Abstract
Aims
Food insecurity is the ‘limited or uncertain availability of nutritionally adequate and safe foods’. Our objective was to examine the association between food insecurity, diabetes self-care and glycaemic control.
Methods
We conducted a cross-sectional analysis of baseline data from adult patients with Type 2 diabetes who were enrolled in a randomized trial evaluating a health literacy-focused diabetes intervention in safety net primary care clinics in middle Tennessee. Food insecurity was assessed with three items from the U.S. Household Food Security Survey. Diabetes self-care behaviours were assessed with the Summary of Diabetes Self-Care Activities Scale, Personal Diabetes Questionnaire and Adherence to Refills and Medication Scale. Glycaemic control was assessed with HbA1c.
Results
The sample consisted of 401 participants, 73% of whom reported some level of food insecurity. Food insecurity was significantly associated with self-care behaviours including less adherence to a general diet (AOR 0.9, P = 0.02), less physical activity (AOR 0.9, P = 0.04) and with a greater occurrence of medication non-adherence (AOR 1.2, P = 0.002) and calorie restriction (AOR 1.1, P = 0.02). Food insecurity was also associated with worse glycaemic control (adjusted β = 0.1, P = 0.03). None of the self-care behaviours were significantly associated with HbA1c, limiting the ability to test for self-care as a mechanism linking food insecurity to glycaemic control.
Conclusions
There was a high rate of food insecurity in a sample of patients with Type 2 diabetes who were of low socio-economic status. Food insecurity was associated with less adherence to recommended self-care behaviours and worse glycaemic control.
Introduction
The prevalence of Type 2 diabetes remains high among adults in the USA at nearly 10% [1]. Moreover, both having low socio-economic status and being a member of a racial/ethnic minority group have been associated with adverse outcomes from diabetes [2–5]. Therefore, developing novel strategies to ameliorate the significant morbidity and mortality attributed to poor glycaemic control is a pressing public health priority, especially among low-income, minority populations [6].
Food insecurity has been identified as a potentially modifiable risk factor that can be associated with both the development of Type 2 diabetes [7] and with poor glycaemic control [8]. Food insecurity is defined as the ‘limited or uncertain availability of nutritionally adequate and safe foods or limited or uncertain ability to acquire acceptable foods in socially acceptable ways’ [9]. Based on the U.S. Household Food Security Survey Module, food insecurity is predicated on both financial instability and a sense of anxiety surrounding access to healthy food [9]. However, studies that have identified food insecurity as a potential risk factor for poor glycaemic control [7,8] have not examined the relationship between food insecurity and diabetes self-care behaviours, or other factors that may help explain its relationship to more distal outcomes such as glycaemic control. Furthermore, the relationship between food insecurity and glycaemic control has not been studied in low-income or minority populations – those who are at highest risk of complications from poor glycaemic control.
Several hypotheses have been generated implicating either a potential association between food insecurity and diabetes self-care behaviours (e.g. meal skipping) or an association between food insecurity and the substitution of calorie-dense, less-nutritious foods for healthy foods [8]. Therefore, we examined the relationships between food insecurity, diabetes self-care behaviours and glycaemic control using cross-sectional data collected from a racially/ethnically diverse, low-income population with Type 2 diabetes.
Methods
Study design and data sources
Baseline data were collected from patients with Type 2 diabetes participating in a cluster-randomized controlled trial known as the PRIDE study (Clinical Trials.gov identifier NCT01344668). This ongoing study is designed to examine the effect of addressing health literacy and numeracy on improved care for adults with diabetes. Ten State health department safety net primary care clinics in the Mid-Cumberland Region of middle Tennessee were randomized to receive training for providers in enhanced low-literacy/numeracy communication techniques for diabetes management or a standard diabetes educational intervention. After providing informed consent, patients at participating health clinics gave a blood sample and completed a survey. The Institutional Review Boards at Vanderbilt University and the Tennessee Department of Health approved all study procedures.
