Abstract
Objectives
To characterize the clinical course of mucopolysaccharidosis type IIIA (MPS IIIA), and identified potential endpoints for future treatment trials.
Study design
Children with a confirmed diagnosis of MPS IIIA, functioning above a developmental age of 1 year, were followed for up to 2 years. Cognitive status and brain atrophy were assessed by standardized tests and volumetric MRI, respectively. Liver and spleen volumes, CSF and urine biomarker levels were measured.
Results
Twenty-five children, from 1.1 to 18.4 years old, were enrolled, and 24 followed for at least 12 months. 19 exhibited a rapidly progressing form of MPS IIIA (RP), and 5, a more slowly progressing form (SP). Children with RP plateaued in development by 30 months, followed by rapid regression after 40 to 50 months. Cognitive developmental quotients (DQ) in patients with RP showed consistent steep declines associated with progressive cortical gray matter atrophy. Children with SP had a similar but more prolonged course. Liver and spleen volumes were approximately double normal size, and CSF and urine HS levels were elevated and relatively constant over time.
Conclusions
DQ and cortical gray matter volumes are sensitive markers of disease progression in MPS IIIA, and may have utility as clinical endpoints in treatment trials. For optimal outcomes, treatment may need to be instituted in children before the onset of steep cognitive decline and brain atrophy.
The purpose of this longitudinal, observational study of the rare lysosomal storage disease, mucopolysaccharidosis type IIIA (MPSIIIA; Sanfilippo syndrome type A), was to gather standardized data on the natural course of this neurodegenerative disease over two years, assessing brain function and structure, and identifying potential endpoints for future treatment trials.
MPS IIIA is a progressive lethal disease caused by deficiency of lysosomal N-sulphoglucosamine sulphohydrolase (SGSH) which degrades heparan sulfate (HS). This autosomal recessive disorder has an incidence of 0.27 – 1.89 per 100,000 births.1 MPS IIIA is the least rare of four subtypes (Types A, B C and D) each resulting from an enzyme deficiency in the catabolic pathway for HS. SGSH is localized to 17q25.3 with more than 100 mutations described2,3 associated with either rapid or slower progression.1,3 Developmental slowing begins in the second year of life followed by cognitive decline and behavioral disturbances.1,3 No therapy is known that to modify disease.
A challenge in monitoring new treatments is the lack of knowledge about rate and variability of clinical disease progression. Although there are retrospective studies1,3-5 and studies of the behavioral phenotype,6,7 prospective studies examining the sequence and timing of cognitive decline and symptom emergence are absent. We hypothesize that cognitive, imaging, and biomarker changes in a two-year time interval will be associated with disease stage and severity.
Methods
Twenty-five patients with MPS IIIA were recruited to this single center study. Inclusion criteria were: (1) confirmed diagnosis of MPS IIIA; (2) calendar age of ≥1 year; and (3) developmental age ≥1 year assessed by the Vineland Adaptive Behavior Scales–Second Edition (VABS-II).8 Exclusion criteria were: (1) history of hematopoietic cell transplant treatment; (2) presence of significant non-MPS IIIA-related central nervous system impairment; and (3) impairment of vision or hearing sufficient to preclude developmental assessment. An ethics committee-approved informed consent form was signed by parents/guardians.
Assessments were performed at baseline, 6 months, one year and two years during a 3 day visit; developmental testing was done the day before procedures requiring anesthesia.
Cerebrospinal fluid (CSF) opening pressure was measured via routine manometry at each lumbar puncture. SGSH mutational analysis was performed for each patient. Adaptive behavior was measured by parent report using the VABS-II.
Neurocognitive assessment was performed for each patient. Two standard instruments, the Bayley Scales of Infant Development, Edition III (BSID-III)9 or the Kaufman Assessment Battery for Children, Edition II (KABC-II)10 were selected to cover anticipated age and ability range. Age equivalent scores (AEqs) were generated from published normative data9,10 and a Development Quotient (DQ) was derived by dividing the AEqs by chronological age and then multiplying by 100. This accepted approach avoids the “floor” effects of standardized scores applied to severely cognitively impaired children.11-13 Either the BSID-III or the nonverbal scale on the KABC-II was chosen for all of each child's visits according to a specific algorithm as previously described.14
The Four Point Scoring System (FPSS),4 an MPS III-specific parent-completed disability questionnaire, which rates motor, expressive language, and cognitive function on a 3 point scale (normal=3, beginning regression=2, severe regression=1, and lost skill=0) was administered. A Total Disability Score was then determined by the average of these three scores.
The Children's Sleep Habits Questionnaire was completed by the parents.15 Based on our experience with MPS III patients, three additional items were added to the original questionnaire that included disruptive behavior at night, dangerous behavior at night, and sleeping during the day.
Head magnetic resonance images (MRI) were acquired on a 3-T Siemens Trio scanner (Siemens, AG, Erlangen, Germany) with a 12-channel RF head coil. The protocol included a 3-dimensional, T1-weighted, magnetization-prepared rapid acquisition with gradient-echo (MPRAGE) sequence. Volumetric analysis of gray and white matter and CSF ventricles was conducted using FreeSurfer (FS) Image Analysis Suite version 5.1.16 The resulting segmentations were visually inspected for mislabeling of anatomy and manually adjusted if necessary.
Liver and spleen volumetry was performed with the same instrument as the head MRI. Liver and spleen volumes were normalized to body surface area and weight, respectively, as described.17,18
Biomarker analysis included urinary glycosaminoglycan (GAG) concentration, heparan sulfate (HS) in CSF, and tau (T-tau) and phospho-tau (p-tau) proteins in CSF. The urinary glycosaminoglycan (GAG) concentration was determined by a 1, 9-dimethylmethylene blue dye binding assay using Blyscan assay kit, and was reported relative to creatinine concentration (mg GAG/mmol creatinine). The level of total HS in CSF was determined by liquid chromatography- tandem mass spectrometry (LC-MS/MS). HS in the CSF was first extracted using an anion-exchange resin and then digested by a combination of enzymes including heparinase I, II, and III. The resultant HS disaccharides were labeled with 12C-4-N-butylaniline by reductive amination and then analyzed by LC-MS/MS. The disaccharides were quantified based on a calibration curve generated using 6 commercially available disaccharide standards that are the most abundant in human CSF HS.19 CSF levels of total tau (T-tau) and phospho-tau (p-tau) proteins were measured by immunoassay.
The level of total HS in CSF was determined by liquid chromatography- tandem mass spectrometry (LC-MS/MS). HS in the CSF was first extracted using an anion-exchange resin and then digested by a combination of enzymes including heparinase I, II, and III. The resultant HS disaccharides were labeled with 12C-4-N-butylaniline by reductive amination and then analyzed by LC-MS/MS. The disaccharides were quantified based on a calibration curve generated using 6 commercially available disaccharide standards that are the most abundant in human CSF HS.19
CSF levels of total tau (T-tau) and phospho-tau (p-tau) proteins were measured by immunoassay.
Control patient data was provided by the University of Innsbruck. These patients/parents provided consent and the samples were anonymized.
