Abstract
Animal and human studies have found that males and females show distinct stress responses. Recent studies suggest the contribution of estrogen in the brain to this sexual dimorphism. Repeated stress has been found to impair cognitive behaviors via suppressing glutamatergic transmission and glutamate receptor surface expression in pyramidal neurons of prefrontal cortex (PFC) in male rats. On the contrary, female rats exposed to the same stress paradigms show normal synaptic function and PFC-mediated cognition. The level of aromatase, the enzyme for the biosynthesis of estrogen, is significantly higher in the PFC of females than males. The stress-induced glutamatergic deficits and memory impairment are unmasked by blocking estrogen receptors or aromatase in females, suggesting a protective role of estrogen against the detrimental effects of repeated stress.
Sexually Dimorphic Effects of Stress and Role of Estrogen
Corticosteroid stress hormones serve as a key regulator of cognitive and emotional processes (de Kloet et al., 2005; Joëls, 2006; McEwen, 2007). It has been proposed that there is an “inverted U” relationship of stress to cognitive function (Diamond et al., 1992), such that a moderate level of corticosteroid has pro-cognitive effects, while too low or too high corticosteroid levels are detrimental to cognitive processing (Joels, 2006). Our group has found that stress exerts dual effects on cognition through bi-directional modulation of glutamatergic transmission in prefrontal cortex (PFC), a key target region of stress hormones. In young (~4 weeks old) male rats, acute stress significantly enhances glutamate receptor-mediated synaptic currents and improves working memory (Yuen et al., 2009; 2011). Conversely, young male rats exposed to one-week repeated restraint or unpredictable stress show the diminished PFC glutamatergic transmission and the impaired PFC-mediated cognitive function, temporal order recognition memory (TORM, Yuen et al., 2012).
While these findings support the notion that short-term (acute) stressors elicit adaptive and beneficial changes, whereas long-term (chronic) stress results in maladaptive and deleterious effects, this pattern of stress responses appears to apply to only males. In response to one acute stressful event of intermittent tail-shocks, spine density is enhanced in the male hippocampus but reduced in the female hippocampus (Shors et al., 2001). When the subchronic stress challenge, which induces cognitive impairment in males (Yuen et al., 2012), is introduced to young female rats, their glutamatergic transmission in PFC and TORM function are unaffected (Wei et al., 2014, Fig. 1A). Similar sex differences to chronic stress have also been reported by other groups. For example, in male rats, restraint stress (6 h/day, 21-day) impairs performance on a variety of spatial memory tasks including radial arm maze, object placement, Y-maze, water maze, and a nonspatial, recognition memory test (Beck and Luine, 1999; 2002; Conrad et al., 1996; Kitraki et al., 2004). In contrast, females exposed to the same stress paradigm show enhanced cognition and memory in almost all of these tasks (Beck and Luine, 2002; Bowman et al., 2001; 2002; 2003, Bowman, 2005; McLaughlin et al., 2005, Conrad et al., 2003; Kitraki et al., 2004). These animal studies suggest that females are more resilient to chronic stress than males, at least in terms of the measured cognitive behaviors (Cohen and Yehuda, 2011).
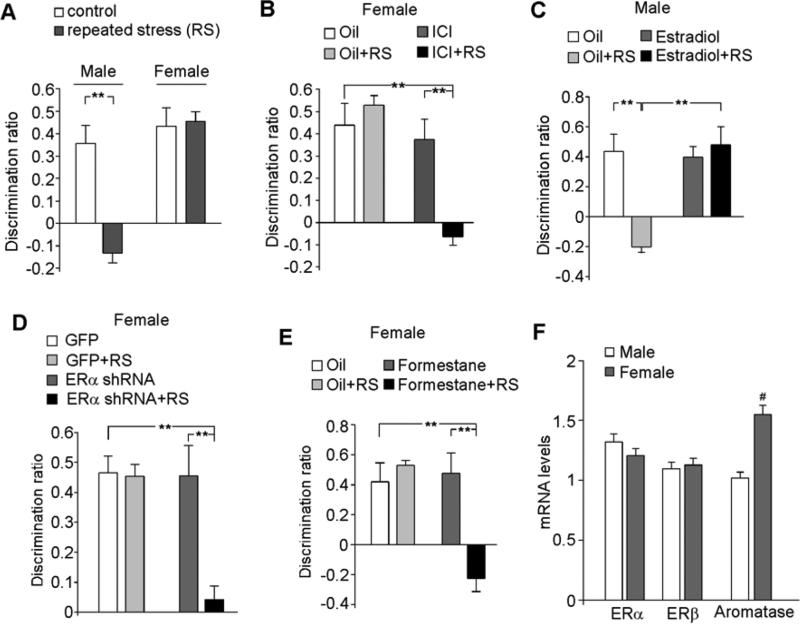
Figure 1. Estrogen protects against the detrimental effects of repeated stress on cognition.
