Abstract
Inflammatory bowel disease (IBD) is a chronic intestinal illness of autoimmune origin affecting millions across the globe. The most common subtypes include ulcerative colitis (UC) and Crohn’s disease. While many medical treatments for IBD exist, none come without the risk of significant immunosuppression and in general do not have benign side effect profiles. Surgical intervention exists only as radical resection for medically refractory UC. There exists a dire need for novel treatments that target the inherent pathophysiologic disturbances of IBD, rather than global immune suppression. One avenue of investigation that could provide such an agent is the interaction between certain dietary elements and the aryl hydrocarbon receptor (AHR). The AHR is a cytosolic transcription factor with a rich history in environmental toxicant handling, however, recently a role has emerged for the AHR as a modulator of the gastrointestinal immune system. Studies have come to elucidate these effects to include the enhancement of Th cell subset differentiation, interactions between enteric flora and the luminal wall, and modulation of inflammatory interleukin and cytokine signaling. This review highlights advancements in our understanding of AHR activity in the digestive tract and how this stimulation may be wrought by certain dietary “micronutriceuticals”, namely indole-3-carbinol (I3C) and its derivatives. Greater clarity surrounding these dynamics could lead to a novel diet-derived agonist of the AHR which is not only non-toxic, but also efficacious in the amelioration of clinical IBD.
Keywords: Inflammatory bowel diseases, Aryl hydrocarbon receptor, Mucosal immunity, Dietary phytochemicals, Autoimmune diseases
Core tip: Inflammatory bowel disease (IBD) is a chronic illness with a paucity of safe and effective treatments, either medically or surgically. The aryl hydrocarbon receptor represents a novel target for future treatments of IBD using dietary ligands of the receptor. Many studies have examined the interplay between the aryl hydrocarbon receptor and gastrointestinal mucosal immunity, though there remains a gap in the understanding of how dietary ligands can modulate this activity. Our objective was to highlight elements of current literature focusing on aryl hydrocarbon receptor biology, IBD, and how their interplay can be activated with dietary “micronutriceuticals”.
INTRODUCTION
The incidence of inflammatory bowel disease (IBD) such as ulcerative colitis (UC) and Crohn’s disease (CD) has been increasing worldwide; it is now estimated that between 1 and 1.3 million Americans are currently diagnosed with IBD[1,2]. This increased incidence is possibly due to currently unidentified environmental factors, which interact with an inherent genetic predisposition[3]. IBD is a family of chronic inflammatory conditions primarily involving the digestive tract, and often having additional extra-intestinal manifestations. The unique chronic inflammatory milieu maintained by IBD predisposes patients to non-adenomatous colorectal cancer as well as small bowel adenocarcinoma[4]. To date there is no accepted etiology or preventive measures for these conditions. Even more, there exists no cure aside from radical surgery for refractory ulcerative colitis[5].
The medical management of IBD currently stands at topical intestinal anti-inflammatories, systemic immunosuppression/immunomodulation, and novel biologic agents. The response rates, or rather the percentage of IBD patients experiencing true and deep remission using currently available treatment, is notoriously low. Only just recently have gut-specific monoclonal antibody inhibitors such as vedolizumab, which targets the integrin α4β7 receptor, been approved for the treatment of IBD, possibly ushering in an age of targeted therapies[6]. However, many if not all of the current treatment modalities for IBD have significant side effect profiles, exorbitant cost, or both[7,8]. A prospective avenue of treatment for IBD that avoids many of the pitfalls of current therapy involves modulating mucosal inflammation using bioactive phytochemicals delivered by the diet. In fact, it has been reported that diets rich in fruits and vegetables are protective of IBD, which may indicate a role for future diet-derived treatments[9,10]. The ideal treatment would have influences on gut barrier permeability, innate GI inflammation, and mucosal immunity, all pathophysiological hallmarks of IBD.
One potential mediator of anti-inflammatory dietary compounds is the aryl hydrocarbon receptor (AHR). The AHR is a chaperoned cytosolic protein that has been found to influence transcription after binding to an exogenous ligand[11]. It is a member of the basic helix-loop-helix transcription factor family as well as the Per-Arnt-Sim protein homology that regulates environmental adaptation to ligand exposure[12,13]. Once bound, the AHR can shed its cytosolic chaperones, heterodimerize with the aryl hydrocarbon receptor nuclear translocator, bind to specific xenobiotic response elements within the genome, and induce downstream genes via transcriptional activation (Figure 1)[14,15]. The canonical function of the AHR exists as an environmentally responsive “sensor” which acts to detoxify its own ligands via upregulation of phase I and phase II enzymes, most notably the cytochrome P450 superfamily[16]. Its biology has been most famously attributed to the metabolism of dioxin, or 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)[17]. In addition to its roles in toxin handling, recently the AHR has been implicated in inflammatory pathways, tumorigenesis, and immune regulation within the intestines[18-20]. These downstream effects of AHR activity have been linked to manipulations of T-cell response, interleukin (IL) production, as well as altered cytokine function[21]. All of these phenomena have been found to contribute in some way to regulation of intestinal immunity, mucosal integrity, and alterations to the microvasculature of the intestine, which are all pathological disturbances inherent to IBD[22]. While it is known that AHR biology is linked to the development and progression of IBD, it is yet to be determined if the AHR can be manipulated in such a way to exert a preventative, protective, or even therapeutic role in IBD via dietary ligands[23].
Figure 1.