Descriptive statistics were summarized overall and by subgroups described below. Mean (SD) was used for continuous covariates with frequency and percentage for categorical variables. Cortex volumes at later visits could not be obtained on 5 patients due to extreme atrophy and so were imputed based on each individual's prior trajectory to diminish bias from missing data. Sensitivity analyses for the imputation were also conducted. The curved mean developmental trajectory was based on local polynomial smoothing.20 Longitudinal associations were estimated with generalized estimating equations and robust variance estimates for 95% confidence intervals and P-values to account for the correlated nature of longitudinal measurements. All analyses were conducted using R v3.0.1.21
Results
Sixteen males and 9 females had a median age of 4.8 years (range 1.1 to 18.4) at enrollment between February 2010, and May 2011. One patient dropped out after the baseline visit for personal reasons; 24 were followed for 12 months and 20 for 24 months. Six sets of siblings were enrolled, including one set of dizygotic twins. The youngest two children were identified because of an older sibling diagnosis.
Patients were sorted post-hoc into groups of RP or SP based on diagnosis before or after the age of 6 years, reported to be a reliable indicator of disease severity,1 and/or known severe genotype. All analyses were conducted separately for these two groups. Six novel mutations were discovered (Table I). Thirteen of 19 patients with RP were either homozygous or compound heterozygous for known severe mutations; 5 patients were compound heterozygous for a known mutation associated with severe disease and 1 of 3 novel mutations; 1 patient was homozygous for a novel mutation, N389S. Among the patients with SP, all were compound heterozygous for mutations, among whom 2 had a known severe mutation associated with S298P, previously reported to confer a relatively mild phenotype.21 Three patients with SP, including 1 pair of siblings, possessed a “severe” mutation in association with 1 of 2 new mutations, L59F or A311D. These two new mutations are therefore associated with slower progressing disease.
Table 1.
Genetic and Demographic Data for Study Subjects
| ID# | Allele 1 | Allele 2 | Phenotypic association in literature (references) | Phenotype observed | Sex | Age at diagnosis (months) | Age at baseline (months) | Cognitive Age Equivalent Baseline/One year/Two years * | DQ Baseline/One year/Two years | FPSS Total score Baseline/One year/Two years |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | R245H | L12P | Severe/New (1, 2) | RP | F | 42 | 79 | 14/10/NA | 18/11/NA | 2.3/1.3/1.3 |
| 2 | N389S | N389S | New/New | RP | M | 34 | 38 | 21/16/14 | 55/31/21 | 2.3/2.0/2.0 |
| 4 | R245H | R245H | Severe/Severe (1, 2, 3) | RP | M | 24 | 28 | 25/27/30 | 89/66/53 | 3.0/2.3/1.7 |
| 5a | S66W | Q380R | Severe/Severe (1, 3, 4) | RP | M | 27 | 29 | 23/23/23 | 79/53/40 | 1.7/2.0/2.0 |
| 6a | S66W | Q380R | Severe/Severe (1, 3, 4) | RP | M | 28 | 29 | 22/23/18 | 76/53/31 | 1.7/2.0/2.0 |
| 7 | S66W | R245H | Severe/Severe (1, 2, 3) | RP | M | 65 | 95 | 8/8/8 | 8/7/7 | 1.0/1.0/1.0 |
| 9b | R245H | R433Q | Severe/Severe (1, 2, 5, 6) | RP | F | 50 | 67 | 27/24/22 | 40/30/23 | 1.7/2.0/1.7 |
| 10b | R245H | R433Q | Severe/Severe (1, 2, 5, 6) | RP | M | 23 | 40 | 27/27/27 | 68/52/40 | 2.0/2.0/2.0 |
| 11 | R245H | R377H | Severe/Severe (1, 2, 4, 7) | RP | M | 52 | 88 | 12/12/13 | 14/12/11 | 2.0/1.7/1.7 |
| 12c | S66W | M376R | Severe/New (1, 3) | RP | F | 56 | 80 | 9/8/NA | 11/9/NA | 2.0/1.3/1.3 |
| 13c | S66W | M376R | Severe/New (1, 3) | RP | M | 19 | 43 | 25/17/9 | 58/30/13 | 2.0/2.0/1.3 |
| 15d | E447K | int7 +1G>C | Severe/New (1, 7) | RP | M | 48 | 51 | 14/7/14 | 27/11/18 | 1.7/1.3/1.3 |
| 16d | E447K | int7 +1G>C | Severe/New (1, 7) | RP | M | 9 | 13 | 11/16/16 | 85/62/41 | 3.0/2.3/2.0 |
| 19 | 1272del 11bp | 1272del 11bp | Severe/Severe (1, 7) | RP | F | 36 | 37 | 25/28/27 | 68/57/44 | 2.3/2.0/2.0 |
| 21 | c.197C>G | c.734G>A | Severe/Severe (1, 2, 3) | RP | F | 29 | 50 | 25/15/10 | 50/24/14 | 1.7/2.0/1.7 |
| 23 | c.197C>G | c.1080delC | Severe/Severe (1, 3, 8, 9) | RP | M | 29 | 45 | 35/28/NA | 78/47/NA | 1.7/2.0/2.3 |
| 24e | R74C | R74C | Severe/Severe (7, 9, 10) | RP | F | 18 | 22 | 20/27/31 | 91/82/67 | 3.0/2.7/2.3 |
| 25e | R74C | R74C | Severe/Severe (7, 9, 10) | RP | M | 52 | 57 | 17/13/13 | 30/19/16 | 1.7/1.7/1.7 |
| 22g | R74C | P293S | Severe/Severe (7, 9, 10, 11) | RP | M | 99 | 105 | 10/11/11 | 10/9/8 | 1.3/1.3/1.3 |
| 3 | R245H | L59F | Severe/New (1, 2 | SP | M | 77 | 81 | 46/50/55* | 57/53/50 | 2.0/2.3/2.3 |
| 14 | P293S | S298P | Severe/Mild (3, 10, 11) | SP | F | 128 | 201 | 45/51/NA* | 22/24/NA | 2.3/2.0/2.0 |
| 17f | 1079delC | A311D | Severe/New (4, 9) | SP | M | 127 | 132 | 28/27/27 | 21/19/17 | 1.7/2.0/2.0 |
| 18f | 1079delC | A311D | Severe/New (4, 9) | SP | F | 150 | 155 | 61/70/69* | 39/42/38 | 2.0/2.3/2.3 |
| 20 | P293S | S298P | Severe/Mild (3, 10, 11) | SP | F | 187 | 220 | 6/6/5 | 3/3/2 | 1.0/1.0/1.0 |
RP : rapid progressor; SP : slow progressor; FPSS: Four Point Scoring System
6 month cognitive data not shown
dizygotic twins
sibling pairs
sibling pairs
sibling pairs
sibling pairs
sibling pairs
patient diagnosed after age six; diagnosed with autism since he was 3, classified as severe / RP.
Blanch L, Weber B, Guo XH, Scott HS, Hopwood JJ. Molecular defects in Sanfilippo syndrome type A. Hum Mol Genet 1997;6:787-91.
Weber B, van de Kamp JJ, Kleijer WJ, et al. Identification of a common mutation (R245H) in Sanfilippo A patients from The Netherlands. J Inherit Metab Dis 1998;21:416-22.