A, Bar graphs (mean ± SEM) showing the discrimination ratio (DR) of temporal order recognition memory (TORM) tasks in control or repeatedly stressed (restraint, 7-day, RS) male or female rats (4-wk-old). B,C, Bar graphs showing the DR of TORM tasks in control vs. repeatedly stressed females with injections of the ER antagonist ICI182,780 (B, 0.05 mg/kg, s.c.), or males with the injections of the ER agonist estradiol (C, 0.1 mg/kg, s.c.). D,E, Bar graphs showing the DR of TORM tasks in control vs. repeatedly stressed females with the PFC injection of GFP or ERα shRNA lentivirus (D), or with the injections of aromatase inhibitor formestane (2 mg/kg, s.c.). **: p < 0.005, ANOVA (A-E). F, Quantitative real-time RT-PCR data on the mRNA level of ERα, ERβ and aromatase in PFC from male vs. female rats. #: p < 0.05, T-test. Adapted from Wei J, et al., Mol. Psychiatry, 19: 588-598, 2014.
Interestingly, when estrogen receptor (ER) function is blocked in female rats, the detrimental effects of repeated stress (2-hr restraint, 7-days) on PFC glutamatergic transmission and TORM function are unmasked (Wei et al., 2014, Fig. 1B). On the other hand, when estradiol is administered in male rats, they become resilient to the same stressor (Wei et al., 2014, Fig. 1C). It suggests that an estrogen-mediated mechanism makes females less susceptible to the deleterious effects of repeated stress than males.
However, the role of estrogen in stress responses is not without controversy. There are also reports suggesting that estrogen may amplify the stress responses in females. Activating stress systems pharmacologically by FG7142, a benzodiazepine inverse agonist, induces impaired PFC working memory in females during proestrus (high estrogen), but not during estrus (low estrogen), suggesting that estrogen may increase the sensitivity to stress in females (Shansky et al., 2004). Estrogen replacement in ovariectomized female rats exposed to a behavoral stressor (2-h immobilization for 10 days) also induces the greater dendritic remodeling in PFC neurons projecting to the basolateral nucleus of the amygdala (BLA) (Shansky et al., 2010). Preclinical studies using fear conditioning and extinction paradigms have found that females with low estrogen levels exhibit impaired extinction retrieval (Milad et al, 2009, 2010), which can be reversed by stimulation of D1 dopamine receptors (Rey et al., 2014). It suggests that estrogen might influence PFC-BLA function in part through dopaminergic mechanisms.
Converging evidence supports that females and males exhibit different biochemical, cellular and behavioral effects of stress (Shors et al., 2001; Luine et al., 2007; Bowman et al., 2009; McEwen, 2010; Bangasser et al., 2010). However, the observed sex differences of stress responses and role of estrogen could be affected by a number of factors, such as animal strains, animal ages, stress paradigms, estrogen regimen, and measured outcomes.
