Abstract
Purpose
To describe a series of Stargardt disease (STGD1) patients exhibiting a phenotype usually associated with hydroxychloroquine (HCQ) retinopathy on spectral domain-optical coherence tomography (SD-OCT).
Methods
Observational case series from Columbia University Medical Center involving 8 patients with genetically-confirmed STGD1. Patients selected for the study presented no history of HCQ use. Horizontal macular SD-OCT scans and accompanying 488nm autofluorescence (AF) images, color fundus photographs, and full-field electroretinograms were analyzed.
Results
All study patients exhibited an abrupt thinning of the parafoveal region or disruption of the outer retinal layers on SD-OCT resembling the transient HCQ retinopathy phenotype. Funduscopy and AF imaging revealed variations of bull’s eye maculopathy (BEM). Five patients exhibited local fleck-like deposits around the lesion. Genetic screening confirmed two disease-causing ABCA4 mutations in 5 patients and one mutation in 3 patients.
Conclusions
A transient SD- OCT phenotype ascribed to patients with HCQ retinopathy is associated with an early subtype of STGD1. This finding may also present with HCQ retinopathy-like BEM lesions on AF imaging and funduscopy. A phenotypic overlap may not be surprising given certain shared mechanistic disease processes between the two conditions. A thorough work-up, including screening of genes that are causal in retinal dystrophies associated with foveal sparing, may prevent the misdiagnoses of more ambiguous cases.
Keywords: Stargardt disease, ABCA4, hydroxychloroquine toxicity, phenocopy
Introduction
Stargardt disease (STGD1, OMIM#248200) is an autosomal recessive macular dystrophy affecting between 1 in 8,000 and 1 in 10,000 people world-wide.[1] The disease is caused by mutations in the ABCA4 gene, which encodes the ATP-binding cassette transporter in photoreceptors.[2] A dysfunctional ABCA4 protein results in the inadequate handling of vitamin A aldehyde in the outer segments of photoreceptor cells which, after shedding and subsequent phagocytosis, leads to an over-accumulation of phototoxic bisretinoids (including A2E) in retinal pigment epithelium (RPE) cells.[3,4]
Accurately diagnosing STGD1 can often be challenging as it encompasses a wide phenotypic spectrum.[5–9] The early expression of STGD1 is typically characterized by a centralized or bull’s eye maculopathy (BEM) lesion after which pisciform fleck formation and/or concurrent atrophic expansion may occur depending on the severity of the disease.[10] Clinically distinguishing STGD1 from other phenotypically similar diseases, such as pattern dystrophy (for example, the subset caused by RDS/PRPH2 mutations), can be difficult especially given that similarities between these conditions exist across different imaging modalities such as 488nm autofluorescence (AF) imaging and spectral domain-optical coherence tomography (SD-OCT). Furthermore, BEM is a relatively common finding in several other retinal dystrophies and can occur in metabolic and drug toxicities such as hydroxychloroquine (HCQ) toxicity.[11] This anti-malaria drug, commonly used in the treatment of various systemic autoimmune disorders, may result in toxic retinopathy and regular screening of retinal function and structure using different tests to detect early signs of retinal toxicity is recommended.[12–15]
Several studies have reported a unique characteristic on SD-OCT in patients with HCQ retinopathy which has been thinning of the outer retinal layers around a preserved region of the ellipsoid zone (EZ), colloquially termed the “flying saucer” sign.[12–17] This term refers to an abrupt disruption of the EZ band in the parafoveal region and thinning of the outer nuclear layer with relative sparing of the foveal region.[13,15–17] This feature has been cited as a contributing element in the diagnosis of HCQ retinopathy;[14,15] however, it is uncertain whether or not its presentation is pathognomonic to this condition.
This report describes eight genetically confirmed STGD1 patients phenocopying the transient HCQ retinopathy phenotype on SD-OCT. A multi-modal assessment of concurrent fundus lesions, ranging from classical BEM to uncharacteristic flecking, is also described. All patients in this study presented to the clinic without a medical history of HCQ use.
Materials and Methods
Patients and clinical evaluation
A retrospective review of 437 patients with a clinical diagnosis and genetic confirmation of STGD1, found to have one or two (expected) disease causing mutations in the ABCA4 gene, was conducted at the Department of Ophthalmology, Columbia University. From this cohort, SD-OCT and AF (488 nm) imaging data were available for 200 patients. Eight of the 200 patients exhibiting the parafoveal outer retina thinning phenotype in HCQ on SD- OCT were identified. All patients (n = 437) were consented before enrolling in the study under the IRB protocol #AAAI9906 approved by the Institutional Review Board at Columbia University. The study adhered to tenets set out in the Declaration of Helsinki. Each patient underwent a complete ophthalmic examination by a retinal physician (SHT), which included slit-lamp and dilated funduscopy examination, best corrected visual acuity (BCVA; Snellen), color fundus photography, short-wavelength fundus autofluorescence (SW-FAF) and SD-OCT imaging. In addition, full-field electroretinograms were obtained on 7 of the 8 patients.
