Abstract
Interventions to assist reproductive health decision-making in populations affected by sickle cell disease (SCD) or trait (SCT) lack proven efficacy over time. Our aim was to compare effects of CHOICES, a Web-based multimedia education program on implementing informed reproductive plans, and usual care education (e-Book) on reproductive knowledge, intention, and behavior over 24 months. We randomized 234 participants with SCD (n=138) or SCT (n=96) (age 18–35 years, 35% male, 94% African American) to CHOICES and e-Book groups. Participants completed a sickle cell-specific reproductive measure before and four times after the intervention (6, 12, 18 and 24 months). Compared to the e-Book group the CHOICES group had significantly more improvement in knowledge over time (p=.004) but not intention (p=.18) or behavior (p=.69). At baseline, 114 (48.7%) participants reported having partners who would not put the couple at risk for their children inheriting SCD. Of the 116 (49.6%) at-risk participants, a higher poroportion of those who were in the CHOICES group chose partners that reduced their risk by the last visit than the e-Book group (p=.04). Study findings provide important insights for designing a national trial of the CHOICES intervention focusing on subjects whose partner status puts them at risk for having a child with SCD.
Keywords: sickle cell disease, sickle cell trait, reproductive behavior, young adult, randomized controlled trial, longitudinal follow-up
Introduction
Single gene inheritance patterns of sickle cell disease (SCD) or sickle cell trait (SCT) is a well-known fact, but at risk young adults with SCD or SCT of childbearing age often lack sufficient knowledge for interpreting the facts into their personal actions regarding reproduction (Gallo, Knafl, & Angst, 2009; Long, Thomas, Grubs, Gettig, & Krishnamurti, 2011; Taylor, Kavanagh, & Zuckerman, 2014). To address this issue, we demonstrated feasibility and immediate posttest efficacy of a web-based, multimedia, targeted, and interactive intervention (CHOICES) to foster informed reproductive health decisions by people with SCD or SCT, but proven efficacy over time is needed (Gallo et al., 2010; Gallo et al., 2014; Wilkie et al., 2013). In this article, we present results from our 2-year longitudinal randomized controlled trial (RCT) of the CHOICES intervention for young adults with SCD or SCT.
Surprising little is known about reproductive health knowledge and decisions among the 100,000 Americans with SCD, and the 3.5 million Americans of African descent with SCT or others of Mediterranean, Middle Eastern, or Indian descent with hemoglobinopathies (Modell & Darlison, 2008; Yawn et al., 2014; Yusuf et al., 2011). There are about 2,000 American infants born each year with SCD (Modell & Darlison, 2008) and 1 of 500 Black or African American infants are born with SCD (CDC, 2015). One of 12 African Americans are carriers of the SCT (CDC, 2015) and in 2010, over 60,000 infants were born with SCT (Ojodu, et al., 2014). Infants with SCT inherit one sickle cell hemoglobin gene from one parent and a normal hemoglobin gene from the other parent and typically are healthy and do not exhibit major symptoms, but they can transmit the gene to their children.
Infants with SCD inherit two sickle hemoglobin genes, one from each parent (Yawn et al., 2014). SCD causes abnormal oxygen-carrying hemoglobin molecules in red blood cells (RBCs) to form crescent shapes and cluster together, adhere to the walls of the blood vessels and obstruct blood flow. This process triggers attacks of pain in the chest, back, arms, legs, and abdomen, severe infections, and can damage major body organs including kidneys, spleen, liver, and brain from silent and overt strokes (DeBaun, 2014b; Pleasants, 2014); the destruction of RBCs leads to severe anemia.
The devastating effects of SCD has contributed to intense intrusion and strain for individuals with SCD and their families (DeBaun, 2014b). Depending on the type or severity of SCD, people with SCD have to carry out demanding daily medical treatments, cope with fatigue, pain, infections, and disability, observe symptoms and decide when to get immediate treatment, undergo possible blood transfusions, and endure frequent hospitalizations (DeBaun, 2014b). Parents and other family members often need to provide and oversee this care for their children with SCD, too. Even though survival rates have improved over the last 4 decades because of newer treatment advances, adults with SCD experience decreased quality of life (McClish et al., 2005) and a shortened life span (Hamideh & Alvarez, 2013) compared to healthy adults; it is estimated that median survival rate was 42 years for males and 48 years for females (Platt, et al., 1994; Powars, et al., 1988). Pregnant women with SCD have a high risk of mortality and morbidity, and perinatal outcomes such as bleeding, infections, preterm labor, preeclampsia (Rogers & Molokie, 2010), and are worsened by sickle cell acute pain crises (Alayed, Kezouk, Oddy, & Abenhaim, 2014). Fortunately, with improved newborn screening for SCD, the preventive care measures and treatments for SCD have reduced childhood mortality but young adulthood mortality remains high (Powars, Chan, Hiti, Ramicone, & Johnson, 2005; Quinn, Rogers, & Buchanan, 2004). Because of these issues, young adults with SCD or SCT face serious decisions about childbearing. Yet, to our knowledge only one quasi-experimental (Gallo et al., 2014), one short-term RCT (Wilkie et al., 2013), three descriptive studies (Acharya, Lang, & Ross, 2009; Gallo et al., 2010; Hill, 1994), and one review (DeBaun, 2014) have been published on reproductive health knowledge of people with SCD or SCT or their intention and behavior related to becoming a parent (Asgharian & Anie, 2003). The RCT was from the sample reported in this article and showed significant differences in knowledge but insignificant differences in intentions and planned behavior immediately after the intervention (Wilkie et al., 2013). However, these findings did not include the effects of the two booster intervention sessions that were delivered 6 and 12 months after the initial intervention. A recent expert panel review of the management of sickle cell disease (Yawn et al., 2014) included recommendations from the World Health Organization about reproduction and contraceptive counseling, which lack sufficient specificity for young adults with SCD or SCT to implement an informed plan to become parents. Taylor et al. (2014) acknowledged that genetic screening has been insufficient to prevent SCD because adolescents and young adults lack knowledge about their sickle cell status.
For individuals at risk of their children inheriting a serious genetic condition such as SCD, the reproductive health decisions and behaviors relate to disease burden, individuals’ right to decide for themselves, and the right to make informed decisions. However, implementing these rights requires reproductive health knowledge specific to SCD and SCT. Although extensive research is available about sex education in general, CHOICES is the first educational intervention specific to SCD or SCT and the reproductive health knowledge that young adults with SCD or SCT need to make decisions about becoming parents (Acharya et al., 2009; Hill, 1994). In a sample of young adults with SCD or SCT, our specific aim was to compare e-Book (control) and CHOICES groups for changes from baseline to 6, 12, 18, and 24 months on reproductive health knowledge, intention and behavior scores. We hypothesized that, controlling for baseline scores, over the 24-months the CHOICES group would have larger reproductive health knowledge gains and higher intention, and behavior scores than the e-Book group.