Resilience vs. Vulnerability of Females to Stress-related Mental Disorders
Epidemiological studies indicate that women are more likely to develop stress-associated mental disorders, such as depression and PTSD (Weissman et al., 1996; Breslau et al, 1999). Therefore, it is easy to assume that females have higher stress susceptibility. However, it is important to note that gender vulnerability in stress responses is different from gender vulnerability in mental disorders. Despite the stress exposure for almost everyone, only a small population develops stress-associated mental disorders, including depression and PTSD. Genetic risk factors carried by the susceptible individuals are likely to play a causal role, while stress may only serve as a trigger to precipitate a variety of emotional and cognitive difficulties. Genetic factors probably also directly influence the intrinsic sensitivity to stress, which contributes to the pathogenesis of psychiatric diseases (Karatsoreos and McEwen, 2011). Recent studies suggest that individuals carrying Vall66met allele of the BDNF (brain derived trophic factor) gene have the altered vulnerability to stress and antidepressant responses (Yu et al., 2012). Epigenetic mechanisms involving chromatin remodeling and gene expression could also influence stress sensitivity (Sterrenburg et al., 2011; Vialou et al., 2013).
There are two peaks of depression prevalence in women: postpartum depression, and perimenopausal depression, both of which are associated with large drops (postpartum) or excessive fluctuations (perimenopausal) of serum estradiol levels (Steiner et al. 2003). Human PET imaging studies suggest that estrogen decline during perimenopausal age elevates the level of monoamine oxidase A, a neurobiological change that also presents during major depressive episodes (Rekkas et al., 2014). The critical role of estrogen in mood disorders is also supported by animal studies. It has been found that injecting estrogen reverses helplessness in animal depression models and enhances hippocampus plasticity (Hajszan et al., 2010; Bredemann and McMahon, 2014). Females are more responsive to the action of antidepressants than males (Gomez et al., 2014). Corroborating with the role of estrogen in females’ stress resilience (Wei et al., 2014), estrogen also shows antianxiety and antidepression effects in animal models, which are dependent upon the utilized regimen of estrogen and interactions with the hypothalamic-pituitary-adrenal axis (Walf and Frye, 2006).
Role of Estrogen in Synaptic Regulation and Brain Diseases
One surprising finding is that the stress resistance in females is unchanged by ovaridectomy that only terminates the ovarian estrogen, but is blocked by knockdown of ERα only in the PFC region (Wei et al., 2014, Fig. 1D) or global inhibition of aromatase, the estrogen synthesis enzyme (Wei et al., 2014, Fig. 1E), implying that CNS-synthesized estrogen (Woolley, 2007; Konkle and McCarthy, 2011) may determine the sexually dimorphic vulnerability to stress. Consistently, it has been shown that estrogen can be synthesized by aromatase localized in neurons from endogenous cholesterol (Hojo et al., 2004). Moreover, ischemic neuroprotection in females has been attributed to the local, nongonadal estrogen, which may be aromatized from precursor androgens (McCullough et al., 2003). The expression of aromatase is significantly higher in PFC neurons of female than male rats (Wei et al., 2014, Fig. 1F), suggesting that prepubertal female PFC has an endogenous capacity to generate estrogen that provides protection against repeated stress.
The protective effect of estrogen against stress is in line with the critical role of estrogen in neurogenesis (Ormerod et al., 2004), synaptic spine growth (Hajszan et al., 2010) and memory consolidation (Sellers et al., 2014). Depriving estrogen in rats induces postpartum-depression symptoms, and altered expression of genes involved in learning and memory (Suda et al., 2008). It has been found that estrogen protects females from several neurological diseases, including seizure (Pottoo et al., 2014), ischemia (Perez-Alvarez et al., 2014) and Alzheimer's disease (Lan et al., 2015). Studies suggest that estrogen receptor (ERα), which is expressed at synapses of hippocampus (Adams et al., 2002) and PFC (Wang et al., 2010), influences synaptic function through a non-genomic mechanism (McEwen et al., 2001; 2012). For example, estrogen potentiates the activation of NMDA receptors and voltage-gated calcium channels, which results in the increased calcium influx and new excitatory synapses in hippocampus (Woolley et al., 1997; McEwen et al., 2001). Intracellularly, estrogen rapidly activates synaptic Akt that facilitates local synthesis of PSD-95, a scaffolding protein required for spinogenesis (Akama and McEwen, 2003). Moreover, estrogen activates RhoA-ROCK-LIMK-cofilin signalling pathway that controls actin polymerization involved in spine growth (Spencer et al., 2008; Kramár et al., 2009). At circuitry level, estrogen suppresses inhibitory GABAergic interneuron by downregulating BDNF synthesis, which indirectly increases excitatory synaptic transmission of pyramidal neurons in hippocampus (Murphy et al., 1998).
