Abstract
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is a novel approach in liver surgery that allows for extensive resection of liver parenchyma by inducing a rapid hypertrophy of the future remnant liver. However, recent reports indicate that not all patients eligible for ALPPS will benefit from this procedure. Therefore, careful patient selection will be necessary to fully exploit possible benefits of ALPPS. Here, we provide a comprehensive overview of the technical evolution of ALPPS with a special emphasis on safety and oncologic efficacy. Furthermore, we review the contemporary literature regarding indication and benefits, but also limitations of ALPPS.
Keywords: Liver tumor, Resection, Hepatectomy, Staged, Portal vein embolization, Future liver remnant, Liver hypertrophy, Liver failure, Morbidity, Mortality
Core tip: We provide a comprehensive overview of the technical evolution of Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) with a special emphasis on safety and oncologic efficacy. Furthermore, we review the contemporary literature regarding indication and benefits, but also limitations of ALPPS.
INTRODUCTION
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), initially known as “in situ split”, was first performed in September 2007, and until today, more than 600 procedures have been performed worldwide[1-4]. ALPPS has shown to have a promising efficacy regarding the induction of a rapid liver hypertrophy, thereby increasing the resectability of previously unresectable liver malignancy[5]. Furthermore, a sufficient volume increase can also be achieved by liver partition after unsuccessful portal vein embolization (PVE)[6,7].
Since the pioneering publication of Schnitzbauer et al[1] in 2012 with the first 25 cases in a multi-centric study, considerable experiences have been obtained. A technical evolution of this novel procedure has been observed during the last four years. This was accompanied by a better understanding of the importance of patient selection, not only to minimize morbidity and mortality, but also to achieve the most oncological benefit[8].
This review was performed to report a current overview on the development of the ALPPS procedure. The review is based on personal experience from our institute as well as a detailed analysis of the international literature of the last four years.
LANDMARKS
The pioneer case and the first multicenter study
The pioneer case of ALPPS was performed in September 2007 by Dr. Schlitt at the University Hospital Regensburg, Germany in a young patient with hilar cholangiocarcinoma (CCA)[2]. During the exploration, the surgeon decided to perform a left hepaticojejunostomy to relief the cholestasis of the future liver remnant (FLR), which was considered too small for a one stage right trisectionectomy. To provide an access to the left bile duct, the liver was transected along the falciform ligament. The right portal vein was ligated to enhance the hypertrophy of the remnant liver. The patient recovered so well that a computer tomography (CT) was performed at the postoperative day (POD) 8, showing a 94% gain of the future remnant liver volume. Thus the second stage operation was successfully performed on POD 9.
This method was found to be reproducible and was soon adopted by many other surgeons around the world. In 2012, Dr. Schnitzbauer reviewed the first 25 cases of this novel concept of 2-staged hepatectomy in five German university hospitals between September 2007 and January 2011[1]. The indications were patients with either primary or secondary liver malignancy, who underwent a right trisectionectomy with a preoperative left lateral lobe to body weight ratio of less than 0.5%. After a median interval of 9 d (range, 5 to 28 d) from in situ splitting and right portal vein ligation (PVL), a CT volumetry was performed, indicating a median increase in volume of 74% (range: 21% to 192%). The procedure was then completed on the same or following day without drop-out. None of the patients developed irreversible liver failure after surgery. Sixteen patients (68%) experienced perioperative complications[1]. In-hospital mortality was 12%, the six-month median overall survival was 86%.
The above procedure was considered as a novel concept representing one of the most promising advances in oncological liver surgery by the editors of the Annals of Surgery[3]. The new strategy was found to elegantly address the most feared complication following major hepatectomy, postoperative liver failure (PHLF). The amount of hypertrophy induced by this procedure is unparalleled by any other techniques. Moreover, the rapid regenerative response offers additional significant advantages. For example, tumor progression is unlikely during this short period, and there are less adhesions during the second stage operation. Furthermore, this procedure thereby allows a faster recovery for the patient, with the possibility to resume chemotherapy earlier. The acronym “ALPPS” was proposed to describe this novel approach: “Associating Liver Partition and Portal vein ligation for Staged hepatectomy”[3].
ALPPS registry
The ALPPS registry was initiated by Dr. De Santibanes, Dr. Lang and Dr. Clavien in 2012 to achieve a more systematic exploration of this new surgical procedure[9]. It is an internet-based international registry for cases performed using the above method. The headquarter is located at the Department of Surgery, University Hospital Zurich, Switzerland. To establish the registry, an electronic case report form using the clinical trials software SECUTRIAL (Interactive System, Berlin, Germany) was presented to selected experts worldwide for approval (Scientific Committee of the ALPPS Registry). Any center willing to report patients in the registry is given access through the internet. The aim of the registry is to systematically and uniformly collect information from multiple centers worldwide[10]. Despite of a possible reporting bias, the registry enables surgeons to study a larger population to overcome shortcomings inherent to small case series reports. In 2014, the first report by the registry consisted of a total of 202 patients from 41 centers, provided complete data sets of procedures and 90 d survival status[10]. Till July 8th, 2015, 583 cases performed worldwide were enrolled into the registry.
The first consensus meeting on ALPPS
In February 2015, the first ALPPS consensus meeting was held by Dr. Oldhafer and Dr. van Gulik in Hamburg, Germany. Nearly all groups with vast experience in the ALPPS approach were invited to participate as faculty. The key points consisted of indications, preparations, techniques and outcomes. The two-day meeting provided the community a scientific base for future decision-making. The video and slides are available at the official website (www.alpps.com). The meeting not only summarized the development and the limitations of ALPPS, but also inspired the ideas and promoted the cooperation between international centers. The summary of the consensus meeting is yet waiting for publication.
EVOLUTION OF THE SURGICAL TECHNIQUE
Classical ALPPS
The first operation (right portal vein transection and in situ liver splitting): During the first operation, an exploration is carried out to exclude extrahepatic tumour dissemination. Resectability is determined if the remnant segments 2 and 3 have adequate inflow as well as outflow. Tumour involvement of segments 2 and 3 is no contraindication as long as it could be safely resected without tumour residual. The next step is the dissection of the hepatoduodenal ligament. A cholecystectomy is optional. In patients without tumour infiltration of the gall bladder, a cholecystectomy is usually carried out. After lifting the common bile duct and right hepatic artery by a lid retractor, the right portal vein and main portal vein is exposed (Figure 1). At this stage, the main right portal vein branch could be transected after suture ligation at the distal end and continuous suture, e.g., with 5/0 Prolene at the proximal end. In patients with trifurcation of the portal vein with separate entry of the right anterior and posterior sectional branches, the anterior and posterior portal veins should be divided separately.
