang et al., 2014). Egan et. al. found that Met allele carriers have poorer episodic memory than subjects with the Val/Val genotype, regardless of whether they were healthy volunteers, persons with schizophrenia, or their unaffected siblings. In the same study, subjects with the Val/Met genotype also had lower left hippocampal N-acetyl aspartate levels. Healthy volunteers with the Met allele also have altered hippocampal functional MRI blood oxygenation level while performing a declarative memory task (Egan et al., 2003) and have smaller hippocampal and prefrontal GM volumes (Pezawas et al., 2004). Numerous studies have reported in the adult general population that individuals with the BDNF 66Met allele show poorer performance when challenged with cognitive and motor learning tasks, suggestive of a decrease in plasticity (Gong et al., 2009; Kleim et al., 2006). However, other studies in healthy young adults have not found this relationship (Karnik et al., 2010; Richter-Schmidinger et al., 2011). Similarly, in children born very preterm, we found that the BDNF Val66Met genotype was unrelated to cognitive and visual-motor performance. However, we did find that lower cognitive and visual-motor performance was associated with greater salivary reactivity cortisol in preterm children with the BDNF 66Met allele, and higher reactivity cortisol was predicted by more neonatal pain exposure in very preterm boys with a the BDNF 66Met variant. The relationship between greater neonatal stress/pain and higher salivary reactivity was consistent with our previous work suggesting long-term altered programming of the HPA axis (Brummelte et al., 2011). BDNF Met/Met mice have been shown to have reduced NMDA receptor-dependent synaptic transmission and plasticity in the infralimbic medial prefrontal cortex (Siobhan et al, 2012) and hippocampus (Ninan et al, 2012). Therefore, it appears plausible that very preterm children with the BDNF 66Met allele have reduced neural plasticity and have greater vulnerability to neonatal pain exposure, especially for boys.
Our results showed that in preterm children with the BDNF 66Met allele, higher salivary cortisol reactivity to a cognitive stressor was related to poorer cognitive performance, consistent with previous work in healthy adults and elders (e.g. (Lee et al., 2007; Bohnen et al., 1990; Minkley and Kirchner, 2012). This relationship was only found for cortisol reactivity, not to chronic stress reflected in hair cortisol. Moreover, lower hair cortisol was related to greater exposure to neonatal pain/stress in boys with the BDNF 66Met allele, similar to the lower hair cortisol found in patients with posttraumatic stress disorder (PTSD) (Steudte et al., 2013) and generalized anxiety disorder (GAD) (Steudte et al., 2011). Both hair and salivary cortisol have been used as biomarkers for stress hormone regulation, showing complementary information about stress axis function in healthy adults (Sauve et al., 2007; Steudte et al., 2011; van Holland et al., 2012); to our knowledge this has not been studied in children. Unexpectedly, we found sex differences in the association between hair and salivary cortisol levels, with only girls showing a relationship. Since hair cortisol is an integrated index of longer-term stress, concurrent social stress exposure and perception of stress may contribute to the differences. We tried to adjust for this variability by adjusting statistically for parental stress and mood in this study, however further research on chronic stress and stress reactivity in children is warranted
Sex-specific effects of early stress on the HPA axis are widely reported in the animal literature. Early life stress can have sex-dependent long-term effects on the responsiveness of the HPA axis (Darnaudery and Maccari, 2008; Viveros et al., 2009; Kaiser and Sachser, 2005), with some studies reporting higher vulnerability or sensitivity in males to the early disturbances compared to females (e.g. (Mueller and Bale, 2008; Llorente et al., 2011), while others report changes in HPA axis function predominantly in females (McCormick et al., 1995; Weinstock, 2007). These differences may be due to different types and timing of the stressors (Bale, 2011). Also, HPA axis function is mediated by many factors such as age, hormones levels (e.g. menstrual phase), genetic and environmental factors and psychopathology (Kudielka et al., 2009), which might explain some of the discrepancies seen in the literature. Nonetheless, overall the literature suggests that males and females may either have different developmental windows of vulnerability and/or adapt differently to an early adverse environment.