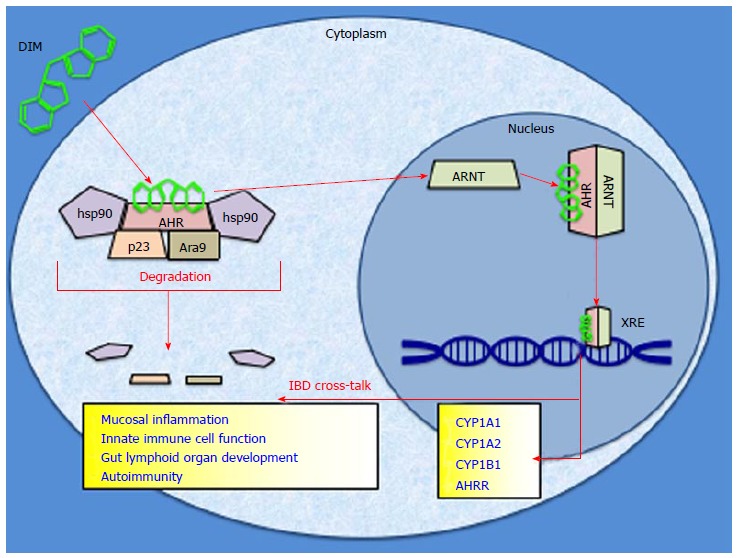
The aryl hydrocarbon receptor signaling pathway is depicted with 3,3′-diindolylmethane as a model agonist. Upon binding to a ligand, aryl hydrocarbon receptor (AHR) sheds its cytosolic chaperones and translocates to the nucleus to heterodimerize with aryl hydrocarbon receptor nuclear translocator (ARNT). This complex binds to the xenobiotic response element (XRE) within the genome and drives transcription of cytochrome P450 detoxifying enzymes. Proposed avenues of cross-talk with inflammatory bowel disease pathology are listed. IBD : Inflammatory bowel disease.
The well-studied dietary component indole-3-carbinol (I3C) has been recognized as a precursor to a host of AHR ligands that are active in the gut. The compound glucobrassicin (precursor to I3C) is found in high concentrations in the Brassica family of vegetables which includes broccoli, cabbage, and Brussels sprouts (Figure 2)[24]. Mastication-induced enzymatic hydrolysis of glucobrassicin produces I3C in the mouth. I3C then dimerizes to 3,3′-diindolylmethane (DIM) in the presence of gastric HCl as well as indole [3,2-b] carbazole (ICZ) among others further down in the GI tract[25]. It is known that DIM is the molecule which exerts more robust effects on the AHR, not its parent I3C[24]. AHR activation has been found to modulate activity of intraepithelial lymphocytes, preserve lymphoid organs in the gut, and maintain mucosal homeostasis[26,27]. Moreover, DIM-supplemented diets have been shown to attenuate colonic inflammation as well as suppress colitis-associated tumorigenesis in mice[28]. This effect may be due to the ability of DIM to modulate various inflammatory cell actions in the gut lining[29]. What is known for certain is that dietary AHR ligands are able to induce the receptor within the gut epithelium as well as globally[30]. These recent advances in the understanding of the effects of AHR stimulation via dietary ligands may lead to diet-derived novel anti-inflammatory agents which combat the inherent disturbances of IBD.
Figure 2.
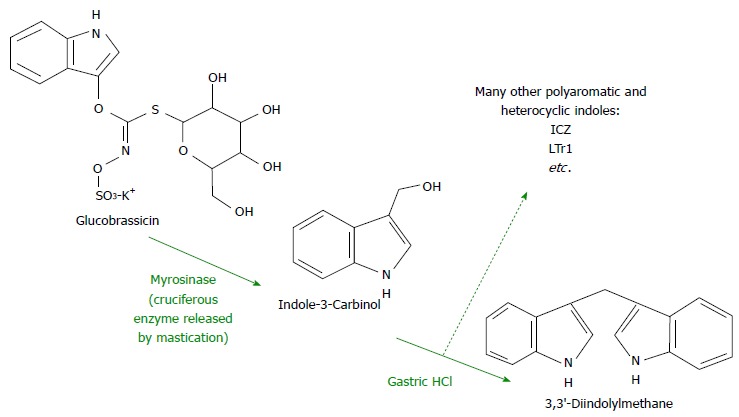
Presented is a simplified model for glucobrassicin digestion. I3C is freed from glucobrassicin by the mastication-released enzyme myrosinase. Gastric HCl drives dimerization of I3C to 3,3′-diindolylmethane, as well as other indole complexes that are released to the duodenum and distal digestive tract. I3C: Indole-3-Carbinol.
This review highlights current knowledge on AHR stimulation in the context of IBD, especially as it relates to dietary stimulation of the receptor. Continued study of the manipulation of the unique gastrointestinal inflammatory milieu associated with IBD could eventually lead to both novel therapeutics as well as diet-modifying strategies. Due to the apparent benign side effect profile of dietary AHR ligands, clinical application of this knowledge could reduce iatrogenic immunosuppressive morbidities associated with current IBD treatment as well as improve overall disease control.
LITERATURE SEARCH
A systematic literature search was conducted using PubMed and Google Scholar for “aryl hydrocarbon receptor”, “AHR”, “IBD”, “ulcerative colitis”, “3,3′-diindolylmethane”, “indole-3-carbinol”, and “mucosal immunity”. Searches containing relevant synonyms and combinations of the above terms were also utilized. Eighty-nine relevant references were identified and cited within this review. Included studies ranged from basic science investigations to clinical trials.
