Abstract
Arabinocytosine (AraC, also known as Cytarabine) is one of the mainstays of AML therapy, but like other DNA damaging therapeutic agents it is rarely curative by itself. There is an emerging realization that the therapeutic outcomes may be improved by combining AraC with other compounds. Here we report that the addition of a differentiating agent combination immediately following AraC damage to AML blasts, selectively increases the cell kill. The experiments were performed using cultured cells from established cell lines of AML (HL60 and U937). The cells were exposed to 100 nM AraC, a concentration which produced approximately 25–50% cell kill, followed by a combination of 100 nM 1alpha-hydroxyvitamin D2 (1-D2) and 10 uM carnosic acid (CA), which together can serve as a powerful differentiating agent combination for AML cells, but are not toxic alone. AraC-induced cell death, measured by Annexin V/Propidium Iodide, was significantly (p<0.01) increased by the 1-D2/CA combination in both cell lines, but not by 1-D2 or CA alone. The enhancement of cell death occurred by both apoptosis and necrosis, was associated with increased DNA damage and with higher levels of DNA damage response (DDR) activated marker Chk1, but the expression of p27, a cell cycle inhibitor protein, was not enhanced by 1-D2/CA. The principal finding is that a vitamin D analog 1-D2 combined with a plant-derived antioxidant CA can markedly augment the cytotoxic action of AraC, an anti-leukemia therapeutic agent.
Keywords: Vitamin D analog, AraC, Plant antioxidant, Acute Myeloid leukemia (AML), Apoptosis, VDR, Chk1
1. Introduction
Poor long-term outcomes from standard therapy continue to be a major problem for patients with Acute Myeloid Leukemia (AML). The physiologically active form of vitamin D3, 1α,25-dihydroxyvitamin D3 (calcitriol), has been known for over three decades to effectively overcome the blocked differentiation of AML cells. However, recent clinical trials of vitamin D derivatives (VDDs) showed unremarkable results, suggesting that approaches to the application of this knowledge to the clinic are currently sub-optimal, as recently articulated [1]. In addition to the problems cited in that review, it is also difficult to obviate the hypercalcemic effect of VDDs, the principal cause of patient toxicity [2, 3]. Repeated attempts to synthesize and clinically test analogs of calcitriol, in which the hypercalcemic activity of the analog is dissociated from its differentiation-inducing activity, have as yet produced no tangible results [3, 4]. An alternative approach to improve AML therapy is to combine VDDs, including calcitriol, with other compounds. Numerous preclinical studies, principally in vitro, have shown greater differentiation potency of VDDs combined with diverse compounds, when compared to VDDs as sole agents, and were often associated with cell death [5, 6]. However, to date, most clinical trials of these combinations have not been encouraging [7, 8]. Of note, most of these trials did not take into account the reported dependence on “time-sequencing”, ie the time of exposure to the other agent relative to the VDD addition [9].
Our laboratories have shown that in order to obtain a potential beneficial therapeutic effect of calcitriol combined with a DNA damaging agent (DDA), arabinocytosine (AraC) or hydroxyurea on HL60 cells, an established AML-M2 cell line, calcitriol has to be administered after the DDA [9, 10]. In contrast, if the exposure to calcitriol takes place before DNA damage is established, calcitriol can protect the cells from damage, possibly due to the up-regulation of hKSR-2 by calcitriol, which facilitates the functioning of survival-enhancing downstream targets of the MAPK pathway [11], and by the AKT pathway [12]. A recent report of survival improvement in patients with AML or MDS, who were in remission achieved with standard chemotherapy, and who then received maintenance treatment with agents that included differentiating agents [13], is consistent with the hypothesis that the exposure to a VDD after cellular DNA damage is necessary for improved outcome of therapy.
In the current study we found that Doxercalciferol (1α-hydroxyvitamin D2; 1-D2), a low calcemic analog of vitamin D2 [2, 14], combined with carnosic acid (CA), a plant derived polyphenol with anti-oxidant properties [15, 16], increases the cytotoxicity of AraC to AML cells.
2. Materials and Methods
2.1. Chemicals and antibodies
Arabinocytosine and Doxercalciferol were purchased from Sigma-Aldrich (St. Louis, MO). Carnosic acid (CA) was purchased from Enzo Life Sciences, Inc. (Farmingdale, NY). The following antibodies: Crk-L (sc-319), VDR (sc-1008), C/EBP beta (sc-150), and p27Kip1 (sc-528) were obtained from Santa Cruz Biotechnology (Dallas, TX). Phospho-Ser139-H2AX (#9718), phospho-Chk1 (#2348), and HRP-linked anti-rabbit (#7074) antibodies were purchased from Cell Signaling Technologies (Danvers, MA).
2.2. Cell culture and NSE staining
AML cell lines, HL60 (acute promyeloblastic leukemia) [17], and U937 (monocytes from histiocytic lymphoma) [18], were cultured in suspension with RPMI 1640 medium supplemented with 10% heat-inactivated bovine calf serum in a humidified atmosphere at 37°C. Cell cultures were passaged two to three times a week to maintain log phase growth. The absence of Mycoplasma was confirmed periodically in each cell line. For experiments, cells were seeded in 6-well plates at densities of 3×105/mL followed by the addition of combinations of the agents under study for the indicated times. For instance, to determine cell death, the cultures were divided into a group exposed for 72 h to only the vehicle for AraC (0.1% DMSO), and a group treated with 100 nM AraC. After 72 h the cells were washed with fresh medium, and each of these groups was further divided for treatment for 96 h with 1-D2 (100 nM), or CA (10 uM), or the combination of these two agents. Cell number and cell viability were determined using the Trypan blue exclusion counts in a Neubauer hemocytometer. Cell differentiation was assessed by non-specific esterase (NSE) staining of smears made by resuspending 1 × 106 cells in 100 µl 1×PBS and spreading on slides. The air-dried smears were fixed in formalin acetone mixture buffer for 30 sec, then washed with distilled water and stained for 45 min at room temperature with the following solution: 67 mM phosphate buffer, pH 7.6, 8.9 ml, hexazotized pararosaniline, 0.6 ml, 10 mg alpha-naphtyl acetate, and 0.5 ml ethylene glycol monomethyl ether. The NSE-positive cells were enumerated by counting at least 500 cells in each group.
