Abstract
Objective
Humans and animals exposed to undernutrition (UN) during development often experience accelerated “catch-up” growth when food supplies are plentiful. Little is known about the mechanisms regulating early growth rates. We previously reported that actions of leptin and presynaptic inputs to orexigenic NPY/AgRP/GABA (NAG) neurons in the arcuate nucleus of the hypothalamus are almost exclusively excitatory during the lactation period, since neuronal and humoral inhibitory systems do not develop until after weaning. Moreover, we identified a critical step that regulates the maturation of electrophysiological responses of NAG neurons at weaning – the onset of genes encoding ATP-dependent potassium (KATP) channel subunits. We explored the possibility that UN promotes subsequent catch-up growth, in part, by delaying the maturation of negative feedback systems to neuronal circuits driving food intake.
Methods
We used the large litter (LL) size model to study the impacts of postnatal UN followed by catch-up growth. We evaluated the maturation of presynaptic and postsynaptic inhibitory systems in NAG neurons using a combination of electrophysiological and molecular criteria, in conjunction with leptin's ability to suppress fasting-induced hyperphagia.
Results
The onset of KATP channel subunit expression and function, the switch in leptin's effect on NAG neurons, the ingrowth of inhibitory inputs to NAG neurons, and the development of homeostatic feedback to feeding circuits were delayed in LL offspring relative to controls. The development of functional KATP channels and the establishment of leptin-mediated suppression of food intake in the peri-weaning period were tightly linked and were not initiated until growth and adiposity of LL offspring caught up to controls.
Conclusions
Our data support the idea that initiation of KATP channel subunit expression in NAG neurons serves as a molecular gatekeeper for the maturation of homeostatic feeding circuits.
Keywords: NPY, AgRP, Leptin, Undernutrition, KATP channel, Feeding circuits
Abbreviations: AgRP, agouti-related peptide; ARH, arcuate nucleus of the hypothalamus; EPSC, excitatory postsynaptic current; GABA, gamma-aminobutyric acid; IPSC, inhibitory postsynaptic current; KATP, ATP-sensitive potassium channel; Kir, potassium inward rectifiying channel subunit; Lepr, leptin receptor; LL, large litter; NAG, NPY, AgRP, GABA, NPY, neuropeptide Y; P, postnatal day; Pomc, pro-opiomelanocortin; PVH, paraventricular nucleus of the hypothalamus; pSTAT3, phosphorylated signal transducer and activator of transcription 3; SUR, sulfonylurea receptor; UN, undernutrition
Highlights
-
•
Undernutrition delays pre- and post-synaptic inhibitory signaling in NAG neurons.
-
•
KATP channel expression is initiated when UN offspring reach the weight of controls.
-
•
Onset of leptin's ability to suppress feeding coincides with KATP channel expression.
-
•
KATP channels may act as gatekeepers for the maturation of circuits delayed by UN.
1. Introduction
Suboptimal maternal nutrition is associated with a delay in the maturation of circuits regulating diverse physiological process, including growth, reproduction and cognition [1], [2], [3], [4]. Although “developmental delay” has negative connotations, a delay may beneficial if it provides a window during which the normal developmental program can be reinstated if adequate nutritional stores are available. Studies in humans and rodent models support the idea that early nutritional supplementation and rapid catch-up growth can mitigate impacts of maternal UN on cognitive function [5], [6], [7], [8], but at the cost of increased risk of metabolic disease [9], [10], [11], [12].
Little is known about mechanisms that regulate growth rates following developmental exposure to UN. Rodents exposed to moderate restriction (<50%) during lactation increase food intake in the post-weaning period such that they ultimately attain the body weight of controls; however, more severe restriction usually leads to persistent decreases in food intake and body weight [13], [14], [15], [16]. In cases of moderate postnatal UN, there is evidence that the accelerated growth rate is supported, in part, by increased orexigenic drive in the post-weaning period. Neurons in the arcuate nucleus of the hypothalamus (ARH) that co-express neuropeptide Y (NPY), agouti-related peptide (AgRP) and gamma-aminobutyric acid (GABA) exert a powerful orexigenic influence on central circuits regulating food intake [17], [18]. NAG neurons are an important node of homeostatic regulation of feeding, as they are activated by signals of negative energy balance (i.e. ghrelin) and are inhibited by circulating signals of an energy replete state (i.e. leptin) [19], [20]. Projections from NAG neurons that regulate food intake are formed during the suckling period [21], [22], and postnatal UN due to lactation in a large litter (LL) leads to increases in the number of projections to downstream targets at 3–4 weeks of age [23], [24], [25].
Leptin is well-positioned to serve as a conduit for impacts of UN on developing feeding circuits. There is a surge in leptin levels in the first two weeks of lactation [26] that precedes its ability to modulate food intake [27]. During this period, leptin promotes axonal outgrowth from NAG neurons [24], [28], [29]. This neurotrophic effect is observed in immature NAG neurons that are activated by leptin [30], while leptin's actions in mature NAG neurons are inhibitory [19], [20], [30]. As UN can alter the timing and/or levels of leptin during the surge [24], [31], [32], we hypothesized that the effects of postnatal UN on orexigenic drive are mediated through impacts on leptin signaling in NAG neurons.
We previously used a combination of genetic, immunohistochemical and electrophysiological criteria to characterize the onset of leptin-mediated signaling in NAG neurons across the postnatal period. In contrast to the well-characterized effect of leptin to hyperpolarize adult NAG neurons via KATP channels [33], [34], we found that all of the Npy-GFP + neurons [35] that responded to leptin on postnatal days 13–15 (P13–15) were depolarized [30]. Starting at P21, there was a gradual increase in the number of hyperpolarized neurons at the expense of depolarized neurons, such by P30, all of the leptin-responsive Npy-GFP + neurons were hyperpolarized. Moreover, we discovered that the onset of leptin-mediated hyperpolarization at weaning coincided with the onset of KATP channel subunit expression [30]. The maturation of the electrophysiological properties of NAG neurons coincides with the onset of homeostatic regulation of food intake [21], [27].
In these studies, we explored whether effects of moderate postnatal UN to increase post-weaning growth rates are associated with impairments in the maturation of systems that provide negative feedback to the orexigenic actions of NAG neurons. We found that UN delays the development of homeostatic regulation of feeding, which is tightly correlated with the onset of KATP channel expression and the maturation of electrophysiological properties of NAG neurons.
