Abstract
AIM: To compare 2-deoxy-2-(18F)fluoro-D-glucose(18F-FDG) and 18F-sodium (18F-NaF) positron emission tomography/computed tomography (PET/CT) accuracy in breast cancer patients with clinically/radiologically suspected or known bone metastases.
METHODS: A total of 45 consecutive patients with breast cancer and the presence or clinical/biochemical or radiological suspicion of bone metastatic disease underwent 18F-FDG and 18F-fluoride PET/CT. Imaging results were compared with histopathology when available, or clinical and radiological follow-up of at least 1 year. For each technique we calculated: Sensitivity (Se), specificity (Sp), overall accuracy, positive and negative predictive values, error rate, and Youden’s index. McNemar’s χ2 test was used to test the difference in sensitivity and specificity between the two diagnostic methods. All analyses were computed on a patient basis, and then on a lesion basis, with consideration ofthe density of independent lesions on the co-registered CT (sclerotic, lytic, mixed, no-lesions) and the divergent site of disease (skull, spine, ribs, extremities, pelvis). The impact of adding 18F-NaF PET/CT to the work-up of patients was also measured in terms of change in their management due to 18F-NaF PET/CT findings.
RESULTS: The two imaging methods of 18F-FDG and 18F-fluoride PET/CT were significantly different at the patient-based analysis: Accuracy was 86.7% and 84.4%, respectively (McNemar’s χ2 = 6.23, df = 1, P = 0.01). Overall, 244 bone lesions were detected in our analysis. The overall accuracy of the two methods was significantly different at lesion-based analysis (McNemar’s χ2 = 93.4, df = 1, P < 0.0001). In the lesion density-based and site-based analysis, 18F-FDG PET/CT provided more accurate results in the detection of CT-negative metastasis (P < 0.002) and vertebral localizations (P < 0.002); 18F-NaF PET/CT was more accurate in detecting sclerotic (P < 0.005) and rib lesions (P < 0.04). 18F-NaF PET/CT led to a change of management in 3 of the 45 patients (6.6%) by revealing findings that were not detected at 18F-FDG PET/CT.
CONCLUSION: 18F-FDG PET/CT is a reliable imaging tool in the detection of bone metastasis in most cases, with a diagnostic accuracy that is slightly, but significantly, superior to that of 18F-NaF PET/CT in the general population of breast cancer patients. However, the extremely high sensitivity of 18F-fluoride PET/CT can exploit its diagnostic potential in specific clinical settings (i.e., small CT-evident sclerotic lesions, high clinical suspicious of relapse, and negative 18F-FDG PET and conventional imaging).
Keywords: 18F-sodium positron emission tomography/computed tomography, Breast cancer, Bone lesion, 2-deoxy-2-(18F)fluoro-D-glucose
Core tip: 18F-fluorodeoxyglucose (18F-FDG) and 18F-sodium positron (18F-NaF) positron emission tomography/computed tomography (PET/CT) is undoubtedly an accurate and validated imaging tool in the general population of breast cancer patients for the detection of bone metastasis in most cases. However, thanks to its extremely high sensitivity, 18F-NaF PET/CT could have an adjunctive value in selected patients, significantly impacting their management (i.e., small CT-evident sclerotic lesions, high clinical suspicious of relapse, and negative 18F-FDG PET and conventional imaging). This sensitivity might be particularly relevant for patients who are candidates for surgery or radiotherapy.
INTRODUCTION
Breast cancer is the most prevalent form of cancer in women of Western countries[1-3], with the skeleton being the most common site of distant metastases. Presence, distribution, and type of bone localizations have relevant prognostic implications[4,5]. In particular, with the growing availability of new therapeutic strategies which could potentially improve survival, the early detection of bone metastases has gained pivotal importance[6,7].
Conventional bone scintigraphy (BS) remains the most suitable technique for whole-body screening of bone metastasis due to its low cost and high availability. However, BS has several important limitations, and so additional imaging procedures are often necessary to determine the real significance of scintigraphic abnormalities[8].
During the last decade, positron emission tomography (PET) has evolved from a research tool to an established imaging modality for the staging of different types of malignant tumors, owing to its better spatial resolution and superior image quality with respect to conventional single-photon imaging. Among PET tracers, glucose analogue 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG) has become the most widely used in clinical routine, resulting in a major impact on the practice of oncology[9]. In breast cancer patients, 18F-FDG-PET enables the detection of neoplastic lesions on the basis of their increased glucose metabolism, potentially allowing for an accurate assessment of local disease, lymph nodes, and visceral metastases in a single imaging study. Furthermore, by directly reflecting tumor cell viability in bone metastases, this technique can potentially be used for therapy response assessments. In fact, changes in 18F-FDG activity after therapy may reflect an early response to therapy that could be potentially prognostic[10].
Characterization of bone metastases is also possible with 18F-sodium fluoride (18F-NaF), which reflects the increased regional blood flow and osteoblastic bone reaction[8]. Specifically, greater activity of remodeling and bone turnover determines greater blood flow and exchange surface for 18F-fluoride ion absorption and subsequent irreversible incorporation into the bone matrix as fluorapatite[11-13].
Both PET tracers have shown a better diagnostic value compared to BS in detecting bone metastases in patients with breast cancer and several other malignancies[14-20]. Conversely, very limited and controversial information exists in comparing the diagnostic accuracy of 18F-FDG and 18F-NaF PET[19,21,22]. The different uptake mechanisms of these two tracers might be complementary in the context of evaluating lytic and sclerotic lesions, which can both coexist in bone localizations of breast cancer patients[23].
