Abstract
Blunt pancreatic trauma is an uncommon injury but has high morbidity and mortality. In modern era of trauma care, pancreatic trauma remains a persistent challenge to radiologists and surgeons alike. Early detection of pancreatic trauma is essential to prevent subsequent complications. However early pancreatic injury is often subtle on computed tomography (CT) and can be missed unless specifically looked for. Signs of pancreatic injury on CT include laceration, transection, bulky pancreas, heterogeneous enhancement, peripancreatic fluid and signs of pancreatitis. Pan-creatic ductal injury is a vital decision-making parameter as ductal injury is an indication for laparotomy. While lacerations involving more than half of pancreatic parenchyma are suggestive of ductal injury on CT, ductal injuries can be directly assessed on magnetic resonance imaging (MRI) or encoscopic retrograde cholangio-pancreatography. Pancreatic trauma also shows temporal evolution with increase in extent of injury with time. Hence early CT scans may underestimate the extent of injures and sequential imaging with CT or MRI is important in pancreatic trauma. Sequential imaging is also needed for successful non-operative management of pancreatic injury. Accurate early detection on initial CT and adopting a multimodality and sequential imaging strategy can improve outcome in pancreatic trauma.
Keywords: Computed tomography, Magnetic resonance imaging, Pancreatic trauma, Complications, Magnetic resonance cholangiopancreatography, Management, Pancreatic injury, Review
Core tip: Pancreatic trauma is an uncommon injury in blunt trauma abdomen. Despite improved multidetector computed tomography (CT) technology, early diagnosis of pancreatic trauma remains difficult. Moreover, pancreatic injury shows evolution with time which affects CT performance in early stages after injury. Diagnosis of pancreatic ductal injury is vital to decide operative vs non-operative management. Magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography has superseded encoscopic retrograde cholangio-pancreatography (ERCP) in evaluation of duct in acute injury. This review discusses injury mechanisms, laboratory diagnosis, CT and MRI evaluation, role of ERCP and contrast-enhanced ultrasound, management and complications of pancreatic trauma. Evolution of pancreatic injury has been specifically discussed as it has important management implications.
INTRODUCTION
Pancreatic trauma in blunt trauma abdomen is an uncommon injury with an incidence of 2%-5%[1]. Despite its relatively uncommon incidence, diagnosis and management of pancreatic trauma remains a persistent challenge and generates continuous debate and search for new paradigms in trauma literature. While the deep retroperitoneal location of pancreas protects it from less severe trauma, it also renders diagnosis of injury more difficult. In an acute setting, pancreatic injury produces severe physiologic dysfunction and traumatic pancreatitis; chiefly due to leakage of enzymes from pancreatic ductal injury while in the chronic setting, duct injury leads to pseudocyst and pancreatic fistula formation[2,3]. Computed tomography (CT) remains the mainstay for diagnosis of pancreatic trauma. Recently, with emphasis on early detection of ductal injury and an increasing trend towards non-operative management of low-grade pancreatic injuries, magnetic resonance imaging (MRI), encoscopic retrograde cholangio-pancreatography (ERCP) and endoscopic stenting have also been incorporated into pancreatic trauma management protocols[2,4-8].
This article provides a review of pancreatic injury and discusses the mechanisms of injury, clinical and laboratory diagnosis, classification, imaging techniques, management, outcome and complications of blunt pancreatic trauma.
MECHANISMS OF INJURY
The common mechanisms of blunt pancreatic trauma are motor vehicle accidents (steering wheel and seat-belt impact injuries) in adults and impact due to bicycle handlebar injuries in children[9-11]. Other mechanisms include fall of heavy objects over abdomen, fall from height and direct blunt assault to abdomen. Injury occurs due to the anteroposterior force compressing the pancreas against the spine with injury most commonly occurring just left to the mesenteric vessels at the junction of neck and body[10]. A slightly left-sided force of impact directed at left upper quadrant causes injury to distal pancreas along with spleen, left kidney and stomach. Similarly right sided forces injure the head or uncinate process of pancreas along with liver, gall bladder and duodenum[9,12]. Hence concomitant injuries of adjacent organs are not uncommon in blunt pancreatic trauma and should be actively sought for while analysing CT scans. Children are more susceptible to pancreatic injury because of the minimal protective retroperitoneal fat mantle unlike adults[11].
CLINICAL AND LABORATORY DIAGNOSIS
Pancreatic injury should be suspected in all polytrauma patients or in patients with history of any high-risk mechanism of injury. Due to the deep retroperitoneal location of pancreas, early diagnosis of pancreatic injury may be missed. Isolated pancreatic trauma may be clinically occult initially and can present later with complications while in polytrauma patients, pancreatic trauma may be masked by signs of more severe other organ injuries[13]. Clinically, patients may present with diffuse abdominal or epigastric pain, epigastric ecchymosis , abdominal guarding, tenderness and absent bowel sounds and along with metabolic acidosis and leucocytosis secondary to the inflammatory response induced by leakage of pancreatic enzymes[13,14].
Both serum amylase and lipase are unreliable markers for pancreatic trauma. While serum amylase is usually elevated after pancreatic trauma, it can also be normal in up to 40% of patients[15]. Thus initial serum amylase levels are neither sensitive nor specific for diagnosis of pancreatic trauma and can also be elevated in non-pancreatic abdominal and bowel injuries[16,17]. In a retrospective study of 1821 pediatric trauma patients by Adamson et al[16], 116 (23%) had elevated amylase or lipase levels while only eight patients had pancreatic injury. Seventy-four of 116 (64%) patients with elevated amylase/lipase levels underwent abdominal and pelvic CT scanning, yet 38 (51%) of these had completely normal scans. Many patients with elevated levels underwent screening CT scans based on amylase/lipase levels alone and had no evidence of pancreatic injury. Hence serum amylase determinations may support clinical suspicion in the diagnosis of pancreatic trauma but are not reliable or cost effective as screening tools. Moreover, serum amylase levels are also time-dependent and in two studies by Matsuno et al[18] and Takishima et al[19], statistically significant increased serum amylase levels were seen only two and three hours after trauma respectively.
Determination of pancreatic amylase isoenzyme also does not add to the diagnosis as demonstrated by Bouwman et al[20]. The major concern raised by an elevated amylase level is differentiation between pancreatic trauma and small bowel injury, which cannot be differentiated by measurement of amylase isoenzyme also[9].
While absolute values of serum amylase do not correspond to the grade and severity of injury, hyperamylasemia in general, is an indicator of development of complications, pancreatic fistula and pseudocyst formation[21]. Also while initial amylase may be normal, repeat amylase measurements at later intervals, persistent or significant hyperamylasemia (more than three times baseline) are suggestive. Thus the trend of serum amylase/lipase levels (increase/decrease) rather than any absolute value are helpful indicators of pancreatic involvement and development of subsequent complications[18,19].
CLASSIFICATION OF PANCREATIC INJURY
The classification of pancreatic trauma has evolved over the years. The earlier used clinical (grade I-IV) and CT based grading systems[22-24] have given way to an universally accepted American Association of Surgery for Trauma (AAST)-organ injury scale (OIS) grading of pancreatic trauma (Table 1). This is a surgical grading and has management implications. First proposed by Moore et al[25,26] in 1990, the grading system has stood the test of time and remains unchanged in the latest revision.
Table 1.
The American Association of Surgery for Trauma-organ injury scale pancreatic injury scale[25]
| Grade | Description |
| I | Hematoma: Minor contusion without duct injury |
| Laceration: Superficial laceration without duct injury | |
| II | Hematoma: Major contusion without duct injury or tissue loss |
| Laceration: Major laceration without duct injury or tissue loss | |
| III | Laceration: Distal transection or parenchymal injury with duct injury |
| IV | Laceration: Proximal transection1 or parenchymal injury involving ampulla or bile duct |
| V | Laceration: Massive disruption of pancreatic head |
Proximal injury is defined as lying to the right of the superior mesenteric vein.
IMAGING IN PANCREATIC TRAUMA
The objectives of imaging are: (1) to detect pancreatic trauma as early as possible to mitigate the consequences of delayed diagnosis; (2) to identify ductal injury; i.e., identify grade 3 and above injuries as ductal involvement has higher morbidity and mortality; (3) to evaluate evolution of pancreatic trauma; and (4) to diagnose complications and facilitate image-guided interventions. With these objectives in mind, CT is the workhorse of imaging in pancreatic trauma. MRI with magnetic resonance cholangiopancreatography (MRCP) and ERCP are useful in definitive diagnosis of ductal injury both in early and late cases while a newer modality like contrast-enhanced ultrasound (CEUS) has also been evaluated in pancreatic trauma.