Recent studies have uncovered new functions of brain-derived estrogen (17αestradiol). This neurosteroid can be produced in hippocampus (Kimoto et al., 2001). It is proposed that estrogen exerts its action in various brain regions through a combination of genomic and nongenomic mechanisms. Unlike the sustained effect of ovarian estrogen, injection of 17α estradiol in ovariectomized animals rapidly induces hippocampal spine formation without increasing the expression of synaptic proteins (Spencer et al., 2008). Electrophysiological data suggests that 17α estradiol exerts an immediate effect on glutamatergic transmission by enhancing presynaptic glutamate release (Smejkalova and Woolley, 2010).
Sexual Dimorphism in Developing Brain
Evaluation of the distribution of ERα and ERβ has revealed their presence in diverse brain regions (cerebral cortex, basal forebrain, amygdala, etc) through early postnatal periods (p3-p14), supports a potential role for estrogens in neural development (Pérez et al., 2003). Our study shows that estrogen protects prepubertal females from the detrimental effects of repeated stress (Wei et al., 2014), suggesting that estrogen plays a role in developing female brains, rather than being a typical sex hormone for reproduction. In agreement with this, mounting studies pinpoint the sexual dimorphism in brain development. For instance, comparing to neurogenesis in females, males have a higher proliferation rate and a better survival rate of de novo neurons (Zhang et al., 2008). Imaging study indicates that white matter development is strongly influenced by hormonal environment of estrogen during early postnatal period, but is minimally affected later in life (Kranz et al., 2014). Significantly decreased levels of ERβ and aromatase (the enzyme converting testosterone to estrogen) have been found in PFC of human subjects with autism spectrum disorders (Crider et al., 2014), which may be linked to the elevated testosterone effects on arousal and social anxiety (Pfaff et al., 2011), and contribute to male predominance in autism.
Why are there gender differences in brain development and function? Genetic information encoded in the sex chromosome has been suggested as an underlying reason. For example, the SRY gene on Y chromosome underlies sex-dependent neuroanatomical structures in various brain regions, as well as behavioral phenotypes, such as social exploration and aggression (De Vries et al., 2002; Hensbroek et al., 1995). Other theories suggest that females’ additional X-chromosome may provide the pattern of X-linked gene effects that is different from males (Davis and Pfaff, 2014).
Besides genomic influence, epigenetic factors also play a key role. Estradiol regulates its own receptor function via DNA methylation on the promotors of ERs, which is thought to contribute to the sexual dimorphism of neuronal anatomy and behavior (Nugent et al., 2011). Consistent with this, knockdown of ER with antisense oligonucleotides disrupts sexual differentiation of the brain (McCarthy et al., 1993). ER knockout female mice display diminished maternal behaviors and exhibit male-like aggressive behaviors (Ogawa et al., 1996).
Postnatal stress is thought to affect the ability to cope with adversity in adulthood (Bagot et al., 2009). Emerging evidence suggests that the vulnerability to stress in developing brains is also gender-dependent. Study of prenatal stress shows that male, but not female, offsprings exposed to stress in early pregnancy have maladaptive stress responses, which is associated with alterations in corticotrophin-releasing factor (CRF) and glucocorticoid receptor (GR) expression (Mueller and Bale, 2008). Female offspring is more resilient to various early life stress challenges than male offspring (Lajud and Torner, 2015). When exposed to postnatal stress, such as early weaning from maternal lactation or limited nesting & bedding materials, neurogenesis is perturbed in male, but not female, animals (Kikusui and Mori, 2009; Naninck et al., 2015). Such gender-dependent susceptibility to stress during development may be linked to mental illnesses in adult life (Bale, 2009; Davis and Pfaff, 2014).
In summary, animal and human studies have found that males and females show distinct stress responses and have different vulnerability to stress-related mental disorders. Recent studies have suggested the contribution of estrogen in the brain to this sexual dimorphism. The differential effects of stress on glutamatergic transmission in males vs. females, which are attributed to the influence of estrogen on synaptic plasticity (Fig. 2, Yuen et al., 2012; Wei et al., 2014), may underlie the sex-specific impact of stress on cognitive processes. More detailed genetic, epigenetic and molecular mechanisms await to be elucidated.
