Abstract
Object sharing abilities of infants at risk for autism (AR infants) and typically developing (TD) infants were compared from 9 to 15 months of age. Specifically, we examined the effects of infants’ locomotor abilities on their object sharing skills. 16 TD infants and 16 AR infants were observed during an “object sharing” paradigm at crawling and walking ages. Overall, AR walking infants demonstrated lower rates of object sharing with caregivers compared to TD walking infants. Specifically, AR walking infants had lower rates of giving and approaches toward caregivers compared to TD walking infants. AR walking infants also had lower step rates toward task-appropriate targets, i.e. caregivers and objects compared to TD walking infants. No group differences in object sharing were observed at crawling ages. Object sharing could be a valuable context for early identification of delays in infants at risk for developing ASD.
Keywords: Object sharing, Locomotion, At-risk infants, Perceptuo-motor impairments, Social impairments, Autism spectrum disorder
1. Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by social communication impairments such as lack of eye contact or response to name, reduced sharing of interests with others, and delayed or atypical language development as well as presence of repetitive and stereotyped behaviors and interests (American Psychiatric Association, 2013). In addition, children demonstrate comorbidities in the perceptuo-motor domains (Bhat, Landa, & Galloway, 2011) including poor postural control, clumsy walking patterns, and poor manual dexterity skills (Esposito, Venuti, Apicella, & Muratori, 2011; Minshew, Sung, Jones, & Furman, 2004; Sacrey, Germani, Bryson, & Zwaigenbaum, 2014; Bhat et al., 2011). Currently, ASD is the most common pediatric developmental disorder in the United States, with a prevalence of 1 in every 68 children (Baio, 2014). Therefore, there is a growing emphasis on early detection of and interventions for infants presenting with early signs of ASD in order to facilitate positive future outcomes (Robins, Fein, Barton, & Green, 2001; Messinger et al., 2013). Recent research on early detection has involved prospective studies that follow the development of infant siblings of children diagnosed with ASD who are at a greater risk to develop autism and other related delays (Messinger et al., 2013). For example, approximately 18.7% of the infant siblings develop ASD at 3 years of age (Ozonoff et al., 2011). Moreover, an additional 20–30% of the infant siblings exhibit subtle delays and difficulties consistent with a Broader Autism Phenotype including multiple motor, social, and language delays (Landa, Gross, Stuart, & Bauman, 2012; Yirmiya, Gamliel, Shaked, & Sigman, 2007; Bhat et al., 2011). Another special population at risk for developing ASD includes preterm infants with 26% receiving the ASD diagnosis and up to 59% presenting general developmental delays (Limperopoulos et al., 2008; Mwaniki, Atieno, Lawn, & Newton, 2012). Currently, ASD can be diagnosed as early as the end of the second year of life (Robins et al., 2001; Shattuck et al., 2009). Given the aforementioned evidence among various sub-groups at-risk for ASD, there is growing recognition for developmental surveillance of at-risk infants in the first year of life to identify early markers of autism risk and other developmental delays.
1.1. Social communication delays in AR infants
Prospective studies have identified several social communication delays within the first two years in AR infants including reduced social smiles, poor social engagement, impaired joint attention skills, and difficulties engaging in social interactions (Presmanes, Walden, Stone, & Yoder, 2007; Goldberg et al., 2005; Stone, McMahon, Yoder, & Walden, 2007; Cassel et al., 2007; Ibanez, Messinger, Newell, Lambert, & Sheskin, 2008; Toth, Dawson, Meltzoff, Greenson, & Fein, 2007; Chawarska, Klin, Paul, & Volkmar, 2007; Landa & Garrett-Mayer, 2006; Ozonoff et al., 2010; Bhat, Galloway, & Landa, 2010). For example, during an associative learning task 6-month-old AR infants engaged in greater bouts of non-social attention and spent less time looking at their caregivers compared to TD infants (Bhat et al., 2010). Similarly, in contrast to TD infants who demonstrated an increase in frequencies of social attention, smiling, and directed vocalizations from 12 to 18 months, AR infants demonstrated a decline in all three behaviors over time (Ozonoff et al., 2010). By 14 months, AR infants diagnosed with ASD demonstrated robust impairments in joint attention (JA), the ability to share attention about interesting events or objects with social partners using eye gaze and gestures (Landa, Holman, & Garrett-Mayer, 2007). Specifically, AR infants demonstrated difficulties in both initiating as well as responding to joint attention bids compared to TD infants in the second year of life (Cassel et al., 2007; Goldberg et al., 2005). In a different study, AR toddlers between 18 and 27 months demonstrated delays in expressive and receptive language, used fewer communicative words and gestures, and had lower IQ scores compared to TD peers (Toth et al., 2007). Specifically, parents reported social impairments in their infants as early as 13 months of age (Toth et al., 2007). Overall, as a group, AR infants demonstrate significant delays in social interaction skills within the first 2 years of life.
1.2. Perceptuo-motor delays in AR infants
There is growing research on perceptuo-motor delays in gross and fine motor skills in AR infants. Some of the earliest delays in AR infants who later developed ASD have been reported in the perceptuo-motor domain and not the diagnostic social communication domain (Bhat et al., 2011; Bryson et al., 2007; Landa & Garrett-Mayer, 2006). AR infants demonstrate gross and fine motor delays including hypotonicity, poor postural control, reduced movement repertoire, poor object exploration skills, and perseverative object play (Flanagan, Landa, Bhat, & Bauman, 2012; Bhat, Galloway, & Landa, 2012; Libertus, Sheperd, Ross, & Landa, 2014; Nickel, Thatcher, Keller, Wozniak, & Iverson, 2013; Ozonoff et al., 2008). For example, at 3 and/or 6 months, AR infants demonstrated poor head control during a pull-to-sit task (Flanagan et al., 2012) and poor postural skills on a standardized motor assessment (Bhat et al., 2012) compared to low-risk TD infants. Similarly, a longitudinal assessment of postural skills of AR and TD infants during naturalistic play-based activities from 6 to 14 months suggested that AR infants showed delays in the acquisition of sitting and standing skills compared to TD infants (Nickel et al., 2013). Moreover, AR infants who went on to develop ASD diagnoses initiated fewer postural changes and were delayed in acquiring more advanced postures compared to TD peers (Nickel et al., 2013). In terms of fine motor skills, AR infants also demonstrated poor grasping and immature object manipulation skills within an object play context as well as on a standardized developmental assessment (Libertus et al., 2014). Overall, there is growing evidence for the presence of delayed and atypical gross and fine motor skills in AR infants within the first year.
1.3. Links between motor and social development in infancy
Although there is substantial evidence on delayed/atypical motor and social skills in AR infants, interestingly, there is a lack of literature exploring links between motor and social domains in AR infants over the first two years of life. In contrast, there is a large body of developmental literature in TD infants exploring how motor development impacts social and communication development (Biringen, Emde, Campos, & Appelbaum, 2008; Campos, 1990; Campos et al., 2000; Campos, Kermoian, Witherington, Chen, & Dong, 1997; Campos, Kermoian, & Zumbahlen, 1992; Clearfield, 2011; Karasik, Tamis-LeMonda, & Adolph, 2011; Walle & Campos, 2014). Toward the end of the first year, as infants expand their motor repertoire in terms of their postural and locomotor skills as well as grasping and object manipulation abilities, they are able to explore their physical and social environment in novel and diverse ways, thereby facilitating concurrent advances in social communication skills. For example, compared to prelocomotor infants, parents of locomotor infants reported that infants showed greater instances of engagement in interactive play, increased back-and-forth checking back with caregivers during exploratory play, frequent displays of intense forms of affection toward caregivers, and greater attention to distal events in the environment (Campos et al., 1992). Similarly, Karasik et al. (2011) examined changes in infants’ actions on objects and people during their transition from crawling to walking. With the onset of walking, TD infants accessed more distal objects and used their locomotor skills more often to approach their caregivers and share objects with them (Karasik et al., 2011). Given the substantial perceptuo-motor and social impairments in AR infants, it would be critical to examine delays or differences in relationships between subsystems over the course of development in AR infants compared to TD infants. A better understanding of multisystem links in AR infants could provide us with potential early markers for autism diagnosis and may also serve as targets for early intervention.
1.4. Present study
The current diagnosis of ASD is based on clinical judgment as well as standardized assessments such as the Autism Diagnostic Interview, Revised (ADI-R) (Lord, Rutter, & Couteur, 1994), a structured parent interview to obtain the developmental history of the subject and the Autism Diagnostic Observation Schedule (ADOS-2) (Lord et al., 2012), that involves a series of structured and semi-structured interactions between the examiner and the subject. During these interactions, the examiner provides a number of social presses or opportunities for the child to demonstrate age-appropriate social communication behaviors as well as restricted and repetitive behavioral patterns specific to autism (Lord et al., 2012). Social communication skills include spontaneous and reciprocal interactions (non-verbal and verbal) during play, motor imitation, response to name, social smiles, and gestural use (Lord et al., 2012). Along the same lines, in the current study, we assessed object sharing, another early emerging social skill that is frequently observed during object play between caregivers and infants within the first two years of life.
