Abstract
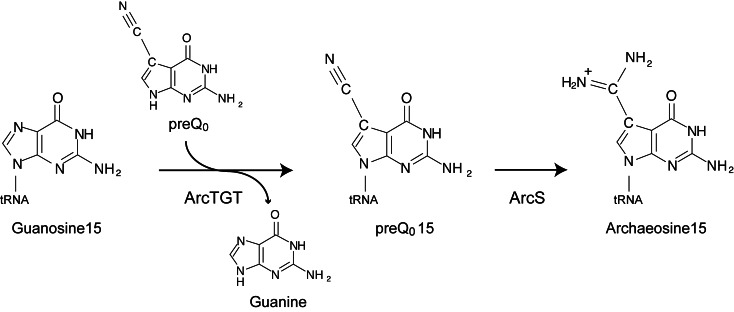
Archaeosine (G+), which is found only at position 15 in many archaeal tRNA, is formed by two steps, the replacement of the guanine base with preQ0 by archaeosine tRNA-guanine transglycosylase (ArcTGT) and the subsequent modification of preQ0 to G+ by archaeosine synthase. However, tRNALeu from Thermoplasma acidophilum, a thermo-acidophilic archaeon, exceptionally has two G+13 and G+15 modifications. In this study, we focused on the biosynthesis mechanism of G+13 and G+15 modifications in this tRNALeu. Purified ArcTGT from Pyrococcus horikoshii, for which the tRNA recognition mechanism and structure were previously characterized, exchanged only the G15 base in a tRNALeu transcript with 14C-guanine. In contrast, T. acidophilum cell extract exchanged both G13 and G15 bases. Because T. acidophilum ArcTGT could not be expressed as a soluble protein in Escherichia coli, we employed an expression system using another thermophilic archaeon, Thermococcus kodakarensis. The arcTGT gene in T. kodakarensis was disrupted, complemented with the T. acidophilum arcTGT gene, and tRNALeu variants were expressed. Mass spectrometry analysis of purified tRNALeu variants revealed the modifications of G+13 and G+15 in the wild-type tRNALeu. Thus, T. acidophilum ArcTGT has a multisite specificity and is responsible for the formation of both G+13 and G+15 modifications.
INTRODUCTION
To date, more than 100 modified nucleosides have been identified in tRNA (1,2). Among them, queosine (Q) and archaeosine (G+) are unique because their structures contain the 7-deazaguanine: Q is [7-(4, 5-cis-dihydroxy-2-cyclopenten-1-yl) amino] methyl-7-deazaguanosine (3), while G+ is 7-formamidino-7-deazaguanosine (2-amino-4, 7-dihydro-4-oxo-7-β-D-ribofuranosyl-1H-pyrro [2, 3-d] pyrimidine-5- carboximidamide) (Figure 1 and ref. 4).
Figure 1.
Archaeosine biosynthesis pathway. The guanine base at position 15 in tRNA is replaced with preQ0 by ArcTGT. The resultant preQ015 is further modified to G+15 by ArcS.
Q and G+ are introduced into tRNA by the base replacement reaction, which is catalyzed by tRNA-guanine transglycosylases (TGT) (5,6). Q and its derivatives have been identified at position 34 in a subset of tRNAs (tRNAAsp, tRNAAsn, tRNAHis and tRNATyr), which have the GUN anticodons, from eubacteria and eukaryotes (1). The introduced Q and its derivatives reinforce the anticodon-codon interaction and prevent the frameshift error (7–9). In contrast, G+ has been identified at position 15 in tRNAs from archaea (1). A bioinformatics study predicted that G+15 stabilizes the L-shaped tRNA structure through reinforcement of the G15-C48 tertiary base pair (10).
In eubacterial tRNAs, eubacterial TGT (QueTGT) replaces the G34 base with 7-aminomethyl-7-deazaguanine (preQ1) (11), and the resultant preQ134 is further modified to Q34 via epoxyqueosine34 by QueA (12) and QueG (13). In eukaryotes, Q base from a salvage system is directly used for the formation of Q34 by eukaryotic TGT (14). In archaea, archaeosine TGT (ArcTGT) exchanges the G15 base by 7-cyano-7-deazaguanine (preQ0) (6,15) and the resultant preQ015 is further modified to G+15 by archaeosine synthase (ArcS) (16–18) (Figure 1).
G+ was first identified at position 15 in tRNAMetm from Thermoplasma acidophilum, a thermo-acidophilic archaeon (19,20) as an unknown modified nucleoside (21) and then found in tRNAs from archaea such as Haloferax volcanii (22), Thermoproteus neutrophilus (23), Sulfolobus acidocaldarius (4) and Haloarcula marismortui (24,25). ArcTGT proteins and their genes have been experimentally identified in several archaea such as H. volcanii (6,26), Methanococcus janaschii (15), Pyrococcus horikoshii (27–29), Pyrococcus furiosus (30), Methanosarcina barkeri (30) and Methanosarcina acetivorans (31), consistent with the wide spread of G+ in archaeal tRNAs.
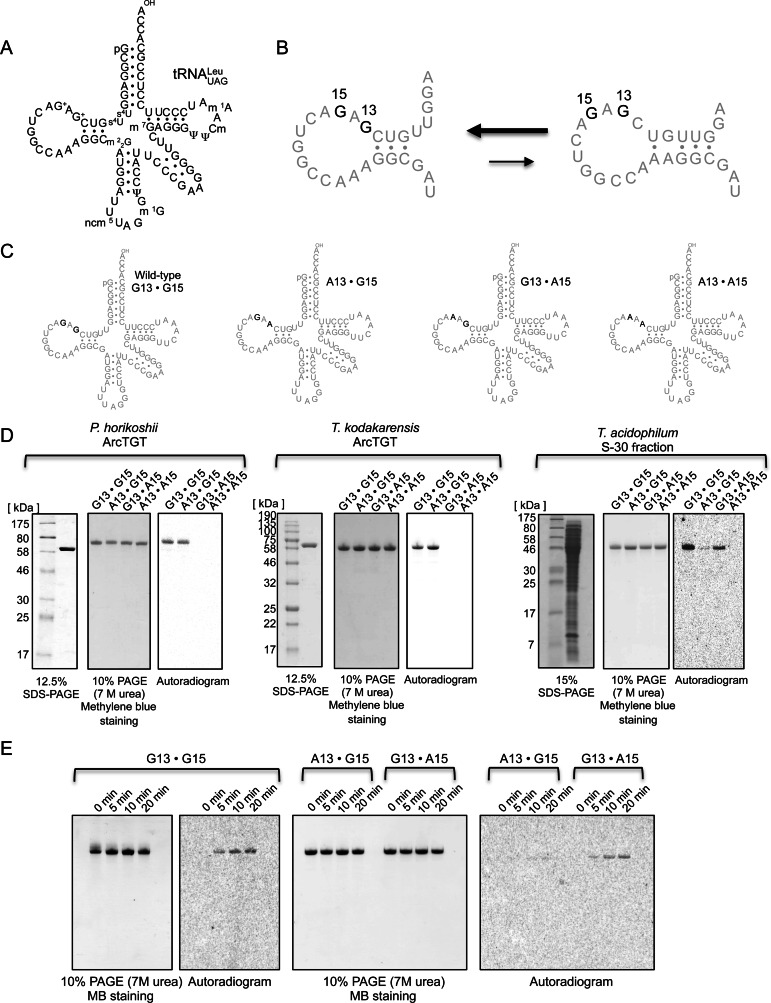
In a recent study, we found that tRNAMeti from T. acidophilum contains G+ modification similar to that of tRNAMetm (32). Furthermore, we found that tRNALeuUAG from T. acidophilum has two G+ modifications at positions 13 and 15 (33) (see Figure 4A). Until now, the G+13 modification system has not been reported. Given that tRNA modification enzymes generally act on only one position in tRNA, the different positions are modified by different enzymes. For example, N2-methylguanine at position 6 (m2G6) in archaeal tRNA is conferred by Trm14 (34), while m2G10 (35) and m2G26 (32,36,37) in archaeal tRNA are formed by Trm-G10 and Trm1, respectively. In contrast, a few tRNA modification enzymes act on multiple sites in tRNA. For example, archaeal TrmI catalyzes N1-methyladenosine modifications at positions 57 and 58 (38). Furthermore, Trm1 from Aquifex aeolicus, a thermophilic eubacterium, brings about N2, N2-dimethylguanosine modifications at positions 26 and 27 (39). Thus, these limited numbers of enzymes have multisite-specificity. In this study, we focused on the biosynthesis of G+13 and G+15 in tRNALeu. Because ArcTGT from T. acidophilum could not be expressed as a soluble protein in Escherichia coli, we employed the genetic manipulation system using another archaeon, Thermococcus kodakarensis. Furthermore, the structural role of G+13 and G+15 modifications in tRNA is discussed.
Figure 4.
In vitro guanine exchanging activities of purified ArcTGT and S-30 fraction from T. acidophilum. (A) Cloverleaf structure of tRNALeuUAG from T. acidophilum. Two G+ (G+13 and G+15) modifications are present in this tRNA. Abbreviations of other modified nucleosides are as follows: 4-thiouridine, s4U; N2, N2-dimethylguanosine, m22G; 5-carbamoylmethyuridine, ncm5U; N1-methylguanosine, m1G; 7-methylguanosine, m7G; pseudouridine, Ψ; 2′-O-methylcytidine, Cm; N1-methyladenosine, m1A. (B) Possibility of structural change in the D-arm of tRNALeu. (C) The cloverleaf structures of wild-type and mutant tRNALeu transcripts. The G13 and/or G15 in the wild-type tRNALeu were substituted by A in mutant tRNALeu transcripts. The nucleosides at positions 13 and 15 are highlighted in black. (D) In vitro guanine exchanging activity of purified ArcTGT and T. acidophilum S-30 fraction. To verify whether the purified ArcTGTs and S-30 fraction from T. acidophilum exchange the guanine base at position 13, ArcTGTs from P. horikoshii (left) and T. kodakarensis (middle) were purified and the S-30 fraction was prepared from T. acidophilum cells (right). The proteins were analyzed by SDS-PAGE. The gels were stained with Coomassie Brilliant Blue. 14C-guanine exchanging activities were tested using these proteins and tRNALeu transcripts. After the reaction, tRNA transcripts were extracted with phenol-chloroform, recovered by ethanol precipitation and separated by 10% PAGE (7 M urea). The RNAs were visualized by staining with methylene blue. Autoradiograms of the gels were acquired. (E) Time-course experiments of 14C-guanine exchanging activity in T. acidophilum S-30 fraction were performed. The samples were taken at 0, 5, 10 and 20 min-periods and loaded onto 10% polyacrylamide gels, which contained 7 M urea. The RNAs were visualized by methylene blue staining and autoradiograms of the gels were acquired.