2.3. Annexin V and propidium iodide staining
Experimental samples were collected, washed twice with 1×PBS, then resuspended in the binding buffer, containing 0.14 M NaCl and 2.5 mM CaCl2, pH 7.5, and stained using an Annexin V-FITC Kit (Sigma). The cells were incubated with 50 µg/ml Annexin V and 20 µg/ml propidium iodide in 1× binding buffer at room temperature in the dark for 15 min, and immediately analyzed by flow cytometry (EPICS XL). Annexin V-positive/ PI-negative cells were considered as early apoptotic, cells with both Annexin V and PI positive, as late apoptotic, and Annexin negative but PI positive as “necrotic”, most likely a variety of caspase independent modes of cell death [19].
2.4. Comet assay of DNA damage
HL60 and U937 cells were treated with AraC, 1-D2/CA or AraC/1-D2/CA for indicated times. DNA damage within cells was measured by comet assay [20]. This assay was performed according to the manufacturer's recommended procedure (Cell Biolabs, San Diego, CA). Briefly, AML cells were washed twice with 1×PBS, then 10,000 cells were mixed with low melting-point agarose gel and pipetted on the “Comet Slide”. The cells transferred to the slide were maintained for 15 minutes at 4°C in the dark. The slide was then immersed in the lysis buffer for 30 minutes at 4°C in the dark, to relax and denature the nuclear DNA. Next, the slide was transferred into a horizontal electrophoresis tank in TBE buffer, and ran for 15 min at 30 volts. The slide was rinsed three times with distilled water and once with 70% ethanol. Finally, the dried slide was stained with Vista Green DNA Dye for 15 minutes at room temperature. The cells on the slide were then visualized and photographed using a fluorescent microscope. The images were analyzed with the Opencomet Software, which was used as a plug-in for the image processing platform, ImageJ [21]. The percentage of DNA in tails was selected as a measure of DNA damage. In each slide, obtained from three or more independent experiments, at least 50 stained cells were measured and analyzed.
2.5. Western blotting
Western blotting was performed using 50 µg of whole cell extracts as described before [22]. Briefly, membranes were incubated with primary antibodies of interest for 2 h, and then blotted with a HRP-linked secondary antibody for 1 h. The protein bands were visualized using the chemiluminescence detection system (Pierce Biotechnology, Rockford, IL). Each membrane was stripped and reprobed for Crk-L as an internal loading control. The optical density (OD) of each band was quantitated using ImageQuant 5.0 (Molecular Dynamics, Sunnyvale, CA).
2.6. Statistical analysis
Each experiment was repeated 3–6 times. The results are presented as the mean ± SD. Significance of the differences between mean values was assessed by a 2-tailed Student's T-test. All computations were done with an IBM-compatible personal computer using Microsoft EXCEL program.
3. Results
3.1. Combination of 1-D2 and CA increases the cytotoxicity of AraC to AML cells
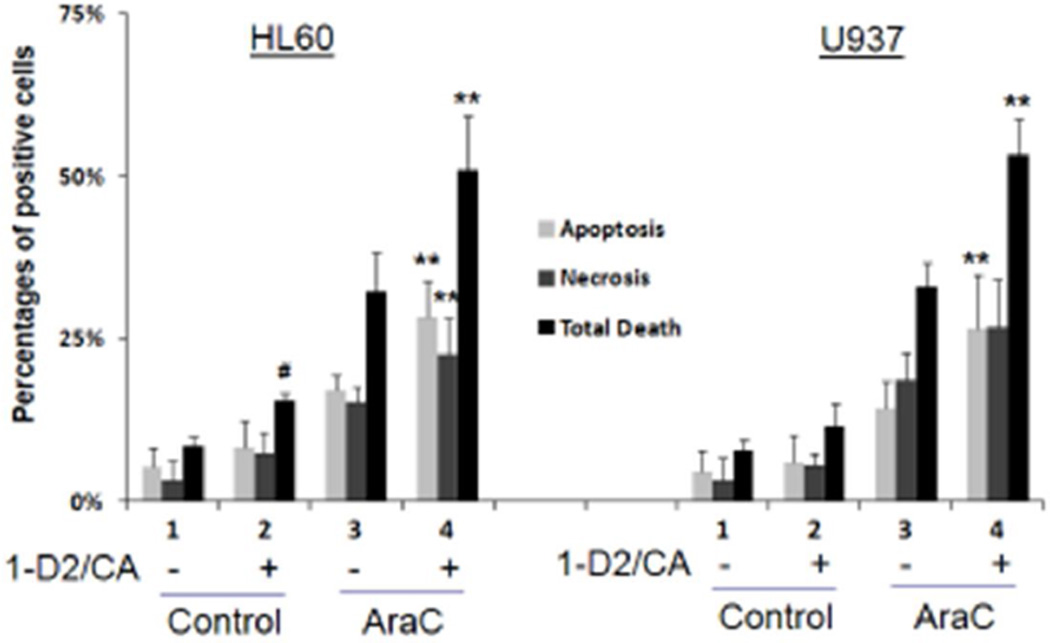
Previous studies of the effects of differentiating agents on AML cells with damaged DNA used calcitriol as the sole differentiating agent at concentrations that were likely to induce hypercalcemia in vivo. Accordingly, it seemed important to perform similar studies using a differentiation–inducing agent or agents which are safe for human use at effective concentrations. Such agents are Doxercalciferol, a vitamin D2 derivative already approved for human use in dialysis patients, and Carnosic acid, a food flavoring botanical which has anti-oxidant properties and increases the differentiation activity of VDDs [14, 15]. As shown in Fig 1 and Table 1, when either HL60 or U937 cells were exposed to AraC for 4 days and then treated with the 1-D2/CA combination there is a highly significant increase in cell death, due to both apoptosis and necrosis, as determined by flow cytometry of Annexin V stained cells (Fig 1) and confirmed by Trypan blue exclusion (data not shown).