2. Materials and methods
2.1. Animals
All mouse protocols were overseen and approved by the Columbia University Medical Center or the Oregon Health and Science University Institutional Animal Care and Use Committees. Mice were maintained in a temperature (22+/− 1 °C) and light controlled (12 h light: 12 h dark) barrier facility. Mice were weaned at P21 and had ad libitum access to chow (13.2% calories from fat, 5053; PicoLab Rodent Diet 20) and autoclaved drinking water. Npy-hrGFP mice (B6.FVB-Tg(Npy-hrGFP)1Lowl/J, Stock No: 006417) were purchased from The Jackson Laboratory. The number of pups per litter was adjusted at P3 to n = 8 (control litter) or n = 12 (LL litter) in a randomized way. Main exclusion criteria for any experiment were based on: a) any sign of sickness and b) body weight outliers: mice with a deviation of 2 times the standard deviation from the mean value of the group. Data from males and females were combined, as we did not detect any gender differences in the endpoints examined. Mice were allocated to saline vs. leptin treatment groups on the basis of body weight, ensuring that the distribution of body weights was similar. Experimenters were not blinded to the experimental groups of animals. Mice were weighed 3 times per week. Body composition was assessed on a biweekly basis using nuclear magnetic resonance imaging (Minispec, Bruker). Naso-anal length was measured bi-weekly on anesthetized animals (4% isoflurane).
2.2. Immunohistochemistry
Mice were injected with 0.9% saline or leptin (4 mg/kg, i.p., National Hormone Peptide Program) and after 45 min were transcardially perfused with saline followed by 4% paraformaldehyde in 0.1M PB under avertin (2.5 mg/10 g, Sigma) anesthesia. Pups under P21 were injected and anesthetized in their breeding cage. Mice older than P21 were fasted for 3 h before injection, to minimize fasting-induced NAG neuronal activation. After perfusion, brains were removed, postfixed overnight, washed with cold PBS and submerged in 30% sucrose before embedding in O.C.T medium. Brains were kept frozen at −80 °C until sectioning. 10 μm-thick coronal sections were acquired from the paraventricular nucleus of the hypothalamus (PVH) to the caudal ARH (Allen Brain Atlas coordinates: −0.08 to −2.0 mm from bregma). For ARH immunohistochemistry, sections from the medial ARH area were incubated with rabbit anti-c-Fos (1:500, Calbiochem, #PC38 or 1:500, Cell Signaling # 2250) or rabbit anti-phospho-STAT3 (pSTAT3) (1:500, Cell Signaling Technology, #9131) [30], followed by a goat anti-rabbit-Cy3 secondary antibody (1:500, Jackson ImmunoResearch Laboratories, #115-165-205), and nuclear staining with DAPI (1:500). An additional antigen retrieval step was performed before pSTAT3 staining; slides were incubated in 1% NaOH, 1% H2O2 for 20 min before primary antibody incubation. Digital images were captured using a Nikon Eclipse 80i equipped with a Retiga EXi camera and X-Cite 120 fluorescent illumination system. Cell counts were performed using Adobe Photoshop CS5, and experimenters were blinded to the experimental groups. Npy-GFP+, c-Fos+ and pSTAT3+ cells were only counted if they colocalized with a DAPI signal from at least 12–18 hemisections per mouse.
2.3. Quantification of fibers in the PVH
Sections were incubated with α-AgRP [36] (1:500, Phoenix Pharmaceuticals #H0035-57) or α-melanocyte stimulating hormone (α-MSH) (1:1000 rabbit A 2-7-83, kindly provided by Sharon Wardlaw). Fluorescent images were captured from 8 to 12 sections per mouse, and the Fiji version of the ImageJ program was used for quantitative analyses of immunoreactive AgRP or α-MSH fibers in the PVH. Briefly, all images were taking using the same settings and adjusted with same threshold to eliminate background. Images were binarized and the percent of the total area that contained fluorescent signals >2 pixels2 was calculated.
2.4. Leptin-induced suppression of food intake
Mice were isolated at P25 and daily food intake and body weight were measured until P29 (Baseline). At P29, food was removed at lights out, and, the next morning, mice were injected with 0.9% saline or leptin (4 mg/kg, i.p.) and provided with ad libitum access to food. Food intake and body weight changes were measured after 24 h.
2.5. Quantitative expression analysis
Brains from 4 to 6 animals per group and age were extracted and maintained in cold artificial cerebrospinal fluid (aCSF). Brains were sectioned in 300 μm coronal slices in a vibratome (Leica, VT1200). Sections containing Npy-GFP+ fluorescence in the ARH were carefully microdissected under a fluorescent scope, using a scalpel to cut a triangular area encompassing the GFP+ neurons from both sides of the ARH, while the median eminence was discarded. Following mRNA extraction (RNeasy Microkit; Quiagen, #74004), 500 ng of RNA was transcribed to cDNA with random hexamers (First Strand Transcription Kit, Roche) and was assayed by quantitative PCR (LightCycler 480 SYBRGreen I Master, Roche). Levels of expression were calculated by the 2ΔΔCt method, using Rplp0 gene expression as a housekeeping gene. For calculations between different time-points in control offspring, expression of each gene was related to AgRP expression within each group and then normalized according to the figure legend. The following primers were designed: SUR1: Forward 5′- CAGCGTCAGCGAATCAGTGTA-3′, reverse 5′- TGCATTAGGTGGTCACTCAGATG-3′; Sur2, forward 5′- GGAGACCAAACTGAAATCGG-3′, reverse 5′- TGCGTCACAAGAACAACCG-3′; Kir6.1, forward 5′- AGGCACCATGGAGAAGAGTC-3′, reverse 5′- TGCGTCACAAGAACAACCG-3′; Kir6.2, forward 5′- GGAGCCTGTACCGGGTTATT-3′, reverse 5′- AAACCAGTCCCGAGCTGAG-3′; AgRP 5′- GGCCTCAAG AAGACAACTGC-3′, reverse 5′- TGCGACTACAGAGGTTCGTG-3′; Pomc, forward 5′- GTGCCAGGACCTCACCA-3′, reverse 5′- CAGCGAGAGGTCGAGTTTG-3′; Rplp0, forward 5′- GAGACTGAGTACACCTTCCCAC-3′, reverse 5′- ATGCAGATGGATCAGCCAGG-3′.
2.6. Electrophysiology
All recordings were performed in Npy-GFP+ neurons in the ARH from P30–33. Coronal slices containing ARH were prepared as previously described [30]. Briefly, brain slices (300 μM) containing ARH were maintained with constant flow (1–2 ml/min) of artificial cerebrospinal fluid (aCSF) containing (in mM): 124 NaCl, 5 KCl, 2.6 NaH2PO4, 1 MgSO4, 1 CaCl2, 26 NaHCO3, 10 Hepes, 10 glucose; oxygenated (95% O2, 5% CO2) osmolarity ∼305 Osm at 32–33 °C. Inhibitory postsynaptic currents (IPSCs) were recorded with a cesium chloride based solution containing (in mM): 140 CsCl, 5 MgCl2, 1 BAPTA, 5 ATP, 0.3 GTP, pH∼7.35 with CSOH, osmolarity ∼295 mosM. The N-methyl-d-aspartate (NMDA) receptor antagonist (DL)-2-amino-5-phosphonovaleric acid (APV) (50 μM) and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor antagonist CNQX (10 μM) were added to the bath to isolate inhibitory postsynaptic currents (IPSCs). Tetrodotoxin (TTX) (1 μM) was added to the bath to isolate miniature inhibitory postsynaptic currents (mIPSCs). Excitatory postsynaptic currents (EPSCs) were recorded with a cesium-methanesulfonate based solution (in mM): 125 CsMeSO3, 10 CsCl, 5 NaCl, 2 MgCl2, 10 HEPES, 1 EGTA, 5 ATP, 0.3 GTP, pH∼7.35 with CSOH, osmolarity ∼295 Osm. Bicuculline (5 μM) was added to the bath to block IPSCs. TTX (1 μM) was added to the bath to isolate miniature excitatory postsynaptic currents (mEPSCs). Both IPSCs and EPSCs were recording at holding potential of −60 mV in whole-cell patch clamp mode and pipettes have a resistance of 2–4 ΩM. Series resistance values were generally <20 MΩ during the experiments. Data analysis was performed using Clampfit 10 and only synaptic events >5 pA were analyzed.