Figure 1.
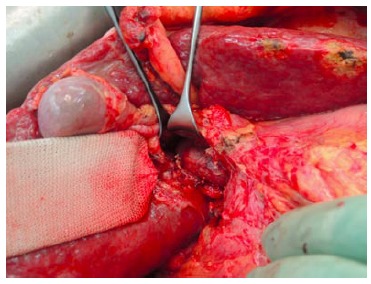
Exposure of the portal vein by lifting the common bile duct and right hepatic artery using a lid retractor. Here the right portal vein branches were transected.
The umbilical portion of the left portal vein is exposed by dissecting the umbilical fissure. The portal branches of segment 4 are ligated and divided at its origin. The hepatic artery, the bile duct and the right hepatic vein are dissected and identified with rubber bands. Subsequently, transection of the liver parenchyma along the falciform ligament is performed (Figure 2). The falciform ligament could also be kept in the future remnant side for re-fixation of the left lateral lobe at the diaphragmatic dome if technically possible. Intraoperative ultrasound should be performed to confirm the absence of right portal flow at the end of the operation. Silicone sheeting or drainage could be applied to separate the two parts of the liver and the surrounding organs in order to prevent strong adhesion among the above mentioned structures. Closed drainage is placed in the liver hilum. An intraabdominal swab should be taken for microbiological analysis at the end of the operation.
Figure 2.
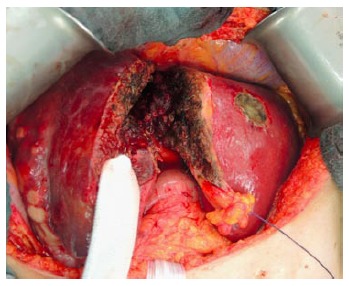
Liver parenchyma transection along the falciform ligament.
Postoperative management after the first operation: The patient is usually transferred to the intermediate care unit and discharged to the normal ward according to the postoperative course. Prophylactic antibiotics are given as single shot intraoperatively. If any bacteria are isolated from the intraoperative swab, the antibiotics should be given until the second operation. In patients with stented bile duct, antibiotics and antimycotics are administered during the whole postoperative phase.
One week after the first operation, depending on the logistics, an abdominal CT scan (native phase) is performed for re-evaluation of the liver volume (Figure 3). When the future liver remnant/total liver volume ratio (FLR/TLV) is more than 30%, the second operation, i.e., right trisectionectomy, could be carried out on the next available operation day. If the FLR/TLV is less than 30%, a repeat CT scan would be carried out in an interval of seven days, and the second operation being postponed accordingly.
Figure 3.
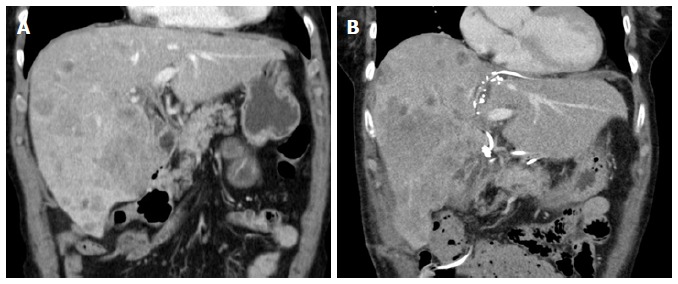
Computed tomography scan before associating liver partition and portal vein ligation for staged hepatectomy in a patient with intrahepatic cholangiocarcinoma and on day 10 after liver partition. A: The future liver remnant consisted of segment 2 and 3 with volume of 347 mL (23% of the standardized total liver volume); B: Showing the hypertrophy of the segment 2 and 3 with volume of 610 mL (41% of the standardized total liver volume).
The second operation (right trisectionectomy): After relaparotomy, the silicone sheeting or drainage is removed. An intraabdominal swab is taken for microbiological analysis for orientated antibiotic therapy if indicated. The hilar structures are easily identified by the rubber bands, and the right hepatic artery, right hepatic ducts (or the left hepatic duct when extrahepatic bile duct should be resected) and the right and middle hepatic veins are transected (Figure 4). Liver segment 1 could be preserved in patients with non-perihilar CCA without tumour involvement.
Figure 4.
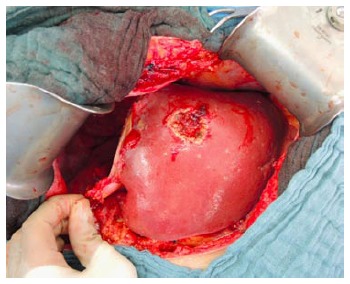
Completion of right trisectionectomy.
After removal of the transected liver, a lymphadenectomy could be conducted at this stage. Biliodigestive anastomosis is followed when resection of the extrahepatic bile duct is indicated in patients with perihilar CCA. The postoperative treatment after the second operation is the same as for the patients undergoing any major hepatectomy.
ALPPS variations
Improvement of patient safety by different approaches of in situ liver splitting: One of the major differences between traditional PVE and ALPPS in liver partition is that the latter, has a liver splitting along the transection line of the FLR. To simplify the first operation, three methods were developed to achieve liver partition without physically splitting the liver: Tourniquet compression, radiofrequency ablation (RFA) or microwave ablation.
The use of a tourniquet to ensure parenchymal compression and intrahepatic collateral occlusion along the future transection line was first described by Robles et al[11]. He used a 1 cm deep groove to place and tighten a 3 mm Vicryl tourniquet, after which ultrasound confirmed occlusion of the vessels between the two parts. This technique was termed Associating Liver Tourniquet and Portal Ligation for Stage Hepatectomy (ALTPS)[11]. In 22 patients undergoing ALTPS procedure, FLR at 7 d increased by a median of 61% (range: 33% to 189%).
