Abstract
Background
Therapeutic effects of antiretroviral therapy (ART) in patients with multidrug resistant tuberculosis (MDR-TB) and HIV infection have not been established.
Objective
The objective of this study was to assess therapeutic outcomes of ART integration with MDR-TB treatment.
Design
A subgroup of MDR-TB patients from the SAPiT study, a randomized controlled trial, conducted in an out-patient clinic in Durban, South Africa from 2008–2012
Methods
Clinical outcomes at 18 months were compared in patients randomized to receive ART within 12 weeks of standard first-line tuberculosis treatment initiation with those who commenced ART after completing tuberculosis treatment.
Results
Mycobacterium tuberculosis drug susceptibility was available in 489 (76%) of 642 SAPiT patients; 23 had MDR-TB, 14 in the integrated treatment arm and 9 in the sequential treatment arm. At 18 months, the mortality rate was 11.9/100 person-years (95% confidence interval (CI): 1.4–42.8) in the combined integrated treatment arm and 56.0/100 person-years (95%CI: 18.2–130.8) in the sequential treatment arm, (Hazard Ratio adjusted for baseline CD4 count and whether MDR-TB treatment was initiated: 0.14; 95% CI: 0.02–0.94; P=0.04).
Conclusion
Despite the small sample size, the 86% reduction in mortality due to early initiation of ART in MDR-TB patients was statistically significant.
Keywords: MDR-TB, HIV treatment
INTRODUCTION
Multi-drug resistant tuberculosis (MDR-TB), defined as resistance to at least isoniazid and rifampicin is a major cause of mortality in tuberculosis-HIV co-infected patients. The number of reported MDR-TB cases in South Africa rose from 6795 in 2008(1) to 10 085 in 2011,(2) among the highest in the world. Of the 6795 reported MDR-TB cases in 2008, 28% (N=1866) occurred in the province of KwaZulu-Natal.(3) 76% were co-infected with HIV. (4, 5)
The SAPiT trial(6) showed that the initiation of antiretroviral therapy (ART) during tuberculosis treatment in patients with mostly drug susceptible tuberculosis and HIV co-infection reduced mortality by 56%. Based on these and other findings,(7, 8) the 2010 South African National ART guidelines were changed; stating that patients co-infected with MDR-TB and HIV should be commenced on ART irrespective of CD4+ cell count.(9)
Empiric evidence for survival benefit of early initiation of ART in patients with MDR-TB is lacking. The SAPiT trial offers an opportunity to provide such information.(6) The purpose of this subgroup analysis of the SAPiT trial was to assess the impact, through a randomized controlled trial design, of early ART initiation on survival, in HIV-infected patients with MDR-TB.
METHODS
Design Overview
This is a secondary analysis of 23 patients diagnosed with MDR-TB from the SAPiT trial, an open-label, randomized controlled trial between June 2005 and July 2010, which has been described in detail elsewhere.(6)
Setting and Participants
The study was conducted at the CAPRISA eThekwini clinic in Durban, South Africa. Ambulatory patients, 18 years or older with pulmonary tuberculosis and HIV co-infection were enrolled. For this analysis, only patients with Mycobacterium tuberculosis (M tuberculosis) resistant to at least rifampicin and isoniazid were classified as MDR-TB cases. Other resistance patterns were classified as ‘non-MDR’.
Randomization and interventions
Participants were randomized to start ART either during the first 12 weeks of tuberculosis treatment (combined integrated treatment arm) or upon tuberculosis treatment completion (sequential treatment arm). The once daily ART regimen contained didanosine, lamivudine and efavirenz. ART adherence was assessed by pill counts. CD4+ cell counts and HIV RNA levels were performed at screening, randomization and at 6-monthly intervals. All patients received anti-tuberculosis therapy comprising rifampicin, isoniazid, ethambutol and pyrazinamide during the intensive phase and rifampicin and isoniazid during the continuation phase unless they were diagnosed with MDR-TB. Patients treated for MDR-TB received the standard MDR-TB regimen of kanamycin, ofloxacin, pyrazinamide, ethambutol/ cycloserine and ethionamide. M tuberculosis was cultured on MGIT and 7H11 Middlebrook medium with drug susceptibility testing by the 1% proportion method.
Outcomes and follow-up
Prior to 2007, in accordance with the existing South African National Tuberculosis Control Programme guidelines,(10) drug susceptibility testing for M. tuberculosis was only performed when drug resistance was suspected. From 2007 onwards, drug susceptibility testing became routinely available and was performed at enrolment and retrospectively for those already enrolled but not previously tested. The median time from specimen collection to receipt of drug susceptibility result was 3 months (range 2–9 months). The follow-up period was 18 months.