Methods
Design/Setting
Stratified by SCD or SCT status in permuted blocks (Matts & Lachin, 1988), the RCT had baseline and multiple posttest measurements (immediately posttest, 6, 12, 18 and 24 months posttest). We have reported the randomization procedures and the immediate posttest results elsewhere (Wilkie et al., 2013). The institutional review boards at the University of Illinois at Chicago (UIC) and the Ann and Robert H. Lurie Children’s Hospital of Chicago (formerly Children’s Memorial Hospital) approved the study.
We identified participants from a variety of settings, including the adult and pediatric sickle cell clinics at the University of Illinois Hospital and Health Sciences System (UI) in Chicago and the pediatric clinic at the Ann and Robert H. Lurie Children’s Hospital of Chicago, community organizations, public settings (e.g., college student centers, public libraries, drug and grocery stores), and online networks (e.g., Backpage.com, Craigslist.org, facebook.com, Twitter.com). We collected data at locations convenient to the participants (e.g., clinical or academic settings, participants’ homes, public libraries, coffee shops, fast-food outlets).
Participants
Young adults (18 to 35 years of age) who reported having SCD or SCT with the capability and wish to have children in the future, and fluent in English were recruited to the sample. Excluded were persons legally blind or physically unable to have children or to complete the study, or who admitted knowing a participant enrolled in the study or being a friend or relative of a participant (to reduce study contamination by diffusion of the intervention to control subjects).
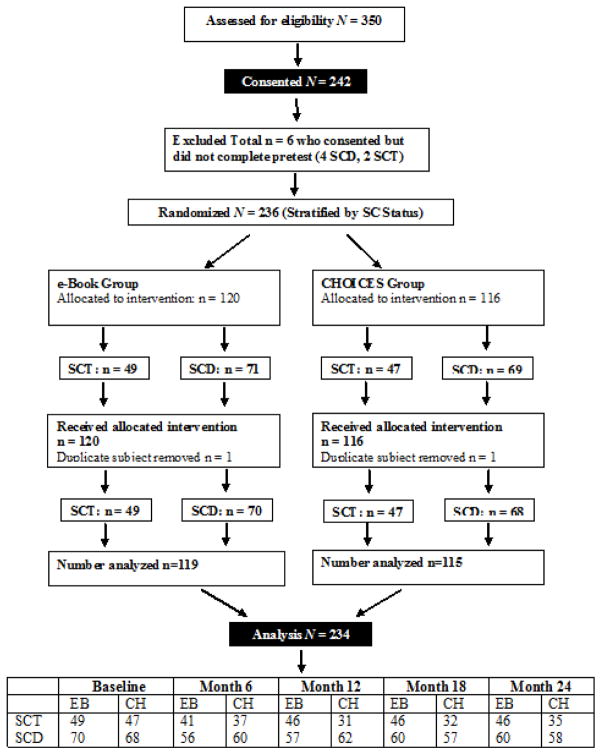
We recruited and obtained consents from 242 eligible participants, and 234 participants completed the baseline measures (see Consort Figure I). The 234 participants had either SCD (n=138) or SCT (n=96) and were 65% female and 94% African American. The CHOICES group mean age was 25.3 years (SD=4.9) and the e-Book group mean age was 26.4 years (SD=4.9); the difference was not statistically significant (p=.09). Other sample demographic characteristics appear in Table I and were not significantly different by CHOICES and e-Book groups.
Figure I.
CHOICES Study Consort Flowchart
Key: SC = sickle cell, SCD = sickle cell disease, SCT = sickle cell trait, EB = e-Book, CH = CHOICES
Table I.
Baseline Demographics for the Total Sample (N=234) and by Intervention and Control Groups with Group Comparisons
| Demographic variable | Category | Total (N = 234) | CHOICES (n = 115) | e-Book (n = 119) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N | % | N | % | N | % | p | ||
| Sex | Female | 152 | 65.0 | 71 | 61.7 | 81 | 68.1 | .38 |
| Male | 82 | 35.0 | 44 | 38.3 | 38 | 31.9 | ||
| Education | High school (grades 9–12) | 78 | 33.3 | 40 | 34.8 | 38 | 31.9 | .97 |
| Some college | 114 | 48.7 | 55 | 47.8 | 59 | 49.6 | ||
| 4-year college degree | 28 | 12.0 | 13 | 11.3 | 15 | 12.6 | ||
| Graduate degree | 14 | 6.0 | 7 | 6.1 | 7 | 5.9 | ||
| Employment | Part-time | 43 | 18.4 | 21 | 18.3 | 22 | 18.5 | .99 |
| Full-time | 47 | 20.0 | 23 | 20.0 | 24 | 20.2 | ||
| No, full-time student | 35 | 15.0 | 18 | 15.7 | 17 | 14.3 | ||
| Not employed | 109 | 46.6 | 53 | 46.1 | 56 | 47.1 | ||
| Income | Less than $10,000 | 89 | 38.0 | 43 | 37.4 | 46 | 38.7 | .26 |
| $10,000 to $29,999 | 64 | 27.4 | 31 | 27.0 | 33 | 27.7 | ||
| $30,000 to $49,999 | 39 | 16.7 | 18 | 15.7 | 21 | 17.6 | ||
| $50,000 or more | 37 | 15.8 | 18 | 15.7 | 19 | 16.0 | ||
| Unknown | 5 | 2.1 | 5 | 4.3 | 0 | 0.0 | ||
| Health Insurance | Employer or union | 46 | 19.7 | 23 | 20.0 | 23 | 19.3 | .98 |
| Medicaid/Medicare | 148 | 63.2 | 74 | 64.3 | 74 | 62.2 | ||
| Private insurance | 4 | 1.7 | 2 | 1.7 | 2 | 1.7 | ||
| No insurance | 29 | 12.4 | 13 | 11.3 | 16 | 13.4 | ||
| Unknown | 7 | 3.0 | 3 | 2.6 | 4 | 3.4 | ||
| Ethnicity | Hispanic or Latino | 10 | 4.3 | 6 | 5.2 | 4 | 3.4 | .53 |
| Not Hispanic or Latino | 224 | 95.7 | 109 | 94.8 | 115 | 96.6 | ||
| Racea | Black | 110 | 47.0 | 51 | 44.3 | 59 | 49.6 | .75 |
| African American | 110 | 47.0 | 56 | 48.7 | 54 | 45.4 | ||
| Multi-racial | 8 | 3.4 | 4 | 3.5 | 4 | 3.4 | ||
| Other | 6 | 2.6 | 4 | 3.5 | 2 | 1.7 | ||
| Sickle Cell Status | Sickle cell disease | 138 | 59.0 | 68 | 59.1 | 70 | 58.8 | .86 |
| Sickle cell trait | 96 | 41.0 | 47 | 40.9 | 49 | 41.2 | ||
| Marital Status | Never married | 193 | 82.5 | 96 | 83.5 | 97 | 81.5 | .77 |
| Married | 31 | 13.2 | 13 | 11.3 | 18 | 15.1 | ||
| Separated | 2 | 0.9 | 1 | 0.9 | 1 | 0.8 | ||
| Divorced | 8 | 3.4 | 5 | 4.3 | 3 | 2.5 | ||
Note.
self-reported race: the participants identified themselves as either Black or African American.