For current clamp experiments, microelectrodes had resistances of 3–5 ΩM and were filled with an internal solution containing (in mM): 125 K-gluconate, 2 KCl, 10 EGTA, 5 HEPES, 1 ATP, 0.3 GTP; pH∼7.25 with KOH, osmolarity ∼295 mosM. The liquid junction potential of −5 mV was corrected in the analysis. TTX (1 μM) and bicuculline (5 μM) were used to block presynaptic inputs. Data acquisition was performed using a multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Data were sampled at 20 KHz using a computer interface Digidata 1322 and pClamp 9.2 software (Molecular Devices).
2.7. Statistical analysis
Data are presented as mean ± standard error of the mean (s.e.m) per experimental condition, age and litter group. Experiments were replicated using at least 3 different litters at the indicated time points. Significance was tested using Prism 5.0 software (Graphpad Software Inc). Normality tests were conducted (Kolmogorov–Smirnov test) and unpaired t-test, one-way and two-way ANOVA followed by Bonferroni post hoc test were used, with P < 0.05 considered as statistically significant. For data that did not meet the normal distribution, Mann–Whitney test was used for further analysis (Figure 3C).
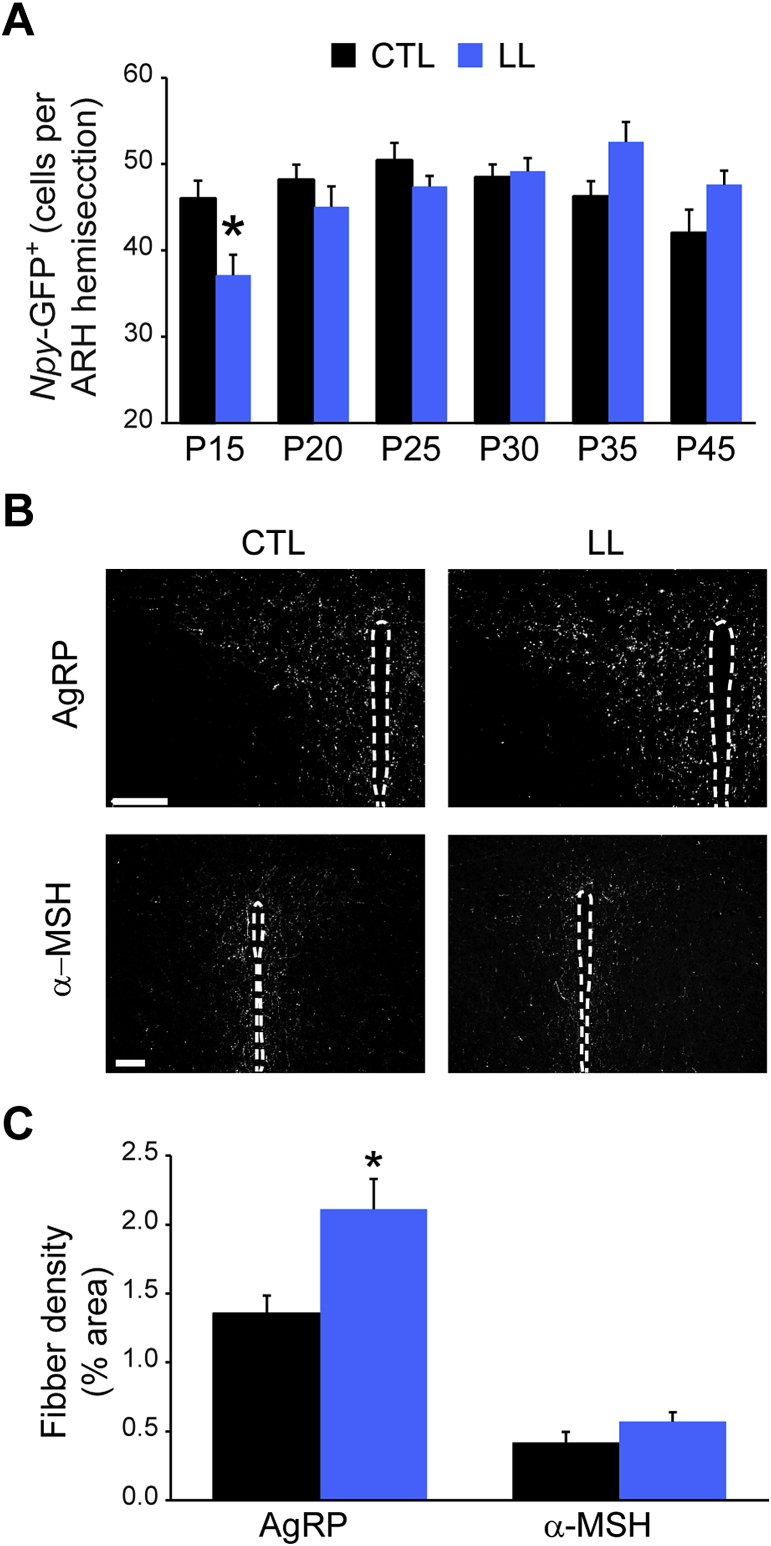
Figure 3.
AgRP projections to the PVH are increased in pups exposed to postnatal undernutrition. (A) Total number of Npy-GFP+ cells in arcuate sections from control (CTL, black bars) and postnatally undernourished (LL, blue bars) mice from P15 to P45. Data are presented as mean ± s.e.m cell number/hemisection; n = 6–12 hemisections per mouse, 4–6 mice per group and treatment, 4–5 different litters; *p < 0.05 CTL vs LL, two-way ANOVA followed by Bonferroni post hoc test. (B) Representative images and (C) quantification of AgRP+ and α-MSH+ fibers in the PVH from control (black bars) and LL (blue bars) mice at P30–35. Data are presented as the mean ± s.e.m of % area occupied by AgRP+/α-MSH+ fibers. n = 8–12 PVH hemisections per mouse, n = 3–4 mice from 3 litters; *p < 0.05 CTL vs. LL, Mann–Whitey test. Scale bar = 100 μm.