Procedures
Following pupil dilation with tropicamide (1%) and phenylephrine hydrochloride (2.5%) macular SD-OCT volume and high resolution horizontal line scan through the fovea and corresponding fundus images (infrared reflectance and 488nm AF) were acquired with the Spectralis HRA+OCT (Heidelberg Engineering, Heidelberg, Germany). Full-field scotopic and photopic electroretinograms (ERGs) were obtained according to the International Society for Clinical Electrophysiology of Vision (ISCEV) standards[18] using the Espion Visual Electrophysiology System (Diagnosys LLC, Littleton, MA, USA) and silver impregnated fiber electrodes (DTL; Diagnosys LLC, Littleton, MA).
Genetic analyses
Screening of the ABCA4 gene was performed by next-generation sequencing (NGS) as described before[19] or with a different method where all 50 exons and exon-intron boundaries of the ABCA4 gene were amplified using Illumina TruSeq Custom Amplicon protocol (Illumina, San Diego, CA), followed by sequencing on Illumina MiSeq platform. The next-generation sequencing reads were analyzed and compared to the reference genome GRCh37/hg19, using the variant discovery software NextGENe (SoftGenetics LLC, State College, PA). All detected possibly disease-associated variants were confirmed by Sanger sequencing and analyzed with Alamut software (http://www.interactive-biosoftware.com). Segregation of the new variants with the disease was analyzed in families if family members were available. The allele frequencies of all variants were compared to the Exome Variant Server (EVS) dataset, NHLBI Exome Sequencing Project, Seattle, WA, USA (http://snp.gs.washington.edu/EVS/; accessed December 2014).
Results
Clinical and Genetics Evaluation
A retrospective analysis of 8 unrelated STGD1 patients exhibiting the transient HCQ retinopathy phenotype on SD-OCT was performed. Demographic, clinical and genetic characteristics are summarized in Table 1. The cohort (age range: 10–57 years; 4 females and 4 males) consisted of ethnically diverse individuals who presented to the clinic for a retinal evaluation. With the exception of P1 and P3, patients presented with a negative systemic medical history. Patient 1 (P1) reported a history of Hashimoto thyroiditis and P3 had been treated for hypertension and hypercholesterolemia for over 10 years (hydrochlorothiazide 12.5 mg, amlodipine 10 mg, lovastatin 40 mg).
Table 1.
Clinical and Genetics Characteristics of the Study Cohort
| Patient | Age, Gender | Race/Ethnicity | Snellen BCVA | Fundus Appearance | ABCA4 Mutation(s) | ||
|---|---|---|---|---|---|---|---|
| OD | OS | Color | AF | ||||
| P1 | 53, M | Caucasian | 20/20 | 20/20 | Mottling + Flecks | Mottling + Flecks | c.[5461-10T>C] |
| P2 | 55, F | Caucasian | 20/20 | 20/20 | Mottling + Flecks | Mottling + Flecks | p. [A1357V]; [G1961E] |
| P3 | 57, M | African-American | 20/20 | 20/20 | BEM + Flecks | BEM + Flecks | p. [R2107H] |
| P4 | 10, F | Caucasian | 20/30 | 20/25 | BEM + Flecks | BEM + Flecks | p. [E160*]; [R1108C] |
| P5 | 26, F | African-American | 20/30 | 20/20 | Mottling + Flecks | Mottling + Flecks | p. [R2107]; [E526A] |
| P6 | 19, F | Asian-Caucasian | 20/25 | 20/25 | BEM | BEM | p. [R602W] |
| P7 | 26, M | African-Arab | 20/20 | 20/20 | BEM | BEM | p. [R1300*]; [R2106C] |
| P8 | 25, M | Caucasian | 20/20 | 20/40 | BEM | BEM | p. [Q1003*]; [G1961E] |
Abbreviations: M, male; F, female; BCVA, best-corrected visual acuity; OD, right eye; OS, left eye; BEM, bull’s eye maculopathy
Five patients (P2, P4, P5, P6 and P7) reported no visual symptoms but were referred for a retinal evaluation after routine optometric visits, while P3 complained of mild, bilateral metamorphopsia and P8 complained of halos in front of both eyes. BCVAs were unilaterally decreased in P5 and P8 (P5: 20/30 in the right eye and P8: 20/40 in the left eye) and mildly decreased bilaterally in P4 and P6 (P4: 20/30 and 20/25; P6: 20/25 in both eyes); P1-P3 and P7 BCVAs were 20/20 in both eyes (Table 1). All patients exhibited either a confined or bull’s eye maculopathy (BEM)-type lesion restricted within the vascular arcades on funduscopy (Figure 1 and 2). Yellow fleck deposits around the central lesions were observed in 5 patients (P1-P5). Full-field ERG results showed no generalized rod or cone dysfunction. Genetic screening of both the ABCA4 and RDS genes by complete sequencing of the coding regions confirmed two (expected) disease-causing ABCA4 mutations in 5 patients and one mutation in the remaining 3 patients.