Jiao et al[12] used in-line radio frequency (Habib Sealer, LH4X, Rita) to create a virtual liver partition in combination with portal vein ligation. The RFA produce a precise avascular area up to 1 cm wide. In the initial report of five patients, Radiofrequency-Assisted Liver Partition with Portal Vein Ligation could significantly increase the FLR by a median of 62.3% (range: 53.1% to 95.4%) after 21.8 ± 9.4 d.
Similar to RFA, Cillo et al[13] used microwave ablation on segment 4 in the first stage operation to complete the liver partition. The authors reported that this technique could minimize the risk of neoplastic left lobe invasion and limit portoportal shunts. They observed a 78% FLR growth, performing the second stage after 10 d.
All three techniques could be performed laparoscopically[13,14]. However, superiority of these procedures to the classic ALPPS approach regarding safety has not been confirmed apart from case reports.
Improvement of patient safety by partial ALPPS: To avoid bile leak from incidental transection of the segment 4 bile duct and to avoid segment 4 ischemia due to transection of the segment 4 artery or middle hepatic vein occlusion, non-total parenchymal transection was carried out systematically in the author’s institute (Figure 5) and selectively in other institutes[15-17].
Figure 5.
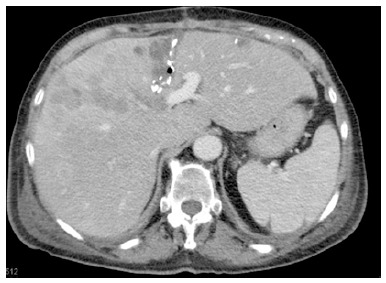
Partial associating liver partition and portal vein ligation for staged hepatectomy. The non-total liver parenchymal transection is indicated by the clips, left along the liver split area in a computed tomography scan performed on day 10 after the liver partition.
The group of De Santibanes identified total parenchymal transection as an independent predictor of postoperative complications during ALPPS. They found that most complications in patients with total parenchymal transection were surgical complications following the first stage. Avoiding total parenchymal transection might be related to the better outcomes in terms of liver-related complication in these patients[15]. The Zurich group observed in an experimental model that partial (75%-80%) transection of the liver triggered a similar degree of hypertrophy of the FLR compared to complete transection. On the basis of experimental observation and clinical implications, they switched from a complete to a well-defined partial transection (> 50% of the transection surface) in 2013[16]. In partial-ALPPS, a median hypertrophy of 60% was observed, compared to 61% after classic ALPPS approach, within a median time of 7 d. To facilitate communication among clinicians, Petrowsky et al[16] proposed to standardize the name of ALPPS with non-total parenchymal transection at stage 1 operation as “partial-ALPPS”.
Improvement of patient safety by selecting different planes of liver splitting: Various modifications of ALPPS that alter the specific segments comprising the FLR have been described, including right hepatectomy ALPPS (segment 2-4 as FLR), left hepatectomy ALPPS (segment 5-8 as FLR), central hepatectomy ALPPS (segment 4, 5 and 8 as FLR)[18]. Liver partition in different extent of hepatectomy is aimed to increase the FLR, thereby avoiding post-hepatectomy liver failure.
Recently, the concept of a monosegmental ALPPS has been addressed[19,20]. The authors proposed to name such procedures, leaving only a one-segmental FLR in the context of ALPPS, according to the remnant liver segment using third-order segment terms, for example ‘‘Segment 2 ALPPS’’, ‘‘Segment 3 ALPPS’’, ‘‘Segment 4 ALPPS’’ and ‘‘Segment 6 ALPPS’’. Among 333 patients, 12 underwent monosegment ALPPS hepatectomies in six centers, all for extensive bilobar colorectal liver metastases (CRLM). Four patients experienced liver failure, but all recovered. There was no mortality. Complications higher than Dindo-Clavien IIIa occurred in four patients with no long-term sequelae. The authors concluded that extreme liver resections for CRLM based on a single segment liver remnant are feasible and safe using the novel monosegmental ALPPS technique, a new surgical tool in the management of extensive CRLM[20].
Improvement of patient safety by imaging study and liver function test: The estimation of the postoperative liver function is mainly based on the remaining liver volume and liver function blood tests. Volumetric measurement of the intended FLR by CT or MRT is routinely carried out prior to the second stage operation. A FLR/TLV ratio exceeding 30% in patients with normal liver or higher than 40% in patients with parenchymal disease is preferred[8]. In the author’s institute, a FLR to body weight ratio over 0.6% in patients with normal liver, or more than 0.8% in patients with preexisting parenchymal damage is used as a threshold, additionally to FLR/TLV ratio for performing the second stage operation. Otherwise, the operation is postponed for another week or even cancelled.
Tanaka et al[21] performed technetium-99 m galactosyl human serum albumin (99mTc-GSA) scintigraphy single-photon emission computed tomography (SPECT)/CT with 3-dimensional volume-rendering fused images preoperatively and at 7 d after the first surgical procedure. They found that the increase in functional FLR calculated at 7 d after the liver partition by ALPPS was similar to functional FLR at 3 wk after the liver partition by PVE alone (52.1% vs 59.2%). In the group of De Santibanes, hepatobiliary scintigraphy was performed in patients with borderline sufficient FLR volume after first stage operation, or when there were doubts regarding functional sufficiency. The regional FLR function was determined by quantifying 99mTc-dimethyl iminodiacetic acid uptake during 10 min (liver uptake phase) after intravenous injection[15]. They found this method to be helpful to decide the best timing of the second stage operation in four patients of this series. In those four patients with delayed hypertrophy, an increase of the FLR function over time was observed, although there was no significant volume increase. These findings suggested that in some patients, the recommended waiting time until second stage operation may be shorter than indicated by volumetric parameters alone.
Lau et al[22] described an intraoperative indocyanine green (ICG) clearance assessment to estimate the function of the future liver remnant. After complete parenchymal transection, Bulldog vascular clamps were applied to occlude the right hepatic artery and the portal vein, and ICG clearance was carried out. They found the plasma disappearance rate was 7.9%/min and with a 15 min residual (R15) amount of 30.6% during the first stage operation. During the second stage operation 14 d later, the plasma disappearance rate increased to 12.1%/min and an R15 of 16.3% was observed. They concluded that intraoperative ICG clearance allows for the direct assessment of the actual future liver remnant function. However, since no safe cut-off levels were suggested, future validation studies would be necessary.