3. Results
3.1. Model to study catch-up growth following moderate postnatal undernutrition
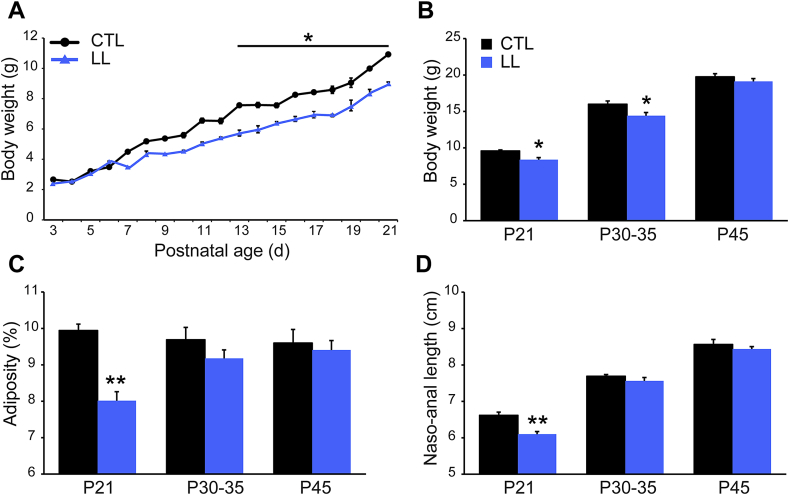
Several different strategies involving reductions in maternal and/or offspring access to nutrition have been used to study the consequences of developmental exposure to UN. We studied postnatal undernutrition caused by increasing the litter size from 8 to 12 pups, because this model is consistently associated with effects on NAG neurons [23], [24], [25], [37]. We found that body weights of LL pups diverged from controls at P7, reaching statistical significance at P13 (Figure 1A; n = 6 litters; F(1.11) = 52.10, p < 0.05 CTL vs. LL, two-way ANOVA plus Bonferroni post hoc test). At weaning LL offspring were 13% lighter than controls (Figure 1B; n = 15–21 animals, 6–8 litters, t(27) = 3.02, p < 0.05, unpaired t-test), which was accompanied by a 20% reduction in adiposity and an 8% reduction in naso-anal length (Figure 1C,D; t(28) = 5.59 and t(29) = 6.91, p < 0.01, unpaired t-test, respectively). However, all of these parameters were normalized by P45 (Figure 1B–D; p > 0.05, unpaired t-test).
Figure 1.
Catch-up growth following postnatal undernutrition. (A) Body weight across the lactation period in pups from control litters (CTL, circles on a black line) vs. large litters (LL, triangles on a blue line); n = 6 litters per group; *p < 0.05, ANOVA followed by Bonferroni post-hoc test. (B) Body weight, (C) adiposity and (D) naso-anal length at postnatal day 21, 30 and 45 from control (CTL, black bars) and undernourished mice (LL, blue bars). Data are presented as mean ± s.e.m.; n = 15–21 mice from 6 to 8 litters; *p < 0.05, **p < 0.01, unpaired t-test.
3.2. Delayed onset of homeostatic feeding regulation in LL offspring
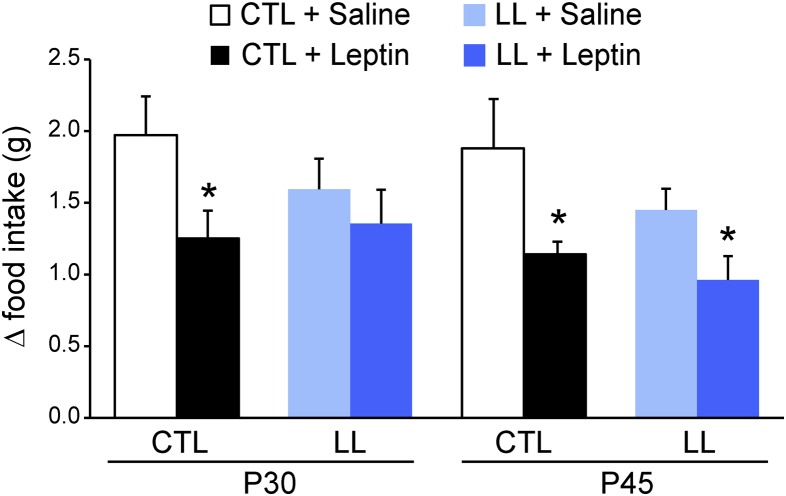
We first considered whether catch-up growth in post-weaning LL offspring is associated with a failure to appropriately develop homeostatic negative feedback to circuits regulating food intake at 4 weeks of age [21], [27]. To this end, we assessed the extent to which peripheral injections of leptin (4 mg/kg, i.p.) vs. saline suppressed food intake after an overnight fast at P30. Leptin reduced fasting-induced food intake by 36.5% in controls (Figure 2; 2.0 ± 0.3 saline vs. 1.3 ± 0.2 leptin; n = 4–5 animals per condition, 4–5 litters t(25) = 2.21 p = 0.04, unpaired t-test), while its effect in LL offspring was not significantly different from saline (Figure 2; 1.6 ± 0.2 saline vs. 1.4 ± 0.2 leptin; t(25) = 0.214, p = 0.8, unpaired t-test). As LL offspring reached the body weight and adiposity of controls at P45, we next examined whether the end of the catch-up growth period is accompanied by the onset of homeostatic regulation of food intake. We found that leptin suppressed fasting-induced re-feeding to a similar degree in both control and LL offspring (Figure 2; 39.3% ± 4.7 vs. saline, controls, and 33.8% ± 11.5 vs. saline, LL; t(21) = 2.5, p = 0.042 and t(22) = 2.36, p = 0.027, respectively; unpaired t-test).
Figure 2.
The onset of leptin's effect on food intake is delayed by postnatal undernutrition. Fasting-induced hyperphagia was assessed by calculating the increase in food intake (Δ food intake) following an overnight fast over baseline levels of intake measured on the previous day. The impact of saline (light colored bars) vs. leptin (4 mg/kg, i.p.; dark colored bars) on fasting-induced hyperphagia in mice from control litters (CTL, black bars) vs. large litters (LL, blue bars) at P30 and P45 was compared. Data are presented as mean ± s.e.m.; n = 4–5 mice per condition, 4–5 different litters tested at each age and group; *p < 0.05, unpaired t-test.
3.3. Failure of leptin to suppress feeding in LL offspring is not due to hard-wired deficits in NAG neuronal circuitry
We next explored whether leptin's failure to affect fasting-induced food intake in P30 LL offspring might be due to structural impairments in NAG neuronal circuits, which are activated by fasting and inhibited by leptin [38], [39], [40], [41]. We first determined whether the number of NAG neurons in the ARH is impacted by postnatal UN. We found that there was a transient reduction in the number of ARH neurons expressing an Npy-GFP reporter [35] in LL offspring at P15, but there was no difference in the size of the NAG neuronal population from P20 onward (Figure 3A; n = 4–6 mice per group from 4 to 5 different litters; F(1,5) = 1.6, p > 0.05, except P15: p < 0.05. Two-way ANOVA followed by Bonferroni post hoc test).
