Abstract
Inflammatory bowel disease (IBD) is a lifelong condition with waxing and waning disease course that requires reassessment of disease status as well as screening for complications throughout a patient’s lifetime. Laboratory testing, endoscopic assessment, and fecal biomarkers are often used in the initial diagnosis and ongoing monitoring of a patient with IBD. Imaging plays an integral role in the diagnosis and evaluation of IBD. Different imaging modalities can be used over the course of a patient’s lifetime, from the initial screening and diagnosis of IBD, to determining the extent of intestinal involvement, monitoring for disease activity, and evaluating for complications of uncontrolled IBD. The various imaging modalities available to the provider each have a unique set of risks and benefits when considering cost, radiation exposure, need for anesthesia, and image quality. In this article we review the imaging techniques available for the evaluation of IBD including fluoroscopic small bowel follow-through, computed tomography enterography, magnetic resonance enterography, and transabdominal ultrasound with particular focus on the judicious use of imaging and the risks and benefits of each option. We also review the risks of ionizing radiation, strategies to reduce exposure to ionizing radiation, and current imaging guidelines among pediatric and adult patient with IBD.
Keywords: Inflammatory bowel disease, Ultrasound, Fluoroscopy, Magnetic resonance imaging, Computed tomography
Core tip: Imaging plays a key role in the diagnosis and lifelong evaluation of a patient with inflammatory bowel disease (IBD). Several imaging modalities are available, each with a unique set of risks and benefits when considering cost, anesthesia risk in the pediatric population, ionizing radiation, image quality, and availability. In this article, we review the imaging techniques available for evaluation of IBD, with particular focus on judicious use of ionizing radiation. We also review current imaging guidelines among pediatric and adult patients with IBD.
INTRODUCTION
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a relapsing and remitting lifelong illness often diagnosed in childhood or early adulthood that is increasing in prevalence. Over 3 million people have inflammatory bowel disease worldwide[1,2]. The prevalence is thought to be as high as 249 per 100000 in North America and 505 per 100000 in Europe[3]. There is a global rise in the incidence of pediatric onset inflammatory bowel disease; approximately 25% of patients with IBD are diagnosed in childhood or adolescence[4,5]. Though the exact etiology of IBD remains unclear, it is thought to be a combination of immune dysregulation, environmental factors, and dysbiosis in a genetically predisposed host.
The diagnosis of IBD involves a detailed history and physical exam, laboratory testing, imaging, and endoscopic evaluation. Serum blood tests, fecal biomarkers, and imaging are important noninvasive tools to distinguish IBD from non-inflammatory conditions with similar clinical presentations. Imaging can be especially helpful in the screening and evaluation of possible IBD to rule out other abdominal pathology. Low cost, limited radiation, and feasibility are particularly important during the screening and evaluation of patients with possible IBD. Previously used imaging techniques such as enteroclysis and nuclear medicine studies (positron emission topography scan or tagged white blood cell scan) have fallen out of favor due to newer cross sectional imaging techniques such as magnetic resonance imaging (MRI) without the risk of ionizing radiation. It can be challenging to identify an imaging modality with sufficient sensitivity while limiting cost and radiation to the patient.
Imaging also plays a pivotal role in monitoring disease activity, determining extent of small bowel involvement, and identifying complications such as abscesses or bowel obstruction. The additional information imaging provides beyond endoscopic assessment may alter therapeutic decisions and impact future disease course. In this review, we will discuss the increasingly important role imaging plays as a noninvasive measure of disease activity in the long term management of IBD patients. We will summarize the different imaging modalities available with an emphasis on the risks and benefits as well as the sensitivity in detecting IBD activity. Lastly, we will review the hazards of ionizing radiation and discuss how this may impact the optimal timing and type of imaging in the best interest of the patient.
TYPES OF IMAGING
Fluoroscopic small bowel follow-through
Small bowel imaging with fluoroscopic barium small bowel follow-through (SBFT) was at one time considered the gold standard in evaluating pediatric IBD and is still used in the initial diagnosis of IBD despite the increasing availability of magnetic resonance enterography (MRE) and computed tomography enterography (CTE). This fluoroscopic study involves drinking barium contrast with serial X-ray images as the contrast progresses through the small intestine to the cecum. SBFT can provide an assessment of the small intestinal luminal anatomy by evaluating for strictures or wall thickening but generally does not provide information on colonic inflammation (Figure 1). SBFT has many benefits including relatively low cost, wide availability, and the ability to complete the study without sedation in the pediatric population. The downsides of SBFT include radiation exposure, length of study, operator dependent quality of images, and lack of extraintestinalevaluation (Table 1). The effective dose of radiation for SBFT in a pediatric patient is estimated to be 1.8-2.2 millisieverts (mSv)[6] with an average effective dose of 5 mSv in the adult population[7], though the actual radiation exposure can increase based on number of films obtained and radiologist technique.