CT
CT is the modality of choice for evaluating pancreatic injury in polytrauma patients. CT has a reportedly variable sensitivity (65%-80%) and specificity for detecting pancreatic trauma[9,27,28]. With older generation single slice and helical CT scanners, diagnosis of pancreatic trauma was unreliable and detection of subtle signs of early pancreatic injury was difficult[13,29]. Newer multidetector CT (MDCT) scanners allow volumetric data acquisition and isovoxel reconstruction, thereby improving the sensitivity and the standard of diagnosis[30-33]. Applications such as curved multiplanar reconstruction (MPR) reconstruction are helpful in evaluating an anatomically curved and obliquely located organ like the pancreas[33]. Improved ductal visualisation has also been noted by MPR and minimum intensity projections[34,35].
Teh et al[30] were the first ones to publish data regarding evaluation of blunt pancreatic injuries with modern-era high resolution CT scanners. In a cohort of 50 patients with pancreatic trauma, operative correlation was available in 33 patients. CT findings corresponded precisely to the operative findings in 18 patients (55%). In the subset of 11 patients with confirmed pancreatic ductal injury (PDI), CT scan was truly positive in 10 patients, falsely positive in 2 patients, and falsely negative in 1 patient. Thus while CT was 55% sensitive for pancreatic injury, it was 91% sensitive and 91% specific for pancreatic ductal injury[30].
A multicentre study by Phelan et al[32] involving 20 centres and both 16 detector and 64 detector scanners found that sensitivity of CT in detecting pancreatic injury varied between 47%-60% (depending on type of scanner used). For PDI, the sensitivity was 52%-54% and specificity was 90%-95%.
In a study published by us[33], operative correlation was available in 24 patients and MDCT correctly identified the surgical grade in 22 out of 24 patients (91.7%). In the subset of 19 patients with PDI, CT correctly identified ductal injury in 18/19 patients (true positives) and correctly ruled out ductal injury in all 5/5 patients (true negatives) giving a sensitivity, specificity and accuracy of 94.7%, 100.0% and 95.8% respectively for PDI. The one patient, in whom CT did not identify ductal injury, had imaging appearances of contusion (grade II) on CT while MRI performed 15 h later showed laceration (grade III injury). Discrepancy in CT and operative findings in this patient was more likely due to evolution of injury rather than failure of MDCT technology[33].
Thus, MDCT scanners have improved accuracy for detecting pancreatic injury as compared to older generation scanners.
CT imaging technique
Wong et al[31] assessed overall accuracies of multiphasic CT in detecting main duct injuries and found that accuracies were 97.9% (pancreatic parenchymal phase), 100.0% (portal venous phase), and 96.8% (equilibrium phase) respectively. Thus the portal venous phase CT was the most accurate scan to detect pancreatic duct injurie.
In our level 1 apex trauma centre, we currently perform dual-phase protocol (arterial and venous phase) in all adult patients with focussed abdominal sonography for trauma (FAST) positive status and a portal venous phase scan in pediatric patients or patients with FAST negative status. Thus, pancreas is primarily assessed in portal venous phase. Both thin-section axial images and MPR images in sagittal, coronal and oblique planes are routinely viewed on 3D workstation. We also generate curved MPR images to estimate depth of laceration in equivocal cases to comment on ductal injury. As generally accepted in trauma CT protocols[36] oral contrast is not administered prior to initial CT scanning while oral contrast is given in patients come for routine follow-up CT scans.
CT signs of pancreatic trauma
Signs of pancreatic trauma can be divided into “hard” signs and “soft” signs (Table 2). “Hard” signs are specific and definitive CT evidence of pancreatic injury. “soft” signs are basically due to associated pancreatitis and, though non-specific, are supportive and should make one raise a possibility of pancreatic involvement in a patient with an appropriate mechanism of injury and associated injuries.
Table 2.
| "Hard" signs/specific signs | "Soft" signs/suggestive signs |
| Fracture of the pancreas | Fluid separating the splenic vein from posterior aspect of pancreas |
| Pancreatic laceration | Fluid surrounding the superior mesenteric artery/(SMV cuff sign) |
| Focal or diffuse pancreatic enlargement/edema | Fluid in the anterior and posterior pararenal spaces |
| Pancreatic hematoma | Fluid in transverse mesocolon and lesser sac |
| Active bleeding/extravasation of intravenous contrast | Inflammatory changes in peripancreatic fat and mesentery |
| Thickening of the left anterior renal fascia | |
| Delayed signs | |
| Pancreatic ductal dilatation | |
| Pseudocyst formation/peripancreatic fluid collection |
SMV: Superior mesenteric vein.
Hard signs (for grading pancreatic injuries)
Pancreatic laceration (AAST grade III and above) is seen as a low-attenuating line oriented perpendicular to the long-axis of pancreas. The line ideally represents separation of fragments with fluid or blood within the fragments (Figures 1 and 2). However in early stage, a laceration may only be seen as a low-attenuation band without separation of fragments and may be underestimated as a contusion (Figure 3). Also a laceration may be seen on only one or two sections and can be missed if not carefully looked for. Pancreatic lacerations should also be differentiated from clefts. Usually the presence of fluid within the gap along with associated signs of inflammation favours laceration while a cleft is lined by fat with clear surrounding area[9,10,12,27,37-41].
Figure 1.
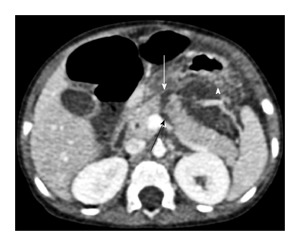
A 3-year-old girl with history of fall of heavy object over abdomen. CECT axial image shows full thickness laceration (grade III injury) of neck of pancreas at level of splenoportal confluence (white arrow). Also note peripancreatic fluid (arrowhead) and fluid between splenic vein and body of pancreas (black arrow). Patient underwent a distal pancreatectomy. CECT: Contrast enhanced computed tomography.
Figure 2.
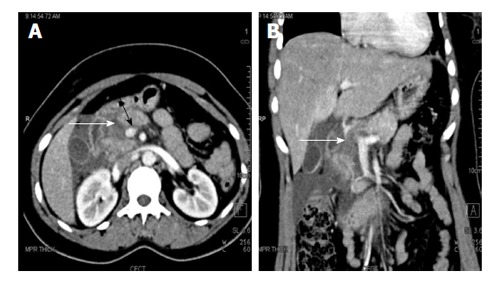
A 27-year-old woman with road traffic accident. CECT axial (A) and coronal (B) images show full thickness laceration in the head of pancreas (white arrows). The laceration is located to the right of splenoportal confluence suggestive of grade IV injury. There is also fluid around the superior mesenteric vein (black arrow) and adjacent superior mesentery artery; the so-called “SMV cuff” sign seen in proximal injuries of pancreas. CECT: Contrast enhanced computed tomography; SMV: Superior mesenteric vein.
Figure 3.
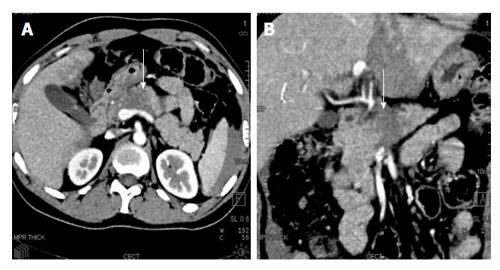
A 35-year-old truck driver with history of steering wheel impact injury. CECT axial (A) and coronal oblique (B) images show a full thickness hypoattenuating band involving neck and body of pancreas (white arrow). On imaging, it was considered as a major contusion/grade II injury. Intraoperatively, full thickness laceration with duct injury was found suggestive of grade III injury and distal pancreatectomy was done. CECT: Contrast enhanced computed tomography.
Lacerations can be divided into superficial or deep. Superficial lacerations involve less than 50% of the gland thickness and imply non-involvement of the duct. Deep lacerations involve more than 50% of the gland and imply duct disruption. This 50% depth of laceration is used as a substitute marker for ductal involvement as the duct often cannot be made out or traced on CT. A full thickness laceration involves the whole thickness of gland and is termed as transection or fracture (Figure 1).
Pancreatic contusion (AAST grade I and II injury) is characterised by: (1) diffuse or focal enlargement; (2) heterogeneously attenuating pancreas; or (3) focal area of hypoattenuation against the background of normally enhancing pancreas (Figure 4). Usually less than involvement of one anatomical division of pancreas (head, neck, body or tail) is considered as minor contusion (AAST grade I) or more than one anatomical division is considered a major contusion (AAST Grade II).
Figure 4.