We specifically aimed to explore motor-social links by extending Karasik’s work and comparing object sharing skills in TD and AR infants at crawling and walking ages. We were specifically interested in assessing group differences in object sharing skills and understanding how these differences are influenced by the transition from crawling to walking. We compared the total rates and types of bids initiated by crawling and walking infants toward their caregivers during a play-based “object sharing” paradigm within naturalistic settings. The onset of walking is associated with more mature and sophisticated forms of interactions with objects and people (Karasik et al., 2011). Therefore, we hypothesized that both TD and AR infants would share more at walking compared to crawling ages. However, we expected that AR infants would have lower rates of sharing compared to TD infants. To explore group differences in the influence of locomotor abilities on object sharing skills following the onset of walking, we coded step rates of infants in both groups during their walking visits. We expected that TD infants would step more toward targets related to the task, i.e. steps toward caregivers for sharing objects with them, whereas AR infants would step more frequently toward other non-social cues in the room.
2. Materials and methods
2.1. Participants
Sixteen infants at risk for ASD or AR infants (13 males and 3 females, 15 Caucasian and 1 African-American, 14 infant siblings of children with ASD and 2 preterm infants who developed ASD at 2 years) and sixteen typically developing infants (TD infants; 11 males and 5 females, 15 Caucasian and 1 of mixed ethnicity) with no family history of ASD, prematurity, or significant birth history participated. Infants were observed at 9 months (TD infants—M = 9.92, SD = 0.58; AR infants—M = 9.70, SD = 0.56, t(28) = 1.09, p = 0.29), 12 months (TD infants—M = 12.88, SD = 0.54; AR infants—M = 12.97, SD = 0.89, t(30) =−0.34, p = 0.74), and 15 months of age (TD infants—M = 15.85, SD = 0.53; AR infants—M = 15.66, SD = 1.13, t(28) = 0.59, p = 0.59) during the object sharing paradigm. All families belonged to the upper-middle or upper class based on socioeconomic status (TD infants—M = 55.32, SD = 9.22; AR infants—M = 52.03, SD = 12.66, p = 0.44) (Hollingshead, 1975). Participants were recruited through phone calls and fliers distributed to autism centers, schools, early intervention centers, ASD advocacy groups, and online announcements. We excluded infants with significant birth history including low birth weight, head injury, birth trauma, known genetic disorder, hearing or vision impairment, or any orthopedic diagnoses. Older siblings of all AR infants and the two preterm infants met diagnostic criteria for ASD based on the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994), expert clinical judgment, and/or medical records. All infants were recruited in the study following written parental consent as approved by the Institutional Review Board at the University of Connecticut.
2.2. Future outcomes
We assessed infants’ future outcomes and delays using parent screening questionnaires at 18 months and by emailing parents around infants’ 2nd birthday to obtain information regarding any developmental delays or diagnoses and services received by infants (see Tables 1 and 2). Parents in both groups were asked to fill out the Ages and Stages Questionnaire, Third Edition (ASQ-3™) (Squires & Bricker, 2009) and the Modified checklist for Autism in Toddlers (M-CHAT) (Robins et al., 2001) at 18 and 24 months. The ASQ-3™ is a valid and reliable screener for developmental delays in children between 1 month and 5.5 years of age (Squires & Bricker, 2009). Standard scores were obtained for the various sub-domains of motor, social, and cognitive development (see Tables 1 and 2). Scores falling 2 SDs below the mean were considered a severe delay. The M-CHAT is a 23-item screening questionnaire used to assess risk for ASD. Failure on a total of 3 or more items or 2 or more critical items is suggestive of greater risk for ASD (Robins et al., 2001). We have ASQ-3 and M-CHAT data from 14 TD infants and 14 AR infants at 18 months (see Tables 1 and 2). In the TD group, none of the infants reported severe delays on the ASQ-3 or failed on the M-CHAT at 18 and 24 months, and none of the parents reported any diagnoses or intervention services at 2 years of age (see Table 1). In the AR group, 7 infants reported delays on the ASQ-3™ and 6 infants failed on the M-CHAT at 18 months (see Tables 1 and 2). Note: that the two preterm infants who later developed ASD did not complete the ASQ-3™. Based on follow-up email inquiries, 3 infants were diagnosed with ASD and an additional 5 infants were diagnosed with language delays. Moreover, six AR infants were receiving early intervention at two years of age (see Tables 1 and 2). Overall, 10 out of the 16 AR infants developed future developmental delays based on questionnaire data or received diagnoses/services based on parent reports (see Table 2). Due to small sample sizes, we will not be distinguishing the performance of toddlers who developed ASD later; however, individual data are shown in Fig. 3B and C.
Table 1.
Future outcomes of TD infants and AR infants based on parent questionnaires collected at 18 months and parent reports of diagnoses or delays and early intervention services received at 2 years of age.
| Group | Delays on ASQ-3™ | Failed on M-CHAT | Email follow-up of current delays or diagnoses and services received |
||
|---|---|---|---|---|---|
| Social communication | Motor | Cognitive | |||
| TD infants | 0/14 | 0/14 | 0/14 | 0/14 | 0/16 |
| AR infants | 5/14 | 3/14 | 2/14 | 6/14 | 8/16 (5 language delays, 3 ASD diagnosis) |
Note: We used the communication and personal-social sub-scales to assess infants’ social communication delays, the gross motor and fine motor sub-scales to assess motor delays, and the problem solving sub-scale to assess for cognitive delays. Delays were defined as performance 2SD below the mean.
Table 2.
Individual data on outcomes, diagnoses, and intervention services received in the AR group.
| Infant no. (gender) | Age at crawling visit |
Age at walking visit |
M-CHAT 18 mos. |
M-CHAT 24 mos. |
ASQ-3 18 and 24 mos. |
Diagnosis | Intervention services |
|---|---|---|---|---|---|---|---|
| 1 (M) | 14.75 | 18.37 | No data | No data | No data | ASD | Multiple |
| 2 (M) | 14.75 | 18.37 | No data | No data | No data | ASD | Multiple |
| 3 (M) | 9.10 | 14.95 | Fail | Fail | Communication & social: SD | ASD | Multiple |
| 4 (M) | 12.91 | 15.61 | No data | No data | Communication: SD | LD | Early intervention |
| 5 (M) | 9.33 | 15.08 | Pass | Pass | None | LD | None |
| 6 (M) | 9.53 | 15.51 | Fail | Pass | Communication, social, gross motor, & problem solving: SD | LD | Speech |
| 7 (M) | 13.67 | 15.44 | Fail | Fail | Communication & social: SD | LD | Speech |
| 8 (M) | 9.20 | 15.28 | Fail | Fail | Gross motor & fine motor: SD | LD | None |
| 9 (M) | 9.76 | 15.70 | Fail | Pass | Problem solving: SD | None | None |
| 10 (M) | 9.17 | 15.33 | Fail | Pass | Fine motor, social, & problem solving: SD | None | None |
| 11 (F) | 9.69 | 14.42 | Pass | Pass | None | None | None |
| 12 (M) | 9.63 | 15.93 | Pass | Pass | None | None | None |
| 13 (M) | 9.53 | 15.24 | Pass | Pass | None | None | None |
| 14 (M) | 9.26 | 15.11 | Pass | Pass | None | None | None |
| 15 (F) | 10.58 | 15.50 | Pass | Pass | None | None | None |
| 16 (F) | 12.09 | 15.15 | Pass | Pass | None | None | None |
| TD M(SD) | 11.09 (1.65) | 15.57 (1.23) | All passed | All passed | No delays | None | None |
Note: SD: Severe delay (<2SD below mean), ASD: Autism Spectrum Disorder, LD: Language Delay.
A total of 10 AR infants developed future delays or received diagnoses or services, see shaded rows.
Fig. 3.