Procedures
Clinic staff referred parents of children with SCD or patients with SCD to well trained, experienced, and culturally competent research specialists (RS). Participants also self-referred through recruitment efforts online and in community settings and public events. RS verified eligibility of referred people, including coordinating the necessary laboratory screening to verify reported SCD or SCT status, if needed. RS obtained signed informed consents. Using a pen-tablet computer for all measures and intervention sessions, the participants completed baseline measures and afterwards received the assigned intervention, followed by the posttest. The participants received booster interventions tailored to their knowledge deficits after completing posttest measures at 6 and 12 months. The participants also completed posttest measures at 18 and 24 months. The RSs gave the participant $25 in cash for their time and travel expenses each time they completed the posttest measures and $50 for completing the 24-month measure ($150 total for the 24-month study).
Intervention
CHOICES
We published extensive validation of the CHOICES intervention for the study population (Gallo et al., 2010; Gallo et al., 2014; Wilkie et al., 2013). Our initial focus group study laid the foundation for the development of the SCKnowIQ measure and the CHOICES intervention by conducting item content validation with 14 older adults with SCD or SCT, and specified participants’ personal beliefs and attitudes about informed decisions and becoming a parent (Gallo et al., 2010). Using cognitive interviews with 20 young adults with SCD or SCT, we learned that the SCKnowIQ items were appropriate and the CHOICES intervention was understandable, balanced, and shared important information about SCD and SCT (Gallo et al., 2014). In our pre- and immediate post-intervention analysis, we found immediate posttest efficacy of CHOICES compared to the e-Book for increased knowledge (Wilkie et al., 2013).
We used Kolb’s Experiential Learning Theory (ELT, Kolb, Boyatzis, & Mainemelis, 2000), 4-stage theory that combines experience, perception, cognition, and behavior, to guide delivery of the CHOICES content, and the Theory of Reasoned Action (TRA, Ajzen & Fishbein, 1980) to guide the content related to reproductive knowledge, reproductive health intentions and reproductive health behaviors. Topics addressed include: Having a Child with SCT or SCD or Normal Hemoglobin, Getting Sickle Cell Disease and Trait, Testing for Sickle Cell Disease and Sickle Cell Trait, Living with Sickle Cell Disease, Talking about Sickle Cell Disease and Trait, Decision Making Options, and Talking about Your Wishes. Table II shows the elements of the CHOICES intervention. Table III shows the length of time participants required to complete CHOICES initially and at each of the two booster sessions. Booster session content was tailored to the CHOICES group’s reproductive health knowledge deficits after completing posttest measures at 6 and 12 months. Knowledge deficits were defined as the knowledge items incorrectly answered. After completing the measures at the 6- and 12-month sessions, the booster content was provided before the end of the session.
Table II.
Elements of the CHOICES and e-Book Interventions
| Intervention | Kolb ELT Component | Content |
|---|---|---|
| CHOICES | Concrete experience | Begins with a video of two young men talk about one man’s daughter born with SCD; the parents and other man are not aware of their sickle cell status. |
| Reflective observation | After viewing the video, participants entered their responses to three related questions about their ideas about SCD. | |
| Abstract conceptualization | Information about genetic risk of SCT and SC, and detailed information about reproductive options for people with SCD or SCT. | |
| Active experimentation | In a selection of videos, couples talk over their decisions made based on their SC status. Participants choose the video that best characterized their situation and decision. At the end of the program, the computer program generates a parenting plan that recaps the participant’s responses. The participant then indicates if the parenting plan is accurate or inaccurate. The participant receives a copy of the parenting plan. | |
| 57 web pages are presented 14 video clips of couples sharing their choices of reproductive options. 17 graphical animations that display, for instance, SCD genetic inheritance and the risks, and a variety of reproductive options. |
||
| e-Book | 9 web pages are presented The content includes information about SCD and SCT that staff from the Chicago-area sickle cell program usually share with parents, patients, and the community using graphics and photographs. |
|
| Both CHOICES and e-Book | The participant either selected the male or female voice text narration or opts for no narration. 8th grade reading level. |
Table III.
Minutes Needed to Complete Tests and Interventions over the five Data Collection Points
| CHOICES (n=115) | e-Book (n=119) | ||
|---|---|---|---|
|
| |||
| Mdn (Median absolute deviation) | Mdn (Median absolute deviation) | ||
| Visit 1 | Baseline | 26.7 (10.8) | 27.8 (12.1) |
| Intervention | 71.8 (28.7) | 8.6 (2.8) | |
| Posttest | 19.9 (8.3) | 20.8 (9.0) | |
| Visit 2 | Test | 26.0 (11.5) | 25.0 (12.7) |
| Booster | 7.9 (7.9) | 7.6 (2.8) | |
| Visit 3 | Test | 24.7 (11.4) | 23.5 (13.2) |
| Booster | 7.0 (7.0) | 7.3 (3.6) | |
| Visit 4 | Test | 23.3 (10.3) | 23.2 (11.8) |
| Visit 5 | Test | 23.4 (9.8) | 21.6 (11.8) |
Attention Control Usual Care
Cognitive interview methods validated the e-Book’s cultural appropriateness and literacy level for the target audience (Gallo et al., 2010; Gallo et al., 2014). Table II shows the elements of the e-Book intervention, which was intended as an attention control condition. Table III shows the length of time participants required to complete the e-Book initially and at each of the two booster sessions. When there were knowledge deficits in the e-Book group at 6 and 12 months, the participants received the entire e-Book content again.