Improvement of patient safety by other modifications: In the International ALPPS Registry, 35% of centers did not use any coverage on the raw surface after liver transection, 26% used a plastic sheet, 26% TachoSil, and 16% of centers still used a plastic bag (of a total 192 patients). The use of a plastic bag or plastic sheeting to cover the cut area and prevent adhesions is not an essential component of ALPPS[10,23]. In the author’s institute, Penrose drainages are routinely used to separate the raw surface after liver transection as well as to avoid collections in case of a bile leak (Figure 6). However, the most important aspect to avoid bile leak and consecutive infection is not to perform in situ splitting in patients with dilated bile duct or cholangitis[24].
Figure 6.
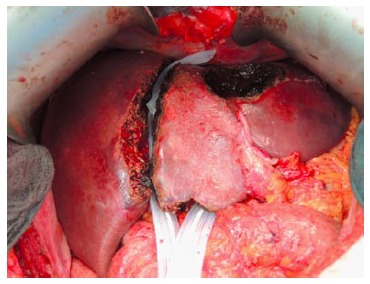
The future liver remnant was separated by two Penrose drains from the right liver lobe in a patient with bilobar colorectal liver metastases during the first stage operation. Three lesions at the left hemi-liver were resected.
Improvement of surgical approach under oncological aspects: The classic approach of ALPPS includes full mobilization of the liver and dissection of the liver hilum[1]. Aloia et al[25] criticized that ALPPS was supposed to be an “all-touch” technique that would reduce the oncological efficacy to treat liver malignancy. This comment was addressed by two technique refinements: “anterior approach” and “hybrid ALPPS”, to improve the efficacy of surgical oncology as well as to reduce the adhesion at the second stage of the operation[26,27]. An analysis of the registry data found that in 37% (66/175) of patients that underwent transection during the first stage of ALPPS, an anterior approach was applied However, caution has to been taken while applying the anterior approach due to the inability to achieve optimal vascular control during this technically complex procedure[23]. “Hybrid ALPPS” was developed in authors’ institute to treat advanced gall bladder carcinoma in two patients[27]. In situ split of the left lateral liver lobe was combined with postoperative right-PVE as a hybrid procedure. The authors concluded that hybrid ALPPS provided rapid hypertrophy of the FLR for a right trisectionectomy in case of tumor infiltration of the RPV or biliary bifurcation, while allowing to adhere to the non-touch principles. A similar procedure was performed by Robles et al[28] by using a tourniquet-technique and sequential PVE to achieve liver partition in a patient with perihilar tumor burden.
Despite lacking sufficient data for a statistical analysis of disease-free or overall survival, non-touch technique is possible, and should therefore be applied for the resection of hepatic malignancy in ALPPS. Furthermore, hybrid ALPPS combining non-physical liver split and sequential PVE could be employed to reduce the rate of bile leak in patients with dilated bile duct.
Summary: Current results on safety and efficacy
Among the preliminary reports, ALPPS showed a high morbidity (59% to 68%) and mortality (12% to 12.8%)[1,29]. In the first report of the international ALPPS registry, 90 d mortality was 19/202 (9%). Severe complications including mortalities (Clavien-Dindo ≥ IIIb) occurred in 27% of patients[10]. In experienced centers, including the authors’ institute, a much lower rate of major morbidity (13.6% to 14%) and mortality (0% to 6.6%) have been reported[15,17]. As is the case with many new techniques, there will be an inherent learning curve, and lower rates of morbidity and mortality will be observed, along with further technical improvements and standardization of the ALPPS procedure.
ALPPS has been found to result in faster FLR growth in comparison with PVE alone[30]. In a recent systemic review with a total of 295 patients, the FLR hypertrophy was 84%, with a confidence interval (CI) of 78%-91%[31]. This high efficacy in inducing FLR hypertrophy was confirmed universally by the published case series and the international ALPPS registry. Moreover, in contrast to a failure rate of 20%-30% after PVE due to inadequate hypertrophy or disease progression[32] (97%CI: 94%-99%) of all patients underwent stage one operation of ALPPS completed the procedure. Furthermore, histological complete resection (R0) was achieved in (91%CI: 87%-94%) of these patients[31].
BENEFITS OF ALPPS
To decrease the risk of grade C PHLF
Similar to PVE and 2-stage hepatectomy, the aim of ALPPS is to decrease the risk of grade C PHLF after major liver resection in otherwise too small FLR. Beside a more rapid FLR hypertrophy induced by ALPPS, this approach can also be used in cases of failed portal vein occlusion (PVO) or an anticipated extremely small FLR[33]. For an early prediction whether a patient will obtain a sufficient FLR, the concept of kinetic growth rate (KGR) or degree of hypertrophy has been introduced[34]. Growth rate was shown to be a predictor of PHLF. Patients with low KGRs are unlikely to benefit from PVO only and could thus be candidates for ALPPS. Another group of patients who might be especially suitable for ALPPS are those with “extremely low” FLRs, who, given the boundaries of growth achieved with PVO, are unlikely to reach a FLR volume deemed necessary for resection.
In the first report of the international ALPPS registry, a median KGR of 2% FLR or 30 mL FLR per day have been observed[10]. The second stage operations were performed at 10 d (interquartile ranges, 8 to 15) after the liver partition. Only 9% (16/202) of patients experienced liver failure according to the 50-50 criteria[35]. Within them PHLF was regarded as the main cause of mortality in 8 patients[10].
To provide more chance of R0 resection
Resection of a large tumor load in the liver may result in an excessive removal of hepatic parenchyma leading to PHLF and associated complications[36]. ALPPS not only allows for resection in patients with very small anticipated FLR that would not be possible with conventional techniques, but also enables surgeons to proceed with multi-staged resections in a short interval before a substantial tumor progression[17]. PVO is burdened with a considerable failure rate, and only about two thirds of patients will eventually be eligible for a subsequent curative resection due to tumor progression during the waiting interval between the two stages, or failure of the FLR to grow[37-39]. A retrospective multicenter study was carried out to compare the rate of complete tumor resection after ALPPS vs conventional two-stage approaches[5]. Eighty-three percent (40/48) of ALPPS patients achieved complete resection compared with 66% (55/83) in the PVO group. Seventeen percent (8/48) of ALPPS patients failed to achieve the primary endpoint due to mortality (n = 7) or incomplete resection (R1, n = 1). The author concluded that ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumours[5].