Furthermore, it has been suggested[24] that anatomical localization of the lesions could also influence the accuracy of each technique; this finding is likely to be related to the morphology of bone metastasis. In fact, the involvement of different skeletal segments could determine different degrees of osteoblastic reaction[25,26]. At the time of writing, controversial results have been reported about the accuracy of these two tracers in breast cancer patients[19,21,22], with some authors even proposing their combined use[27,28]. In particular, 18F-FDG PET/computed tomography (CT) can provide information about the presence/absence of disease in the skeleton, as well as in non-skeletal districts. In this context, it not been clearly investigated whether 18F-NaF PET/CT can provide incremental information for the management of breast cancer patients that have already been evaluated by means of 18F-FDG PET/CT.
The current study aims to evaluate the role of the two imaging modalities in the restaging of breast cancer patients with clinically/radiologically suspected or known metastatic bone lesions. In particular, we planned to verify whether the accuracy of the two imaging methods could be influenced by lesion density and location.
MATERIALS AND METHODS
Patient population
Between January 2010 and June 2012, 45 breast cancer patients were referred to our institutions for the execution of both 18F-NaF and 18F-FDG PET/CT for the restaging of clinically/radiologicallysuspected or proven metastatic bone lesions. All study participants, or their legal guardians, provided informed written consent prior to study enrollment, and practices were performed in accordance with the ethical standards laid down in the Declaration of Helsinki. We included only patients who performed the two PET scans within 1 mo and did not received chemotherapy or radiotherapy between the two examinations. By contrast, chemotherapy administration in the month before the two PET/CT exams was not an exclusion criterion. Patient characteristics are listed in Table 1.
Table 1.
Patient characteristics (n = 45)
| Demography | |
| Age (yr) | 61 ± 10 |
| Stage at diagnosis | |
| I | 5 |
| II | 21 |
| III | 14 |
| IV | 5 |
| Histology | |
| Ductal | 35 |
| Lobular | 8 |
| Other | 2 |
| Tumor receptor (+/-/unknown) | |
| Estrogen receptor | 39/2/4 |
| PgR | 29/11/5 |
| c-erb B2 | 14/24/7 |
| Site of metastatic disease other than bone | |
| Lung | 27% |
| Liver | 20% |
| Lymph nodes | 30% |
| Systemic therapy | |
| First-line | 81% |
| Second-line | 62% |
| Third-line | 36% |
| Bisphosphonates | 22% |
| Follow-up (mean 28 mo range 22-39) | |
| Patients with disease progression | 58% |
| Patients dead from disease | 30% |
PET/CT protocols
Image acquisition was performed according to standard procedures and international guidelines[29,30].
Patients were submitted to 18F-NaF PET/CT using two 16 slices PET/CT hybrid systems: (1) Biograph 16 (Siemens Medical Solutions, Knoxville TN, United States); and (2) Discovery LS (GE Medical Systems, Milwaukee, WI, United States) according to the standard procedure as previously detailed[31].
Image interpretation
Each 18F-FDG-PET/CT and 18F-NaF-PET/CT scan were independently evaluated by two nuclear medicine physicians aware of the patient’s clinical history but blinded to the results of the other PET/CT scan and that of other cross-sectional morphological imaging modalities [magnetic resonance imaging (MRI)/CT]. In cases of disagreement,a consensus obtained among readers was used for the final decision. For both 18F-NaF and 18F-FDG PET/CT, scans were interpreted as negative for bone lesions when no pathologic tracer uptake was present within the skeleton. In cases of increased uptake within the joints, the exam was also considered negative. Similarly, for both 18F-NaF and 18F-FDG, avid lesions were diagnosed as benign when degenerative changes or fractures were detected on non-diagnostic CT. Conversely, the presence of focal tracer uptake associated with suspicious or indeterminate morphological changes on non-diagnostic CT were considered as positive. Similarly, for both 18F-NaF and 18F-FDG, high and focal uptake in the absence of lesions on the non-diagnostic CT was considered likely to be “micro-scleroses”, and thus classified as positive/malignant.
For each lesion, density on the co-registered CT was recorded and lesions were divided into four groups: Sclerotic, lytic, mixed, and no-lesions. Similarly, lesion localizations were also recorded to assess the impact of the divergent disease sites (skull, spine, ribs, extremities, and pelvis).
Standard references
Since a bone biopsy of all lesions for histology was not considered appropriate for obvious ethical reasons, the radiological and clinical follow-up at 12 mo served as the standard of reference for the final evaluation of the results as true-positive, true-negative, false-positive, and false-negative. Follow-up information included physical examination, laboratory tests, tumor markers, and other independent imaging studies (CT, MRI, 18F-FDG PET/CT, X-ray studies, and bone scans).
Statistical analysis
For statistical analysis we used the “R” software program[32] and DiagnosisMed software package[33]. We compared 18F-NaF PET/CT and 18F-FDG PET/CT results through patient-, lesion density-, and site-based analyses.
Cochran Q test followed by multiple comparisons using McNemar’s test with continuity correction and Bonferroni adjustment were used in order to assess differences among imaging modalities. P values less than 0.05 were considered statistically significant. The impact of adding 18F-NaF PET/CT to the work-up of patients was also measured in terms of changes to their management due to findings related to this functional imaging.