Instrumentation
Guided by the TRA, we created the SCKnowIQ measure (Gallo et al., 2014; Gallo et al., 2010; Wilkie et al., 2013), by selecting or modifying items from other existing tools (Kaslow et al., 2000; Koontz, Short, Kalinyak, & Noll, 2004; Rosengard, Phipps, Adler, & Ellis, 2004, 2005) or by creating items or scales to measure the three study outcomes in Table IV and demographic characteristics. The Sickle Cell Reproductive Knowledge outcome includes sickle cell inheritance, etiology and risks; the Reproductive Health Intentions outcome includes intentions to implement a parenting plan; and the Reproductive Health Behavior outcome relates to implementing the parenting plan. For each subscale, response options vary: the knowledge subscale had multiple choice options with a single correct option; 0-to-5 Likert-type scales regarding how important, likely, influenced, happy, or concerned; 0-to-4 agree/disagree scale; or other item-specific options. The average subject required 27 minutes to complete the SCKnowIQ at pretest and 20 minutes at immediate posttest. More detailed statistics of completion times by group appear in Table III. Validity and reliability of the SCKnowIQ are adequate for a new instrument (Gallo et al., 2010; Wilkie et al., 2013).
Table IV.
Study Outcome Measures Derived from the SCKnowIQ Measure
| Outcome Measure | Synopsis |
|---|---|
| Knowledge | 18 items on SCD and SCT genetic inheritance (4 hypothetical items; 3 participant-specific items), SCD etiology and risks, and parenting options for people with SCD or SCT. Multiple choice response options with one correct answer scored as 0 = not correct or 1= correct and summed to create a total knowledge score that ranges from 0 to 18. The Cronbach’s alpha in this sample ranged from .70 to .71. Test-retest reliability in the e-Book group was .66. |
| Reproductive Health Intention | 8 items on intention to avoid having children with SCD or SCT, to have a child without SCD or affected by SCD, to abort a pregnancy due to health concern or to prevent SCD or SCT, to use a variety of advanced reproductive technologies, or to seek other non-childbearing options (e.g., foster, adopt). Response options ranged from 0 (not at all likely) to 4 (extremely likely); the total intention score ranges from 0 to 32. The Cronbach’s alphas in this sample ranged from .60 to .69. |
| Reproductive Behavior | 10 items on behaviors engaged in ever/during the past 6 months (baseline) or during the past 6 months (6, 12, 18, 24 mo posttest) to implement the parenting plan. Behaviors include frequency using birth control, talking with partner, prenatal testing, adopting/fostering a child, seeking other options such as advanced reproductive technologies, and agreeing or disagreeing that the individual is doing all things to help avoid having a child with SCD or SCT. Response options were descriptive for each item, but coded based on consistency with the parenting plan. Codes were binary for nine items (0 inconsistent with parenting plan or 1 consistent with parenting plan) and tertiary for one item that was coded as 0 inconsistent, 0.5 somewhat consistent, 1 consistent with the parenting plan. Total behavior outcome score ranges from 0 to 10. The Cronbach’s alphas in this sample ranged from .62 to .67. |
Data Analysis
We generated descriptive statistics for demographics of the study participants as well as their scores in knowledge, intention, and behavior scales at baseline and at the last posttest (month 24). We compared demographic characteristics of the two study groups using chi-square or Fisher’s exact test. When computing descriptive statistics for scores, we used multiple imputations to impute the missing entries. For regression analysis, we used maximum likelihood estimation. Since the amount of missing data was relatively small (11%), we expect these two approaches to work well even if some data were not missing at random (Collins, Schafer, & Kam, 2001). We did not include block in the analysis because all the participants were recruited within 10 months and the intrablock correlations for the knowledge, intention, and behavior outcomes are all close to 0, indicating there would be little to gain from using a blocked analysis. For longitudinal analysis of knowledge, intention, and behavior scores, we utilized a two-level linear growth curve model with random effect terms accounting for between participant differences. For exploratory analysis, we used logistic regression to predict partner status at the last study visit. Statistical significance was set at a two-sided alpha level of .05. We performed all statistical analyses using the statistical software package R (Team, 2011).
Results
Table V shows the descriptive statistics for the knowledge, intention, and behavior outcomes at baseline and final posttest by e-Book and CHOICES groups. Inferential analyses appear in Table VI. Tables VII and VIII show exploratory analyses.
Table V.
Mean and Standard Deviation for the Mean Score by Intervention Group and Time for Knowledge, Intention, and Behavior Outcomes (N = 234)
| Group | Time | Outcomes | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Knowledge | Intention | Behavior | |||||
|
| |||||||
| M | SD | M | SD | M | SD | ||
| e-Book | Baseline | 9.6 | 3.1 | 5.9 | 4.3 | 5.1 | 2.0 |
| Visit 5 | 11.1 | 3.1 | 6.8 | 4.7 | 5.1 | 1.8 | |
| CHOICES | Baseline | 9.4 | 3.1 | 5.6 | 4.6 | 4.8 | 1.9 |
| Visit 5 | 12.0 | 3.0 | 6.8 | 4.8 | 4.8 | 1.5 | |
Table VI.
Regression Effects of the Change in Outcome Variables over Time by Intervention Group and Sickle Cell Status (N = 234)
| Variable and Effect | Estimate | SE | Z | P |
|---|---|---|---|---|
| Knowledge Scores | ||||
| Time | 0.34 | 0.06 | 5.60 | <.001 |
| Group (reference = e-Book) | −0.30 | 0.38 | −0.80 | .43 |
| Group x Time | 0.25 | 0.09 | 2.88 | .004 |
| Intention Scores | ||||
| Time | 0.14 | 0.12 | 1.21 | .23 |
| Group (reference = e-Book) | −0.53 | 0.55 | −0.97 | .34 |
| Group x Time | 0.23 | 0.17 | 1.34 | .18 |
| Reproductive Health Behaviors | ||||
| Time | −0.02 | 0.05 | −0.37 | .72 |
| Group (reference = e-Book) | −0.56 | 0.27 | −2.10 | .04 |
| Group x Time | 0.03 | 0.07 | 0.40 | .69 |
Note. intervention group reference is e-Book; SC = sickle cell; SC status reference is sickle cell trait
Table VII.
Predictors of Partner Status at the Last Study Visit
| Predictor | Estimate | SE | z | p | |
|---|---|---|---|---|---|
| Status at first visit (ref=at risk) | −1.498 | 0.426 | −3.512 | <.001 | |
| Group (ref=e-Book) | At risk at first visit | −0.915 | 0.444 | −2.060 | 0.039 |
| Not at risk at first visit | 0.098 | 0.445 | 0.221 | 0.825 |
Table VIII.