Figure 1. Comparison of the effects of 1-D2/CA on AraC-induced cell death in cell lines.
The treatment groups were: 1. Vehicle control- for 96 h; 2. 1-D2 96 h; 3. AraC-72 h- medium only −96 h; 4. AraC-72 h-1-D296 h. The concentrations and other experimental details are described in Materials and Methods. # = p<0.05 when compared to control, *= p < 0.05, and ** = p < 0.01, compared with AraC treated group; n=3 for both HL60 and U937 cells.
Table 1.
Statistical significance of the effects of AraC vs combinations of AraC, CA and 1-D2.
| HL60 cells | U937 cells | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment groups | Average±SD Apoptosis |
P value vs AraC |
P value vs AraC-CA |
P value vs AraC-1-D2 |
Average Apoptosis |
P value vs AraC |
P value vs AraC-CA |
P value vs AraC-1-D2 |
| Ara-C-100 nM-Cotnrol | 17.1% ± 5.8% | 14.3% ± 4.7% | ||||||
| Ara-C-72hr-CA-96hr | 19.2% ± 6.1% | 0.083 | 15.9% ± 2.3% | 0.252 | ||||
| Ara-C-72hr-1-D2-96hr | 18.3% ± 8.3% | 0.573 | 17.8% ± 4.0% | 0.081 | ||||
| Ara-C-72hr-CAD2-96hr | 28.3% ± 5.6% | 0.008 | 0.004 | 0.003 | 25.5% ± 5.9% | 0.002 | 0.011 | 0.024 |
| Necrosis | Necrosis | |||||||
| Ara-C-100 nM-Cotnrol | 15.3% ± 7.9% | 18.6% ± 3.9% | ||||||
| Ara-C-72hr-CA-96hr | 16.1% ± 6.9% | 0.540 | 20.5% ± 7.2% | 0.272 | ||||
| Ara-C-72hr-1-D2-96hr | 18.5% ± 8.2% | 0.158 | 21.0% ± 5.9% | 0.119 | ||||
| Ara-C-72hr-CAD2-96hr | 22.5% ± 9.8% | 0.010 | 0.008 | 0.073 | 25.8% ± | 0.118 | 0.232 | 0.156 |
| Total death | Total death | |||||||
| Ara-C-100 nM-Cotnrol | 32.4% ± 9.4% | 32.9% ± 2.5% | ||||||
| Ara-C-72hr-CA-96hr | 35.3% ± 10.3% | 0.012 | 36.4% ± 5.3% | 0.208 | ||||
| Ara-C-72hr-1-D2-96hr | 36.8% ± 9.6% | 0.192 | 38.8% ± 4.1% | 0.042 | ||||
| Ara-C-72hr-CAD2-96hr | 50.8% ± 10.2% | 0.001 | 0.002 | 0.001 | 51.3% ± 5.7% | 0.003 | 0.002 | 0.004 |
While the enhancing effect of the 1-D2 and CA combination on apoptosis and necrosis was supra-additive (or synergistic) of the individual actions of its components, when these were used alone only a minimal effect on AraC- induced cell death was observed (Table 1). Also, in HL60 cells the 1-D2/CA combination had only a marginal effect on survival if the cells were not exposed to AraC, while in U937 cells no significant effect of the 1-D2/CA combination was noted without AraC (Fig 1). Collectively, the data show that the 1-D2/CA combination effectively enhances AraC cytotoxicity.
3.2. The enhancement of AraC-induced cell death parallels increased DNA damage and higher levels of a DNA damage response (DDR) marker
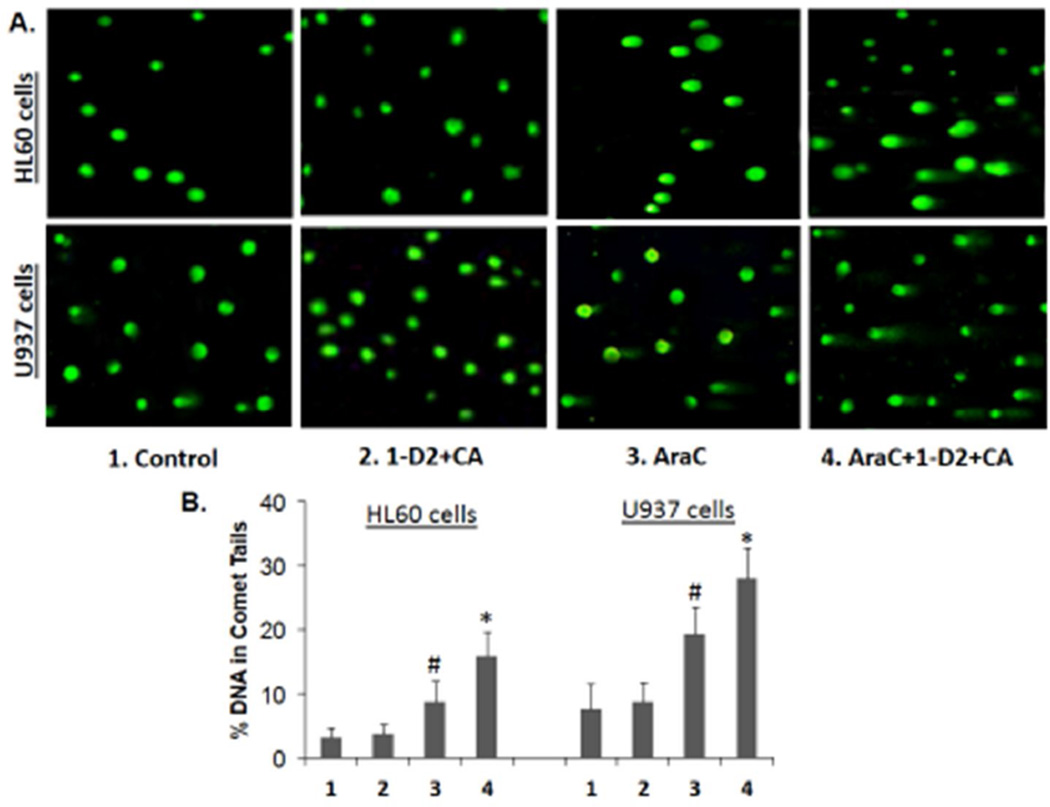
DNA damage can be recognized by various methods. In previous studies the effects of differentiation agents on toxic AML cells were demonstrated by the reduced rate of maturation of DNA replication intermediates and the development of DNA double strand breaks [10, 23]. To establish that the basis of the cell death enhancement by the 1- D2/CA combination is the result of increased DNA damage, we used here the “comet” assay [20]. Exposure of HL60 or U937 cells to 1-D2/CA in the absence of AraC had no discernible effect, while AraC alone produced only moderate DNA damage as shown by DNA “tails” on agarose micro-electrophoresis (Fig 2). However, after the enhancement of AraC cytotoxicity by 1-D2/CA combination the comet tails markedly increased, not only in the numbers of affected cells but also in tail length (Fig 2, A and B).
