Abstract
AIM: To analyze outcomes in patients who underwent liver transplantation (LT) for hepatocellular carcinoma (HCC) and received autologous intraoperative blood salvage (IBS).
METHODS: Consecutive HCC patients who underwent LT were studied retrospectively and analyzed according to the use of IBS or not. Demographic and surgical data were collected from a departmental prospective maintained database. Statistical analyses were performed using the Fisher’s exact test and the Wilcoxon rank sum test to examine covariate differences between patients who underwent IBS and those who did not. Univariate and multivariate Cox regression models were developed to evaluate recurrence and death, and survival probabilities were estimated using the Kaplan-Meier method and compared by the log-rank test.
RESULTS: Between 2002 and 2012, 158 consecutive patients who underwent LT in the same medical center and by the same surgical team were identified. Among these patients, 122 (77.2%) were in the IBS group and 36 (22.8%) in the non-IBS group. The overall survival (OS) and recurrence free survival (RFS) at 5 years were 59.7% and 83.3%, respectively. No differences in OS (P = 0.51) or RFS (P = 0.953) were detected between the IBS and non-IBS groups. On multivariate analysis for OS, degree of tumor differentiation remained as the only independent predictor. Regarding patients who received IBS, no differences were detected in OS or RFS (P = 0.055 and P = 0.512, respectively) according to the volume infused, even when outcomes at 90 d or longer were analyzed separately (P = 0.518 for both outcomes).
CONCLUSION: No differences in RFS or OS were detected according to IBS use. Trials addressing this question are justified and should be designed to detect small differences in long-term outcomes.
Keywords: Cell saver, Cancer, Hepatocellular carcinoma, Liver transplantation, Recurrence
Core tip: This study addresses an alternative option for allogeneic blood transfusion during liver transplantation (LT) for hepatocellular carcinoma (HCC). The autologous blood salvage in LT, in our series, did not impact recurrence or death. This suggests that autologous blood transfusion should be considered an option avoiding the deleterious effects of allogeneic blood transfusion. Overall, we do believe that our data claim for trials looking for non-inferiority comparing the two modalities of blood transfusion in patients who underwent LT for HCC. We do believe that further studies are justified and should be designed to detect small differences in long-term outcomes.
INTRODUCTION
Autologous intraoperative blood salvage (IBS) is used routinely in many surgical specialties to minimize the effects of intraoperative bleeding, avoiding the risks of allogeneic red blood cell (RBC) transfusion. A recent cochrane review showed a 40% reduction in the requirements for allogeneic blood transfusion with cell salvage[1]. IBS has been generally used in liver transplantation (LT), although it is not usually recommended in patients with hepatocellular carcinoma (HCC) since there is a putative risk of reinfusion of neoplastic cells. The IBS is an alternative to allogeneic blood transfusion but it remains a controversial technique in oncologic procedures since it could represent an uncertain risk of malignant cell reinfusion[2-5].
The circulation of viable neoplastic cells in the IBS device and their detection in the leukocyte depletion filter (LDF) have been proved, and LDF has been used as an effective method to clean the RBC component before infusing it back[5-9]. Although the rationale to use LDF to block neoplastic cells back by the IBS device has been investigated on experimental studies, the clinical relevance analysis over patients who underwent LT for HCC has been restricted to a single study[5]. In the latter case, no differences were observed in recurrence between patients who received IBS and those who did not. However, it was not possible to rule out the possibility that this result was a consequence of a small sample size.
The aim of this study was to evaluate if the use of IBS for HCC patients who underwent LT increases the risk of tumor recurrence. To our knowledge, this is the largest series addressing this question in this population.
MATERIALS AND METHODS
Subjects and data collection
Patients submitted to LT for HCC at Hospital das Clínicas of University of São Paulo Medical School (HCFMUSP) were analyzed from a prospectively maintained database containing demographic, clinical, operative, pathological, and follow-up data and studied retrospectively. Permission was obtained from the informed consent statement and institutional review board according to the institutional policy for protected health information.