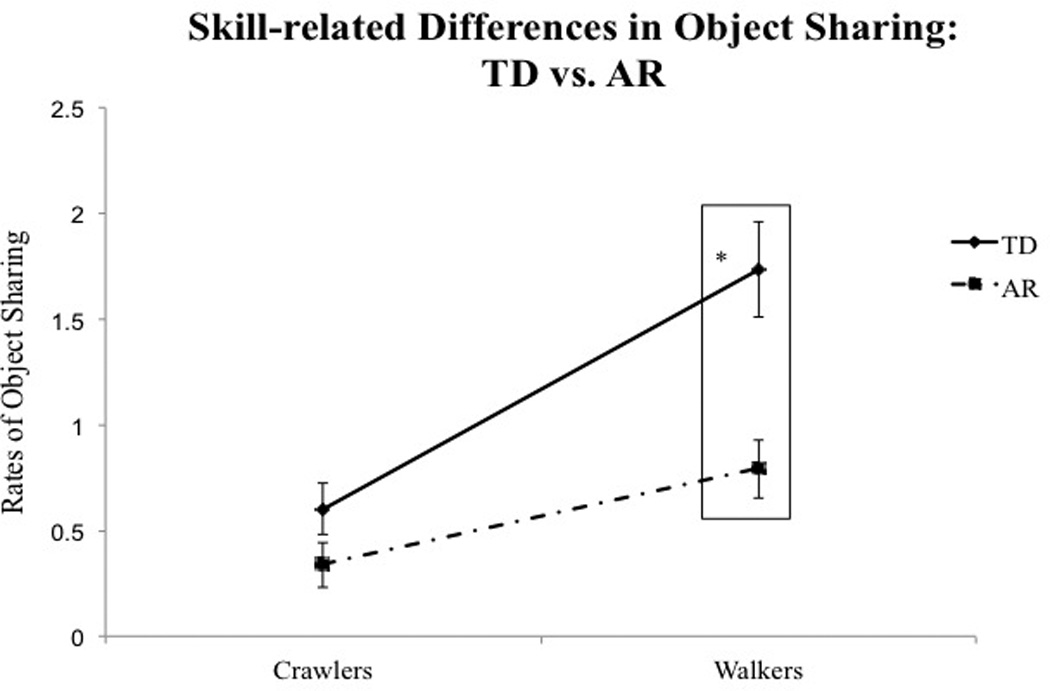
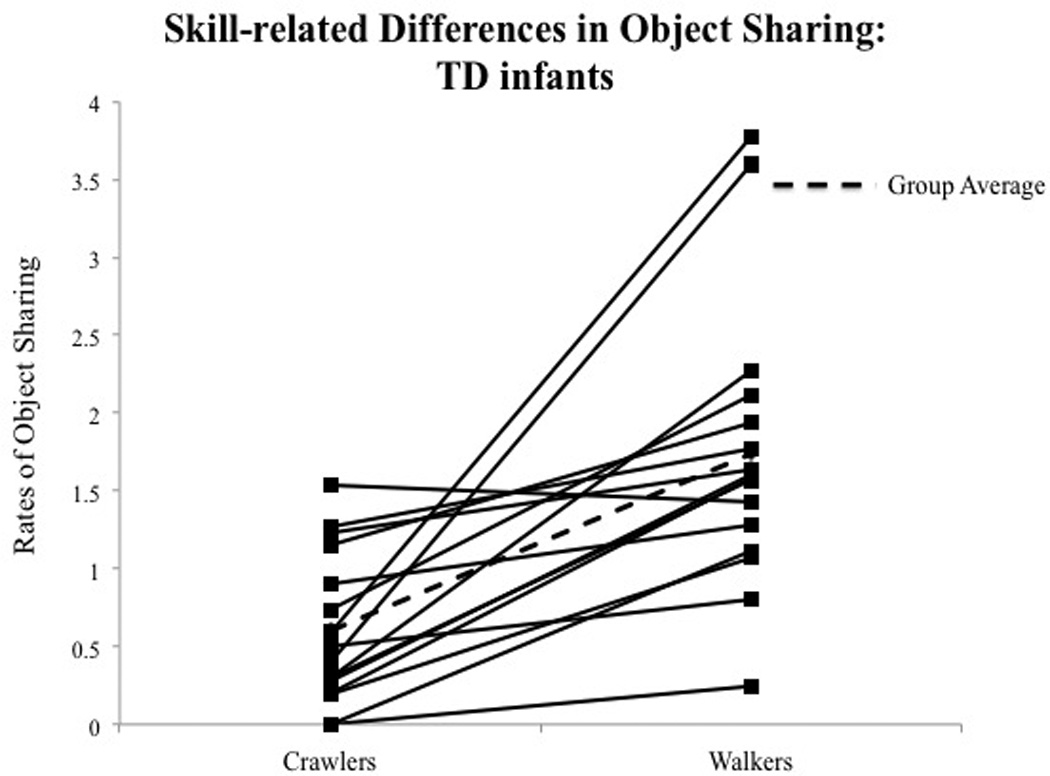
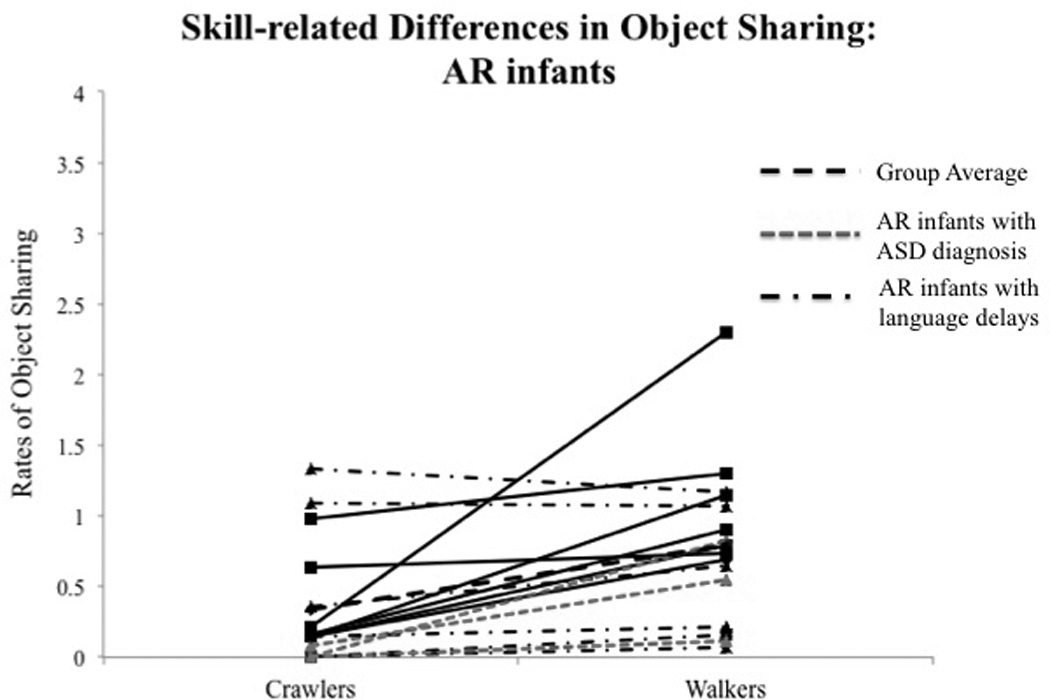
(A) Skill-related group differences in total rates of object sharing. Error bars represent standard error of the mean. (B) Individual data on total rates of object sharing at crawling and walking ages in TD infants. (C) Individual data on total rates of object sharing at crawling and walking ages in AR infants. Each solid line represents an individual infant. Dash lines represent group averages. Dot-dashed lines represent AR infants with future diagnoses or delays.
2.3. Experimental procedures
During each testing visit that took place at the infants’ home, we assessed their locomotor status and observed them within the “object sharing” paradigm (adapted from Karasik et al., 2011) (see Fig. 1).
Fig. 1.

Experimental setup for spontaneous and social conditions of the object sharing task.
2.3.1. Locomotor status
An infant had to demonstrate >5 independent and successive crawl or step cycles to be classified as a crawling or walking infant respectively. There were no significant group differences in the mean age of infants at crawling (TD infants—M = 11.09, SD = 1.65; AR infants—M = 10.81, SD = 2.08, t(30) = 0.42, p = 0.68) and walking visits (TD infants—M = 15.57, SD = 0.90; AR infants—M = 15.56, SD = 1.23, t(30) = 0.02, p = 0.98). In the TD group, 1 infant did not walk at the 15-month visit, whereas, in the AR group, 4 infants did not walk at 15 months. In addition, 2 at-risk infants showed crawling asymmetries (with excessive hip abduction and leg trailing) whereas none of the TD infants demonstrated any locomotor asymmetries.
2.3.2. Object sharing task
The task lasted for 14 min. Infants were seated on the floor with 20 age-appropriate toys such as rattles, balls, and books, at a distance of approximately four feet from their caregivers (see Fig. 1). In the spontaneous condition (first 7 min), infants were encouraged to play freely with the toys, and caregivers were asked to wait for infants to initiate interactions (Fig. 1) (Mean duration in minutes of spontaneous condition—TD infants: M = 5.96, SD = 1.25, AR infants: M = 6.28, SD = 1.05). In the social condition (next 7 min), caregivers were asked to initiate a cleanup activity (Fig. 1) (Mean duration in minutes of social condition—TD infants: 4.51, SD = 1.81, AR infants:M = 5.33, SD = 1.94). Specifically, caregivers would point to the toy and with a show of their hand ask for the toy by saying “It’s cleanup time. Let’s put the toy away.” The task was considered complete when infants shared all the toys or at the end of the social condition, whichever occurred earlier. Behavioral coding was done using OpenSHAPA (GitHub, Inc.) video coding software.
2.4. Behavioral coding of the object sharing task
2.4.1. Object sharing bids
We coded for the total rates and types of sharing bids per minute that infants initiated toward their caregivers during the spontaneous and social conditions of the task. Specifically, we coded for the following caregiver-directed object sharing bids—throws (infant flings object in the direction of caregiver from a stationary position), gives (infant hands object to caregiver from a stationary position), and approaches (infant crawls or walks to caregiver to share objects with them). Approaches were coded if infants used ≥2 crawl cycles or steps in the forward direction toward the caregiver. A single coder blinded to the grouping of the child coded the data after establishing intra- and inter-rater reliability based on videos from 20% of the entire dataset. Intra-class correlations (ICCs) were used to calculate reliability for all behaviors (throw ≥ 0.99, reach ≥ 0.97, approach = 0.99).
2.4.2. Step target
All walking visits of infants were coded for step rates i.e. steps taken per minute toward caregivers, objects, and elsewhere in the room, for example, toward furniture or other toys unrelated to the task. For infants who were not walking independently at their 15-month visit, we coded for steps taken by infants with external support (for example, steps taken while holding onto furniture etc. The step target analysis was based on 15 TD and 15 AR infants, since 2 infants in our sample did not demonstrate supported walking at the 15-month visit.
2.5. Statistical analyses
We assessed infants at 9, 12, and 15 months. However, since we were interested in examining changes in object sharing skills over the transition from crawling to walking, out of the three visits, we analyzed sharing data from one crawling and one walking visit for each infant (Note, Fig. 2 shows object sharing data from all 3 visits for TD and AR infants). Moreover, to control for the effect of age on object sharing, we will include age at walking visit as a covariate in our statistical analysis. One TD infant and 4 AR infants did not walk by their 15-month visit, so we used data from their 15-month crawling visit as their best performance.