The statistical methods of this study were reviewed by a biomedical statistician.
RESULTS
Overall diagnostic accuracy and patient-based analysis
Sixteen patients were negative and 16 patients were positive at both imaging modalities. Eleven and two patients were positive only ata single tracer (18F-FDG and 18F-NaF, respectively). Histology was used as standard references in two patients (specifically in one patient who was true positive for bone marrow involvement at 18F-FDG PET and in one patient who was true positive for the presence of an osteosclerotic lesion in the ribs detected by 18F-NaF only).
The two imaging methods were significantly different in the patient-based analysis, with anaccuracy of 86.7% and 84.4%, respectively (McNemar’s χ2 = 6.23, df = 1, P = 0.01.). See Table 2 for details on sensitivity, specificity, and predictive values of the two PET/CT modalities.
Table 2.
Patient-based analysis: Performance comparisons between 2-deoxy-2-(18F)fluoro-D-glucose and 18F-sodium positron emission tomography/computed tomography
| 18F-FDG | 18F-NaF | |
| Sensitivity (%) | 75.00 (55.10-88.00) | 91.67 (74.15-97.68) |
| Specificity (%) | 99.00 (84.54-100) | 76.19 (54.91-89.37) |
| Positive predictive value (%) | 99.00 (82.41-100) | 81.48 (63.3 91.82) |
| Negative predictive value (%) | 77.78 (59.24-89.39) | 88.89 (67.2-96.90) |
| Error rate (%) | 13.33 (6.26-26.18) | 15.56 (7.75-28.78) |
| Accuracy (%) | 86.67 (73.82-93.74) | 84.44 (71.22-92.25) |
| Youden's index | 0.75 (0.75-0.74) | 0.6786 (0.68-0.6718) |
Estimated parameters corresponding to each technique are presented with 95%CI between square brackets. 18F-FDG: 2-deoxy-2-(18F)fluoro-D-glucose; 18F-NaF: 18F-sodium.
Overall lesion-based analysis
Overall, 244 bone lesions were detected in our analysis. The overall accuracy of the two methods was significantly different in the lesion-based analysis (McNemar’s χ2 = 93.4, df = 1, P < 0.0001). 18F-NaF showed high sensitivity (90.5%), but a very low specificity (17.5%). By contrast, although 18F-FDG PET/CT showed a lower sensitivity (66%), it was characterized by a significantly higher specificity (96.2%).
Lesion density- and site-based analysis
Significant differences were highlighted in the lesion density- and site- based analysis, as 18F-FDG PET/CT was more accurate in the detection of CT-negative metastasis (P < 0.002),vertebral localizations (P < 0.002), and sclerotic (P < 0.005) and rib lesions (P < 0.04). No significant differences were highlighted with respect to accuracy in evaluating lytic and mixed lesions, or in lesions localized in the skull, distal extremities or pelvis. See Table 3 for details on sensitivity, specificity, and accuracy. Figures 1-3 show representative examples of the different performance of the two imaging modalities.
Table 3.
Sites and density characteristics showing different performance between 2-deoxy-2-(18F)fluoro-D-glucose and 18F-sodium positron emission tomography/computed tomography
| n | 18F-FDG | 18F-NaF | P | |
| Density | ||||
| Osteosclerotic | 89 | 0.005 | ||
| Sensitivity (%): | 42.86 (15.82-74.95) | 99.00 (64.57-99.00) | ||
| Specificity (%): | 97.00 (60.97-97.00) | 48.15 (35.39-61.15) | ||
| No lesion/bone marrow | 29 | 0.002 | ||
| Sensitivity(%): | 100.00 (60.97-100.00) | 48.15 (30.74-66.01) | ||
| Specificity(%): | 100.00 (34.24-100.00) | 100.00 (34.24-100.00) | ||
| Site | ||||
| Spine | 81 | 0.002 | ||
| Sensitivity (%): | 65.38 (51.80-76.85) | 100.00 (93.12-100.00) | ||
| Specificity (%): | 98.00 (88.30-98.00) | 13.45 (0.61-27.18) | ||
| Ribs | 118 | 0.04 | ||
| Sensitivity (%): | 83.96 (75.81-89.74) | 96.23 (90.70-98.52) | ||
| Specificity (%): | 78.37 (52.33-92.50) | 78.57 (52.41-92.43) |
18F-FDG: 2-deoxy-2-(18F)fluoro-D-glucose; 18F-NaF: 18F-sodium.
Figure 1.
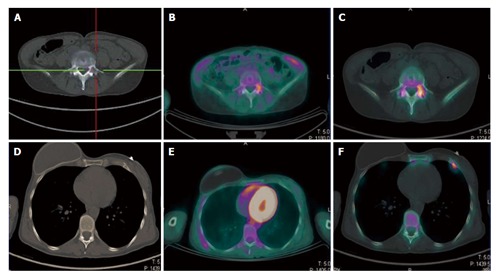
A 49-year-old breast cancer patient with bone relapse. A lytic lesion on the fifth lumbar vertebra was detected with computed tomography in absence of local pain (A). The lesion showed high uptake both at 18F-FDG PET/CT (B) and 18F-NaF PET/CT (C), thus confirming its malignant nature. No further 18F-FDG avid metastasis was highlighted (E). By contrast a focal area of high 18F-NaF uptake was evident in the anterior branch of the seventh rib on the left side (F). This area was indeed corresponding to a small sclerotic indeterminate lesion on the CT and was considered as a further site of disease (D). The patient started systemic therapy rather than a targeted radiotherapy on the fifth lumbar vertebra. 18F-FDG: 2-deoxy-2-(18F)fluoro-D-glucose; 18F-NaF: 18F-sodium; PET/CT: Positron emission tomography/computed tomography.