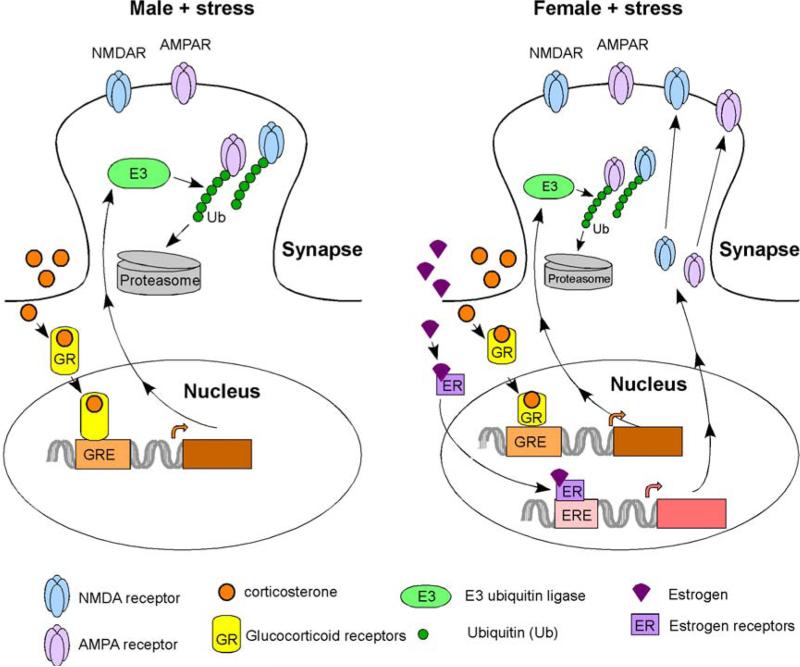
Figure 2. A diagram showing the role of estrogen in determining the sexually dimorphic effects of stress on glutamatergic synaptic function in prefrontal cortical neurons.
In males, repeated stress triggers the activation of glucocorticoid receptors (GR). GR bind to glucocorticoid response element (GRE) on the promoters of downstream genes, triggering the increased glutamate receptor ubiquitination and degradation. In females, estrogen activates estrogen receptors (ER), which bind to estrogen response element (ERE) and enhance the transcription of synaptic plasticity genes that promote the synthesis and exocytosis of glutamate receptors, therefore counteracting the stress-induced depressing effects.
Highlights.
Stress exerts sexually dimorphic effects on synaptic and cognitive function.
Estrogen protects females against the detrimental effects of repeated stress.
The level of aromatase is higher in prefrontal cortex of females than males.
Acknowledgements
This work was supported by NIH grant (R01-DA037618) to Z.Y.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. Journal of neuroscience. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. Journal of neuroscience. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joels M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiology of learning and memory. 2009;92:292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Bale TL. Neuroendocrine and immune influences on the CNS: it's a matter of sex. Neuron. 2009;64:13–16. doi: 10.1016/j.neuron.2009.09.036. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Molecular psychiatry. 2010;15:877, 896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids. Brain Res. 1999;830:56–71. doi: 10.1016/s0006-8993(99)01380-3. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol Behav. 2002;75:661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Bowman RE. Stress-induced changes in spatial memory are sexually differentiated and vary across the lifespan. J Neuroendocrinol. 2005;17:526–535. doi: 10.1111/j.1365-2826.2005.01335.x. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory. Sex differences in performance and monoamines. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Micik R, Gautreaux C, Fernandez L, Luine VN. Sex-dependent changes in anxiety, memory, and monoamines following one week of stress. Physiol Behav. 2009;97:21–29. doi: 10.1016/j.physbeh.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Bredemann TM, McMahon LL. 17beta Estradiol increases resilience and improves hippocampal synaptic function in helpless ovariectomized rats. Psychoneuroendocrinology. 2014;42:77–88. doi: 10.1016/j.psyneuen.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Chilcoat HD, Kessler RC, Peterson EL, Lucia VC. Vulnerability to assaultive violence: further specification of the sex difference in post-traumatic stress disorder. Psychol Med. 1999;29:813–821. doi: 10.1017/s0033291799008612. [DOI] [PubMed] [Google Scholar]
- Crider A, Thakkar R, Ahmed AO, Pillai A. Dysregulation of estrogen receptor beta (ERβ), aromatase (CYP19A1), and ER co-activators in the middle frontal gyrus of autism spectrum disorder subjects. Mol Autism. 2014;5:46. doi: 10.1186/2040-2392-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Yehuda R. Gender differences in animal models of posttraumatic stress disorder. Disease markers. 2011;30:141–150. doi: 10.3233/DMA-2011-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KD, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol Learn Mem. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Davis EP, Pfaff D. Sexually dimorphic responses to early adversity: implications for affective problems and autism spectrum disorder. Psychoneuroendocrinology. 2014;49:11–25. doi: 10.1016/j.psyneuen.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. Journal of neuroscience. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Gomez ML, Martinez-Mota L, Estrada-Camarena E, Fernandez-Guasti A. Influence of the brain sexual differentiation process on despair and antidepressant-like effect of fluoxetine in the rat forced swim test. Neuroscience. 