Valstar MJ, Neijs S, Bruggenwirth HT, et al. Mucopolysaccharidosis type IIIA: clinical spectrum and genotype-phenotype correlations. Ann Neurol 2010;68:876-87.
Weber B, Guo XH, Wraith JE, et al. Novel mutations in Sanfilippo A syndrome: implications for enzyme function. Hum Mol Genet 1997;6:1573-9.
Chabas A, Montfort M, Martinez-Campos M, et al. Mutation and haplotype analyses in 26 Spanish Sanfilippo syndrome type A patients: possible single origin for 1091delC mutation. Am J Med Genet 2001;100:223-8.
Montfort M, Garrido E, Hopwood JJ, Grinberg D, Chabas A, Vilageliu L. Expression and functional characterization of human mutant sulfamidase in insect cells. Mol Genet Metab 2004;83:246-51.
Yogalingam G, Hopwood JJ. Molecular genetics of mucopolysaccharidosis type IIIA and IIIB: Diagnostic, clinical, and biological implications. Hum Mutat 2001;18:264-81.
Weber B, Hopwood JJ, Yogalingam G. Expression and characterization of human recombinant and alpha-N-acetylglucosaminidase. Protein Expr Purif 2001;21:251-9.
Pollard LM, Jones JR, Wood TC. Molecular characterization of 355 mucopolysaccharidosis patients reveals 104 novel mutations. J Inherit Metab Dis 2013;36:179-87.
Meyer A, Kossow K, Gal A, et al. The mutation p.Ser298Pro in the sulphamidase gene (SGSH) is associated with a slowly progressive clinical phenotype in mucopolysaccharidosis type IIIA (Sanfilippo A syndrome). Hum Mutat 2008;29:770.
Lee-Chen GJ, Lin SP, Ko MH, et al. Identification and characterization of mutations underlying Sanfilippo syndrome type A (mucopolysaccharidosis type IIIA). Clin Genet 2002;61:192-7.
Neurocognitive Assessment
Either of two experienced psychometrists tested 21 patients (19 RP and 2 SP) with the BSID and 3 (SP) with the KABC (Table I).
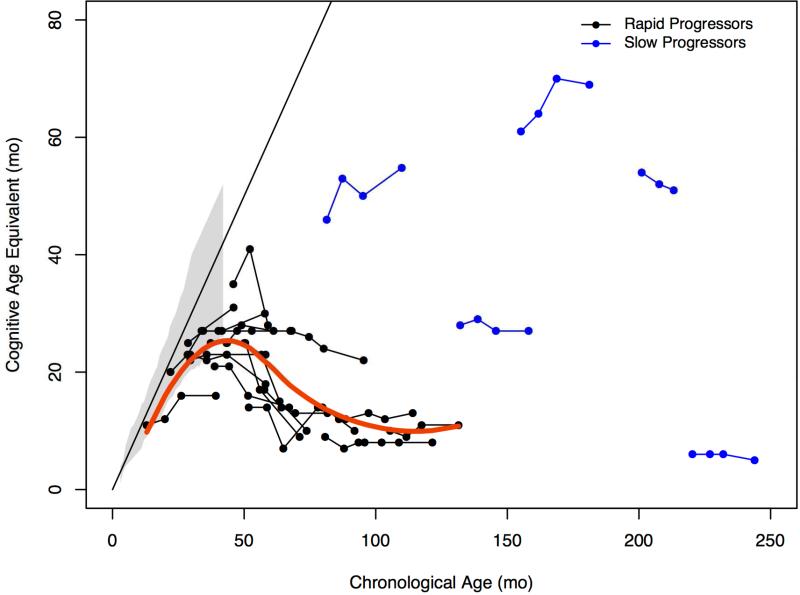
Using AEqs, the 3 youngest children, <28 months of age, continued to acquire skills as reflected in an upward trajectory (Figure 1, A). A slowing in development between 36 and 40 months and a loss of skills after 48 months was noted in children with RP. After 66 months, cognitive decline appeared to reach a nadir. The 5 patients with SP displayed no consistent trends over time.
Figure 1.
A. Trajectory of cognitive growth by age in months for rapid and slow progressors compared with published normative growth data (gray shaded area), with a ceiling at 42 months. The Bayley was administered for all rapid progressors and 2 of 5 slow progressors. The KABC-II was administered for 3 slow progressors whose baseline age equivalent was above 42 months. Red line is growth trajectory for rapid progressors only. B, Change in developmental quotient (100 X age equivalent/chronological age) and age expressed in months for rapid and slow progressors.
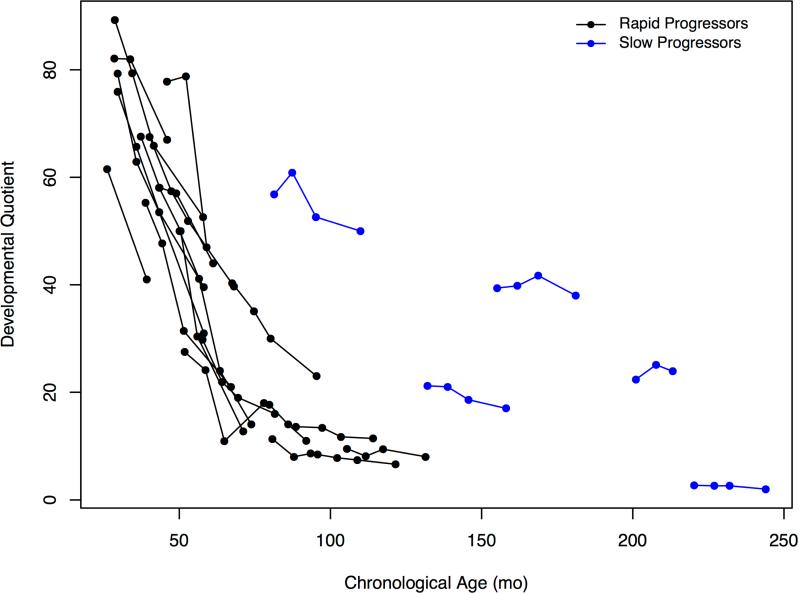
Using DQ, patients with RP showed a dramatic decline over time estimated at −9.8 points/year (95%CI: −11.8, −7.7), P<0.001 (Figure 1, B). This was most notable up to age 6 where decline was −14.6 points/year (−17.5, −11.8). In comparison with the group with RP, DQ decline in the SP, was −3.7 points/year (−5.0, −2.4), P=0.012.