Figure 3.
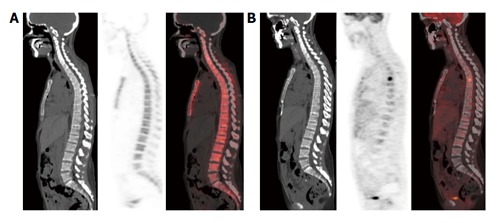
This area is likely to correspond to a bone marrow-confined metastasis not yet characterized by bone remodeling and thus falsely negative in both multidetector computed tomography and 18F-sodium positron emission tomography/computed tomography. No structural lesions or area of 18F-NaF uptake are evident in the vertebral column of this breast cancer patient (A); by contrast an area of high focal 18F-FDG uptake was present in the sixth dorsal vertebra (B). 18F-NaF: 18F-sodium; 18F-FDG: 2-deoxy-2-(18F)fluoro-D-glucose.
Figure 2.
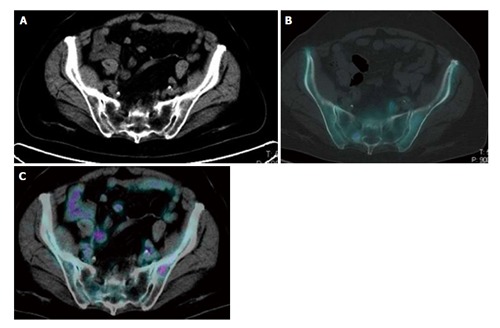
Evidence of the complementary features of 2-deoxy-2-(18F)fluoro-D-glucose and 18F-sodium positron emission tomography/computed tomography in the pelvis of the same breast cancer patient. An 18F-NaF avid sclerotic lesion was detected in the right sacrum in absence of significant 18F-FDG uptake. By contrast high uptake of 18F-FDG was present in a small lytic lesion in the left iliac bone. Due to the absence of a significant local bone reaction, this small lesion did not show any uptake of 18F-NaF. Both lesions corresponded to metastatic sites of disease and disappeared after chemotherapy. 18F-FDG: 2-deoxy-2-(18F)fluoro-D-glucose; 18F-NaF: 18F-sodium; PET/CT: Positron emission tomography/computed tomography.
Impact on patient management
Findings that were found only in 18F-NaF PET/CT imaging led to a change of management for 3 of the 45 patients (6.6%). In particular, two patients underwent chemotherapy rather than targeted radiotherapy due to the detection of further skeletal lesions. One patient was excluded from surgical treatment of lung metastasis due to the presence of bone involvement.
DISCUSSION
In this study, we aimed to elucidate the role of 18F-FDG and 18F-NaF PET/CT in restaging breast cancer patients with bone lesions by means of patient-, density-, and site-based analyses.
Slight, but significant, differences were highlighted between 18F-FDG and 18F-NaF in the patient-based analysis, with the former showing higher specificity and the latter being characterized by higher sensitivity. These differences were more markedly evident at the lesion-based analysis, where 18F-FDG showed higher accuracy for detecting CT-negative (likely bone marrow confined) metastasis and lesions located in the spine, while 18F-NaF PET/CT performed better with respect to osteosclerotic and rib lesions. Our results support the view that, when a functional method is needed, information derived by 18F-FDG can correctly classify most breast cancer patients with suspected or known bone metastasis. However, the lesion-based analysis highlighted significant differences between the two imaging methods, which emphasize the different complementary information provided by the two tracers. In fact, these data fit with the different distribution mechanisms of the two tracers into bone metastases. More specifically, 18F-FDG accumulates into viable, metabolically-active tumor cells[34,35], while 18F-NaF is incorporated into bone crystals within the forming fluorapatite matrix, and thus tends to preferentially accumulate at sites of actively mineralizing bone[36,37]. Osseous metastases seed into the red bone marrow rather than the cortical bone, and this might explain the extremely high accuracy of 18F-FDG PET in detecting metastases confined in the bone marrow, especially at an earlier stage before the occurrence of bone reaction[38,39]. Accordingly, it has been suggested that 18F-FDG PET/CT can be assensitive as magnetic resonance imaging in this setting[40,41]. The relatively poor cellularity that may characterize sclerotic metastases, with relatively smaller volumes of tumor tissue in individual lesions, may influence the degree of 18F-FDG uptake given the small number of elements able to trap it[42].