Evidences of oncological benefits compared to other two-stage liver resection when R0 achieved
Colorectal liver metastasis: Colorectal liver metastasis (CRLM) is the most common indication for ALPPS as indicated in the first report of the international ALPPS registry[10]. To compare the benefits of ALPPS to conventional 2-stage hepatectomy by PVO, evaluation of resection rate, postoperative mortality, as well as disease-free survival (DFS) or/and overall survival (OS) are necessary.
Resection rate, the resection rate for CRLM by traditional two-stage liver resection, either PVE or PVL, was reported to be 52%-80%[40-42]. Non-resectability was mainly due to progression of metastasis[40,41]. ALPPS avoided this type of drop-out by effectively inducing a sufficient liver hypertrophy within 6 to 15 d[33]. A retrospective study carried out by Tanaka et al[21] found that at first hepatectomy, Ki67 expression was evident in 28.2% ± 42.7% of tumor cells in the ALPPS group and 51.7% ± 35.6% in the conventional 2-stage group (P = 0.09). However, at second hepatectomy, expression of Ki67 was detected in 20.5% ± 24.7% and 54.5% ± 26.9% of patients in the ALPPS and in the conventional 2-stage group respectively (P = 0.01)[21]. Therefore, the reduced expression of Ki67 in tumors resected during the second stage in the ALPPS group may indicate an oncologic benefit from ALPPS, as the short period between the two interventions helps to avoid the risk of tumor progression. Of note, in a recent review on the treatment of CRLM, the resection rate by ALPPS was reported to be about 97.1%[33].
Postoperative mortality, PVO is a well-established, state of art procedure for patients with insufficient FLR whereas ALPPS is still among the phase of exploration. Therefore, a comparison of these two procedures should be done when the learning curve of ALPPS is overcome. According to the first report of the international registry data, mortality of ALPPS for CRLM is 8%, and 5.1% in CRLM- patients younger than 60 years of age[10]. In experienced centers, including the authors’ institute, nil mortality after ALPPS for CRLM has been reported[17].
DFS/OS, the 1 and 2-year DFS for patients undergoing ALPPS for CRLM from the ALPPS registry is 59% and 41% respectively[10]. Overall survival is 86% at six months postoperatively, dropping to 59% at 2 years[10]. Similar to the high recurrence rate despite a survival advantage observed in patients with advanced CRLM (> 4 metastases) undergoing traditional resection[43-45], high recurrence rates have also been reported in patients undergoing ALPPS[5,33,46]. In some case series, the recurrence of CRLM after ALPPS was quite early. For example, in the 7 patients reported by Oldhafer et al[41] recurrence was observed after 3, 6, 7, 8, 11, 13 and 13 morespectively following ALPPS procedure[46]. However, to date there is no direct comparison of DFS in patients undergoing PVE or ALPPS. Of note, two RCTs investigating ALPPS vs conventional two-stage hepatectomies for CRLM were recently launched (clinicaltrials.gov-identifier NCT01775267 and NCT01842971).
To get the best benefit of ALPPS, Hernandez-Alejandro et al[17] proposed selecting the group of patients with biologically favorable CRLM. The inclusion criteria for ALPPS in their group were (1) no evidence of extrahepatic disease; (2) good functional capacity Eastern Cooperative Oncology Group performance status grade 0 or 1 and (3) complete or partial response to systemic chemotherapy after 6 cycles. In the 14 patients reported in this series, recurrence developed in 2 patients after a median follow-up of 9.4 mo. Overall survival at the time of follow-up was 100%[17].
Hepatocellular carcinoma: An aggressive surgical approach in patients with locally advanced hepatocellular carcinoma (HCC) has been reported to yield an acceptable long term outcome that is significantly better than that of patients with unresectable HCC treated with Sorafenib[47-49]. In this view, the ALPPS procedure could yield a better outcome and further expand the number of patients undergoing radical major liver resection for HCC in liver cirrhosis that were previously considered non-resectable, compared to non-surgical treatment[49].
Chan et al[50] reported the largest case series with 17 patients having HCC on the basis of chronic hepatitis B infection. Selection criteria included Child-Pugh A liver cirrhosis, indocyanine green retention rate < 20% at 15 min, FLR/sTLV (standardized total liver volume) < 40%, and platelet count ≥ 100 × 109/L. After a median of 6 d, a hypertrophy of the left FLR by 48.7% with a FLR/sTLV ratio of 38.5% (preoperative FLR/sTLV 24.2%) was noted. All patients proceeded to second-stage hepatectomy. Major surgical complications (Clavien–Dindo grade III or above) occurred in 11.8% of patients (n = 2), and in-hospital mortality rate was 5.9% (n = 1). No follow-up data were reported. Chan et al[50] concluded that ALPPS could also promote liver hypertrophy in patients with chronic liver diseases, with a similar safety profile compared to other established series. Another case series by Vennarecci et al[51] suggested that the ALPPS procedure could be very useful in a subgroup of patients with HCC and venous thrombosis. In their series, portal hypertension or liver cirrhosis more than Child-Pugh A was considered as a contraindication[49,52]. However, data regarding the long term outcome of ALPPS in patients with HCC are still very limited, and further reports on the use of the ALPPS in this setting are expected[49].
Perihilar cholangicarcinoma: Although the first case of ALPPS was successfully performed in a patient with hilar CCA, high rates of major postoperative complication and mortality were found in this population after ALPPS[10]. Li et al[24] first questioned the benefit of ALPPS in treatment of perihilar CCA. The authors found that patients undergoing ALPPS for perihilar CCA were at a high risk of intraabdominal infection and bacteraemia as the diseased liver and stented biliary system were not removed between the two operations. Two of three patients with hilar CCA received ERCP and a stent before referral. Both of them had postoperative intraabdominal bacterial infections, and eradication of bacteria failed. The deaths of those two patients account for the 22% mortality observed in the cohort of 9 ALPPS patients from this series. Thus, the authors considered the combination of a stented biliary system and cholestatic liver with low potential of regeneration as a contraindication for ALPPS. This opinion has been shared with the HPB community, in which caution has aroused against the use of ALPPS for hilar tumors[8,53].
Other indications: The other indications of ALPPS comprised of intrahepatic cholangiocarcinoma, gallbladder cancer, neuroendocrine tumors and other liver metastases. Because of the limited number of cases, no high quality evidence on the oncological benefit other than increased resectability is available[5,8,10].