Figure 1.
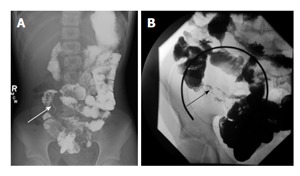
Small bowel follow-through examination in two patients with Crohn’s disease. A and B demonstrate mucosal irregularity and luminal narrowing of the terminal ileum (arrows).
Table 1.
Summary of risks and benefits of imaging studies for evaluation of inflammatory bowel disease
| Imaging study | Utility | Approximate radiation | Length of study | Pediatric sedation | Relative cost | Contrast | Sensitivity diagnosing IBD | Specificity diagnosing IBD |
| SBFT | Baseline diagnosis | Pediatric 2 mSv | Total: 1-3 h | None | $ | Oral < 1 L | 45%-76%[8-10] | 67%-100%[8-10] |
| Adult 5 mSv | Scan time: 1 h | |||||||
| CTE | Baseline diagnosis, follow-up, contraindication to MRE | Pediatric 3-16 mSv | Total: 1 h | None | $$ | Oral 1-2 L, intravenous | 84%[23] | 95%[23] |
| Adult 5-20 mSv | Scan time: Several minutes | |||||||
| MRE | Baseline diagnosis, follow-up, complications of IBD | None | Total: 1-2 h | Often depending on hospital protocol | $$$ | Oral 1-2 L, intravenous | 93%[23] | 93%[23] |
| Scan time 1-2 h | ||||||||
| Ultrasound | Screening if low suspicion for IBD, monitoring of disease activity | None | Total: 30-60 min | None | $ | Oral < 1 L | 90%[23] | 96%[23] |
| Scan time: 30 min |
SBFT: Small bowel follow through; CTE: Computed tomography enterography; MRE: Magnetic resonance enterography; mSv: Millisieverts; IBD:Inflammatory bowel disease.
Based on several retrospective studies among pediatric patients with IBD, the sensitivity of SBFT in detecting terminal ileum inflammation using histology as a gold standard is 45%-76% with specificity of 67%-96%[8-10]. A prospective study among adults with newly diagnosed IBD similarly showed the sensitivity and specificity of SBFT detecting terminal ileum inflammation was 67%-72% and 100% respectively[11]. This study found that there was not a statistically significant difference in the sensitivity in detecting terminal ileitis between SBFT, CTE and MRE, but CTE and MRE had significantly greater sensitivity for detection of extraintestinal complications[11]. Overall, SBFT is an imaging technique that has a role in identifying small bowel inflammation given its low cost and ease of performing the study. However, it has been falling out of favor given risks of radiation and improved sensitivity in detecting extraintestinal complications with newer cross sectional imaging techniques.
CTE
CTE was first described in 1997 as a modification of standard abdominal computed tomography (CT) to better evaluate the small bowel in CD[12]. Patients typically drink 1-2 Lofa neutral or low-density oral contrast mixture and receive intravenous (IV) contrast during the study to optimize luminal distention and assessment of the bowel wall[13]. Diagnostic criteria for IBD disease activity using CTE include bowel wall thickening, bowel hyperemia, submucosal fat deposition, and lymphadenopathy[14,15] (Figure 2). This cross-sectional imaging technique can evaluate for complications of IBD including bowel obstruction, fistula, perforation, or abscess[14,15]. The advantages of CTE include rapid scan time, cross-sectional imaging for evaluation of extraintestinal complications, relatively lower cost compared to MRE, and ability to perform the study without sedation in children (Table 1).
Figure 2.
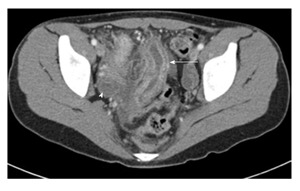
Axial computed tomography image of the pelvis in an adolescent boy with Crohn’s disease. It demonstrates bowel wall thickening of the distal small bowel and enhancement of the mucosa (arrow). There is surrounding free fluid (arrowhead).