Fig. 2.
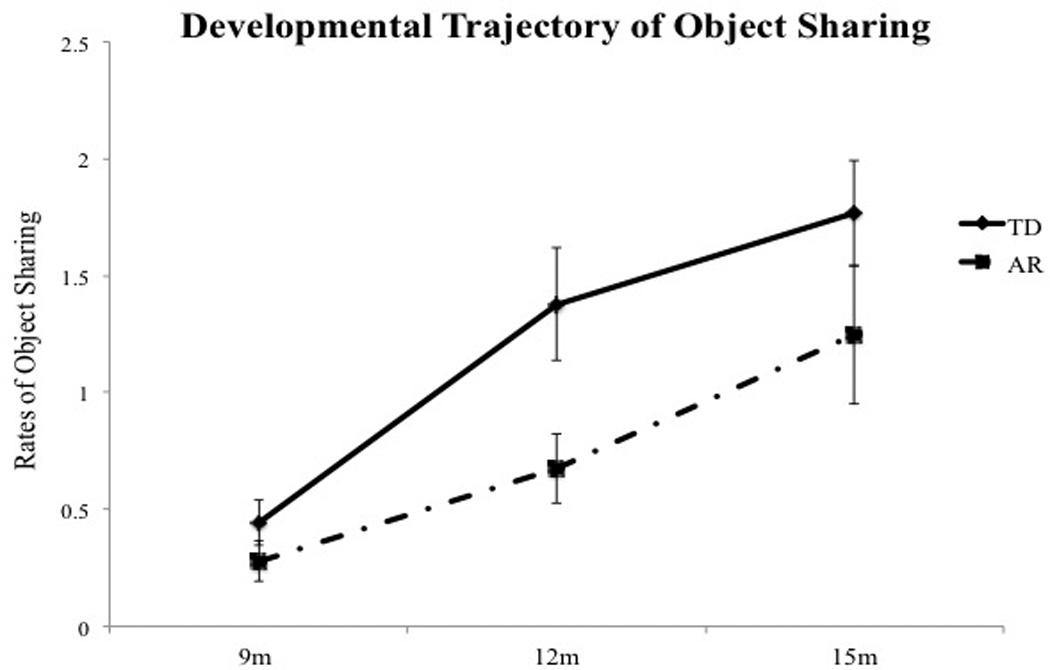
Developmental trajectory of object sharing in TD and AR infants.
We analyzed the total rates and types of object sharing using multivariate ANCOVAs with condition (spontaneous and social) and skill level (crawling and walking) as within-subjects factors, group (TD and AR) as a between-subjects factor, and age at walking visit as a covariate. For types of sharing, bid type (throw, reach, approach) was an additional within subjects factor. For analyzing group differences in step target during the walking visits, we used a 3 × 2 factorial ANOVA with step target (to caregiver, to objects, and to elsewhere) and condition (spontaneous, social) as the within-subjects factors and group as the between-subjects factor. For all analyses, if there was a significant main effect and an interaction effect for a given factor, we conducted further post-hoc t-tests to assess the significant interaction. In case of 2-way and 3-way interactions involving the same factors, we analyzed the 3-way interactions further. For all analyses, p ≤ 0.05 was considered significant. Following Bonferroni corrections, significance was set at the adjusted p value of ≤ 0.01 and statistical trends were reported when p values were between 0.05 and 0.01. Effect sizes are reported as partial eta-squared (ηp2) and standardized mean difference (SMD) (Hedges, 1981) values.
3. Results
3.1. Object sharing bids
3.1.1. Total rates of object sharing
Multivariate ANCOVA revealed significant main effect of Group (F(1, 29) = 12.82, p = 0.001, ηp2 = 0.31) and interaction effects of Skill level × Group (Pillai’s trace = 0.19, F(1, 29) = 6.61, p = 0.016, ηp2 = 0.19) and Condition × Group (Pillai’s trace = 0.19, F(1, 29) = 6.62, p = 0.015, ηp2 = 0.19).
Post-hoc analysis of the Skill level × Group interaction suggested that TD infants shared more than AR infants at the walking visit (p < 0.001, see Table 3A and Fig. 3A), with no significant group differences observed at the crawling visit. Fig. 3B and C show individual data on the total rates of sharing during the transition from crawling to walking, suggesting that a majority of the AR infants showed lower rates of sharing compared to TD infants at walking visits.
Table 3.
| A | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group differences in object sharing at crawler and walker visits. | |||||||||
| Group | Crawlers | Walkers | |||||||
| Throws | Gives | Approaches | Total | Throws | Gives | Approaches | Total | ||
| TD M(SD) & range | 0.16(0.21) (0–0.64) | 0.39(0.41) (0–1.23) | 0.05(0.09) (0–0.3) | 0.60(0.48) (0–1.54) | 0.21(0.36) (0–1.39) | 0.72(0.59) (0–1.77) | 0.81(0.69) (0–2.6) | 1.74(0.91) (0–3.77) | |
| AR M(SD) & range | 0.10(0.23) (0–0.90) | 0.17(0.30) (0–0.89) | 0.06(0.08) (0–0.27) | 0.34(0.43) (0–1.33) | 0.21(0.25) (0–0.82) | 0.33(0.31) (0–1.17) | 0.25(0.40) (0–1.5) | 0.79(0.56) (0.11–2.3) | |
| Significant group differences | – | – | – | – | – | p = 0.03† | p = 0.01† | p < 0.001* | |
| Effect sizes with confidence intervals | – | – | – | – | – | 0.81 (0.09–1.53) | 0.97 (0.24–1.70) | 1.23 (0.47–1.98) | |
| B | |||
|---|---|---|---|
| Group differences in object sharing in the spontaneous and social conditions of the task. | |||
| Group | Spontaneous | Social | |
| TD M(SD) & range | 0.51(0.70) (0–3.57) | 2.63(2.57) (0–10.4) | |
| AR M(SD) & range | 0.21(0.25) (0–1.14) | 1.07(1.17) (0–5) | |
| Significant group differences | p = 0.03* | p < 0.001* | |
| Effect sizes with Confidence intervals | 0.56 (−0.15–1.26) | 0.76 (0.04–1.48) | |
| C | |||
|---|---|---|---|
| Group differences in step rates to different targets during object sharing task. | |||
| Group | To objects | To caregiver | To elsewhere |
| TD M(SD) & range | 5.21(4.22) (0–12.71) | 6.05(4.00) (0–13.5) | 5.87(5.67) (0–16.79) |
| AR M(SD) & range | 1.82(3.43) (0–13.4) | 1.45(1.80) (0–5.8) | 8.22(7.41) (0.78–28.11) |
| Significant group differences | p = 0.02* | p < 0.001* | – |
| Effect sizes with confidence intervals | 0.86 (0.14–1.58) | 1.45 (0.67–2.22) | – |
Significant difference.
Statistical trend.
Post-hoc analysis of the Condition × Group interaction suggested that TD infants shared more than AR infants during the social condition of the task (p = 0.001) with a similar trend seen during the spontaneous condition of the task (p = 0.03) as well. Moreover, the magnitude of group difference was greater during the social compared to the spontaneous condition (see Table 3B and Fig. 4).
Fig. 4.
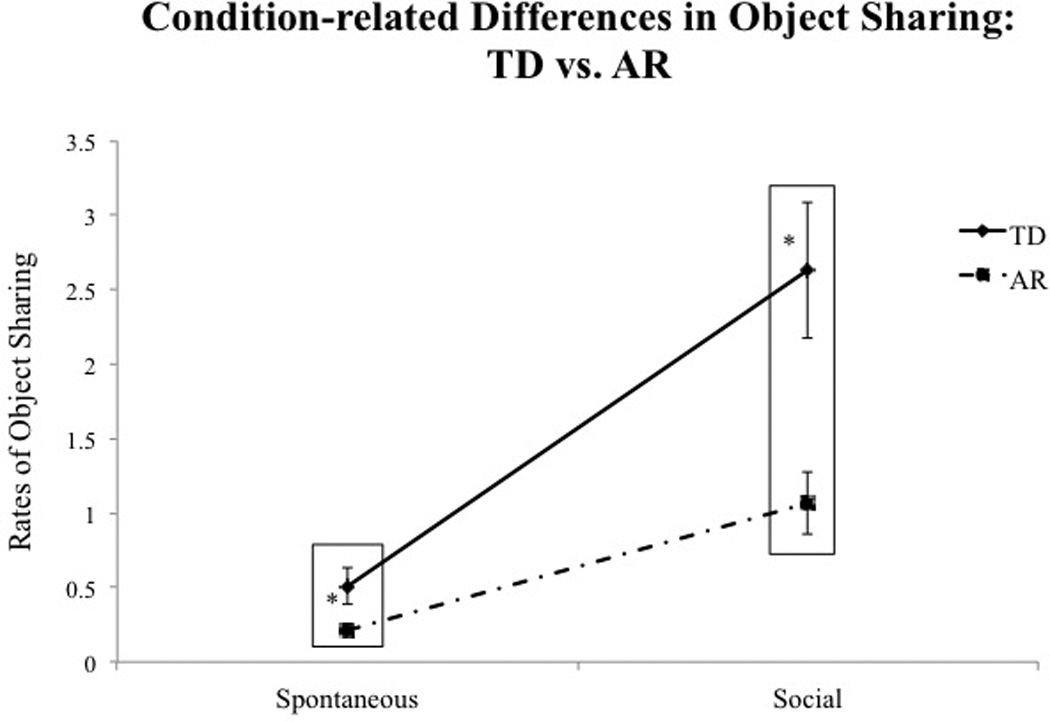
Condition-related group differences in total rates of object sharing. Error bars represent standard error of the mean.