Next we examined whether insensitivity of LL offspring to leptin's effects on food intake is also reflected in reductions in another leptin-mediated process – axonal outgrowth to the PVH [28], [29], a downstream mediator of NAG neuronal effects on feeding behavior [42], [43]. We observed a 56% increase in the density of AgRP-immunoreactive fibers in the PVH of LL offspring at P30 (Figure 3B,C; 1.35 ± 0.13% control vs. 2.12 ± .21% LL; n = 12–16 sections, 4 animals, p < 0.001, Mann–Whitney test). In contrast, we did not detect a difference in PVH innervation by fibers expressing α-MSH, a marker of projections from an intermingled population of ARH neurons that expresses pro-opiomelanocortin (POMC) (Figure 3C; p = 0.096; Mann–Whitney test). Thus, our findings argue against the hypothesis that deficits in leptin's effects on food intake are caused by structural defects in NAG neuronal circuitry.
3.4. Altered leptin responsiveness in NAG neurons of LL offspring
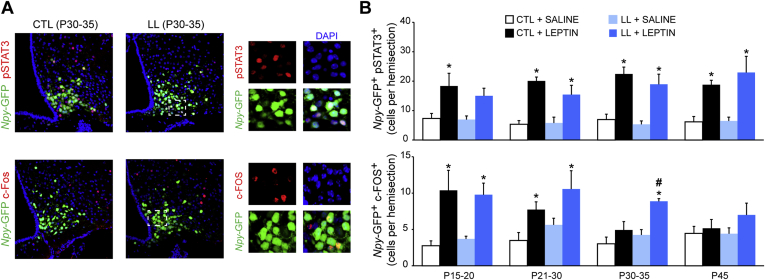
As leptin promotes axonal outgrowth from NAG neurons [24], [28], [29] and NAG neuronal projections were increased in LL offspring at P30 (Figure 3C), it raised the possibility that leptin sensitivity was not diminished by undernutrition. To confirm our suspicion, we evaluated the impact of postnatal UN on the number of NAG neurons that respond to a peripheral leptin injection by activating the STAT3 signaling cascade [44]. To this end, we assessed the number of Npy-GFP+ neurons that were pSTAT3-immunoreactive after treatment with saline or leptin (4 mg/kg, i.p.) from P15 through P45 (Figure 4A). We did not detect any differences between the number of pSTAT3+Npy-GFP+ neurons in control vs. LL offspring following an injection of saline or leptin (Figure 4B; n = 6–12 hemisections, 4–6 animals per group, 4–5 litters; F(1,30) = 0.79, p > 0.05, two-way ANOVA followed by Bonferroni post hoc test) at any point during development.
Figure 4.
Delayed switch in leptin responses in a subset of NAG neurons from pups exposed to postnatal undernutrition. (A) Representative images of the ARH from leptin-treated offspring of control and large litters at P30 showing co-localization between Npy-GFP+ (green) and pSTAT3+ (upper panel, red) or c-Fos+ (lower panel, red) from mice of control or large litters at P30–35. Magnified views of the individual merged layers from the boxed areas in the composite are shown at the right. Scale bar = 100 μm 3V: Third ventricle. (B) Quantification of Npy-GFP+ neurons per ARH hemisection that co-stain for pSTAT3+ (upper panel) or c-Fos+ (lower panel) from offspring of control litters (CTL, white/black bars) or large litters (LL, blue bars) treated with saline (light colored bars) or leptin (4 mg/kg, i.p.; dark colored bars). Data are presented as mean ± s.e.m cell number/hemisection; n = 6–12 hemisections per mouse, 4–6 mice per age and group, 3 different litters; *p < 0.05 leptin vs saline, #p < 0.05 CTL vs LL, two-way ANOVA followed by Bonferroni post hoc test.
Since the total size of the leptin-sensing NAG neuronal population was not impacted by LL, we next considered whether responses to leptin might be altered at the intracellular level. We previously discovered that there is a subset of immature NAG neurons that is activated by leptin in the postnatal period [30]. The disappearance of this subpopulation in the post-weaning period coincides with the onset on homeostatic regulation of feeding at P28 [27], [30]. To evaluate the impact of postnatal UN on the subpopulation of leptin-activated NAG neurons, we used c-Fos immunoreactivity as a surrogate measure of neuronal activation in response to peripheral injections of leptin, as compared to saline-injected controls (Figure 4A). At P15, when the number of c-Fos+Npy-GFP+ is maximal, we did not observe any difference between LL and control offspring (Figure 4B; F(1,3) = 0.5341, p > 0.05; two-way ANOVA followed by Bonferroni post hoc test). In controls, number of leptin-induced c-Fos+Npy-GFP+ neurons steadily declined after P21, so that by P30–35, it was the same as saline-injected controls (Figure 4B; F(1,3) = 9.44, p > 0.05 at P30 and P45). This process was delayed by UN so that leptin-induced c-Fos+Npy-GFP+ neurons were still detected at P30–35 (Figure 4B; 4.9 ± 2.3 control vs. 8.9 ± 0.4 LL; F(1,3) = 24.95, p < 0.05), but were absent by P45 (Figure 4B; p > 0.05), coincident with the end of the catch-up growth phase (Figure 1) and the onset of leptin's effects on food intake (Figure 2).
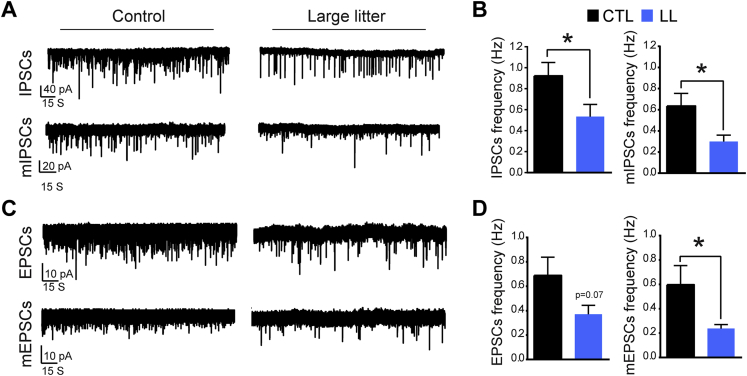
The progressive loss of c-Fos+Npy-GFP+ neurons in the post-weaning period occurs during a period of increased inhibitory inputs to NAG neurons and the maturation of electrophysiological properties of NAG neurons [30], [45]. Thus we evaluated whether impacts of UN on either of these parameters might contribute to the delay in the development of homeostatic regulation of feeding in LL offspring. To this end, we recorded IPSCs and EPSCs in Npy-GFP+ neurons at P30–33. Consistent with our hypothesis, we found that the frequency of IPSCs was decreased by 42% in NAG neurons from LL offspring as compared to controls (Figure 5A,B; IPSCs: 0.92 ± 0.1 control vs. 0.53 ± 0.1 LL; n = 17, 12 animals, t(15) = 2.2, p < 0.05, unpaired t-test). This reduction was still observed when action potentials from presynaptic neurons were blocked with TTX (1 μM) (Figure 5A,B; mIPSCs: 0.63 ± 0.1 control vs. 0.29 ± 0.06 LL; n = 15, 12 animals, t(13) = 2.3, p < 0.05, unpaired t-test). There was no difference in the amplitude of any postsynaptic currents (data not shown).