The primary care clinics in the Mid-Cumberland Region provide care for over 2500 patients with diabetes, and ~ 20% of all visits are related to diabetes. Approximately 20% of the population is Spanish-speaking. Clinic sites that participated in the trial met the following criteria: (1) at least 30 patients were recruited per site; (2) physician(s), nurse practitioner(s), diabetes educator(s) or dietician(s) at each site agreed to participate in the intervention; (3) the site agreed to participate for a minimum of 2.5 years; and (4) the site agreed to be randomized to either arm of the study. Inclusion criteria at the patient level were: (1) patient had a clinical diagnosis of Type 2 diabetes; (2) age 18–85 years; (3) English- or Spanish-speaking; (4) most recent HbA1c ≥ 7.5%; and (5) patient agreed to participate in the study for 2 years. Exclusion criteria at the patient level were: (1) poor visual acuity (vision worse than 20/50 using Rosenbaum Pocket Screener); (2) significant dementia or psychosis (by health provider report or chart review); or (3) terminal illness with an anticipated life expectancy < 2 years (per health provider or patient report).
At enrolment, trained bilingual research assistants collected baseline measures from consenting patients through oral administration of surveys and abstraction of medical records from the local clinic. Surveys were administered in English or Spanish based on patient preference.
Measures
Demographics included age, gender, race/ethnicity (White, non-Hispanic; Other, non-Hispanic; Hispanic), education level, income, BMI, insurance status and duration of diabetes.
Food insecurity was measured using a version of the U.S. Household Food Security Survey Module which includes the following three items: (1) I worried whether my food would run out before I got money to buy more; (2) The food that I bought just didn’t last, and I didn’t have money to get more; and (3) I couldn’t afford to eat balanced meals. Each item is scored with the following response options: never true (0), sometimes true (1) or often true (2). Although most prior research has used some form of the U.S. Household Food Security Survey Module to assess household and personal food insecurity, a wide range of subscales has been used [10]. For the primary analysis, food insecurity was treated as a continuous variable with a range of 0–6, with higher scores indicating more food insecurity. This resulted in a scale with high internal consistency (Cronbach’s alpha = 0.83). In our descriptive analyses and for ease of interpretation, we a priori chose to dichotomize responses into those who were ‘food secure’ and those who were ‘food insecure’ to be consistent with previous studies [11–13]. Participants were categorized as food insecure if they answered ‘sometimes true’ or ‘often true’ to any of the three items.
Diabetes self-care behaviours were measured using three previously validated scales: the Summary of Diabetes Self-Care Activities (SDSCA), a short form of the Personal Diabetes Questionnaire (PDQ-11), and the Adherence to Refills and Medications Scale (ARMS). The SDSCA is a 14-item measure that is scored based on the number of days that a respondent has complied with recommended behaviours (scored 0–7) [14]. There are six subscales that have been validated previously: General Diet, Specific Diet, Exercise, Blood Glucose Testing, Foot Care and Medication Adherence [14].
The PDQ also measures diabetes self-care behaviours [15] and its short form, PDQ-11, is an 11-item instrument developed specifically for this study that addresses several domains including: Poor Eating Behavior, Use of Data to Modify Diet, Skipping Meals, Reducing Portion Size, Routine Physical Activity, Stage of Change for Weight Management and Stage of Change for Exercise. Items have either five or six response options indicating the frequency of adherence to diabetes self-care behaviours or the degree of readiness to change. Based on factor analysis (data not shown), four subscales are presented: (1) Poor Eating Behaviors combines three items that ask about overeating, eating unplanned snacks and making poor food choices with a range of 0–18; (2) Use of Data to Modify Diet combines three items that ask about making decisions using information about the number of calories, the number of carbohydrates and the number of grams of fat with a range of 0–18; (3) Calorie Restriction Strategies combines three items that ask about skipping meals, taking small portion sizes to cut calories, sugar or fat, and plans for losing weight with a range of 0–17; and (4) Activity/Exercise Behaviors combines two items that ask about level of daily routine activity and plans for exercising with a range of 0–11.
Medication non-adherence was measured with the ARMS, which is a 12-item scale, with four response options (ranging from none of the time to all of the time), indicating how often a respondent misses taking medications or fails to refill prescriptions [16,17]. Higher scores on the ARMS (range 12–48) indicate having more problems with medication adherence.
HbA1c was measured prospectively at study baseline to characterize glycaemic control. Samples were submitted as a part of routine clinical care to the state laboratory, which is used in the clinical practice of each of the community health department clinics.