Adaptive Behavior
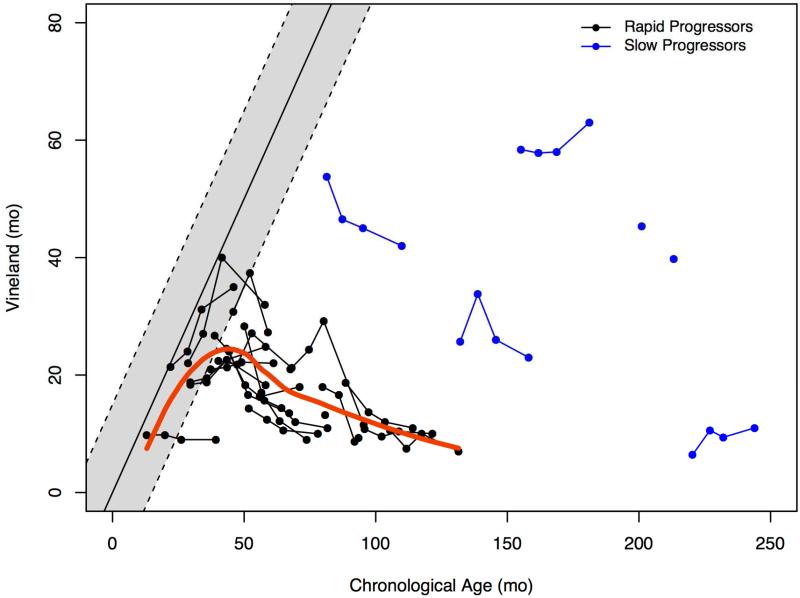
AEqs on the VABSII indicated continuing acquisition of skills in the group with RP before the age of 50 months with subsequent loss of skills (Figure 2). The scores of patients with SP were notably different with no clear trends.
Figure 2.
Developmental growth trajectory of mean age equivalent scores in rapid and slow progressors for composite score compared to normal published data on the Vineland Adaptive Behavior Scales – II. Initial assessment was on the survey interview form with parents and subsequent ones were on the parent rating form.
Disability
FPSS total disability scores were tightly associated with DQ at the high and low ends but showed a lack of association of DQ with FPSS scores in the middle of the range (Table I). The FPSS was able to classify correctly some of the least impaired children (n=4) whose score was 2.7 or 3.0 (mean DQ was 87 ranging from 82 to 91). The FPSS was also sensitive to the most impaired children (scores of 1 or 1.3) (mean DQ was 11 with a range from 2 to 31) but misclassifying a few. However, the FPSS was insensitive in the midrange with many overlapping DQ scores at all three FPSS scores of 1.7 (mean DQ=37, range: 11 to 79), 2 (mean DQ 44; range: 11 to 79), or 2.3 (mean DQ is 55; range: 18 to 82).
Sleep Habits Questionnaire
Analysis indicated that although many sleep problems were reported, no pattern was found nor was there change over time (data not shown). The data found were insensitive for use in a clinical trial even as a secondary marker.
Imaging
Volumetric analysis was completed for all but 10 scans. Two children, < 24 months exhibited lack of gray-white matter differentiation, thought to reflect immaturity of myelination,23 which precluded volumetric assessment at baseline and 6 months for one, and baseline for the other. In 5 children, progressive atrophic changes resulted in analytic failure, even with manual adjustment, at 24 month visits. Values were imputed from the slope of previous visits. In one child with a ventriculo-peritoneal shunt, artifact precluded volumetric analysis at all 4 time points.
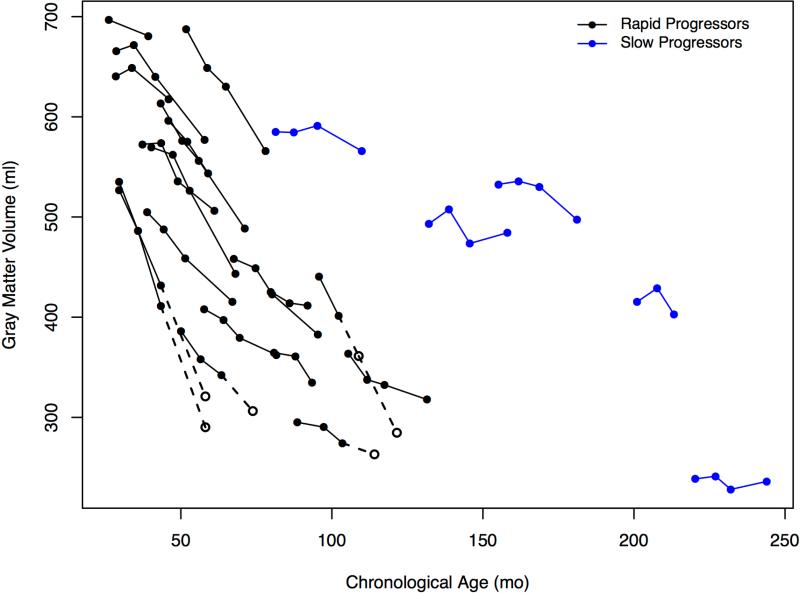
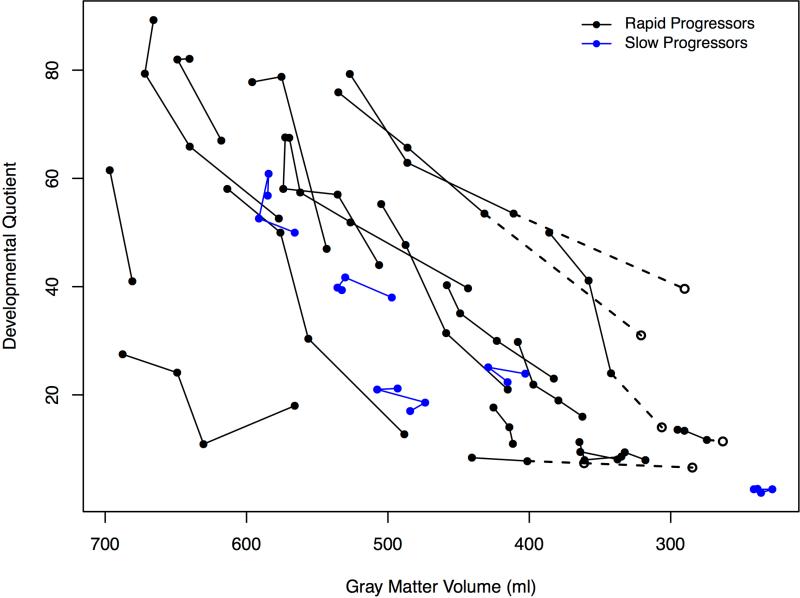
Two trajectories of cortical gray matter volumes are evident in Figure 3, A between the RP and SP groups over time. A striking decline among patients with RP and consistent at −41.1 ml/year (−52.7, −29.4), P<0.001, and the decline for patients with SP is attenuated at −26.4 ml/year (−37.4, −15.4), P<0.001. The estimates for patients with RP were not significantly different when imputed values were not used.
Figure 3.
A, Change in gray matter volumes in milliliters for rapid and slow progressors and age expressed in months. B, Association of decline in developmental quotient with gray matter volumes and age expressed in months for rapid and slow progressors. Open circles indicate the imputed values which are connected with dotted lines (values imputed from slope of previous visits) for 4 patients at the 24 month visit only and 1 patient at the 12 month visit and the 24 month visit.