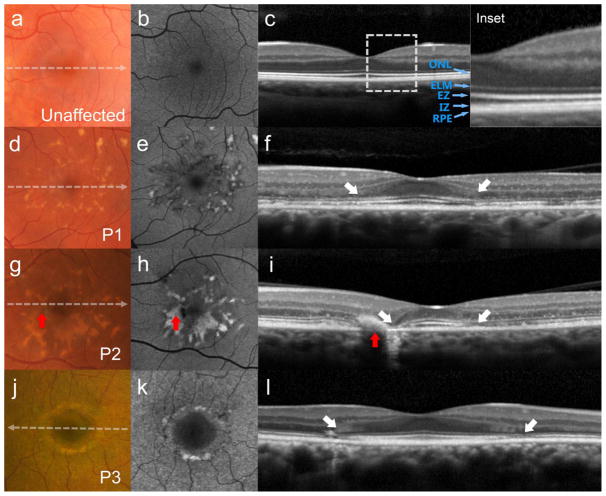
Figure 1.
Thinning of the parafoveal region with relative foveal sparing presenting as the hydroxychloroquine retinopathy-associated parafoveal outer retina thinning phenotype in patients with recessive Stargardt disease (STGD1). (a) Color photograph, (b) autofluorescence (AF), and (c) spectral domain-optical coherence tomography (SD-OCT) of an unaffected individual with outer retinal layers defined: outer nuclear layer (ONL), external limiting membrane (ELM), ellipsoid zone (EZ), interdigitation zone (IZ) and retinal pigment epithelium (RPE) (Inset). Yellow pisciform flecks accompanying mottling over in the central macula are apparent on color, AF and SD-OCT (g, h and i; red arrows). Corresponding SD-OCT scans (f, i and l) reveal abrupt disruptions of the outer retinal layers in the parafoveal regions of each patient (white arrows).
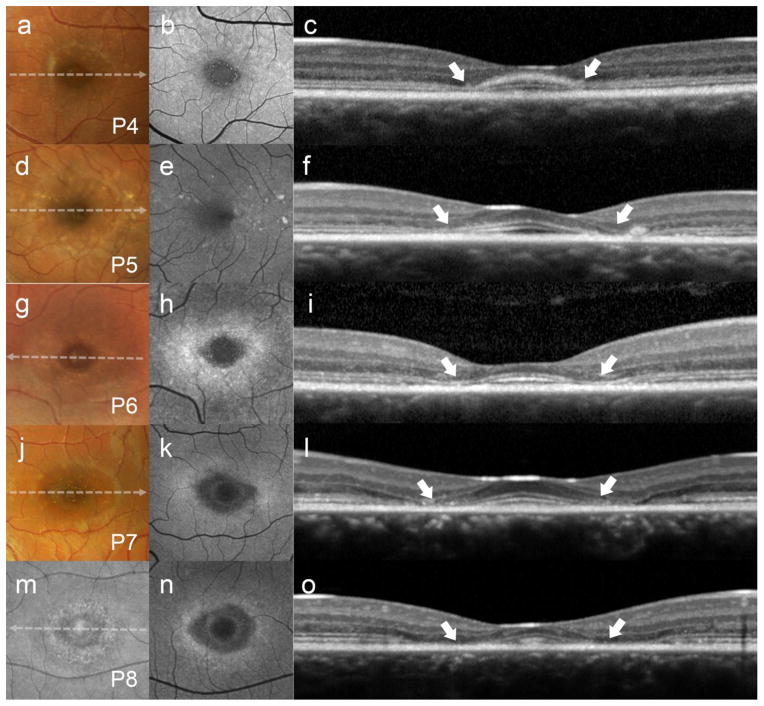
Figure 2.