MATERIALS AND METHODS
Materials
Guanine hydrochloride [8–14C] (2.19 MBq/mmol) was purchased from Moravek Biochemicals (Brea, CA, USA). Hitrap Q-Sepharose and Hitrap Heparin-Sepharose were bought from GE Healthcare (Tokyo, Japan). DNA oligomers were obtained from Invitrogen Japan (Tokyo, Japan). Other chemical reagents were of analytical grade.
Strain, media and culture
The culture source of T. acidophilum strain HO-62 was a gift from Dr Akihiko Yamagishi (Tokyo University of Pharmacy and Life Science) (20). The strain was cultured at 56°C as described previously (32).
Solid-phase DNA probe method for tRNA purification
Total RNA was prepared as described previously (40). The tRNA fraction was further purified by 10% PAGE (7 M urea). Transfer RNACys and tRNALeu were purified from tRNA mixtures by the solid-phase DNA probe method (40,41). The sequences of the DNA probes were complimentary to G15-A36 in tRNACys: 5′- TGC AGT CCC ATG CAT GAC CTC -3′ and A16-G36 in tRNALeu: 5′- CTA AAT CCA TTG CCT TTG GCC AGT - biotin 3′. Because m22G modifications were expected at position 26 in both tRNA, T was used instead of C as the complimentary nucleotide (the T are underlined).
MALDI-MS spectrometry
Desalting of the tRNALeu samples was performed with a ZipTipC18 (Merck Millipore Ltd.). Briefly, RNA solution containing 0.1 A260 units of tRNACys or tRNALeu was aspirated and dispensed through a ZipTipC18. The ZipTipC18 was washed with 20 mM triethylamine acetate (pH 6.9), and the tRNA was eluted with 20 μl acetonitrile. The sample was then dried with a centrifugal evaporator and dissolved in 5 μl water. An aliquot (1.5 μl) of the sample was mixed with 1.5 μl RNase T1 solution [50 mM triethylammonium bicarbonate (pH 7.0) and 4 units/μl RNase T1] or RNase A solution [50 mM triethylammonium bicarbonate (pH 7.0) and 100 μg/ml RNase A (Roche)] and incubated at 37°C for 2 h. The reaction mixture was then incubated at 65°C for 5 min and then further incubated at 37°C for 2 h. After the digestion, 1 μl of the RNA digest was mixed with 1 μl MALDI matrix [20 mg/ml 3-hydroxypicolinic acid and 5 mg/ml diammonium hydrogen citrate in 45% (v/v) acetonitrile, and 0.045% (v/v) trifluoroacetic acid] and the mixture was spotted onto a MALDI plate. The RNA fragments on the plate were analyzed in the positive ion mode using an AXIMA ResonanceTM MALDI-QIT-TOF mass spectrometer system (Shimadzu).
Preparation of T. acidophilum tRNALeu transcript
The transcripts were prepared using T7 RNA polymerase, and purified by Q-Sepharose column chromatography and 10% polyacrylamide gel containing 7 M urea electrophoresis [PAGE (7 M urea)].
Purification of P. horikoshii ArcTGT
ArcTGT from P. horikoshii was expressed in E. coli BL21 (DE3) Rosetta 2 strain (Novagen) and purified as described previously (27).
Cloning, expression and purification of T. kodakarensis ArcTGT
The T. kodakarensis arcTGT (TK0760) gene was amplified by polymerase chain reaction (PCR) from T. kodakarensis genomic DNA using the following primers : TK0760F primer, 5′- GGA GAT ATA CAT ATG GTC GAT TTC AGG TTT GAG GT -3′; TK0760R primer, 5′- GAA TTC GGA TCC TCA TAA CTA CTT CTC GAC TCC CCT CCT A -3′. Underlined regions show restriction enzyme sites (NdeI and BamHI). The PCR product was cloned into the expression vector pET30a (Novagen). The expression of recombination protein in E.coli BL21 (DE3) Rosetta 2 strain was performed according to the manufacture's manual. ArcTGT from T. kodakarensis was purified by heat treatment at 70°C for 30 min, followed by successive rounds of column chromatography through HiTrap Q-Sepharose and HiTrap Heparin-Sepharose. The final eluted sample was dialyzed against buffer A [50 mM Tris-HCl (pH7.6), 600 mM KCl, 10 μM ZnCl2 and 1 mM DTT]. Glycerol was added to the sample to a final concentration of 50% v/v and the sample was stored at –30°C.
Preparation of T. acidophilum S-30 fraction
Thermoplasma acidophilum cell extract was prepared as previously described (42). In brief, frozen cells (0.5 g) were suspended in 5 ml H2O supplemented with 50 μl of an EDTA-free protease inhibitor cocktail (Thermo Scientific), and then collected by centrifugation at 4000 × g at 4ºC for 10 min. The cells were resuspended in 1 ml DNase I buffer [40 mM Tris-HCl (pH7.9), 10 mM NaCl, 6 mM MgCl2 and 1 mM CaCl2] supplemented with 10 μl of an EDTA-free protease inhibitor cocktail, and the pH was then adjusted to 7.5 with 2 M Trizma base. After the pH adjustment, 100 units DNase I (Roche) was added, and the sample was incubated on ice for 1 h. The sample was centrifuged at 30 000 × g at 4ºC for 10 min. The supernatant fraction was used as the supernatant fraction of centrifugation at 30 000 × g (S-30).
Measurement of 14C-guanine base exchanging activity
Guanine exchanging activity was measured as follows: 30 μg of proteins from the S-30 fraction (or 1 μg purified ArcTGT from P. horikoshii or T. kodakarensis), 0.1 A260 units tRNALeu transcript and 1.69 nmol 14C-guanine in 20 μl buffer B [50 mM Tris-HCl (pH7.6), 50 mM KCl, 5 mM MgCl2 and 6 mM 2-mercaptoethanol] were incubated at 55°C for 30 min. The RNA was extracted with phenol-chloroform and then recovered by ethanol precipitation. The RNA pellet was dissolved in 5 μl H2O, and then separated by 10% PAGE (7 M urea). The gel was stained with methylene blue, and dried. The incorporation of 14C-guanine base into the tRNA was monitored with a Typhoon FLA 7000 laser scanner (GE Healthcare).
Genetic manipulations using T. kodakarensis
The outline of genetic manipulations using T. kodakarensis in this study, the construction of T. kodakarensis strain KUWA, the T. kodakarensis ΔarcTGT strain, and Ta1493 gene complimentary (KTA1493) strain, and the expression of tRNALeu in the KTA1493 strain are described in the Supplementary data (Supplementary Figures S1–S8).
Nucleoside analysis
Nucleoside analysis was performed after complete digestion of tRNA with phosphodiesterase, RNase A and bacterial alkaline phosphatase as described previously (43). The elution point of G+ was confirmed by MS analysis.
Preparation of the anti-Ta1493 gene product polyclonal antibody fraction and western blotting analysis
The Ta1493 coding region was amplified by PCR from T. acidophilum genomic DNA using the following primers: Ta1493F 5′- GGA GAT ATA CAT ATG AAG ATA GAG GAA AGG GAC GG -3′; Ta1493R 5′- GAA TTC GGA TCC TCA CTA TTT CTC TGA TTG ATC TCT GCC -3′. Underlines indicate the restriction enzyme sites (NdeI and BamHI). The PCR product was inserted into the linker of pET30a expression vector. The Escherichia coli BL21 (DE3) Rosetta 2 strain was used for the expression. The cells (1 g) were suspended in 10 ml buffer B, and sonicated in an ultrasonic disruptor (model VCX-500, Sonics and Materials Inc, USA) at 4ºC. The cell debris was collected by centrifugation at 6000 x g at 4ºC for 15 min. This precipitate was dissolved in 5 ml buffer B containing 6 M guanidine-HCl. The sample was centrifuged at 16 000 x g at 4ºC for 15 min and the supernatant fraction was diluted by addition of 45 ml buffer B. The diluted sample was centrifuged at 16 000 x g at 4ºC for 15 min, and then the precipitant was dissolved in 6 ml buffer B containing 2 M urea. The sample was centrifuged at 16 000 x g at 4ºC for 15 min and the supernatant fraction was used as the antigen. Customized rabbit anti-Ta1493 gene product serum was prepared by Kitayama Labes (Nagano, Japan). Polyclonal antibody fractions were prepared using an Econo-pac serum IgG purification kit (Bio-Rad). Western blotting analysis was performed as described previously (44). The cell extracts of T. kodakarensis were prepared as follows. The ΔarcTGT and KTA1493 strains were cultured at 60 or 85ºC. When cell densities were reached at 0.7 A660, the cells in 250 μl medium were collected, added 10 μl of 2 × SDS-PAGE loading buffer [100 mM Tris–HCl (pH 6.8), 200 mM dithiothreitol, 2.5% SDS, 0.2% bromophenol blue and 20% glycerol], boiled for 5 min and then used for 15% SDS-PAGE.
RESULTS
The Ta1493 gene product was the only candidate for ArcTGT from T. acidophilum.