Statistical Analysis
All analyses were by intention-to-treat in the subgroup of patients who had drug susceptibility results. Fisher’s exact test was used for categorical data and unpaired t-tests or the Wilcoxon two-sample test for continuous data. The time on study was calculated as the time from randomization to death, or termination from the trial, or 18 months on the study, whichever occurred first. All patients were censored at 18 months if still in follow-up at that point. Poisson approximations were used to calculate confidence intervals (CIs) for incidence rates. The CIs for the incidence rate ratios (IRRs) were calculated using the F-distribution. The proportional hazards assumption was checked by fitting a model with the interaction between each covariate and time. Only one interaction was statistically significant. In the instance where this assumption was violated we reported the IRR instead of the hazard ratio. Missing data was not imputed, and because of the small sample size, pattern mixture models were not employed for missing data. The total number of data points analysed is given and available data is presented and analysed. The missing data mechanism is likely to be missing at random. The statistical analysis was performed using SAS version 9.2
Ethics
The trial was approved by the University of KwaZulu-Natal Biomedical Research Ethics Committee (E:07/05).
RESULTS
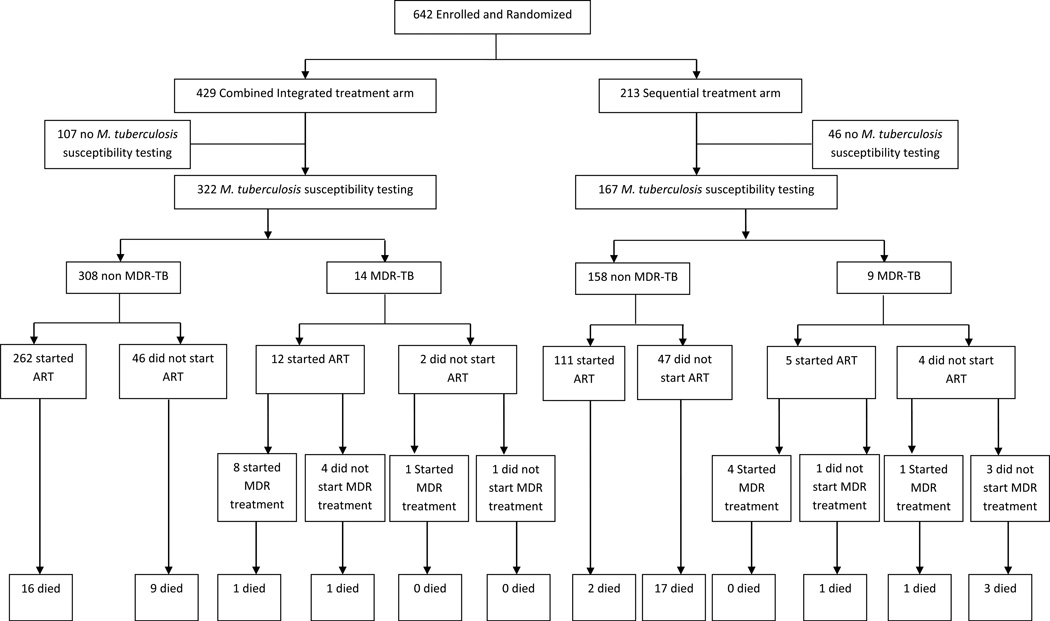
The SAPiT trial enrolled 642 HIV-tuberculosis co-infected patients and 489 (76%) had sputum culture and drug susceptibility testing for M tuberculosis performed. Prior to 2007, 56 patients had susceptibility testing performed when drug resistance was suspected. After 2007, when drug susceptibility testing was routinely available, 389 were tested for resistance at enrolment and 44 were tested at a follow-up visit as part of the routine testing. 23 of 489 (5%) patients were diagnosed with MDR-TB (Figure 1), of which 11 of 23 had no past history of tuberculosis. At baseline, patients with MDR-TB had lower Karnofsky scores(11), were more often WHO stage 4 and presented more frequently with extra-pulmonary tuberculosis (Table 1).
Figure 1.
Enrolment and outcomes of patients with MDR-TB and non MDR-TB in the SAPiT trial
Table 1.