These findings are thus coherent with the fact that 18F-FDG uptake is more specific for malignant lesions than bone metabolism tracers, while 18F-NaF is characterized by an extremely high sensitivity, rather than specificity, for both sclerotic and lytic lesions[43].Surprisingly, both radiotracers showed high accuracy in the detection of lytic localizations, and no differences were highlighted between the accuracy of 18F-FDG or 18F-NaF in the evaluation of this type of lesion. Previous studies compared the accuracy of 18F-FDG PET/CT and BS with respect to lytic lesions and found that 18F-FDG PET/CT is superior to BS in this setting[42,44]. Accordingly, the present findings support the concept that, although 18F-NaF and BS highlight the same pathophysiological mechanisms (increased osteoblastic activity), the greater spatial resolution of PET accounts for the better diagnostic accuracy of 18F-NaF with respect to BS[24,43,45]. In fact, thanks to its better spatial resolution, this tracer is even capable of capturing the increased mineral metabolism related to the thin reactive border that may surround a lytic lesion. By contrast, this subtle reaction is generally too small to be detected by the limited spatial resolution of BS. However, it must be underlined that the extremely high sensitivity of 18F-NaF PET/CT in the detection of both lytic and sclerotic metastases is paralleled by a relatively low specificity[30].
This behavior might represent an important limitation in the use of 18F-NaF PET and strongly advise in favor of the use of hybrid PET/CT imaging, thus increasing the specificity of 18F-NaF PET thanks to the CT-based characterization of bone remodeling (i.e., exclusion of clearly degenerative lesions)[31].
Significant differences between the two tracers were also found in the site-based analysis. In particular, the 18F-FDG results were more accurate in detecting lesions located in the spine, while 18F-NaF provided a more accurate characterization of rib lesions. This could be related to the different structural modification induced by metastases as a function of their anatomical localization. Small lesions in the ribs can show an intense osteoblastic response, even in the presence of poor cellularity, and can therefore be easily identified by means of 18F-NaF[25]. By contrast, the highlighted superiority of 18F-FDG PET in the evaluation of spine lesions can be explained by the fact that many lesions located in the spine were, in this study, characterized by an absence of structural correlates in the co-registered CT. On the other hand, the age of our patient population (mean 60 years) may have also influenced the low accuracy of 18F-NaF for spine lesions. In fact, the presence of areas of non-specific 18F-NaF uptake due to age-related degenerative changes may partially explain the relatively lower accuracy of 18F-NaF in this site. This finding is in line with the notion that 18F-NaF is more accurate than BS, especially for evaluating vertebral localizations[46]. In fact, thanks to the greater resolution and fusion with CT, 18F-NaF can reduce the number off alse positive/indeterminate findings due to degenerative lesions. Although 18F-FDG can also be influenced by degenerative changes, the intensity and focality of these uptakes are lower with respect to bone metastasis; the glucose analogue is thus superior to both bone metabolism tracers in this setting.
Finally, when the specific influence of 18F-NaF was evaluated with respect to patient management, we found that adding 18F-NaF PET to patient work-up led to a change in management in 3 out of 45 patients, due to it revealing metastases undetected by 18F-FDG scan. These findings may underline that the extremely high sensitivity of 18F-NaF uptake can be useful in evaluating patients who are candidates for regional therapy (i.e., surgery or radiotherapy) with the aim of excluding patients with further occult metastases.
The present study has some limitations. It is a two center, retrospective study whose results may have been influenced by its patient population’s high pre-test probability of bone metastasis. Although the number of included patients was relatively small, it was comparable, or even higher, with respect to similar studies on the impact of different functional imaging techniques in breast cancer patients with bone metastasis[47,48]. Histological confirmation of metastases was not obtained in the majority of patients, for both practical and ethical reasons. A clinical, biochemical, and radiological follow-up of 12 mo was used as the standard of reference. Obviously, 12-mo follow-up findings might not be sufficient to exhaustively depict disease status. However, sclerotic and/or lytic bone lesions on CT are mostly accepted as metastases[49,50].Additionally, in many other studies, clinical biopsy was performed only in a minority of patients and comparative imaging modalities were used as standard in order to assess metastatic bone involvement[51].
In conclusion, 18F-FDG PET/CT is a reliable imaging tool in the detection of bone metastasis in most cases, with a high diagnostic accuracy and superior specificity with respect to 18F-NaF PET/CT in the general population of breast cancer patients. However, the extremely high sensitivity of 18F-NaF PET/CT can exploit its diagnostic potential in specific clinical settings, such as small CT-evident sclerotic lesions, possibly changing patient staging or management. Similarly, given the hereby proven complementary role of the two tracers, breast cancer patients could be candidates for 18F-NaF when, despite negative results in 18F-FDG and other imaging methods, they have suggestive clinical and biochemical sign of disease. Therefore 18F-NaF PET/CT emerges as a powerful “second-line” functional imaging tool, which may be of use in selected patients on the basis of their specific clinical history, in order to identify a priori in those patients in which 18F-NaF PET/CT may significantly impact their management.
COMMENTS
Background
Early detection of bone metastases is of pivotal importance in breast cancer patients. To this purpose, besides conventional bone scintigraphy, positron emission tomography has become an established imaging modality, with better spatial resolution and superior image quality. Among positron emission tomography (PET) tracers, 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG) represents the most widely used tracer in clinical routine, and can provide information about the presence or absence of disease in the skeleton, as well as in non-skeletal districts. However, characterization of bone metastases is also possible with 18F-sodium fluoride (18F-NaF). In this context, it has not yet been clearly investigated whether 18F-NaF PET/computed tomography (CT) can provide incremental information concerning breast cancer patients that have already been evaluated by means of FDG PET/CT.
Research frontiers
To date, controversial results have been reported about the accuracy of the two PET tracers in breast cancer patients,with some authors even proposing their combined use. This work aims to clarify whether, at least in specific conditions, these two tracers could be complementary in order to improve diagnostic accuracy in bone lesion characterization.