All patients presented in this analysis were initially considered to meet the Milan criteria or UCSF criteria[10,11]. Patients who had detectable extra-hepatic disease during the pre- or intraoperative course and patients with a concurrent second neoplasm were not included. Patients who did not present HCC in the specimen were excluded with the exception of those previously treated with radiofrequency or chemoembolization. Pre-operative imaging modalities to evaluate the extent of intrahepatic disease and to exclude extra-hepatic metastatic sites included computed tomography and/or magnetic resonance imaging of the chest, abdomen, and pelvis. Model of end-stage liver disease (MELD) scores were calculated using laboratory results collected prior to the LT. The MELD score was calculated using the standard UNOS formula: MELD = 3.78 × ln (bilirubin) + 11.2 × ln (INR) + 9.57 × ln (creatinine) + 6.43, where bilirubin and creatinine are in mg/dL units and INR is the international normalized ratio. The MELD score was analyzed separately as both continuous and categorical variables (i.e., ≥ 20 vs <20).
The estimated blood loss was not fully available and thus it was not described and analyzed. The intraoperative decision to transfuse either allogeneic or autologous blood was consensual between the surgeon and the anesthesiologist. It was based on hemodynamic status, blood loss, hemoglobin concentration and patient’s comorbidities.
Follow-up time was calculated from the date of LT to the date of last clinical encounter captured by the HCFMUSP medical record system or the date of death. Recurrence-free survival (RFS) was calculated from the LT to the first detected recurrence or last follow-up without recurrence. Overall survival (OS) was calculated based on the survivorship status (deceased or alive) at last follow-up.
Blood salvage processing
The blood from the surgical field was collected using a Cell Saver auto-transfusion device (Fresenius C.A.T.S, Terumo Cardiovascular Systems, Germany) and anti-coagulated with heparinized saline and stored. The RBC component of aspirated blood was centrifuged and washed with heparinized saline. The RBC concentrates were filtered through an LDF (FTS-RC202, Shuangweibio Corp., Nanjing, China). Processed RBCs were transfused back to the patient when appropriate.
Statistical analysis
Statistical analyses were performed using the Fisher’s exact test and the Wilcoxon rank sum test to examine covariate differences between patients who underwent IBS and those who did not. Values are expressed as median (interquartile) or percentage, as appropriate. Survival probabilities were estimated using the Kaplan-Meier method and compared using the Log-Rank test. A Cox regression model was developed to determine factors independently associated with death. The use of IBS was included in the multivariate analysis regardless of its univariate significance. Other factors that were significantly associated with outcomes by univariate analysis (inclusion criterion, P ≤ 0.1) were entered into a multivariate analysis to test for significance of IBS adjusting for possible confounders. For recurrence assessment, no Cox regression was used since the number of events per variable was not appropriated[12,13]. A P value < 0.05 was considered significant for univariate and multivariate analyses. All statistical analyses were conducted using STATA v 9.0 (Stata Corp, College Station, TX).
RESULTS
Between January 2002 and September 2012, 158 consecutive patients who underwent potentially curative LT for HCC were included. One hundred and twenty-two (77.2%) patients in the IBS group and 36 (22.8%) patients in the non-IBS group were compared. Patients and clinicopathological presentation were compared between groups and are summarized in Table 1. Briefly, the demographic and clinicopathological characteristics were comparable between the two groups. The only significant difference was the presence of liver cirrhosis, which was more prevalent in the non-IBS group (100% vs 84.8%, P = 0.014).
Table 1.