Figure 2. Comet assays of DNA damage.
A. Representative images of tailing of damaged DNA show that 1-D2/CA combination alone has no apparent effect on AML cells. As expected, there is evidence of DNA damage when the cells are exposed to AraC alone, 100 nM for 72 h and maintained in normal medium for additional 96 h, (center panels). DNA damage is increased when AraC is followed by 1-D2 (100 nM) and CA (10 uM) for 96 h (right side panels). B. Quantitation of comet tails, as described in M & M. # = p <0.05 when compared to control; * = p < 0.01, compared with AraC-treated group; n=3.
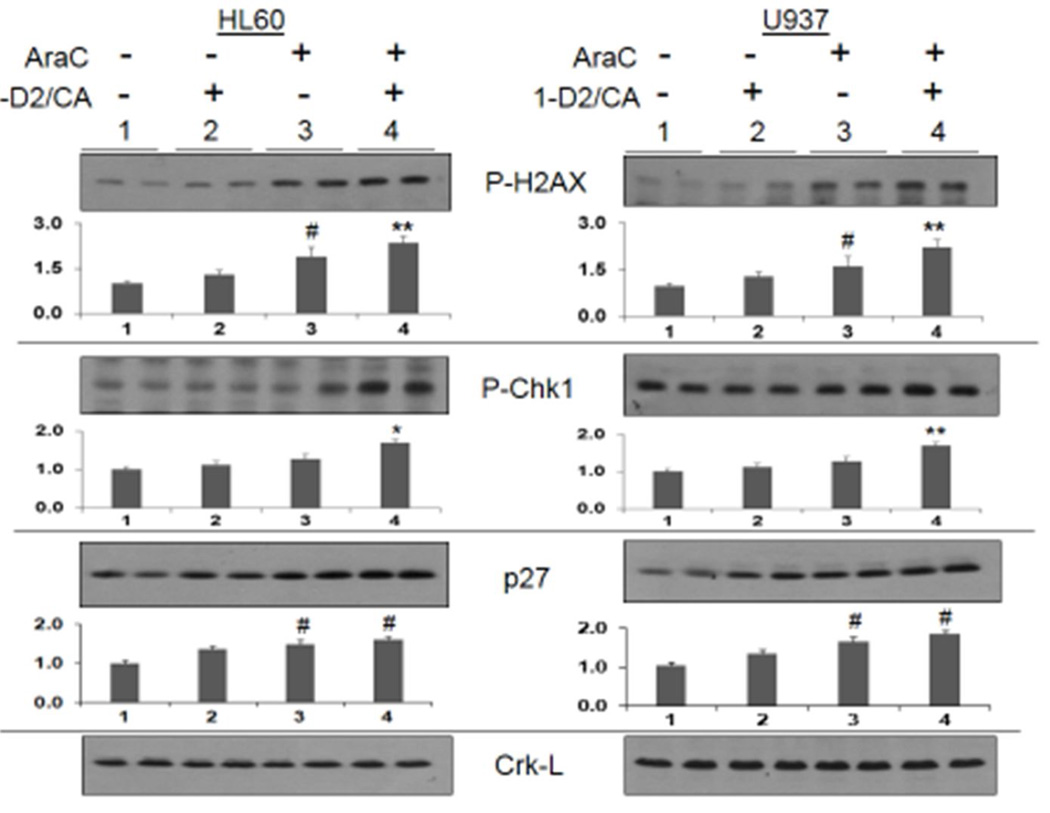
Phosphorylated histone 2 variant AX (gamma H2AX, P-H2AX, Ser139) is an established marker of DNA double strand breaks [24, 25], while Chk1 is an important component of the complex network known as DDR [26, 27]. We have therefore monitored the levels of these proteins to further confirm that cell death enhancement by 1-D2/CA involves DNA damage. As shown in Fig 3, top panel, 1-D2/CA further increased P-H2AX levels in AraC-treated AML cells, already elevated by AraC exposure. Thus, the comet assay and P-H2AX levels clearly indicate that the enhancement of cell death is due to the synergistic induction of dsDNA breaks by AraC followed by differentiation agents. Interestingly, in these experiments the expression of activated Chk1, an integrator of DNA damage checkpoints [28, 29], was not increased by AraC alone, yet the addition of 1-D2/CA resulted in its significant increase (Fig 3). Thus, the higher levels of dsDNA breaks in the AraC/1-D2/CA indicated by P-H2AX may be required to be recognized by P-Chk1, or by its upstream target, and provide the initial trigger for a cascade of further molecular events that can lead to the activation of cell death pathways.