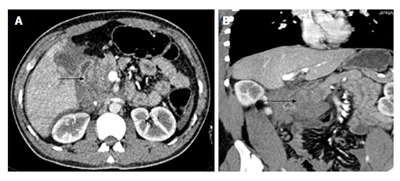
A 30-year-old man with road traffic accident. CECT axial (A) and coronal MPR (B) images show the hypoattenuating and bulky head of pancreas suggestive of major contusion with fluid in pancreaticoduodenal groove (arrow). Intra-operatively a hematoma was found in head and neck of pancreas (grade II injury). No active surgical intervention was done for pancreatic injury. CECT: Contrast enhanced computed tomography; MPR: Multiplanar reconstruction.
An area of hyperattenuation within the substance of the gland is suggestive of pancreatic hematoma which is a very specific sign of pancreatic trauma (Figure 5). Similarly active extravasation within the gland, i.e., contrast leak which increases on delayed scan, is specific for pancreatic injury[9] (Figure 6).
Figure 5.
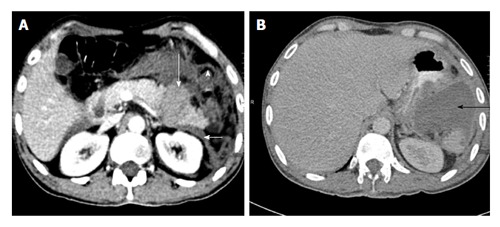
A 35-year-old man with road traffic accident. CECT axial image at time of trauma (A) shows distal transection with fragments that separated by hyperattenuating fluid suggestive of hematoma (long white arrow). The left anterior renal fascia is also thickened (arrowhead). Patient underwent distal pancreatectomy and 4 wk follow up CECT axial image (B) show a post-operative collection in lesser sac (black arrow). CECT: Contrast enhanced computed tomography.
Figure 6.
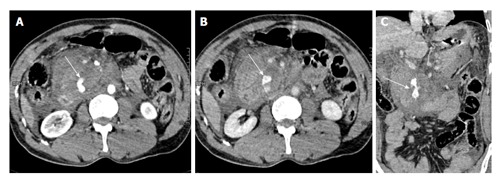
A 40-year-old man with history of fall of heavy object over abdomen. CECT axial images, initial scan (A) and delayed scan (B) show complete disruption of head of pancreas with a large retroperitoneal hematoma replacing the head region. There is active extravasation of contrast (arrows). On coronal oblique image (C), the disrupted head of pancreas (arrow) with active contrast extravasation can be seen. Surgically, a crush injury (grade V) was confirmed and patient underwent Whipple’s procedure. The patient eventually died due to sepsis and multiorgan failure. CECT: Contrast enhanced computed tomography.
Soft signs (reflective of pancreatic injury-associated inflammatory changes)
Fluid between distal pancreas and splenic vein was first described by Lane et al[39] and was found in 90% of cases of pancreatic injury in their study. Normally the splenic vein is closely apposed to the posterior aspect of the pancreas or is separated from the pancreas by a thin layer of fat. In a patient with fluid insinuating between the splenic vein and the pancreas and a history of abdominal trauma, a pancreatic injury should be suspected. The fluid is believed to represent either a leak from transected duct or blood tracking into peripancreatic tissues and is more commonly seen in distal pancreatic injuries (Figures 1 and 5). Similarly, SMV cuff sign may be seen in more proximal injuries involving neck region (Figure 2).
Peripancreatic fat stranding and fluid collections around the pancreas in combination with one or more signs to be strongly predictive of pancreatic injury. Peripancreatic fluid collections in lesser sac, pararenal spaces and transverse mesocolon are seen in 70%-90% in patients with pancreatic injury[39,42]. Similarly inflammatory changes such as thickening of anterior renal facia was seen in 44% of patients with pancreatic trauma[42] (Figures 1 and 5).
While in isolation, soft signs may not be diagnostic of pancreatic injury, they are often found in combination with each other or with a hard sign such as pancreatic laceration/transection. Patients with only soft signs on CT should be closely monitored clinically, biochemically and radiologically with follow-up CT scan or MRI for confirmation of pancreatic injury.
MAGNETIC RESONANCE IMAGING
MRI with MRCP serves as a problem solving tool in pancreatic trauma. In acute pancreatic trauma wherein diagnosis of ductal injury is imperative, MRI is a non-invasive alternative to ERCP to evaluate pancreatic duct. The other advantages of MRI over ERCP include its ability to demonstrate the status of the duct upstream of the laceration, better definition of parenchymal injury and the extent and location of peripancreatic fluid collections.
MRI also has good correlation with CT and can well demonstrate features of pancreatic parenchymal injuries such as pancreatic contusion, lacerations and hematomas[33,38]. Pancreatic contusions are seen as focal T2 hyperintense areas (Figure 7) while lacerations are seen as linear T2 hyperintense areas within the gland (Figure 8). On MRI, the lacerations can be seen directly extending to the duct unlike CT. Pancreatic hematoma is seen as intrapancreatic T1 hyperintense area which has variablesignal intensity on T2 weighted images[43]. Apart from confirming ductal injury, MRI is useful in confirming ductal integrity so that surgeons can safely proceed with conservative management (Figure 9). MRI is also useful in evaluating evolution of pancreatic injury as described later. Thus in our institution, MRI is performed if CT findings are equivocal or if conservative management is planned to evaluate the MPD. The MRI protocol in our institution include axial T1 and T2 weighted images, axial and coronal fast spoiled gradient echo imaging with steady state precession (TRUFISP) and single shot fast spin-echo (SSFSE) T2-weighted MR imaging (T2 HASTE) sequences and heavily T2-weighted 3D sequences for MRCP.
Figure 7.
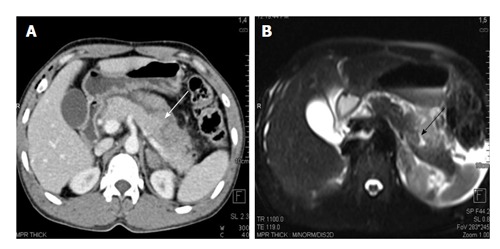
A 25-year-old man with history of blunt trauma abdomen. CECT axial image (A) shows injury of distal pancreas (white arrow). MRI T2 HASTE axial image show T2 weighted hyperintensity in pancreatic body suggestive of contusion/edema with a small laceration (black arrow). CECT: Contrast enhanced computed tomography; MRI: Magnetic resonance imaging.
Figure 8.
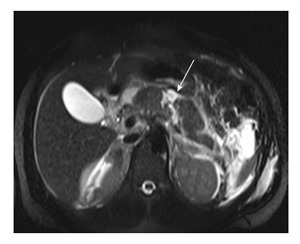
Magnetic resonance imaging T2 HASTE axial image of a 23-year-old man with road traffic accident show linear full thickness laceration in proximal body (arrow) suggestive of grade III injury.
Figure 9.
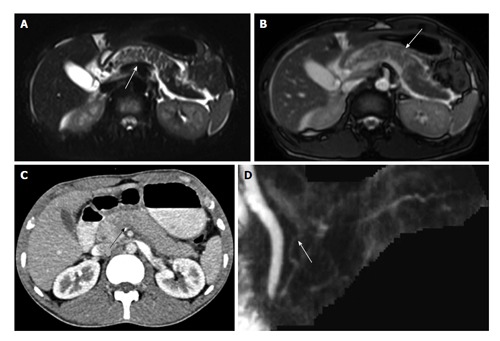
A 25-year-old man was involved in road traffic accident. CECT axial image (A) shows ill-defined hypoattenuating area in neck of pancreas suggestive of contusion (arrow). MRI done 10 h after CT shows extent of contusion better with wider area of involvement on both T2 HASTE (B) and TRUFISP (C) images. MRCP thick MPR (D) image shows ductal integrity. Patient was conservatively managed and follow-up imaging showed decrease in area of contusion. CECT: Contrast enhanced computed tomography; MPR: Multiplanar reconstruction; TRUFISP: True fast imaging with steady-state free precession; MRI: Magnetic resonance imaging; MRCP: Magnetic resonance pancreatography; CT: Computed tomography.
MRI is useful in follow-up of conservatively managed cases or to diagnose sequelae of pancreatic trauma such as pseudocysts, pancreatic strictures and chronic pancreatitis[6,44-46]. MRI is also useful for follow-up evaluation in children as it provides a non-radiation alternative to CT.
Secretin-enhanced MRCP, i.e., MRCP obtained after intravenous injection of secretin may be helpful to further characterise pancreatic ductal injury. Secretin increases the output of pancreatic secretions and can be used to actively demonstrate leak from the disrupted pancreatic duct[47].