Clinicopathological distribution according to the use of autologous intraoperative blood salvage for patients with hepatocellular carcinoma who underwent liver transplantation
| Total (%) n = 158 |
Intraoperative blood salvage |
P | ||
| Yes (%) n = 122 (77.2) | No (%) n = 36 (22.8) | |||
| Age1 | 58 (51-62) | 58 (51-62) | 58 (51-62) | 0.958 |
| Male gender | 122 (77.2) | 95 (77.9) | 27 (75) | 0.821 |
| BMI12 | 25.7 (23.6-27.8) | 25.7 (23.6-27.8) | 25.5 (23.5-2.3) | 0.712 |
| Pre-op AFP3 | 9.2 (3.7-35.4) | 8.9 (3.5-3.6) | 10.9 (6.7-33.7) | 0.175 |
| Cirrhosis4 | 135 (88.3) | 100 (84.8) | 35 (100) | 0.014 |
| Alcohol4 | 22 (14.4) | 18 (15.3) | 4 (11.4) | 0.785 |
| Hepatitis4 | ||||
| B | 20 (13.1) | 12 (10.2) | 8 (22.9) | 0.082 |
| C | 97 (63.4) | 73 (61.9) | 24 (68.6) | 0.551 |
| Others4 | 8 (5.2) | 8 (6.8) | 0 | 0.199 |
| Blood type | 0.420 | |||
| A | 60 (37) | 42 (34.4) | 18 (50) | |
| B | 21 (13.3) | 17 (13.9) | 4 (11.1) | |
| AB | 14 (8.9) | 11 (9) | 3 (8.3) | |
| O | 63 (39.9) | 52 (42.6) | 11 (30.6) | |
| Rhesus5 | 123 (86.6) | 93 (86.1) | 30 (88.3) | 1.000 |
| MELD1 | 10 ( 8-15) | 10.5 (9-17) | 9 (8-13.5) | 0.058 |
| Radiofrequency4 | 4 (2.6) | 3 (2.6) | 1 (2.8) | 1.000 |
| Chemoembolization4 | 69 (45.1) | 53 (45.3) | 16 (44.5) | 1.000 |
| Alcoholization4 | 7 (4.6) | 5 (4.3) | 2 (5.6) | 0.668 |
| Graft/body proportion12 | 1.75 (1.5-2.2) | 1.8 (1.5-2.2) | 1.7 (1.4– 2.2) | 0.454 |
| Number of lesions1 | 2 (1-3) | 2 (1-3) | 2 (1-3) | 0.715 |
| Largest lesion, mm1 | 25 (19-31) | 25 (19-30) | 25 (18-35) | 0.384 |
| Edmond-steiner degree (III and IV)6 | 88 (59.9) | 67 (58.8) | 21 (63.5) | 0.689 |
| Vascular invasion | 53 (33.6) | 44 (36.1) | 9 (25) | 0.236 |
| Microsatellite lesions | 26 (16.5) | 19 (15.6) | 7 (19.4) | 0.612 |
| Cholangiocarcinoma | 6 (3.8) | 6 (4.9) | 0 | 0.338 |
| Recurrence | 14 (8.9) | 10 (8.2) | 4 (11.1) | 0.525 |
| Death | 52 (32.9) | 41 (33.6) | 11 (30.6) | 0.841 |
Expressed as median (p25-p75);
N = 150;
N = 148;
N = 153;
N = 142;
N = 147. BMI: Body mass index; AFP: Alpha-feto protein; MELD: Model of end-stage liver disease.
Survival analysis
The median follow-up time for all patients was 27 mo; 25 mo for the group who received IBS and 32 mo for the group who did not (P = 0.049). The median follow-up time for survivors was 38 mo; 37 mo for the group who received IBS and recurred and 41 mo for the group who did not (P = 0.017). The estimated 3- and 5-year OS rates were 68% and 59.7%, respectively. When OS was adjusted for the use of IBS or not, no difference was detected (P = 0.51), as depicted in the Figure 1A. The univariate and multivariate analyses for death were performed and are shown in Table 2. Briefly, no differences were detected according to MELD either as a continuous variable (recurrence, P = 0.633; death, P = 0.286) or as binominal, as demonstrated in Tables 2 and 3. Only elevated Edmond-Steiner degree of tumor differentiation (III-IV) remained significant for the risk of death, as shown in Table 2. The estimated 3- and 5-year RFS rates were 87.7% and 83.3%, respectively. When RFS was adjusted for the use of IBS or not, no difference was detected (P = 0.953; Figure 1B). The univariate analysis for recurrence is shown in Table 3. Briefly, elevated Edmond-Steiner degree of tumor differentiation (III-IV), pre-operative alpha-feto protein level equal to or higher than 100 ng/dL and presence of microsatellite lesions were independent predictors of recurrence, as demonstrated in Table 3.
Figure 1.