Figure 5.
Presynaptic tone is reduced in Npy-GFP neurons by exposure to postnatal undernutrition. (A) Representative traces from measurements of IPSCs and mIPSCs in Npy-GFP+ neurons from offspring of control litters and large litters at P30–33. (B) The mean frequency of IPSCs and mIPSCs in Npy-GFP+ neurons from offspring of control litters (CTL, black bars) vs. large litters (LL, blue bars) at P30–33. *p < 0.05, unpaired t-test. (C) Representative traces from measurements of EPSCs and mEPSCs in Npy-GFP+ neurons from offspring of control litters and large litters (LL) at P30–33. (D) The mean frequency of EPSCs and mEPSCs in Npy-GFP+ neurons from offspring of control litters (CTL, black bars, n = 9 cells, 6 animals) vs. large litters (LL, blue bars, n = 7 cells, 6 animals) at P30–33. *p < 0.05, unpaired t-test.
We found that EPSCs and mEPSCs were also diminished in LL offspring; however, these differences only reached significance in TTX-treated slices (Figure 5C,D; EPSCs: 0.68 ± 0.1 control vs. 0.37 ± 0.07 LL; mEPSCs: 0.59 ± 0.1 control vs. 0.23 ± 0.03 LL; n = 15, 12 animals, t(13) = 2.3, p = 0.07 and p < 0.05, respectively; unpaired t-test). In addition, we observed that the frequency of excitatory currents in NAG neurons was significantly reduced by exposure to TTX, consistent with the idea that most excitatory postsynaptic currents in NAG neurons are generated by spontaneous vesicular fusion in controls [45], [46], [47], but from presynaptic action potentials in LL offspring. In summary, as we observed that excitatory and inhibitory postsynaptic currents are reduced to a similar degree in NAG neurons of LL offspring, leptin's failure to suppress feeding is not likely caused by changes in the balance of excitatory and inhibitory inputs onto NAG neurons.
3.5. Postnatal UN delays the onset of KATP channel subunit expression and the maturation of electrophysiological properties of NAG neurons
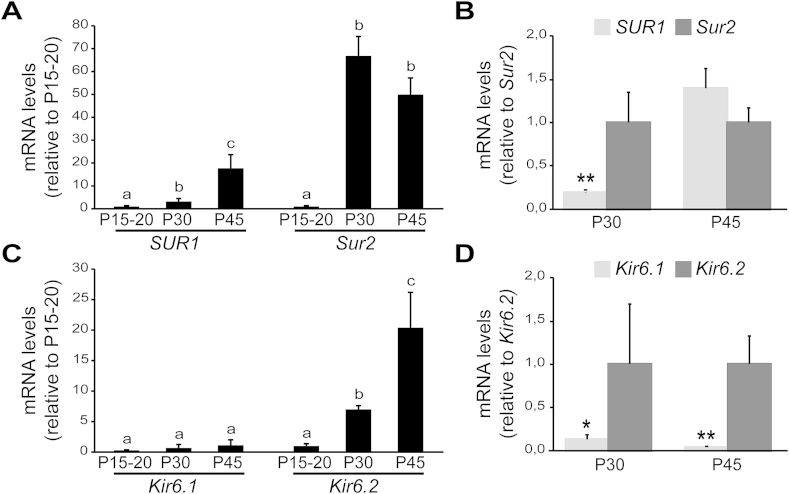
As we previously discovered that the switch to leptin-induced hyperpolarization in the peri-weaning period coincided with onset of KATP channel subunit expression, we asked whether these processes are impacted by postnatal UN. First we performed quantitative PCR on microdissected Npy-GFP+ tissue from P15 to P45 in control offspring to characterize the temporal pattern of the expression of genes encoding the two pore-forming subunits – Kir6.1 (also known as Kcnj8) and Kir6.2 (also known as Kcnj11) – and two sulfonylurea receptor regulatory subunits – SUR1 (also known as Abcc8) and Sur2 (also known as Abcc9). Consistent with our previous results [30], expression of all four subunits was barely detectable at P15-20 Figure 6A,C; n = 4 samples, 4–6 mice from independent litters; p = 0.029 (SUR1), 0.0005 (Sur2), 0.908 (Kir6.1) and 0.004 (Kir6.2); One-way ANOVA for temporal analysis, followed by Bonferroni post hoc test). By P45, we observed robust expression of SUR1, Sur2 and Kir6.2, while Kir6.1 expression remained low throughout the entire period (Figure 6A,C). We found differences in the timing of subunit expression upregulation; Sur2 reached maximal levels at P30 (Figure 6A; p < 0.01 P15 vs. P30 and vs. P45), while SUR1 and Kir6.2 expression continued to increase between P30 and P45 (Figure 6A,C; p < 0.05 P15 vs. P30; p < 0.01 P15 vs. P45). Due to these temporal differences, Sur2 expression is 5.5-fold higher than SUR1 at P30, while they are expressed at similar levels at P45 (Figure 6B; n = 4 samples, 4–6 mice from independent litters, t(6) = 3.14, p = 0.019 at P30; t(8) = 1.16, p = 0.27 at P45; unpaired t-test)). On the other hand, Kir6.2 is the principal pore-forming subunit in the mediobasal hypothalamus, as Kir6.1 is not expressed (Figure 6D; t(4) = 4.14, p = 0.014 for P30; t(8) = 2.7, p = 0.017 for P45, unpaired t-test).
Figure 6.
Temporal dynamics of KATPchannel subunit expression during postnatal development. (A, C) Temporal dynamics of the expression of genes encoding sulfonylurea subunits, SUR1 and Sur2(A) and pore-forming subunits Kir6.1 and Kir6.2(C) of KATP channels in the microdissected ARH of controls. Age-associated changes in the expression of each gene were calculated relative to its own expression levels at P15. Data are expressed as mean ± s.e.m.; n = 4 samples, 4–6 mice per sample, 4 litters. Different lowercase letters above bars denote statistical significance of p < 0.05, ANOVA followed by Bonferroni post-hoc test. (B, D) Relative expression of SUR1 (light gray bars) vs. Sur2 (dark grey bars) (B) and Kir6.1 (light grey bars) vs. Kir6.2 (dark grey bars) (D) in the ARH region of control mice at P30 and P45. n = 4 samples, 4–6 mice, 4 litters; *p < 0.05 and **p < 0.01, unpaired t-test.