CEUS
CEUS using SonoVue® (sulphur hexafluoride, Bracco, Milan, Italy) has also been described for pancreatic trauma[48]. Unlike conventional US which performs poorly in detecting pancreatic injuries, CEUS provides better contrast between normal and contused pancreas due to differential blood supply. Pancreatic injuries appear as anechoic or hypoechoic irregular perfusion defects in both arterial and parenchymal phases. In a study by Lv et al[49], in comparison to CT, CEUS detected pancreatic injuries in 21/22 patients with a detection rate of 95.5%. Because of its portability, CEUS can be employed as a part of initial US protocol during resuscitation to detect solid organ injuries. CEUS may also serve as a non-radiation alternative to CT for follow-up in known cases of pancreatic trauma to assess pancreatic disruptions, peripancreatic collections and pseudocysts. The disadvantages include cost, learning curve, short window time to obtain useful information and limited information regarding extent of other injuries sustained by the patients compared to CT[48,49].
ERCP
ERCP is considered the traditional gold standard for pancreatic ductal injury but has been superseded by MRCP in acute pancreatic trauma. While ERCP can directly visualise ductal injury, disadvantages include its invasive nature, high rate of complications (5%-15%) such as pancreatitis, cholangitis and duodenal perforation and the lack of availability of the technique or trained personnel to do this procedure on emergent basis[50,51]. Because of its tendency to induce iatrogenic pancreatitis, most trauma surgeons are wary of subjecting critical polytrauma patients to ERCP in an acute setting. However, in subacute cases and in chronic follow-up cases, ERCP provides therapeutic options such as duct stenting and pancreatic sphincterotomies for pancreatic fistula, pseudocysts and strictures[52-54]. Also with recent emphasis on non-operative management, endoscopic trans papillary drainage and ERCP guided stenting can also be done for partial duct disruptions and in isolated grade 3 injuries respectively to avoid laparotomy[5].
EVOLUTION OF PANCREATIC INJURY
Another factor affecting diagnostic performance in pancreatic trauma is the evolution of pancreatic injury. Findings can be subtle in early cases leading to a low CT sensitivity. In the study by Arkovitz et al[11], CT had an 85% sensitivity within the initial 24 h after pancreatic injury while overall sensitivity was 90%. The pancreas can appear normal in 20%-40% of patients with acute blunt pancreatic injuries, especially when imaging is done within the first 12 h after injury. This is due to the obscuration of the fracture plane, hemorrhage, and close apposition of the pancreatic fragments. On repeat scanning at 12 to 24 h; an abnormality which was initially ambiguous or subtle becomes more evident. Findings become more radiologically apparent over time with the development of post-traumatic pancreatitis, edema, leakage of pancreatic enzymes, and subsequent auto-digestion of the surrounding parenchyma[9,29]. The delay in CT findings of pancreatic injury is especially pronounced in pediatric or thin patients who often lack the contrast provided by surrounding adipose tissue to appreciate pancreatic injuries[11,55]. CT can either miss or underestimate depth of laceration too in very early stage because accumulation of fluid within the gap and separation of fragments is a time-dependent phenomenon[32] (Figure 10). Thus, the inability to detect early pancreatic trauma even with advanced multidetector CT technology is not a reflection of failure of technology but due to the natural history and evolution of trauma[32].
Figure 10.
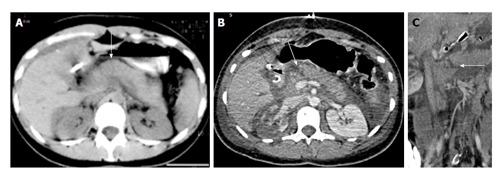
A 23-year-old woman with history of road traffic accident. Day 1 CECT axial image (A) shows ill-defined contusion in pancreatic neck (arrow). No obvious laceration was seen. Day 3 CECT axial (B) and coronal oblique (C) images show a full-thickness laceration in neck of pancreas s/o grade III injury with ductal involvement. Patient was operated and distal pancreatectomy was done. Thus there was evolution of injury from contusion to laceration. CECT: Contrast enhanced computed tomography.
Delayed diagnosis or the missed early diagnosis is more likely in patients with isolated pancreatic injuries, absent or minimal other associated abdominal injuries or in those undergoing non-operative management without any follow-up imaging[56]. Thus it is recommended to do a sequential imaging along with correlation with clinical and laboratory parameters to avoid a missed diagnosis[57].
Another scenario where sequential imaging plays a role is in non-operative management of pancreatic injury. The key factor in non-operative management is identification of low grade pancreatic injury (grade 2 or less) like contusion and superficial laceration not involving duct as these injuries can be managed conservatively. An inability to correctly estimate the grade of injury on first day imaging and absence of follow-up imaging with either CT, MRI, MRCP or ERCP is associated with higher incidence of failure of non-operative management in pancreatic injury[8]. Thus, once a pancreatic trauma is identified and the patient is considered for non-operative management, follow-up imaging with either CT or MRI should be done again to look for evolution of findings and guide management[58]. Follow-up imaging with MRI is preferred because of its superior soft tissue resolution, lack of radiation exposure and its ability to directly evaluate the duct. If a follow-up MRI reveals ductal integrity, then conservative management can be continued while evidence of ductal involvement on follow-up would necessitate surgical intervention (Figures 11 and 12).
Figure 11.
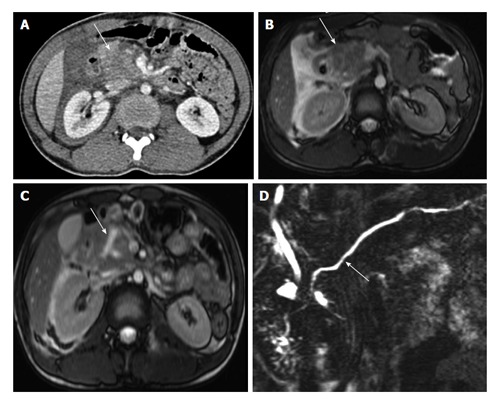
A 22-year-old man with history of fall of heavy object over abdomen. CECT axial image (A) shows bulky heterogeneously attenuating head of pancreas (arrow). MRI done 28 h after CT (B) shows similar findings with bulky head and altered signal intensity with adjacent fluid (arrow B). No definite laceration seen. Follow-up MRI on day 6 (C) shows a Y shaped laceration in inferior part of head and uncinate process. However the main pancreatic duct was normal (arrow D). Since the MPD was not involved, conservative management was continued. CECT: Contrast enhanced computed tomography; MRI: Magnetic resonance imaging; CT: Computed tomography.
Figure 12.
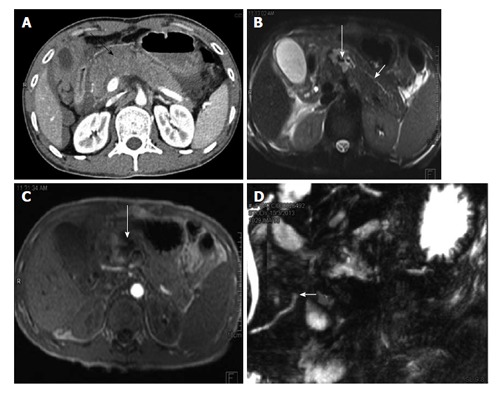
A 35-year-old man with history of road traffic accident. CECT axial image (A) shows bulky hypoattenuating pancreas (black arrow) with peripancreatic fluid. No definite laceration was seen on CT and patient was kept on conservative management. MRI done 4 d later show hematoma/collection in neck of pancreas (long white arrows B and C). The duct was seen to communicate with the hematoma and MRCP showed cut off of duct at site of injury (short white arrow D). The patient subsequently underwent distal pancreatectomy. CECT: Contrast enhanced computed tomography; MRI: Magnetic resonance imaging; MRCP: Magnetic resonance pancreatography; CT: Computed tomography.
MANAGEMENT
Management of pancreatic trauma depends on: (1) grade/ severity of injury; (2) location of injury; (3) other associated abdominal injuries; and (4) time elapsed after injury[2,9,14]. If CT shows ductal involvement (more than 50% depth of laceration), the operative management is preferred. If CT is equivocal, MRI (or ERCP) should be done to look for ductal involvement followed by laparotomy in presence of ductal involvement.
Low grade pancreatic injuries are usually managed conservatively. If laparotomy is indicated in these patients for other associated abdominal injuries, then simple external drainage of pancreatic bed can be done concomitantly. The management options based on grade of pancreatic injury have been summarised in Table 3.
Table 3.