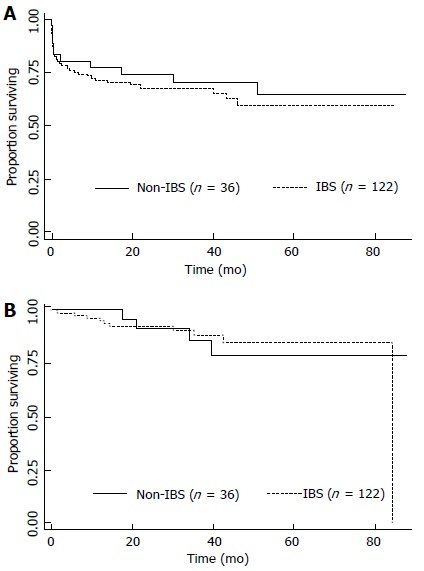
Kaplan-Meier estimates of survival from the date of liver transplantation according to the use of autologous intraoperative blood salvage. A: Overall survival (P = 0.51); B: Recurrence free survival (P = 0.953). IBS: Intraoperative blood salvage.
Table 2.
Univariate and multivariate analyses for predictors of overall survival
| Total | 5-yr survival (%) | Median survival (mo) | Univariate analysis P | HR | 95%CI | Multivariate analysis P | |
| Overall | 158 | 59.7 | - | - | |||
| Age (≥ 60 yr) | - | - | - | 0.133 | |||
| Gender | - | - | 0.097 | ||||
| Male | 122 | 61.5 | 0.88 | 0.45-1.74 | 0.714 | ||
| Female | 36 | 55.4 | - | ||||
| BMI (≥ 28) | 0.080 | ||||||
| Yes | 37 | 48.2 | 46 | 1.55 | 0.81-2.98 | 0.186 | |
| No | 113 | 63.6 | - | ||||
| Pre-op AFP (≥ 100 ng/dL) | 0.087 | ||||||
| Yes | 19 | 51.8 | - | 1.50 | 0.68-3.32 | 0.316 | |
| No | 129 | 60.8 | - | ||||
| Cirrhosis | - | - | - | 0.950 | |||
| Alcohol related | 0.048 | ||||||
| Yes | 22 | 86.4 | - | 0.30 | 0.09-1 | 0.051 | |
| No | 131 | 55.5 | - | ||||
| Hepatitis B infection | - | - | - | 0.156 | |||
| Hepatitis C infection | - | - | - | 0.130 | |||
| Others | - | - | - | 0.281 | |||
| Blood type | - | - | - | 0.470 | |||
| Rhesus | - | - | - | 0.554 | |||
| Radiofrequency | - | - | - | 0.821 | |||
| MELD (≥ 15) | - | - | - | 0.721 | |||
| Chemo-embolization | - | - | - | 0.877 | |||
| Tumor alcoholization | - | - | - | 0.118 | |||
| Graft/body % (≥ 2) | - | - | - | 0.163 | |||
| No. of lesions (> 3) | - | - | - | 0.819 | |||
| Largest lesion (≥ 30 mm) | - | - | - | 0.640 | |||
| Edmond-Steiner degree | 0.013 | ||||||
| III-IV | 88 | 48.9 | 51 | 2.19 | 1.07-4.47 | 0.031 | |
| 0-II | 59 | 74.4 | - | ||||
| Vascular invasion | - | - | - | 0.290 | |||
| Microsatellite lesions | - | - | - | 0.283 | |||
| Cholangiocarcinoma | - | - | - | 0.957 | |||
| IBS | 0.510 | ||||||
| Yes | 122 | 59.5 | - | 1.56 | 0.74-3.30 | 0.237 | |
| No | 36 | 64.5 | - | ||||
BMI: Body mass index; AFP: Alpha-feto protein; IBS: Intraoperative blood salvage; MELD: Model of End-Stage Liver Disease. The number of patients included in multivariate model is 141.
Table 3.