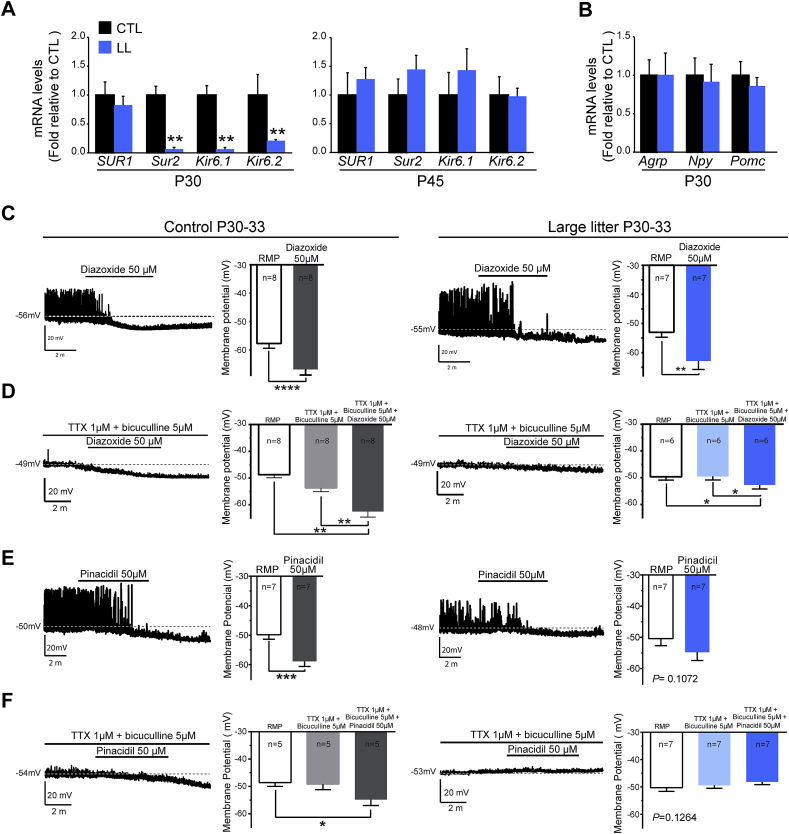
Next we evaluated the impact of postnatal UN on the expression of genes encoding KATP channel subunits at P30, when all leptin-responsive NAG neurons are hyperpolarized [30]. We found that the expression of Sur2, Kir6.1 and Kir6.2 was markedly reduced in LL as compared to controls (Figure 7A; 90.1% ± 2.7, 81.6% ± 3.9 and 70.1% ± 4.4 lower than controls, respectively; n = 4 samples, 4–6 mice from independent litters. t(7) = 2.735, p = 0.0291; t(6) = 2.80, p = 0.03; t(7) = 2.735, p = 0.0291, respectively; unpaired t-test), consistent with persistence of a leptin-induced c-Fos+ subset of NAG neurons (Figure 4B). However, expression of SUR1, Agrp, Npy and Pomc was not altered at either age (Figure 7B and data not shown; SUR1: t(5) = 2.05, p = 0.09 at P30; t(9) = 1.25, p = 0.24 at P45; unpaired t-test). By P45 we found that LL offspring expressed normal levels of Sur2, Kir6.1 and Kir6.2 (Figure 7A; t(9) = 0.53, p = 0.6; t(8) = 0.16, p = 0.87; t(9) = 0.77, p = 0.46, respectively; unpaired t-test), concomitant with the onset of leptin-induced suppression of food intake (Figure 2).
Figure 7.
Onset of the expression and function of KATPchannels is delayed by exposure to postnatal undernutrition. (A) Relative expression of genes encoding KATP channel subunits and (B) neuropeptides in the ARH region of offspring of large litters (LL, blue bars) as compared to control litters (CTL, black bars) at P30 and P45. n = 4 samples per age and group, 4–6 mice per sample; **p < 0.01 CTL vs. LL, unpaired t-test. (C–F) Representative traces and average changes in membrane potential in Npy-GFP+ neurons in acute slices from P30–35 treated with SUR1 (C, D) or SUR2 (E, F) agonists in the absence (C, E) or presence (D, F) of presynaptic blockers (TTX 1 μM + bicuculine 5 μM). Data from control litters are shown on the left (grey bars) and LL litters on the right (blue bars). (C) SUR1 response to 50 μM diazoxide treatment in control (n = 7 cells from 5 animals) and LL offspring (n = 7 cells from 7 animals). (D) Response to 50 μM diazoxide in the presence of presynaptic blockers (n = 8 cells from 6 CTL animals and n = 6 cells from 6 LL animals). (E) SUR2 response to 50 μM pinacidil in control (n = 7 cells from 5 animals) and LL offspring (n = 7 cells from 6 animals). (F) Response to 50 μM pinacidil in the presence of presynaptic blockers in control (n = 5 cells from 4 animals) and LL offspring (n = 7 cells from 7 animals). Results are shown as mean ± s.e.m. *p < 0.05, ***p < 0.001, ****p < 0.0001, paired t-test and ANOVA followed by Bonferroni post hoc test.
We next confirmed that impact of postnatal UN on KATP channel subunit expression at P30 is reflected in the electrophysiological properties of NAG neurons in acute brain slices. To this end, we performed current clamp recordings in control and LL slices in the presence of the SUR1 agonist diazoxide (50 μM) or the SUR2 agonist pinacidil (50 μM). To exclude possible contributions from inhibitory presynaptic inputs [45], [47], we also evaluated the effects of diazoxide and pinacidil in the presence of the sodium channel blocker TTX and the GABAA receptor blocker bicuculline. As reported previously [30], [48], treating acute slices with 50 μM of the SUR1 agonist diazoxide caused membrane hyperpolarization of Npy-GFP + neurons from controls (Figure 7C; −57 ± 1.7 mV baseline vs. −66 ± 1.9 mV diazoxide; n = 7 cells, 5 animals, t(7) = 13.4, p < 0.0001; paired t-test), which was diminished, but still detectable, when presynaptic inputs were blocked with bicuculline and TTX (Figure 7D; −53 ± 1.4 mV baseline vs. −62 ± 2 mV diazoxide + TTX + bicuculine; n = 8 cells, 6 animals, F(1.7, 12.5) = 25.8, p < 0.001; RMP vs. TTX + bicuculline + diazoxide q(7) = 8.7, p < 0.01; TTX + bicuculline vs. TTX + bicuculline + diazoxide q(7) = 7.6, p < 0.01; ANOVA with post hoc Tukey's shows significant changes in the membrane potential by diazoxide in the presence of presynaptic blockers). In the absence of presynaptic blockers, diazoxide hyperpolarized NAG neurons in LL mice to a similar degree as controls (Figure 7C; n = 7 cells, 7 animals, t(6) = 3.8, p < 0.01, paired t-test). However, diazoxide-mediated effects in NAG neurons were decreased in LL mice in the presence of TTX and bicuculline (Figure 7D; −10 mV in controls to −3 mV in LL; n = 6 cells, 6 animals, F(1.6, 8.4) = 6.8, p < 0.001, RMP vs. TTX + bicuculline + diazoxide q(5) = 5, p < 0.01; TTX + bicuculline vs. TTX + bicuculline + diazoxide q(5) = 5.1, p < 0.01; ANOVA with post hoc Tukey's shows significant changes in the membrane potential by diazoxide in the presence of presynaptic blockers). Together, these observations support the idea that the modest reduction in SUR1-containing KATP channel in LL offspring is largely mediated through direct effects on NAG neurons.