Innovations and breakthroughs
This work aims to compare the role of 18F-FDG and 18F-NaF PET/CT in restaging breast cancer patients with bone lesions through patient-, lesion density-, and site-based analyses. A more prompt and accurate characterization of bone alterations could lead to more accurate patient management.
Applications
Besides 18F-FDG, 18F-NaF PET/CT emerges as a powerful “second-line” functional imaging tool, which may be useful in selected patients on the basis of their specific clinical history.
Terminology
Glucose analogue 18F-FDG PET enables the detection of neoplastic lesions on the basis of their increased glucose metabolism directly reflecting tumor cell viability, thereby allowing forthe characterization of skeletal and extra-skeletal lesions. On the other hand, 18F-NaF reflects the increased regional blood flow and osteoblastic bone reaction being irreversibly incorporated into the bone matrix as fluorapatite.
Peer-review
An agreement on which is the best PET tracer in the characterization of bone lesions has not been yet been reached. In this study, the authors compared 18F-FDG and 18F-NaF PET/CT accuracy in the restaging of breast cancer patients. They observed that, despite 18F-FDG PET/CT possibly being considered the most reliable tool in the general population of breast cancer patients, it can exploit its diagnostic potential in specific clinical settings. These results were interesting and provided important information concerning the most appropriate management of breast cancer patients with suspected bone metastases.
Footnotes
Institutional review board statement: The Internal Review Board (Comitato Etico Regionale della Liguria) evaluated and approved this retrospective study.
Informed consent statement: All study participants, or their legal guardians, provided informed written consent prior to study enrollment. We did not report any details that might disclose the identity of the subjects under study.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 31, 2015
First decision: September 18, 2015
Article in press: December 11, 2015
P- Reviewer: Vinh-Hung V S- Editor: Qiu S L- Editor: Rutherford A E- Editor: Liu SQ
References
- 1.Viadana E, Cotter R, Pickren JW, Bross ID. An autopsy study of metastatic sites of breast cancer. Cancer Res. 1973;33:179–181. [PubMed] [Google Scholar]
- 2.Scheid V, Buzdar AU, Smith TL, Hortobagyi GN. Clinical course of breast cancer patients with osseous metastasis treated with combination chemotherapy. Cancer. 1986;58:2589–2593. doi: 10.1002/1097-0142(19861215)58:12<2589::aid-cncr2820581206>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamashita K, Koyama H, Inaji H. Prognostic significance of bone metastasis from breast cancer. Clin Orthop Relat Res. 1995;(312):89–94. [PubMed] [Google Scholar]
- 5.Yamashita K, Ueda T, Komatsubara Y, Koyama H, Inaji H, Yonenobu K, Ono K. Breast cancer with bone-only metastases. Visceral metastases-free rate in relation to anatomic distribution of bone metastases. Cancer. 1991;68:634–637. doi: 10.1002/1097-0142(19910801)68:3<634::aid-cncr2820680332>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Bergh J, Jönsson PE, Glimelius B, Nygren P. A systematic overview of chemotherapy effects in breast cancer. Acta Oncol. 2001;40:253–281. doi: 10.1080/02841860151116349. [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi GN. Overview of treatment results with trastuzumab (Herceptin) in metastatic breast cancer. Semin Oncol. 2001;28:43–47. [PubMed] [Google Scholar]
- 8.Schirrmeister H. Detection of bone metastases in breast cancer by positron emission tomography. Radiol Clin North Am. 2007;45:669–676, vi. doi: 10.1016/j.rcl.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Hillner BE, Siegel BA, Liu D, Shields AF, Gareen IF, Hanna L, Stine SH, Coleman RE. Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: initial results from the National Oncologic PET Registry. J Clin Oncol. 2008;26:2155–2161. doi: 10.1200/JCO.2007.14.5631. [DOI] [PubMed] [Google Scholar]
- 10.Specht JM, Tam SL, Kurland BF, Gralow JR, Livingston RB, Linden HM, Ellis GK, Schubert EK, Dunnwald LK, Mankoff DA. Serial 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) to monitor treatment of bone-dominant metastatic breast cancer predicts time to progression (TTP) Breast Cancer Res Treat. 2007;105:87–94. doi: 10.1007/s10549-006-9435-1. [DOI] [PubMed] [Google Scholar]
- 11.Hsu WK, Virk MS, Feeley BT, Stout DB, Chatziioannou AF, Lieberman JR. Characterization of osteolytic, osteoblastic, and mixed lesions in a prostate cancer mouse model using 18F-FDG and 18F-fluoride PET/CT. J Nucl Med. 2008;49:414–421. doi: 10.2967/jnumed.107.045666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blau M, Nagler W, Bender MA. Fluorine-18: a new isotope for bone scanning. J Nucl Med. 1962;3:332–334. [PubMed] [Google Scholar]