IN VITRO INVESTIGATION OF THE AHR, DIETARY LIGANDS, AND IBD
The biology of the AHR is well studied in numerous in vitro models, however, recently the common understanding of the AHR solely acting as a toxicological sensor that upregulates detoxification enzymes has been challenged[31]. Interactions between the receptor and dioxin (TCDD) have always been the cornerstone of mechanistic and physiologic AHR studies, however it is now known that there is a wide compendium of exogenous chemicals that operate via the AHR[32-34]. In fact, it is micronutritional chemicals such as the indole family including I3C and DIM that have recently been identified as the bridge between AHR signaling and anti-inflammatory as well as chemoprotective effects in the gastrointestinal system[35]. These chemicals have been found to enhance mucosal integrity, maintain intraepithelial lymphocyte populations, as well as sensitize the GI tract to certain populations of enteric flora[36,37]. In contrast, TCDD treatment has been found to weaken mucosal immunity in the gut[38]. This would present a possible bifunctional role for the AHR and IBD. Further investigation of these actions is warranted to elucidate their role within the inherent disturbances of IBD. An important step in understanding the role of both the AHR and its dietary ligands is to examine their roles modulating inflammation in vitro.
Research surrounding the aryl hydrocarbon receptor and various aspects of immunity has recently exploded, especially concerning the effects of dietary ligands. First, it is important to note that not only has DIM been found to activate the AHR in vitro, but has also been found to elicit multiple chemoprotective responses in various intestinal cell lines[39-42]. In addition to its ability to upregulate the AHR in cells of the digestive tract, DIM treatment also modulates immune cell activity. For instance, DIM treatment suppresses the inflammatory response of murine macrophages in vitro via downregulation of TNF-α, IL-6, and IL-1β[43]. These effects and more were also found when murine dendritic cells were treated with I3C. This protocol prompted a downregulation of TNF-α, IL-6, and IL-1β as well as an upregulation of IL-10[44]. These are important findings as activated macrophages as well as these inflammatory cytokines, especially TNF-α, play key roles in the pathogenesis of IBD[45-49]. In fact, many of the most widely used biologic agents for the treatment of IBD are anti-TNF-α antibodies[50].
In addition to various cytokine and interleukin abnormalities, IBD has also been linked to various T-cell populations and their relative size and function in the GI tract[51]. Two distinct populations that have been linked to IBD disease activity are T-regulatory cells (Treg) and Th17 T-cells[52-54]. The action of Treg cells has been found to be protective, while Th17 cell activity has propagated inflammatory damage in IBD. It is well established that the aryl hydrocarbon receptor modulates various populations of immune cells, which has implications for the future treatment of IBD[55]. In fact, AHR stimulation via natural ligands has been linked specifically to upregulated Treg cell activity and inhibition of Th17 cell activity[27,55-57]. These effects have been further proven to be AHR-dependent[58]. Another immunomodulatory effect of in vitro AHR stimulation comes as a consequence of Treg cell biology. AHR activity enhances Treg differentiation and thus increases the population of immunoregulatory/anti-inflammatory cell populations that are responsive to IL-10[58]. This is not only important because, as mentioned earlier, DIM treatment of murine immune cells leads to the induction of IL-10 , but also because IL-10 has a firmly seated role in the pathophysiology of IBD. Mice null for IL-10 have been found to be deficient in various immunoregulatory functions in the GI tract[59]. Even more, in a small trial, patients with CD responded favorably to treatment with recombinant IL-10 producing microbes[60]. Further study of the interaction between dietary AHR ligands and immune cell function could lead to a better and more targeted understanding of their interplay.
There exists a large battery of cellular cascades and signaling pathways enhanced, inhibited, or modulated by the actions of dietary indoles such as I3C and DIM, though there remains a gap concerning a full understanding of their anti-inflammatory effects[61]. Further in vitro protocols focusing solely on the interaction between the AHR and certain “micronutriceuticals” like I3C and DIM could one day lead to a better understanding of their cellular effects in the context of IBD.
IN VIVO INVESTIGATION OF THE AHR, DIETARY LIGANDS, AND IBD
The AHR has been extensively studied in vivo, mainly through the use of murine models null for the AHR to better understand its unique role in toxicology. Previous research has suggested the need to better understand the potential immunological function of AHR across various disciplines[62]. The AHR has been previously implicated as an important autoimmune target in vivo as it alters expression of the Th17 cell subset and associated cytokines in response to environmental toxins in the intestine[56,63]. Perhaps one of the most interesting avenues of research linking environmental exposures to altered immune response via the AHR can be found in the pathogenesis of IBD[21]. To best study the complex interaction of environmental factors and AHR expression in the context of immune function in the gut, many in vivo models have been developed to pick apart this inflammatory environment.
Due to the historical classification of AHR as the dioxin receptor, many models have been developed using TCDD treatment after induction of IBD. Dextran sulfate sodium (DSS) is a commonly employed agent to induce colitis in murine models, and multiple studies have shown that pre-treatment with low dose TCDD can prevent inflammation associated with colitis and/or reduce inflammation when administered after the onset of colitis in mice[57,64]. A similar study using trinitrobenzenesulfonic acid (TNBS)-induced colitis in mice as a model for CD, showed that animals treated with TCDD recover quicker and experience less colonic damage than those that are untreated[43]. While these studies show promise for the role of AHR in IBD, dioxin is a carcinogen that is highly persistent in tissue, leading to efforts to identify novel AHR ligands with low toxicity for use in vivo.