Human studies examining sex differences in pre-pubertal children in regards to HPA axis programming due to early adverse experiences are rare. We previously reported in the same cohort that very preterm boys have lower hair cortisol than girls at age 7 years (Grunau et al., 2013). In that study, we found that girls appeared to be more sensitive to concurrent stressors while boys were more sensitive to neonatal pain-related stress. To further highlight the different sex response to early adversity, we recently showed that in preterm boys only, the overall methylation level in the promoter region of serotonin transporter (SLC6A4) was associated with neonatal pain and stress exposure (Chau et al., 2014). A previous study from another group (Jones et al., 2006) found that in 7–9 year old children, lower birth weight was significantly associated with a higher salivary cortisol response to the Trier Social Stress Test (TSST) in boys, but not in girls. In line with this, a blunted cortisol response to the TSST was reported in young adults with early trauma, which was more prominent in men than in women (Elzinga et al., 2008). Similarly, Carpenter et al., (Carpenter et al., 2007) revealed a flattened adrenocorticotropin (ACTH) response to the TSST in men with early maltreatment. Moreover, a recent study (DeSantis et al., 2011) found that early traumatic experiences were strongly positively associated with baseline ACTH levels and with ACTH response to corticotropic releasing hormone (CRH) in men but not or less so in women. Further, parental loss or separation is differently associated with HPA axis function in men and women (Tyrka et al., 2008; Pesonen et al., 2010). However, it is important to note that other studies did not observe differences between males and females in neuroendocrine regulation after early stress or trauma (for review see: (Kajantie and Raikkonen, 2010) and that the direction of effect of early stress on cortisol or ACTH baseline or reactivity levels (i.e. positive or negative association) is inconsistent in the literature. Furthermore there is a large body of studies showing that effects of early life adversity on HPA programming and clinical outcomes are greater in women (e.g. Klengel et al 2013; Heim et al 2001). It is important that we and others (Jones et al. 2006) have found sex differences in prepubertal children, thus differences in concurrent sex steroid concentrations cannot sufficiently explain the observed sex differences (Kajantie and Raikkonen, 2010). Instead, evidence from animal studies suggests that there might be an important interaction between early stress and regulation of gonadal hormone production, thus early adverse experiences may interfere with gonadal programming of a sexually dimorphic brain (Bale, 2011). However, less is known about the disturbances of the hormonal milieu and surges due to a premature birth and early stress exposure. Therefore more research is needed to better understand the impact of gender on neuroendocrine outcomes after stress early in life.
5. Limitations
Our findings in this study reflect associations rather than causal relationships, which is a limitation. However, in human infants, prospective longitudinal cohort studies are the only way to investigate the mechanisms underlying long-term effects of repetitive procedural pain/stress exposure. Secondly, although we found statistically significant relationships in this study, we have limited power to address sex differences. Therefore we view this study as exploratory; our results will suggest new research directions.
6. Conclusion
Our results further the understanding of how early neonatal pain/stress exposure may influence later HPA functioning, and how genetic variation may affect the resilience to early adversity, in a sex-specific manner. In very preterm boys only, the BDNF Val66Met variant interacted with procedural pain-related stress in the NICU (adjusted for medical confounders) to predict altered stress programming of the HPA axis at age 7 years, and greater neonatal pain/stress exposure was associated with poorer cognitive and visual-motor performance.
Highlights.
BDNF rs6265 modulates the association between neonatal pain/stress and cortisol levels in children born very preterm
In boys with a Met allele, greater pain/stress is associated with lower hair cortisol and higher reactivity salivary cortisol
Higher salivary cortisol reactivity was negatively correlated with IQ and visual-motor integration
Presence of the Met allele may increase vulnerability to neonatal pain/stress in a sex-dependent way
Acknowledgments
This study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health R01 HD039783 to REG. REG holds a Senior Scientist salary award for the Child and Family Research Institute. CMYC was supported by a graduate stipend for the Pain in Child Health (PICH) CIHR Strategic Training Program. AMD holds a Scientist salary award from the Child and Family Research Institute.
We thank the families who participated, Gisela Gosse for coordinating the study, Wayne Yu for determination of salivary cortisol levels. We would also like to thank Drs. Evan Russell, Gideon Koren and Stan Van Uum for hair cortisol measurement.
Footnotes
Conflict of interest statement
None declared.
Contributors
REG and JW designed the study, CMYC, IC contributed to collecting and analyzing the data and all authors contributed to interpreting the data. CMYC wrote the first draft of the manuscript and all authors reviewed and approved the final manuscript.