The main disadvantage to CTE is exposure to ionizing radiation, though the need to ingest a large volume of contrast and cost may also be prohibitive. The estimated effective dose of radiation is approximately 10 mSv for standard abdominal CT and 10-20 mSv for CTE in the adult population[7,16]. In the pediatric population, estimated effective doses as low as 2.9-4 mSv have been reported for abdominal CT using multiple detector computed tomography[6] and varied 64-320 detector CT scanners[17]. Newer adaptive iterative dose reduction techniques have been described that greatly reduce the radiation exposure among pediatric patients undergoing CTE from 16.7 milligray (mGy) to 6.1 mGy, with minimal reduction in diagnostic sensitivity and specificity[18]. Similarly, newly proposed CTE imaging techniques using low-dose radiation and noise reduction techniques among adults can reduce effective dose radiation exposure by 53%-60% from 15-20 mSv to 5-7 mSv[19]. Ultimately, effective doses of radiation from abdominal CT and CTE are dependent on protocols at individual institutions and can vary greatly, but promising new techniques may be able to reduce the radiation exposure to levels equivalent to SBFT.
CTE was previously recommended as the imaging study of choice in initial diagnosis and suspected complications of CD among adults and children[20], but it has fallen out of favor as MRE has become more widely available with faster scanning protocols for pediatrics[20-22]. According to a recent meta-analysis,sensitivity and specificity of CTE in diagnosing IBD is 84% and 95% respectively[23], with growing evidence that CTE is more sensitive than SBFT in diagnosing IBD among adults and children[11,24-26]. CTE is useful both in the initial diagnosis of IBD as well as monitoring disease activity and screening for complications over the course of a patient’s lifetime. CTE findings including unsuspected penetrating disease, fistula, abscess, or stricture have been shown to alter medical management plans in 61% of patients and lead to interventional procedures in 18% of patients with known or suspected CD[27]. CTE remains an instrumental study in diagnosing IBD, monitoring disease activity, and identifying complications; though the risk of ionizing radiation often limits its use to emergency situations when MRE is not feasible.
MRE
MRE has become an increasingly important cross sectional imaging modality in the initial diagnosis of IBD as well as disease activity monitoring. Patients typically drink 1-2 L of a hyper osmolar oral contrast material to distend the bowel lumen, which can be difficult for younger patients and is often not well tolerated. Intravenous gadolinium contrast and spasmolytic medications such as glucagon are often administered during the study[28,29]. The imaging procedure generally takes 1-2 h to complete, and patients must comply with instructions to hold their breath intermittently. Historically, young children undergo anesthesia for this procedure, but newer protocols to reduce scan time, limit oral contrast, and enlist child life team support have made MRE without anesthesia more feasible[30-32]. Signs of active IBD using MRE include bowel wall thickening, increased T2 bowel wall signal, bowel wall hyperemia, and creeping fat[29,33] (Figure 3). The major benefit of MRE is the absence of ionizing radiation.Other advantages include the ability to evaluate extraintestinal manifestations of disease activity and to obtain a dynamic assessment of the bowel with real time imaging sequences. The potential disadvantages of MRE are lack of availability at certain centers, longer scan time with possible need for sedation in younger children, and higher cost than other imaging techniques (Table 1).
Figure 3.
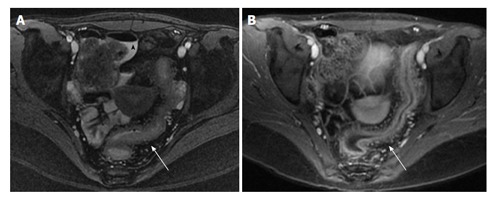
Magnetic resonance enterography study of the pelvis in an adolescent patient with active Crohn’s disease. T2 weighted image (A) demonstrating free fluid (arrowhead) and bowel wall thickening (arrow); T1 weighted image (B) with contrast demonstrating enhancement of the bowel and increased mesenteric vascularity (arrow).
MRE is now recommended as the imaging modality of choice for the diagnosis of IBD among children, monitoring disease activity among children and adults, and evaluation of perianal disease among children and adults[20-22]. A meta-analysis of prospective studies shows MRE has a sensitivity of 93% and specificity of 93% in diagnosing IBD[23]. MRE is the preferred study for evaluation of perianal disease and possible fistulas[34,35]. There is not a statistically significant difference between CTE and MRE in diagnostic accuracy for detecting active inflammation in IBD[36]. However, MRE is superior to CTE for differentiating bowel fibrosis from active inflammation (sensitivity 57% and 42%, specificity 82% and 68% respectively)[36]. The addition of diffusion weighted imaging on MRE has been shown to aid in identifying colonic inflammation and improve diagnostic confidence among children with IBD without the need for IV contrast[37,38]. Newer techniques such as automated motility mapping analysis can improve the identification of inflammatory lesions among patients with IBD[39]. MRE has also been shown to detect endoscopic remission with 83% accuracy and aphthous ulcer healing with 90% accuracy in a prospective multicenter study[40]. The ability of MRE to confirm the absence of disease rather than to identify inflammation or complications of IBD is novel. Future studies are needed to determine if this imaging modality will play a larger role in noninvasive routine CD monitoring.