As a non-toxic agonist of the AHR, β-naphthoflavone (βNF) has shown great potential in attenuating colitis through reducing the histological score in both wild-type and AHR null mice with varying severities of DSS-induced colitis[65]. Perhaps even more interesting is the use of an endogenous mammalian AHR ligand such as the non-toxic tryptophan byproduct 6-formylindolo(3,2-b)carbazole (FICZ), which has been shown to protect mice from DSS-, TNBS-, and T-cell transfer-induced colitis through reduced inflammatory cytokine levels and lack of IL-22 induction[18]. While these compounds attenuate colitis without the potential toxic side effects of TCDD, research into the use of dietary phytochemicals as AHR ligands is of even greater interest for the treatment of IBD[66]. One study showed that the AHR is induced by phytochemicals derived from plants of the Brassicaceae family, which includes broccoli, cabbage, kale, and others. It was found that this AHR activation is required for development of RORγt+-expressing innate lymphoid cells (ILCs), as shown by increasing pools of these cells when mice are fed a diet supplemented with I3C, a product of glucobrassicin breakdown[26]. Another study established a role for I3C in controlling bacterial colonization of the gut, sustaining immune function, and protecting epithelial barrier organization as it pertains to colitis severity[27]. I3C remains a compound of great interest for the treatment of IBD, but the activity of I3C in the diet is most likely dependent on the activity of DIM, the dimer product of I3C hydrolysis by gastric acid. DIM would make up the majority of the indole load that reaches portions of the intestinal tract distal to the duodenum.
DIM has previously been shown to alleviate hepatic inflammation through shifting of diet-induced Th17 dominance to Treg dominance[67]. These data were further supported in studies where DIM was shown to attenuate experimental colitis as determined by pathological findings in mouse models, including evidence that DIM works through the AHR to decrease the Th17 cell population while increasing the number of Treg cells[29,68]. In DSS-induced colitis experiments, DIM has been shown to attenuate the disease by reducing the clinical severity of colitis, including prevention of colonic shortening and weight loss in addition to dramatically decreasing the number of tumors in AOM/DSS treated mice, which provide a common model of colitis-associated colorectal cancer[28].
In vivo models to study the AHR and IBD remain warranted, as there are numerous unidentified factors that affect progression of the disease. In particular, the interaction between immune cells and the gut microbiome is of growing interest to the research community. For example, AHR null mice succumb to infection by Citrobacter Rodentium because the absence of AHR signaling leads to a lack of RORγt+ ILCs that consequently do not produce enough IL-22[69]. Furthermore, the balance between ILCs and Th17 cells regulated by AHR has been shown to control the composition of commensal flora[69]. In fact, the menaquinone precursor 1,4-dihydroxy-2-naphthoic acid, an AHR ligand produced by Propionibacterium freudenreichii has been shown to inhibit DSS-induced colitis in mice and is even commercially available in Japan as a dietary supplement that holds promise as an IBD treatment agent[70]. These findings are critical to the continued study of IBD, as interactions between dietary factors and various states of colonic dysbiosis have been shown to contribute to disease progression[71,72].
HUMAN AND CLINICAL INVESTIGATION OF THE AHR, DIETARY LIGANDS, AND IBD
While there is a wealth of data and analysis surrounding the aryl hydrocarbon receptor and DIM in both tissue culture and murine models, there are few studies in humans related to IBD, clinical or otherwise. Some correlations have been made however, and these have prepared the way for many potential future studies. Arsenescu et al[23] found that AHR activity is upregulated in colonic biopsy tissue in IBD patients when compared to healthy controls. Even more, this increased activity mirrored that of IL-8, a neutrophil chemotactic that is elevated in IBD patient tissues[23,73]. Conversely, it has also been reported that biopsies from patients with CD exhibit downregulated levels of AHR, which is thought to be due to Th17 cell infiltration of inflamed tissue in CD[18]. This underscores the inherent bifunctionality of the AHR. Even though there are few studies which investigate human tissue, they do provide some exciting evidence to a role for the AHR in human IBD.
There are not currently any clinical trials using DIM for any form of IBD. However, there are multiple chemopreventive and chemotherapeutic trials using both DIM and its parent I3C in the context of a variety of neoplasms. These trials aimed to treat, prevent, or modulate hormone response in breast cancer, vulvar epithelial neoplasia, cervical intraepithelial neoplasia, and recurrent respiratory papillomatosis[74-77]. Again, while these examples are outside the realm of IBD, they do serve to prove that a clinical trial using I3C and/or DIM is biologically feasible in humans. In addition, there have been multiple studies which have analyzed the pharmacodynamics of these compounds in humans, which provide groundwork to one day optimize dosing protocols for trials aimed at IBD[78-80]. One pharmacokinetic and safety investigation established that not only are I3C and DIM non-toxic at doses ranging from 200-800 mg daily, but also that in most of the participants tissue concentrations over 1 mmol/L were observed[81].