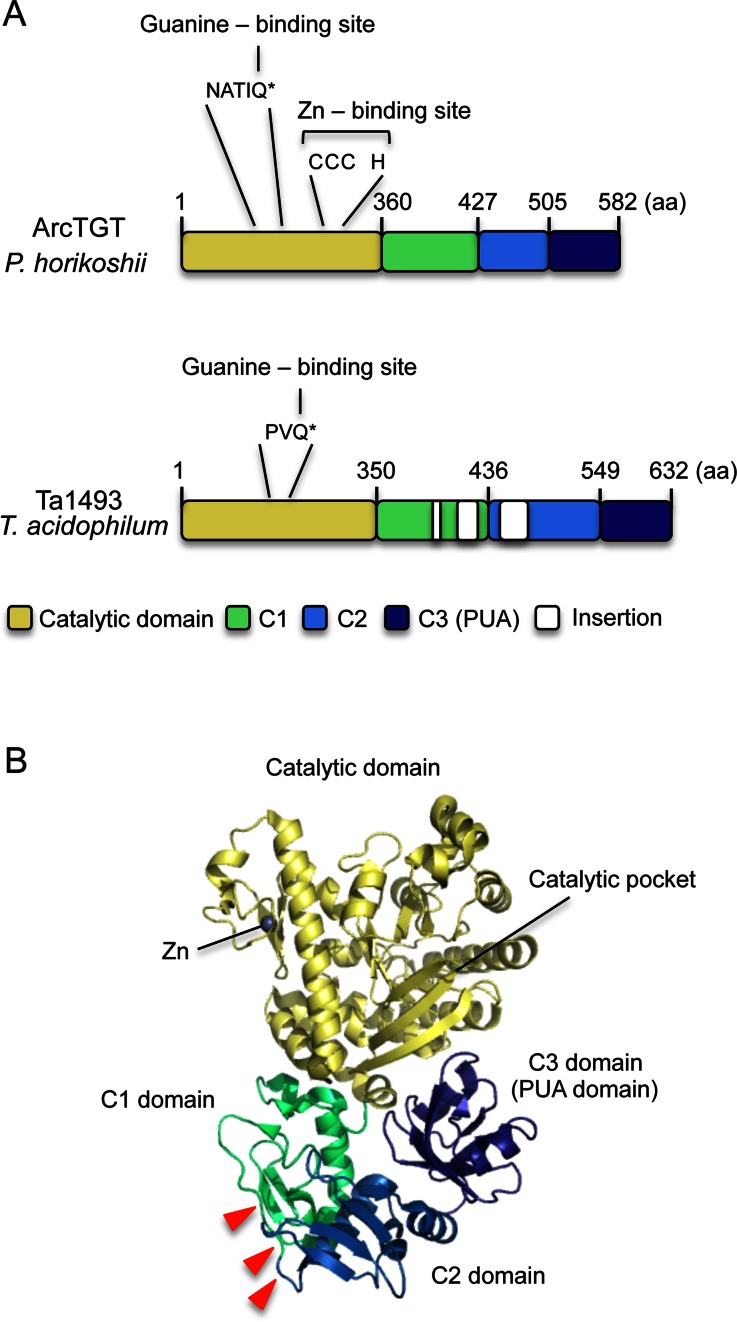
Genome sequencing demonstrated that T. acidophilum genome contains only around 1500 open reading frames (45). Our previous BLAST-search suggested that only one set of genes for ArcTGT (Ta1493) and ArcS (Ta0924) is encoded in the T. acidophilum genome (33). Comparison of the amino acid sequence of Ta1493 gene product with that of ArcTGT from P. horikoshii showed a high sequence similarity between the two proteins except for the insertions in the Ta1493 gene product (Figure 2A). However, the Ta1493 gene product has three insertions in the C1 and C2 domains, and the CXCX2CX22H (CCCH) motif, which binds a Zn atom (Figure 2B and ref. 28), is missing. In addition, the amino acid sequence around the guanine binding site, Gln169, in P. horikoshii ArcTGT is different from that in the Ta1493 gene product (Figure 2A). In the crystal structure of P. horikoshii ArcTGT-tRNA complex, tRNA was captured by three parts, the catalytic domain, the C-terminal region of C2 domain and the C3 (PUA) domain (29). Thus, three insertions in the Ta1493 gene product were predicted not to be located in the tRNA binding sites. Furthermore, split-type ArcTGTs from H. volcanii (26), M. barkeri (30) and M. acetivorans (31) lack a connection region between the C1 and C2 domains. Nevertheless, these split-type ArcTGTs have enzymatic activity (26,30,31). Moreover, the deletion of C3 (PUA) domain in P. furiosus ArcTGT decreased the affinity for tRNA but did not cause the loss of enzymatic activity (30). Therefore, we considered that the three insertions in the C1 and C2 domains in the Ta1493 gene product do not cause the loss of enzymatic activity. In addition, the Zn binding site is located opposite to the catalytic pocket in P. horikoshii ArcTGT (Figure 2B). Thus, the bound Zn is not involved in the catalytic reaction (28). Based on these observations, we considered that the presence of three insertions and absence of the Zn binding site in Ta1493 gene product did not cause the loss of enzymatic activity. Thus, the Ta1493 gene product was a candidate for ArcTGT from T. acidophilum.
Figure 2.
(A) Comparison of the domain structures of P. horikoshii ArcTGT and T. acidophilum Ta1493 gene product. ArcTGT from P. horikoshii is composed of four domains, catalytic, C1, C2 and C3 (PUA) domains. The guanine- and Zn-binding sites in the catalytic domain are highlighted: Q* in the NATIQ sequence binds the G15 base. In contrast, the Ta1493 gene product possesses three insertions in the C1 and C2 domains and the Zn-binding site (CCCH motif) is missing. In addition, the amino acid sequence around the guanine binding site is different from that in P. horikoshii ArcTGT. (B) Structure of P. horikoshii ArcTGT. Three insertion sites are shown by red triangles.
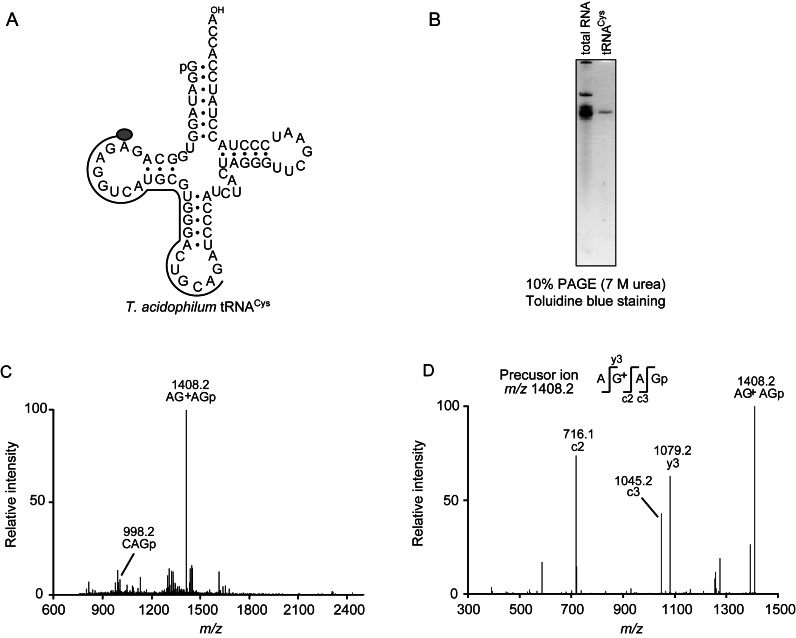
Thermoplasma acidophilum tRNACys possesses unmodified G13 and G+15
The G13 sequence is often observed in tRNALeu, tRNASer and tRNACys not only from archaea, but also from eubacteria and eukaryotes (1). However, the RNA sequences of only three tRNA species (tRNACys, tRNALeuUAA and tRNASerGGA from H. volcanii) apart from T. acidophilum tRNALeu are available in archaeal tRNA and these tRNAs have unmodified G13 (1). In the T. acidophilum tRNAs, tRNACys possesses G13 and G15 in addition to tRNALeu (Figure 3A). We considered that both G13 and G15 in tRNACys may be modified to G+13 and G+15 like tRNALeu in living T. acidophilum cells. To confirm this idea, we purified tRNACys from total RNA by the solid-phase DNA probe method (Figure 3B). In T. acidophilum tRNAs, the amount of tRNACys is considerably low: only 0.12 A260 units tRNACys was purified from 50.0 A260 units T. acidophilum total RNA. This tRNACys was digested with RNase T1 and then analyzed by MS spectrometry. Figure 3C shows the 600–2400 m/z region. In this region, AG+AGp (m/z = 1408.2) could be detected: the sequence was determined by MS/MS analysis (Figure 3D). Thus, tRNACys possessed unmodified G13 and G+15, showing that the G+13 and G+15 modifications is tRNALeu-specific in T. acidophilum cells. Although we focused on the G13 and G15 modifications in this experiment, MS analysis detected Cm32, m1G37, Cm56 and m1A58 modifications in tRNACys (data not shown).
Figure 3.
(A) Cloverleaf structure of T. acidophilum tRNACys. The DNA probe for purification was hybridized with the G15-A36 region in this tRNA. (B) T. acidophilum total RNA (left, 0.3 A260 units) and purified tRNACys (right, 0.015 A260 units) were analyzed by 10% PAGE (7 M urea). The gel was stained with toluidine blue. (C) The purified tRNACys (0.03 A260 units) was digested with RNase T1 and its fragments were analyzed by MS spectrometry. The sequence of fragment (m/z = 1408.2) was determined by MS/MS analysis (D) as AG+AGp.
G+13 formation is not explainable by the structural equilibrium of tRNALeu and the activity of already-known ArcTGTs
To confirm whether Ta1493 is involved in the G+13 formation, we attempted to express the Ta1493 gene product in Escherichia coli. However, the recombinant protein could not be expressed in a soluble form (data not shown). To overcome this problem, we devised an expression system of Ta1493 gene product in another archaeon, Thermococcus kodakarensis.
Before constructing the expression system, we checked whether the G+13 formation was not caused by the structural equilibrium (change) of tRNALeu and the activity of an already-known ArcTGT. Figure 4A shows the cloverleaf structure of tRNALeu from T. acidophilum. If there is a structural equilibrium, in which the location of G13 in the D-loop is changed as shown in Figure 4B, an already-known ArcTGT may catalyze the exchange reaction of G13. Furthermore, the crystal structural study of ArcTGT-tRNA complex revealed that the L-shaped tRNA structure was changed to the λ-form in the complex (29). Therefore, the D-loop structure in tRNALeu might be changed by an already-known ArcTGT during the complex formation. To exclude these possibilities, we performed in vitro guanine base exchanging experiments. Four types of tRNALeu transcripts were prepared (Figure 4C). The wild-type tRNALeu transcript possessed G13 and G15. The G13 and/or G15 were replaced by A in the other mutant tRNALeu transcripts. As shown in Figure 4D, we purified P. horikoshii ArcTGT as an already-known ArcTGT because its tRNA recognition mechanism (27) and structure (28,29) have been well-characterized. Furthermore, T. kodakarensis ArcTGT was also expressed in E. coli and purified (Figure 4D) because the Ta1493 gene product was planned to be expressed in T. kodakarensis cells. We prepared the cell extract (S-30 fraction) from T. acidophilum instead of the Ta1493 gene product (Figure 4D). 14C-guanine incorporation into tRNALeu transcripts was tested using purified ArcTGTs or the T. acidophilum S-30 fraction. As shown in Figure 4D, P. horikoshii ArcTGT exchanged only the guanine base at position 15 with 14C-guanine. Similarly, T. kodakarensis ArcTGT exchanged only the guanine base at position 15. In contrast, T. acidophilum S-30 exchanged guanine bases at both positions 13 and 15. These results clearly showed that the G+13 formation was not explainable by the structural change of tRNALeu and the activity of an already-known ArcTGT. Thus, the guanine base exchanging activity for G13 exists in the T. acidophilum S-30 fraction. The time-course experiments revealed that the T. acidophilum S-30 fraction preferentially exchanged the G13 by 14C-guanine as compared to the G15 (Figure 4E).