Univariate analysis for predictors of recurrence
| Total | 5-yr survival (%) | Median survival (mo) | Univariate analysis P | |
| Overall | 158 | 83.3 | - | - |
| Age (≥ 60 yr) | - | - | - | 0.319 |
| Male gender | - | - | - | 0.410 |
| BMI (≥ 28) | - | - | - | 0.166 |
| Pre-op AFP (≥ 100 mg/dL) | 0.001 | |||
| Yes | 19 | 59.4 | 84.5 | |
| No | 129 | 85 | - | |
| Cirrhosis | - | - | - | 0.163 |
| Alcohol related | - | - | - | 0.207 |
| Hepatitis B infection | - | - | - | 0.911 |
| Hepatitis C infection | - | - | - | 0.568 |
| Others | - | - | - | 0.794 |
| Blood type | - | - | - | 0.912 |
| Rhesus | - | - | - | 0.494 |
| MELD (≥ 15) | - | - | - | 0.694 |
| Radiofrequency | - | - | - | 0.758 |
| Chemoembolization | - | - | - | 0.133 |
| Tumor alcoholization | - | - | - | 0.373 |
| Graft/body % (≥ 2) | - | - | - | 0.605 |
| Number of lesions (> 3) | - | - | - | 0.496 |
| Largest lesion mm (≥ 30) | - | - | - | 0.429 |
| Edmond-Steiner degree | 0.0162 | |||
| III-IV | 88 | 73 | 84.5 | |
| 0-II | 59 | 94.3 | - | |
| Vascular invasion | 0.071 | |||
| Yes | 26 | 74.8 | 84.5 | |
| No | 132 | 86.3 | - | |
| Microsatellite lesions | 0.007 | |||
| Yes | 26 | - | - | |
| No | 132 | 86.5 | - | |
| Cholangiocarcinoma | - | - | - | 0.375 |
| IBS | 0.953 | |||
| Yes | 122 | 85 | 84.5 | |
| No | 36 | 78.8 | - |
BMI: Body mass index; AFP: Alpha-feto protein; IBS: Intraoperative blood salvage; MELD: Model of End-Stage Liver Disease.
Regarding the group of patients who received IBS (122 patients), the infusion volume was additionally analyzed as a continuous variable, and no differences were found in either recurrence (P = 0.512) or death (P = 0.055), as demonstrated in Figure 2A and B. Analyses of outcomes at 90 d or longer were performed and no differences in recurrence (P = 0.518) or death (P = 0.518) were detected (Figure 2C and D).
Figure 2.
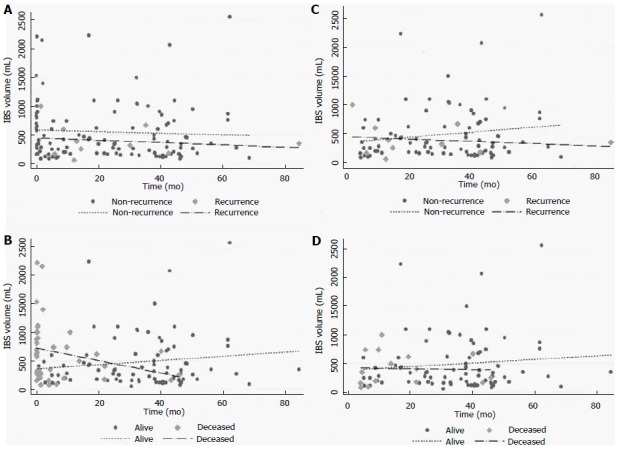
Scatter plots of the infusion volume of autologous intraoperative blood salvage over time. Overall distribution (total, n = 122) according to the time for recurrence (A: Recurrence, 10/91) and death (B: Death, 41/92). Distribution at 90 d and longer according to the time for recurrence (C: Recurrence, 9/91) and death (D: Death, 15/92). IBS: Intraoperative blood salvage.
DISCUSSION
The IBS is largely accepted as an option for blood transfusion. However, the contra-indications are based on the use of contaminated blood as in chronic diseases like hepatitis or other viral infections, bile infection or colonization, and intra-operative contamination[4,8]. The same rationale is applied to avoid tumor dissemination in patients with liver cancer already identified. Although this apprehension has been justifying its practice, no clear relation between the use of IBS and cancer recurrence has already been proved. Operations with high blood loss including cancer surgery have been demanding IBS use, however retrospective series did not show any suggestive association between the increase of recurrence and IBS use[14].
Concerning HCC patients, IBS use was described in a few series for resection and LT. One series described no increase in recurrence with IBS, showing no differences in higher stages and even better results for patients who used IBS in early stage disease[15,16]. Two series of LT, respectively, with 31 and 40 patients in the IBS groups vs 16 and 96 patients as control group, were described[17,18]. Despite the theoretical risk of tumor cell dissemination, the recurrence rates were not increased by IBS use in both series[17,18].