Analyses
Descriptive statistics were calculated as frequencies and proportions, means and standard deviations (SD) or medians with interquartile ranges (IQR) according to the distribution of the variables. Wilcoxon rank-sum tests were used to test whether dichotomous food insecurity status was associated with diabetes self-care behaviours. Linear regression models assessed the unadjusted and adjusted associations between the continuous measure of food insecurity and the continuous measure of HbA1c. Because the distribution of HbA1c was approximately normal, and the distribution of the residuals from the linear regression was also approximately normal, no transformation of HbA1c was necessary. Proportional odds logistic regression models were used to test the association between diabetes self-care behaviours and the continuous food insecurity measure.
Covariates were selected a priori based on their potential association with food insecurity, diabetes self-care or glycaemic control. We controlled for age, gender, race/ethnicity (White, non-Hispanic; other, non-Hispanic; Hispanic), education level, income, BMI and duration of diabetes. Education was treated continuously as years of schooling. Household income was categorized into four groups: < $10 000; $10 000–$19 999; $20 000–$39 999; and ≥ $40 000. BMI reported in kg/m2 was obtained from self-reported height and weight. Length of time with diabetes was calculated as the difference in time (months) between study enrolment and self-reported date of diabetes diagnosis. We did not adjust for insurance status because the vast majority of participants (88%) were uninsured.
If there was a total effect of food insecurity and self-care on HbA1c, we examined the indirect effect of food insecurity on HbA1c via self-care (i.e. a test of mediation) [18,19]. All tests were two-tailed and a P-value of < 0.05 was considered significant. Only participants with complete data were included. Data were analysed using Stata v. 12.1 (StataCorp LP, College Station, TX, USA), SPSS for indirect effects and R, v. 3.1.1(http://www.rstudio.com/).
Results
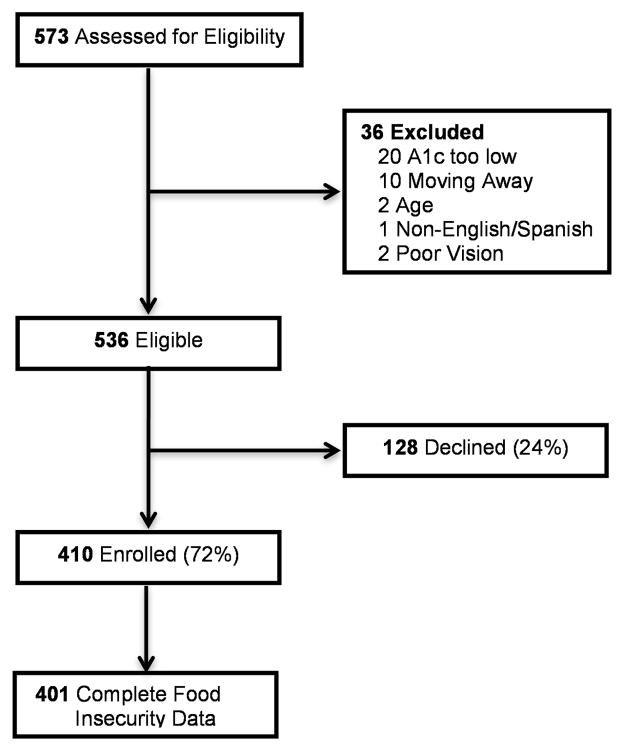
We identified 401 participants from participating State health department clinics that had complete data (Fig. 1). Baseline characteristics of this sample are presented in Table 1. The median age at study enrolment was 52 years (IQR 45–58 years), with a median BMI of 34.8 kg/m2 (IQR 29.3–41.5 kg/m2). More than half of the participants were women (61%), with 57% self-identifying as White, non-Hispanic and 24% as Hispanic.
FIGURE 1.
Study enrolment.
Table 1.