What all of this work has done is prove that both I3C and DIM have some form of biologic and therapeutic activity in humans. Whether or not this activity is the result of AHR stimulation remains to be seen. Moving forward, a clinical trial utilizing these phytochemicals to combat IBD is warranted. The concept of using natural chemicals to treat intestinal inflammation is not new. Curcumin, the biologically active derivative of the spice turmeric, has been found to modulate numerous inflammatory, oxidative, and tumorigenic pathways in various tissues, including the colorectum[82-85]. Numerous in vitro and in vivo studies have propelled curcumin into multiple IBD-related clinical trials. The first of these investigations was a very small pilot study which discovered that in IBD patients curcumin treatment lowered both erythrocyte sedimentation rates and CD Activity Index scores vs placebo[86]. More recently, Hanai et al[87] found in their RCT that curcumin performed well vs placebo for maintenance therapy of mild-moderate ulcerative colitis. While oral delivery proved to have therapeutic activity, curcumin enemas have also been employed in the treatment of distal colitis with similarly efficacious results[88]. In relevance to the paradigm of I3C/DIM acting via the aryl hydrocarbon receptor, curcumin as well as other dietary phytochemicals have been found to modulate AHR activity[89]. These trials of curcumin provide relevance to investigating the therapeutic potential of natural dietary chemicals such as I3C and DIM in the context of IBD.
CONCLUSION AND FUTURE DIRECTIONS
The complex and often dangerous treatment of IBD is a dilemma faced by gastroenterologists and colorectal surgeons alike. The intricate inflammatory milieu of IBD presents many avenues for potential targets to attenuate the inherent autoimmunity of the condition. In order to better understand the role that dietary ligands of the AHR play in attenuating IBD, potential avenues of study should focus on the aryl hydrocarbon receptor as it pertains to intestinal barrier function, immune regulation, and inflammation. To achieve this, portions of the IBD phenotype would be isolated and measured under AHR stimulation by a dietary agonist such as I3C or DIM. Also, the binding affinities of these compounds to the AHR in an array of gastrointestinal tissues must be established in order to localize the cell and tissue types where these agents will achieve the most robust response. Another important line of inquiry is to delineate the molecular cross-talk between AHR stimulation and the numerous other pathways previously identified as those that drive IBD. More globally, tissue-specific AHR activity should be investigated in order to ascertain off-target effects of treatment with a dietary AHR agonist. Finally, the most rigorous examination of these agents would be a randomized controlled trial of I3C or DIM for the treatment of IBD within the Phases set by the FDA. However, incorporation of dietary AHR ligands into human clinical studies demands a crystal clear picture put forth by exhaustive in vitro and in vivo murine models as to how these compounds exert their effects. Throughout these various investigations, it would remain important to delineate additional molecular pathways engaged by these dietary ligands in addition to the AHR in order to better understand their complete mechanisms of action.
Further investigation of how IBD-related cascades can be manipulated exogenously, perhaps via the AHR, could one day lead to diet-derived and well-tolerated regimens for those with ulcerative colitis and CD. That being said, it must be appreciated that the AHR is only one of many potential signaling cascades that may influence the IBD phenotype in humans. The characterization of a diet-derived agent, AHR agonist or not, that targets the hallmark imbalances in IBD without compromising host immune function would revolutionize current medical treatment modalities and save many from radical surgical intervention.
Footnotes
Conflict-of-interest statement: None of the authors have any conflicts, financial or otherwise.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 1, 2015
First decision: November 6, 2015
Article in press: December 11, 2015
P- Reviewer: Rajendran VM, Soares RL, Tsai HH S- Editor: Wang JL L- Editor: A E- Editor: Wu HL
References
- 1.Kappelman MD, Rifas-Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, Finkelstein JA. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 3.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12 Suppl 1:S3–S9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 4.Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727–2737. [PubMed] [Google Scholar]
- 5.Cima RR, Pemberton JH. Medical and surgical management of chronic ulcerative colitis. Arch Surg. 2005;140:300–310. doi: 10.1001/archsurg.140.3.300. [DOI] [PubMed] [Google Scholar]
- 6.Gilroy L, Allen PB. Is there a role for vedolizumab in the treatment of ulcerative colitis and Crohn’s disease? Clin Exp Gastroenterol. 2014;7:163–172. doi: 10.2147/CEG.S45261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engel MA, Neurath MF. New pathophysiological insights and modern treatment of IBD. J Gastroenterol. 2010;45:571–583. doi: 10.1007/s00535-010-0219-3. [DOI] [PubMed] [Google Scholar]