Construction of T. kodakarensis arcTGT gene disruption strain
In this study, we developed a new T. kodakarensis strain, KUWA, to construct an arcTGT gene disruption (ΔarcTGT) strain (Supplementary Figure S1). This strain was derived from T. kodakarensis strain KUW1 (46,47). Because the strain KUWA is auxotrophic for uracil, tryptophan, and agmatine, multiple gene selection is possible. At the beginning of this study, we assumed that unknown factor(s) for structural change of tRNALeu in the S-30 fraction and ArcS might be required for the G+13 modification in addition to ArcTGT. Therefore, we constructed the strain KUWA for multiple gene selection. In this study, we used the auxotrophy for uracil to construct the ΔarcTGT (Supplementary Figure S2) and Ta1493 gene complimentary (TKA1493) (Supplementary Figure S3) strains, and auxotrophy for agmatine to supply tRNALeu genes by plasmid vectors. Details are available in the Supplementary information.
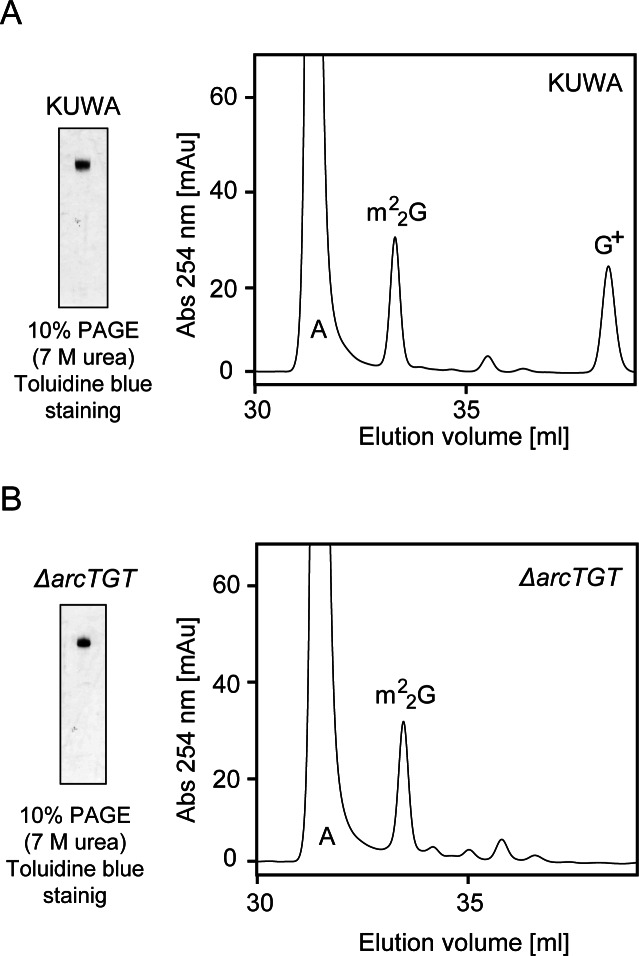
The arcTGT (Tk0760) gene in the genome of T. kodakarensis strain KUWA was disrupted by the method described in the Supplementary information. We successfully isolated candidate clones and their DNA sequences in the recombinant regions were verified (data not shown). As shown in Figure 5A, nucleosides derived from tRNA mixture of T. kodakarensis strain KUWA contained G+. In contrast, the peak of G+ disappeared in the sample from the ΔarcTGT strain (Figure 5B). Taking these results together, we concluded that the ΔarcTGT strain was successfully constructed.
Figure 5.
Construction of the T. kodakarensis ΔarcTGT strain. (A) Nucleoside analysis of tRNA mixtures from the KUWA strain. The tRNA fraction (0.2 A260 units) from KUWA strain was analyzed by 10% PAGE (7 M urea) (left panel). The gels were stained with toluidine blue. The tRNA mixtures were digested to nucleosides and then analyzed by HPLC C18-reverse-phase column chromatography (right panel). (B) Modified nucleosides in the tRNA fraction from the ΔarcTGT strain were analyzed by the same method.
Complementation of ΔarcTGT strain with the Ta1493 gene
Next, we constructed the strain complementary to the ΔarcTGT strain expressing the Ta1493 gene (KTA1493 strain) (Supplementary Figure S2). The expression of Ta1493 gene in the KTA1493 strain was verified by western blotting analysis (Figure 6A). We prepared a rabbit anti-Ta1493 gene product polyclonal antibody. The precipitated Ta1493 gene product in E. coli cells was dissolved in 6 M guanidine-HCl and then used as the antigen. When the KTA1493 strain was cultured at 60ºC, the band corresponding to the Ta1493 gene product was clearly observed (Figure 6A right panel). In contrast, this band was not observed in the sample from the ΔarcTGT strain, demonstrating that this band was derived from the complimented Ta1493 gene. When the KTA1493 and ΔarcTGT strains were cultured at 85ºC, the band disappeared, suggesting that the Ta1493 gene product was denatured and degraded at 85ºC. This result is in line with the fact that T. acidophilum grows optimally at 56ºC (20).
Figure 6.
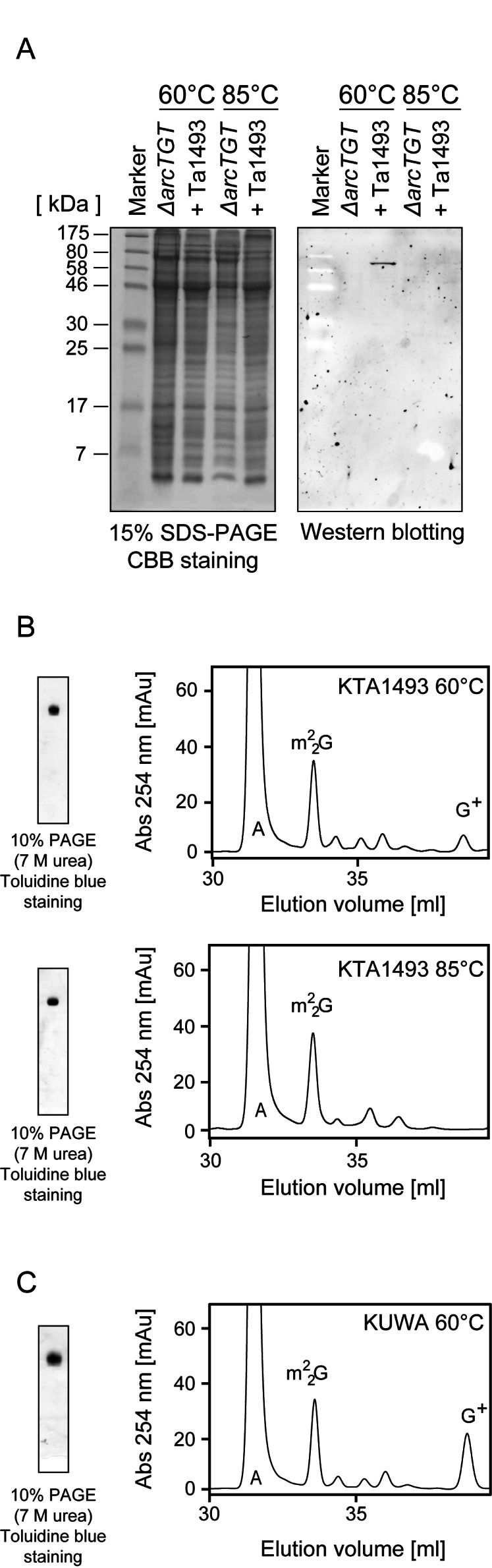
Construction of the KTA1493 strain. (A) Western blotting analysis. The expression of Ta1493 gene product was assessed by western blotting analysis. Proteins from the ΔarcTGT and KTA1493 strains cultured at 60 or 85°C were separated by 15% SDS-PAGE (left) and western blotting analysis was performed (right). (B) Nucleoside analysis in tRNA fractions from the KTA1493 strain, which was cultured at 60ºC (upper panels) and 85ºC (lower panels). 0.2 A260 units of tRNA fractions were analyzed by 10% PAGE (7 M urea) (left panels). The gels were stained with toluidine blue. Modified nucleosides in tRNA fractions were analyzed (right panels). (C) Nucleoside analysis in tRNA fraction from the KUWA strain, which was cultured at 60ºC. 0.2 A260 units of tRNA fractions were analyzed by 10% PAGE (7 M urea) (left panels). The gels were stained with toluidine blue. Modified nucleosides in the tRNA fraction were analyzed (right panel).
The nucleoside analysis of tRNA mixture from the KTA1493 strain cultured at 60ºC revealed the presence of G+ (Figure 6B upper panel). In contrast, the peak of G+ disappeared in the sample from the KTA1493 strain cultured at 85ºC (Figure 6B lower panel), consistent with the results of western blotting analysis. Thus, these results showed that the Ta1493 gene product was expressed in the T. kodakarensis ΔarcTGT strain at 60ºC. It is also clear that the Ta1493 gene product is ArcTGT from T. acidophilum. Furthermore, a fragment containing preQ0 was not detected (data not shown). Therefore, the base exchanging reaction by T. acidophilum ArcTGT seemed to be the rate-limiting step of G+13 and G15+ formations. It should be mentioned that the activity of T. acidophilum ArcTGT in the KTA1493 strain was considerably weak as compared to the activity of T. kodakarensis ArcTGT in the KUWA (wild-type) strain (Figure 6C). Therefore, in the KTA1493 strain, tRNAs are not fully modified by introduced T. acidophilum ArcTGT.