- 13.Narita N, Kato K, Nakagaki H, Ohno N, Kameyama Y, Weatherell JA. Distribution of fluoride concentration in the rat’s bone. Calcif Tissue Int. 1990;46:200–204. doi: 10.1007/BF02555045. [DOI] [PubMed] [Google Scholar]
- 14.Liu T, Xu JY, Xu W, Bai YR, Yan WL, Yang HL. Fluorine-18 deoxyglucose positron emission tomography, magnetic resonance imaging and bone scintigraphy for the diagnosis of bone metastases in patients with lung cancer: which one is the best?--a meta-analysis. Clin Oncol (R Coll Radiol) 2011;23:350–358. doi: 10.1016/j.clon.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Chang MC, Chen JH, Liang JA, Lin CC, Yang KT, Cheng KY, Yeh JJ, Kao CH. Meta-analysis: comparison of F-18 fluorodeoxyglucose-positron emission tomography and bone scintigraphy in the detection of bone metastasis in patients with lung cancer. Acad Radiol. 2012;19:349–357. doi: 10.1016/j.acra.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Liu T, Cheng T, Xu W, Yan WL, Liu J, Yang HL. A meta-analysis of 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with breast cancer. Skeletal Radiol. 2011;40:523–531. doi: 10.1007/s00256-010-0963-8. [DOI] [PubMed] [Google Scholar]
- 17.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–297. [PubMed] [Google Scholar]
- 18.Ben-Haim S, Israel O. Breast cancer: role of SPECT and PET in imaging bone metastases. Semin Nucl Med. 2009;39:408–415. doi: 10.1053/j.semnuclmed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Iagaru A, Mittra E, Dick DW, Gambhir SS. Prospective evaluation of (99m)Tc MDP scintigraphy, (18)F NaF PET/CT, and (18)F FDG PET/CT for detection of skeletal metastases. Mol Imaging Biol. 2012;14:252–259. doi: 10.1007/s11307-011-0486-2. [DOI] [PubMed] [Google Scholar]
- 20.Withofs N, Grayet B, Tancredi T, Rorive A, Mella C, Giacomelli F, Mievis F, Aerts J, Waltregny D, Jerusalem G, et al. 18F-fluoride PET/CT for assessing bone involvement in prostate and breast cancers. Nucl Med Commun. 2011;32:168–176. doi: 10.1097/MNM.0b013e3283412ef5. [DOI] [PubMed] [Google Scholar]
- 21.Krüger S, Buck AK, Mottaghy FM, Hasenkamp E, Pauls S, Schumann C, Wibmer T, Merk T, Hombach V, Reske SN. Detection of bone metastases in patients with lung cancer: 99mTc-MDP planar bone scintigraphy, 18F-fluoride PET or 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:1807–1812. doi: 10.1007/s00259-009-1181-2. [DOI] [PubMed] [Google Scholar]
- 22.Chan SC, Wang HM, Ng SH, Hsu CL, Lin YJ, Lin CY, Liao CT, Yen TC. Utility of 18F-fluoride PET/CT and 18F-FDG PET/CT in the detection of bony metastases in heightened-risk head and neck cancer patients. J Nucl Med. 2012;53:1730–1735. doi: 10.2967/jnumed.112.104893. [DOI] [PubMed] [Google Scholar]
- 23.Hortobagyi GN. Bone metastases in breast cancer patients. Semin Oncol. 1991;18:11–15. [PubMed] [Google Scholar]
- 24.Schirrmeister H, Guhlmann A, Elsner K, Kotzerke J, Glatting G, Rentschler M, Neumaier B, Träger H, Nüssle K, Reske SN. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623–1629. [PubMed] [Google Scholar]
- 25.Langsteger W, Heinisch M, Fogelman I. The role of fluorodeoxyglucose, 18F-dihydroxyphenylalanine, 18F-choline, and 18F-fluoride in bone imaging with emphasis on prostate and breast. Semin Nucl Med. 2006;36:73–92. doi: 10.1053/j.semnuclmed.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Käkönen SM, Mundy GR. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer. 2003;97:834–839. doi: 10.1002/cncr.11132. [DOI] [PubMed] [Google Scholar]
- 27.Iagaru A, Mittra E, Yaghoubi SS, Dick DW, Quon A, Goris ML, Gambhir SS. Novel strategy for a cocktail 18F-fluoride and 18F-FDG PET/CT scan for evaluation of malignancy: results of the pilot-phase study. J Nucl Med. 2009;50:501–505. doi: 10.2967/jnumed.108.058339. [DOI] [PubMed] [Google Scholar]
- 28.Iagaru A, Mittra E, Mosci C, Dick DW, Sathekge M, Prakash V, Iyer V, Lapa P, Isidoro J, de Lima JM, et al. Combined 18F-fluoride and 18F-FDG PET/CT scanning for evaluation of malignancy: results of an international multicenter trial. J Nucl Med. 2013;54:176–183. doi: 10.2967/jnumed.112.108803. [DOI] [PubMed] [Google Scholar]
- 29.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segall G, Delbeke D, Stabin MG, Even-Sapir E, Fair J, Sajdak R, Smith GT. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med. 2010;51:1813–1820. doi: 10.2967/jnumed.110.082263. [DOI] [PubMed] [Google Scholar]