Role of the funding source
The funding sources had no further role in the study design; in the collection, analysis and interpretation of the data; in the writing of the report and in the decision to submit the paper for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarnoudse-Moens CS, Duivenvoorden HJ, Weisglas-Kuperus N, Van Goudoever JB, Oosterlaan J. The profile of executive function in very preterm children at 4 to 12 years. Dev Med Child Neurol. 2012;54:247–253. doi: 10.1111/j.1469-8749.2011.04150.x. [DOI] [PubMed] [Google Scholar]
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–637. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14:348–356. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111:52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Beery KE, Beery MA. The Beery-Buktenica Developmental-Test of Visual-Motor Integration. Minneapolis, MN: NCS Pearson Inc; 2004. [Google Scholar]
- Bohnen N, Houx P, Nicolson N, Jolles J. Cortisol reactivity and cognitive performance in a continuous mental task paradigm. Biol Psychol. 1990;31:107–116. doi: 10.1016/0301-0511(90)90011-k. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Chau CM, Cepeda IL, Degenhardt A, Weinberg J, Synnes AR, Grunau RE. Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology. 2015;51:151–163. doi: 10.1016/j.psyneuen.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Chau CMY, Synnes AR, Cepeda IL, Weinberg J, Grunau RE. Neonatal pain predicts cortisol levels at age 7 years in children born preterm. Pediatric Academic Societies’ 2000–2012 Archive 2012 [Google Scholar]
- Brummelte S, Grunau RE, Zaidman-Zait A, Weinberg J, Nordstokke D, Cepeda IL. Cortisol levels in relation to maternal interaction and child internalizing behavior in preterm and full-term children at 18 months corrected age. Dev Psychobiol. 2011;53:184–195. doi: 10.1002/dev.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Krieger S, Wilkes C, Rauh W, Weiss S, Hellhammer DH. Hypothalamic-pituitary-adrenal axis function and the cellular immune response in former preterm children. J Clin Endocrinol Metab. 2007;92:3429–3435. doi: 10.1210/jc.2006-2223. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau CMY, Ranger M, Sulistyoningrum D, Devlin AM, Oberlander TF, Grunau RE. Neonatal pain and COMT Val158Met genotype in relation to serotonin transporter (SLC6A4) promoter methylation in very preterm children at school age. Front Behav Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM. Modulation of pain by estrogens. Pain. 2007;132(Suppl 1):S3–12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Darnaudery M, Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev. 2008;57:571–585. doi: 10.1016/j.brainresrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- DeSantis SM, Baker NL, Back SE, Spratt E, Ciolino JD, Moran-Santa Maria M, Dipankar B, Brady KT. Gender differences in the effect of early life trauma on hypothalamic-pituitary-adrenal axis functioning. Depress Anxiety. 2011;28:383–392. doi: 10.1002/da.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Gong P, Zheng A, Chen D, Ge W, Lv C, Zhang K, Gao X, Zhang F. Effect of BDNF Val66Met polymorphism on digital working memory and spatial localization in a healthy Chinese Han population. J Mol Neurosci. 2009;38:250–256. doi: 10.1007/s12031-009-9205-8. [DOI] [PubMed] [Google Scholar]
- Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci U S A. 2006;103:13208–13213. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE. Neonatal pain in very preterm infants: long-term effects on brain, neurodevelopment and pain reactivity. Rambam Maimonides Med J. 2013;4:e0025. doi: 10.5041/RMMJ.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE. Long term effects of pain in infants. In: Schmidt RF, Willis WD, editors. Encyclopedic Reference of Pain. New York, NY: Springer Berlin/Heidelberg; 2007. pp. 1055–1057. [Google Scholar]
- Grunau RE, Cepeda IL, Chau CMY, Brummelte S, Weinberg J, Lavoie P, Ladd M, Hirschfeld AF, Russell E, Koren G, Van Uum S, Brant R, Turvey SE. Neonatal Pain-Related Stress and NFKBIA Genotype are Associated with Altered Cortisol Levels in Preterm Boys at School Age. PLoS ONE. 2013;8:e73926. doi: 10.1371/journal.pone.0073926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150:151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, Keidar A, Rogers M, Mackay M, Hubber-Richard P, Johannesen D. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143:138–146. doi: 10.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. Neurobiology of early life stress: clinical studies. Semin Clin Neuropsychiatry. 2002;7:147–159. doi: 10.1053/scnp.2002.33127. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Henderson YO, Victoria NC, Inoue K, Murphy AZ, Parent MB. Early life inflammatory pain induces long-lasting deficits in hippocampal-dependent spatial memory in male and female rats. Neurobiol Learn Mem. 2015;118:30–41. doi: 10.1016/j.nlm.