Baseline characteristics of the study population
| All participants (n = 401) | Food secure (n = 109) | Food insecure (n = 292) | P | |
|---|---|---|---|---|
| Age (years), Median (IQR) | 52 (45, 58) | 55 (47, 59) | 51 (44, 57) | 0.02* |
| BMI (kg/m2), Median (IQR) (n = 398) | 34.8 (29.3, 41.5) | 33.3 (29.2, 39.2) | 34.8 (29.3, 41.5) | 0.1* |
| Duration of diabetes (years), Median (IQR) | 7.0 (3.0, 13.0) | 8.0 (3.0, 14.2) | 7.0 (3.2, 12.0) | 0.9* |
| Gender (female), n (%) | 244 (61) | 64 (59) | 180 (62) | 0.6† |
| Race/ethnicity, n (%) | ||||
| White, Non-Hispanic | 229 (57) | 57 (52) | 172 (59) | |
| Black, Non-Hispanic | 70 (17) | 25 (23) | 45 (15) | 0.4† |
| Other, Non-Hispanic | 7 (2) | 2 (2) | 5 (2) | |
| Hispanic | 95 (24) | 25 (23) | 70 (24) | |
| Highest educational attainment, n (%) | ||||
| Less than high school | 146 (37) | 42 (39) | 104 (36) | |
| High school graduate/equivalent | 142 (36) | 39 (36) | 103 (36) | 0.9† |
| Some college | 88 (22) | 22 (20) | 66 (23) | |
| College or greater | 23 (6) | 6 (6) | 17 (6) | |
| Household income, n (%) | ||||
| < $10 000 | 214 (54) | 50 (46) | 164 (57) | |
| $10 000–$19 000 | 113 (29) | 30 (28) | 83 (29) | 0.03† |
| $20 000–$39 000 | 60 (15) | 24 (22) | 36 (12) | |
| ≥ $40 000 | 10 (3) | 5 (5) | 5 (2) | |
| Uninsured, n (%) | 353 (88) | 93 (85) | 260 (89) | 0.3† |
Wilcoxon’s rank-sum test.
Pearson χ2.
Associations between food insecurity and covariates
Using a dichotomous measure of food insecurity, 73% of the participants (292/401) were classified as food insecure. Participants who experienced some level of food insecurity in the past year had a lower median age at study enrolment (51 vs. 55 years, P = 0.02) and lower incomes (< $10,000; 57% vs. 46%, P = 0.048) than participants classified as food secure. Univariate relationships between the dichotomous measure of food insecurity and all of the covariates are presented in Table 1. When evaluating food insecurity using the continuous scale, food insecurity was associated with age (Spearman ρ = −0.10, P = 0.047), BMI (ρ = 0.15, P = 0.003) and higher HbA1c (Spearman ρ = 0.14, P = 0.01), but not with duration of diabetes or education level (all P > 0.05).
Associations between food insecurity and diabetes self-care behaviours
The relationships between food insecurity and diabetes self-care behaviours are summarized in Table 2. In the adjusted proportional odds regression models, food insecurity was associated with General Diet as measured by the SDSCA [OR 0.9 (95% CI 0.8–0.9), P = 0.02], Activity/Exercise Behaviours [OR 0.9 (95% CI 0.8–0.9, P = 0.04] and Calorie Restriction Strategies subscales of the PDQ [OR = 1.1 (95% CI: 1.01–1.2), P = 0.02) and the Adherence to Refills and Medications Scale (OR = 0.86 (95% CI: 0.78 to 0.94), P = 0.02].
Table 2.
Unadjusted and adjusted association between diabetes self-care behaviours and food insecurity
| All participants (n=401) | Correlation with food insecurity | Adjusted* proportional odds regression | |||
|---|---|---|---|---|---|
| Median (IQR) | Spearman ρ | P | Odds ratio (95% CI) | P | |
| Summary of Diabetes Self-Care Activities (range 0–7) | |||||
| General Diet | 3.5 (1.5, 5.0) | −0.1 | 0.004 | 0.9 (0.8, 0.9) | 0.02 |
| Specific Diet | 3.5 (2.5, 4.5) | −0.01 | 0.8 | 0.9 (0.9, 1.1) | 0.8 |
| Exercise | 2.5 (0.5, 4.0) | −0.04 | 0.4 | 0.9 (0.9, 1.1) | 0.5 |
| Blood Glucose Testing | 6.0 (2.0, 7.0) | 0.05 | 0.03 | 1.0 (0.9, 1.1) | 0.5 |
| Foot Care | 3.5 (2.0, 7.0) | 0.04 | 0.5 | 1.0 (0.9, 1.1) | 0.5 |
| Personal Diabetes Questionnaire | |||||
| Poor Eating Behavior (range 0–18) | 7.0 (5.0, 9.0) | 0.06 | 0.1 | 1.0 (0.9, 1.1) | 0.5 |
| Use of Data to Modify Diet (range 0–18) | 5.0 (0, 9.0) | −0.01 | 0.8 | 0.9 (0.9, 1.0) | 0.3 |
| Activity/Exercise Behaviors (range 0–11) | 5.0 (3.0, 7.0) | −0.1 | 0.03 | 0.9 (0.8, 0.9) | 0.04 |
| Calorie Restriction Strategies (range 0–17) | 6.0 (3.0, 8.0) | 0.13 | 0.006 | 1.1 (1.01, 1.2) | 0.02 |
| Non-adherence to Refills and Medication Scale (range 12–48) | 17.0 (15.0, 20.0) | 0.17 | < 0.001 | 1.2 (1.1, 1.3) | 0.002 |
Odds ratio was calculated using proportional odds logistic regression model with adjustment for age, gender, race/ethnicity, BMI, household income, highest education and duration of diabetes.