Ultrasound
Transabdominal ultrasound is a well-established imaging technique for the evaluation of IBD among children and adolescents; though primarily used in Europe, it is gaining popularity in North America. More recently, intraluminal contrast enhanced ultrasound (SICUS) has been used with improved bowel visualization. It involves drinking a relatively small volume (200-500 mL) of non-absorbable contrast solution approximately 30 min prior to abdominal ultrasound, and the scan time generally takes less than an hour. Conventional ultrasound is typically performed first using a 3.5-5 MHz probe to evaluate for extraintestinal abnormalities; then a high frequency 7.5-17 Hz probe is used to evaluate bowel wall thickness as well as Doppler assessment of blood flow to the intestine[41]. Sonographic evidence of active IBD include bowel wall thickening greater than 3 mm and bowel wall hyperemia[41] (Figure 4). Abdominal ultrasound can also assess for extraintestinal complications such as abscess, lymphadenopathy, or other complications of active IBD such as stricture or fistula. Bowel ultrasound has many advantages including low cost, lack of ionizing radiation, dynamic real-time bowel assessment, and no need for sedation in the pediatric population (Table 1). The potential disadvantage of bowel ultrasound is the need for a skilled operator to provide optimal sensitivity and imaging, which may not be available in all centers.
Figure 4.
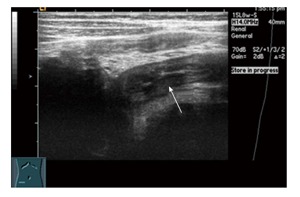
Transabdominal ultrasound in a 12-year-old boy with Crohn’s disease. It demonstrates bowel wall thickening of the terminal ileum.
Ultrasound has been shown to have similar sensitivity and specificity in identifying IBD compared to MRE and CTE[23]. Though most studies use bowel wall thickening greater than 3 mm as a marker of active inflammation, studies have also demonstrated that bowel hyperemia as measured by Doppler blood flow is associated with active bowel inflammation[41,42]. A meta-analysis showed abdominal ultrasound had a sensitivity of 89.7% and a specificity of 95.6% for diagnosing IBD[23]. One group demonstrated 57% sensitivity in detecting undiagnosed CD among adults[43] and 76% sensitivity among undiagnosed children[44] with transabdominal ultrasound. Addition of enteral contrastdemonstrated improved sensitivity to as high as 94% among adults and 100% among children. Further studies are needed to confirm these findings. SICUS has excellent accuracy in diagnosing complications of CD such as stricture, abscess, and fistula compared to surgical findings[45]. Abdominal ultrasound findings have also been shown to correlate with endoscopic severity in moderate to severe UC[46]. Though still an experimental technique, some studies have shown that IV microbubble contrast can also be used to better detect vascular density and predict IBD disease activity[47].
Ultrasound is cost-effective, highly accurate in detecting bowel inflammation, and does not involve ionizing radiation or sedation. It is particularly beneficial in the pediatric population and in monitoring disease activity over time given these attributes. The routine use of bowel ultrasound has not been adopted in North America though it is widely used in Europe. There is concern that the diagnostic accuracy is operator dependent, and this may impact the utility in more widespread use.
IONIZING RADIATION
One of the strongest arguments for the prudent use of certain imaging techniques such as SBFT and CTE is the long term risks of ionizing radiation. Much of what we understand about the risks of ionizing radiation comes from studies among atomic bomb survivors.Studies have demonstrated a linear no-threshold relationship between radiation exposure dose and risk of solid tumors; the risk is greatest among those exposed during childhood[48]. Some epidemiological data suggests that cumulative radiation exposure as low as 50 mSv may increase risk of certain solid tumors[49]. A recent study demonstrated multiple CT scans in childhood with cumulative radiation exposure of 50 mSv tripled the relative risk of leukemia and brain cancer[50]. Retrospective data among the pediatric IBD population suggests the average cumulative effective dose (CED) over an extended period of time was 20.5 mSv among children with CD and 11.7 mSv with UC[51]. Retrospective data among adults with IBD estimates CED was 20.1 mSv for patients with CD and 15.1 mSv with UC[52]. Approximately 5.8% of children and 7.1%-13% of adults with IBD had an estimated CED > 50 mSv[51-53]. Children and adults with CD, history of prior surgery, and prednisone use are more likely to have increased radiation exposure[51,52]. Adults with IBD are also at risk for increased radiation exposure within the first year after diagnosis[52].