2014;261:11–22. doi: 10.1016/j.neuroscience.2013.12.035. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Szigeti-Buck K, Sallam NL, Bober J, Parducz A, Maclusky NJ, Leranth C, Duman RS. Effects of estradiol on learned helplessness and associated remodeling of hippocampal spine synapses in female rats. Biological psychiatry. 2010;67:168–174. doi: 10.1016/j.biopsych.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensbroek RA, Sluyter F, Guillot PV, Van Oortmerssen GA, Crusio WE. Y chromosomal effects on hippocampal mossy fiber distributions in mice selected for aggression. Brain research. 1995;682:203–206. doi: 10.1016/0006-8993(95)00270-z. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori T-a, Enami T, Furukawa A, Suzuki K, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. USA. 2003;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M. Corticosteroid effects in the brain: U-shape it. Trends in pharmacological sciences. 2006;27:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, McEwen BS. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci. 2011;15:576–84. doi: 10.1016/j.tics.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Mori Y. Behavioural and neurochemical consequences of early weaning in rodents. Journal of neuroendocrinology. 2009;21:427–431. doi: 10.1111/j.1365-2826.2009.01837.x. [DOI] [PubMed] [Google Scholar]
- Kimoto T, Tsurugizawa T, Ohta Y, Makino J, Tamura H, Hojo Y, Takata N, Kawato S. Neurosteroid synthesis by cytochrome p450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology. 2001;142:3578–3589. doi: 10.1210/endo.142.8.8327. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152:223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen's acute effects on synaptic transmission and plasticity. Journal of neuroscience. 2009;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz GS, Hahn A, Kaufmann U, Kublbock M, Hummer A, Ganger S, Seiger R, Winkler D, Swaab DF, Windischberger C, et al. White matter microstructure in transsexuals and controls investigated by diffusion tensor imaging. Journal of neuroscience. 2014;34:15466–15475. doi: 10.1523/JNEUROSCI.2488-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajud N, Torner L. Early life stress and hippocampal neurogenesis in the neonate: sexual dimorphism, long term consequences and possible mediators. Frontiers in molecular neuroscience. 2015;8:3. doi: 10.3389/fnmol.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan YL, Zhao J, Li S. Update on the neuroprotective effect of estrogen receptor alpha against Alzheimer's disease. J Alzheimers Dis. 2015;43:1137–48. doi: 10.3233/JAD-141875. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Schlenker EH, Pfaff DW. Enduring consequences of neonatal treatment with antisense oligodeoxynucleotides to estrogen receptor messenger ribonucleic acid on sexual differentiation of rat brain. Endocrinology. 1993;133:433–439. doi: 10.1210/endo.133.2.8344188. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J. Neurosci. 2003;23:8701–05. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126:4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc. Natl. Acad. Sci. USA. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McLaughlin KF, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction. Possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. Journal of neuroscience. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Segal M. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proc. Natl. Acad. Sci. USA. 1998;95:11412–11417. doi: 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naninck EF, Hoeijmakers L, Kakava-Georgiadou N, Meesters A, Lazic SE, Lucassen PJ, Korosi A. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25:309–328. doi: 10.1002/hipo.22374. [DOI] [PubMed] [Google Scholar]