3.1.2. Types of object sharing
Multivariate ANCOVA indicated main effect of group (F(1, 29) = 12.82, p = 0.001, ηp2 = 0.31) as well as significant interaction effects of Skill level × Group (Pillai’s trace = 0.19, F(1, 29) = 6.61, p = 0.016, ηp2 = 0.19), Condition × Group (Pillai’s trace = 0.19, F(1, 29) = 6.62, p = 0.015, ηp2 =0.19), and Skill level × Bid type × Group (Pillai’s trace = 0.21, F(2, 28) = 3.61, p = 0.04, ηp2 = 0.21).
Post-hoc analysis of the Skill level × Bid type × Group suggested that TD walking infants demonstrated significantly greater approaches (p = 0.01) and a trend for greater giving (p = 0.03) toward caregivers compared to AR walking infants (see Table 3A and Fig. 5); no significant group differences were seen at crawling visits.
Fig. 5.
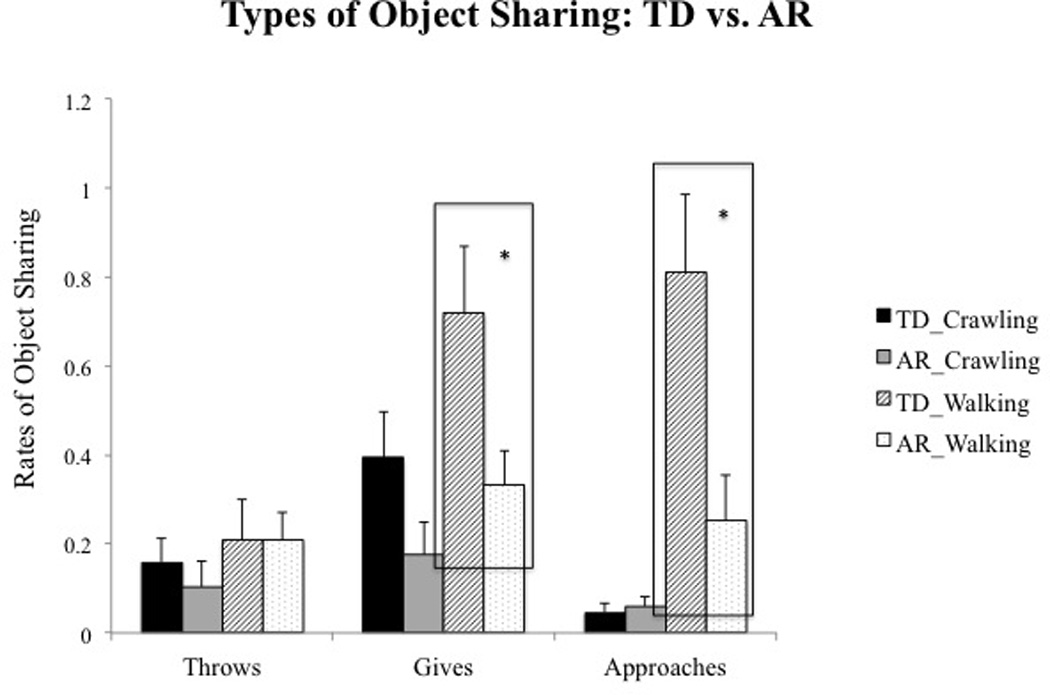
Group differences in types of object sharing bids at crawling and walking ages. Error bars represent standard error of the mean.
3.2. Step target
A 3 × 2 factorial ANOVA revealed significant main effect of Condition (F(1, 28) = 11.49, p = 0.002, ηp2 = 0.29) and Step target (F(2, 56) = 3.77, p = 0.029, ηp2 =0.12), as well as a Step target × Group (F(2, 56) = 6.19, p = 0.004, ηp2 = 0.18) interaction.
Analysis of the Step target × Group interaction revealed that TD infants took significantly more steps toward the caregivers (p = 0.02) and objects (p < 0.001) compared to the AR infants (see Table 3C and Fig. 6).Within the AR group, infants took greater steps toward elsewhere in the room compared to steps taken toward caregivers and objects (p values < 0.008). Overall, 12 out of the 15 AR infants followed the group trends.
Fig. 6.
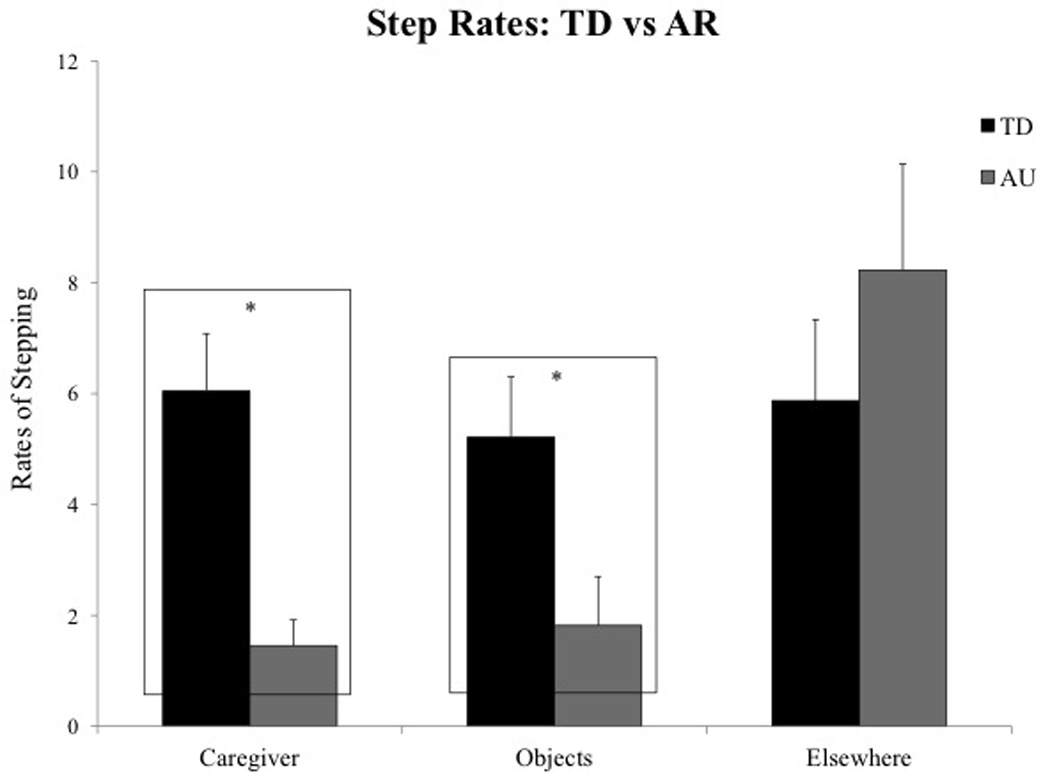
Group differences in step rates toward caregiver, objects, and elsewhere during the sharing task.
4. Discussion
4.1. Summary of results
In the current study, we found that the onset of walking was associated with a developmental surge in object sharing skills in both groups. However, group differences in object sharing were evident at walking visits only, with TD walking infants sharing objects at significantly greater rates compared to AR walking infants. Specifically, TD walking infants reached out and approached their caregivers more often than AR walking infants. Moreover, group differences in sharing were evident in the spontaneous and social conditions of the task, although the magnitude of the difference was greater in the social compared to the spontaneous condition of the task. Our step target analysis suggested that TD walking infants used their locomotor skills to engage in task-appropriate exploration involving steps taken toward objects and caregivers for the purpose of sharing. In contrast, AR walking infants took fewer steps toward task-appropriate targets and instead took greater steps toward elsewhere in the room suggesting that they used their locomotor skills for non-social exploration. Next, we will identify several factors that may contribute to the observed increase in object sharing skills over the transition from crawling to walking. Furthermore, we will also discuss potential reasons for group differences in sharing skills within the first 2 years of life.
4.2. Developmental changes and group differences in object sharing over the transition from crawling to walking
Our findings fit with previous literature suggesting a reorganization of infants’ perceptuo-motor and social communication systems following the onset of walking. Previous studies showed that walking infants were able to engage in greater and more sophisticated object sharing bids with their caregivers including pointing at objects, offering distal toys, and vocalizing to caregivers compared to age-matched crawling infants (Clearfield, 2011; Karasik et al., 2011). In our study, we found greater reaches and approaches with objects directed toward the caregivers in walking infants than crawling infants within both groups. However, reduced object sharing in AR infants compared to TD infants has been reported in other studies that have used similar tasks in children with ASD (Lemanek, Stone, & Fishel, 1993; Sigman et al., 1986). During a cleanup task, children with ASD were less likely to show, point at, or share objects with caregivers compared to TD children (Sigman et al., 1986). Similarly, children with autism ignored caregiver bids during cooperative play activities compared to TD children and children with other developmental disabilities (Lemanek et al., 1993).
To engage in object sharing, infants must perceive objects in their environment, attend to caregiver bids, move efficiently toward objects and caregivers, and shift attention smoothly and fluidly between objects and caregivers. Below, we will briefly discuss each of the above-mentioned factors and how they could contribute to group differences between TD and AR infants.