Baseline characteristics of patients with MDR-TB and non MDR-TB in the SAPiT trial
| MDR-TB N=23 |
All treatment arms N=489 |
|||||
|---|---|---|---|---|---|---|
| Combined Integrated treatment arm |
Sequential treatment arm |
p- value |
MDR-TB | non MDR-TB | p- value |
|
| Number (%) n=14 |
Number (%) n=9 |
Number (%) N=23 |
Number (%) N=466 |
|||
| Age (year) | 0.98$ | 0.23$ | ||||
| Mean | 36.8 | 36.7 | 36.7 | 34.6 | ||
| Range | 26–72 | 23–49 | 23–72 | 19–68 | ||
| Male sex | 7 (50) | 8 (89) | 0.09 | 15 (65) | 222 (48) | 0.13 |
| History of tuberculosis |
8 (57) | 4 (44) | 0.68 | 12 (52) | 185 (40) | 0.28 |
| Karnofsky score | ||||||
| 100 or 90 | 9 (64) | 3 (33) | 0.21 | 12 (52) | 332 (71) | 0.15 |
| 80 or 70 | 5 (36) | 6 (67) | 11 (48) | 131(28) | ||
| 60 | 0 | 0 | 0 | 2 (0.4) | ||
| World Health Organization (WHO) stage 4 at baseline§ |
3 (21) | 2 (22) | 1.00 | 5 (22) | 27 (6) | 0.01 |
| Presence of extra- pulmonary tuberculosis |
3 (21) | 2 (22) | 1.00 | 5 (22) | 18 (4) | 0.003 |
| Median (interquartile range) |
Median (interquartile range) |
Median (interquartile range) |
Median (interquartile range) |
|||
| log viral load (copies/ml)‡ |
5.1 (3.4 – 5.4) |
5.2 (4.8 – 5.4) |
0.42 | 5.1 (3.8 – 5.4) |
5.2 (4.6 – 5.6) |
0.34 |
| CD4+ count (cells/mm3) |
123.5 (88 – 399) |
146.0 (72 – 304) |
0.85# | 135.0 (72 – 341) |
146.5 (73 – 245) |
0.93# |
The remainder of patients had WHO stage 3 infection
Baseline viral load was not available for 14 patients in the combined integrated susceptible group and 6 patients in the sequential susceptible group. It was available for all patients with multidrug resistant tuberculosis
p-value calculated using unpaired t-test comparing means
p-value calculated using Wilcoxon two sample test comparing medians
All other p-values calculated using Fisher’s exact test
MDR-TB: Multidrug resistant tuberculosis
At baseline, MDR-TB patients in the combined integrated treatment arm (14/322 = 4.3%) and the sequential treatment arm (9/167 = 5.4%; Table 1) were similar. Of the 14 patients in the combined integrated treatment arm, nine were diagnosed with MDR-TB on their enrolment sputum specimen, one during the study (but had no enrolment susceptibility testing) and four had susceptible M tuberculosis at enrolment and subsequently developed MDR-TB. Of the nine patients in the sequential treatment arm, two were diagnosed with MDR-TB on their enrolment sputum specimen, 3 during the course of treatment (but had no enrolment susceptibility testing) and four had susceptible M tuberculosis at enrolment and subsequently developed MDR-TB. One of the 14 patients in the combined integrated treatment arm and none in the sequential treatment arm had extensively drug resistant tuberculosis.
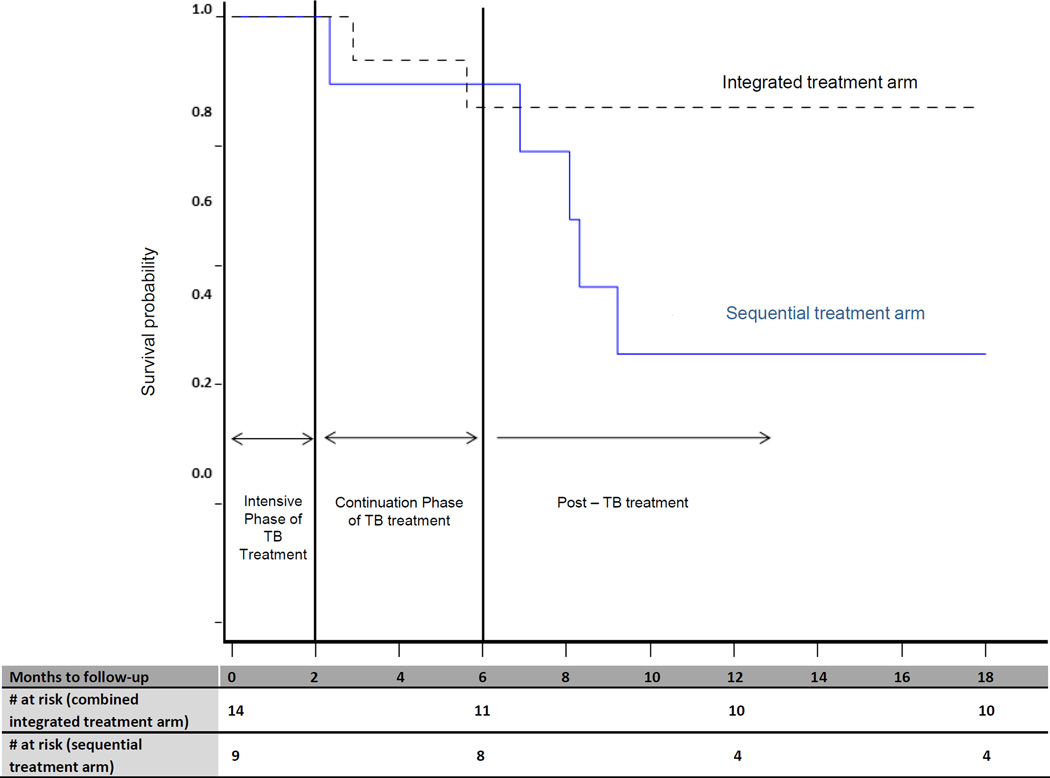
Seven of the 23 (30%) MDR-TB patients died; two in the combined integrated treatment arm and five in the sequential treatment arm. Death rates were similar in the two treatment arms until 6 months (Figure 2). After 6 months, more patients died in the sequential treatment arm compared to the integrated treatment arm (Figure 2). The mortality rate in MDR-TB patients was 11.9 per 100 person-years (95%CI: 1.4–42.8) in the combined integrated treatment arm compared to 56.0 per 100 person-years (95%CI: 18.2–130.8) in the sequential treatment arm (HR: 0.14; 95% CI: 0.02–0.94; P=0.04, adjusting for baseline CD4+ cell count and whether MDR-TB treatment was initiated). The mortality rate in MDR-TB patients was approximately 4-fold higher (P<0.001) than mortality in patients who did not have MDR-TB (Table 2a). Irrespective of whether patients received specific treatment for MDR-TB, mortality rates were lower in the combined integrated treatment arm (Table 2b).