The purpose of our study was to compare long-term outcomes for patients undergoing LT for HCC who received IBS or not. In our study population, the groups were comparable except for the remarkable presence of cirrhosis in the IBS group. As expected, patients with cirrhosis are technically challenging and the blood loss is usually elevated, more justifying IBS. With regard to oncologic outcomes, the use of IBS or not was not significantly associated with recurrence or death. The predictors associated with recurrence were presence of satellite lesions and elevated Edmond-Steiner tumor degree. This was also an independent predictor of death in the multivariate model. The principal finding of this study is that in a large patient population from a single institution there were no measurable differences in outcomes based on the IBS use for patients who underwent LT for HCC.
Regarding only the IBS group, differences in the volumes infused were associated with death but not with recurrence, as depicted in Figure 2. The volume infused changed when the time point of 90 d was used. In the earlier period, higher volumes were associated with death but not with recurrence. This performance translates the IBS volume as surrogate of estimated blood loss, which is an independent predictor of mortality and transfusion as well[19]. Patients in the earlier period died in a short follow-up period and they could not have presented recurrence. With regard to longer follow-up (90 d or longer), the IBS volumes fit similarly for the distribution of recurrence or death. Long-term outcomes were not affected for the IBS volume in our series.
The limitations of the study are those associated with the immeasurable biases seen in all retrospective studies. We recognize that selection bias based on several nonobjective, undocumented criteria may have contributed to some of the differences between the two study groups. The estimated blood loss was not fully available and thus it was not described or analyzed.
The major finding of this analysis is the lack of any association between the use of IBS and oncologic outcomes. The results of this study should not be misinterpreted as an endorsement for the IBS use for all cancer patients. On the contrary, our data claim for more translational and clinical investigations of this issue. The operative hemorrhage in LT remains significant and blood transfusion is often demanded. The IBS should be applied as much as necessary, however the rationale of tumor cell reinfusion is a common concern[4,14,17,18,20-22]. Studies in vitro and retrospective series suggest that the use of LDF is effective enough to avoid tumor cell recirculation[5-7]. We believe that this finding is convincing and perhaps it is a reasonable explanation for no differences in recurrence or death in our series, since the LDF was used in all cases.
Moreover, a recent meta-analysis, including only non-randomized trials, showed an increase of risk for death and recurrence in patients with HCC who received allogeneic blood transfusion during hepatic resection[23]. Patients in the allogeneic group had a 16% more chance of recurrence at 5 years as well as a 60% more chance of all-case death in the same period. The reasons for the worse outcomes remain uncertain but it has been assumed that suppressive effects in the host immune system may have been responsible. The postulated mechanisms are allogeneic mononuclear cells; leucocytes-derived soluble mediators; and soluble HLA peptides circulating in allogeneic plasma inducing the host immune suppression[24]. These effects could be prevented by the autologous transfusion[24].
In summary, the present study shows that in a large consecutive series of patients undergoing LT for HCC in this single institution, there were no measurable differences in RFS or OS between patients who received IBS or not. With the lack of randomized clinical trials comparing the use of IBS for oncologic patients, its use could be considered a reasonable option for individualized patients. Based on these data, a trial looking for no inferiority comparing the use of IBS and conventional blood transfusion for LT for HCC is justified and should be designed to detect small differences in outcomes.
COMMENTS
Background
Blood transfusion is usually necessary for liver transplantation (LT). Intra-operative blood salvage has generally been used in LT to avoid deleterious effect of allogeneic blood transfusion. However, autologous blood transfusion has not been recommended in patients with hepatocellular carcinoma (HCC) since there is a putative risk of reinfusion of neoplastic cells.
Research frontiers
Although there is a putative risk of reinfusion of cancer cells into circulation during surgery, there is no data yet demonstrating that it would really impact on oncologic outcomes. This study did not demonstrate impact on clinical and oncologic outcomes. However, since the data are retrospective, our finding claims for trials looking for no inferiority comparing the two modalities of blood transfusion in patients who underwent LT for HCC, to detect small differences in outcomes.
Application
This study addresses an alternative option for allogeneic blood transfusion during LT for HCC. The autologous blood salvage in LT, in this series, did not impact recurrence or death. This suggests that autologous blood transfusion should be considered an option avoiding the deleterious effects of allogeneic blood transfusion.