- Nugent BM, Schwarz JM, McCarthy MM. Hormonally mediated epigenetic changes to steroid receptors in the developing brain: implications for sexual differentiation. Hormones and Behavior. 2011;59:338–344. doi: 10.1016/j.yhbeh.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Taylor JA, Lubahn DB, Korach KS, Pfaff DW. Reversal of sex roles in genetic female mice by disruption of estrogen receptor gene. Neuroendocrinology. 1996;64:467–470. doi: 10.1159/000127154. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT, Galea LA. Estradiol enhances neurogenesis in the dentate gyri of adult male meadow voles by increasing the survival of young granule neurons. Neuroscience. 2004;128:645–54. doi: 10.1016/j.neuroscience.2004.06.039. [DOI] [PubMed] [Google Scholar]
- Pérez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145:117–39. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Perez-Alvarez MJ, Mateos L, Alonso A, Wandosell F. Estradiol and Progesterone Administration After pMCAO Stimulates the Neurological Recovery and Reduces the Detrimental Effect of Ischemia Mainly in Hippocampus. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8963-7. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Rapin I, Goldman S. Male predominance in autism: neuroendocrine influences on arousal and social anxiety. Autism research. 2011;4:163–176. doi: 10.1002/aur.191. [DOI] [PubMed] [Google Scholar]
- Pottoo FH, Bhowmik M, Vohora D. Raloxifene protects against seizures and neurodegeneration in a mouse model mimicking epilepsy in postmenopausal woman. Eur J Pharm Sci. 2014;65C:167–173. doi: 10.1016/j.ejps.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Rekkas PV, Wilson AA, Lee VW, Yogalingam P, Sacher J, Rusjan P, Houle S, Stewart DE, Kolla NJ, Kish S, Chiuccariello L, Meyer JH. Greater monoamine oxidase a binding in perimenopausal age as measured with carbon 11-labeled harmine positron emission tomography. JAMA Psychiatry. 2014;71:873–9. doi: 10.1001/jamapsychiatry.2014.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey CD, Lipps J, Shansky RM. Dopamine D1 receptor activation rescues extinction impairments in low-estrogen female rats and induces cortical layer-specific activation changes in prefrontal-amygdala circuits. Neuropsychopharmacology. 2014;39:1282–9. doi: 10.1038/npp.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers K, Raval P, Srivastava DP. Molecular signature of rapid estrogen regulation of synaptic connectivity and cognition. Frontiers in neuroendocrinology. 2014 doi: 10.1016/j.yfrne.2014.08.001. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Shansky RM, Glavis-Bloom C, Lerman D, McRae P, Benson C, Miller K, Cosand L, Horvath TL, Arnsten AF. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Molecular psychiatry. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20:2560–7. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–7. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. Journal of neuroscience. 2010;30:16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Frontiers in neuroendocrinology. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Elliott E, Chen A, Peeters BW, Roubos EW, Kozicz T. Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PloS one. 2011;6:e28128. doi: 10.1371/journal.pone.0028128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda S, Segi-Nishida E, Newton SS, Duman RS. A postpartum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biological psychiatry. 2008;64:311–319. doi: 10.1016/j.biopsych.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Feng J, Robison AJ, Nestler EJ. Epigenetic mechanisms of depression and antidepressant action. Annu Rev Pharmacol Toxicol. 2013;53:59–87. doi: 10.1146/annurev-pharmtox-010611-134540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH. Synaptic estrogen receptor-alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. Journal of neuroscience. 2010;30:12770–12776. doi: 10.1523/JNEUROSCI.3192-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Molecular Psychiatry. 2014;19:588–598. doi: 10.1038/mp.2013.83. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. Journal of neuroscience. 2012;32:4092–4101. doi: 10.1523/JNEUROSCI.5048-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc. Natl. Acad. Sci. USA. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol. Psychiatry. 2011;16:156–70. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]