4.2.1. Changes in perception of objects in the physical environment
With the onset of upright locomotion, infants begin to observe objects and people within their distal environment from an elevated vantage point. Walking also provides a perceptual advantage compared to crawling by allowing infants to continuously monitor their dynamic distal environment as they move (Kretch, Franchak, & Adolph, 2014). Therefore, compared to crawling infants who predominantly engage with proximal objects, walking infants primarily access and share distal objects with their caregivers (Karasik et al., 2011). In our study, similar perceptual changes could have contributed to the greater rates and variety of sharing bids in the walking infants compared to the crawling infants. In contrast, the reduced object sharing in AR infants at walking ages could have been the result of heightened object perception or perseveration in AR compared to TD infants. AR infants and children with ASD have a substantial bias toward non-social versus social cues and engage in high levels of perseveration and non-functional play with objects compared to TD peers (Bhat et al., 2010; Maestro et al., 2002; Ozonoff et al., 2008; Wolff et al., 2014). For example, during a novel social-object learning task that required infants to spontaneously interact with objects and caregivers, AR infants spent greater time looking at objects than toward caregivers compared to TD infants (Bhat et al., 2010). In a different study, using a caregiver rating scale, both AR infants who eventually developed ASD and AR infants who did not develop autism engaged in greater repetitive behaviors compared to their TD peers at 12 and 24 months (Wolff et al., 2014). Overall, AR infants in our study may have spent greater time attending to objects and engaging in repetitive sensorimotor exploration with objects, that may have affected their ability to engage in functional, socially directed play with caregivers. Currently, we are coding the attention patterns observed in both groups of infants to validate our hypotheses.
4.2.2. Changes in perception of caregivers in the social environment
Independent locomotion provides infants with a myriad of opportunities to explore their environment at their will without having to depend on caregivers (Adolph & Robinson, 2013). This autonomy is further boosted with the onset of walking. Weeks of independent exploration of the distal space and physical separation from caregivers may foster a sense of self-other distinction in infants (Campos et al., 2000; Clearfield, 2011). Despite this sense of independence, walking infants frequently seek out caregivers to share their exploration and actually become more sensitive to the distal communicative verbal and gestural bids of caregivers (Biringen et al., 2008; Campos et al., 2000; Adolph & Robinson, 2013; Clearfield, 2011; Karasik et al., 2011). This enhanced social perception with the onset of walking may facilitate typically developing infants’ ability to respond to caregiver-initiated bids during object-based play. In contrast to this typical developmental trajectory, AR infants and children with ASD have difficulties attending to social cues probably due to their lack of social motivation to engage with caregivers (Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998; Shic, Bradshaw, Klin, Scassellati, & Chawarska, 2011; Dawson et al., 1998, 2004; Mundy & Newell, 2007). In our study, we observed group differences during both, the spontaneous and social conditions even though the magnitude of the group difference was greater during the social condition. Previous research suggests that AR infants who later develop ASD demonstrate impairments in both initiating and responding to joint attention (Landa et al., 2007; Bakeman & Adamson, 1984; Mundy, 2003; Mundy, Sigman, Ungerer, & Sherman, 1986). Developmentally, infants begin to respond to bids initiated by others before they are able to spontaneously initiate bids to share their interests with others (Bakeman & Adamson, 1984; Mundy & Newell, 2007). In the present study, both groups demonstrated lower levels of self-initiated sharing in the spontaneous condition possibly leading to a low group difference. In contrast, as mentioned earlier, the impairments in responsive joint attention and lack of social motivation reported in AR infants could have contributed to the low rates of sharing in the social condition.
4.2.3. Changes in access to objects and caregivers in the environment
Typically developing walking infants engage in greater moving bids to engage with caregivers compared to crawling infants who despite their ability to move chose to engage in stationary sharing bids (Karasik et al., 2011). Walking is a more efficient mode of locomotion allowing quicker, easier access to distal objects and people (Karasik et al., 2011; Sparrow & Irizarry-Lopez, 1987). Moreover, given their hands-free position, walkers are able to carry objects more often while moving compared to crawlers (Karasik, Adolph, Tamis-LeMonda, & Zuckerman, 2012). In contrast to TD infants, poor postural control in a variety of postures may significantly limit AR infants’ ability to share with caregivers using stationary and moving bids (Bhat et al., 2011, 2012; Nickel et al., 2013; Flanagan et al., 2012). For example, AR infants were delayed in achieving proficiency in sitting and standing postures; moreover, infants who eventually developed ASD initiated fewer posture changes and spent greater time in less advanced postures such as sitting and lying even at 14 months of age (Nickel et al., 2013). In our sample, roughly 25% AR infants did not walk even at 15 months compared to 6% TD infants that could have affected infants’ ability to share with caregivers. Moreover, as a group, AR infants seemed unable to use their locomotor skills to interact in meaningful ways with caregivers. While TD infants stepped more toward task-appropriate targets, i.e. toys and caregivers for the purpose of sharing, AR infants stepped more toward elsewhere in the room. Lösche (1990) reported similar trends with the onset of walking, where TD children used their newfound abilities to engage in diverse actions on objects in their surroundings, whereas children with ASD walked aimlessly and did not use their motor skills to explore the environment. Overall, postural and locomotor delays as well as inability to use reaching and locomotor skills for social engagement could have also contributed to poor sharing skills in AR compared to TD infants.
4.2.4. Changes in attention shifting abilities between objects and people
Locomotor experiences can enhance typically developing infants’ abilities to focus on the distal environment and shift attention smoothly and fluidly between multiple targets. For example, prelocomotor infants with walker experience and locomotor crawling infants looked at objects in their distal space, whereas prelocomotor infants without such experiences did not look at anything in particular and instead fixated on vacant parts of the room (Campos et al., 1992). Furthermore, with the onset of upright locomotion there is a transition from primarily dyadic interactions with caregivers to more sophisticated triadic interactions involving objects (Campos et al., 1992). For example, a toddler playing catch with mom frequently shifts attention between mom and the ball. Attention regulation might thus develop as a function of locomotor experiences in typically developing infants. In contrast to this trajectory, AR infants demonstrate significant difficulties in attention shifting that may adversely affect their ability to engage in triadic sharing episodes with caregivers (Landry & Bryson, 2004). For instance, children with ASD have difficulty disengaging attention and rapidly shifting attention between stimuli; instead they fixate on stimuli in their current focus of attention (Landry & Bryson, 2004). Difficulties in social orienting, increased perseveration on objects, and impaired shifting of attention between objects and people may adversely affect children’s ability to engage in joint attention episodes to share their play with caregivers (Landry & Bryson, 2004; Dawson et al., 2004; Mundy & Newell, 2007). Overall, attention-shifting impairments in AR infants may have also interfered with infants’ ability to spontaneously engage with caregivers as well as respond to the attentional bids initiated by their caregivers.
4.3. Clinical implications for assessment and treatment
In terms of early detection, a diagnosis of ASD can be made as early as 18 to 24 months (Robins et al., 2001; Shattuck et al., 2009). Our findings show that autism-related delays in AR infants are observed during object sharing within the first year. Therefore, caregivers should look for specific red flags during social-object play contexts such as low frequencies of giving and physical approaches with objects, lack of functional play, high rates of perseveration, as well as lack of engagement with caregivers.
In terms of treatment implications, social-object play is a valuable context to facilitate social communication, cognitive, and imitation skills in at-risk infants within the first 2 years of life. Through object sharing, infants learn social skills such as JA and imitation, communication skills such as verbal labels of objects, and cognitive skills such as object affordances. Current early intervention programs for toddlers and young children with ASD are based on behavioral and developmental principles (Corsello, 2005; Warren et al., 2011; Boyd, Odom, Humphreys, & Sam, 2010; Reichow, 2012). Multiple child-directed, parent-delivered, developmental approaches such as the Early Start Denver Model that facilitate social interactions and age-appropriate play during child-preferred activities have led to improvements in IQ, adaptive behavior, and autism symptomatology in children with ASD (Dawson et al., 2010; Diggle & McConachie, 2002). With parents as “co-therapists,” children with ASD are provided a multitude of learning opportunities throughout the day and this also allows for early initiation of intervention within the first year of life. Furthermore, parent training is also associated with lower caregiver stress and increased confidence in addressing the child’s symptoms (McConachie & Diggle, 2007). Along these lines, object play episodes may serve as early therapeutic opportunities for parents to promote critical skills in at-risk infants. Within the first year of life, typically developing infants frequently engage in object-based play with caregivers involving pointing, showing, as well as sharing of objects (Williams, 2003; Bakeman & Adamson, 1984; Girolametto, Verbey, & Tannock, 1994). Parents typically facilitate infants’ exploration of objects as well as their understanding of object properties and object affordances, often by using elements of imitative play, wherein they encourage infants to imitate actions on objects. Our study suggests that triadic object sharing games could serve as promising contexts to teach social interactions and functional skills to at-risk infants. Moreover, our study also underscores the importance of providing infants with a variety of postural and locomotor movement experiences that will allow them to explore their world in sophisticated ways, which in turn may have cascading effects on their social communication skills (Bhat et al., 2011, 2012).