Frequency of Partner Status at Baseline and Last Study Visits by Study Groups†
| Partner Status at: | e-Book (n=111) | CHOICES (n=104) | |
|---|---|---|---|
|
| |||
| Baseline | Last Visit | f (%) | f (%) |
| Normal | Normal | 42 (74%) | 33 (72%) |
| No partner | 6 (11%) | 10 (22%) | |
| At risk | 9 (16%) | 3 (7%) | |
| No partner | Normal | 5 (18%) | 7 (26%) |
| No partner | 20 (71%) | 17 (63%) | |
| At risk | 3 (11%) | 3 (11%) | |
| At risk | Normal | 5 (19%) | 14 (45%) |
| No partner | 7 (27%) | 5 (16%) | |
| At risk | 14 (54%) | 12 (39%) | |
Note:
not all last visits were at 24 months
Normal = partner reported as having normal hemoglobin AA
At risk = partner reported as having sickle cell trait or sickle cell disease
Knowledge Outcome
There was no significant difference between groups at baseline. At 24 months, both e-Book and CHOICES had higher knowledge scores than at baseline (Table V). The coefficient estimates for effects of intervention group, time, and their interaction on the knowledge scores appear in Table VI. Participants receiving the CHOICES intervention had a significantly higher knowledge improvement rate over time (baseline, 6, 12, 18, and 24 months) than the e-Book group (p=.004), but e-Book participants did show significant improvement over time (p<.001).
Reproductive Health Intention Outcome
Table V shows that both e-Book and CHOICES participants had higher intention scores at final posttest than at baseline and that the two groups had the same average score at final posttest. Regression outcomes in Table VI show that there was no significant group difference on intention scores at baseline and that the intervention effect is not significant on the intention scores between groups over time.
Reproductive Health Behavior Outcome
There was a statistically significant group difference at baseline with e-Book subjects having a higher average behavior score. From Table V, we observed that the average behavior scores of both e-Book and CHOICES at the final posttest were practically the same as those at baseline, with e-Book scoring slightly higher at both occasions. Regression analysis presented in Table VI confirmed that there was no significant improvement over time and that the intervention effect was not significant.
Exploratory Analysis
An ultimate behavior outcome for a reproductive health study reasonably could focus on pregnancy or birth-related outcomes. Excluding pregnancies that occurred before study enrollment, 18 children were born during the study period: 5 with normal hemoglobin, 11 with SCT, 1 with SCD, and 1 with unknown SC status. Additionally, 14 participants reported pregnancy at their last visit, 7 of whom communicated the SC status of their babies after the study ended (4 babies had SCT, 3 babies had normal hemoglobin) and 7 of whom did not communicate (SC status is unknown).
For a population at risk for genetic inheritance of disease like SCD, another behavior outcome for an individual could focus on partner change over the study period that reduced risk of a child inheriting the disease (Table VII). At baseline, 114 (48.7%) participants, who reported that their partners had normal hemoglobin, were not at risk of their children inheriting SCD, whereas 116 (49.6%) participants had a partner with SCD or SCT or did not have a current partner (but a future partner could have SCD or SCT) and therefore were at risk of their children inheriting SCD. A higher proportion of those at risk who were in the CHOICES group chose partners that reduced their risk by the last visit than the e-Book group (p=.04) (Table VII). Based on the partner status at baseline, the partner status at the last study visit for the e-Book and CHOICES groups appears in Table VIII and suggests the CHOICES group changed to partners that reduced their risk for their children inheriting SCD.
In an analysis that was respectful of individual autonomy, 221 participants reported that it was important for their child to be SCD free and 8 participants (5 with SCD and 3 with SCT) reported that it was “not at all important” for their child to be SCD free. In exploratory analysis, we focused on participants satisfying the following criteria at baseline: 1) had no partner, or a partner with SCD, SCT, or unknown partner SC status; 2) did not select “Not at all important” when asked whether it is important for their children to be SCD free; 3) belong to the 21–29 years of age group at baseline, the ages with the highest pregnancy rate, and analyzed the percentage of time they were at risk based on their answers to our questionnaire in subsequent visits. We defined a participant as at risk at a given visit if their behavior at the time could lead to conceiving a baby with SCD (e.g., a participant’s partner had SCD or SCT and they were not using birth control). We identified 70 participants (37 in CHOICES and 33 in e-Book groups) satisfying the above criteria. On average across the 24-month study with data collection every 6 months, participants in the CHOICES group were at risk 20% (SD=0.28) of the time and participants in the e-Book group were at risk 26% (SD=0.34) of the time; the 6% difference was not statistically significant (p=.44).
Discussion
This study is the first to test longitudinally the effects of a Web-based intervention focused on improving knowledge, intention, and behavior related to reproductive health in young adults with SCD or SCT. As hypothesized, findings showed that the previously reported significant gain in knowledge immediate posttest (Wilkie et al., 2013) was retained over 24 months and was significantly larger for the intervention group (CHOICES) than the attention control group (e-Book). Contrary to our hypotheses, controlling for baseline scores the intervention group effect was not significant for intention and behavior scores. However, the exploratory analyses showed that nearly half of the participants reported having a partner who did not put them at risk for having a child with SCD. In subgroup analysis, the participants who were at risk of and wanted to avoid having a child with SCD at baseline were more likely to lower their risk over time if they were in the CHOICES rather than the e-Book groups. People in the CHOICES group lowered their risk by partnering with people with normal hemoglobin, which was one of the behaviors suggested in the CHOICES intervention.
A major study finding was the statistically significant knowledge gain for both groups that was sustained for 24 months but showing the CHOICES intervention was more effective than the e-Book usual care intervention. Knowledge of SCD inheritance at the general and individual risk level is necessary but not sufficient for informed decision making to develop a reproductive health (risk) plan and implement actions consistent with the plan. This set of complex behaviors is especially important when a person’s genotype conveys risk of disease to the next generation, such as SCD. Other researchers provided a context for the complexity of reproductive health (Acharya et al., 2009; Asgharian & Anie, 2003; Siddiqui et al., 2012), family planning (Okunlola, Olutayo, Okonkwo, & Akingbola, 2010), and pregnancy termination (Wonkam & Hurst, 2014) issues among people with SCD or SCT, and others have implemented educational programs about SCD transmission (Alswaidi et al., 2012; Serjeant, 2010) and screening of parents (Brown, Dormandy, Reid, Gulliford, & Marteau, 2011). The necessity of improving knowledge was a common thread in the scientific research base related to reproductive health for people with SCD or SCT, which supports the importance of our findings that the online e-Book education improved knowledge of SCD and SCT and that online, multimedia CHOICES education was superior to the e-Book education among a sample of predominately African American young adults with SCD or SCT.
Other researchers used different approaches to education about inheritance of SCD and SCT. Serjeant (2010) described a school-based program being implemented in Jamaica that is targeted at 16–18 year old adolescents and involves genetic testing and provision of a laminated risk card and genetic counseling for carriers of the SCT; we were unable to locate published outcomes. Alswaidi et al. (2012) found minimal effects of compulsory premarital testing and counseling that resulted in 88% of the incompatible Saudi Arabian couples continuing with marriage, but no findings were reported on births among affected persons in the sample. CHOICES is a Web-based application that has potential for adaptation and efficient use in other countries with policies different than those in the U.S.