Figure 3. Western blots showing protein levels of molecular markers of DNA damage (P-H2AX), DNA damage signaling (PChk1), and cell cycle inhibitor p27Kip1.
Duplicate lanes of each experimental group were run, as shown by the horizontal line above the signals. Note that AraC alone increases the P-H2AX signal, but the addition of 1-D2/CA significantly increases the AraC effect, while the 1-D2 /CA combination alone has no significant effect on vehicle–treated control cells. In contrast, p27 protein levels are increased by AraC but this increase is not further enhanced by1-D2/CA combination. Crk-L signal provided a loading control for the Westerns, which was used to determine the corrected optical densities (OD) for each signal. Below each blot the bar chart shows the mean OD and SD of 3 replicate blots. # = p < 0.05, when compared with control group; * = p < 0.05, when compared with AraC alone treated cells; ** = p < 0.01, when compared with AraC alone; n=3.
The p27 cell cycle inhibitor is known to be the principal regulator of cell cycle arrest in VDD-treated AML cells [30]. In contrast to P-H2AX and P-Chk1, we observed no enhancement by 1-D2/CA of its AraC induced levels (Fig 3, bottom row). Since AraC is an S-phase specific drug [31], this suggests that a relaxation of cell cycle controls, and thus a prolongation of the S phase, is not a contributing factor to the observed AraC cytotoxicity enhancement.
3.3. The 1-D2/CA combination stimulates differentiation-inducing pathways in cells with DNA damage
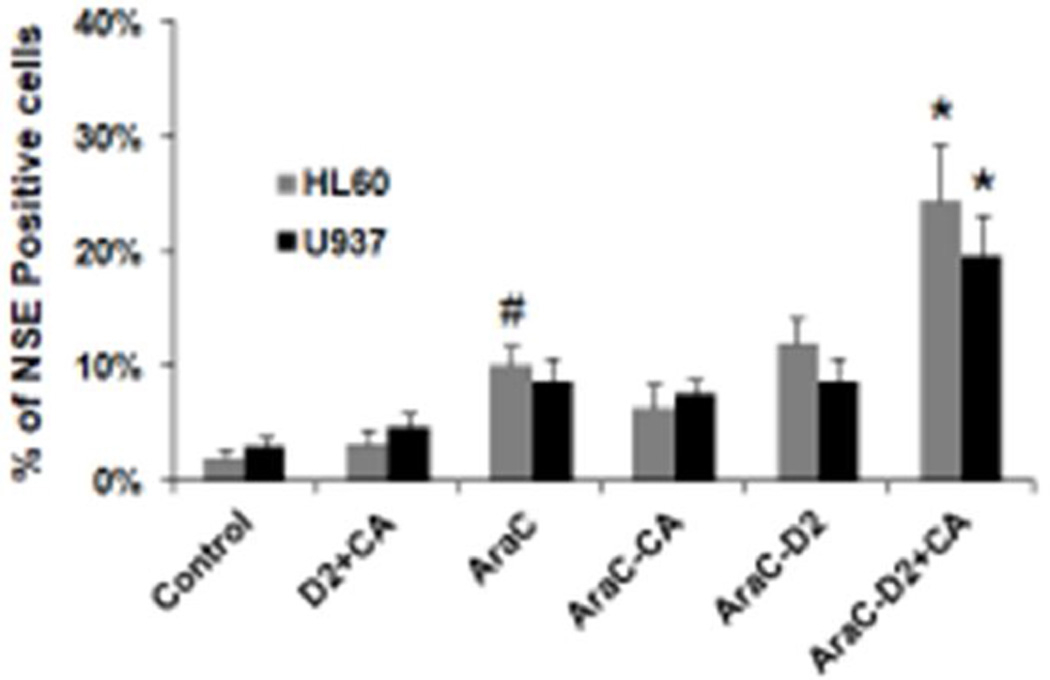
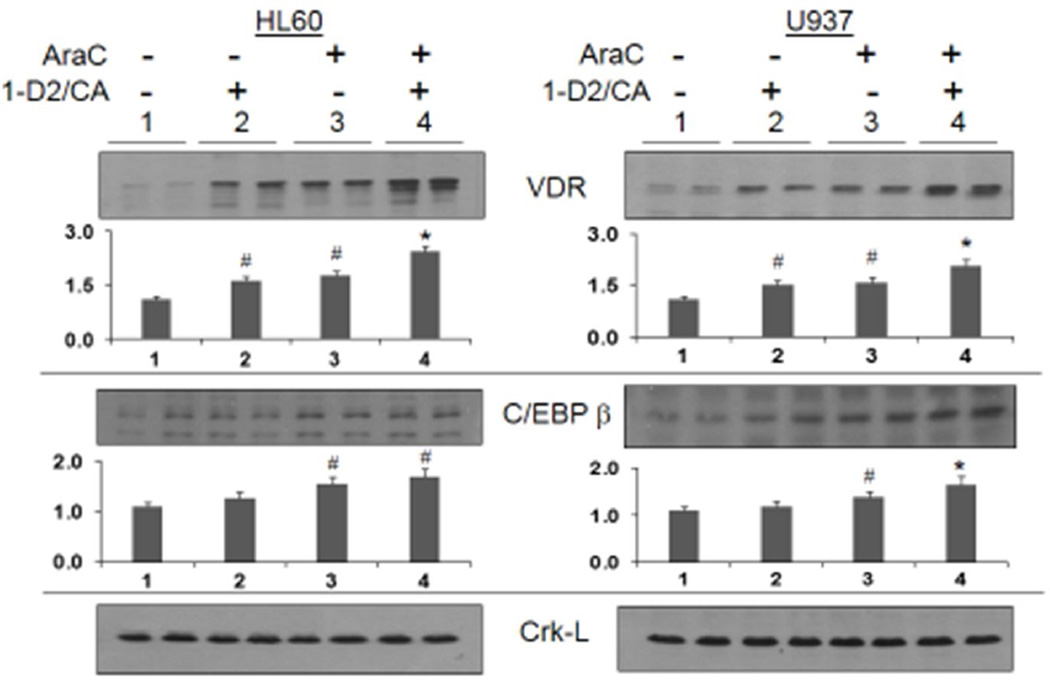
The underlying hypothesis of these studies is that the attempt to differentiate cells arrested at a primitive or an intermediate stage of development (such as HL60 and U937 cells) enhances and modifies DDR signaling, which then activates cell death pathways rather than the DNA repair. In order to confirm that 1-D2 and CA function as differentiating agents in AML cells with serious DNA damage, we demonstrated that the 1-D2/CA combination can indeed enhance AraC-induced differentiation. This was determined by the NSE reaction specific for the monocyte phenotype in hematopoietic cells, and the differentiated cells represented a considerable proportion of nontoxic cells (compare the cell numbers in Fig 1 and Fig 4). Cell differentiation was confirmed by molecular markers, the calcitriol-regulated transcription factors VDR and C/EBPbeta in AML cells (Fig 5). Interestingly, we also found that AraC alone increases protein levels of VDR in the AML cells studied (Fig 5, duplicate lanes 3), and therefore AraC-induced VDR is likely to participate in the initiation of differentiation in these studies when 1-D2 is added. Collectively, the results described here show that AraC, as well as the cytotoxicity enhancers used here, has some properties similar to differentiation agents. Thus, unsuccessful or interrupted differentiation can lead to activation of cell death pathways.