Figure 2.
Kaplan Meier curve of the survival probability of patients diagnosed with MDR-TB
Table 2a.
Mortality in patients with MDR-TB stratified by the Combined Integrated and Sequential treatment arms and compared to non MDR-TB patients in the SAPiT trial
| MDR-TB patients N =23 |
All patients both treatment arms N=489 |
|||||
|---|---|---|---|---|---|---|
| Combined Integrated treatment arm N=14 |
Sequential treatment arm N=9 |
p-value | MDR-TB N=23 |
non MDR-TB N=466 |
p-value | |
| Mortality Events | 2 | 5 | 7 | 40 | ||
| Person-years | 16.9 | 8.9 | 25.8 | 638.6 | ||
| Incidence per 100 person-years (95%CI) |
11.9 (1.4 – 42.8) |
56.0 (18.2 – 130.8) |
27.1 (10.9 –55.9) |
6.3 (4.5 – 8.5) |
||
| Hazard ratio (95%CI) comparing combined integrated to sequential: 0.14 (0.02 – 0.94) § |
0.04§ | Incidence rate ratio (95% CI): 0.23 (0.10 – 0.52)‖ |
<0.001‖ | |||
adjusted for baseline CD4+ cell count and whether MDR-TB treatment was started
Proportional hazards regression was not done, because the proportional hazards assumption did not hold. The HR is 0.24 (95% CI: 0.11 to 0.54: P=0.0006), which is similar to the IRR of 0.23.
CI: Confidence interval
MDR-TB: Multidrug resistant tuberculosis
Table 2b.
Mortality rates of patients with MDR-TB in the Combined Integrated and Sequential SAPiT trial arms stratified by MDR-TB treatment
| Started MDR-TB treatment during the study period N=14 |
Did not start MDR-TB treatment during the study period N=9 |
|||
|---|---|---|---|---|
| Combined Integrated treatment arm |
Sequential treatment arm |
Combined Integrated treatment arm |
Sequential treatment arm |
|
| N | 9 | 5 | 5 | 4 |
| Person-years of follow up | 11.8 | 6.7 | 5.1 | 2.2 |
| Mortality events | 1 | 1 | 1 | 4 |
| Mortality incidence rate per 100 person-years (95%CI) |
8.5 (0 – 25.1) |
14.9 (0 – 44.2) |
19.6 (0 – 58.0) |
181.2 (3.6 – 360) |
| Hazard ratio (95% CI) | 0.59 (0.04 – 9.43) |
P = 0.71 | 0.15 (0.02 – 1.41) |
P = 0.10 |
MDR-TB: Multidrug resistant tuberculosis
CI: Confidence interval
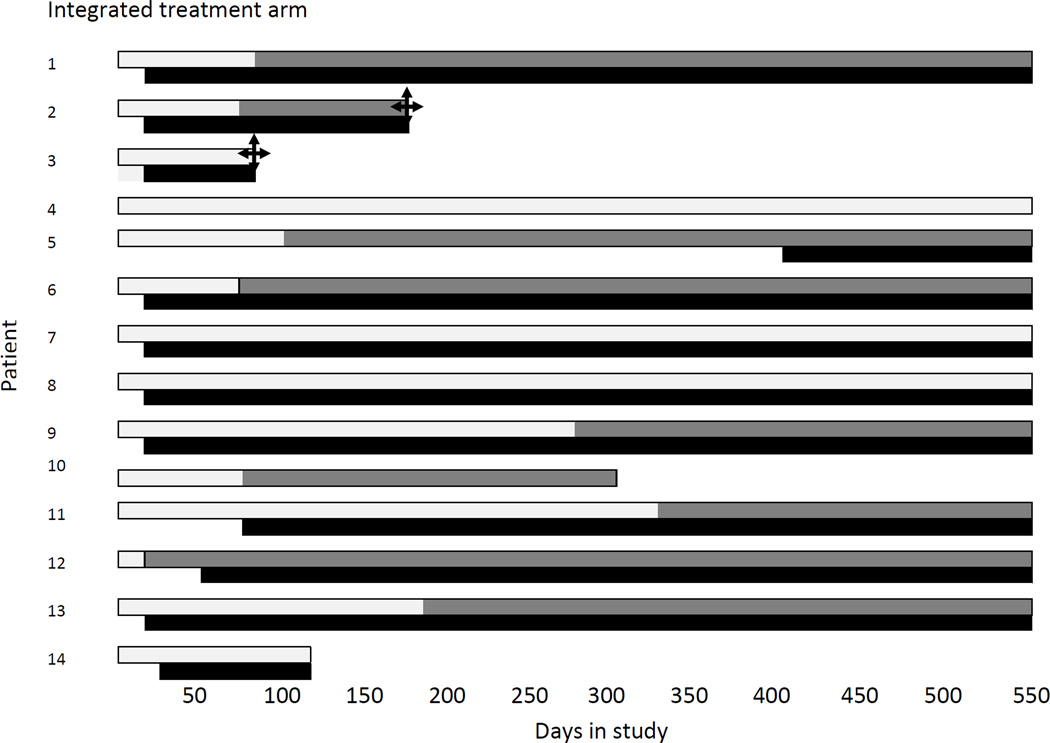