Strategies for reducing ionizing radiation exposure include limiting unnecessary imaging studies and choosing an imaging modality without ionizing radiation when possible. The use of CT has increased particularly in the emergency department (ED) in the past 10 years[54]. Despite the increasing use of abdominal CT among adults with CD in the ED from 47% of encounters to 78% of encounters over an 8-year period, there was no significant difference in the detection of complications of IBD including perforation, obstruction, or abscess[55]. Alternative imaging techniques without the risk of ionizing radiation such as ultrasound and MRI are preferred in patients who are clinically stable to undergo such evaluation. Imaging forms without ionizing radiation are particularly beneficial among the pediatric population who are at greater risk of the harmful effects of ionizing radiation and have a lifetime of periodic imaging for possible complications of IBD or assessment of disease activity ahead of them.
IMAGING GUIDELINES
There are many clinical scenarios from initial presentation and diagnosis to assessment for disease complications years after diagnosis where imaging is necessary to evaluate a patient with IBD. When considering the potential risks of imaging-including ionizing radiation, cost, and potential need for sedation in the pediatric population-it is prudent to consider the minimum imaging all patients with IBD require. Pediatric and adult guidelines in the United States and Europe recommend small bowel imaging for any patient with newly diagnosed CD or newly diagnosed UC with atypical endoscopic appearance of colonic inflammation[21,22,56]. It has been proposed that ultrasound or MRE are the preferred imaging modalities in pediatric patients with suspected IBD; MRE or CTE are recommended for complete assessment of the small bowel in newly diagnosed pediatric patients with IBD; and MRE is the modality of choice for assessment of complications of IBD[56]. Adult and pediatric European guidelines recommend MRE as the imaging modality of choice for assessment of the small bowel in newly diagnosed CD or atypical UC as well as for monitoring therapeutic response and screening for complications of IBD[21,22]. There are no evidence-based guidelines currently that describe the minimum necessary frequency of abdominal imaging in IBD.Studies have shown MRE to be accurate in detecting mucosal healing and therapeutic response[40], but it remains unclear what the optimal interval for repeat imaging may be and how to implement in clinical practice.
CONCLUSION
Imaging plays a pivotal role in the diagnosis and ongoing evaluation of IBD activity over the course of a patient’s life. Noninvasive imaging techniques without ionizing radiation such as ultrasound and MRE are likely to become increasingly important in monitoring for disease activity. This may be particularly true for the growing pediatric IBD population given concerns for potential risks of repeated anesthesia and invasive procedures to assess for disease activity during childhood. No imaging modality is perfect, but each option has a potential role in the evaluation of IBD. It is the clinician’s responsibility to weigh the risks and benefits in each unique clinical scenario while considering patient stability, availability, and what information is needed. We advocate for the judicious use of imaging studies that require ionizing radiation, and to consider an alternative method of evaluation when possible.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest to report.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 8, 2015
First decision: September 8, 2015
Article in press: January 7, 2016
P- Reviewer: Sipahi AM S- Editor: Gong ZM L- Editor: A E- Editor: Liu SQ
References
- 1.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 2.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17:423–439. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 5.Abraham BP, Mehta S, El-Serag HB. Natural history of pediatric-onset inflammatory bowel disease: a systematic review. J Clin Gastroenterol. 2012;46:581–589. doi: 10.1097/MCG.0b013e318247c32f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaca AM, Jaffe TA, Delaney S, Yoshizumi T, Toncheva G, Nguyen G, Frush DP. Radiation doses from small-bowel follow-through and abdomen/pelvis MDCT in pediatric Crohn disease. Pediatr Radiol. 2008;38:285–291. doi: 10.1007/s00247-007-0702-z. [DOI] [PubMed] [Google Scholar]