Innovations and breakthroughs
The use of intra-operative blood salvage would have immunological and economic impact during postoperative course. Circulating cancer cells were already demonstrated, however it also seems that leucocyte filters are safe enough to block those cells. Then, the use of auto-transfusion devices associated with leucocytes filters seems to be a potential resource to help patients who undergo LT for HCC
Terminology
IBS: Autologous intraoperative blood salvage; HCC: Hepatocellular carcinoma; LDF: Leukocyte depletion filter; LT: Liver transplantation; MELD: Model of End-Stage Liver Disease; OS: Overall survival; RFS: Recurrence free survival; RBC: Red blood cell.
Peer-review
Autologous IBS is generally used in liver transplantation to minimize the effect of intraoperative bleeding. However, the peripheral blood of HCC patients may be contaminated with cancer cells or cancer-inducing virus, which can lead to potential risks of recurrence. In this study, authors investigated the association between the intraoperative use of IBS and survival of HCC patients. According to the data of a postoperative follow-up cohort, they reported that the use of IBS cannot influence the survival of HCC patients. This is an interesting study and is useful for clinicians.
Footnotes
Institutional review board statement: This study was approved by the Ethics Committee in Research of University of São Paulo School of Medicine.
Informed consent statement: All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement: None of the authors has received fees for serving as a speaker, consultant or advisory board member for any organization that might have a stake in the results of this study; nor do any of the authors owns stocks or shares of any such organization. None of the authors owns any patents related to the materials, devices or procedures mentioned in the manuscript.
Data sharing statement: The original anonymous dataset is available on request from the corresponding authors at wellington@usp.br and raphael-araujo@usp.br.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 10, 2015
First decision: September 17, 2015
Article in press: December 2, 2015
P- Reviewer: Cao GW, Silva R S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Carless PA, Henry DA, Moxey AJ, O’connell DL, Brown T, Fergusson DA. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2006;(4):CD001888. doi: 10.1002/14651858.CD001888.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Hansen E, Wolff N, Knuechel R, Ruschoff J, Hofstaedter F, Taeger K. Tumor cells in blood shed from the surgical field. Arch Surg. 1995;130:387–393. doi: 10.1001/archsurg.1995.01430040049007. [DOI] [PubMed] [Google Scholar]
- 3.Oefelein MG, Kaul K, Herz B, Blum MD, Holland JM, Keeler TC, Cook WA, Ignatoff JM. Molecular detection of prostate epithelial cells from the surgical field and peripheral circulation during radical prostatectomy. J Urol. 1996;155:238–242. doi: 10.1016/S0022-5347(01)66603-5. [DOI] [PubMed] [Google Scholar]
- 4.Kudo H, Fujita H, Hanada Y, Hayami H, Kondoh T, Kohmura E. Cytological and bacteriological studies of intraoperative autologous blood in neurosurgery. Surg Neurol. 2004;62:195–199; discussion 195-199. doi: 10.1016/j.surneu.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 5.Liang TB, Li DL, Liang L, Li JJ, Bai XL, Yu W, Wang WL, Shen Y, Zhang M, Zheng SS. Intraoperative blood salvage during liver transplantation in patients with hepatocellular carcinoma: efficiency of leukocyte depletion filters in the removal of tumor cells. Transplantation. 2008;85:863–869. doi: 10.1097/TP.0b013e3181671f2e. [DOI] [PubMed] [Google Scholar]
- 6.Edelman MJ, Potter P, Mahaffey KG, Frink R, Leidich RB. The potential for reintroduction of tumor cells during intraoperative blood salvage: reduction of risk with use of the RC-400 leukocyte depletion filter. Urology. 1996;47:179–181. doi: 10.1016/S0090-4295(99)80411-7. [DOI] [PubMed] [Google Scholar]