- 8.Kappelman MD, Rifas-Shiman SL, Porter CQ, Ollendorf DA, Sandler RS, Galanko JA, Finkelstein JA. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135:1907–1913. doi: 10.1053/j.gastro.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Souza S, Levy E, Mack D, Israel D, Lambrette P, Ghadirian P, Deslandres C, Morgan K, Seidman EG, Amre DK. Dietary patterns and risk for Crohn’s disease in children. Inflamm Bowel Dis. 2008;14:367–373. doi: 10.1002/ibd.20333. [DOI] [PubMed] [Google Scholar]
- 10.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 11.Wilson CL, Safe S. Mechanisms of ligand-induced aryl hydrocarbon receptor-mediated biochemical and toxic responses. Toxicol Pathol. 1998;26:657–671. doi: 10.1177/019262339802600510. [DOI] [PubMed] [Google Scholar]
- 12.McIntosh BE, Hogenesch JB, Bradfield CA. Mammalian Per-Arnt-Sim proteins in environmental adaptation. Annu Rev Physiol. 2010;72:625–645. doi: 10.1146/annurev-physiol-021909-135922. [DOI] [PubMed] [Google Scholar]
- 13.Tian J, Feng Y, Fu H, Xie HQ, Jiang JX, Zhao B. The Aryl Hydrocarbon Receptor: A Key Bridging Molecule of External and Internal Chemical Signals. Environ Sci Technol. 2015;49:9518–9531. doi: 10.1021/acs.est.5b00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes JD, Dinkova-Kostova AT, McMahon M. Cross-talk between transcription factors AhR and Nrf2: lessons for cancer chemoprevention from dioxin. Toxicol Sci. 2009;111:199–201. doi: 10.1093/toxsci/kfp168. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji N, Fukuda K, Nagata Y, Okada H, Haga A, Hatakeyama S, Yoshida S, Okamoto T, Hosaka M, Sekine K, et al. The activation mechanism of the aryl hydrocarbon receptor (AhR) by molecular chaperone HSP90. FEBS Open Bio. 2014;4:796–803. doi: 10.1016/j.fob.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miao W, Hu L, Scrivens PJ, Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J Biol Chem. 2005;280:20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- 17.Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta. 2003;1619:263–268. doi: 10.1016/s0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- 18.Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, MacDonald TT, Pallone F, Monteleone G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–248, 248.e1. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Kawajiri K, Kobayashi Y, Ohtake F, Ikuta T, Matsushima Y, Mimura J, Pettersson S, Pollenz RS, Sakaki T, Hirokawa T, et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc Natl Acad Sci USA. 2009;106:13481–13486. doi: 10.1073/pnas.0902132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteleone I, MacDonald TT, Pallone F, Monteleone G. The aryl hydrocarbon receptor in inflammatory bowel disease: linking the environment to disease pathogenesis. Curr Opin Gastroenterol. 2012;28:310–313. doi: 10.1097/MOG.0b013e328352ad69. [DOI] [PubMed] [Google Scholar]
- 22.Cromer WE, Mathis JM, Granger DN, Chaitanya GV, Alexander JS. Role of the endothelium in inflammatory bowel diseases. World J Gastroenterol. 2011;17:578–593. doi: 10.3748/wjg.v17.i5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arsenescu R, Arsenescu V, Zhong J, Nasser M, Melinte R, Dingle RW, Swanson H, de Villiers WJ. Role of the xenobiotic receptor in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1149–1162. doi: 10.1002/ibd.21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderton MJ, Manson MM, Verschoyle RD, Gescher A, Lamb JH, Farmer PB, Steward WP, Williams ML. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin Cancer Res. 2004;10:5233–5241. doi: 10.1158/1078-0432.CCR-04-0163. [DOI] [PubMed] [Google Scholar]
- 26.Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 28.Kim YH, Kwon HS, Kim DH, Shin EK, Kang YH, Park JH, Shin HK, Kim JK. 3,3’-diindolylmethane attenuates colonic inflammation and tumorigenesis in mice. Inflamm Bowel Dis. 2009;15:1164–1173. doi: 10.1002/ibd.20917. [DOI] [PubMed] [Google Scholar]
- 29.Huang Z, Jiang Y, Yang Y, Shao J, Sun X, Chen J, Dong L, Zhang J. 3,3’-Diindolylmethane alleviates oxazolone-induced colitis through Th2/Th17 suppression and Treg induction. Mol Immunol. 2013;53:335–344. doi: 10.1016/j.molimm.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Ito S, Chen C, Satoh J, Yim S, Gonzalez FJ. Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J Clin Invest. 2007;117:1940–1950. doi: 10.1172/JCI31647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol Chem. 2010;391:1235–1248. doi: 10.1515/BC.2010.128. [DOI] [PubMed] [Google Scholar]
- 32.Mandal PK. Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J Comp Physiol B. 2005;175:221–230. doi: 10.1007/s00360-005-0483-3. [DOI] [PubMed] [Google Scholar]
- 33.Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007;581:3608–3615. doi: 10.1016/j.febslet.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 34.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 35.Leavy O. Mucosal immunology: the ‘AHR diet’ for mucosal homeostasis. Nat Rev Immunol. 2011;11:806. doi: 10.1038/nri3115. [DOI] [PubMed] [Google Scholar]