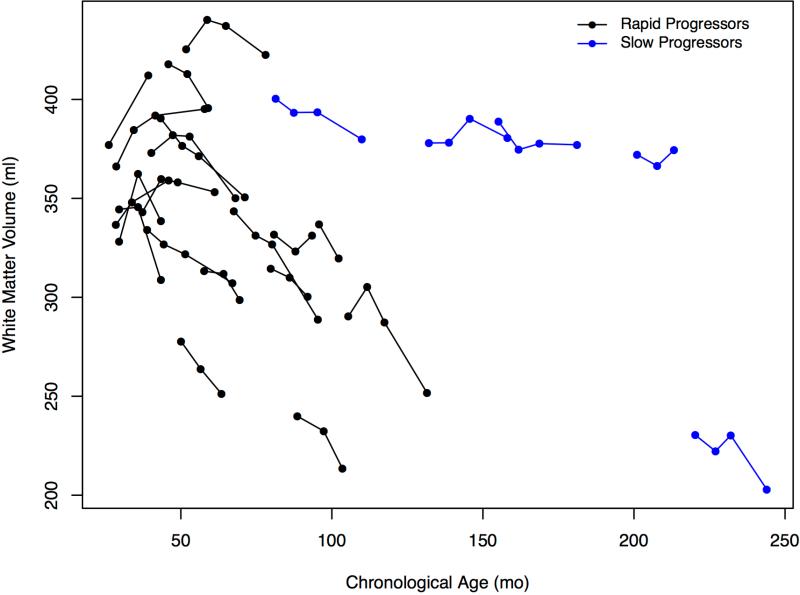
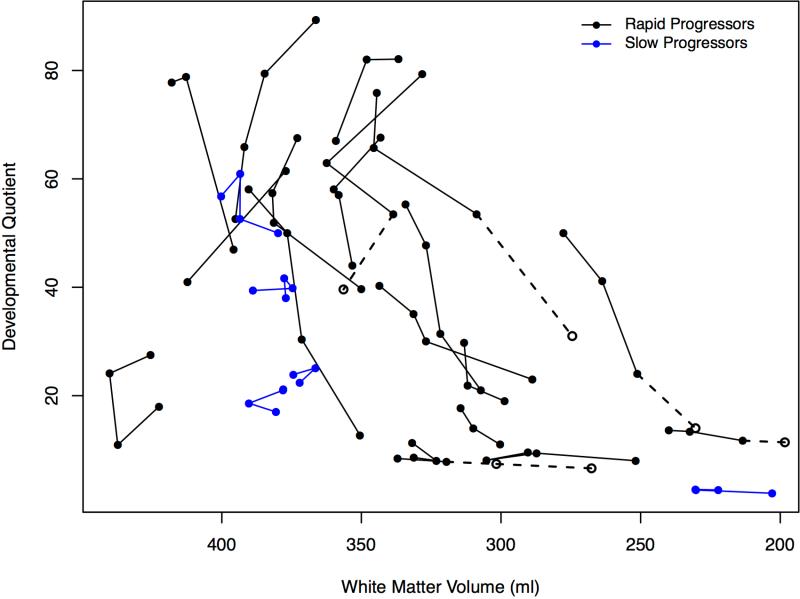
In the youngest patients, white matter volumes showed an upward trajectory until ~40 months, with subsequent declines evident in most children with RP. Patients with SP showed little or no change (Figure 4, A; available at www.jpeds.com).
Figure 4.
A, White matter volumes and age in months. B, White matter volumes for rapid and slow progressors and DQ. Imputed values are the same subjects as in gray matter volumes. All volumes were converted from mm3 to milliliters.
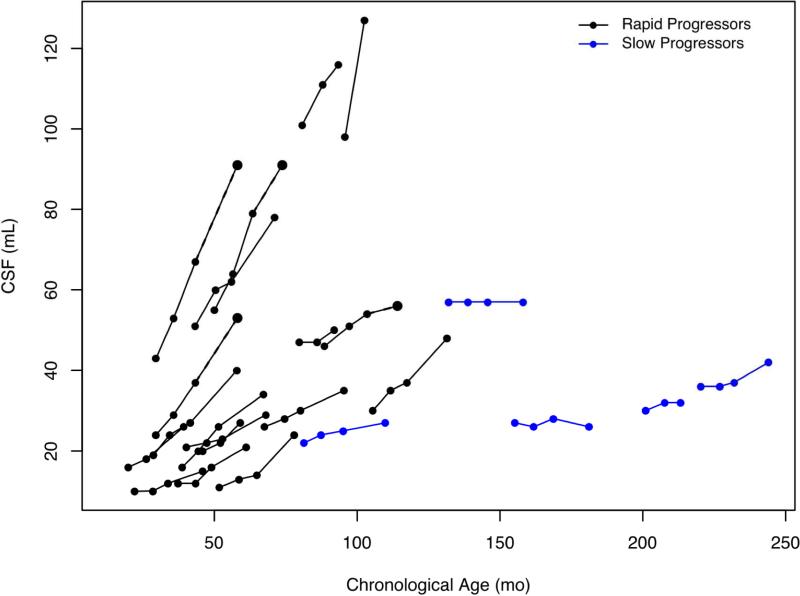
Ventricular volumes showed increases in all age groups and both groups, although the upward trajectory in the group with RP was steeper (Figure 5; available at www.jpeds.com).
Figure 5.
Volumes of cerebrospinal fluid in ventricles in milliliters for rapid and slow progressors and age expressed months.
The relationship of decline in DQ to gray matter volumes over all visits can be seen in Figure 3B. No relationship of DQ to white matter volumes was observed (Figure 4, B).
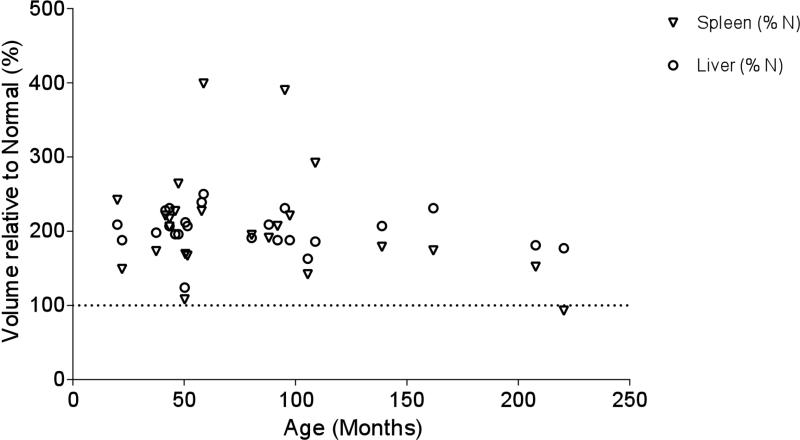
Liver and spleen volumes were increased at baseline in most patients, across ages, with an approximate doubling in organ volume compared with expected values (Figure 6; available at www.jpeds.com).17,18 No trends were apparent over the period of observation (data not shown).
Figure 6.
Liver and spleen size relative to normal controls by age based on percent of normal and based on first reading only for each patient.