Treatment options for isolated pancreatic injuries based on the American Association of Surgery for Trauma pancreas organ injury scale[2,14,60]
| AAST grade | Treatment options |
| I | Observation/conservative management |
| Simple external drainage | |
| Omental pancreatorrhaphy and drainage | |
| II | Observation/conservative management |
| Simple external drainage | |
| Omental pancreatorrhaphy and drainage | |
| III | Distal pancreatectomy +/- splenectomy |
| Roux-en-Y distal pancreatojejunostomy | |
| IV | Simple drainage in damage control situations |
| Pancreatoduodenectomy (Whipple procedure) | |
| Distal Roux-en-Y pancreatojejunostomy | |
| Anterior Roux-en-Y pancreatojejunostomy | |
| Endoscopically placed stent | |
| V | Pancreatoduodenectomy |
| Drainage in damage control situations |
AAST: American Association of Surgery for Trauma.
For grade I and II injuries, placement of suction drain suffices to promote external drainage of pancreatic secretions and promote natural healing of minor ductal injuries. Omental pancreatorrhaphy may be done after repair of superficial lacerations and placement of omental graft over site of injury to promote healing[2].
For grade III injuries, distal pancreatectomy is the standard surgery of choice. Associated splenic injuries may necessitateconcomitant splenectomy. If the injury occurs at the neck, then pancreaticojejunostomy may be done as an alternative to distal pancreatectomy to preserve the intact entire distal pancreas[59].
For grade IV injuries, currently pancreatic drainage is recommended as part of damage control surgery. When clinical condition improves, then either resection or reconstruction with pancreatic enteric anastomosis may be done[60,61].
For grade V injuries, Whipple’s procedure (pancreaticoduodenectomy) may be done at first stage[62]. However since most patients with grade V injuries are poor candidates to withstand extensive surgeries, initial damage control with drainage followed by resection-anastomosis may be done[63,64]. Grade IV and V injuries are often associated with duodenal injuries which may be subjected to primary repair and diversion or duodenum is resected along with pancreas.
Non-operative management
Literature on non operative management of injuries (NOMI) mostly pertains to pediatric patients with reported outcomes similar to operative management[65,66]. However this approach can also be extended to adults[8]. Proper patient selection (patients with low-grade injuries, isolated pancreatic injuries and absence of ductal involvement on MRI or ERCP), continuous patient monitoring and radiological follow-up and availability of radiological or endoscopic interventions for management of local/pancreatic complications are keystones to successful NOMI[8,67]. In case of clinical and radiological progression of injury, subsequent surgical management is preferred over endoscopy as the laparotomy has better outcomes with lesser complications[2,3].
COMPLICATIONS, MORBIDITY AND MORTALITY
Despite the relatively low incidence of pancreatic trauma, morbidity and mortality are high. While isolated pancreatic trauma has an incidence of less than 30% and a lower mortality of 3%-10%[68], the overall morbidity is 30%-50% and mortality is 10%-30%. There is a proportionately direct increase in adverse outcome with: (1) increasing grade of injury; (2) associated organ injuries; and (3) delay in diagnosis with failure to identify ductal injuries[3,9,13,24,29,69,70].
Approximately, one-third of the patients survive the first 48 h develop complications due to pancreatic injury. Complications include traumatic pancreatitis, pancreatitis induced vascular complications such as pseudoaneurysms, pseudocysts, pancreatic fistulas, intraabdominal abscesses, pancreatic strictures and chronic obstructive pancreatitis, wound complications, septicaemia and multiorgan failure[14,69,71-73].
Post-traumatic pancreatitis occurs due to missed or delayed diagnosis of ductal injury. The incidence of pancreatitis is 17% after pancreatic injury[74]. Patients present with abdominal pain and hyperamylasemia. CT demonstrates typical imaging features of pancreatitis with bulky, heterogeneously enhancing pancreas, intrapancreatic and peripancreatic collections and can lead to sepsis and multiorgan failure (Figure 13). Treatment is usually conservative while pancreatectomy, debridement and drainage may be done for failure of conservative treatment. Patients may also present with recurrent episodes of pancreatitis months after trauma due to persistent duct leak. This may require surgical intervention or endoscopic stenting[74].
Figure 13.
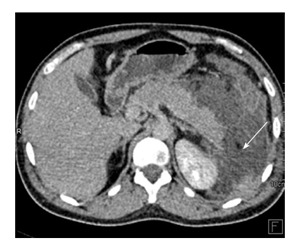
A 22-year-old man with history of road traffic accident, presenting on day 17 after trauma. CECT axial image shows injury to distal body and tail of pancreas with peripancreatic collection (arrow). No imaging was done at time of trauma. Features are suggestive of traumatic pancreatitis following initial missed injury. Patient was conservatively managed with percutaneous drainage. CECT: Contrast enhanced computed tomography.
Pancreatic fistula is one of the commonest complications after pancreatic trauma. Its incidence varies from 20% in isolated pancreatic trauma to 35% in combined pancreaticoduodenal injuries[61,75,76]. Fistula output more than 200 mL/d is a low output fistula while output more than 500 mL/d is a high output fistula. Conservative management with CT guided drainage of fistula over weeks is the treatment of choice[77]. In case of persistently high output drainage or internal communication with a hollow viscus or pleural cavity, ERCP may be done to delineate the fistulous anatomy followed by surgery or endoscopic stenting[5]. Proximal fistulas are better treated by stenting or Roux-en-Y procedures while distal fistulas are treated by pancreatectomy[3,14].
Pancreatic pseudocysts more commonly occur after missed injuries to distal pancreas or as a sequelae of NOMI[14,66]. These are commonly located anterior to body and tail of pancreas. MRCP or ERCP should be done to look for communication with pancreatic duct. If communication is present, endoscopic stenting along with CT guided percutaneous drainage is done[78,79]. If there is no communication with pancreatic duct, drainage alone is sufficient. If closely apposing stomach or bowel walls, surgical or endoscopic cystogastostomy or cystoenterosotmy are other therapeutic options[77].
Peripancreatic abscess/infected walled-off collections usually occur secondary to contamination from hollow viscus or from skin flora through the external drain. These increase morbidity and mortality due to ensuing sepsis[61,69,80]. On imaging, air foci within peripancreatic collections are suggestive of infection (Figure 14). However, if external drainage is maintained, presence of air foci may be normal. In such cases MRI can show debris within the collections while positive culture of fluid in the presence of fever, leucocytosis and acidosis are diagnostic.
Figure 14.
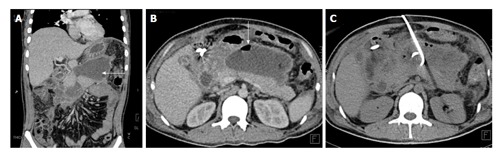
A 45-year-old man with missed pancreatic injury. CECT coronal (A) and axial (B) image done weeks after injury shows an ill-defined walled off necrosis (arrow A) with air foci within (arrow B). CT guided pigtail drainage of collection was done (C) with antibiotic coverage. CECT: Contrast enhanced computed tomography; CT: Computed tomography.
Vascular complications such as pseudoaneurysms either occur due to complications of surgery or secondary to erosion of vessel wall by pancreatic enzymes[81,82]. Post-pancreatitis and post-traumatic pancreatic pseudoaneurysms commonly involve splenic, gastroduodenal and common hepatic arteries. Pseudoaneurysms are potentially life threatening events and if untreated can rupture leading to haemorrhagic death. Imminent rupture or bleeding pseudoaneuryms manifest as upper gastrointestinal bleed (hematemesis/melena) or hemobilia. If patient is hemodynamically stable, CT angiography is the modality of choice to diagnose site and size of pseudoaneurysms followed by angio-embolization with coils, glue or thrombin. If hemodynamically unstable, patients can directed be taken for embolization[83-85]. In cases of failure of embolization or in cases non-amenable to embolization, surgical management is done (Figure 15).
Figure 15.
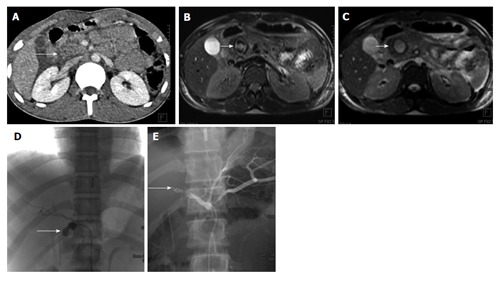
Post-traumatic common hepatic artery pseudoaneurysm 22 years old man with blunt trauma abdomen. CECT axial image shows injury to head of pancreas (arrow A). MRI done 3 d later showed well defined lesion in head of pancreas with heterogeneous signal intensity on T1 weighted image (arrow B) and hyperintense on TRUFISP image (arrow C). A possibility of pseudoaneurysm was given. Angiogram showed pseudoaneurysm arising from proximal common hepatic artery (arrow D) which was embolised with Nester coils (arrow E). CECT: Contrast enhanced computed tomography; MRI: Magnetic resonance imaging; TRUFISP: True fast imaging with steady-state free precession.