Analysis of the wild-type and mutant tRNALeu expressed in the T. kodakarensis Ta1493 complementary strain suggested that G13 was modified to G+13 by T. acidophilum ArcTGT
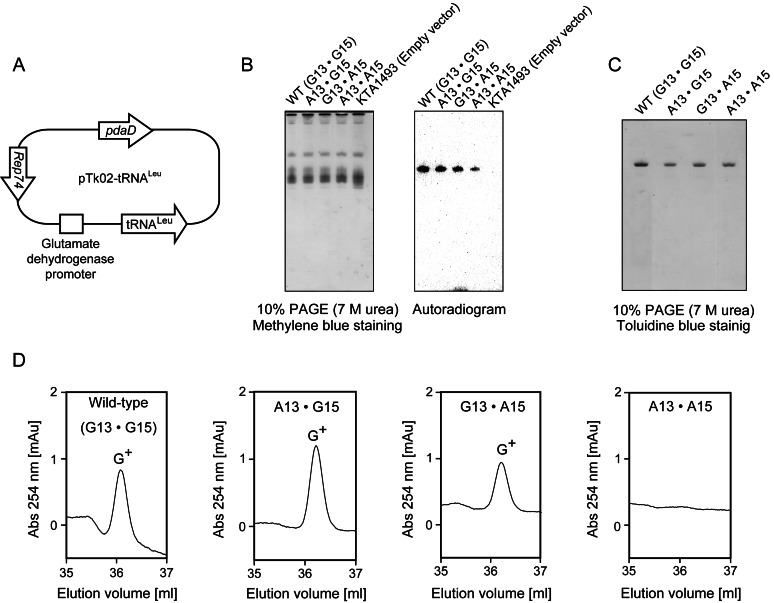
To verify whether T. acidophilum ArcTGT (Ta1493 gene product) was specific toward G13 and G15, we expressed T. acidophilum wild-type and mutant tRNALeu (Figure 4C) using plasmids in the KTA1493 strain at 60ºC: tRNALeu was constitutively expressed under the glutamate dehydrogenase promoter (Figure 7A). The expression of these tRNA was assessed by northern hybridization (Figure 7B). The probe sequence was complimentary to G36-A16 in the wild-type tRNALeu. Fortunately, this probe did not hybridize with tRNAs from T. kodakarensis strain KTA1493 (with empty vector), but hybridized with the expressed tRNALeu (Figure 7B right panel). Thus, T. acidophilum wild-type and mutant tRNALeu were successfully expressed in the KTA1493 strain. The expressed tRNALeu variants were purified by the solid-phase DNA probe method (40,41) as shown in Figure 7C. The nucleoside analysis revealed that G+ was formed in the wild-type, the A13G15 mutant, and the G13A15 mutant tRNALeu (Figure 7D). In contrast, G+ was not formed in the A13A15 mutant tRNALeu (Figure 7D). These results strongly suggested that G+ was formed at both positions 13 and 15.
Figure 7.
G+ formation in the wild-type and mutant tRNALeu expressed in the KTA1493 strain. (A) Schematic representation of the wild-type and mutant tRNALeu expression system. Plasmid vectors, expressing the wild-type or mutant tRNALeu gene were constructed. The wild-type and mutant tRNALeu were constitutively expressed under the glutamate dehydrogenase promoter. The transformants were selected by culture in medium without agmatine, which is synthesized by the pdaD gene product (pyruvoyl-dependent arginine decarboxylase). The Rep74 region is the plasmid origin for maintenance in T. kodakarensis cells. (B) Northern blotting analysis. Transfer RNA mixtures (0.2 A260 units each) from the KTA1493 strain expressing plasmids were separated by 10% PAGE (7 M urea) (left). The tRNA mixture from the T. kodakarensis KTA1493 strain with empty vector was prepared as a negative control (right side). The gel was stained with methylene blue. Northern blotting analysis was performed with a 32P-labeled DNA probe, which was complimentary to G36-A16 in the wild-type tRNALeu. (C) The wild-type and mutant tRNALeu were purified by the solid-phase DNA probe method. The probe sequence was the same as the probe used for northern blotting analysis. 0.04 A260 units of purified tRNAs were analyzed by 10% PAGE (7 M urea). The gel was stained with toluidine blue. (D) G+ formation in the purified tRNALeu. The purified wild-type and mutant tRNALeu were digested to nucleosides, and G+ formation in the samples was analyzed.
Mass spectrometry analysis revealed that G+ was formed at both positions 13 and 15 in the wild-type tRNALeu
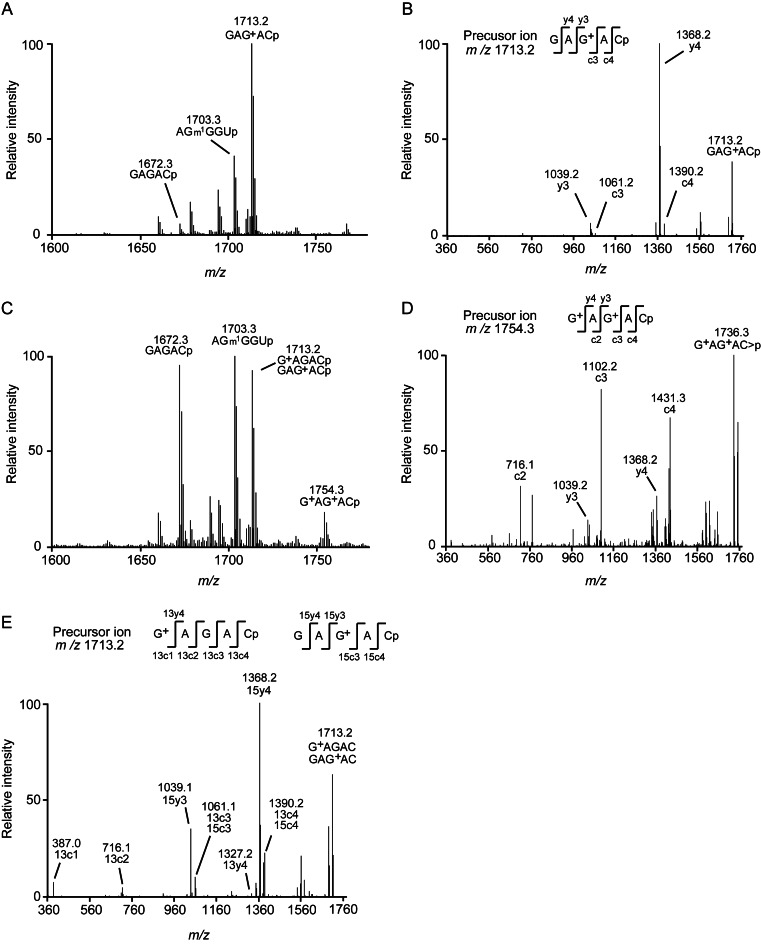
The position(s) of G+ in the wild-type tRNALeu expressed in the KTA1493 strain was determined by MALDI-MS analysis. In this experiment, we prepared the wild-type tRNALeu expressed in the T. kodakarensis strain, KUWA, as a control. This control tRNALeu was purified, digested with RNase A, and its fragments were analyzed. Figure 8A shows the 1600–1800 m/z value region. In this region, a GAG+ACp fragment (m/z = 1713.2) corresponding to G13-C17 in tRNALeu could be detected: the sequence was determined by the MS/MS analysis of this fragment (Figure 8B). Furthermore, MS/MS analysis revealed other fragments (m/z = 1672.3 and 1703.3) in Figure 8A were derived from the unmodified G13-C17 fragment and anticodon-loop, respectively (data not shown). Thus, G+ was formed only at position 15 in the wild-type tRNALeu expressed in the T. kodakarensis strain, KUWA, consistent with the result of the in vitro guanine exchanging activity of T. kodakarensis ArcTGT (Figure 4D).
Figure 8.
MALDI-MS analysis of the wild-type tRNALeu. (A) The wild-type tRNALeu was expressed in T. kodakarensis strain KUWA and purified as a control. The purified tRNALeu was digested with RNaseA and its fragments were then analyzed by MALDI-MS spectrometry. In this region, a fragment (m/z = 1713.2), which coincided with the expected m/z value of the G13-C17 fragment (GAG+ACp) in the wild-type tRNALeu, was detected. (B) The fragment sequence (m/z = 1713.2) was determined by MS/MS analysis as GAG+ACp. (C) The RNaseA-digested fragments derived from the wild-type tRNALeu expressed in the KTA1493 strain were analyzed by the same method as (A). In the 1600–1800 m/z region, a new fragment (m/z = 1754.3) appeared. (D) The sequence of this fragment (m/z = 1754.3) was determined as G+AG+ACp, which corresponded to the G+13-C17 fragment in the wild-type tRNALeu. (E) Two fragments (G+AGACp and GAG+ACp; m/z = 1713.2) were detected by MS/MS analysis.
In contrast, when the wild-type tRNALeu was expressed in the KTA1493 strain, a new fragment (m/z = 1754.3) appeared in this region (Figure 8C). MS/MS analysis revealed that this fragment was G+AG+ACp corresponding to G+13-C17 in tRNALeu (Figure 8D). Thus, G+ was formed at both positions 13 and 15 in the wild-type tRNALeu. Taking these experimental results together, we concluded that T. acidophilum ArcTGT (Ta1493 gene product) has a multisite specificity and is responsible for the formation of both G+13 and G+15 in tRNALeu. Furthermore, G+AGACp and GAG+ACp (m/z = 1713.2) appeared in addition to G+AG+ACp in the wild-type tRNALeu expressed in the KTA1493 strain (Figure 8E), demonstrating that there is no order in G13 and G15 modification by the expressed T. acidophilum ArcTGT in T. kodakarensis cells. This is in line with the time-course experiments by the T. acidophilum S-30 fraction: the S-30 fraction exchanged the G13 and G15 in T. acidophilum tRNALeu transcript by 14C-guanine independently although the speed of exchanging of G13 was faster than that of G15 (Figure 4E). Moreover, two tRNALeu species from T. kodakarensis possess G13 and G15 (Supplementary Figure S9A). The difference of these two tRNALeu species is only one position (the first letter of anticodon). Although the DNA probe for purification was designed to be complimentary to tRNALeuUAG (Supplementary Figure S9A), the separation of these tRNALeu species was difficult. Therefore, we purified two tRNALeu species as the mixture (Supplementary Figure S9B). MS analysis revealed that the positions 13 and 15 in tRNALeu species were identified as unmodified G13 and G15 (Supplementary Figure S9C and D). This phenomenon is probably caused by the weak activity of T. acidophilum ArcTGT in the T. kodakarensis cells. Given that the formation of G+ was observed in the tRNA mixture (Figure 6B), the other tRNA species seemed to be modified preferentially. This result showed that T. acidophilum tRNALeu is a very good substrate for T. acidophilum ArcTGT. The sequence (architecture) of T. acidophilum tRNALeu is required for the multisite specificity of T. acidophilum ArcTGT.
DISCUSSION
In this study, we demonstrated that ArcTGT from T. acidophilum possesses specificity not only for G15, but also for G13 T. acidophilum tRNALeu. This multisite specificity brings two G+ modifications, G+13 and G+15, in T. acidophilum tRNALeu.
The ArcTGT from T. acidophilum possesses three insertions and does not have the Zn-binding site as compared to the P. horikoshii ArcTGT. Given that many variations exist in the C1 and C2 domains of ArcTGTs, the insertions in T. acidophilum ArcTGT do not seem to be directly related to its multisite-specificity. The Zn-binding site is missing in some ArcTGTs. For example, ArcTGT from Ferroplasma acidarmanus does not have the CCCH motif, the Zn-binding site (48). Although T. acidophilum and F. acidarmanus are acidophilic archaea, the absence of Zn-binding site is not explainable by the availability of Zn under the acidic environment: the solubility of ZnSO4 is very high (540 g/1 L water at 20ºC). Indeed, ArcTGT from S. acidocaldarius, an acidophilic archaeon, has the CCCH motif (49). Therefore, the absence of Zn-binding site is not specific for ArcTGTs from acidophilic archaea.