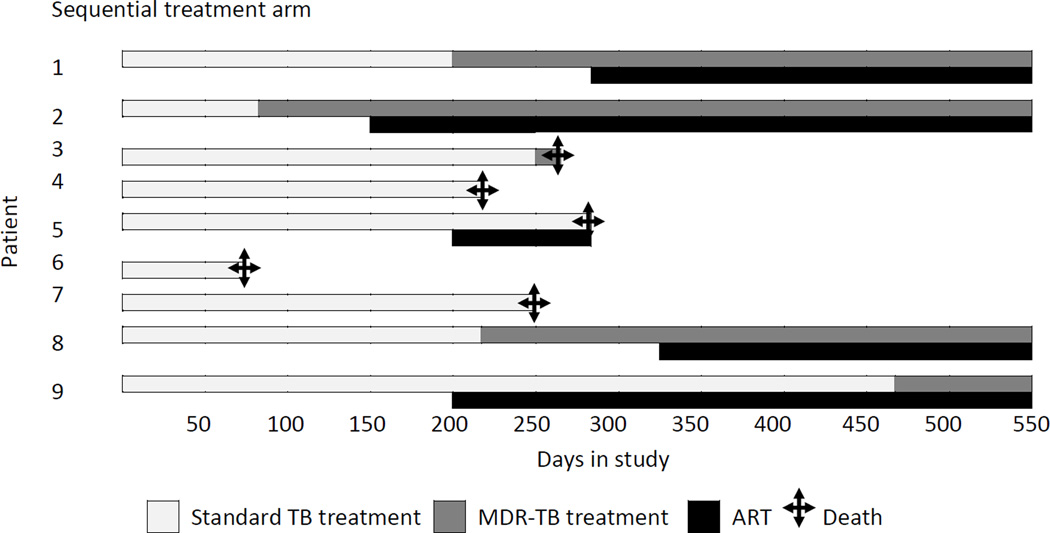
Of the 23 patients with MDR-TB, 14 (61%) started MDR-TB treatment, 64% (9/14) in the combined integrated treatment arm, and 56% (5/9) in the sequential treatment arm (Figure 3). The overall mortality rate in patients who initiated MDR-TB treatment was 10.8 per 100 person-years (95%CI: 1.3–39.0) and overall mortality rate in patients who did not initiate MDR-TB treatment was 68 per 100 person-years (95%CI: 22.3–160.4, P=0.03). After adjustment for baseline CD4+ cell count, patients with MDR-TB who had received MDR-TB treatment had a 93% lower mortality rate at 18 months post-randomization, compared to patients who had not initiated MDR-TB treatment (P=0.02). Initiating MDR-TB treatment (in those patients who survived long enough to receive their MDR-TB diagnosis) or being in the combined integrated treatment arm reduced the risk of dying by 97%, adjusting for baseline CD4+ cell count (P=0.003).
Figure 3.
Time to initiation of MDR-TB treatment and ART in relation to standard TB treatment per patient
Seven patients initiated MDR-TB treatment within 6 months of starting standard tuberculosis treatment and only 1/7 (14%) died (Figure 3). In contrast, 16 patients either did not start MDR-TB treatment or started MDR-TB treatment more than 6 months after standard tuberculosis treatment, and 6/16 (38%) died (RR 0.38; 95% CI: 0.06–2.60; P=0.37). In this latter group, 1/8 (13%) patients in the combined integrated treatment arm died and 5/8 (63%) patients in the sequential treatment arm died (RR: 0.20; 95%CI: 0.03–1.35; P=0.12).
In the combined integrated treatment arm, the median time from ART to MDR-TB treatment was 113 (range: 40–239) days(n=6) and in the Sequential treatment arm it was 258 days - only one patient started ART before MDR-TB treatment (Figure 3)
The reasons for not commencing MDR-TB treatment in the combined integrated arm were: lost to follow up (n=2), death(n=1) and response to 1st line TB drugs before commencing treatment for MDR-TB(n=2); in the sequential arm, 4 patients died before commencing treatment for MDR-TB.