4.4. Limitations and conclusions
Our study has several limitations including a small sample size, diverse at-risk group, lack of a developmentally delayed control group, the preliminary nature of the study, and lack of additional follow-up. Although promising, our study results should be interpreted with caution and require replication using larger sample sizes. Although we controlled for the age at walking visit, we could not control for amount of walking experiences. Furthermore, our sample was also slightly diverse since we included two preterm infants in our study. However, preterm infants are a known population at-risk for ASD (Limperopoulos et al., 2008; Mwaniki et al., 2012) and the two preterm infants included in the present study received ASD diagnoses after their second birthday. This study was a preliminary study to assess the presence of object sharing deficits in AR infants. However, given the small sample size and the lack of a developmentally delayed control group, we were not able to assess the feasibility of using object sharing as a paradigm to identify future developmental delays in AR infants. We found that AR infants as a group, irrespective of their outcome diagnoses, demonstrated poor sharing skills. However, we were not able to conduct detailed sub-group analyses to understand the differences in developmental trajectories of infants who developed ASD versus language delays. Future studies should aim at rigorously and carefully examining the sensitivity and specificity of this paradigm for predicting developmental delays in AR infants. Our study was also not designed to understand the individual contributions of component motor and social skills to object sharing behaviors. Furthermore, the small sample size precluded the statistical examination of relationships between outcome measures and specific motor-social impairments in AR infants. In terms of the study coding, we have restricted our discussion in the current paper to infant behaviors only. However, given that object sharing is a dyadic activity, it would be equally important to code for caregiver behaviors. Currently, we are systematically coding caregiver behaviors including proximity to infants, use of gestures, and caregiver verbalization to examine potential group differences in caregiver behaviors and their contribution to infant object sharing skills.
Overall, our study suggested that AR infants shared less than TD infants following the onset of walking. Furthermore, AR infants had difficulties in using their locomotor skills to engage in meaningful ways with caregivers within the second year of life. Despite the considerable limitations in the study, we think that object sharing may be a valuable paradigm to identify delays in AR infants within the first year of life and social-object play episodes may be a promising intervention context for infants at risk for developing autism.
Highlights.
Infants at-risk for autism had less object sharing than typically developing infants at 12 and 15 months.
Infants at-risk for autism had fewer reaches and physical approaches to caregivers than typically developing infants at 12 and 15 months.
Acknowledgments
We would like to thank all the infants and families who participated in the study. We thank Dr. Deborah Fein and her student Alyssa Orinstein for their support of this research through completion of ADI-R interviews with participating families. We would also like to thank graduate student, Maninderjit Kaur and undergraduate students, Lyly Tran, Esther Cha, Irene Cheng, Isabel Park, Kathleen Lynch at University of Connecticut who helped with data collections and analysis. We are very grateful to Dr. Lana Karasik & Dr. Karen Adolph for sharing information about their work on object sharing during the early development phase of this study. Lastly, AB thanks the National Institutes of Child Health and Human Development (NICHHD) for support of this research through a RO3 award (R03HD060809).
References
- Adolph KE, Robinson SR. The road to walking: What learning to walk tells us about development. Oxford Handbook of Developmental Psychology. 2013;1:403–443. http://dx.doi.org/10.1093/oxfordhb/9780199958450.013.0015. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Baio J. Prevalence of autism spectrum disorders among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morbidity and Mortality Weekly Report Surveillance Summaries. 2014;63(2):1–21. [PubMed] [Google Scholar]
- Bakeman R, Adamson LB. Coordinating attention to people and objects in mother–infant and peer–infant interaction. Child Development. 1984;55:1278–1289. [PubMed] [Google Scholar]
- Bhat A, Galloway J, Landa R. Social and non-social visual attention patterns and associative learning in infants at risk for autism. Journal of Child Psychology and Psychiatry. 2010;51(9):989–997. doi: 10.1111/j.1469-7610.2010.02262.x. http://dx.doi.org/10.1111/j.1469-7610.2010.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat A, Landa R, Galloway JC. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Physical Therapy. 2011;91(7):1116–1129. doi: 10.2522/ptj.20100294. http://dx.doi.org/10.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- Bhat A, Galloway JC, Landa R. Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behavior & Development. 2012;35:838–846. doi: 10.1016/j.infbeh.2012.07.019. http://dx.doi.org/10.1016/j.infbeh.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biringen Z, Emde RN, Campos JJ, Appelbaum M. Development of autonomy: Role of walking onset and its timing. Perceptual and Motor Skills. 2008;106(2):395–414. doi: 10.2466/pms.106.2.395-414. http://dx.doi.org/10.2466/pms.106.2.395-414. [DOI] [PubMed] [Google Scholar]
- Boyd BA, Odom SL, Humphreys BP, Sam AM. Infants and toddlers with autism spectrum disorder: Early identification and early intervention. Journal of Early Intervention. 2010;32:75. http://dx.doi.org/10.1177/1053815110362690. [Google Scholar]
- Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, et al. A prospective case series of high-risk infants who developed autism. Journal of Autism and Developmental Disorders. 2007;37(1):12–24. doi: 10.1007/s10803-006-0328-2. http://dx.doi.org/10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- Campos JJ. Crawling onset organizes affective development in infancy. Research & Clinical Center for Child Development. 1990;12:41–47. [Google Scholar]
- Campos JJ, Anderson DI, Barbu-Roth MA, Hubbard EM, Hertenstein MJ, Witherington D. Travel broadens the mind. Infancy. 2000;1(2):149–219. doi: 10.1207/S15327078IN0102_1. http://dx.doi.org/10.1207/S15327078IN0102_1. [DOI] [PubMed] [Google Scholar]
- Campos JJ, Kermoian R, Witherington D, Chen H, Dong Q. Activity, attention, and developmental transitions in infancy. In: Lang PJ, Simons RF, editors. Attention and orienting: Sensory and motivational processes. Mahwah, NJ: Lawrence Erlbaum Associates Inc.; 1997. pp. 393–415. [Google Scholar]
- Campos JJ, Kermoian R, Zumbahlen MR. Socioemotional transformations in the family system following infant crawling onset. New Directions for Child and Adolescent Development. 1992;1992(55):25–40. doi: 10.1002/cd.23219925504. [DOI] [PubMed] [Google Scholar]
- Cassel TD, Messinger DS, Ibanez LV, Haltigan JD, Acosta SI, Buchman AC. Early social and emotional communication in the infant siblings of children with autism spectrum disorders: An examination of the broad phenotype. Journal of Autism and Developmental Disorders. 2007;37(1):122–132. doi: 10.1007/s10803-006-0337-1. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Volkmar F. Autism spectrum disorder in the second year: Stability and change in syndrome expression. Journal of Child Psychology and Psychiatry. 2007;48(2):128–138. doi: 10.1111/j.1469-7610.2006.01685.x. http://dx.doi.org/10.1111/j.1469-7610.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- Clearfield MW. Learning to walk changes infants’ social interactions. Infant Behavior and Development. 2011;34(1):15–25. doi: 10.1016/j.infbeh.2010.04.008. http://dx.doi.org/ 10.1016/j.infbeh.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Corsello CM. Early intervention in autism. Infants & Young children. 2005;18(2):74–85. [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. http://dx.doi.org/10.1023/A:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, et al. Randomized controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010;125:e17–e23. doi: 10.1542/peds.2009-0958. http://dx.doi.org/10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, et al. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. http://dx.doi.org/10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Diggle TJ, McConachie HR. Parent-mediated early intervention for young children with autism spectrum disorder. Cochrane Database of Systematic Reviews. 2002:2. doi: 10.1002/14651858.CD003496. http://dx.doi.org/10.1002/146651858.CD003496. [DOI] [PubMed] [Google Scholar]
- Esposito G, Venuti P, Apicella F, Muratori F. Analysis of unsupported gait in toddlers with autism. Brain and Development. 2011;33(5):367–373. doi: 10.1016/j.braindev.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Flanagan JE, Landa R, Bhat A, Bauman M. Head lag in infants at risk for autism: A preliminary study. The American Journal of Occupational Therapy. 2012;66(5):577–585. doi: 10.5014/ajot.2012.004192. http://dx.doi.org/10.5014/ajot.2012.004192. [DOI] [PubMed] [Google Scholar]
- Girolametto L, Verbey M, Tannock R. Improving joint engagement in parent–child interaction: An intervention study. Journal of Early Intervention. 1994;18:155–167. [Google Scholar]
- Goldberg WA, Jarvis KL, Osann K, Laulhere TM, Straub C, Thomas E, et al. Brief report: Early social communication behaviors in the younger siblings of children with autism. Journal of Autism and Developmental Disorders. 2005;35(5):657–664. doi: 10.1007/s10803-005-0009-6. [DOI] [PubMed] [Google Scholar]
- Hedges LV. Distribution theory for glass’s estimator of effect size and related estimators. Journal of Educational and Behavioral Statistics. 1981;6(2):107–128. [Google Scholar]
- Hollingshead A. Four factor index of social status. New Haven, CT: Yale University; 1975. (Unpublished Manuscript) [Google Scholar]