Our longitudinal study is the first to use the TRA to investigate reproductive health behaviors over time in young adults with SCD or SCT. TRA is a common theoretical framework for research among other populations focused on reproductive-related health behaviors (Baker, Morrison, Carter, & Verdon, 1996; Doswell, Braxter, Cha, & Kim, 2011; Koniak-Griffin, Lesser, Uman, & Nyamathi, 2003), reproductive decision-making (Koniak-Griffin, Lesser, Nyamathi, et al., 2003; Koniak-Griffin & Stein, 2006; Pivetti & Melotti, 2012; Wesley et al., 2000), or diabetes (Wang, Charron-Prochownik, Sereika, Siminerio, & Kim, 2006).
TRA concepts are relevant to three issues that Hershberger et al. (June 15 2015, Epub ahead of print) identified in this sample: (1) strong value and inclination for having one’s own biological child, (2) religious beliefs related to abortion and advanced reproductive technologies, and (3) sexual orientation perspectives. Hershberger et al. also noted that individuals with SCD often lead meaningful lives and contribute significantly to their families, communities, and our society generally and specifically. It is important that interventions such as CHOICES portray a balanced view as information about reproductive options is presented to assure each person sees their value as a human being. We were mindful of these issues when we created CHOICES, but we recommend re-review of the content with this added sensitivity before the intervention is evaluated in another study.
Reasons for lack of CHOICES and e-Book group differences for the intention and behavior outcomes are unknown. One glaring possibility is that at baseline, 49% of our participants were not at risk of having a child with SCD, and consequently they did not change their intentions or behaviors because they knew that they did not need to take action to avoid having a child with SCD. This finding has implications for the sample size and eligibility criteria for future studies of reproductive health interventions for people with genetically inherited diseases. Investigators will need to screen for participants at risk not only by virtue of their own genotype but also their partner’s genotype and with consideration of the likelihood of partner changes during the study period.
Another possible reason for similar intention and behavior scores for both intervention groups is that the SCKnowIQ measure may lack sufficient sensitivity to the intervention effects. Or it is possible that measurement of intention and behaviors helped the control group to change their intention and behaviors (e.g., a learning effect from completing the study instruments). This learning effect may have been enhanced further by the effect of the attention control intervention in that it increased knowledge, intention and behavior. For ethical reasons, we thought it was important to provide the control group with the basic information about SCD and SCT and its inheritance that comprehensive sickle cell program personnel typically communicate to their patients with SCD. However, people with SCT do not receive usual care from sickle cell program personnel, which means that the e-Book would not be an appropriate attention control intervention for them. As reported elsewhere (Hershberger et al., June 15 2015, Epub ahead of print), evidence from qualitative interviews with e-Book participants at the end of the study indicated that the study assisted them to think and feel differently and expanded their thinking about reproduction. For example, a female e-Book participant with SCT who did not have a current partner said, “… my sickle cell trait had always been in the back of my mind, but this study has just made me a little more conscious of talking to partners before I get serious with them just to kind of figure out, ‘Do you have sickle cell trait or disease?’ [and] ‘What options would you consider if we were to have children?’”. Therefore, although we were cautious about overlap in CHOICES and e-Book content, the little information about SCD inheritance may have been sufficient when combined with the possible instrumentation effects to overshadow the effects of the CHOICES intervention on changing intention and behaviors.
This possibility of results being influenced by study design issues is supported further by the subgroup analysis that those participants who were at risk of having a child with SCD at the beginning of the study were more likely to change to partners who lowered their risk, which was content in the CHOICES intervention but not the e-Book. There was no specific question about changing partners to lower risk. Instead, that variable was derived from several questions including demographic items. These findings have important implications for the study design and selection of the comparison group and outcomes for future studies.
Research Recommendations
Based on a careful review of our study findings, for a future study of the CHOICES intervention, we recommend (1) selection of research designs that will allow evaluation of the instrumentation effects, (2) use of health promotion education as an attention control condition, (3) recruitment of young adults with SCD or SCT who are between 18 and 35 years old without a partner or whose current partner has either SCD or SCT, and (4) selection of risk-reduction behavioral outcomes that take into consideration the participant’s values. We expect that major sites for future recruitment would be colleges and universities and clinics caring for patients with SCD who are transitioning to adult care.
Our exploratory findings indicate that generally the participants’ risk status at baseline was the same at the last study visit for those with no partner or whose partner did not put them at risk for having a child with SCD regardless of their intervention group (CHOICES or e-Book). The exception was the CHOICES participants who were at risk at baseline who changed partners to reduce their risk for having a child with SCD at the last visit. Slightly more than half of the e-Book participants who were at risk at baseline remained at risk at their last visit. Considering their partner at risk status every 6 months, 20% of the time the CHOICES group was at risk of having a child with SCD compared to 26% of the time for the e-Book group, a small but important difference that was not statistically significant in the small subsample. This information is important for projecting the sample size needed for an adequately powered national trial of the CHOICES intervention compared to a health promotion control condition.
Practice Implications
If the intervention shows efficacy in a future trial of people at risk for having a child with SCD, CHOICES can be offered to people with SCD or SCT to increase their knowledge and assist in making informed decisions based on a computer-delivered, interactive program that educates about SCD, its inheritance and reproduction options. CHOICES with its computer-tailored and engaging experience focus can reach a larger population at convenient times and can contribute to greater user satisfaction than many other education approaches.
A clinical implication of our study could be clinicians using parts or all of the intervention in face-to-face encounters with people with SCD or SCT, providing a kiosk in hematology, medical, and gynecological clinics so that individuals have access to the educational program, or promoting the Web address for individuals to access the program from home or another location. Including CHOICES in college and high school health education programs might provide broad access to individuals with SCT.
Study Limitations
A few limitations detract from the study findings. Conducted in one U.S. geographical location, there is a possibility, in locations where cultural norms are different, that the effects of the intervention will be different. Although SCD and hemoglobinopathies occur in other ethnic populations, it is unknown if the intervention effects would apply to them since predominantly individuals of African descent participated in this study. It is unknown if learning was influenced by participants with SCD who may have had cognitive impairments since cognitive ability was not an eligibility criterion.
Conclusion
In conclusion, these findings are the first to show efficacy for an intervention over two years to help young adults with SCD or SCT to be informed as they make decisions about becoming parents. In general, the participants reported that they wanted to avoid having a child with SCD. To do so, those at risk need to implement reproductive behavior choices that are consistent with their decisions for becoming parents. Compared to the e-Book attention control group, our CHOICES intervention provided information about such options and did so in a manner that was not only acceptable to the participants, but also was effective in significantly increasing and sustaining their knowledge. There were, however, no significant differences between the intervention groups for reproductive health intentions and behaviors. For the 51% of the participants at risk for and wanting to avoid having a child with SCD at baseline, change of partners to reduce the risk was more prevalent in the CHOICES group than the e-Book group. Of the 25 births, one child with SCD was born during the study period and the status of 7 pregnancies unknown at the last contact with the participant. Study findings provide important insights for planning a national trial of the CHOICES intervention.