Abrupt thinning of the parafoveal region (white arrows) with relative foveal sparing presenting as the hydroxychloroquine retinopathy-associated parafoveal outer retina thinning phenotype with recessive Stargardt disease (STGD1) continued. (a, d, g and j) Color photographs, (b, e, h, k and n) autofluorescence (AF), and (c, f, i, l and o) spectral domain-optical coherence tomography (SD-OCT) in patients 4, 5, 6, 7 and 8. (m) Infrared reflectance imaging in P8.
Spectral Domain-Optical Coherence Tomography and Autofluorescence Imaging
Single horizontal line scans through the fovea and volume SD-OCT scans in each patient revealed an abrupt disruption of the EZ band in the parafoveal region and thinning of outer nuclear layer (ONL) with relative sparing of the foveal region. Posterior displacement of the parafoveal inner retinal layers and relatively spared fovea with slightly anteriorly bowing ELM and EZ bands forming so-called “flying saucer” configuration associated with HCQ-induced retinal toxicity[13] was evident in all patients (see Figure 1 and Figure 2, white arrows).
Regions of relative sparing were found to consistently occupy a central area ~0.5 mm in diameter over the foveal center in each patient except P3 whose spared region extended further out (~1.5 mm). Despite the apparent sparing of the fovea, SD-OCT scans showed some abnormalities within this region in all patients. All patients had loss of the interdigitation zone (IZ), thinning of ONL and EZ, except P3, who exhibited normal apparent thickness of all retinal layers and P1 who showed no apparent decrease in ONL thickness in the fovea (Fig 1 and 2). Thinning of the RPE- Bruch’s membrane complex in the fovea was observed in 3 patients (P2, P7 and P8). Patient 4 exhibited a thickened ELM protuberance that delimited a thin layer of spared EZ in the fovea.
The degree of outer retina involvement in the parafoveal region was variable. Three patients (P1-3) showed discontinuity/disruption of the EZ band and thinning of ONL preserving the ELM. Ellipsoid zone disruption in P1 was seen throughout a speckled AF lesion. Total loss of parafoveal EZ was seen in P4-8, while P7 and P8 had the most prominent changes resembling most advanced parafoveal atrophy with ELM loss and RPE thinning (Fig 2, P7 and P8). RPE thinning in this region also was noted in P1 and P2, while P2 had a very confined area of geographic atrophy in the nasal side of the fovea (Fig 1, P2). Interdigitation zone loss was present in all patients becoming visible in this parafoveal region in the majority of patients (P1, P2 and P6-8).
Accompanying macular lesions in SW-AF imaging varied between patients. Round or elliptical BEM lesions with a dark center and hyperautofluorescent border were noted in P6, P7 and P8 while mottled fleck patterns where observed in P1, P2, P3, P4 and P5 (Fig 1 and 2). Patient 3 (P3) and P4 also exhibited a ring of fluorescent granular deposits surrounding the hypoautofluorescent ovoid foveal lesion (Figures 1 and 2, P3 and P4).
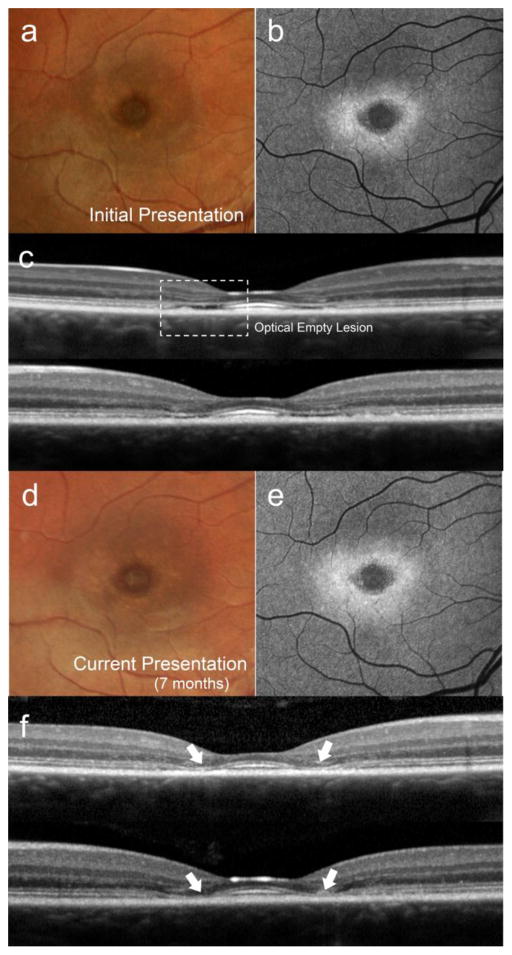
A preceding stage of the transient HCQ retinopathy phenotype in STGD1 was observed in P6 who exhibited EZ band loss (optically empty space) in the parafoveal region around the central island of preserved and hyper-reflective photoreceptor layer (Fig 3c). Seven months later, the optically empty space appeared to collapse, forming the lesion resembling the transient HCQ- induced retinal toxicity SD- OCT phenotype (Fig 3f, white arrows).