- 7.Perseghin P, Viganò M, Rocco G, Della Pona C, Buscemi A, Rizzi A. Effectiveness of leukocyte filters in reducing tumor cell contamination after intraoperative blood salvage in lung cancer patients. Vox Sang. 1997;72:221–224. doi: 10.1046/j.1423-0410.1997.7240221.x. [DOI] [PubMed] [Google Scholar]
- 8.Elias D, Lapierre V, Billard V. Perioperative autotransfusion with salvage blood in cancer surgery. Ann Fr Anesth Reanim. 2000;19:739–744. doi: 10.1016/S0750-7658(00)00310-5. [DOI] [PubMed] [Google Scholar]
- 9.Catling S, Williams S, Freites O, Rees M, Davies C, Hopkins L. Use of a leucocyte filter to remove tumour cells from intra-operative cell salvage blood. Anaesthesia. 2008;63:1332–1338. doi: 10.1111/j.1365-2044.2008.05637.x. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 11.Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8:765–774. doi: 10.1053/jlts.2002.34892. [DOI] [PubMed] [Google Scholar]
- 12.Bagley SC, White H, Golomb BA. Logistic regression in the medical literature: standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol. 2001;54:979–985. doi: 10.1016/S0895-4356(01)00372-9. [DOI] [PubMed] [Google Scholar]
- 13.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 14.Waters JH, Yazer M, Chen YF, Kloke J. Blood salvage and cancer surgery: a meta-analysis of available studies. Transfusion. 2012;52:2167–2173. doi: 10.1111/j.1537-2995.2011.03555.x. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto J, Okamoto E, Yamanaka N, Oriyama T, Furukawa K, Kawamura E, Tanaka T, Tomoda F. Efficacy of autotransfusion in hepatectomy for hepatocellular carcinoma. Arch Surg. 1993;128:1065–1069. doi: 10.1001/archsurg.1993.01420210129021. [DOI] [PubMed] [Google Scholar]
- 16.Hirano T, Yamanaka J, Iimuro Y, Fujimoto J. Long-term safety of autotransfusion during hepatectomy for hepatocellular carcinoma. Surg Today. 2005;35:1042–1046. doi: 10.1007/s00595-005-3082-8. [DOI] [PubMed] [Google Scholar]
- 17.Foltys D, Zimmermann T, Heise M, Kaths M, Lautem A, Wisser G, Weiler N, Hoppe-Lotichius M, Hansen T, Otto G. Liver transplantation for hepatocellular carcinoma--is there a risk of recurrence caused by intraoperative blood salvage autotransfusion? Eur Surg Res. 2011;47:182–187. doi: 10.1159/000330746. [DOI] [PubMed] [Google Scholar]
- 18.Muscari F, Suc B, Vigouroux D, Duffas JP, Migueres I, Mathieu A, Lavayssiere L, Rostaing L, Fourtanier G. Blood salvage autotransfusion during transplantation for hepatocarcinoma: does it increase the risk of neoplastic recurrence? Transpl Int. 2005;18:1236–1239. doi: 10.1111/j.1432-2277.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- 19.Sima CS, Jarnagin WR, Fong Y, Elkin E, Fischer M, Wuest D, D’Angelica M, DeMatteo RP, Blumgart LH, Gönen M. Predicting the risk of perioperative transfusion for patients undergoing elective hepatectomy. Ann Surg. 2009;250:914–921. doi: 10.1097/SLA.0b013e3181b7fad3. [DOI] [PubMed] [Google Scholar]
- 20.Ubee S, Kumar M, Athmanathan N, Singh G, Vesey S. Intraoperative red blood cell salvage and autologous transfusion during open radical retropubic prostatectomy: a cost-benefit analysis. Ann R Coll Surg Engl. 2011;93:157–161. doi: 10.1308/003588411X561044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engle DB, Connor JP, Morris PC, Bender DP, De Geest K, Ahmed A, Goodheart MJ. Intraoperative autologous blood transfusion use during radical hysterectomy for cervical cancer: long-term follow-up of a prospective trial. Arch Gynecol Obstet. 2012;286:717–721. doi: 10.1007/s00404-012-2351-1. [DOI] [PubMed] [Google Scholar]
- 22.Zhai B, Sun XY. Controversy over the use of intraoperative blood salvage autotransfusion during liver transplantation for hepatocellular carcinoma patients. World J Gastroenterol. 2013;19:3371–3374. doi: 10.3748/wjg.v19.i22.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Wang Z, Jiang S, Shao B, Liu J, Zhang S, Zhou Y, Zhou Y, Zhang Y. Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8:e64261. doi: 10.1371/journal.pone.0064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–348. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]