- 31.Piccardo A, Altrinetti V, Bacigalupo L, Puntoni M, Biscaldi E, Gozza A, Cabria M, Iacozzi M, Pasa A, Morbelli S, et al. Detection of metastatic bone lesions in breast cancer patients: fused (18)F-Fluoride-PET/MDCT has higher accuracy than MDCT. Preliminary experience. Eur J Radiol. 2012;81:2632–2638. doi: 10.1016/j.ejrad.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 32.R Development Core Team. The R project for statistical computing. [Accessed: 2013. p. Jan 21]. Available from: http//www.R-project.org. [Google Scholar]
- 33.Brasil P. Diagnosis Med: Diagnostic test accuracy evaluation for medical professionals. [Accessed: 2013. p. Jan 21]. Available from: http//cran.r-project.org/src/contrib/Archive/DiagnosisMed. [Google Scholar]
- 34.Beheshti M, Vali R, Waldenberger P, Fitz F, Nader M, Hammer J, Loidl W, Pirich C, Fogelman I, Langsteger W. The use of F-18 choline PET in the assessment of bone metastases in prostate cancer: correlation with morphological changes on CT. Mol Imaging Biol. 2010;12:98–107. doi: 10.1007/s11307-009-0239-7. [DOI] [PubMed] [Google Scholar]
- 35.Cook GJ. PET and PET/CT imaging of skeletal metastases. Cancer Imaging. 2010;10:1–8. doi: 10.1102/1470-7330.2010.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiepers C, Nuyts J, Bormans G, Dequeker J, Bouillon R, Mortelmans L, Verbruggen A, De Roo M. Fluoride kinetics of the axial skeleton measured in vivo with fluorine-18-fluoride PET. J Nucl Med. 1997;38:1970–1976. [PubMed] [Google Scholar]
- 37.Cook GJ, Fogelman I. The role of positron emission tomography in the management of bone metastases. Cancer. 2000;88:2927–2933. doi: 10.1002/1097-0142(20000615)88:12+<2927::aid-cncr8>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- 38.Basu S, Alavi A. Bone marrow and not bone is the primary site for skeletal metastasis: critical role of [18F]fluorodeoxyglucose positron emission tomography in this setting. J Clin Oncol. 2007;25:1297; author reply 1297–1299. doi: 10.1200/JCO.2006.10.0123. [DOI] [PubMed] [Google Scholar]
- 39.Basu S, Tiwari BP. Complimentary role of FDG-PET imaging and skeletal scintigraphy in the evaluation of patients of prostate carcinoma. Indian J Cancer. 2011;48:513–514. doi: 10.4103/0019-509X.92247. [DOI] [PubMed] [Google Scholar]
- 40.Yang HL, Liu T, Wang XM, Xu Y, Deng SM. Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol. 2011;21:2604–2617. doi: 10.1007/s00330-011-2221-4. [DOI] [PubMed] [Google Scholar]
- 41.Basu S, Torigian D, Alavi A. Evolving concept of imaging bone marrow metastasis in the twenty-first century: critical role of FDG-PET. Eur J Nucl Med Mol Imaging. 2008;35:465–471. doi: 10.1007/s00259-007-0593-0. [DOI] [PubMed] [Google Scholar]
- 42.Abe K, Sasaki M, Kuwabara Y, Koga H, Baba S, Hayashi K, Takahashi N, Honda H. Comparison of 18FDG-PET with 99mTc-HMDP scintigraphy for the detection of bone metastases in patients with breast cancer. Ann Nucl Med. 2005;19:573–579. doi: 10.1007/BF02985050. [DOI] [PubMed] [Google Scholar]
- 43.Schirrmeister H, Guhlmann A, Kotzerke J, Santjohanser C, Kühn T, Kreienberg R, Messer P, Nüssle K, Elsner K, Glatting G, et al. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J Clin Oncol. 1999;17:2381–2389. doi: 10.1200/JCO.1999.17.8.2381. [DOI] [PubMed] [Google Scholar]
- 44.Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998;16:3375–3379. doi: 10.1200/JCO.1998.16.10.3375. [DOI] [PubMed] [Google Scholar]
- 45.Hoegerle S, Juengling F, Otte A, Altehoefer C, Moser EA, Nitzsche EU. Combined FDG and [F-18]fluoride whole-body PET: a feasible two-in-one approach to cancer imaging? Radiology. 1998;209:253–258. doi: 10.1148/radiology.209.1.9769840. [DOI] [PubMed] [Google Scholar]
- 46.Fogelman I, Cook G, Israel O, Van der Wall H. Positron emission tomography and bone metastases. Semin Nucl Med. 2005;35:135–142. doi: 10.1053/j.semnuclmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Uematsu T, Yuen S, Yukisawa S, Aramaki T, Morimoto N, Endo M, Furukawa H, Uchida Y, Watanabe J. Comparison of FDG PET and SPECT for detection of bone metastases in breast cancer. AJR Am J Roentgenol. 2005;184:1266–1273. doi: 10.2214/ajr.184.4.01841266. [DOI] [PubMed] [Google Scholar]
- 48.Damle NA, Bal C, Bandopadhyaya GP, Kumar L, Kumar P, Malhotra A, Lata S. The role of 18F-fluoride PET-CT in the detection of bone metastases in patients with breast, lung and prostate carcinoma: a comparison with FDG PET/CT and 99mTc-MDP bone scan. Jpn J Radiol. 2013;31:262–269. doi: 10.1007/s11604-013-0179-7. [DOI] [PubMed] [Google Scholar]
- 49.Haubold-Reuter BG, Duewell S, Schilcher BR, Marincek B, von Schulthess GK. The value of bone scintigraphy, bone marrow scintigraphy and fast spin-echo magnetic resonance imaging in staging of patients with malignant solid tumours: a prospective study. Eur J Nucl Med. 1993;20:1063–1069. doi: 10.1007/BF00173484. [DOI] [PubMed] [Google Scholar]
- 50.Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]