Pancreatic duct strictures and chronic obstructive pancreatitis can occur as sequelae of NOMI wherein fibrosis at injury site can lead to pancreatic duct strictures. Chronic obstruction and raised intraductal pressure leads to chronic obstructive pancreatitis, presenting months to years after trauma[72] (Figure 16). MRI is useful in diagnosis while ERCP and endoscopic stenting are therapeutic. Other options include surgical pancreaticojejunostomy and distal pancreatectomy for distal strictures[77].
Figure 16.
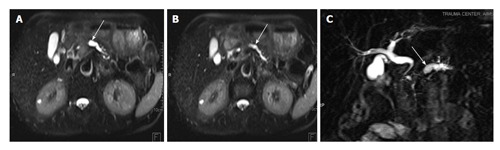
A 40-year-old male with previous history of pancreatic trauma. Injury was missed at time of presentation. Follow up MRI T2 HASTE images (A and B) and MRCP image (C) show dilated main pancreatic duct and side branches (arrows A and B) with cut off in proximal body region (arrow C) suggestive of post-traumatic pancreatic stricture. MRI: Magnetic resonance imaging; MRCP: Magnetic resonance pancreatography.
SUMMARY
Pancreatic trauma remains a difficult diagnosis with high morbidity and mortality. While MDCT is the mainstay for diagnosing pancreatic injury, early scans may miss pancreatic trauma, especially if not carefully looked for. Thus radiologists should have a very high index of suspicion for pancreatic injury and should carefully evaluate all CT scans for signs of pancreatic involvement. Early diagnosis of ductal injury is essential to improve outcomes. If ductal involvement is equivocal on CT, MRI should be done to comment on ductal injury vs integrity and guide management. ERCP has selective role in management of complications of pancreatic trauma and its complications. Since pancreatic injury is an evolving process, serial imaging with CT or MRI should be done to look for temporal evolution and for follow-up in non-operative management of pancreatic trauma. Radiology also plays a crucial role in follow-up and management of complications in pancreatic trauma.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest regarding this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 4, 2015
First decision: July 31, 2015
Article in press: December 15, 2015
P- Reviewer: Chow J, Li YZ, Nouh MR S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
References
- 1.Stawicki SP, Schwab CW. Pancreatic trauma: demographics, diagnosis, and management. Am Surg. 2008;74:1133–1145. [PubMed] [Google Scholar]
- 2.Biffl WL, Moore EE, Croce M, Davis JW, Coimbra R, Karmy-Jones R, McIntyre RC, Moore FA, Sperry J, Malhotra A, et al. Western Trauma Association critical decisions in trauma: management of pancreatic injuries. J Trauma Acute Care Surg. 2013;75:941–946. doi: 10.1097/TA.0b013e3182a96572. [DOI] [PubMed] [Google Scholar]
- 3.Lin BC, Chen RJ, Fang JF, Hsu YP, Kao YC, Kao JL. Management of blunt major pancreatic injury. J Trauma. 2004;56:774–778. doi: 10.1097/01.ta.0000087644.90727.df. [DOI] [PubMed] [Google Scholar]
- 4.Fisher M, Brasel K. Evolving management of pancreatic injury. Curr Opin Crit Care. 2011;17:613–617. doi: 10.1097/MCC.0b013e32834cd374. [DOI] [PubMed] [Google Scholar]
- 5.Bhasin DK, Rana SS, Rawal P. Endoscopic retrograde pancreatography in pancreatic trauma: need to break the mental barrier. J Gastroenterol Hepatol. 2009;24:720–728. doi: 10.1111/j.1440-1746.2009.05809.x. [DOI] [PubMed] [Google Scholar]
- 6.Nirula R, Velmahos GC, Demetriades D. Magnetic resonance cholangiopancreatography in pancreatic trauma: a new diagnostic modality? J Trauma. 1999;47:585–587. doi: 10.1097/00005373-199909000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Kong Y, Zhang H, He X, Liu C, Piao L, Zhao G, Zhen Y. Endoscopic management for pancreatic injuries due to blunt abdominal trauma decreases failure of nonoperative management and incidence of pancreatic-related complications. Injury. 2014;45:134–140. doi: 10.1016/j.injury.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Duchesne JC, Schmieg R, Islam S, Olivier J, McSwain N. Selective nonoperative management of low-grade blunt pancreatic injury: are we there yet? J Trauma. 2008;65:49–53. doi: 10.1097/TA.0b013e318176c00d. [DOI] [PubMed] [Google Scholar]
- 9.Cirillo RL, Koniaris LG. Detecting blunt pancreatic injuries. J Gastrointest Surg. 2002;6:587–598. doi: 10.1016/s1091-255x(01)00028-2. [DOI] [PubMed] [Google Scholar]
- 10.Daly KP, Ho CP, Persson DL, Gay SB. Traumatic Retroperitoneal Injuries: Review of Multidetector CT Findings. Radiographics. 2008;28:1571–1590. doi: 10.1148/rg.286075141. [DOI] [PubMed] [Google Scholar]
- 11.Arkovitz MS, Johnson N, Garcia VF. Pancreatic trauma in children: mechanisms of injury. J Trauma. 1997;42:49–53. doi: 10.1097/00005373-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Linsenmaier U, Wirth S, Reiser M, Körner M. Diagnosis and classification of pancreatic and duodenal injuries in emergency radiology. Radiographics. 2008;28:1591–1602. doi: 10.1148/rg.286085524. [DOI] [PubMed] [Google Scholar]
- 13.Bradley EL, Young PR, Chang MC, Allen JE, Baker CC, Meredith W, Reed L, Thomason M. Diagnosis and initial management of blunt pancreatic trauma: guidelines from a multiinstitutional review. Ann Surg. 1998;227:861–869. doi: 10.1097/00000658-199806000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chrysos E, Athanasakis E, Xynos E. Pancreatic trauma in the adult: current knowledge in diagnosis and management. Pancreatology. 2002;2:365–378. doi: 10.1159/000065084. [DOI] [PubMed] [Google Scholar]
- 15.Moretz JA, Campbell DP, Parker DE, Williams GR. Significance of serum amylase level in evaluating pancreatic trauma. Am J Surg. 1975;130:739–741. doi: 10.1016/0002-9610(75)90432-8. [DOI] [PubMed] [Google Scholar]
- 16.Adamson WT, Hebra A, Thomas PB, Wagstaff P, Tagge EP, Othersen HB. Serum amylase and lipase alone are not cost-effective screening methods for pediatric pancreatic trauma. J Pediatr Surg. 2003;38:354–357; discussion 354-357. doi: 10.1053/jpsu.2003.50107. [DOI] [PubMed] [Google Scholar]
- 17.Olsen WR. The serum amylase in blunt abdominal trauma. J Trauma. 1973;13:200–204. doi: 10.1097/00005373-197303000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Matsuno WC, Huang CJ, Garcia NM, Roy LC, Davis J. Amylase and lipase measurements in paediatric patients with traumatic pancreatic injuries. Injury. 2009;40:66–71. doi: 10.1016/j.injury.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Takishima T, Sugimoto K, Hirata M, Asari Y, Ohwada T, Kakita A. Serum amylase level on admission in the diagnosis of blunt injury to the pancreas: its significance and limitations. Ann Surg. 1997;226:70–76. doi: 10.1097/00000658-199707000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouwman DL, Weaver DW, Walt AJ. Serum amylase and its isoenzymes: a clarification of their implications in trauma. J Trauma. 1984;24:573–578. [PubMed] [Google Scholar]
- 21.Herman R, Guire KE, Burd RS, Mooney DP, Ehlrich PF. Utility of amylase and lipase as predictors of grade of injury or outcomes in pediatric patients with pancreatic trauma. J Pediatr Surg. 2011;46:923–926. doi: 10.1016/j.jpedsurg.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Wong YC, Wang LJ, Lin BC, Chen CJ, Lim KE, Chen RJ. CT grading of blunt pancreatic injuries: prediction of ductal disruption and surgical correlation. J Comput Assist Tomogr. 1997;21:246–250. doi: 10.1097/00004728-199703000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Lucas CE. Diagnosis and treatment of pancreatic and duodenal injury. Surg Clin North Am. 1977;57:49–65. doi: 10.1016/s0039-6109(16)41133-3. [DOI] [PubMed] [Google Scholar]