- 7.Mettler FA, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 8.Batres LA, Maller ES, Ruchelli E, Mahboubi S, Baldassano RN. Terminal ileum intubation in pediatric colonoscopy and diagnostic value of conventional small bowel contrast radiography in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2002;35:320–323. doi: 10.1097/00005176-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Giles E, Barclay AR, Chippington S, Wilson DC. Systematic review: MRI enterography for assessment of small bowel involvement in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2013;37:1121–1131. doi: 10.1111/apt.12323. [DOI] [PubMed] [Google Scholar]
- 10.Stenerson M, Vittinghoff E, Heyman MB, Kim GE, Gupta N. Role of small bowel follow-through in diagnosing inflammation of the terminal ileum in pediatric patients. J Pediatr Gastroenterol Nutr. 2010;51:433–436. doi: 10.1097/MPG.0b013e3181d67ea7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SS, Kim AY, Yang SK, Chung JW, Kim SY, Park SH, Ha HK. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751–761. doi: 10.1148/radiol.2513081184. [DOI] [PubMed] [Google Scholar]
- 12.Raptopoulos V, Schwartz RK, McNicholas MM, Movson J, Pearlman J, Joffe N. Multiplanar helical CT enterography in patients with Crohn’s disease. AJR Am J Roentgenol. 1997;169:1545–1550. doi: 10.2214/ajr.169.6.9393162. [DOI] [PubMed] [Google Scholar]
- 13.Ilangovan R, Burling D, George A, Gupta A, Marshall M, Taylor SA. CT enterography: review of technique and practical tips. Br J Radiol. 2012;85:876–886. doi: 10.1259/bjr/27973476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gore RM, Balthazar EJ, Ghahremani GG, Miller FH. CT features of ulcerative colitis and Crohn’s disease. AJR Am J Roentgenol. 1996;167:3–15. doi: 10.2214/ajr.167.1.8659415. [DOI] [PubMed] [Google Scholar]
- 15.Duigenan S, Gee MS. Imaging of pediatric patients with inflammatory bowel disease. AJR Am J Roentgenol. 2012;199:907–915. doi: 10.2214/AJR.11.7966. [DOI] [PubMed] [Google Scholar]
- 16.Swanson G, Behara R, Braun R, Keshavarzian A. Diagnostic medical radiation in inflammatory bowel disease: how to limit risk and maximize benefit. Inflamm Bowel Dis. 2013;19:2501–2508. doi: 10.1097/MIB.0b013e31828dc6b6. [DOI] [PubMed] [Google Scholar]
- 17.Johnston JH, Podberesky DJ, Yoshizumi TT, Angel E, Toncheva G, Larson DB, Egelhoff JC, Anderson-Evans C, Nguyen GB, Barelli A, et al. Comparison of radiation dose estimates, image noise, and scan duration in pediatric body imaging for volumetric and helical modes on 320-detector CT and helical mode on 64-detector CT. Pediatr Radiol. 2013;43:1117–1127. doi: 10.1007/s00247-013-2690-5. [DOI] [PubMed] [Google Scholar]
- 18.Wallihan DB, Podberesky DJ, Sullivan J, Denson LA, Zhang B, Salisbury SR, Towbin AJ. Diagnostic Performance and Dose Comparison of Filtered Back Projection and Adaptive Iterative Dose Reduction Three-dimensional CT Enterography in Children and Young Adults. Radiology. 2015;276:233–242. doi: 10.1148/radiol.14140468. [DOI] [PubMed] [Google Scholar]
- 19.Del Gaizo AJ, Fletcher JG, Yu L, Paden RG, Spencer GC, Leng S, Silva AM, Fidler JL, Silva AC, Hara AK. Reducing radiation dose in CT enterography. Radiographics. 2013;33:1109–1124. doi: 10.1148/rg.334125074. [DOI] [PubMed] [Google Scholar]
- 20.Huprich JE, Rosen MP, Fidler JL, Gay SB, Grant TH, Greene FL, Lalani T, Miller FH, Rockey DC, Sudakoff GS, et al. ACR Appropriateness Criteria on Crohn’s disease. J Am Coll Radiol. 2010;7:94–102. doi: 10.1016/j.jacr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, Kolho KL, Veres G, Russell RK, Paerregaard A, Buderus S, Greer ML, Dias JA, Veereman-Wauters G, Lionetti P, Sladek M, Martin de Carpi J, Staiano A, Ruemmele FM, Wilson DC; European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806. doi: 10.1097/MPG.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 22.Panes J, Bouhnik Y, Reinisch W, Stoker J, Taylor SA, Baumgart DC, Danese S, Halligan S, Marincek B, Matos C, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556–585. doi: 10.1016/j.crohns.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Horsthuis K, Bipat S, Bennink RJ, Stoker J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology. 2008;247:64–79. doi: 10.1148/radiol.2471070611. [DOI] [PubMed] [Google Scholar]