Biomarkers
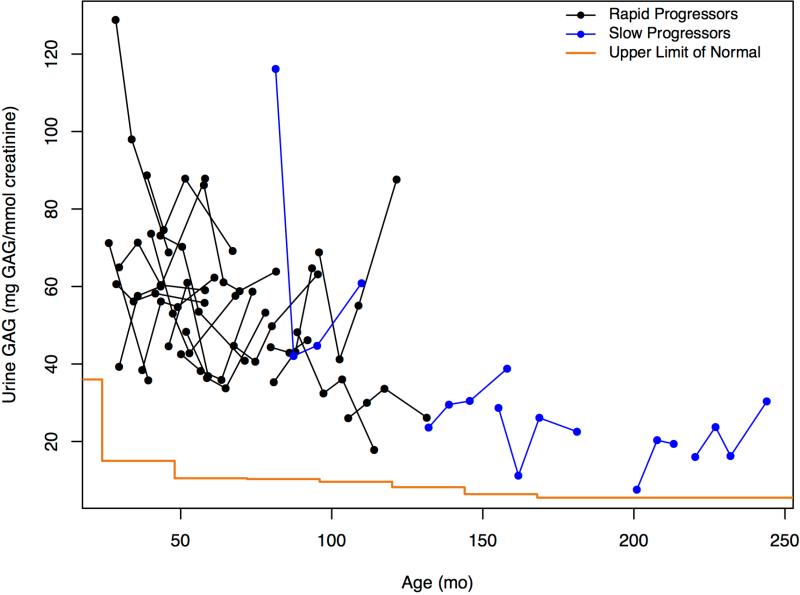
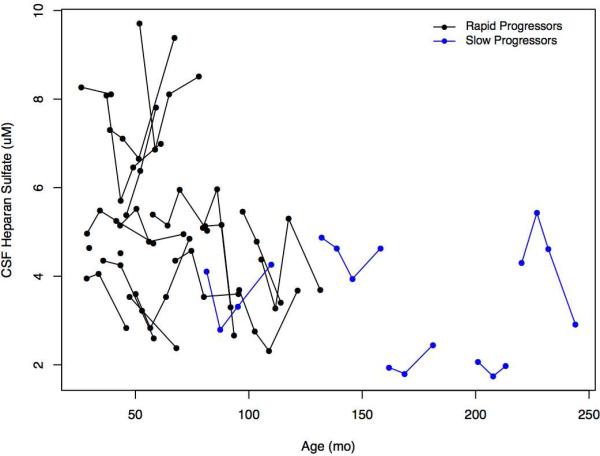
Total urine GAGs remained elevated in all patients relative to control values (Figure 7, A). The apparent overall decline with increasing age confounded interpretation of lower levels in patients with SP. Similarly, CSF HS levels were elevated in all patients compared with controls (Table II; available at www.jpeds.com) with RP increased but overlapping compared with patients with SP (Figure 7, B). Neither CSF HS nor urine GAG levels exhibited within-patient time-dependent increases associated with disease progression. CSF HS levels were strikingly similar in 4 out of 6 of the sibling pairs (data not shown).
Figure 7.
A, Change in urine GAGs in mg GAG/mmol creatinine and age expressed in months for rapid and slow progressors. Upper limit of normal values were taken from Naimy et al,19 which provide values in two year increments from 0 to 14 years and above with the step at the end of each increment. B, Change in CSF heparan sulfate in μM and age expressed in months for rapid and slow progressors. Control values can be found in Table II.
Table 2.
Control CSF heparan sulfate levels
| Age Group | 0-27 days | 1-23 months | 2-11 years | 12-18 years |
|---|---|---|---|---|
| N (Total) | 24 | 52 | 41 | 31 |
| N (<LLOQ) | 0 | 0 | 13 (44%) | 27 (87%) |
| Min (μM) | 0.229 | 0.248 | <0.251 | <0.300 |
| Max (μM) | 0.463 | 0.648 | 0.443 | 0.426 |
Heparan sulfate levels were measured by tandem mass spectrometry in de-identified CSF samples collected from children without mucopolysaccharidosis, obtained from the National Children Medical Center biorepository. Samples were assayed from the following age groups: birth to 27 days (24 samples), 1 to 23 months (52 samples), 2 to 11 years (41 samples, of which 44% were below the limits of quantitation), 12 to 18 years (31 samples, of which 87% were below the limits of quantitation). The increasing frequency of samples with levels of HS below the limits of quantitation with increasing age suggests age-related declines in CSF HS levels. The maximum level observed was 0.648 μM in a child in the 1 to 23 month age range.
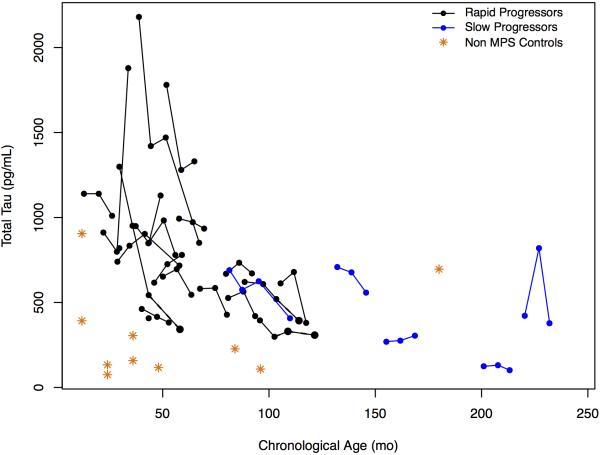
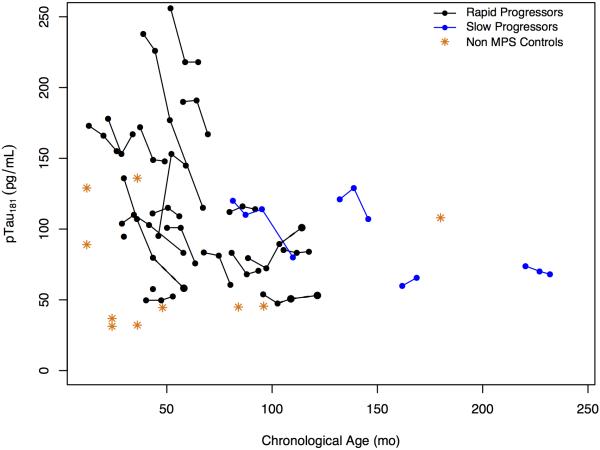
CSF T-tau and p-tau levels were modestly elevated relative to controls and appeared to decline with increasing age, so no relationship with disease progression was evident (Figure 8; available at www.jpeds.com).
Figure 8.
A, CSF total tau protein levels and B, CSF total p-Tau protein levels (grams per milliliter) over time for rapid and slow progressors compared with controls.
In all but 4 patients CSF opening pressures were normal (< 20 cm water) (data not shown). Among the four, pressures were normal in 2 or 3 out of 4 lumbar punctures. The highest CSF opening pressure observed was 30 cm water followed by 3 who were between 20 and 25 cm.
Discussion
Our patients sorted into two distinctive groups with differing natural histories of cognitive and brain changes with a difference in disease cadence.3 Classifying patients into RP or SP will enhance understanding of the patient's disease stage.
One exception to accurate classification by age of diagnosis1 was a patient previously diagnosed with autism. This diagnosis, although frequently observed in MPS IIIA,7 may have contributed to the delayed recognition of MPS IIIA in this patient, who was diagnosed at 99 months of age. Although by age (greater than 72 months of age) he should be classified as SP, his known severe mutations classified him as RP. Both age of diagnosis and genetic criteria will be needed to accurately classify patients but will be limited by the frequent identification of new mutations.