Figure 3.
Structural development of the lesion resembling the HCQ- associated parafoveal outer retina thinning phenotype in patient 6. Color (a) and autofluorescence (b) images presented with corresponding spectral domain-optical coherence tomography (SD-OCT) scans in both eyes (c). Parafoveal optically empty lesions in each eye (dotted box) are apparent bilaterally. A subsequent visit 7 months later (d, e and f) reveals an apparent collapse of the inner retinal layers forming abrupt thinning of the parafoveal region (white arrows) consistent with an HCQ- induced retinal toxicity presentation.
Discussion
A retrospective analysis of 8 patients with at least one disease-causing ABCA4 mutation exhibited a variant of foveal sparing phenotype resembling a transient HCQ retinopathy phenotype on SD-OCT. None of these patients had been treated with chloroquine or HCQ.
Majority of the patients did not report any visual symptoms, while 1 patient complained of decreased vision, one patient of metamorphopsia and another patient of halos at the time of presentation. The relatively restricted retinal area of photoreceptor loss and the preservation of the photoreceptors at the fovea is consistent with good visual acuity with no or minimal visual symptoms. The AF images exhibited variable flecks, making AF imaging an important method to distinguish STGD1 from HCQ retinopathy, although three of the patients had classical bull’s eye autofluorescence pattern without any apparent hyperautofluorescent flecks. With regard to the HCQ retinopathy phenotype on SD-OCT, three patients exhibited a ring of EZ granularity and ONL thinning surrounding relatively spared fovea. Three patients showed loss of photoreceptors in the parafoveal region but preserved ELM, while two others, who were at a more advanced stage, exhibited a more extensive zone of atrophy with ELM loss. Despite being relatively spared in all cases, the foveal region in each patient exhibited some apparent abnormalities, not common in early stages of HCQ retinopathy. All patients had loss of IZ and majority had noticeable ONL thinning. Slight thinning of EZ was noticed in all patients, except that P3 appeared unaffected. Noticeable RPE thinning in the fovea was observed in P2, P7 and P8. Together, the varying degrees of disease severity in each case may represent various stages of HCQ retinopathy phenotype development in STGD1. The majority of patients represent early stages of STGD1 with foveal sparing, a confined area of photoreceptor loss, while P7 and P8 had a fully developed form of foveal sparing phenotype with advanced atrophic changes occurring in the parafoveal region of the retina.
Early Foveal Sparing in STGD1
Patients with foveal sparing retain partial or full functioning of the foveal region and typically maintain good visual acuity as both the EZ and RPE layers remain relatively intact in the fovea.[9,20] The precise etiology of foveal sparing in STGD1 is unknown but certain theories point to the anatomical factors within this region that impart protective effects--for instance, given the damaging effects of light in the pathogenesis of STGD1[21,22] it has been suggested that the presence of luteal pigment, which absorbs short-wavelength and ultraviolet light, protects this region.[23] Another hypothesis points to the ability of cones to receive 11-cis-retinal from Muller cells enabling the alternative visual cycle, whereas the chromophore in rods is solely derived from the RPE cells which have been found to be significantly affected in STGD1.[24,25] The role of genetic etiology in foveal sparing has been explored and it has been reported that the ABCA4 variant p.R2030Q is more prevalent in these patients[9], however, this rare variant was not found in our cohort. An etiological connection between foveal sparing and ABCA4 is further weakened by the incidence of foveal sparing phenotype in other genetically distinct retinal degenerative diseases such as RDS/PRPH2 pattern dystrophy and AMD, among others.[26,27] Further more extensive study will be necessary to elucidate its precise mechanism.