Odds ratios with 95% CI indicate the relative odds of getting higher scores on each of the respective self-care scales for one interquartile range increase in food insecurity.
Associations between food insecurity and HbA1c
Median HbA1c was 9.3% (IQR 8.2, 11.0) for food insecure patients compared with 8.6% (IQR 7.8, 10.6) for food secure patients (P = 0.02). HbA1c was correlated with the continuous measure of food insecurity (Spearman ρ = 0.14, P = 0.01). This relationship remained significant [0.12 (95% CI 0.01–0.23), P = 0.03] after adjustment for age, gender, race/ethnicity, income, education, BMI and duration of diabetes (Table 3).
Table 3.
Linear regression predicting HbA1c
| Variable | β | 95% CI | P |
|---|---|---|---|
| Food insecurity | 0.12 | (0.01, 0.23) | 0.03 |
| Age at enrolment | −0.06 | (−0.08, −0.04) | < 0.001 |
| Gender (Ref = female) | −0.5 | (−0.9, −0.05) | 0.03 |
| Race/ethnicity | |||
| Non-Hispanic, White | Ref | Ref | Ref |
| Non-Hispanic, Other | 0.7 | (−0.8, 2.2) | 0.4 |
| Non-Hispanic, Black | 0.5 | (−0.01, 1.1) | 0.06 |
| Hispanic | 0.1 | (−0.5, 0.7) | 0.8 |
| BMI | −0.02 | (−0.05, 0.0) | 0.049 |
| Household income | |||
| < $10 000 | Ref | Ref | Ref |
| $10 000–$19 000 | −0.2 | (−0.6, 0.3) | 0.5 |
| $20 000–$39 000 | 0.5 | (−0.04, 1.1) | 0.07 |
| ≥ $40 000 | 0.9 | (−0.4, 2.2) | 0.2 |
| Highest education | −0.03 | (−0.1, 0.03) | 0.3 |
| Duration of diabetes | 0.04 | (0.01, 0.07) | 0.02 |
Food insecurity is statistically significantly associated with higher HbA1c with adjustment for age, gender, race/ethnicity, BMI, household income, highest education and duration of diabetes (P = 0.03).
Ref = referent group in the regression.
Of the self-care behaviours associated with food insecurity, none were also associated with HbA1c. Thus, there was no evidence for self-care behaviours mediating the relationship between food insecurity and higher HbA1c.
Discussion
In this study of predominately uninsured patients with Type 2 diabetes, we found significant relationships between being food insecure and being less adherent to various diabetes self-care behaviours. Being food insecure was associated with lower adherence to general dietary recommendations, including eating poorly and skipping meals more often. It was also associated with being less physically active and having more problems with medication adherence. In addition, being food insecure was associated with worse glycaemic control; those participants who were food insecure had a median HbA1c level that was 0.7% higher than participants who were food secure. The fact that this relationship remained significant after controlling for several factors known to be associated with HbA1c (e.g. age, diabetes duration) suggests that food insecurity may have a modest, but meaningful contribution to suboptimal glycaemic control in this population, replicating previous research. In our sample, the diabetes self-care behaviours associated with food insecurity were not associated with glycaemic control, limiting our ability to examine one plausible causal pathway between food insecurity and glycaemic control.