A positive trajectory of cognitive development was evident in patients with RP who were under 28 months of age at baseline. A ceiling of development at 42-48 months was observed in all but 1 patient with RP (Figure 1, A). That patient, who was 45 months at baseline, acquired cognitive skills for the first 6 months of the study, but lost significant function by the 12 month follow up visit. This slowing of development between 2 and 4 years of age has previously been described in MPS III5,24 and also has been found in other untreated MPS disorders.25-27 As the development is very rapid in typically developing children between ages 2 and 4, slowing during this age range should prompt further diagnostic studies.
Disease progression has been described as slowing of speech/language acquisition (phase 1) followed by a halt in cognitive development with emerging behavioral abnormalities (phase 2), and then a loss of mobility progressing to a vegetative state (phase 3).28 Median age of death is in the second decade usually due to neurological disease.1,3,4 At baseline, two patients in our study were in the first phase (both diagnosed early because of an affected sibling), and the remaining 23 were in the second. Although designed to track this progression, the FPSS4 lacked sensitivity to disease progression during the second stage compared with DQ.
When cognitive status was expressed as a DQ, all patients with RP exhibited marked declines over 1 or 2 years (Figure 1, B). Even in the youngest patients who had slower than normal development, the BSID-III (used in all patients with RP) had the requisite sensitivity for measuring disease progression within a time interval that might correspond to the duration of a therapeutic trial. Mean loss of 15 DQ points over one year in children < 6 is similar to findings in MPS IH, of a loss of 15-20 points per year at ages 1 to 3.24,25
Changes in cognition were mirrored dramatically by quantitative neuroimaging findings. Brain atrophy and characteristic perivascular spaces have been reported clinically29,30 but using quantitative volumetrics the consistent declines in cortical gray matter volume starting at ~30 months in patients with RP demonstrated a close association with rate of cognitive decline (Figure 3, B). This atrophy was accompanied by a compensatory increase in ventricle volumes (Figure 5). White matter volumes were less dramatically affected.
Volumetric analysis was not possible in the baseline scans in the 2 youngest patients, owing to lack of gray-white contrast resulting from early changes in gray matter water content and lack of myelination.31 Gray matter growth to age 9 with a small decline thereafter and continued growth in white matter through adolescence has been shown in typical development.32,33 Hence, our findings of steep declines in gray matter volume represent gross pathological changes associated with MPS IIIA. The primacy of gray matter volume loss suggests that cognitive decline is associated with loss or damage to cortical neurons. The finding that a quantitative measure of brain structure is associated with neurocognitive assessment points to the validity of the latter as a clinical measure of disease progression in this patient population.
Liver and spleen volumes were approximately double the predicted normal volume for both RP and SP phenotypes (Figure 6).17,18 Although not usually diagnosed clinically, this finding suggests that volumetric analysis could be a useful treatment response marker.
Unsurprisingly, CSF and urine glycosaminoglycan levels were abnormally elevated. In contrast to cognitive and neuroimaging measures, there was no relationship between these disease-associated biomarkers and disease progression. Data from animal studies suggest that MPS III is a “tauopathy”.34,35 However, although there were equivocal elevations in CSF tau levels, these were not useful markers of disease progression. Our data suggest age-related declines in these markers, as may be observed physiologically in early life.36
This study presents a composite picture of the progression of MPS IIIA over the course of childhood. Although the fact that individual children were only followed for up to 2 years may be considered a limitation of the study, the prospective design, together with the striking conformance of the individual patient trajectories to the overall pattern, suggest that this represents an accurate picture of the long term natural history of this disease.
In conclusion, we found that cognitive decline measured by DQ and cortical gray matter volumes are sensitive markers of disease progression in MPS IIIA. The consistent declines in as little as 1 year suggest that these variables could have utility as endpoints in clinical trials in patients with RP. The finding that developmental arrest occurs around the age of 4 years of age suggests that therapeutic interventions should be made before this stage to have the greatest benefit. Similar to MPS IH, where early treatment with hematopoietic cell transplantation benefits cognition,37 treatment should be instituted in children before the onset of steep cognitive decline and brain atrophy. Unlike MPS IH, diagnosis is usually delayed beyond this period in MPS III because of the lack of apparent physical features. Treatments with protein and gene replacement38 are imminent, and once available, earlier diagnosis will be crucial. This leads inevitably to considerations of newborn screening and to developing an increased awareness by community practitioners of the possibility of neurometabolic disease in children with developmental delay.
Acknowledgments
We are grateful to the patients and parents who participated in this study; Brenda Diethelm-Okita for administrative assistance; the Center for Neurobehavioral Development, the Center for Magnetic Resonance Research, and the Minnesota Supercomputer Center for the provision of infrastructure for this research; and Dr Kevin Rostasy for providing control data from his biorepository at the University of Innsbruck.
Supported by Shire, Lexington MA and the National Center for Advancing Translational Sciences, the National Institutes of Health (NIH), through University of Minnesota (UL1TR000114 [to K.R.]). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. E.S. and I.N. received investigator-initiated grants (<grant numbers> [to E.S.] and <grant number> [to I.N.) and personal fees outside of the submitted work from Shire. K.D. received personal fees from Shire. V.K. received salary support and an investigator-initiated grant (<grant number>) from Shire. N.N., C.R., and P.H. were employees of Shire during the study. C.W. received an institutional trial contract for this study from Shire.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration ClinicalTrials.gov: NCT01047306
References
- 1.Heron B, Mikaeloff Y, Froissart R, et al. Incidence and natural history of mucopolysaccharidosis type III in France and comparison with United Kingdom and Greece. Am J Med Genet A. 2011;155A:58–68. doi: 10.1002/ajmg.a.33779. [DOI] [PubMed] [Google Scholar]
- 2.Yogalingam G, Hopwood JJ. Molecular genetics of mucopolysaccharidosis type IIIA and IIIB: Diagnostic, clinical, and biological implications. Hum Mutat. 2001;18:264–281. doi: 10.1002/humu.1189. [DOI] [PubMed] [Google Scholar]