Common Pathways between STGD1 and HCQ Retinopathy
Chloroquine and its derivative HCQ is known to bind with melanin and preferentially accumulate in the pigmented tissues of the eye (iris, ciliary body, choroid and RPE).[28] As weak bases, both chloroquine and HCQ, elevate lysosomal pH disrupting its degradative capacity. This effect in RPE reduces the efficient degradation of photoreceptor outer segments ultimately leading to the formation and oxidation of lipofuscin.[29,30] The pathophysiology of STGD1 results from the dysfunction of the ABCA4 protein permitting the accumulation of retinaldehydes in the outer segments and lipofuscin in the RPE cells. Lipofuscin accumulation in the RPE is believed to be the impetus of STGD1.[3,4] Increased levels of AF from lipofuscin accumulation in STGD1 have been reported[31] and the areas of increased AF around the developed BEM lesion observed in our cohort (P6, P7 and P8) may reflect local accumulation of lipofuscin as has been previously speculated upon.[32] However, in previous studies of patients with HCQ retinopathy the increased AF has been attributed to photoreceptor outer segment-derived AF.[33,34,15] Despite the possible similarities between these two retinopathies and early disruption of RPE metabolism in HCQ retinopathy, several imaging studies with OCT in HCQ retinopathy have shown that photoreceptor loss precedes RPE cells atrophy,[15,34,12,13] while in STGD1, the precise sequence of RPE/photoreceptor loss is less clear.[35,36,6] All patients from our cohort with the described HCQ retinopathy phenotype on SD- OCT had a bull’s eye pattern or confined patterns of mottling on AF suggesting RPE involvement, while in HCQ retinopathy bull’s eye atrophy occurs at a relatively late stage.[14] The observation that RPE is more affected and that this occurs at an earlier stage of the disease process in STGD1 patients than in patients with HCQ retinopathy serves as an important distinguishing characteristic between these two disease phenotypes.
An ABCA4-associated genetic predisposition to retinopathy following chloroquine or HCQ treatment was proposed by Shroyer et al [37] where disease-associated missense variants in ABCA4 were found in two of 8 patients with the history of HCQ use and apparent HCQ maculopathy. These variants were not present in a control group, suggesting that carrying an ABCA4 mutation may increase the risk of HCQ retinopathy. In fact, one of the 2 patients was homozygous for the missense mutation p.R2107H, but was thought to have HCQ maculopathy due to classical appearance to this retinopathy in addition to lack of dark choroid and flecks characteristic to STGD1. Interestingly, the p.R2107H mutation was also present in two of the 8 patients in this study with phenotypes resembling HCQ retinopathy. Both of these patients are of African descent. Furthermore, this variant has been recently described as a highly prevalent disease-causing mutation in African-American patients with STGD1, usually with a later onset, milder phenotype.[38]
In conclusion we present 8 STGD1 patients who exhibited an early and transient stage of foveal sparing which resembles the parafoveal thinning phenotype associated with HCQ retinopathy. A conspicuously distinct feature in the retinal appearance of our STGD1 cohort was the presence of pisciform flecks in 5/8 patients (P1, P2, P3, P4 and P5) which was apparent on funduscopy, AF and SD-OCT imaging. The development of such flecks is characteristic to STGD1; however, certain cases may present only with a centralized lesion or BEM (e.g., patients P6, P7 and P8 in this study). Conversely, the fundus appearance of early HCQ retinopathy has been described to be less variable across cases.[14] A thorough examination of the fundus, and in more difficult, ambiguous cases, genetic screening of retinal dystrophies associated with foveal sparing, should accompany the evaluation of retinal toxicity to avoid the misdiagnosis of masquerading inherited retinal dystrophies, particularly Stargardt disease.
Acknowledgments
Financial support
This study was supported, in part, by grants from the National Eye Institute/NIH EY021163, EY019861, EY018213 and EY019007 (Core Support for Vision Research), Foundation Fighting Blindness (Owings Mills, MD), and unrestricted funds from Research to Prevent Blindness (New York, NY) to the Department of Ophthalmology, Columbia University.