Acknowledgments
The research and this publication were made possible by Grant Numbers U54HL090513 and R01HL114404 from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NHLBI. The final peer-reviewed manuscript is subject to the National Institutes of Health Public Access Policy. We extend special thanks to the Lay Advisory Board members who guided the development of the CHOICES intervention and all the study participants who showed extraordinary commitment to increasing knowledge about sickle cell conditions.
Drs. Wilkie and Molokie are co-investigators on an unrelated grant funded by Pfizer. Dr. Wilkie is Chairman and Founder of eNURSING llc. All other authors declare no conflicts.
‘All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.’
‘No animal studies were carried out by the authors for this article’
Footnotes
Compliance with Ethical Standards
Drs. Wilkie and Molokie are co-investigators on an unrelated grant funded by Pfizer. Dr. Wilkie is Chairman and Founder of eNURSING llc. All other authors declare no conflicts. “All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.”
References
- Acharya K, Lang CW, Ross LF. A pilot study to explore knowledge, attitudes, and beliefs about sickle cell trait and disease. Journal of the National Medical Association. 2009;101(11):1163–1172. doi: 10.1016/s0027-9684(15)31113-5. [DOI] [PubMed] [Google Scholar]
- Ajzen I, Fishbein M. Predicting behavior from intentions. In: Ajzen I, editor. Understanding attitudes and predicting social behavior. Englewood Cliffs, N.J: Prentice-Hall, Inc; 1980. pp. 46–52. [Google Scholar]
- Alayed N, Kezouh A, Oddy L, Abenhaim HA. Sickle cell disease and pregnancy outcomes: Population-based study on 8.8 million births. Journal of Perinatal Medicine. 2014;42(4):487–492. doi: 10.1515/jpm-2013-0275. [DOI] [PubMed] [Google Scholar]
- Alswaidi FM, Memish ZA, O’Brien SJ, Al-Hamdan NA, Al-Enzy FM, Alhayani OA, Al-Wadey AM. At-risk marriages after compulsory premarital testing and counseling for beta-thalassemia and sickle cell disease in Saudi Arabia, 2005–2006. Journal of Genetic Counseling. 2012;21(2):243–255. doi: 10.1007/s10897-011-9395-4. [DOI] [PubMed] [Google Scholar]
- Asgharian A, Anie KA. Women with sickle cell trait: Reproductive decision-making. Journal of Reproductive and Infant Psychology. 2003;21(1):23–34. [Google Scholar]
- Baker SA, Morrison DM, Carter WB, Verdon MS. Using the theory of reasoned action (TRA) to understand the decision to use condoms in an STD clinic population. Health Education Quarterly. 1996;23(4):528–542. doi: 10.1177/109019819602300411. [DOI] [PubMed] [Google Scholar]
- Brown K, Dormandy E, Reid E, Gulliford M, Marteau T. Impact on informed choice of offering antenatal sickle cell and thalassaemia screening in primary care: A randomized trial. Journal of Medical Screening. 2011;18(2):65–75. doi: 10.1258/jms.2011.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojodu J, Mulihan MM, Pope SN, Grant AM. Incidence of sickle cell trait-United States, 2010. Centers for Disease Control and Prevention-Morbidity and Mortality Weekly Report (MMWR) 2014;63(49):1155–1158. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities, Division of Blood Disorders. Sickle cell trait toolkit. 2015 Retrieved from http://www.cdc.gov/ncbddd/traits.html.
- Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrctive strategies in modern missing data procedures. Psychological Methods. 2001;6(4):330–351. [PubMed] [Google Scholar]
- DeBaun MR. Hydroxyurea therapy contributes to infertility in adult men with sickle cell disease: A review. Expert Review of Hematology. 2014a;7(6):767–773. doi: 10.1586/17474086.2014.959922. [DOI] [PubMed] [Google Scholar]
- DeBaun MR. Perspectives: Thinking beyond survival. Nature. 2014b;515:S16. doi: 10.1038/515S16a. [DOI] [PubMed] [Google Scholar]
- Doswell WM, Braxter BJ, Cha E, Kim KH. Testing the theory of reasoned action in explaining sexual behavior among African American young teen girls. Journal of Pediatric Nursing. 2011;26(6):e45–54. doi: 10.1016/j.pedn.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Gallo AM, Knafl KA, Angst DB. Information management in families who have a child with a genetic condition. Journal f Pediatric Nursing. 2009;24(3):194–204. doi: 10.1016/j.pedn.2008.07.010. S0882-5963(08)00299-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo AM, Wilkie D, Suarez M, Labotka R, Molokie R, Thompson A, Johnson B. Reproductive decisions in people with sickle cell disease or sickle cell trait. Western Journal of Nursing Research. 2010;32(8):1073–1090. doi: 10.1177/0193945910371482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo AM, Wilkie DJ, Labotka RJ, Molokie RE, Stahl C, Hershberger PE, Thompson A. Evaluation of the SCKnowIQ tool and reproductive CHOICES intervention among young adults with sickle cell disease or sickle cell trait. Clinical Nursing Research. 2014;23(4):421–441. doi: 10.1177/1054773813479377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999–2009) Pediatric Blood & Cancer. 2013;60:1482–1486. doi: 10.1002/pbc.24557. [DOI] [PubMed] [Google Scholar]
- Hershberger PE, Gallo AM, Molokie R, Thompson AA, Suarez ML, Yao Y, Wilkie DJ. Perception of young adults with sickle cell disease or sickle cell trait about participation in the CHOICES randomized controlled trial. Journal of Advanced Nursing. 2015 Jun 15; doi: 10.1111/jan.12702. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SA. Motherhood and the obfuscation of medical knowledge: The case of sickle cell disease. Gender and Society. 1994;8:29–47. [Google Scholar]
- Kaslow NJ, Collins MH, Rashid FL, Baskin ML, Griffith JR, Hollins L, Eckman JE. The efficacy of a pilot family psychoeducational Intervention for pediatric sickle cell disease (SCD) Families, Systems & Health. 2000;18(4):381–404. [Google Scholar]
- Kolb DA, Boyatzis RE, Mainemelis C. Experiential learning theory: Previous research and new directions. In: Sternberg RJ, Zhang LF, editors. Perspectives on cognitive, learning, and thinking styles. Mahwah, N. J: Lawrence Erlbaum; 2000. pp. 227–248. [Google Scholar]
- Koniak-Griffin D, Lesser J, Nyamathi A, Uman G, Stein JA, Cumberland WG. Project CHARM: An HIV prevention program for adolescent mothers. Family & Community Health. 2003;26(2):94–107. doi: 10.1097/00003727-200304000-00003. [DOI] [PubMed] [Google Scholar]