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Godfrey KM, Wood P, Osmond C, Goulden P, Phillips DI. Fetal growth and the adrenocortical response to psychological stress. J Clin Endocrinol Metab. 2006;91:1868–1871. doi: 10.1210/jc.2005-2077. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Sachser N. The effects of prenatal social stress on behaviour: mechanisms and function. Neurosci Biobehav Rev. 2005;29:283–294. doi: 10.1016/j.neubiorev.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Raikkonen K. Early life predictors of the physiological stress response later in life. Neurosci Biobehav Rev. 2010;35:23–32. doi: 10.1016/j.neubiorev.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Karnik MS, Wang L, Barch DM, Morris JC, Csernansky JG. BDNF polymorphism rs6265 and hippocampal structure and memory performance in healthy control subjects. Psychiatry Res. 2010;178:425–429. doi: 10.1016/j.psychres.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9:735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Kulseng S, Jennekens-Schinkel A, Naess P, Romundstad P, Indredavik M, Vik T, Brubakk AM. Very-low-birthweight and term small-for-gestational-age adolescents: attention revisited. Acta Paediatr. 2006;95:224–230. doi: 10.1080/08035250500421568. [DOI] [PubMed] [Google Scholar]
- Lakshminarasimhan H, Chattarji S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS One. 2012;7:e30481. doi: 10.1371/journal.pone.0030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, Bolla KI, Schwartz BS. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Arch Gen Psychiatry. 2007;64:810–818. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- Llorente R, Miguel-Blanco C, Aisa B, Lachize S, Borcel E, Meijer OC, Ramirez MJ, De Kloet ER, Viveros MP. Long term sex-dependent psychoneuroendocrine effects of maternal deprivation and juvenile unpredictable stress in rats. J Neuroendocrinol. 2011;23:329–344. doi: 10.1111/j.1365-2826.2011.02109.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lynn DJ, Winsor GL, Chan C, Richard N, Laird MR, Barsky A, Gardy JL, Roche FM, Chan TH, Shah N, Lo R, Naseer M, Que J, Yau M, Acab M, Tulpan D, Whiteside MD, Chikatamarla A, Mah B, Munzner T, Hokamp K, Hancock RE, Brinkman FS. InnateDB: facilitating systems-level analyses of the mammalian innate immune response. Mol Syst Biol. 2008;4:218. doi: 10.1038/msb.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- Minkley N, Kirchner WH. Influence of test tasks with different cognitive demands on salivary cortisol concentrations in school students. Int J Psychophysiol. 2012;86:245–250. doi: 10.1016/j.ijpsycho.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder H, Pitchford NJ, Hagger MS, Marlow N. Development of executive function and attention in preterm children: a systematic review. Dev Neuropsychol. 2009;34:393–421. doi: 10.1080/87565640902964524. [DOI] [PubMed] [Google Scholar]
- Notaras M, Hill R, van den Buuse M. A role for the BDNF gene Val66Met polymorphism in schizophrenia? A comprehensive review. Neurosci Biobehav Rev. 2015;51C:15–30. doi: 10.1016/j.neubiorev.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen AK, Raikkonen K, Feldt K, Heinonen K, Osmond C, Phillips DI, Barker DJ, Eriksson JG, Kajantie E. Childhood separation experience predicts HPA axis hormonal responses in late adulthood: a natural experiment of World War II. Psychoneuroendocrinology. 2010;35:758–767. doi: 10.1016/j.psyneuen.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Shi L, Duman R. Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology. 2002;27:133–142. doi: 10.1016/S0893-133X(02)00286-5. [DOI] [PubMed] [Google Scholar]
- Richards M, Wadsworth ME. Long term effects of early adversity on cognitive function. Arch Dis Child. 2004;89:922–927. doi: 10.1136/adc.2003.032490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Schmidinger T, Alexopoulos P, Horn M, Maus S, Reichel M, Rhein C, Lewczuk P, Sidiropoulos C, Kneib T, Perneczky R, Doerfler A, Kornhuber J. Influence of brain-derived neurotrophic-factor and apolipoprotein E genetic variants on hippocampal volume and memory performance in healthy young adults. J Neural Transm. 2011;118:249–257. doi: 10.1007/s00702-010-0539-8. [DOI] [PubMed] [Google Scholar]
- Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J Pain. 2012;13:228–234. doi: 10.1016/j.jpain.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Klein MH, Slattery MJ, Kalin NH, Armstrong JM, Essex MJ. Adolescent adrenocortical activity and adiposity: differences by sex and exposure to early maternal depression. Psychoneuroendocrinology. 2014;47:68–77. doi: 10.1016/j.psyneuen.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakowski JK. BDNF gene: functional Val66Met polymorphism in mood disorders and schizophrenia. Pharmacogenomics. 2008;9:1589–1593. doi: 10.2217/14622416.9.11.1589. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn LM, Davis EP. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res. 2013;75:327–335. doi: 10.1016/j.jpsychores.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve B, Koren G, Walsh G, Tokmakejian S, Van Uum SH. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. 2007;30:E183–91. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- Shum D, Neulinger K, O’Callaghan M, Mohay H. Attentional problems in children born very preterm or with extremely low birth weight at 7–9 years. Arch Clin Neuropsychol. 2008;23:103–112. doi: 10.1016/j.acn.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Steudte S, Kirschbaum C, Gao W, Alexander N, Schonfeld S, Hoyer J, Stalder T. Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biol Psychiatry. 2013;74:639–646. doi: 10.1016/j.biopsych.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Steudte S, Stalder T, Dettenborn L, Klumbies E, Foley P, Beesdo-Baum K, Kirschbaum C. Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Res. 2011;186:310–314. doi: 10.1016/j.psychres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory Data Analysis. Don Mills, ON: Addison-Wesley; 1977. [Google Scholar]
- Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holland BJ, Frings-Dresen MH, Sluiter JK. Measuring short-term and long-term physiological stress effects by cortisol reactivity in saliva and hair. Int Arch Occup Environ Health. 2012;85:849–852. doi: 10.1007/s00420-011-0727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventriglia M, Bocchio Chiavetto L, Benussi L, Binetti G, Zanetti O, Riva MA, Gennarelli M. Association between the BDNF 196 A/G polymorphism and sporadic Alzheimer’s disease. Mol Psychiatry. 2002;7:136–137. doi: 10.1038/sj.mp.4000952. [DOI] [PubMed] [Google Scholar]
- Verhagen M, van der Meij A, van Deurzen PA, Janzing JG, Arias-Vasquez A, Buitelaar JK, Franke B. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol Psychiatry. 2010;15:260–271. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- Victoria NC, Inoue K, Young LJ, Murphy AZ. Long-term dysregulation of brain corticotrophin and glucocorticoid receptors and stress reactivity by single early-life pain experience in male and female rats. Psychoneuroendocrinology. 2013a;38:3015–3028. doi: 10.1016/j.psyneuen.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Victoria NC, Inoue K, Young LJ, Murphy AZ. A single neonatal injury induces life-long deficits in response to stress. Dev Neurosci. 2013b;35:326–337. doi: 10.1159/000351121. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Lopez-Gallardo M, Suarez J, Bermudez-Silva F, De la Fuente M, Rodriguez de Fonseca F, Garcia-Segura LM. Sex-dependent alterations in response to maternal deprivation in rats. Psychoneuroendocrinology. 2009;34(Suppl 1):S217–26. doi: 10.1016/j.psyneuen.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Walker CD, Kudreikis K, Sherrard A, Johnston CC. Repeated neonatal pain influences maternal behavior, but not stress responsiveness in rat offspring. Brain Res Dev Brain Res. 2003;140:253–261. doi: 10.1016/s0165-3806(02)00611-9. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Preschool and Primary Scale of Intelligence - Revised. San Antonio, TX: The Psychological Corporation; 1989. [Google Scholar]
- Weikum WM, Brain U, Chau CM, Grunau RE, Boyce WT, Diamond A, Oberlander TF. Prenatal serotonin reuptake inhibitor (SRI) antidepressant exposure and serotonin transporter promoter genotype (SLC6A4) influence executive functions at 6 years of age. Front Cell Neurosci. 2013;7:180. doi: 10.3389/fncel.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res. 2007;32:1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- Xiu MH, Hui L, Dang YF, Hou TD, Zhang CX, Zheng YL, Chen da C, Kosten TR, Zhang XY. Decreased serum BDNF levels in chronic institutionalized schizophrenia on long-term treatment with typical and atypical antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1508–1512. doi: 10.1016/j.pnpbp.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Zhang L, Benedek DM, Fullerton CS, Forsten RD, Naifeh JA, Li XX, Hu XZ, Li H, Jia M, Xing GQ, Benevides KN, Ursano RJ. PTSD risk is associated with BDNF Val66Met and BDNF overexpression. Mol Psychiatry. 2013 doi: 10.1038/mp.2012.180. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Chen DC, Tan YL, Tan SP, Wang ZR, Yang FD, Xiu MH, Hui L, Lv MH, Zunta-Soares GB, Soares JC. Gender difference in association of cognition with BDNF in chronic schizophrenia. Psychoneuroendocrinology. 2014;48:136–146. doi: 10.1016/j.psyneuen.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Zouikr I, Tadros MA, Barouei J, Beagley KW, Clifton VL, Callister RJ, Hodgson DM. Altered nociceptive, endocrine, and dorsal horn neuron responses in rats following a neonatal immune challenge. Psychoneuroendocrinology. 2014;41:1–12. doi: 10.1016/j.psyneuen.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]