Footnotes
Conflict of Interest: All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- 1.Michaelides M, Hunt DM, Moore AT. The genetics of inherited macular dystrophies. J Med Genet. 2003;40(9):641–650. doi: 10.1136/jmg.40.9.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15(3):236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 3.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98(1):13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 4.Quazi F, Molday RS. ATP-binding cassette transporter ABCA4 and chemical isomerization protect photoreceptor cells from the toxic accumulation of excess 11-cis-retinal. Proc Natl Acad Sci U S A. 2014;111(13):5024–5029. doi: 10.1073/pnas.1400780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fishman GA, Stone EM, Grover S, Derlacki DJ, Haines HL, Hockey RR. Variation of clinical expression in patients with Stargardt dystrophy and sequence variations in the ABCR gene. Arch Ophthalmol. 1999;117(4):504–510. doi: 10.1001/archopht.117.4.504. [DOI] [PubMed] [Google Scholar]
- 6.Noupuu K, Lee W, Zernant J, Tsang SH, Allikmets R. Structural and Genetic Assessment of the ABCA4-Associated Optical Gap Phenotype. Invest Ophthalmol Vis Sci. 2014 doi: 10.1167/iovs.14-14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cella W, Greenstein VC, Zernant-Rajang J, Smith TR, Barile G, Allikmets R, Tsang SH. G1961E mutant allele in the Stargardt disease gene ABCA4 causes bull’s eye maculopathy. Exp Eye Res. 2009;89(1):16–24. doi: 10.1016/j.exer.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lois N, Holder GE, Bunce C, Fitzke FW, Bird AC. Phenotypic subtypes of Stargardt macular dystrophy-fundus flavimaculatus. Arch Ophthalmol. 2001;119(3):359–369. doi: 10.1001/archopht.119.3.359. [DOI] [PubMed] [Google Scholar]
- 9.Fujinami K, Sergouniotis PI, Davidson AE, Wright G, Chana RK, Tsunoda K, Tsubota K, Egan CA, Robson AG, Moore AT, Holder GE, Michaelides M, Webster AR. Clinical and molecular analysis of Stargardt disease with preserved foveal structure and function. Am J Ophthalmol. 2013;156(3):487–501. e481. doi: 10.1016/j.ajo.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Fishman GA. Fundus flavimaculatus. A clinical classification. Arch Ophthalmol. 1976;94(12):2061–2067. doi: 10.1001/archopht.1976.03910040721003. [DOI] [PubMed] [Google Scholar]
- 11.Kanski JJBB. A Systemic Approach. 7. Elsevier; Edinburg: 2011. Clinical Ophthalmology. [Google Scholar]
- 12.Rodriguez-Padilla JA, Hedges TR, 3rd, Monson B, Srinivasan V, Wojtkowski M, Reichel E, Duker JS, Schuman JS, Fujimoto JG. High-speed ultra-high-resolution optical coherence tomography findings in hydroxychloroquine retinopathy. Arch Ophthalmol. 2007;125(6):775–780. doi: 10.1001/archopht.125.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen E, Brown DM, Benz MS, Fish RH, Wong TP, Kim RY, Major JC. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the “flying saucer” sign) Clin Ophthalmol. 2010;4:1151–1158. doi: 10.2147/OPTH.S14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF American Academy of O. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):415–422. doi: 10.1016/j.ophtha.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Marmor MF. Comparison of screening procedures in hydroxychloroquine toxicity. Arch Ophthalmol. 2012;130(4):461–469. doi: 10.1001/archophthalmol.2011.371. [DOI] [PubMed] [Google Scholar]
- 16.Ascaso FJ, Rodriguez NA, San Miguel R, Huerva V. The “flying saucer” sign on spectral domain optical coherence tomography in chloroquine retinopathy. Arthritis Rheum. 2013;65(9):2322. doi: 10.1002/art.38063. [DOI] [PubMed] [Google Scholar]
- 17.Tailor R, Elaraoud I, Good P, Hope-Ross M, Scott RA. A case of severe hydroxychloroquine-induced retinal toxicity in a patient with recent onset of renal impairment: a review of the literature on the use of hydroxychloroquine in renal impairment. Case Rep Ophthalmol Med. 2012;2012:182747. doi: 10.1155/2012/182747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M International Society for Clinical Electrophysiology of V. ISCEV Standard for full-field clinical electroretinography (2008 update) Doc Ophthalmol. 2009;118(1):69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- 19.Zernant J, Schubert C, Im KM, Burke T, Brown CM, Fishman GA, Tsang SH, Gouras P, Dean M, Allikmets R. Analysis of the ABCA4 gene by next-generation sequencing. Invest Ophthalmol Vis Sci. 2011;52(11):8479–8487. doi: 10.1167/iovs.11-8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Huet RA, Bax NM, Westeneng-van Haaften SC, Muhamad M, Zonneveld-Vrieling MN, Hoefsloot LH, Cremers FP, Boon CJ, Klevering BJ, Hoyng C. Foveal sparing in Stargardt disease. Invest Ophthalmol Vis Sci. 2014 doi: 10.1167/iovs.13-13825. [DOI] [PubMed] [Google Scholar]