Recent work has used data from the NHANES cohort to identify associations between food insecurity and the prevalence of Type 2 diabetes, as well as between food insecurity and glycaemic control [7,8,20,21]. Consistent with findings from that nationally representative sample, our sample of largely uninsured families from poor socio-economic strata produced similar results. Previous work has postulated that either replacing healthy foods with low-cost calorically dense foods or having poor self-management strategies could mediate the relationship between food insecurity and poor glycaemic control [8,21]. Although our results cannot confirm the pathway by which food insecurity is related to suboptimal glycaemic control, the association between food insecurity and less adherence to diabetes self-care lends credence to the possibility that food insecure patients have difficulty performing these behaviours. Notably, the relationship between food insecurity, poor diet and skipping meals points to the difficulty these patients have in maintaining a consistent caloric intake – a feature that is often associated with suboptimal glycaemic control. Also, our results indicate that food insecurity is associated with more problems with medication adherence. Even though our results cannot confirm this as a mechanism by which food insecurity is associated with suboptimal glycaemic control, the relationship warrants further investigation.
Our study has several limitations. This design was cross-sectional, limiting causal inferences, and relied on self-reported survey data that may not reflect actual diabetes self-care behaviours and can be susceptible to social desirability bias. Furthermore, the SDSCA and the PDQ were not associated with HbA1c, suggesting that our measurement of diabetes self-care may not have been sensitive enough to account for a potential relationship between self-care and HbA1c. The lack of a relationship between diabetes self-care, medication adherence and HbA1c may have also been due to a lack of variation in glycaemic control in this population. It is important to note that previous work has identified associations with the ARMS and HbA1c, and it is unclear why there is a lack of association in this study [16]. Finally, this study focused on a predominantly uninsured population in middle Tennessee and may not be generalizable to all populations. However, patients from low socio-economic strata represent a segment of the population for whom diabetes self-care and glycaemic control are a significant challenge. As such, the conclusions from this study may provide important implications for this population subgroup.
Conclusions
We found an association between food insecurity and less adherence to recommended diabetes self-care behaviours – specifically eating a healthy diet, eating consistent meals, engaging in physical activity and taking medications. Furthermore, our findings are consistent with previously reported associations between food insecurity and having worse glycaemic control among patients with Type 2 diabetes. These findings underscore the importance of food insecurity as a risk factor for glycaemic control in patients with Type 2 diabetes. Future work should use other measures of diabetes self-care behaviours than the ones used here to test whether self-care is a mechanism by which food insecurity contributes to poor glycaemic control. If food insecurity is proven to be causally related to suboptimal glycaemic control, it would represent an important potentially modifiable factor for patients with diabetes.
What’s new?
These data provide the first evidence that food insecurity is associated with diabetes self-care behaviours, suggesting that further work should focus here to understand the mechanism by which food insecurity is associated with the onset of diabetes.
These data show that food insecurity is associated with glycaemic control in a low-income, under-served population.
The associations between food insecurity and glycaemic control, and food insecurity and diabetes self-care behaviours point to food insecurity as a modifiable risk factor for improving diabetes control, especially in low-income populations.
Acknowledgments
Funding sources
This work was supported by the NIDDK (R18DK083264), the Vanderbilt Institute for Clinical and Translational Research NCATS (UL1TR000445), and the Vanderbilt Center for Diabetes Translational Research NIDDK (P30DK092986). Dr Osborn was supported by a career development award NIDDK (K01DK087894). Dr Heerman was supported by the NICHD (T32HD060554). The REDCap software for data management was also used for this work, supported by NCATS (UL1TR000445).
The authors would like to acknowledge Lorainne MacDonald, MD; Laura Harris, RD CDE; Charlene Haynes, RN; and Annette Haley from the Tennessee Department of Health for their contribution to the design and implementation of the study.
Footnotes
Preliminary data from this article were presented as a poster presentation at the Vanderbilt Diabetes Research Day, 2013 and as an oral presentation at the Society for General Internal Medicine, 2014.
Competing interests
None disclosed.