The C3 (PUA) domain deletion mutant of P. furiosus ArcTGT precisely recognizes the target guanine at position 15 (30). ArcTGT from P. horikoshii recognizes the ribose-phosphate backbone in the D-arm and aminoacyl-stem (27). These studies suggest that the interaction between the catalytic domain and ribose-phosphate backbone in tRNA determines the specificity of ArcTGTs. In the current study, we confirmed that P. horikoshii and T. kodakarensis ArcTGTs act only on the G15 base (Figure 3D). In the complex of P. horikoshii ArcTGT and tRNA, the ribose at position 13, the phosphate between positions 12 and 13, and the phosphate between positions 13 and 14 are captured by Glu202, Tyr204 and Arg261 residues, respectively (29). These amino acid residues are highly conserved in ArcTGTs, including T. acidophilum ArcTGT. However, amino acid sequences around the catalytic pockets in ArcTGTs are considerably different from each other (Figure 2). This difference around the catalytic pocket may be involved in the multisite-specificity of T. acidophilum ArcTGT. Several ArcTGTs (for example, F. acidarmanus ArcTGT) share homology with T. acidophilum ArcTGT in this region (48). Given that the G13 sequence is widely observed in tRNALeu, tRNASer and tRNACys (1), the G+13 modification may exist in tRNAs from several archaea in addition to T. acidophilum. ArcTGT recognizes the ribose-phosphate backbone in the D-arm and aminoacyl-stem (27) and changes the tRNA structure during the formation of tRNA-ArcTGT complex (29). In this study, we found that the sequence of T. acidophilum tRNALeu is required for the G+13 formation. In the case of multisite specific Trm1 (39), the distance between the catalytic center and tRNA binding site is longer than that of single site specific Trm1 (50,51). This sequence and rule mechanism may be applicable for the tRNA recognition mechanism of T. acidophilum ArcTGT. Further studies are required to clarify this point.
It should be mentioned that the substrate tRNA recognition mechanism of ArcTGT is completely different from that of QueTGT. QueTGT recognizes the U33G34U35 sequence in the anticodon-loop (52) and the T-arm structure prevents incorrect G53 recognition (53). This tRNA recognition mechanism by QueTGT confers the Q34 modification in specific tRNAs (tRNAHis, tRNAAsn, tRNATyr and tRNAAsp). In contrast, ArcTGT recognizes the ribose-phosphate backbone in the D-arm and aminoacyl-stem (27). This tRNA recognition mechanism by ArcTGT brings the broad substrate tRNA specificity: sixteen of the 33 sequenced tRNAs from H. volcanii possess G+15 modifications (22). QueTGT from Shigella flexneri acts on the mRNA of virulence gene (virF) in addition to tRNA (54,55). Because ArcTGTs have broader substrate specificity than QueTGTs, ArcTGTs may act on RNA(s) other than tRNA.
Because T. acidophilum proteome analysis indicated that many proteins form large protein complexes (56), T. acidophilum ArcTGT may interact with other proteins in living cells. Indeed, we attempted the purification of 6 x His-tag T. acidophilum ArcTGT expressed in the T. kodakarensis ΔarcTGT strain. However, the purified 6 x His-tag T. acidophilum ArcTGT was precipitated during the dialysis (data not shown). To maintain the solubility of T. acidophilum ArcTGT, the interaction with other proteins may be required. The insertions in the C1 and C2 domains might be involved in this interaction. To clarify whether interaction(s) of ArcTGT with other proteins exist in T. acidophilum cells, further study is required. However, it is clear that T. acidophilum ArcTGT possesses a multisite specificity because the introduction of T. acidophilum Ta1493 gene into the T. kodakarensis ΔarcTGT strain caused the formation of both G+13 and G+15.
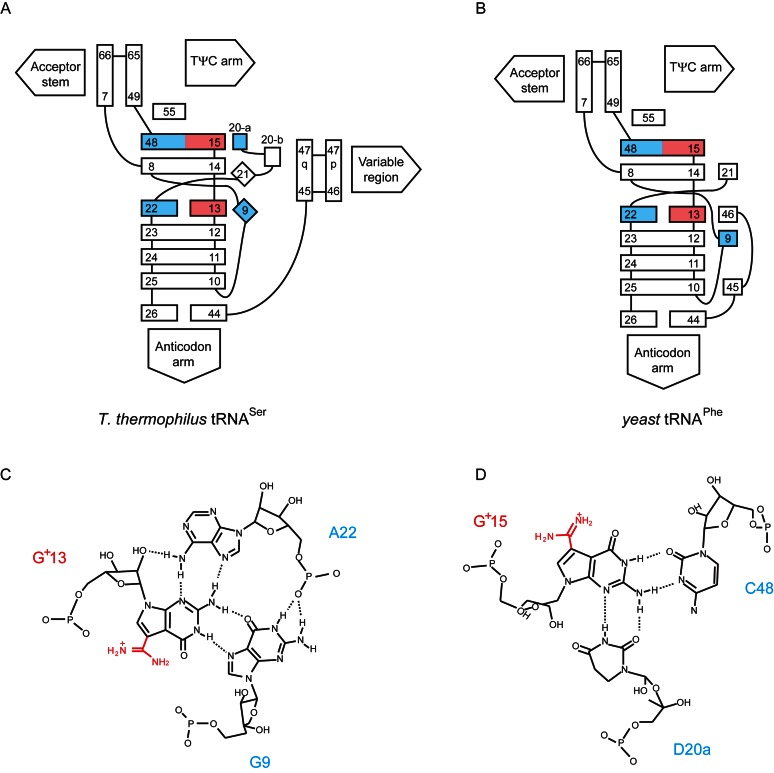
In the L-shaped structure of yeast tRNAPhe, G15 forms a tertiary base pair with C48 and the N7-atom in the G15 constitutes one of the Mg2+ binding sites (57–59). The replacement of guanine base at position 15 by deazaguanine in G+ may abolish the Mg2+ binding site. A bioinformatics study predicted that this replacement gives the positive charge of G+15 and results in the reinforcement of the G+15-C48 tertiary base pair (10). Because tRNALeu and tRNASer have a long variable region (1), the interaction between the D-arm and variable region is different from that in yeast tRNAPhe (Figure 9A and B). The crystal structure of tRNALeu, which contains G13 and G15, has not been reported. However, fortunately, the crystal structure of T. thermophilus tRNASer, which contains G13 and G15, has been reported (Figure 9A and ref. 60): G13 interacts with A22 and G9 (Figure 9C) and G15 forms tertiary base pairs with C48 and D20a (Figure 9D). G9 and D20a in tRNASer are replaced by s4U9 and A20, respectively, in T. acidophilum tRNALeu. Therefore, the accurate interactions of G+13-G9 and G+15-A20 are unknown. However the location of 7-formamidino groups in G+13 and G+15 (indicated in red in Figure 9C and D) can be predicted from the G13-A22 and G15-C48 tertiary base pairs in tRNASer. As shown in Figure 9C and D, introduced 7-formamidino groups in G+13 and G+15 do not cause steric hindrance with tertiary base pairs and may interact with phosphate groups. Therefore, G+13 and G+15 modifications in T. acidophilum tRNALeu do not disrupt the tertiary base pairs and may reinforce the stacking among G+15-C48-A20, A14-s4U8-A21 and G+13-A22-s4U9 tertiary base pairs.
Figure 9.
Predicted tertiary interactions of G+13 and G+15 in tRNALeu. Architecture of the three-dimensional cores of T. thermophilus tRNASer (A) and yeast tRNAPhe (B) are compared. These presentations of tRNA architectures are based on the reference (58). The nucleotides at positions 13 and 15 and at positions 9, 20, 22 and 48 are highlighted in red and cyan, respectively. 7-Formamidino groups (indicated in red) in G+13 (C) and G+15 (D) are manually placed onto the tertiary base pairs in T. thermophilus tRNASer.
In this study, we focused on the multisite-specificity of T. acidophilum ArcTGT. Therefore, the phenotype of T. kodakarensis ΔarcTGT strain was not investigated in details. Under the tested conditions, no growth delay was observed. This growth phenotype is in line with the phenotype of H. volcanii ΔarcTGT strain (26). Therefore, the other modifications in tRNA seem to compensate for the stability of tRNA in the ΔarcTGT strain.
This study revealed that at least T. kodakarensis ArcS acts on both positions 13 and 15. Although the tRNA recognition mechanism by ArcS has not been reported, our current study suggests that ArcS possesses a relatively broad site-specificity. ArcS is composed of four domains, the N-terminal Zn-binding, catalytic, C2 and C3 (PUA) domains (16). The obvious differences between ArcS and ArcTGT are the location of the catalytic domain and the size of the N-terminal domain: the C1 domain in ArcTGT is replaced by the catalytic domain in ArcS and the N-terminal 70–130 amino acid residues in ArcTGT is missing in ArcS. Several conserved residues such as Asp95, Ser96 and Phe99 in the ArcTGT (the numbering is based on P. horikoshii ArcTGT), which interact with the D-loop in tRNA (29), are missing in ArcS. Therefore, the absence of N-terminal region may cause the broad site-specificity of ArcS. To clarify this, studies focusing on ArcS are necessary.
In this study, we developed the ArcTGT expression system in archaea instead of E. coli. Recently, several genetic manipulation systems in archaea have been developed (26,46,47,61–65). Indeed, the arcTGT gene in H. volcanii was experimentally confirmed by using a gene disruption system (26). However, a genetic manipulation system in T. acidophilum has not been reported. Therefore, we used the T. kodakarensis for this study. Thermococcus kodakarensis, a hyperthermophilic archaeon, was isolated from a solfatara on Kodakara Island, Japan (66). The complete genome sequence was determined (67) and several genetic manipulation systems have been devised (46,47,61,64,65). One of advantages of T. kodakarensis genetic manipulation system is that multiple genes can be deleted or altered in the genome. At the beginning of this study, we assumed that T. acidophilum ArcS might be required for the G+13 modification in addition to T. acidophilum ArcTGT. Therefore, we developed the KUWA strain, in which three nutrient markers are available. These markers can also be used for the introduction of a plasmid vector. Indeed, tRNALeu genes were supplied by plasmid vectors in this study. Although tRNA modifications and tRNA modification enzymes from T. kodakarensis have not been reported, the genetic manipulation system of this archaeon can be utilized for studies on many proteins beyond tRNA modification enzymes.