In the combined integrated treatment arm, one patient was diagnosed with MDR-TB posthumously; he had not received MDR-TB treatment but had received ART for 2.5 months before he died from severe diarrhoea. The other death in this arm was due to suicide and occurred after 4 months of MDR-TB treatment and 5 months of ART. In the sequential treatment arm, four patients were diagnosed with MDR-TB posthumously; none had received MDR-TB treatment while one had received ART for 3 months before he died; the fifth patient had not initiated ART and had been treated for MDR-TB for 19 days prior to death. Clinical outcomes of tuberculosis treatment (some on standard tuberculosis treatment and others on MDR-TB treatment) were available in 19 of the 23 MDR-TB patients; four were lost to follow-up in the combined integrated treatment arm. At 18 months, seven patients had died, six were still receiving MDR-TB treatment (four in the combined integrated arm and two in the sequential treatment arm) and a further six were cured [four in the combined integrated treatment arm and two in the sequential treatment arm (P=1.00)].
At 12 months of follow-up, 78% (N=9) in the combined integrated treatment arm and all four in the sequential treatment arm had undetectable viral loads. The mean increase in CD4+ cell count was 108 and 89.8 cells/mm3 in the combined integrated and sequential treatment arms respectively (Table 3).
Table 3.
HIV treatment outcomes in patients with MDR-TB and non MDR-TB in the SAPiT trial
| MDR-TB patients N=23 |
All patients both treatment arms N=489 |
|||||
|---|---|---|---|---|---|---|
| Combined Integrated treatment arm |
Sequential treatment arm |
p- value |
MDR-TB | non MDR- TB |
p- value |
|
|
Viral load < 400 copies/ml | ||||||
| 12 months after randomization: n/N (%) |
7/9 (78) | 4/4 (100) | 1.00 | 11/13 (85) | 277/325 (85) |
1.00 |
| 6 months after initiation of anti- retroviral therapy: n/N (%) |
5/6 (83) | 4/4 (100) | 1.00 | 9/10 (90) | 211/239 (88) |
1.00 |
|
Mean increase in CD4+ count from baseline | ||||||
|
12 months after randomization | ||||||
| N | 9 | 4 | 13 | 327 | ||
| Mean CD4+ cell count increase in cells/mm3 (95% CI) |
108.0 (−30 – 246) |
89.8 (−183 – 362) |
0.55 | 102.4 (−0.1 – 204.9) |
132.4 (118.6 – 146.2) |
0.82 |
|
6 months after initiation of antiretroviral therapy | ||||||
| N | 6 | 4 | 10 | 240 | ||
| Mean CD4+ cell count increase in cells/mm3 (95% CI) |
122.5 (36.9 – 208.1) |
131.8 (−71.4 – 334.9) |
0.59 | 126.2 (57.8 – 194.6) |
117.9 (102.7 – 133.2) |
0.59 |
MDR-TB: Multidrug resistant tuberculosis
CI: Confidence interval
DISCUSSION
Initiation of ART during tuberculosis treatment in patients with MDR-TB was associated with an 86% reduction in mortality. This survival benefit associated with ART initiation was evident even in patients who had not initiated appropriate MDR-TB treatment. Similar survival benefits have also been observed in retrospective studies among XDR-TB patients who were co-infected with HIV and received ART.(12, 13)
The long laboratory delays in the diagnosis of MDR-TB delays initiation of appropriate treatment and contributes to high mortality among MDR-TB patients. Patients with MDR-TB in this study experienced a 4-fold higher rate of mortality compared to those with non MDR-TB. Similar high mortality rates have been observed in patients co-infected with MDR-TB and HIV in rural KwaZulu-Natal, where 40% of MDR-TB cases died within 30 days of sputum collection, most dying before the diagnosis of MDR-TB was confirmed.(5) A retrospective review conducted in Thailand showed that HIV and MDR-TB co-infected patients were 12-times more likely to die than patients without MDR-TB.(14)
A consequence of the diagnostic and treatment initiation delays resulted in MDR-TB confirmation posthumously in this study. This is not unique. In a post mortem study of 240 individuals (94% HIV-infected), 17% had MDR TB that had not been recognized clinically.(15) Therefore, initiating ART during tuberculosis treatment provides an opportunity to improve survival rates until MDR-TB can be confirmed and appropriate treatment instituted. It is quicker to diagnose HIV, institute ART and improve survival until MDR-TB can be diagnosed and MDR-TB treatment initiated. If MDR-TB can be diagnosed early and both ART and MDR-TB treatment initiated mortality is likely to be reduced even further.(12, 13, 16)
Although MDR-TB patients in this study were more often categorized as WHO stage 4 and had extra-pulmonary tuberculosis, these criteria were insufficient indicators of which patients had MDR-TB. Immune markers such as CD4+ cell count and viral load were similar among MDR-TB patients and those with drug susceptible tuberculosis. Other studies have also shown that the clinical and radiological presentation for patients with MDR-TB and drug susceptible tuberculosis is similar.(17)
Another factor contributing to the delay in investigation of possible MDR-TB is that immune reconstitution inflammatory syndrome (IRIS) was considered for MDR-TB patients in the combined integrated treatment arm. In addition to a higher index of suspicion for drug resistance, there is an urgent need for earlier case finding, better diagnostics, as well as integration of tuberculosis and HIV care and treatment.(18)
The MDR-TB case load in KwaZulu-Natal has increased 4-fold between 2002 and 2004, with resultant larger numbers of primary MDR-TB cases presenting for tuberculosis treatment. Extremely high rates of primary MDR-TB have been reported from specific parts of KwaZulu-Natal.(19, 20) A high proportion of MDR-TB patients in these areas with high rates of HIV co-infection had no prior history of tuberculosis treatment, in contrast to a recent multi-country study which showed that a history of previous tuberculosis was the strongest risk factor for MDR-TB.(21, 22) It is also important to note that eight patients in our study initially had confirmed drug susceptible tuberculosis and subsequently developed MDR-TB. This sequence suggests either selection of resistant organisms while on tuberculosis treatment or super-infection with MDR-TB,(23) emphasizing the critical importance of strengthening both treatment adherence and infection control in tuberculosis programs.