Summary - current status of oncological benefit by patient selection
The ALPPS procedure was developed to decrease the morbidity and mortality related to PHLF, to avoid drop-out in patients undergoing conventional two-stage liver resection and to achieve histopathological complete tumor resections (R0) in otherwise non-resectable patients. Although contemporary reports have highlighted the importance of patient selection in avoiding perioperative morbidity and mortality, suitable indications for the ALPPS approach remain to be determined[17]. Till date, there is no clear evidence for the oncological benefit of ALPPS in treatment of CRLM over other procedures as long as R0 resection is achieved. For patients with hilar CCA, ALPPS should be considered with extreme caution due to the aforementioned safety issue. For other indications, there are no ongoing studies comparing ALPPS with non-surgical treatment in term of overall survival.
CONCLUSION
ALPPS is a pertinent alternative approach to the conventional two-stage liver resection after PVE or PVL. In selected cases, it could even increase the resectability of previously unresectable liver malignancy by promising rapid hypertrophy of the FLR (Table 1). With the evolution of surgical technique, proper patient selection for ALPPS has been found to be the key element to achieve the best oncological results.
Table 1.
Recent published studies on associating liver partition and portal vein ligation for staged hepatectomy (only case series with more than 10 patients are listed)
| Ref. | Date (yr) | Total cases (center involved) | Interval1 (d) | FLR hypertrophy (median) | Completion stage 2 | R0 resection | PHLF | Morbidity2 | In-hospital mortality | Follow-up (median, months) | Recurrence | Overall survival |
| Schnitzbauer et al[1] | 2012 | 25 (5) | 9 | 74% | 88% | 96% | - | Overall: 64% ≥ III: 40% | 12% | 6 | 20% | 86% at 6 m |
| Torres et al[29] | 2013 | 39 (9) | 14 | 83% | 94.80% | 100% | - | Overall: 59% | 12.80% | - | - | - |
| Schadde et al[10] | 2014 | 202 (56) | 7 | 80% | 98% | 91% | - | ≥ III: 40% | 9% | 9 | 40% at 12 m | 73% at 12 m |
| Truant et al[4] | 2015 | 62 (9) | 8 | 48.60% | 95.20% | - | 25.8% | ≥ III: 40.3% | 12.90% | - | - | - |
| Robles et al[11] | 2014 | 22 (1) | 7 | 61% | 100% | 100% | 22.7% | Overall: 64% | 9% | 6 | 5% | 91% at 6 m |
| Nadalin et al[53] | 2014 | 15 (1) | 10 | 87.20% | 100% | 87% | - | Overall: 67% | 28.70% | 17 | 29% | 67% at 17 m |
| Alvarez et al[15] | 2015 | 30 (1) | 6 | 89.70% | 93% | 93% | 14% | Overall: 53% ≥ III: 43% | 6.60% | 17 | 22% at 12 m | 67% at 12 m |
| Petrowsky et al[16] | 2015 | 24 (1) | 7 | 61% | 100% | - | - | ≥ IIIb: 33% | 16.70% | - | - | - |
| Hernandez- Alejandro et al[17] | 2015 | 14 (1) | 7 | 93% | 100% | 86% | 29% | Overall: 36% ≥ IIIb: 14% | 0% | 9 | 14% | 100% at 9 m |
| Tanaka et al[21] | 2015 | 11 (1) | 7 | 54% | 100% | 100% (R0/R1) | 18% | Overall: 46% ≥ III: 27% | 9% | - | - | - |
| Chan et al[50] | 2015 | 17 (1) | 6 | 48.70% | 100% | - | - | ≥ III: 11.8% | 5.90% | - | - | - |
Interval: Median days from the stage 1 to CT scan;
Morbidity: Clavien-Dindo classification was applied; FLR: Future liver remnant; PHLF: Post-hepatectomy liver failure according to 50-50 criteria (35).
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest regarding this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 10, 2015
First decision: September 18, 2015
Article in press: December 11, 2015
P- Reviewer: Cerwenka HR, Mizuguchi T S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 2.Lang SA, Loss M, Schlitt HJ. “In situ split” (ISS) liver resection: new aspects of technique and indication. Zentralbl Chir. 2014;139:212–219. doi: 10.1055/s-0032-1328742. [DOI] [PubMed] [Google Scholar]
- 3.de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the “ALPPS” approach. Ann Surg. 2012;255:415–417. doi: 10.1097/SLA.0b013e318248577d. [DOI] [PubMed] [Google Scholar]
- 4.Truant S, Scatton O, Dokmak S, Regimbeau JM, Lucidi V, Laurent A, Gauzolino R, Castro Benitez C, Pequignot A, Donckier V, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): impact of the inter-stages course on morbi-mortality and implications for management. Eur J Surg Oncol. 2015;41:674–682. doi: 10.1016/j.ejso.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Schadde E, Ardiles V, Slankamenac K, Tschuor C, Sergeant G, Amacker N, Baumgart J, Croome K, Hernandez-Alejandro R, Lang H, et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg. 2014;38:1510–1519. doi: 10.1007/s00268-014-2513-3. [DOI] [PubMed] [Google Scholar]
- 6.Knoefel WT, Gabor I, Rehders A, Alexander A, Krausch M, Schulte am Esch J, Fürst G, Topp SA. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg. 2013;100:388–394. doi: 10.1002/bjs.8955. [DOI] [PubMed] [Google Scholar]
- 7.Tschuor Ch, Croome KP, Sergeant G, Cano V, Schadde E, Ardiles V, Slankamenac K, Clariá RS, de Santibaňes E, Hernandez-Alejandro R, et al. Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion -- an extension of the ALPPS approach. Eur J Surg Oncol. 2013;39:1230–1235. doi: 10.1016/j.ejso.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Bertens KA, Hawel J, Lung K, Buac S, Pineda-Solis K, Hernandez-Alejandro R. ALPPS: challenging the concept of unresectability--a systematic review. Int J Surg. 2015;13:280–287. doi: 10.1016/j.ijsu.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Barkun JS, Aronson JK, Feldman LS, Maddern GJ, Strasberg SM, Altman DG, Barkun JS, Blazeby JM, Boutron IC, Campbell WB, et al. Evaluation and stages of surgical innovations. Lancet. 2009;374:1089–1096. doi: 10.1016/S0140-6736(09)61083-7. [DOI] [PubMed] [Google Scholar]