Figure 4. AML cells can differentiate under some cytotoxic conditions.
The effects of 1-D2/CA combination on the expression of non-specific esterase (NSE). # = p < 0.05, when compared with control group; * = p < 0.05, when compared with AraC alone treated group; n=3.
Figure 5. Western blots showing the upregulation of two molecular markers of monocytic differentiation (VDR and C/EBP) following the exposure to AraC alone, 1-D2/CA combination, and those added sequentially.
When added alone AraC and D2/CA combination significantly increase VDR, expression and this is enhanced in both cell lines when the 1-D2/CA combination follows AraC treatment, as expected. C/EBP β expression is also increased by AraC, but is enhanced by 1-D2/CA only in U937 cells. The Crk-L signal provided a loading control for the Westerns, which was used to determine the corrected OD for each signal. The bar chart below each blot shows the Mean and SD of replicate blots, #= p< 0.05 vs untreated control; * = p< 0.05 vs AraC alone; n=3.
Discussion
The principal novel finding in this report is that agents generally used to induce monocytic differentiation can function as cytotoxicity enhancers in a therapeutic setting. This is perhaps akin to the recent realization that the remission-inducing action of ATRA in APL patients results from a blend of differentiation and cytotoxicity [eg, [32]]. However, so far, agents capable of induction of the monocytic lineage have not been considered in this dual capacity, and in depth consideration of such combination has not been proposed for leukemia other than APL.
Specifically, we report here for the first time that in AML cells AraC-induced apoptosis is enhanced by a potent differentiation stimulus, which is essentially nontoxic to untreated AML cells. Although previous studies indicated that anti-proliferative and cell death inducing actions of calcitriol and other VDDs can be augmented by a variety of compounds in numerous neoplastic diseases [eg, [33–35]], this has led to only minor clinical successes [eg, [7, 13, 36]. In general, the optimal combination therapy for the treatment of AML is still a subject of debate [eg [37]]. While various combinations have been suggested, the rationale for those combinations was not evident, apart from the knowledge that each compound has some anti-leukemia cell activity when used alone.
In this study we focus on the mechanisms of initiation of cell death caused by DNA damage and then enhanced by a strong differentiation stimulus provided by the combination of the calcitriol analog 1-D2 and the antioxidant CA. By standard criteria, cell death occurs by both apoptosis and by necrosis-like cell disintegration, though it is unclear if the latter is actually a consequence of the former, since apoptosis can result in time in the so called secondary necrosis.
Although AraC has been reported to have a weak differentiating action on AML cells [38, 39], to our knowledge it has not been previously reported that AraC can upregulate VDR expression. So in addition to its cytotoxic action, a pre-exposure to AraC can have a priming action by increasing VDR levels in the treated cells, and thus makes the cells more responsive to VDD combinations. Our data also suggest that all three agents used in the present studies have the commonality of promoting differentiation. However, the possibility that AraC is not uniquely required for the 1-D2/CA enhancement remains to be investigated, as the effects of other DNA damaging modalities such as irradiation have also been shown to be potentiated by VDDs [eg, [40]].
Our recent studies also implicated the MAP kinase ERK5 in signaling of VDD-induced differentiation of human AML cells. We showed that the inhibition of the activity of the oncogene Cot1/Tlp2 in VDD-treated AML cells reduces ERK5 activation and upregulates p27 [41], resulting in the G1 and G2 cell cycle arrest [42]. Additional studies will clarify if the ERK5-MEF2C axis is involved in the phenomena studied here [43].
In conclusion, while additional extensive studies are needed to further understand the molecular basis for the negative effect on cell survival of the intersection of DDR and differentiation pathways, we provide a coherent rationale, that warrants testing, for the sequential AraC then 1-D2/CA induction of cell death in AML cells.
Highlights.
Exposure of AML cells with DNA damage to differentiation agents increases cell death.
Vitamin D analogs and plant-derived antioxidants can produce synergistic cytotoxicity.
VDR expression is up-regulated by AraC, and further enhanced by 1-D2/CA combination.