- 36.Hooper LV. You AhR what you eat: linking diet and immunity. Cell. 2011;147:489–491. doi: 10.1016/j.cell.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Kinoshita H, Abe J, Akadegawa K, Yurino H, Uchida T, Ikeda S, Matsushima K, Ishikawa S. Breakdown of mucosal immunity in gut by 2,3,7,8-tetraclorodibenzo-p-dioxin (TCDD) Environ Health Prev Med. 2006;11:256–263. doi: 10.1007/BF02898015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hestermann EV, Brown M. Agonist and chemopreventative ligands induce differential transcriptional cofactor recruitment by aryl hydrocarbon receptor. Mol Cell Biol. 2003;23:7920–7925. doi: 10.1128/MCB.23.21.7920-7925.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin XF, Chen J, Mao W, Wang YH, Chen MH. A selective aryl hydrocarbon receptor modulator 3,3’-Diindolylmethane inhibits gastric cancer cell growth. J Exp Clin Cancer Res. 2012;31:46. doi: 10.1186/1756-9966-31-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim EJ, Park SY, Shin HK, Kwon DY, Surh YJ, Park JH. Activation of caspase-8 contributes to 3,3’-Diindolylmethane-induced apoptosis in colon cancer cells. J Nutr. 2007;137:31–36. doi: 10.1093/jn/137.1.31. [DOI] [PubMed] [Google Scholar]
- 41.Bonnesen C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–6130. [PubMed] [Google Scholar]
- 42.Cho HJ, Seon MR, Lee YM, Kim J, Kim JK, Kim SG, Park JH. 3,3’-Diindolylmethane suppresses the inflammatory response to lipopolysaccharide in murine macrophages. J Nutr. 2008;138:17–23. doi: 10.1093/jn/138.1.17. [DOI] [PubMed] [Google Scholar]
- 43.Benson JM, Shepherd DM. Dietary ligands of the aryl hydrocarbon receptor induce anti-inflammatory and immunoregulatory effects on murine dendritic cells. Toxicol Sci. 2011;124:327–338. doi: 10.1093/toxsci/kfr249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zareie M, Singh PK, Irvine EJ, Sherman PM, McKay DM, Perdue MH. Monocyte/macrophage activation by normal bacteria and bacterial products: implications for altered epithelial function in Crohn’s disease. Am J Pathol. 2001;158:1101–1109. doi: 10.1016/S0002-9440(10)64057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plevy SE, Landers CJ, Prehn J, Carramanzana NM, Deem RL, Shealy D, Targan SR. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn’s disease. J Immunol. 1997;159:6276–6282. [PubMed] [Google Scholar]
- 46.Masuda H, Iwai S, Tanaka T, Hayakawa S. Expression of IL-8, TNF-alpha and IFN-gamma m-RNA in ulcerative colitis, particularly in patients with inactive phase. J Clin Lab Immunol. 1995;46:111–123. [PubMed] [Google Scholar]
- 47.Sashio H, Tamura K, Ito R, Yamamoto Y, Bamba H, Kosaka T, Fukui S, Sawada K, Fukuda Y, Tamura K, et al. Polymorphisms of the TNF gene and the TNF receptor superfamily member 1B gene are associated with susceptibility to ulcerative colitis and Crohn’s disease, respectively. Immunogenetics. 2002;53:1020–1027. doi: 10.1007/s00251-001-0423-7. [DOI] [PubMed] [Google Scholar]
- 48.Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–298. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 49.Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5:119–133. doi: 10.1097/00054725-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009;15:199–207. doi: 10.1016/j.molmed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Boschetti G, Nancey S, Sardi F, Roblin X, Flourié B, Kaiserlian D. Therapy with anti-TNFα antibody enhances number and function of Foxp3(+) regulatory T cells in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:160–170. doi: 10.1002/ibd.21308. [DOI] [PubMed] [Google Scholar]
- 52.Wilke CM, Bishop K, Fox D, Zou W. Deciphering the role of Th17 cells in human disease. Trends Immunol. 2011;32:603–611. doi: 10.1016/j.it.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limón P, Paiva RS, Ching T, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H, Wei Y, Yu D. Control of lymphocyte homeostasis and effector function by the aryl hydrocarbon receptor. Int Immunopharmacol. 2015;28:818–824. doi: 10.1016/j.intimp.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 55.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 56.Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One. 2011;6:e23522. doi: 10.1371/journal.pone.0023522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scheinin T, Butler DM, Salway F, Scallon B, Feldmann M. Validation of the interleukin-10 knockout mouse model of colitis: antitumour necrosis factor-antibodies suppress the progression of colitis. Clin Exp Immunol. 2003;133:38–43. doi: 10.1046/j.1365-2249.2003.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, van Deventer SJ, Neirynck S, Peppelenbosch MP, Steidler L. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:754–759. doi: 10.1016/j.cgh.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 61.Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 62.Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127:299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qiu J, Zhou L. Aryl hydrocarbon receptor promotes RORγt⁺ group 3 ILCs and controls intestinal immunity and inflammation. Semin Immunopathol. 2013;35:657–670. doi: 10.1007/s00281-013-0393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takamura T, Harama D, Matsuoka S, Shimokawa N, Nakamura Y, Okumura K, Ogawa H, Kitamura M, Nakao A. Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunol Cell Biol. 2010;88:685–689. doi: 10.1038/icb.2010.35. [DOI] [PubMed] [Google Scholar]
- 65.Furumatsu K, Nishiumi S, Kawano Y, Ooi M, Yoshie T, Shiomi Y, Kutsumi H, Ashida H, Fujii-Kuriyama Y, Azuma T, et al. A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig Dis Sci. 2011;56:2532–2544. doi: 10.1007/s10620-011-1643-9. [DOI] [PubMed] [Google Scholar]