- 10.Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R, Soubrane O, Schnitzbauer AA, Raptis D, Tschuor C, et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014;260:829–836; discussion 836-838. doi: 10.1097/SLA.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 11.Robles R, Parrilla P, López-Conesa A, Brusadin R, de la Peña J, Fuster M, García-López JA, Hernández E. Tourniquet modification of the associating liver partition and portal ligation for staged hepatectomy procedure. Br J Surg. 2014;101:1129–1134; discussion 1134. doi: 10.1002/bjs.9547. [DOI] [PubMed] [Google Scholar]
- 12.Gall TM, Sodergren MH, Frampton AE, Fan R, Spalding DR, Habib NA, Pai M, Jackson JE, Tait P, Jiao LR. Radio-frequency-assisted Liver Partition with Portal vein ligation (RALPP) for liver regeneration. Ann Surg. 2015;261:e45–e46. doi: 10.1097/SLA.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 13.Gringeri E, Boetto R, D'Amico FE, Bassi D, Cillo U. Laparoscopic microwave ablation and portal vein ligation for staged hepatectomy (LAPS): a minimally invasive first-step approach. Ann Surg. 2015;261:e42–e43. doi: 10.1097/SLA.0000000000000606. [DOI] [PubMed] [Google Scholar]
- 14.Cai X, Peng S, Duan L, Wang Y, Yu H, Li Z. Completely laparoscopic ALPPS using round-the-liver ligation to replace parenchymal transection for a patient with multiple right liver cancers complicated with liver cirrhosis. J Laparoendosc Adv Surg Tech A. 2014;24:883–886. doi: 10.1089/lap.2014.0455. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez FA, Ardiles V, de Santibañes M, Pekolj J, de Santibañes E. Associating liver partition and portal vein ligation for staged hepatectomy offers high oncological feasibility with adequate patient safety: a prospective study at a single center. Ann Surg. 2015;261:723–732. doi: 10.1097/SLA.0000000000001046. [DOI] [PubMed] [Google Scholar]
- 16.Petrowsky H, Györi G, de Oliveira M, Lesurtel M, Clavien PA. Is partial-ALPPS safer than ALPPS? A single-center experience. Ann Surg. 2015;261:e90–e92. doi: 10.1097/SLA.0000000000001087. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Alejandro R, Bertens KA, Pineda-Solis K, Croome KP. Can we improve the morbidity and mortality associated with the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) procedure in the management of colorectal liver metastases? Surgery. 2015;157:194–201. doi: 10.1016/j.surg.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 18.Gauzolino R, Castagnet M, Blanleuil ML, Richer JP. The ALPPS technique for bilateral colorectal metastases: three “variations on a theme”. Updates Surg. 2013;65:141–148. doi: 10.1007/s13304-013-0214-3. [DOI] [PubMed] [Google Scholar]
- 19.de Santibañes M, Alvarez FA, Santos FR, Ardiles V, de Santibañes E. The associating liver partition and portal vein ligation for staged hepatectomy approach using only segments I and IV as future liver remnant. J Am Coll Surg. 2014;219:e5–e9. doi: 10.1016/j.jamcollsurg.2014.01.070. [DOI] [PubMed] [Google Scholar]
- 20.Schadde E, Malagó M, Hernandez-Alejandro R, Li J, Abdalla E, Ardiles V, Lurje G, Vyas S, Machado MA, de Santibañes E. Monosegment ALPPS hepatectomy: extending resectability by rapid hypertrophy. Surgery. 2015;157:676–689. doi: 10.1016/j.surg.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka K, Matsuo K, Murakami T, Kawaguchi D, Hiroshima Y, Koda K, Endo I, Ichikawa Y, Taguri M, Tanabe M. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): short-term outcome, functional changes in the future liver remnant, and tumor growth activity. Eur J Surg Oncol. 2015;41:506–512. doi: 10.1016/j.ejso.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Lau L, Christophi C, Nikfarjam M, Starkey G, Goodwin M, Weinberg L, Ho L, Muralidharan V. Assessment of Liver Remnant Using ICG Clearance Intraoperatively during Vascular Exclusion: Early Experience with the ALIIVE Technique. HPB Surg. 2015;2015:757052. doi: 10.1155/2015/757052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ardiles V, Schadde E, Santibanes E, Clavien PA. Commentary on “Happy marriage or “dangerous liaison”: ALPPS and the anterior approach”. Ann Surg. 2014;260:e4. doi: 10.1097/SLA.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Girotti P, Königsrainer I, Ladurner R, Königsrainer A, Nadalin S. ALPPS in right trisectionectomy: a safe procedure to avoid postoperative liver failure? J Gastrointest Surg. 2013;17:956–961. doi: 10.1007/s11605-012-2132-y. [DOI] [PubMed] [Google Scholar]
- 25.Aloia TA, Vauthey JN. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): what is gained and what is lost? Ann Surg. 2012;256:e9; author reply e16–e19. doi: 10.1097/SLA.0b013e318265fd3e. [DOI] [PubMed] [Google Scholar]
- 26.Chan AC, Pang R, Poon RT. Simplifying the ALPPS procedure by the anterior approach. Ann Surg. 2014;260:e3. doi: 10.1097/SLA.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Kantas A, Ittrich H, Koops A, Achilles EG, Fischer L, Nashan B. Avoid “All-Touch” by Hybrid ALPPS to Achieve Oncological Efficacy. Ann Surg. 2016;263:e6–e7. doi: 10.1097/SLA.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 28.Robles Campos R, Brusadin R, López Conesa A, Parrilla Paricio P. Staged liver resection for perihilar liver tumors using a tourniquet in the umbilical fissure and sequential portal vein embolization on the fourth postoperative day (a modified ALTPS) Cir Esp. 2014;92:682–686. doi: 10.1016/j.ciresp.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Torres OJ, Fernandes Ede S, Oliveira CV, Lima CX, Waechter FL, Moraes-Junior JM, Linhares MM, Pinto RD, Herman P, Machado MA. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): the Brazilian experience. Arq Bras Cir Dig. 2013;26:40–43. doi: 10.1590/s0102-67202013000100009. [DOI] [PubMed] [Google Scholar]