In the SAPiT study participants in each arm were monitored and evaluated using the same standardized criteria and practices to minimize bias during the study implementation thus preserving external validity. One of the main limitations of this study is the small sample size. Despite this, the results were statistically significant due to the large effect size observed. This was a post hoc analysis and the study was not designed to screen patients for MDR-TB and randomize these patients to early or sequential ART initiation. Newer technology now makes the earlier diagnosis of drug resistance possible.(24, 25) Even though randomization was not stratified on MDR-TB status, the participants who had undetected MDR-TB at baseline constituted a similar proportion of patients in each treatment arm. Thus studying this subgroup of patients preserves the benefits of randomization. When the SAPiT trial was initiated, it was not possible to conduct such a study because of the delays in obtaining drug susceptibility results and many of the patients died (5 of the 7 deaths) even before they were diagnosed with MDR-TB.
The use of all-cause mortality is also a limitation. For example, the death by suicide is treated as an equivalent primary endpoint to tuberculosis meningitis. The SAPiT trial included 18 months of follow up but some patients with MDR-TB were diagnosed late during the study and their clinical outcomes were not known as they were still on the required long course of MDR-TB treatment.
Despite these limitations, our study provides empirical evidence from a randomized controlled trial of a survival benefit when ART is initiated early in patients with MDR-TB. Even though there may be concerns about patient acceptability of integrated therapy, retrospective studies have shown a decreased mortality with integrated TB-HIV treatment in MDR and XDR-TB despite protracted and complex TB treatment regimens.(5, 12)
In this study, although MDR-TB mortality was high compared to non-MDR-TB cases, survival was significantly improved when ART was initiated, even among those who had not yet been initiated on MDR-TB treatment. Early ART initiation is desirable in settings with high MDR-TB prevalence, especially in sick patients and those with low CD4+ cell counts(26) because treatment initiation even before MDR-TB is diagnosed has a survival benefit.
Acknowledgments
We gratefully acknowledge the participants in the study. We thank Dr Surie Chinappa and Sr Jeanne Liebertrau of the Prince Cyril Zulu Communicable Disease Centre; Dr Wafaa El-Sadr of Columbia University; Dr Ed Tramont, Dr Rod Hoff, Dr Sandi Lerhman and Dr Richard Hafner of the Division of AIDS at the NIH; Dr Gavin Churchyard, Dr Douglas Taylor, and Dr Mark Weaver for serving on the SAPiT Safety Monitoring Committee; Ms Anushka Naidoo, the on-site study pharmacist; members of the Community Advisory Board; Ms Nomapando Barnabas of the CAPRISA Community Programme; Ms Natasha Samsunder and Mr Keith Coetzee for laboratory analysis; Ms Irene van Middelkoop for data management and all the members of the CAPRISA 003 – SAPiT trial team.
Funding:
This work was supported by the US President's Emergency Plan for AIDS Relief (PEPfAR) which funded the care of all the participants in the trial (grant# 5U26PS001350); and the Global Fund to fight AIDS, Tuberculosis and Malaria funded the cost of the drugs used in the trial. The research infrastructure to conduct this trial, including the data management, laboratory and pharmacy cores were established through the Comprehensive International Program of Research on AIDS (CIPRA) (grant # AI51794). CAPRISA was established as part of the CIPRA from the US National Institutes of Health. NP, AG and KN were supported by the Columbia University-Southern African Fogarty AIDS International Training and Research Program (AITRP) funded by the Fogarty International Center, National Institutes of Health (grant # D43TW00231).
Role of Funding Source
The sponsor of the trial had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Disclaimer:
All authors had access to the data, commented on drafts, and approved the final report. The corresponding author had final responsibility for the decision to submit for publication.
Conflict of Interest:
There are no potential conflicts of interest relevant to this article.