- 66.Saxena A, Kaur K, Hegde S, Kalekhan FM, Baliga MS, Fayad R. Dietary agents and phytochemicals in the prevention and treatment of experimental ulcerative colitis. J Tradit Complement Med. 2014;4:203–217. doi: 10.4103/2225-4110.139111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, She W, Wang F, Li J, Wang J, Jiang W. 3, 3’-Diindolylmethane alleviates steatosis and the progression of NASH partly through shifting the imbalance of Treg/Th17 cells to Treg dominance. Int Immunopharmacol. 2014;23:489–498. doi: 10.1016/j.intimp.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 68.Huang Z, Zuo L, Zhang Z, Liu J, Chen J, Dong L, Zhang J. 3,3’-Diindolylmethane decreases VCAM-1 expression and alleviates experimental colitis via a BRCA1-dependent antioxidant pathway. Free Radic Biol Med. 2011;50:228–236. doi: 10.1016/j.freeradbiomed.2010.10.703. [DOI] [PubMed] [Google Scholar]
- 69.Qiu J, Guo X, Chen ZM, He L, Sonnenberg GF, Artis D, Fu YX, Zhou L. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–399. doi: 10.1016/j.immuni.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fukumoto S, Toshimitsu T, Matsuoka S, Maruyama A, Oh-Oka K, Takamura T, Nakamura Y, Ishimaru K, Fujii-Kuriyama Y, Ikegami S, et al. Identification of a probiotic bacteria-derived activator of the aryl hydrocarbon receptor that inhibits colitis. Immunol Cell Biol. 2014;92:460–465. doi: 10.1038/icb.2014.2. [DOI] [PubMed] [Google Scholar]
- 71.Kaur N, Chen CC, Luther J, Kao JY. Intestinal dysbiosis in inflammatory bowel disease. Gut Microbes. 2011;2:211–216. doi: 10.4161/gmic.2.4.17863. [DOI] [PubMed] [Google Scholar]
- 72.Neuman MG, Nanau RM. Inflammatory bowel disease: role of diet, microbiota, life style. Transl Res. 2012;160:29–44. doi: 10.1016/j.trsl.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 73.Daig R, Andus T, Aschenbrenner E, Falk W, Schölmerich J, Gross V. Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut. 1996;38:216–222. doi: 10.1136/gut.38.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF. Pilot study: effect of 3,3’-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer. 2004;50:161–167. doi: 10.1207/s15327914nc5002_5. [DOI] [PubMed] [Google Scholar]
- 75.Naik R, Nixon S, Lopes A, Godfrey K, Hatem MH, Monaghan JM. A randomized phase II trial of indole-3-carbinol in the treatment of vulvar intraepithelial neoplasia. Int J Gynecol Cancer. 2006;16:786–790. doi: 10.1111/j.1525-1438.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 76.Bell MC, Crowley-Nowick P, Bradlow HL, Sepkovic DW, Schmidt-Grimminger D, Howell P, Mayeaux EJ, Tucker A, Turbat-Herrera EA, Mathis JM. Placebo-controlled trial of indole-3-carbinol in the treatment of CIN. Gynecol Oncol. 2000;78:123–129. doi: 10.1006/gyno.2000.5847. [DOI] [PubMed] [Google Scholar]
- 77.Rosen CA, Bryson PC. Indole-3-carbinol for recurrent respiratory papillomatosis: long-term results. J Voice. 2004;18:248–253. doi: 10.1016/j.jvoice.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 78.Reed GA, Peterson KS, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A. A phase I study of indole-3-carbinol in women: tolerability and effects. Cancer Epidemiol Biomarkers Prev. 2005;14:1953–1960. doi: 10.1158/1055-9965.EPI-05-0121. [DOI] [PubMed] [Google Scholar]
- 79.Paltsev M, Kiselev V, Muyzhnek E, Drukh V, Kuznetsov I, Pchelintseva O. Comparative preclinical pharmacokinetics study of 3,3’-diindolylmethane formulations: is personalized treatment and targeted chemoprevention in the horizon? EPMA J. 2013;4:25. doi: 10.1186/1878-5085-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sepkovic DW, Bradlow HL, Bell M. Quantitative determination of 3,3’-diindolylmethane in urine of individuals receiving indole-3-carbinol. Nutr Cancer. 2001;41:57–63. doi: 10.1080/01635581.2001.9680612. [DOI] [PubMed] [Google Scholar]
- 81.Ho GH, Luo XW, Ji CY, Foo SC, Ng EH. Urinary 2/16 alpha-hydroxyestrone ratio: correlation with serum insulin-like growth factor binding protein-3 and a potential biomarker of breast cancer risk. Ann Acad Med Singapore. 1998;27:294–299. [PubMed] [Google Scholar]
- 82.Aggarwal BB, Gupta SC, Sung B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol. 2013;169:1672–1692. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rahmani AH, Al Zohairy MA, Aly SM, Khan MA. Curcumin: a potential candidate in prevention of cancer via modulation of molecular pathways. Biomed Res Int. 2014;2014:761608. doi: 10.1155/2014/761608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patel VB, Misra S, Patel BB, Majumdar AP. Colorectal cancer: chemopreventive role of curcumin and resveratrol. Nutr Cancer. 2010;62:958–967. doi: 10.1080/01635581.2010.510259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dulbecco P, Savarino V. Therapeutic potential of curcumin in digestive diseases. World J Gastroenterol. 2013;19:9256–9270. doi: 10.3748/wjg.v19.i48.9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50:2191–2193. doi: 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
- 87.Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, Tsujikawa T, Fujiyama Y, Mitsuyama K, Sata M, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502–1506. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 88.Singla V, Pratap Mouli V, Garg SK, Rai T, Choudhury BN, Verma P, Deb R, Tiwari V, Rohatgi S, Dhingra R, et al. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis - a randomized, placebo-controlled, pilot study. J Crohns Colitis. 2014;8:208–214. doi: 10.1016/j.crohns.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 89.Mohammadi-Bardbori A, Bengtsson J, Rannug U, Rannug A, Wincent E. Quercetin, resveratrol, and curcumin are indirect activators of the aryl hydrocarbon receptor (AHR) Chem Res Toxicol. 2012;25:1878–1884. doi: 10.1021/tx300169e. [DOI] [PubMed] [Google Scholar]