- 21.Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80(5):595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000;41(7):1981–1989. [PubMed] [Google Scholar]
- 23.Kim SR, Nakanishi K, Itagaki Y, Sparrow JR. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp Eye Res. 2006;82(5):828–839. doi: 10.1016/j.exer.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Lee W, Noupuu K, Oll M, Duncker T, Burke T, Zernant J, Bearelly S, Tsang SH, Sparrow JR, Allikmets R. The external limiting membrane in early-onset Stargardt disease. Invest Ophthalmol Vis Sci. 2014;55(10):6139–6149. doi: 10.1167/iovs.14-15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JS, Kefalov VJ. The cone-specific visual cycle. Prog Retin Eye Res. 2011;30(2):115–128. doi: 10.1016/j.preteyeres.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boon CJ, Jeroen Klevering B, Keunen JE, Hoyng CB, Theelen T. Fundus autofluorescence imaging of retinal dystrophies. Vision Res. 2008;48(26):2569–2577. doi: 10.1016/j.visres.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz-Valckenberg S, Fleckenstein M, Helb HM, Charbel Issa P, Scholl HP, Holz FG. In vivo imaging of foveal sparing in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(8):3915–3921. doi: 10.1167/iovs.08-2484. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein H, Zvaifler N, Rubin M, Mansour AM. The Ocular Deposition of Chloroquine. Invest Ophthalmol. 1963;2:384–392. [PubMed] [Google Scholar]
- 29.Sundelin SP, Terman A. Different effects of chloroquine and hydroxychloroquine on lysosomal function in cultured retinal pigment epithelial cells. APMIS. 2002;110(6):481–489. doi: 10.1034/j.1600-0463.2002.100606.x. [DOI] [PubMed] [Google Scholar]
- 30.Mahon GJ, Anderson HR, Gardiner TA, McFarlane S, Archer DB, Stitt AW. Chloroquine causes lysosomal dysfunction in neural retina and RPE: implications for retinopathy. Curr Eye Res. 2004;28(4):277–284. doi: 10.1076/ceyr.28.4.277.27835. [DOI] [PubMed] [Google Scholar]
- 31.Burke TR, Duncker T, Woods RL, Greenberg JP, Zernant J, Tsang SH, Smith RT, Allikmets R, Sparrow JR, Delori FC. Quantitative fundus autofluorescence in recessive Stargardt disease. Invest Ophthalmol Vis Sci. 2014;55(5):2841–2852. doi: 10.1167/iovs.13-13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kellner U, Kellner S, Weinitz S. Chloroquine retinopathy: lipofuscin- and melanin-related fundus autofluorescence, optical coherence tomography and multifocal electroretinography. Doc Ophthalmol. 2008;116(2):119–127. doi: 10.1007/s10633-007-9105-6. [DOI] [PubMed] [Google Scholar]
- 33.Kellner U, Renner AB, Tillack H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest Ophthalmol Vis Sci. 2006;47(8):3531–3538. doi: 10.1167/iovs.05-1290. [DOI] [PubMed] [Google Scholar]
- 34.Kellner S, Weinitz S, Kellner U. Spectral domain optical coherence tomography detects early stages of chloroquine retinopathy similar to multifocal electroretinography, fundus autofluorescence and near-infrared autofluorescence. Br J Ophthalmol. 2009;93(11):1444–1447. doi: 10.1136/bjo.2008.157198. [DOI] [PubMed] [Google Scholar]
- 35.Gomes NL, Greenstein VC, Carlson JN, Tsang SH, Smith RT, Carr RE, Hood DC, Chang S. A comparison of fundus autofluorescence and retinal structure in patients with Stargardt disease. Invest Ophthalmol Vis Sci. 2009;50(8):3953–3959. doi: 10.1167/iovs.08-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burke TR, Rhee DW, Smith RT, Tsang SH, Allikmets R, Chang S, Lazow MA, Hood DC, Greenstein VC. Quantification of peripapillary sparing and macular involvement in Stargardt disease (STGD1) Invest Ophthalmol Vis Sci. 2011;52(11):8006–8015. doi: 10.1167/iovs.11-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shroyer NF, Lewis RA, Lupski JR. Analysis of the ABCR (ABCA4) gene in 4-aminoquinoline retinopathy: is retinal toxicity by chloroquine and hydroxychloroquine related to Stargardt disease? Am J Ophthalmol. 2001;131(6):761–766. doi: 10.1016/s0002-9394(01)00838-8. [DOI] [PubMed] [Google Scholar]
- 38.Zernant J, Collison FT, Lee W, Fishman GA, Noupuu K, Yuan B, Cai C, Lupski JR, Yannuzzi LA, Tsang SH, Allikmets R. Genetic and clinical analysis of ABCA4-associated disease in African American patients. Hum Mutat. 2014;35(10):1187–1194. doi: 10.1002/humu.22626. [DOI] [PMC free article] [PubMed] [Google Scholar]