Acknowledgments
Grant Support
This study was supported by grants R01CA044722-26 from the National Cancer Institute, NIH, and 10A049 from American Institute for Cancer Research, both to GPS, and by the Nellie B. Smith endowment at the University of Missouri to JSH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Krishnan AV, Trump DL, Johnson CS, Feldman D. The role of vitamin D in cancer prevention and treatment. Rheumatic diseases clinics of North America. 2012;38:161–178. doi: 10.1016/j.rdc.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrich A, Kahl B, Bailey H, Kim K, Turman N, Juckett M. Phase II study of doxercalciferol for the treatment of myelodysplastic syndrome. Leuk Lymphoma. 2008;49:57–61. doi: 10.1080/10428190701713648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison JS, Bershadskiy A. Clinical experience using vitamin d and analogs in the treatment of myelodysplasia and acute myeloid leukemia: a review of the literature. Leuk Res Treatment. 2012;2012:125814. doi: 10.1155/2012/125814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen CM, Hamberg KJ, Binderup E, Binderup L. Seocalcitol (EB 1089): a vitamin D analogue of anti-cancer potential. Background, design, synthesis, pre-clinical and clinical evaluation. Curr Pharm Des. 2000;6:803–828. doi: 10.2174/1381612003400371. [DOI] [PubMed] [Google Scholar]
- 5.Danilenko M, Studzinski GP. Enhancement by other compounds of the anti-cancer activity of vitamin D(3) and its analogs. Exp Cell Res. 2004;298:339–358. doi: 10.1016/j.yexcr.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 6.Studzinski GP, Harrison JS, Wang X, Sarkar S, Kalia V, Danilenko M. Prospect: "Vitamin D Control of Hematopoietic Cell Differentiation and Leukemia". J Cell Biochem. 2015 doi: 10.1002/jcb.25104. [DOI] [PubMed] [Google Scholar]
- 7.Ma Y, Trump DL, Johnson CS. Vitamin D in combination cancer treatment. J Cancer. 2010;1:101–107. doi: 10.7150/jca.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woloszynska-Read A, Johnson CS, Trump DL. Vitamin D and cancer: clinical aspects. Best Pract Res Clin Endocrinol Metab. 2011;25:605–615. doi: 10.1016/j.beem.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Studzinski GP, Bhandal AK, Brelvi ZS. Potentiation by 1-alpha,25-dihydroxyvitamin D3 of cytotoxicity to HL-60 cells produced by cytarabine and hydroxyurea. J Natl Cancer Inst. 1986;76:641–648. doi: 10.1093/jnci/76.4.641. [DOI] [PubMed] [Google Scholar]
- 10.Studzinski GP, Reddy KB, Hill HZ, Bhandal AK. Potentiation of 1-beta-D-arabinofuranosylcytosine cytotoxicity to HL-60 cells by 1,25-dihydroxyvitamin D3 correlates with reduced rate of maturation of DNA replication intermediates. Cancer Res. 1991;51:3451–3455. [PubMed] [Google Scholar]
- 11.Wang X, Patel R, Studzinski GP. hKSR-2, a vitamin D-regulated gene, inhibits apoptosis in arabinocytosine-treated HL60 leukemia cells. Mol Cancer Ther. 2008;7:2798–2806. doi: 10.1158/1535-7163.MCT-08-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhang J, Studzinski GP. AKT pathway is activated by 1, 25-dihydroxyvitamin D3 and participates in its anti-apoptotic effect and cell cycle control in differentiating HL60 cells. Cell Cycle. 2006;5:447–451. doi: 10.4161/cc.5.4.2467. [DOI] [PubMed] [Google Scholar]
- 13.Ferrero D, Crisa E, Marmont F, Audisio E, Frairia C, Giai V, Gatti T, Festuccia M, Bruno B, Riera L, Passera R, Boccadoro M. Survival improvement of poor-prognosis AML/MDS patients by maintenance treatment with low-dose chemotherapy and differentiating agents. Ann Hematol. 2014;93:1391–1400. doi: 10.1007/s00277-014-2047-7. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher JC, Bishop CW, Knutson JC, Mazess RB, DeLuca HF. Effects of increasing doses of 1 alpha-hydroxyvitamin D2 on calcium homeostasis in postmenopausal osteopenic women. J Bone Miner Res. 1994;9:607–614. doi: 10.1002/jbmr.5650090504. [DOI] [PubMed] [Google Scholar]
- 15.Danilenko M, Wang X, Studzinski GP. Carnosic acid and promotion of monocytic differentiation of HL60-G cells initiated by other agents. J Natl Cancer Inst. 2001;93:1224–1233. doi: 10.1093/jnci/93.16.1224. [DOI] [PubMed] [Google Scholar]
- 16.Danilenko M, Wang Q, Wang X, Levy J, Sharoni Y, Studzinski GP. Carnosic acid potentiates the antioxidant and prodifferentiation effects of 1alpha,25-dihydroxyvitamin D3 in leukemia cells but does not promote elevation of basal levels of intracellular calcium. Cancer Res. 2003;63:1325–1332. [PubMed] [Google Scholar]
- 17.Gallagher R, Collins S, Trujillo J, McCredie K, Ahearn M, Tsai S, Metzgar R, Aulakh G, Ting R, Ruscetti F, Gallo R. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54:713–733. [PubMed] [Google Scholar]
- 18.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 19.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostling O, Johanson KJ. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun. 1984;123:291–298. doi: 10.1016/0006-291x(84)90411-x. [DOI] [PubMed] [Google Scholar]