- 24.Smego DR, Richardson JD, Flint LM. Determinants of outcome in pancreatic trauma. J Trauma. 1985;25:771–776. doi: 10.1097/00005373-198508000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Moore EE, Cogbill TH, Malangoni MA, Jurkovich GJ, Champion HR, Gennarelli TA, McAninch JW, Pachter HL, Shackford SR, Trafton PG. Organ injury scaling, II: Pancreas, duodenum, small bowel, colon, and rectum. J Trauma. 1990;30:1427–1429. [PubMed] [Google Scholar]
- 26.Moore EE, Moore FA. American Association for the Surgery of Trauma Organ Injury Scaling: 50th anniversary review article of the Journal of Trauma. J Trauma. 2010;69:1600–1601. doi: 10.1097/TA.0b013e318201124e. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A, Stuhlfaut JW, Fleming KW, Lucey BC, Soto JA. Blunt trauma of the pancreas and biliary tract: a multimodality imaging approach to diagnosis. Radiographics. 2004;24:1381–1395. doi: 10.1148/rg.245045002. [DOI] [PubMed] [Google Scholar]
- 28.Stuhlfaut JW, Anderson SW, Soto JA. Blunt abdominal trauma: current imaging techniques and CT findings in patients with solid organ, bowel, and mesenteric injury. Semin Ultrasound CT MR. 2007;28:115–129. doi: 10.1053/j.sult.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Akhrass R, Kim K, Brandt C. Computed tomography: an unreliable indicator of pancreatic trauma. Am Surg. 1996;62:647–651. [PubMed] [Google Scholar]
- 30.Teh SH, Sheppard BC, Mullins RJ, Schreiber MA, Mayberry JC. Diagnosis and management of blunt pancreatic ductal injury in the era of high-resolution computed axial tomography. Am J Surg. 2007;193:641–643; discussion 643. doi: 10.1016/j.amjsurg.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Wong YC, Wang LJ, Fang JF, Lin BC, Ng CJ, Chen RJ. Multidetector-row computed tomography (CT) of blunt pancreatic injuries: can contrast-enhanced multiphasic CT detect pancreatic duct injuries? J Trauma. 2008;64:666–672. doi: 10.1097/TA.0b013e31802c5ba0. [DOI] [PubMed] [Google Scholar]
- 32.Phelan HA, Velmahos GC, Jurkovich GJ, Friese RS, Minei JP, Menaker JA, Philp A, Evans HL, Gunn ML, Eastman AL, et al. An evaluation of multidetector computed tomography in detecting pancreatic injury: results of a multicenter AAST study. J Trauma. 2009;66:641–646; discussion 646-647. doi: 10.1097/TA.0b013e3181991a0e. [DOI] [PubMed] [Google Scholar]
- 33.Panda A, Kumar A, Gamanagatti S, Bhalla AS, Sharma R, Kumar S, Mishra B. Evaluation of diagnostic utility of multidetector computed tomography and magnetic resonance imaging in blunt pancreatic trauma: a prospective study. Acta Radiol. 2015;56:387–396. doi: 10.1177/0284185114529949. [DOI] [PubMed] [Google Scholar]
- 34.Wu B, Song B. [Curved planar reformations in multi-slice spiral CT in pancreatic adenocarcinoma: prediction of invasion of pancreatic and peripancreatic ductal structures] Zhongguo Yixue Kexueyuan Xuebao. 2006;28:71–75. [PubMed] [Google Scholar]
- 35.Paspulati RM. Multidetector CT of the pancreas. Radiol Clin North Am. 2005;43:999–1020, viii. doi: 10.1016/j.rcl.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Shanmuganathan K. Multi-detector row CT imaging of blunt abdominal trauma. Semin Ultrasound CT MR. 2004;25:180–204. doi: 10.1016/j.sult.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Venkatesh SK, Wan JM. CT of blunt pancreatic trauma: a pictorial essay. Eur J Radiol. 2008;67:311–320. doi: 10.1016/j.ejrad.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Rekhi S, Anderson SW, Rhea JT, Soto JA. Imaging of blunt pancreatic trauma. Emerg Radiol. 2010;17:13–19. doi: 10.1007/s10140-009-0811-0. [DOI] [PubMed] [Google Scholar]
- 39.Lane MJ, Mindelzun RE, Sandhu JS, McCormick VD, Jeffrey RB. CT diagnosis of blunt pancreatic trauma: importance of detecting fluid between the pancreas and the splenic vein. AJR Am J Roentgenol. 1994;163:833–835. doi: 10.2214/ajr.163.4.7503824. [DOI] [PubMed] [Google Scholar]
- 40.Sivit CJ, Eichelberger MR, Taylor GA, Bulas DI, Gotschall CS, Kushner DC. Blunt pancreatic trauma in children: CT diagnosis. AJR Am J Roentgenol. 1992;158:1097–1100. doi: 10.2214/ajr.158.5.1566674. [DOI] [PubMed] [Google Scholar]
- 41.Holalkere NS, Soto J. Imaging of miscellaneous pancreatic pathology (trauma, transplant, infections, and deposition) Radiol Clin North Am. 2012;50:515–528. doi: 10.1016/j.rcl.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 42.Sivit CJ, Eichelberger MR. CT diagnosis of pancreatic injury in children: significance of fluid separating the splenic vein and the pancreas. AJR Am J Roentgenol. 1995;165:921–924. doi: 10.2214/ajr.165.4.7676993. [DOI] [PubMed] [Google Scholar]
- 43.Yang L, Zhang XM, Xu XX, Tang W, Xiao B, Zeng NL. MR imaging for blunt pancreatic injury. Eur J Radiol. 2010;75:e97–101. doi: 10.1016/j.ejrad.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Soto JA, Alvarez O, Múnera F, Yepes NL, Sepúlveda ME, Pérez JM. Traumatic disruption of the pancreatic duct: diagnosis with MR pancreatography. AJR Am J Roentgenol. 2001;176:175–178. doi: 10.2214/ajr.176.1.1760175. [DOI] [PubMed] [Google Scholar]
- 45.Fulcher AS, Turner MA, Yelon JA, McClain LC, Broderick T, Ivatury RR, Sugerman HJ. Magnetic resonance cholangiopancreatography (MRCP) in the assessment of pancreatic duct trauma and its sequelae: preliminary findings. J Trauma. 2000;48:1001–1007. doi: 10.1097/00005373-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Ragozzino A, Manfredi R, Scaglione M, De Ritis R, Romano S, Rotondo A. The use of MRCP in the detection of pancreatic injuries after blunt trauma. Emerg Radiol. 2003;10:14–18. doi: 10.1007/s10140-003-0278-3. [DOI] [PubMed] [Google Scholar]
- 47.Gillams AR, Kurzawinski T, Lees WR. Diagnosis of duct disruption and assessment of pancreatic leak with dynamic secretin-stimulated MR cholangiopancreatography. AJR Am J Roentgenol. 2006;186:499–506. doi: 10.2214/AJR.04.1775. [DOI] [PubMed] [Google Scholar]
- 48.Valentino M, Ansaloni L, Catena F, Pavlica P, Pinna AD, Barozzi L. Contrast-enhanced ultrasonography in blunt abdominal trauma: considerations after 5 years of experience. Radiol Med. 2009;114:1080–1093. doi: 10.1007/s11547-009-0444-0. [DOI] [PubMed] [Google Scholar]
- 49.Lv F, Tang J, Luo Y, Nie Y, Liang T, Jiao Z, Zhu Z, Li T. Emergency contrast-enhanced ultrasonography for pancreatic injuries in blunt abdominal trauma. Radiol Med. 2014;119:920–927. doi: 10.1007/s11547-014-0410-3. [DOI] [PubMed] [Google Scholar]
- 50.Pannu HK, Fishman EK. Complications of endoscopic retrograde cholangiopancreatography: spectrum of abnormalities demonstrated with CT. Radiographics. 2001;21:1441–1453. doi: 10.1148/radiographics.21.6.g01nv101441. [DOI] [PubMed] [Google Scholar]
- 51.Stone A, Sugawa C, Lucas C, Hayward S, Nakamura R. The role of endoscopic retrograde pancreatography (ERP) in blunt abdominal trauma. Am Surg. 1990;56:715–720. [PubMed] [Google Scholar]
- 52.Rogers SJ, Cello JP, Schecter WP. Endoscopic retrograde cholangiopancreatography in patients with pancreatic trauma. J Trauma. 2010;68:538–544. doi: 10.1097/TA.0b013e3181b5db7a. [DOI] [PubMed] [Google Scholar]