Author contributions
All authors are responsible for the reported research and approve of the final submitted version. Dr Heerman defined the study question, performed the primary analysis, and contributed significantly to the drafting and revision of the manuscript. Drs Wallston, Osborn, Schlundt and Rothman participated in the concept and design of the study, assisted with analysis and interpretation of data, and contributed significantly to the drafting and revising the manuscript. Ms Barto assisted with data collection, interpretation of data, and contributed significantly to the drafting and revising the manuscript.
References
- 1.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33:562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heisler M, Faul JD, Hayward RA, Langa KM, Blaum C, Weir D. Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older Americans in the health and retirement study. Arch Intern Med. 2007;167:1853–1860. doi: 10.1001/archinte.167.17.1853. [DOI] [PubMed] [Google Scholar]
- 3.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 4.Everson SA, Maty SC, Lynch JW, Kaplan GA. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. J Psychosom Res. 2002;53:891–895. doi: 10.1016/s0022-3999(02)00303-3. [DOI] [PubMed] [Google Scholar]
- 5.Robbins JM, Vaccarino V, Zhang H, Kasl SV. Socioeconomic status and type 2 diabetes in African American and non-Hispanic white women and men: evidence from the Third National Health and Nutrition Examination Survey. Am J Public Health. 2001;91:76–83. doi: 10.2105/ajph.91.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seligman HK, Bindman AB, Vittinghoff E, Kanaya AM, Kushel MB. Food insecurity is associated with diabetes mellitus: results from the National Health Examination and Nutrition Examination Survey (NHANES) 1999–2002. J Gen Intern Med. 2007;22:1018–1023. doi: 10.1007/s11606-007-0192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkowitz SA, Baggett TP, Wexler DJ, Huskey KW, Wee CC. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care. 2013;36:3093–3099. doi: 10.2337/dc13-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to Measuring Household Food Security. Alexandria, VA: 2000. XXX. [Google Scholar]
- 10.Larson NI, Story MT. Food insecurity and weight status among U.S. children and families: a review of the literature. Am J Prev Med. 2011;40:166–173. doi: 10.1016/j.amepre.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Blumberg SJ, Bialostosky K, Hamilton WL, Briefel RR. The effectiveness of a short form of the Household Food Security Scale. Am J Public Health. 1999;89:1231–1234. doi: 10.2105/ajph.89.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gundersen C, Lohman BJ, Garasky S, Stewart S, Eisenmann J. Food security, maternal stressors, and overweight among low-income US children: results from the National Health and Nutrition Examination Survey (1999–2002) Pediatrics. 2008;122:e529–e540. doi: 10.1542/peds.2008-0556. [DOI] [PubMed] [Google Scholar]
- 13.Metallinos-Katsaras E, Must A, Gorman K. A longitudinal study of food insecurity on obesity in preschool children. J Acad Nutr Diet. 2012;112:1949–1958. doi: 10.1016/j.jand.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23:943–950. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 15.Stetson B, Schlundt D, Rothschild C, Floyd JE, Rogers W, Mokshagundam SP. Development and validation of The Personal Diabetes Questionnaire (PDQ): a measure of diabetes self-care behaviors, perceptions and barriers. Diabetes Res Clin Pract. 2011;91:321–332. doi: 10.1016/j.diabres.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Mayberry LS, Gonzalez JS, Wallston KA, Kripalani S, Osborn CY. The ARMS-D out performs the SDSCA, but both are reliable, valid, and predict glycemic control. Diabetes Res Clin Pract. 2013;102:96–104. doi: 10.1016/j.diabres.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kripalani S, Risser J, Gatti ME, Jacobson TA. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value in Health. 2009;12:118–123. doi: 10.1111/j.1524-4733.2008.00400.x. [DOI] [PubMed] [Google Scholar]
- 18.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Method. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 19.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Meth Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 20.Bawadi HA, Ammari F, Abu-Jamous D, Khader YS, Bataineh S, Tayyem RF. Food insecurity is related to glycemic control deterioration in patients with type 2 diabetes. Clin Nutr. 2012;31:250–254. doi: 10.1016/j.clnu.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Lyles CR, Wolf MS, Schillinger D, Davis TC, Dewalt D, Dahlke AR, et al. Food insecurity in relation to changes in hemoglobin A1c, self-efficacy, and fruit/vegetable intake during a diabetes educational intervention. Diabetes Care. 2013;36:1448–1453. doi: 10.2337/dc12-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]