- 21.Gyori BM, Venkatachalam G, Thiagarajan PS, Hsu D, Clement MV. OpenComet: an automated tool for comet assay image analysis. Redox biology. 2014;2:457–465. doi: 10.1016/j.redox.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Studzinski GP. Activation of extracellular signal-regulated kinases (ERKs) defines the first phase of 1,25-dihydroxyvitamin D3-induced differentiation of HL60 cells. J Cell Biochem. 2001;80:471–482. doi: 10.1002/1097-4644(20010315)80:4<471::aid-jcb1001>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Waxman S, Huang Y, Scher BM, Scher M. Enhancement of differentiation and cytotoxicity of leukemia cells by combinations of fluorinated pyrimidines and differentiation inducers: development of DNA double-strand breaks. Biomed Pharmacother. 1992;46:183–192. doi: 10.1016/0753-3322(92)90081-h. [DOI] [PubMed] [Google Scholar]
- 24.Redon CE, Nakamura AJ, Zhang YW, Ji JJ, Bonner WM, Kinders RJ, Parchment RE, Doroshow JH, Pommier Y. Histone gammaH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers. Clin Cancer Res. 2010;16:4532–4542. doi: 10.1158/1078-0432.CCR-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang X, Traganos F. Darzynkiewicz, DNA damage induced by DNA topoisomerase I- and topoisomerase II-inhibitors detected by histone H2AX phosphorylation in relation to the cell cycle phase and apoptosis. Cell Cycle. 2003;2:614–619. [PubMed] [Google Scholar]
- 26.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai Y, Grant S. New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin Cancer Res. 2010;16:376–383. doi: 10.1158/1078-0432.CCR-09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- 29.Walworth NC, Bernards R. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science. 1996;271:353–356. doi: 10.1126/science.271.5247.353. [DOI] [PubMed] [Google Scholar]
- 30.Wang QM, Jones JB, Studzinski GP. Cyclin-dependent kinase inhibitor p27 as a mediator of the G1-S phase block induced by 1,25-dihydroxyvitamin D3 in HL60 cells. Cancer Res. 1996;56:264–267. [PubMed] [Google Scholar]
- 31.Bhuyan BK, Fraser TJ, Gray LG, Kuentzel SL, Neil GL. Cell-kill kinetics of several S-phase-specific drugs. Cancer Res. 1973;33:888–894. [PubMed] [Google Scholar]
- 32.Hu J, Shen ZX, Sun GL, Chen SJ, Wang ZY, Chen Z. Long-term survival and prognostic study in acute promyelocytic leukemia treated with all-trans-retinoic acid, chemotherapy, and As2O3: an experience of 120 patients at a single institution. Int J Hematol. 1999;70:248–260. [PubMed] [Google Scholar]
- 33.Shiozawa K, Nakanishi T, Tan M, Fang HB, Wang WC, Edelman MJ, Carlton D, Gojo I, Sausville EA, Ross DD. Preclinical studies of vorinostat (suberoylanilide hydroxamic acid) combined with cytosine arabinoside and etoposide for treatment of acute leukemias. Clin Cancer Res. 2009;15:1698–1707. doi: 10.1158/1078-0432.CCR-08-1587. [DOI] [PubMed] [Google Scholar]
- 34.Hidalgo AA, Deeb KK, Pike JW, Johnson CS, Trump DL. Dexamethasone enhances 1alpha,25-dihydroxyvitamin D3 effects by increasing vitamin D receptor transcription. J Biol Chem. 2011;286:36228–36237. doi: 10.1074/jbc.M111.244061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao A, Li Y, Tong Y, Zheng H, Wu W, Wei C. 1,25-Dihydroxyvitamin D(3) and cisplatin synergistically induce apoptosis and cell cycle arrest in gastric cancer cells. International journal of molecular medicine. 2014;33:1177–1184. doi: 10.3892/ijmm.2014.1664. [DOI] [PubMed] [Google Scholar]
- 36.Yamada K, Miyamoto K, Hosoe H, Mizutani M, Shimizu K. Scoliosis associated with Prader-Willi syndrome. The spine journal : official journal of the North American Spine Society. 2007;7:345–348. doi: 10.1016/j.spinee.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Erba HP. Finding the optimal combination therapy for the treatment of newly diagnosed AML in older patients unfit for intensive therapy. Leuk Res. 2015;39:183–191. doi: 10.1016/j.leukres.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 38.Bodner AJ, Ting RC, Gallo RC. Induction of differentiation of human promyelocytic leukemia cells (HL-60) by nucleosides and methotrexate. J Natl Cancer Inst. 1981;67:1025–1030. [PubMed] [Google Scholar]
- 39.Housset M, Daniel MT, Degos L. Small doses of ARA-C in the treatment of acute myeloid leukaemia: differentiation of myeloid leukaemia cells? Br J Haematol. 1982;51:125–129. doi: 10.1111/j.1365-2141.1982.tb07297.x. [DOI] [PubMed] [Google Scholar]
- 40.Sundaram S, Sea A, Feldman S, Strawbridge R, Hoopes PJ, Demidenko E, Binderup L, Gewirtz DA. The combination of a potent vitamin D3 analog, EB 1089, with ionizing radiation reduces tumor growth and induces apoptosis of MCF-7 breast tumor xenografts in nude mice. Clin Cancer Res. 2003;9:2350–2356. [PubMed] [Google Scholar]
- 41.Wang X, Gocek E, Novik V, Harrison JS, Danilenko M, Studzinski GP. Inhibition of Cot1/Tlp2 oncogene in AML cells reduces ERK5 activation and upregulates p27Kip1 concomitant with enhancement of differentiation and cell cycle arrest induced by silibinin and 1,25-dihydroxyvitamin D3. Cell Cycle. 2010;9:4542–4551. doi: 10.4161/cc.9.22.13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Pesakhov S, Weng A, Kafka M, Gocek E, Nguyen M, Harrison JS, Danilenko M, Studzinski GP. ERK 5/MAPK pathway has a major role in 1alpha,25-(OH)2 vitamin D3-induced terminal differentiation of myeloid leukemia cells. J Steroid Biochem Mol Biol. 2014;144(Pt A):223–227. doi: 10.1016/j.jsbmb.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng R, Wang X, Studzinski GP. 1,25-Dihydroxyvitamin D3 induces monocytic differentiation of human myeloid leukemia cells by regulating C/EBPbeta expression through MEF2C. J Steroid Biochem Mol Biol. 2015;148:132–137. doi: 10.1016/j.jsbmb.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]