Supplementary Material
Acknowledgments
The authors thank Dr Akihiko Yamagishi (Tokyo University of Pharmacy and Life Science), Dr Tamotsu Kanai (Kyoto University) and Dr Shinsuke Fujiwara (Kwansei-Gakuin University) for providing T. acidophilum strain HO-62, T. kodakarensis strain KUW1, and a plasmid vector, pTK02, respectively. We also thank Dr Chie Tomikawa (Ehime University), a previous member in our laboratory, for valuable discussion.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Funding for open access charge: Grant-in-Aid for Scientific Research [23350081 to H.H., 24770125 to A.H, 23570208 to T. Y.] from the Japan Society for the Promotion of Science.
Conflict of interest statement. None declared.
REFERENCES
- 1.Jühling F., Mörl M., Hartmann R.K., Sprinzl M., Stadler P.F., Pütz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–D162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machnicka M.A., Milanowska K., Osman Oglou O., Purta E., Kurkowska M., Olchowik A., Januszewski W., Kalinowski S., Dunin-Horkawicz S., Rother K.M., et al. MODOMICS: a database of RNA modification pathways–2013 update. Nucleic Acids Res. 2013;41:D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasai H., Oashi Z., Harada F., Nishimura S., Oppenheimer N.J., Crain P.F., Liehr J.G., von Minden D.L., McCloskey J.A. Structure of the modified nucleoside Q isolated from Escherichia coli transfer ribonucleic acid. 7-(4,5-cis-Dihydroxy-1-cyclopenten-3-ylaminomethyl)-7-deazaguanosine. Biochemistry. 1975;14:4198–4208. doi: 10.1021/bi00690a008. [DOI] [PubMed] [Google Scholar]
- 4.Gregson J.M., Crain P.F., Edmonds C.G., Gupta R., Hashizume T., Phillipson D.W., McCloskey J.A. Structure of the archaeal transfer RNA nucleoside G*-15 (2-amino-4,7-dihydro-4-oxo-7-beta-D-ribofuranosyl-1H-pyrrolo[2,3-d]pyrimidine-5-carboximidamide (archaeosine)) J. Biol. Chem. 1993;268:10076–10086. [PubMed] [Google Scholar]
- 5.Okada N., Nishimura S. Isolation and characterization of a guanine insertion enzyme, a specific tRNA transglycosylase, from Escherichia coli. J. Biol. Chem. 1979;254:3061–3066. [PubMed] [Google Scholar]
- 6.Watanabe M., Matsuo M., Tanaka S., Akimoto H., Asahi S., Nishimura S., Katze J.R., Hashizume T., Crain P.F., McCloskey J.A., et al. Biosynthesis of archaeosine, a novel derivative of 7-deazaguanosine specific to archaeal tRNA, proceeds via a pathway involving base replacement on the tRNA polynucleotide chain. J. Biol. Chem. 1997;272:20146–20151. doi: 10.1074/jbc.272.32.20146. [DOI] [PubMed] [Google Scholar]
- 7.Urbonavicius J., Qian Q., Durand J.M., Hagervall T.G., Björk G.R. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 2001;20:4863–4873. doi: 10.1093/emboj/20.17.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson B.A., Kwon S.Y., Chamorro M., Oroszlan S., Hatfield D.L., Lee B.J. Transfer RNA modification status influences retroviral ribosomal frameshifting. Virology. 1999;255:2–8. doi: 10.1006/viro.1998.9569. [DOI] [PubMed] [Google Scholar]
- 9.Zaborske J.M., DuMont V.L., Wallace E.W., Pan T., Aquadro C.F., Drummond D.A. A nutrient-driven tRNA modification alters translational fidelity and genome-wide protein coding across an animal genus. PLoS Biol. 2014;12:e1002015. doi: 10.1371/journal.pbio.1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliva R., Tramontano A., Cavallo L. Mg2+ binding and archaeosine modification stabilize the G15 C48 Levitt base pair in tRNAs. RNA. 2007;13:1427–1436. doi: 10.1261/rna.574407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okada N., Noguchi S., Nishimura S., Ohgi T., Goto T., Crain P.F., McCloskey J.A. Structure determination of a nucleoside Q precursor isolated from E. coli tRNA: 7-(aminomethyl)-7-deazaguanosine. Nucleic Acids Res. 1978;5:2289–2296. doi: 10.1093/nar/5.7.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slany R.K., Bösl M., Crain P.F., Kersten H. A new function of S-adenosylmethionine: the ribosyl moiety of AdoMet is the precursor of the cyclopentenediol moiety of the tRNA wobble base queuine. Biochemistry. 1993;32:7811–7817. doi: 10.1021/bi00081a028. [DOI] [PubMed] [Google Scholar]
- 13.Miles Z.D., McCarty R.M., Molnar G., Bandarian V. Discovery of epoxyqueuosine (oQ) reductase reveals parallels between halorespiration and tRNA modification. Proc. Natl. Acad. Sci. U.S.A. 2011;108:7368–7372. doi: 10.1073/pnas.1018636108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reuter K., Slany R., Ullrich F., Kersten H. Structure and organization of Escherichia coli genes involved in biosynthesis of the deazaguanine derivative queuine, a nutrient factor for eukaryotes. J. Bacteriol. 1991;173:2256–2264. doi: 10.1128/jb.173.7.2256-2264.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai Y., Fox D.T., Lacy J.A., Van Lanen S.G., Iwata-Reuyl D. Hypermodification of tRNA in Thermophilic archaea. Cloning, overexpression, and characterization of tRNA-guanine transglycosylase from Methanococcus jannaschii. J. Biol. Chem. 2000;275:28731–28738. doi: 10.1074/jbc.M002174200. [DOI] [PubMed] [Google Scholar]
- 16.Phillips G., Chikwana V.M., Maxwell A., El-Yacoubi B., Swairjo M.A., Iwata-Reuyl D., de Crécy-Lagard V. Discovery and characterization of an amidinotransferase involved in the modification of archaeal tRNA. J. Biol. Chem. 2010;285:12706–12713. doi: 10.1074/jbc.M110.102236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips G., de Crécy-Lagard V. Biosynthesis and function of tRNA modifications in Archaea. Curr. Opin. Microbiol. 2011;14:335–341. doi: 10.1016/j.mib.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Phillips G., Swairjo M.A., Gaston K.W., Bailly M., Limbach P.A., Iwata-Reuyl D., de Crécy-Lagard V. Diversity of archaeosine synthesis in crenarchaeota. ACS Chem. Biol. 2012;7:300–305. doi: 10.1021/cb200361w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darland G., Brock T.D., Samsonoff W., Conti S.F. A thermophilic, acidophilic mycoplasma isolated from a coal refuse pile. Science. 1970;170:1416–1418. doi: 10.1126/science.170.3965.1416. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda M., Oyaizu H., Yamagishi A., Oshima T. Morphological variation of new Thermoplasma acidophilum isolates from Japanese hot springs. Appl. Environ. Microbiol. 1995;61:3482–3485. doi: 10.1128/aem.61.9.3482-3485.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilpatrick M.W., Walker R.T. The nucleotide sequence of the tRNAMMet from the archaebacterium Thermoplasma acidophilum. Nucleic Acids Res. 1981;9:4387–4390. doi: 10.1093/nar/9.17.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J. Biol. Chem. 1984;259:9461–9471. [PubMed] [Google Scholar]
- 23.Edmonds C.G., Crain P.F., Gupta R., Hashizume T., Hocart C.H., Kowalak J.A., Pomerantz S.C., Stetter K.O., McCloskey J.A. Posttranscriptional modification of tRNA in thermophilic archaea (Archaebacteria) J. Bacteriol. 1991;173:3138–3148. doi: 10.1128/jb.173.10.3138-3148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandal D., Köhrer C., Su D., Russell S.P., Krivos K., Castleberry C.M., Blum P., Limbach P.A., Söll D., RajBhandary U.L. Agmatidine, a modified cytidine in the anticodon of archaeal tRNA(Ile), base pairs with adenosine but not with guanosine. Proc. Natl. Acad. Sci. U.S.A. 2010;107:2872–2877. doi: 10.1073/pnas.0914869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeuchi Y., Kimura S., Numata T., Nakamura D., Yokogawa T., Ogata T., Wada T., Suzuki T., Suzuki T. Agmatine-conjugated cytidine in a tRNA anticodon is essential for AUA decoding in archaea. Nat. Chem. Biol. 2010;6:277–282. doi: 10.1038/nchembio.323. [DOI] [PubMed] [Google Scholar]
- 26.El Yacoubi B., Phillips G., Blaby I.K., Haas C.E., Cruz Y., Greenberg J., de Crécy-Lagard V. A Gateway platform for functional genomics in Haloferax volcanii: deletion of three tRNA modification genes. Archaea. 2009;2:211–219. doi: 10.1155/2009/428489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe M., Nameki N., Matsuo-Takasaki M., Nishimura S., Okada N. tRNA recognition of tRNA-guanine transglycosylase from a hyperthermophilic archaeon, Pyrococcus horikoshii. J. Biol. Chem. 2001;276:2387–2394. doi: 10.1074/jbc.M005043200. [DOI] [PubMed] [Google Scholar]
- 28.Ishitani R., Nureki O., Fukai S., Kijimoto T., Nameki N., Watanabe M., Kondo H., Sekine M., Okada N., Nishimura S., et al. Crystal structure of archaeosine tRNA-guanine transglycosylase. J. Mol. Biol. 2002;318:665–677. doi: 10.1016/S0022-2836(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 29.Ishitani R., Nureki O., Nameki N., Okada N., Nishimura S., Yokoyama S. Alternative tertiary structure of tRNA for recognition by a posttranscriptional modification enzyme. Cell. 2003;113:383–394. doi: 10.1016/s0092-8674(03)00280-0. [DOI] [PubMed] [Google Scholar]
- 30.Sabina J., Söll D. The RNA-binding PUA domain of archaeal tRNA-guanine transglycosylase is not required for archaeosine formation. J. Biol. Chem. 2006;281:6993–7001. doi: 10.1074/jbc.M512841200. [DOI] [PubMed] [Google Scholar]
- 31.Nomura Y., Onda Y., Ohno S., Taniguchi H., Ando K., Oka N., Nishikawa K., Yokogawa T. Purification and comparison of native and recombinant tRNA-guanine transglycosylases from Methanosarcina acetivorans. Protein Expr. Purif. 2013;88:13–19. doi: 10.1016/j.pep.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Kawamura T., Anraku R., Hasegawa T., Tomikawa C., Hori H. Transfer RNA methyltransferases from Thermoplasma acidophilum, a thermoacidophilic archaeon. Int. J. Mol. Sci. 2014;16:91–113. doi: 10.3390/ijms16010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomikawa C., Ohira T., Inoue Y., Kawamura T., Yamagishi A., Suzuki T., Hori H. Distinct tRNA modifications in the thermo-acidophilic archaeon, Thermoplasma acidophilum. FEBS Lett. 2013;587:3575–3580. doi: 10.1016/j.febslet.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 34.Menezes S., Gaston K.W., Krivos K.L., Apolinario E.E., Reich N.O., Sowers K.R., Limbach P.A., Perona J.J. Formation of m2G6 in Methanocaldococcus jannaschii tRNA catalyzed by the novel methyltransferase Trm14. Nucleic Acids Res. 2011;39:7641–7655. doi: 10.1093/nar/gkr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armengaud J., Urbonavicius J., Fernandez B., Chaussinand G., Bujnicki J.M., Grosjean H. N2-methylation of guanosine at position 10 in tRNA is catalyzed by a THUMP domain-containing, S-adenosylmethionine-dependent methyltransferase, conserved in Archaea and Eukaryota. J. Biol. Chem. 2004;279:37142–37152. doi: 10.1074/jbc.M403845200. [DOI] [PubMed] [Google Scholar]