- Koniak-Griffin D, Lesser J, Uman G, Nyamathi A. Teen pregnancy, motherhood, and unprotected sexual activity. Research in Nursing & Health. 2003;26(1):4–19. doi: 10.1002/nur.10062. [DOI] [PubMed] [Google Scholar]
- Koniak-Griffin D, Stein JA. Predictors of sexual risk behaviors among adolescent mothers in a human immunodeficiency virus prevention program. Journal of Adolescent Health. 2006;38(3):297 e291–211. doi: 10.1016/j.jadohealth.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Koontz K, Short AD, Kalinyak K, Noll R. A randomized, controlled pilot trial of a school intervention for children with sickle cell anemia. Journal of Pediatric Psychology. 2004;29(1):7–17. doi: 10.1093/jpepsy/jsh002. [DOI] [PubMed] [Google Scholar]
- Long KA, Thomas SB, Grubs RE, Gettig EA, Krishnamurti L. Attitudes and beliefs of African-Americans toward genetics, genetic testing, and sickle cell disease education and awareness. Journal of Genetic Counseling. 2011;20(6):572–592. doi: 10.1007/s10897-011-9388-3. [DOI] [PubMed] [Google Scholar]
- Matts JP, Lachin JM. Properties of permuted-block randomization in clinical trials. Controlled Clinical Trials. 1988;9(4):327–344. doi: 10.1016/0197-2456(88)90047-5. [DOI] [PubMed] [Google Scholar]
- McClish DK, Penberthy LT, Bovbjerg VE, Roberts JD, Aisiku IP, Levenson JL, Roseff SD. Health related quality of life in sickle cell patients: The PiSCES project. Health and Quality of Life Outcomes. 2005:3. doi: 10.1186/1477-7525-3-50. Retrieved from http://www.hqlo.com/content/3/1/50ally. [DOI] [PMC free article] [PubMed]
- Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bulletin of the World Health Organization. 2008;86(6):480–487. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojodu J, Hulihan MM, Pope SN, Grant AM. Incidence of sickle cell trait-United States, 2010. CDC, Morbidity and Mortality Weekly Report (MMWR) 2014;63(49):1155–1158. [PMC free article] [PubMed] [Google Scholar]
- Okunlola MA, Olutayo AA, Okonkwo NS, Akingbola TS. Pattern of contraceptive use among women with sickle cell disease in Ibadan, South-west Nigeria. Journal of Obstetrics & Gynaecology. 2010;30(2):171–174. doi: 10.3109/01443610903452799. [DOI] [PubMed] [Google Scholar]
- Pivetti M, Melotti G. Prenatal genetic testing: An investigation of determining factors affecting the decision-making process. Journal of Genetic Counseling. 2013;22(1):76–89. doi: 10.1007/s10897-012-9498-6. [DOI] [PubMed] [Google Scholar]
- Pleasants S. Sickle cell disease epidemiology: A moving target. Nature. 2014;(515):S2–3. doi: 10.1038/515S2a. [DOI] [PubMed] [Google Scholar]
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: A 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84(6):363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- Quinn CT, Rogers ZR, Buchanan GR. Survival of children with sickle cell disease. Blood. 2004;103(11):4023–4027. doi: 10.1182/blood-2003-11-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengard C, Phipps MG, Adler ME, Ellis JM. Adolescent pregnancy intentions and pregnancy outcomes: A longitudinal examination. Journal of Adolescent Health. 2004;35:453–461. doi: 10.1016/j.jadohealth.2004.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengard C, Phipps MG, Adler ME, Ellis JM. Psychosocial correlates of adolescent males’ pregnancy intentions. Pediatrics. 2005;116(3):414–418. doi: 10.1542/peds.2005-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serjeant GR. One hundred years of sickle cell disease. British Journal of Haematology. 2010;151(5):425–429. doi: 10.1111/j.1365-2141.2010.08419.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui S, Schunk K, Batista M, Adames F, Ayala P, Stix B, Green NS. Awareness of sickle cell among people of reproductive age: Dominicans and African Americans in northern Manhattan. Journal of Urban Health. 2012;89(1):53–58. doi: 10.1007/s11524-011-9618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Whitley K. Reproductive issues in SCD. Blood. 2014;124(24):3538–3543. doi: 10.1182/blood-2014-07-577619. [DOI] [PubMed] [Google Scholar]
- Taylor C, Kavanagh P, Zuckerman B. Sickle cell trait--neglected opportunities in the era of genomic medicine. Journal of the American Medical Association. 2014;311(15):1495–1496. doi: 10.1001/jama.2014.2157. [DOI] [PubMed] [Google Scholar]
- Team, R. D. C. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. Retrieved from http://www.R-project.org. [Google Scholar]
- Wang SL, Charron-Prochownik D, Sereika SM, Siminerio L, Kim Y. Comparing three theories in predicting reproductive health behavioral intention in adolescent women with diabetes. Pediatric Diabetes. 2006;7(2):108–115. doi: 10.1111/j.1399-543X.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- Wesley Y, Smeltzer SC, Redeker NS, Walker S, Palumbo P, Whipple B. Reproductive decision making in mothers with HIV-1. Health Care for Women International. 2000;21(4):291–304. doi: 10.1080/073993300245159. [DOI] [PubMed] [Google Scholar]
- Wilkie DJ, Gallo AM, Yao Y, Molokie RE, Stahl C, Hershberger PE, Thompson AA. Reproductive health choices for young adults with sickle cell disease or trait: Randomized controlled trial immediate posttest effects. Nursing Research. 2013;62(5):352–361. doi: 10.1097/NNR.0b013e3182a0316b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonkam A, Hurst S. A call for policy action in sub-Saharan Africa to rethink diagnostics for pregnancy affected by sickle cell disease: Differential views of medical doctors, parents and adult patients predict value conflicts in Cameroon. Omics. 2014;18(7):472–480. doi: 10.1089/omi.2013.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, John-Sowah J. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. Journal of the American Medical Association. 2014;312(10):1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- Yusuf HR, Lloyd-Puryear MA, Grant AM, Parker CS, Creary MS, Atrash HK. Sickle cell disease: The need for a public health agenda. American Journal of Preventive Medicine. 2011;41(6 Suppl 4):S376–383. doi: 10.1016/j.amepre.2011.09.007. [DOI] [PubMed] [Google Scholar]