- Ibanez LV, Messinger DS, Newell L, Lambert B, Sheskin M. Visual disengagement in the infant siblings of children with an autism spectrum disorder (ASD) Autism. 2008;12(5):473–485. doi: 10.1177/1362361308094504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik LB, Adolph KE, Tamis-LeMonda CS, Zuckerman AL. Carry on: Spontaneous object carrying in 13-month-old crawling and walking infants. Developmental Psychology. 2012;48(2):389–397. doi: 10.1037/a0026040. http://dx.doi.org/10.1037/a0026040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik LB, Tamis-LeMonda CS, Adolph KE. Transition from crawling to walking and infants’ actions with objects and people. Child Development. 2011;82(4):1199–1209. doi: 10.1111/j.1467-8624.2011.01595.x. http://dx.doi.org/10.1111/j.1467-8624.2011.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik LB, Tamis-LeMonda CS, Adolph KE. Crawling and walking infants elicit different verbal responses from mothers. Developmental Science. 2014;17(3):388–395. doi: 10.1111/desc.12129. http://dx.doi.org/10.1111/desc.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretch KS, Franchak JM, Adolph KE. Crawling and walking infants see the world differently. Child Development. 2014;85(4):1503–1518. doi: 10.1111/cdev.12206. http://dx.doi.org/10.1111/cdev.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007;64(7):853–864. doi: 10.1001/archpsyc.64.7.853. http://dx.doi.org/10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. http://dx.doi.org/10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry. 2004;45(6):1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. http://dx.doi.org/10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, Bauman M. Latent class analysis of early developmental trajectory in baby siblings of children with autism. Journal of Child Psychology and Psychiatry. 2012;53(9):986–996. doi: 10.1111/j.1469-7610.2012.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemanek KL, Stone WL, Fishel PT. Parent–child interactions in handicapped preschoolers: The relation between parent behaviors and compliance. Journal of Clinical Child Psychology. 1993;22(1):68–77. http://dx.doi.org/10.1207/s15374424jccp2201_7. [Google Scholar]
- Libertus K, Sheperd KA, Ross SW, Landa RJ. Limited fine motor and grasping skills in 6-month-old infants at high risk for autism. Child Development. 2014;85(6):2218–2231. doi: 10.1111/cdev.12262. http://dx.doi.org/10.1111/cdev.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Bassan H, Sullivan NR, Soul JS, Robertson RL, du Plessis AJ, et al. Positive screening for autism in ex-preterm infants: Prevalence and risk factors. Pediatrics. 2008;121:758–765. doi: 10.1542/peds.2007-2158. http://dx.doi.org/10.1542/peds.2007-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. http://dx.doi.org/10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism diagnostic observation schedule, second edition (ADOS-2) manual (Part 1): Modules 1–4. Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- Lösche G. Sensorimotor and action development in autistic children from infancy to early childhood. Journal of Child Psychology and Psychiatry. 1990;31(5):749–761. doi: 10.1111/j.1469-7610.1990.tb00815.x. http://dx.doi.org/10.1111/j.1469-7610.1990.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Lovaas OI. Behavioral treatment and normal educational and intellectual functioning in young autistic children. Journal of Consulting and Clinical Psychology. 1987;55(1):3–9. doi: 10.1037//0022-006x.55.1.3. [DOI] [PubMed] [Google Scholar]
- Maestro S, Muratori F, Cavallaro MC, Pei F, Stern D, Golse B, et al. Attentional skills during the first 6 months of age in autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(10):1239–1245. doi: 10.1097/00004583-200210000-00014. http://dx.doi.org/10.1097/00004583-200210000-00014. [DOI] [PubMed] [Google Scholar]
- McConachie HR, Diggle T. Parent implemented early intervention for young children with autism spectrum disorder: A systematic review. Journal of evaluation in clinical practice. 2007;13(1):120–129. doi: 10.1111/j.1365-2753.2006.00674.x. http://dx.doi.org/10.1111/j.1365-2753.2006.00674.x. [DOI] [PubMed] [Google Scholar]
- Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, et al. Beyond autism: A baby siblings research consortium study of high-risk children at three years of age. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(3):300–308. doi: 10.1016/j.jaac.2012.12.011. http://dx.doi.org/10.1016/j.jaac.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Sung KB, Jones BL, Furman JM. Underdevelopment of the postural control system in autism. Neurology. 2004;63(11):2056–2061. doi: 10.1212/01.wnl.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Brian J, Zwaigenbaum L, Roberts W, Szatmari P, Smith I, et al. Early language and communication development of infants later diagnosed with autism spectrum disorder. Journal of Developmental & Behavioral Pediatrics. 2006;27(2):S69–S78. doi: 10.1097/00004703-200604002-00004. http://dx.doi.org/10.1097/00004703-200604002-00004. [DOI] [PubMed] [Google Scholar]
- Mundy P, Newell L. Attention, joint attention, and social cognition. Current Directions in Psychological Science. 2007;16(5):269–274. doi: 10.1111/j.1467-8721.2007.00518.x. http://dx.doi.org/10.1111/j.1467-8721.2007.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P. Annotation: The neural basis of social impairments in autism: The role of the dorsal medial-frontal cortex and anterior cingulate system. Journal of Child Psychology and Psychiatry. 2003;44(6):793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: The contribution of non-verbal communication measures. Journal of Child Psychology and Psychiatry. 1986;27(5):657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Mwaniki M, Atieno M, Lawn J, Newton C. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: A systematic review. Lancet. 2012;379:445–452. doi: 10.1016/S0140-6736(11)61577-8. http://dx.doi.org/10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel LR, Thatcher AR, Keller F, Wozniak RH, Iverson JM. Posture development in infants at heightened versus low risk for autism spectrum disorders. Infancy. 2013:1–23. doi: 10.1111/infa.12025. http://dx.doi.org/10.1111/infa.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif A, Baguio F, Cook IC, Hill MM, Hutman T, et al. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(3):256–266. doi: 00004583-201003000-00009. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism. 2008;12(5):457–472. doi: 10.1177/1362361308096402. http://dx.doi.org/10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presmanes AG, Walden TA, Stone WL, Yoder PJ. Effects of different attentional cues on responding to joint attention in younger siblings of children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(1):133–144. doi: 10.1007/s10803-006-0338-0. [DOI] [PubMed] [Google Scholar]
- Reichow B. Overview of meta-analyses on early intensive behavioral intervention for young children with autism spectrum disorders. Journal of Autism and Developmental disorders. 2012;42(4):512–520. doi: 10.1007/s10803-011-1218-9. [DOI] [PubMed] [Google Scholar]
- Robins DL, Fein D, Barton ML, Green JA. The modified checklist for autism in toddlers: An initial study investigating the early detection of autism and pervasive developmental disorders. Journal of Autism and Developmental Disorders. 2001;31(2):131–144. doi: 10.1023/a:1010738829569. http://dx.doi.org/10.1023/A:1010738829569. [DOI] [PubMed] [Google Scholar]
- Sacrey LR, Germani T, Bryson SE, Zwaigenbaum L. Reaching and grasping in autism spectrum disorder: A review of recent literature. Frontiers in Neurology. 2014:5. doi: 10.3389/fneur.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck PT, Durkin M, Maenner M, Newschaffer C, Mandell DS, Wiggins L, et al. Timing of identification among children with an autism spectrum disorder: Findings from a population-based surveillance study. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(5):474–483. doi: 10.1097/CHI.0b013e31819b3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shic F, Bradshaw J, Klin A, Scassellati B, Chawarska K. Limited activity monitoring in toddlers with autism spectrum disorder. Brain Research. 2011;1380:246–254. doi: 10.1016/j.brainres.2010.11.074. http://dx.doi.org/10.1016/j.brainres.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow WA, Irizarry-Lopez VM. Mechanical efficiency and metabolic cost as measures of learning a novel gross motor task. Journal of Motor Behavior. 1987;19(2):240–264. doi: 10.1080/00222895.1987.10735410. [DOI] [PubMed] [Google Scholar]
- Stone WL, McMahon CR, Yoder PJ, Walden TA. Early social-communicative and cognitive development of younger siblings of children with autism spectrum disorders. Archives of Pediatrics & Adolescent Medicine. 2007;161(4):384. doi: 10.1001/archpedi.161.4.384. [DOI] [PubMed] [Google Scholar]
- Squires J, Bricker D. Ages & stages questionnaires®. Third. Baltimore, MD: Brookes Publishing; 2009. (ASQ-3™) [Google Scholar]
- Toth K, Dawson G, Meltzoff AN, Greenson J, Fein D. Early social, imitation, play, and language abilities of young non-autistic siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37(1):145–157. doi: 10.1007/s10803-006-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle EA, Campos JJ. Infant language development is related to the acquisition of walking. Developmental Psychology. 2014;50(2):336–348. doi: 10.1037/a0033238. http://dx.doi.org/10.1037/a0033238. [DOI] [PubMed] [Google Scholar]
- Warren Z, McPheeters ML, Sathe N, Foss-Feig JH, Glasser A, Veenstra-VanderWeele J. A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics. 2011;127:e1303–e1311. doi: 10.1542/peds.2011-0426. http://dx.doi.org/10.1542/peds.2011-0426. [DOI] [PubMed] [Google Scholar]
- Williams E. A comparative review of early forms of object-directed play and parent–infant play in typical infants and young children with autism. Autism. 2003;7:361. doi: 10.1177/1362361303007004003. http://dx.doi.org/10.1177/1362361303007004003. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Botteron KN, Dager SR, Elison JT, Estes AM, Gu H, et al. Longitudinal patterns of repetitive behavior in toddlers with autism. Journal of Child Psychology and Psychiatry. 2014;55(8):945–953. doi: 10.1111/jcpp.12207. http://dx.doi.org/10.1111/jcpp.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya N, Gamliel I, Shaked M, Sigman M. Cognitive and verbal abilities of 24-to 36-month-old siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37(2):218–229. doi: 10.1007/s10803-006-0163-5. [DOI] [PubMed] [Google Scholar]