- 3.Valstar MJ, Neijs S, Bruggenwirth HT, et al. Mucopolysaccharidosis type IIIA: clinical spectrum and genotype-phenotype correlations. Ann Neurol. 2010;68:876–887. doi: 10.1002/ana.22092. [DOI] [PubMed] [Google Scholar]
- 4.Meyer A, Kossow K, Gal A, et al. Scoring evaluation of the natural course of mucopolysaccharidosis type IIIA (Sanfilippo syndrome type A). Pediatrics. 2007;120:e1255–61. doi: 10.1542/peds.2007-0282. [DOI] [PubMed] [Google Scholar]
- 5.Buhrman D, Thakkar K, Poe M, Escolar ML. Natural history of Sanfilippo syndrome type A. J Inherit Metab Dis. 2014;37:431–437. doi: 10.1007/s10545-013-9661-8. [DOI] [PubMed] [Google Scholar]
- 6.Potegal M, Yund B, Rudser K, et al. Mucopolysaccharidosis Type IIIA presents as a variant of Kluver-Bucy syndrome. J Clin Exp Neuropsychol. 2013;35:608–616. doi: 10.1080/13803395.2013.804035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rumsey RK, Rudser K, Delaney K, Potegal M, Whitley CB, Shapiro E. Acquired Autistic Behaviors in Children with Mucopolysaccharidosis Type IIIA. J Pediatr. 2014;164:1147–1151. doi: 10.1016/j.jpeds.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparrow SS, Cicchetti DV. Diagnostic uses of the Vineland Adaptive Behavior Scales. J Pediatr Psychol. 1985;10:215–225. doi: 10.1093/jpepsy/10.2.215. [DOI] [PubMed] [Google Scholar]
- 9.Bayley N. Bayley Scales of Infant and Toddler Development. Third Edition Psychological Corporation; San Antonio, TX: 2006. [Google Scholar]
- 10.Kaufman AS. Manual for the Kaufman Assessment Battery for Children - Second Edition (KABC-II) Comprehensive Form. American Guidance Service; Circle Pines MN: 2004. [Google Scholar]
- 11.Volkmar FR, Sparrow SS, Goudreau D, Cicchetti DV, Paul R, Cohen DJ. Social deficits in autism: an operational approach using the Vineland Adaptive Behavior Scales. J Am Acad Child Adolesc Psychiatry. 1987;26:156–161. doi: 10.1097/00004583-198703000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Staba SL, Escolar ML, Poe M, et al. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N Engl J Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- 13.Peters C, Balthazor M, Shapiro EG, et al. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood. 1996;87:4894–4902. [PubMed] [Google Scholar]
- 14.Delaney KA, Rudser KR, Yund BD, Whitley CB, Haslett PA, Shapiro EG. Methods of Neurodevelopmental Assessment in Children with Neurodegenerative Disease: Sanfilippo Syndrome. JIMD Rep. 2013 doi: 10.1007/8904_2013_269. DOI/10.1007/8904_2013_269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2001;23:1043–51. (2001) [PubMed] [Google Scholar]
- 16.Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urata K, Kawasaki S, Matsunami H, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317–1321. [PubMed] [Google Scholar]
- 18.Schlesinger AE, Edgar KA, Boxer LA. Volume of the spleen in children as measured on CT scans: normal standards as a function of body weight. AJR Am J Roentgenol. 1993;160:1107–1109. doi: 10.2214/ajr.160.5.8470587. [DOI] [PubMed] [Google Scholar]
- 19.Naimy H, Powell KD, Moriarity JR, Wu J, McCauley TG, Haslett PAJ, Barbier AJ, Qiu Y. A novel LC–MS/MS assay for heparan sulfate screening in the cerebrospinal fluid of mucopolysaccharidosis IIIA patients. Submitted for publication. [DOI] [PubMed]
- 20.Cleveland WS, Grosse E, Shyu WM. In: Local regression models. Chapter 8 of Statistical Models in S. Chambers JM, Hastie TJ, editors. Wadsworth & Brooks/Cole; 1992. [Google Scholar]
- 21.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. URL http://www.R-project.org/ [Google Scholar]
- 22.Meyer A, Kossow K, Gal A, et al. The mutation p.Ser298Pro in the sulphamidase gene (SGSH) is associated with a slowly progressive clinical phenotype in mucopolysaccharidosis type IIIA (Sanfilippo A syndrome). Hum Mutat. 2008;29:770. doi: 10.1002/humu.20738. [DOI] [PubMed] [Google Scholar]
- 23.van der Knaap MS, Valk J. MR imaging of the various stages of normal myelination during the first year of life. Neuroradiology. 1990;31:459–470. doi: 10.1007/BF00340123. [DOI] [PubMed] [Google Scholar]
- 24.Valstar MJ, Marchal JP, Grootenhuis M, Colland V, Wijburg FA. Cognitive development in patients with Mucopolysaccharidosis type III (Sanfilippo syndrome). Orphanet J Rare Dis. 2011;6:43. doi: 10.1186/1750-1172-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krivit W, Peters C, Shapiro E. Bone marrow transplantation as effective treatment of central nervous system disease in globoid cell leukodystrophy, metachromatic leukodystrophy, adrenoleukodystrophy, mannosidosis, fucosidosis, aspartylglucosaminuria, Hurler, Maroteaux-Lamy, and Sly syndromes, and Gaucher disease type III. Current Opinion in Neurology. 1999;12:167–176. doi: 10.1097/00019052-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro E, Balthazor M. Metabolic and neurodegenerative disorders of childhood. In: Taylor G, Ris D, Yeates K, editors. Pediatric Neuropsychology: Research, Theory and Practice. Guilford Press; 2000. pp. 171–205.pp. 184 [Google Scholar]
- 27.Holt JB, Poe MD, Escolar ML. Natural progression of neurological disease in mucopolysaccharidosis type II. Pediatrics. 2011;127:e1258–1265. doi: 10.1542/peds.2010-1274. [DOI] [PubMed] [Google Scholar]
- 28.Cleary MA, Wraith JE. Management of mucopolysaccharidosis type III. Arch Dis Child. 1993;69:403–406. doi: 10.1136/adc.69.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barone R, Nigro F, Triulzi F, Musumeci S, Fiumara A, Pavone L. Clinical and neuroradiological follow-up in mucopolysaccharidosis type III (Sanfilippo syndrome). Neuropediatrics. 1999;30:270–274. doi: 10.1055/s-2007-973503. [DOI] [PubMed] [Google Scholar]
- 30.Kara S, Sherr EH, Barkovich AJ. Dilated perivascular spaces: an informative radiologic finding in Sanfilippo syndrome type A. Pediatr Neurol. 2008;38:363–366. doi: 10.1016/j.pediatrneurol.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Barkovich AJ, Kjos BO, Jackson DE, Jr., Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166:173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- 32.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 33.Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- 34.Ohmi K, Kudo LC, Ryazantsev S, Zhao HZ, Karsten SL, Neufeld EF. Sanfilippo syndrome type B, a lysosomal storage disease, is also a tauopathy. Proc Natl Acad Sci U S A. 2009;106:8332–8337. doi: 10.1073/pnas.0903223106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohmi K, Zhao HZ, Neufeld EF. Defects in the medial entorhinal cortex and dentate gyrus in the mouse model of Sanfilippo syndrome type B. PLoS One. 2011;6:e27461. doi: 10.1371/journal.pone.0027461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattsson N, Sävman K, Osterlundh G, Blennow K, Zetterberg H. Converging molecular pathways in human neural development and degeneration. Neurosci Res. 2010;66:330–332. doi: 10.1016/j.neures.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Peters C, Shapiro EG, Krivit W. Neuropsychological development in children with Hurler syndrome following hematopoietic stem cell transplantation. Pediatr Transplant. 1998;2:250–253. [PubMed] [Google Scholar]
- 38.Tardieu M, Zerah M, Husson B, et al. Intracerebral Administration of Adeno-Associated Viral Vector Serotype rh.10 Carrying Human SGSH and SUMF1 cDNAs in Children with Mucopolysaccharidosis Type IIIA Disease: Results of a Phase I/II Trial. Hum Gene Ther. 2014;25:506–516. doi: 10.1089/hum.2013.238. [DOI] [PubMed] [Google Scholar]