- 36.Constantinesco F., Benachenhou N., Motorin Y., Grosjean H. The tRNA(guanine-26,N2-N2) methyltransferase (Trm1) from the hyperthermophilic archaeon Pyrococcus furiosus: cloning, sequencing of the gene and its expression in Escherichia coli. Nucleic Acids Res. 1998;26:3753–3761. doi: 10.1093/nar/26.16.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Constantinesco F., Motorin Y., Grosjean H. Characterisation and enzymatic properties of tRNA(guanine 26, N (2), N (2))-dimethyltransferase (Trm1p) from Pyrococcus furiosus. J. Mol. Biol. 1999;291:375–392. doi: 10.1006/jmbi.1999.2976. [DOI] [PubMed] [Google Scholar]
- 38.Roovers M., Wouters J., Bujnicki J.M., Tricot C., Stalon V., Grosjean H., Droogmans L. A primordial RNA modification enzyme: the case of tRNA (m1A) methyltransferase. Nucleic Acids Res. 2004;32:465–476. doi: 10.1093/nar/gkh191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Awai T., Kimura S., Tomikawa C., Ochi A., Ihsanawati, Bessho Y., Yokoyama S., Ohno S., Nishikawa K., Yokogawa T., et al. Aquifex aeolicus tRNA (N2,N2-guanine)-dimethyltransferase (Trm1) catalyzes transfer of methyl groups not only to guanine 26 but also to guanine 27 in tRNA. J. Biol. Chem. 2009;284:20467–20478. doi: 10.1074/jbc.M109.020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazayama A., Yamagami R., Yokogawa T., Hori H. Improved solid-phase DNA probe method for tRNA purification: large scale preparation, and alteration of DNA fixation. J. Biochem. 2015;157:411–418. doi: 10.1093/jb/mvu089. [DOI] [PubMed] [Google Scholar]
- 41.Yokogawa T., Kitamura Y., Nakamura D., Ohno S., Nishikawa K. Optimization of the hybridization-based method for purification of thermostable tRNAs in the presence of tetraalkylammonium salts. Nucleic Acids Res. 2010;38:e89. doi: 10.1093/nar/gkp1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hubert A., Mitani Y., Tamura T., Boicu M., Nagy I. Protein complex purification from Thermoplasma acidophilum using a phage display library. J. Microbiol. Methods. 2014;98:15–22. doi: 10.1016/j.mimet.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Tomikawa C., Yokogawa T., Kanai T., Hori H. N7-Methylguanine at position 46 (m7G46) in tRNA from Thermus thermophilus is required for cell viability through a tRNA modification network. Nucleic Acids Res. 2010;38:942–957. doi: 10.1093/nar/gkp1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kusuba H., Yoshida T., Iwasaki E., Awai T., Kazayama A., Hirata A., Tomikawa C., Yamagami R., Hori H. In vitro dihydrouridine formation by tRNA dihydrouridine synthase from Thermus thermophilus, an extreme-thermophilic eubacterium. J. Biochem. 2015;158:513–521. doi: 10.1093/jb/mvv066. [DOI] [PubMed] [Google Scholar]
- 45.Ruepp A., Graml W., Santos-Martinez M.L., Koretke K.K., Volker C., Mewes H.W., Frishman D., Stocker S., Lupas A.N., Baumeister W. The genome sequence of the thermoacidophilic scavenger Thermoplasma acidophilum. Nature. 2000;407:508–813. doi: 10.1038/35035069. [DOI] [PubMed] [Google Scholar]
- 46.Sato T., Fukui T., Atomi H., Imanaka T. Targeted gene disruption by homologous recombination in the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J. Bacteriol. 2003;185:210–220. doi: 10.1128/JB.185.1.210-220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato T., Fukui T., Atomi H., Imanaka T. Improved and versatile transformation system allowing multiple genetic manipulations of the hyperthermophilic archaeon Thermococcus kodakaraensis. Appl. Environ. Microbiol. 2005;71:3889–3899. doi: 10.1128/AEM.71.7.3889-3899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allen E.E., Tyson G.W., Whitaker R.J., Detter J.C., Richardson P.M., Banfield J.F. Genome dynamics in a natural archaeal population. Proc. Natl. Acad. Sci. U.S.A. 2007;104:1883–1888. doi: 10.1073/pnas.0604851104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen L., Brügger K., Skovgaard M., Redder P., She Q., Torarinsson E., Greve B., Awayez M., Zibat A., Klenk H.P., et al. The genome of Sulfolobus acidocaldarius, a model organism of the Crenarchaeota. J. Bacteriol. 2005;187:4992–4999. doi: 10.1128/JB.187.14.4992-4999.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Awai T., Ochi A., Ihsanawati, Sengoku T., Hirata A., Bessho Y., Yokoyama S., Hori H. Substrate tRNA recognition mechanism of a multisite-specific tRNA methyltransferase, Aquifex aeolicus Trm1, based on the X-ray crystal structure. J. Biol. Chem. 2011;286:35236–35246. doi: 10.1074/jbc.M111.253641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ihsanawati, Nishimoto M., Higashijima K., Shirouzu M., Grosjean H., Bessho Y., Yokohama S. Crystal structure of tRNA N2,N2-guanosine dimethyltransferase Trm1 from Pyrococcus horikoshii. J. Mol. Biol. 2008;383:871–884. doi: 10.1016/j.jmb.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 52.Nakanishi S., Ueda T., Hori H., Yamazaki N., Okada N., Watanabe K. A UGU sequence in the anticodon loop is a minimum requirement for recognition by Escherichia coli tRNA-guanine transglycosylase. J. Biol. Chem. 1994;269:32221–32225. [PubMed] [Google Scholar]
- 53.Kung F.L., Nonekowski S., Garcia G.A. tRNA-guanine transglycosylase from Escherichia coli: recognition of noncognate-cognate chimeric tRNA and discovery of a novel recognition site within the TpsiC arm of tRNA(Phe) RNA. 2000;6:233–244. doi: 10.1017/s135583820099191x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durand J.M., Okada N., Tobe T., Watarai M., Fukuda I., Suzuki T., Nakata N., Komatsu K., Yoshikawa M., Sasakawa C. vacC, a virulence-associated chromosomal locus of Shigella flexneri, is homologous to tgt, a gene encoding tRNA-guanine transglycosylase (Tgt) of Escherichia coli K-12. J. Bacteriol. 1994;176:4627–4634. doi: 10.1128/jb.176.15.4627-4634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hurt J.K., Olgen S., Garcia G.A. Site-specific modification of Shigella flexneri virF mRNA by tRNA-guanine transglycosylase in vitro. Nucleic Acids Res. 2007;35:4905–4913. doi: 10.1093/nar/gkm473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun N., Pan C., Nickell S., Mann M., Baumeister W., Nagy I. Quantitative proteome and transcriptome analysis of the archaeon Thermoplasma acidophilum cultured under aerobic and anaerobic conditions. J. Proteome Res. 2010;9:4839–4850. doi: 10.1021/pr100567u. [DOI] [PubMed] [Google Scholar]
- 57.Robertus J.D., Ladner J.E., Finch J.T., Rhodes D., Brown R.S., Clark B.F.C., Klug A. Structure of yeast phenylalanine tRNA at 3 Å resolution. Nature. 1974;250:546–551. doi: 10.1038/250546a0. [DOI] [PubMed] [Google Scholar]
- 58.Kim S.H., Suddath F.L., Quigley G.J., McPherson A., Sussan J.L., Wang A.H.J., Seeman N.C., Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974;185:435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- 59.Shi H., Moore P.B. The crystal structure of yeast phenylalanine tRNA at 1.93 A resolution: a classic structure revisited. RNA. 2000;6:1091–1105. doi: 10.1017/s1355838200000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biou V., Yaremchuk A., Tukalo M., Cusack S. The 2.9 A crystal structure of T. thermophilus seryl-tRNA synthetase complexed with tRNA(Ser) Science. 1994;263:1404–1410. doi: 10.1126/science.8128220. [DOI] [PubMed] [Google Scholar]
- 61.Atomi H., Imanaka T., Fukui T. Overview of the genetic tools in the Archaea. Front. Microbiol. 2012;3 doi: 10.3389/fmicb.2012.00337. 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang C., Tian B., Li S., Ao X., Dalgaard K., Gökce S., Liang Y., She Q. Genetic manipulation in Sulfolobus islandicus and functional analysis of DNA repair genes. Biochem. Soc. Trans. 2013;41:405–410. doi: 10.1042/BST20120285. [DOI] [PubMed] [Google Scholar]
- 63.Kohler P.R., Metcalf W.W. Genetic manipulation of Methanosarcina spp. Front. Microbiol. 2012;3 doi: 10.3389/fmicb.2012.00259. 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagaoka E., Hidese R., Imanaka T., Fujiwara S. Importance and determinants of induction of cold-induced DEAD RNA helicase in the hyperthermophilic archaeon Thermococcus kodakarensis. J. Bacteriol. 2013;195:3442–3450. doi: 10.1128/JB.00332-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takemasa R., Yokooji Y., Yamatsu A., Atomi H., Imanaka T. Thermococcus kodakarensis as a host for gene expression and protein secretion. Appl. Environ. Microbiol. 2011;77:2392–2398. doi: 10.1128/AEM.01005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morikawa M., Izawa Y., Rashid N., Hoaki T., Imanaka T. Purification and characterization of a thermostable thiol protease from a newly isolated hyperthermophilic Pyrococcus sp. Appl. Environ. Microbiol. 1994;60:4559–4566. doi: 10.1128/aem.60.12.4559-4566.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukui T., Atomi H., Kanai T., Matsumi R., Fujiwara S., Imanaka T. Complete genome sequence of the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1 and comparison with Pyrococcus genomes. Genome Res. 2005;15:352–363. doi: 10.1101/gr.3003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.