- 24.Hara AK, Leighton JA, Heigh RI, Sharma VK, Silva AC, De Petris G, Hentz JG, Fleischer DE. Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy. Radiology. 2006;238:128–134. doi: 10.1148/radiol.2381050296. [DOI] [PubMed] [Google Scholar]
- 25.Wold PB, Fletcher JG, Johnson CD, Sandborn WJ. Assessment of small bowel Crohn disease: noninvasive peroral CT enterography compared with other imaging methods and endoscopy--feasibility study. Radiology. 2003;229:275–281. doi: 10.1148/radiol.2291020877. [DOI] [PubMed] [Google Scholar]
- 26.Jamieson DH, Shipman PJ, Israel DM, Jacobson K. Comparison of multidetector CT and barium studies of the small bowel: inflammatory bowel disease in children. AJR Am J Roentgenol. 2003;180:1211–1216. doi: 10.2214/ajr.180.5.1801211. [DOI] [PubMed] [Google Scholar]
- 27.Booya F, Akram S, Fletcher JG, Huprich JE, Johnson CD, Fidler JL, Barlow JM, Solem CA, Sandborn WJ, Loftus EV. CT enterography and fistulizing Crohn’s disease: clinical benefit and radiographic findings. Abdom Imaging. 2009;34:467–475. doi: 10.1007/s00261-008-9419-1. [DOI] [PubMed] [Google Scholar]
- 28.Mollard BJ, Smith EA, Dillman JR. Pediatric MR enterography: technique and approach to interpretation-how we do it. Radiology. 2015;274:29–43. doi: 10.1148/radiol.14122449. [DOI] [PubMed] [Google Scholar]
- 29.Anupindi SA, Terreblanche O, Courtier J. Magnetic resonance enterography: inflammatory bowel disease and beyond. Magn Reson Imaging Clin N Am. 2013;21:731–750. doi: 10.1016/j.mric.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Courtier J, Cardenas A, Tan C, Towne M, Rhee SJ, Heyman M, MacKenzie JD. Non-Anesthesia MR Enterography in Very Young Children-Feasibility, Technique and Performance. J Pediatr Gastroenterol Nutr. 2015:Epub ahead of print. doi: 10.1097/MPG.0000000000000712. [DOI] [PubMed] [Google Scholar]
- 31.McGee K. The role of a child life specialist in a pediatric radiology department. Pediatr Radiol. 2003;33:467–474. doi: 10.1007/s00247-003-0900-2. [DOI] [PubMed] [Google Scholar]
- 32.MacKenzie JD, Vasanawala SS. Advances in pediatric MR imaging. Magn Reson Imaging Clin N Am. 2008;16:385–402, v. doi: 10.1016/j.mric.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Mentzel HJ, Reinsch S, Kurzai M, Stenzel M. Magnetic resonance imaging in children and adolescents with chronic inflammatory bowel disease. World J Gastroenterol. 2014;20:1180–1191. doi: 10.3748/wjg.v20.i5.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Towbin AJ, Sullivan J, Denson LA, Wallihan DB, Podberesky DJ. CT and MR enterography in children and adolescents with inflammatory bowel disease. Radiographics. 2013;33:1843–1860. doi: 10.1148/rg.337105140. [DOI] [PubMed] [Google Scholar]
- 35.Koelbel G, Schmiedl U, Majer MC, Weber P, Jenss H, Kueper K, Hess CF. Diagnosis of fistulae and sinus tracts in patients with Crohn disease: value of MR imaging. AJR Am J Roentgenol. 1989;152:999–1003. doi: 10.2214/ajr.152.5.999. [DOI] [PubMed] [Google Scholar]
- 36.Quencer KB, Nimkin K, Mino-Kenudson M, Gee MS. Detecting active inflammation and fibrosis in pediatric Crohn’s disease: prospective evaluation of MR-E and CT-E. Abdom Imaging. 2013;38:705–713. doi: 10.1007/s00261-013-9981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kinner S, Blex S, Maderwald S, Forsting M, Gerken G, Lauenstein TC. Addition of diffusion-weighted imaging can improve diagnostic confidence in bowel MRI. Clin Radiol. 2014;69:372–377. doi: 10.1016/j.crad.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 38.Sirin S, Kathemann S, Schweiger B, Hahnemann ML, Forsting M, Lauenstein TC, Kinner S. Magnetic resonance colonography including diffusion-weighted imaging in children and adolescents with inflammatory bowel disease: do we really need intravenous contrast? Invest Radiol. 2015;50:32–39. doi: 10.1097/RLI.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 39.Hahnemann ML, Nensa F, Kinner S, Maderwald S, Umutlu L, Gerken G, Lauenstein TC. Improved detection of inflammatory bowel disease by additional automated motility analysis in magnetic resonance imaging. Invest Radiol. 2015;50:67–72. doi: 10.1097/RLI.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 40.Ordás I, Rimola J, Rodríguez S, Paredes JM, Martínez-Pérez MJ, Blanc E, Arévalo JA, Aduna M, Andreu M, Radosevic A, et al. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology. 2014;146:374–82.e1. doi: 10.1053/j.gastro.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 41.Strobel D, Goertz RS, Bernatik T. Diagnostics in inflammatory bowel disease: ultrasound. World J Gastroenterol. 2011;17:3192–3197. doi: 10.3748/wjg.v17.i27.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drews BH, Barth TF, Hänle MM, Akinli AS, Mason RA, Muche R, Thiel R, Pauls S, Klaus J, von Boyen G, et al. Comparison of sonographically measured bowel wall vascularity, histology, and disease activity in Crohn’s disease. Eur Radiol. 2009;19:1379–1386. doi: 10.1007/s00330-008-1290-5. [DOI] [PubMed] [Google Scholar]
- 43.Pallotta N, Tomei E, Viscido A, Calabrese E, Marcheggiano A, Caprilli R, Corazziari E. Small intestine contrast ultrasonography: an alternative to radiology in the assessment of small bowel disease. Inflamm Bowel Dis. 2005;11:146–153. doi: 10.1097/00054725-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Pallotta N, Civitelli F, Di Nardo G, Vincoli G, Aloi M, Viola F, Capocaccia P, Corazziari E, Cucchiara S. Small intestine contrast ultrasonography in pediatric Crohn’s disease. J Pediatr. 2013;163:778–784.e1. doi: 10.1016/j.jpeds.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 45.Pallotta N, Vincoli G, Montesani C, Chirletti P, Pronio A, Caronna R, Ciccantelli B, Romeo E, Marcheggiano A, Corazziari E. Small intestine contrast ultrasonography (SICUS) for the detection of small bowel complications in crohn’s disease: a prospective comparative study versus intraoperative findings. Inflamm Bowel Dis. 2012;18:74–84. doi: 10.1002/ibd.21678. [DOI] [PubMed] [Google Scholar]
- 46.Parente F, Molteni M, Marino B, Colli A, Ardizzone S, Greco S, Sampietro G, Foschi D, Gallus S. Are colonoscopy and bowel ultrasound useful for assessing response to short-term therapy and predicting disease outcome of moderate-to-severe forms of ulcerative colitis?: a prospective study. Am J Gastroenterol. 2010;105:1150–1157. doi: 10.1038/ajg.2009.672. [DOI] [PubMed] [Google Scholar]
- 47.Romanini L, Passamonti M, Navarria M, Lanzarotto F, Villanacci V, Grazioli L, Calliada F, Maroldi R. Quantitative analysis of contrast-enhanced ultrasonography of the bowel wall can predict disease activity in inflammatory bowel disease. Eur J Radiol. 2014;83:1317–1323. doi: 10.1016/j.ejrad.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950-1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 49.Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl AcadSci USA. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Sir Craft AW, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuchs Y, Markowitz J, Weinstein T, Kohn N, Choi-Rosen J, Levine J. Pediatric inflammatory bowel disease and imaging-related radiation: are we increasing the likelihood of malignancy? J Pediatr Gastroenterol Nutr. 2011;52:280–285. doi: 10.1097/MPG.0b013e3181f57177. [DOI] [PubMed] [Google Scholar]
- 52.Levi Z, Fraser E, Krongrad R, Hazazi R, benjaminov O, meyerovitch J, Tal OB, Choen A, Niv Y, Fraser G. Factors associated with radiation exposure in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2009;30:1128–1136. doi: 10.1111/j.1365-2036.2009.04140.x. [DOI] [PubMed] [Google Scholar]
- 53.Hou JK, Malaty HM, Thirumurthi S. Radiation exposure from diagnostic imaging studies among patients with inflammatory bowel disease in a safety-net health-care system. Dig Dis Sci. 2014;59:546–553. doi: 10.1007/s10620-013-2852-1. [DOI] [PubMed] [Google Scholar]
- 54.Kocher KE, Meurer WJ, Fazel R, Scott PA, Krumholz HM, Nallamothu BK. National trends in use of computed tomography in the emergency department. Ann Emerg Med. 2011;58:452–62.e3. doi: 10.1016/j.annemergmed.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 55.Kerner C, Carey K, Mills AM, Yang W, Synnestvedt MB, Hilton S, Weiner MG, Lewis JD. Use of abdominopelvic computed tomography in emergency departments and rates of urgent diagnoses in Crohn’s disease. Clin Gastroenterol Hepatol. 2012;10:52–57. doi: 10.1016/j.cgh.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anupindi SA, Grossman AB, Nimkin K, Mamula P, Gee MS. Imaging in the evaluation of the young patient with inflammatory bowel disease: what the gastroenterologist needs to know. J Pediatr Gastroenterol Nutr. 2014;59:429–439. doi: 10.1097/MPG.0000000000000475. [DOI] [PubMed] [Google Scholar]