- 53.Kim HS, Lee DK, Kim IW, Baik SK, Kwon SO, Park JW, Cho NC, Rhoe BS. The role of endoscopic retrograde pancreatography in the treatment of traumatic pancreatic duct injury. Gastrointest Endosc. 2001;54:49–55. doi: 10.1067/mge.2001.115733. [DOI] [PubMed] [Google Scholar]
- 54.Kozarek RA. Endoscopic therapy of complete and partial pancreatic duct disruptions. Gastrointest Endosc Clin N Am. 1998;8:39–53. [PubMed] [Google Scholar]
- 55.Bosboom D, Braam AW, Blickman JG, Wijnen RM. The role of imaging studies in pancreatic injury due to blunt abdominal trauma in children. Eur J Radiol. 2006;59:3–7. doi: 10.1016/j.ejrad.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 56.Leppäniemi AK, Haapiainen RK. Risk factors of delayed diagnosis of pancreatic trauma. Eur J Surg. 1999;165:1134–1137. doi: 10.1080/110241599750007649. [DOI] [PubMed] [Google Scholar]
- 57.Brestas PS, Karakyklas D, Gardelis J, Tsouroulas M, Drossos C. Sequential CT evaluation of isolated non-penetrating pancreatic trauma. JOP. 2006;7:51–55. [PubMed] [Google Scholar]
- 58.Horst HM, Bivins BA. Pancreatic transection. A concept of evolving injury. Arch Surg. 1989;124:1093–1095. doi: 10.1001/archsurg.1989.01410090107024. [DOI] [PubMed] [Google Scholar]
- 59.Subramanian A, Dente CJ, Feliciano DV. The management of pancreatic trauma in the modern era. Surg Clin North Am. 2007;87:1515–1532, x. doi: 10.1016/j.suc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 60.Patton JH, Lyden SP, Croce MA, Pritchard FE, Minard G, Kudsk KA, Fabian TC. Pancreatic trauma: a simplified management guideline. J Trauma. 1997;43:234–239; discussion 239-241. doi: 10.1097/00005373-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Sharpe JP, Magnotti LJ, Weinberg JA, Zarzaur BL, Stickley SM, Scott SE, Fabian TC, Croce MA. Impact of a defined management algorithm on outcome after traumatic pancreatic injury. J Trauma Acute Care Surg. 2012;72:100–105. doi: 10.1097/TA.0b013e318241f09d. [DOI] [PubMed] [Google Scholar]
- 62.Asensio JA, Petrone P, Roldán G, Kuncir E, Demetriades D. Pancreaticoduodenectomy: a rare procedure for the management of complex pancreaticoduodenal injuries. J Am Coll Surg. 2003;197:937–942. doi: 10.1016/j.jamcollsurg.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 63.Seamon MJ, Kim PK, Stawicki SP, Dabrowski GP, Goldberg AJ, Reilly PM, Schwab CW. Pancreatic injury in damage control laparotomies: Is pancreatic resection safe during the initial laparotomy? Injury. 2009;40:61–65. doi: 10.1016/j.injury.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 64.Wang GF, Li YS, Li JS. Damage control surgery for severe pancreatic trauma. Hepatobiliary Pancreat Dis Int. 2007;6:569–571. [PubMed] [Google Scholar]
- 65.Paul MD, Mooney DP. The management of pancreatic injuries in children: operate or observe. J Pediatr Surg. 2011;46:1140–1143. doi: 10.1016/j.jpedsurg.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 66.Wood JH, Partrick DA, Bruny JL, Sauaia A, Moulton SL. Operative vs nonoperative management of blunt pancreatic trauma in children. J Pediatr Surg. 2010;45:401–406. doi: 10.1016/j.jpedsurg.2009.10.095. [DOI] [PubMed] [Google Scholar]
- 67.Velmahos GC, Tabbara M, Gross R, Willette P, Hirsch E, Burke P, Emhoff T, Gupta R, Winchell RJ, Patterson LA, et al. Blunt pancreatoduodenal injury: a multicenter study of the Research Consortium of New England Centers for Trauma (ReCONECT) Arch Surg. 2009;144:413–419; discussion 419-420. doi: 10.1001/archsurg.2009.52. [DOI] [PubMed] [Google Scholar]
- 68.Wilson RH, Moorehead RJ. Current management of trauma to the pancreas. Br J Surg. 1991;78:1196–1202. doi: 10.1002/bjs.1800781017. [DOI] [PubMed] [Google Scholar]
- 69.Oláh A, Issekutz A, Haulik L, Makay R. Pancreatic transection from blunt abdominal trauma: early versus delayed diagnosis and surgical management. Dig Surg. 2003;20:408–414. doi: 10.1159/000072708. [DOI] [PubMed] [Google Scholar]
- 70.Silveira HJ, Mantovani M, Fraga GP. [Trauma of pancreas: predictor’s factors of morbidity and mortality related to trauma index] Arq Gastroenterol. 2009;46:270–278. doi: 10.1590/s0004-28032009000400005. [DOI] [PubMed] [Google Scholar]
- 71.Recinos G, DuBose JJ, Teixeira PG, Inaba K, Demetriades D. Local complications following pancreatic trauma. Injury. 2009;40:516–520. doi: 10.1016/j.injury.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 72.Bradley EL. Chronic obstructive pancreatitis as a delayed complication of pancreatic trauma. HPB Surg. 1991;5:49–59; discussion 59-60. doi: 10.1155/1991/73834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akhrass R, Yaffe MB, Brandt CP, Reigle M, Fallon WF, Malangoni MA. Pancreatic trauma: a ten-year multi-institutional experience. Am Surg. 1997;63:598–604. [PubMed] [Google Scholar]
- 74.Fleming WR, Collier NA, Banting SW. Pancreatic trauma: Universities of Melbourne HPB Group. Aust N Z J Surg. 1999;69:357–362. doi: 10.1046/j.1440-1622.1999.01572.x. [DOI] [PubMed] [Google Scholar]
- 75.Jones RC. Management of pancreatic trauma. Am J Surg. 1985;150:698–704. doi: 10.1016/0002-9610(85)90412-x. [DOI] [PubMed] [Google Scholar]
- 76.Balasegaram M. Surgical management of pancreatic trauma. Curr Probl Surg. 1979;16:1–59. doi: 10.1016/s0011-3840(79)80013-1. [DOI] [PubMed] [Google Scholar]
- 77.Ahmed N, Vernick JJ. Pancreatic injury. South Med J. 2009;102:1253–1256. doi: 10.1097/SMJ.0b013e3181c0dfca. [DOI] [PubMed] [Google Scholar]
- 78.Lin BC, Fang JF, Wong YC, Liu NJ. Blunt pancreatic trauma and pseudocyst: management of major pancreatic duct injury. Injury. 2007;38:588–593. doi: 10.1016/j.injury.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 79.Coelho DE, Ardengh JC, Carbalo MT, de Lima-Filho ER, Baron TH, Coelho JF. Clinicopathologic characteristics and endoscopic treatment of post-traumatic pancreatic pseudocysts. Pancreas. 2011;40:469–473. doi: 10.1097/MPA.0b013e31820bf898. [DOI] [PubMed] [Google Scholar]
- 80.Patton JH, Fabian TC. Complex pancreatic injuries. Surg Clin North Am. 1996;76:783–795. doi: 10.1016/s0039-6109(05)70480-1. [DOI] [PubMed] [Google Scholar]
- 81.Pang TC, Maher R, Gananadha S, Hugh TJ, Samra JS. Peripancreatic pseudoaneurysms: a management-based classification system. Surg Endosc. 2014;28:2027–2038. doi: 10.1007/s00464-014-3434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu YP, Ni JJ, Chen RB, Matro E, Xu XW, Li B, Hu HJ, Mou YP. Successful interventional radiological management of postoperative complications of laparoscopic distal pancreatectomy. World J Gastroenterol. 2013;19:8453–8458. doi: 10.3748/wjg.v19.i45.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Otah E, Cushin BJ, Rozenblit GN, Neff R, Otah KE, Cooperman AM. Visceral artery pseudoaneurysms following pancreatoduodenectomy. Arch Surg. 2002;137:55–59. doi: 10.1001/archsurg.137.1.55. [DOI] [PubMed] [Google Scholar]
- 84.De Rosa A, Gomez D, Pollock JG, Bungay P, De Nunzio M, Hall RI, Thurley P. The radiological management of pseudoaneurysms complicating pancreatitis. JOP. 2012;13:660–666. doi: 10.6092/1590-8577/1193. [DOI] [PubMed] [Google Scholar]
- 85.Hur S, Yoon CJ, Kang SG, Dixon R, Han HS, Yoon YS, Cho JY. Transcatheter arterial embolization of gastroduodenal artery stump pseudoaneurysms after pancreaticoduodenectomy: safety and efficacy of two embolization techniques. J Vasc Interv Radiol. 2011;22:294–301. doi: 10.1016/j.jvir.2010.11.020. [DOI] [PubMed] [Google Scholar]