REFERENCES
- 1.WHO. Multidrug and extensively drug-resistant TB(M/XDR-TB): 2010 global report on surveillance and response. Switzerland: Geneva; 2010. [Google Scholar]
- 2.WHO. Global Tuberculosis Report 2012. Switzerland: Geneva; 2013. [Google Scholar]
- 3.Buthelezi S. Situational Analysis of TB Drug Resistance in KwaZulu-Natal Province: Republic of South Africa. 2008. Apr 9, [Google Scholar]
- 4.Brust JC, Gandhi NR, Carrara H, Osburn G, Padayatchi N. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000–2003. Int J Tuberc Lung Dis. 2010;14(4):413–419. [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181(1):80–86. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 6.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362(8):697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scano F, Vitoria M, Burman W, Harries AD, Gilks CF, Havlir D. Management of HIV-infected patients with MDR- and XDR-TB in resource-limited settings. Int J Tuberc Lung Dis. 2008;12(12):1370–1375. [PubMed] [Google Scholar]
- 8.Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. Int J Tuberc Lung Dis. 2007;196(Suppl 1):S86–S107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 9.South African Department of Health. [Accessed on 25 October 2012];Clinical Guidelines for the Management of HIV & AIDS in adults and adolescents. 2010 http://www.hiv911.org.za/wp-content/uploads/2010/04/2010-Adult-ART-Guidelines.pdf.
- 10.South African Department of Health. [Accessed on 25 October 2012];National Tuberculosis Management Guidelines 2008. 2008 http://www.who.int/hiv/pub/guidelines/south_africa_tb.pdf.
- 11.Oxford, Textbook, of, Palliative, Medicine. Oxford University Press; 1993. p. 109. http://www.pennmedicine.org/homecare/hcp/elig_worksheets/Karnofsky-Performance-Status.pdf. [Google Scholar]
- 12.O'Donnell MR, Padayatchi N, Master I, Osburn G, Horsburgh CR. Improved early results for patients with extensively drug-resistant tuberculosis and HIV in South Africa. Int J Tuberc Lung Dis. 2009;13(7):855–861. [PMC free article] [PubMed] [Google Scholar]
- 13.Shenoi SV, Brooks RP, Barbour R, et al. Survival from XDR-TB Is Associated with Modifiable Clinical Characteristics in Rural South Africa. PLoS one. 2012;7(3):e31786. doi: 10.1371/journal.pone.0031786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sungkanuparph S, Eampokalap B, Chottanapund S, Thongyen S, Manosuthi W. Impact of drug-resistant tuberculosis on the survival of HIV-infected patients. Int J Tuberc Lung Dis. 2007;11(3):325–330. [PubMed] [Google Scholar]
- 15.Cohen T, Murray M, Wallengren K, Alvarez GG, Samuel EY, Wilson D. The prevalence and drug sensitivity of tuberculosis among patients dying in hospital in KwaZulu-Natal, South Africa: a postmortem study. PLoS Med. 2010;7(6):e1000296. doi: 10.1371/journal.pmed.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dheda K, Shean K, Zumla A, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 2010;375(9728):1798–1807. doi: 10.1016/S0140-6736(10)60492-8. [DOI] [PubMed] [Google Scholar]
- 17.Wells CD. Global Impact of Multidrug-Resistant Pulmonary Tuberculosis Among HIV-Infected and Other Immunocompromised Hosts: Epidemiology, Diagnosis, and Strategies for Management. Curr Infect Dis Rep. 2010;12(3):192–197. doi: 10.1007/s11908-010-0104-5. [DOI] [PubMed] [Google Scholar]
- 18.Lawn SD, Ayles H, Egwaga S, et al. Potential utility of empirical tuberculosis treatment for HIV-infected patients with advanced immunodeficiency in high TB-HIV burden settings. Int J Tuberc Lung Dis. 2011;15(3):287–295. [PubMed] [Google Scholar]
- 19.Brust JC, Lygizos M, Chaiyachati K, et al. Culture conversion among HIV co-infected multidrug-resistant tuberculosis patients in Tugela Ferry, South Africa. PLoS One. 2011;6(1):e15841. doi: 10.1371/journal.pone.0015841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassim S, Shaw PA, Sangweni P, et al. Detection of a substantial rate of multidrug-resistant tuberculosis in an HIV-infected population in South Africa by active monitoring of sputum samples. Clin Infect Dis. 2010;50(7):1053–1059. doi: 10.1086/651119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalton T, Cegielski P, Akksilp S, et al. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet. 2012;380(9851):1406–1417. doi: 10.1016/S0140-6736(12)60734-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffner S. Unexpected high levels of multidrug-resistant tuberculosis present new challenges for tuberculosis control. Lancet. 2012;380(9851):1367–1369. doi: 10.1016/S0140-6736(12)61069-1. [DOI] [PubMed] [Google Scholar]
- 23.Andrews JR, Gandhi NR, Moodley P, et al. Exogenous reinfection as a cause of multidrug-resistant and extensively drug-resistant tuberculosis in rural South Africa. J Infect Dis. 2008;198(11):1582–1589. doi: 10.1086/592991. [DOI] [PubMed] [Google Scholar]
- 24.Ling DI, Zwerling AA, Pai M. Rapid diagnosis of drug-resistant TB using line probe assays: from evidence to policy. Exp rev resp med. 2008;2(5):583–588. doi: 10.1586/17476348.2.5.583. [DOI] [PubMed] [Google Scholar]
- 25.Perkins MD, Cunningham J. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J Infect Dis. 2007;196(Suppl 1):S15–S27. doi: 10.1086/518656. [DOI] [PubMed] [Google Scholar]
- 26.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365(16):1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]