- 30.Donati M, Stavrou GA, Basile F, Gruttadauria S, Niehaus KJ, Oldhafer KJ. Combination of in situ split and portal ligation: lights and shadows of a new surgical procedure. Ann Surg. 2012;256:e11–e2; author reply e11-e2. doi: 10.1097/SLA.0b013e318265fe36. [DOI] [PubMed] [Google Scholar]
- 31.Schadde E, Schnitzbauer AA, Tschuor C, Raptis DA, Bechstein WO, Clavien PA. Systematic review and meta-analysis of feasibility, safety, and efficacy of a novel procedure: associating liver partition and portal vein ligation for staged hepatectomy. Ann Surg Oncol. 2015;22:3109–3120. doi: 10.1245/s10434-014-4213-5. [DOI] [PubMed] [Google Scholar]
- 32.Turrini O, Ewald J, Viret F, Sarran A, Goncalves A, Delpero JR. Two-stage hepatectomy: who will not jump over the second hurdle? Eur J Surg Oncol. 2012;38:266–273. doi: 10.1016/j.ejso.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Hasselgren K, Sandström P, Björnsson B. Role of associating liver partition and portal vein ligation for staged hepatectomy in colorectal liver metastases: a review. World J Gastroenterol. 2015;21:4491–4498. doi: 10.3748/wjg.v21.i15.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung U, Simpson AL, Araujo RL, Gönen M, McAuliffe C, Miga MI, Parada EP, Allen PJ, D’Angelica MI, Kingham TP, et al. Remnant growth rate after portal vein embolization is a good early predictor of post-hepatectomy liver failure. J Am Coll Surg. 2014;219:620–630. doi: 10.1016/j.jamcollsurg.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828, discussion 828-829. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 37.Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037–1049; discussion 1049-1051. doi: 10.1097/01.sla.0000145965.86383.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kianmanesh R, Farges O, Abdalla EK, Sauvanet A, Ruszniewski P, Belghiti J. Right portal vein ligation: a new planned two-step all-surgical approach for complete resection of primary gastrointestinal tumors with multiple bilateral liver metastases. J Am Coll Surg. 2003;197:164–170. doi: 10.1016/S1072-7515(03)00334-X. [DOI] [PubMed] [Google Scholar]
- 39.Tsai S, Marques HP, de Jong MC, Mira P, Ribeiro V, Choti MA, Schulick RD, Barroso E, Pawlik TM. Two-stage strategy for patients with extensive bilateral colorectal liver metastases. HPB (Oxford) 2010;12:262–269. doi: 10.1111/j.1477-2574.2010.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Worni M, Shah KN, Clary BM. Colorectal cancer with potentially resectable hepatic metastases: optimizing treatment. Curr Oncol Rep. 2014;16:407. doi: 10.1007/s11912-014-0407-z. [DOI] [PubMed] [Google Scholar]
- 41.Shindoh J, Vauthey JN, Zimmitti G, Curley SA, Huang SY, Mahvash A, Gupta S, Wallace MJ, Aloia TA. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg. 2013;217:126–133; discussion 133-134. doi: 10.1016/j.jamcollsurg.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam VW, Laurence JM, Johnston E, Hollands MJ, Pleass HC, Richardson AJ. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB (Oxford) 2013;15:483–491. doi: 10.1111/j.1477-2574.2012.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, Levi F, Bismuth H. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061; discussion 1061-1064. doi: 10.1097/01.sla.0000145964.08365.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pawlik TM, Abdalla EK, Ellis LM, Vauthey JN, Curley SA. Debunking dogma: surgery for four or more colorectal liver metastases is justified. J Gastrointest Surg. 2006;10:240–248. doi: 10.1016/j.gassur.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 45.Kornprat P, Jarnagin WR, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, D’Angelica M. Outcome after hepatectomy for multiple (four or more) colorectal metastases in the era of effective chemotherapy. Ann Surg Oncol. 2007;14:1151–1160. doi: 10.1245/s10434-006-9068-y. [DOI] [PubMed] [Google Scholar]
- 46.Oldhafer KJ, Donati M, Jenner RM, Stang A, Stavrou GA. ALPPS for patients with colorectal liver metastases: effective liver hypertrophy, but early tumor recurrence. World J Surg. 2014;38:1504–1509. doi: 10.1007/s00268-013-2401-2. [DOI] [PubMed] [Google Scholar]
- 47.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Yuan L, Ge RL, Sun Y, Wei G. Survival benefit of surgical treatment for hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: results of a retrospective cohort study. Ann Surg Oncol. 2013;20:914–922. doi: 10.1245/s10434-012-2646-2. [DOI] [PubMed] [Google Scholar]
- 49.Vennarecci G, Grazi GL, Santoro R, Ettorre GM. A room for the alpps procedure in patients with HCC. Int J Surg. 2015;13:90–91. doi: 10.1016/j.ijsu.2014.11.054. [DOI] [PubMed] [Google Scholar]
- 50.Chan AC, Poon RT, Chan C, Lo CM. Safety of ALPPS Procedure by the Anterior Approach for Hepatocellular Carcinoma. Ann Surg. 2016;263:e14–e16. doi: 10.1097/SLA.0000000000001272. [DOI] [PubMed] [Google Scholar]
- 51.Vennarecci G, Laurenzi A, Santoro R, Colasanti M, Lepiane P, Ettorre GM. The ALPPS procedure: a surgical option for hepatocellular carcinoma with major vascular invasion. World J Surg. 2014;38:1498–1503. doi: 10.1007/s00268-013-2296-y. [DOI] [PubMed] [Google Scholar]
- 52.Giannini EG, Savarino V, Farinati F, Ciccarese F, Rapaccini G, Marco MD, Benvegnù L, Zoli M, Borzio F, Caturelli E, et al. Influence of clinically significant portal hypertension on survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Liver Int. 2013;33:1594–1600. doi: 10.1111/liv.12199. [DOI] [PubMed] [Google Scholar]
- 53.Nadalin S, Capobianco I, Li J, Girotti P, Königsrainer I, Königsrainer A. Indications and limits for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Lessons Learned from 15 cases at a single centre. Z Gastroenterol. 2014;52:35–42. doi: 10.1055/s-0033-1356364. [DOI] [PubMed] [Google Scholar]