Postnatal UN had a pronounced effect on the electrophysiological response of NAG neurons to a SUR2 agonist, which paralleled the dramatic reduction in Sur2 expression at P30 (Figure 7A). Treatment of acute brain slices from controls with 50 μm of the SUR2 agonist pinacidil [49] caused a robust membrane hyperpolarization (Figure 7E; −50 ± 1.5 mV baseline vs. −59 ± 1.7 mV pinacidil; n = 7 cells, 5 animals, t(6) = 5.9, p < 0.01; paired t-test), which was diminished somewhat when recordings were performed in the presence of presynaptic blockers (Figure 7F; −49 ± 2 mV baseline vs. −55 ± 2 mV pinacidil + TTX + bicuculine; n = 5 cells, 4 animals, F(1.1, 4.5) = 13.3, p < 0.05, RMP vs. TTX + bicuculline + pinadicil q(4) = 3.8, p < 0.05; ANOVA with post hoc Tukey's shows significant changes in the membrane potential by pinadicil in the presence of presynaptic blockers). In slices from LL offspring, the effect of pinacidil alone to hyperpolarize NAG neurons was reduced (Figure 7E; −50 ± 2 mV baseline vs. −55 ± 2 mV pinacidil; n = 7 cells, 6 animals, p = 0.1072). The response to pinacidil in LL offspring was completely abrogated when recordings were performed in the presence of TTX and bicuculline (Figure 7F; −49 ± 1 mV baseline vs. −48 ± 1 mV pinacidil + TTX + bicuculine; n = 7 cells, 7 animals, p > 0.05). These findings are consistent with the idea that postnatal UN reduces the activity of SUR2-containing KATP channels through direct and effects on NAG neurons and indirect effects on their presynaptic inputs.
4. Discussion
We previously found that the maturation of the electrophysiological properties of NAG neurons coincides with the onset of homeostatic regulation of food intake in the fifth week of life [27], [50] and hypothesized that the delayed maturation could help to promote food intake needed to support rapid growth in the immediate post-weaning period. We tested this hypothesis by studying LL offspring, which have reduced body weight at weaning, and thus need to promote growth for a longer period than controls. We found that LL caused a delay in the switch in leptin responsiveness of NAG neurons from excitatory to inhibitory, as evidenced by the persistence of leptin-induced c-Fos immunoreactive NAG neurons (Figure 4B), increased density of AgRP + projections in the PVH (Figure 3B,C) and a diminished ability of leptin to suppress fasting-induced food intake at P30 (Figure 2B). Our discovery, that the appearance of functional KATP channels and the establishment of presynaptic inhibitory inputs after weaning are suppressed by postnatal UN, provides a mechanism whereby the maturation of homeostatic signals that provide negative feedback to orexigenic circuits is delayed until growth has caught up to controls.
4.1. Maturation of inhibitory systems for NAG neurons in the peri-weaning period
NAG neurons provide an important source of information about metabolic and nutritional status to feeding circuits. Activation of these neurons by neuronal and humoral signals of negative energy balance increases orexigenic drive [19], [20]. Conversely, signals of the energy replete state, such as leptin, inhibit fasting-induced increases in the actions of adult NAG neurons [34], [41], [46], [51]. While the field initially focused on direct action of metabolic signals such as ghrelin and leptin on NAG neuronal activity, there is a growing appreciation that modulation of presynaptic excitatory transmission is an important mechanism for regulating NAG neuronal activity [52], [53].
NAG neuronal differentiation and maturation spans gestational, lactational and peri-weaning periods [54]. The acquisition of a NAG peptidergic identity starts at E14 and extends through P25 [30], [55]. Lepr expression in NAG neurons begins several days after birth and progressively increases across lactation [30]. Leptin depolarizes NAG neurons in the second postnatal week [30], which likely underlies its neurotrophic effects at this developmental stage [28], [29]. The activity of NAG neurons during lactation is also promoted by excitatory inputs from other neurons, which reach mature levels by P13–15 [45].
Neuronal and humoral systems that inhibit NAG neurons do not develop until after weaning. Inhibitory synaptic inputs to NAG neurons increase across the peri-weaning period and reach mature levels in the fifth week of life [30], [45]. KATP channel subunit expression is initiated in the peri-weaning period, after which leptin only inhibits NAG neurons [30]. Kir6.2 is the predominant inward rectifying subunit expressed in the mediobasal hypothalamus throughout the post-weaning period Figure 6D and [56], [57]. SUR2-containing KATP channels appear to develop before SUR1-containing channels (Figure 6A,B), but electrophysiological recordings provide evidence that both types are active in NAG neurons by P30 (Figure 7D–F). Studies to determine whether SUR1-and SUR2-containing KATP channels are co-expressed in the same neuron, or whether they mark distinct subpopulations of NAG neurons is an important area for future research.
4.2. Impact of postnatal undernutrition on the formation of NAG neuronal circuits
While the number of Npy-GFP+ neurons in controls reached adult-like levels by P15, this did not occur until weaning in LL offspring (Figure 3A). We did not detect significant apoptosis in Npy-GFP+ neurons in this period (A.J.D.S. and L.M.Z., data not shown). Thus, the transient difference in the size of the Npy-GFP+ population is likely due to effects in LL pups. Since litter size manipulations are too late to impact the main period of NAG neurogenesis during gestation [55], reductions in the size of the Npy-GFP+ population in LL pups at P15 likely reflect a delay in the acquisition of an NPYergic identity in a subset of ARH neurons.
Undernutrition due to maternal caloric restriction [24], [31], [32], [58] or LL rearing [25], [59] leads to reduced serum leptin in pups during lactation, a period when leptin normally acts to promote axonal outgrowth from NAG neurons [28], [29]. The density of NPY/AgRP fibers in the PVH of undernourished pups is initially lower in the first two weeks of life [58], [60] but attains the level of controls by weaning [58]. Consistent with our observation that AgRP fiber density was higher in the PVH of LL pups at P28 (Figure 3B,C), studies that evaluated NPY/AgRP immunoreactivity in the PVH in the post-weaning period found that fiber density was the same [58] or increased [23], [24], [25]) in undernourished offspring. The transient decrease in PVH projections in the early postnatal period could reflect a developmental delay in the maturation of some NAG neurons, as defined by the acquisition of an NPYergic identity (Figure 3A). It is also possible that reduced leptin impairs outgrowth from differentiated NAG neurons that project to pre-autonomic neurons in the PVH [29]. We hypothesize that persistent activation of NAG neurons by leptin in LL pups in the peri-weaning period, albeit at lower levels, promotes the accumulation of AgRP+ fibers in the PVH. Determining whether increased AgRP+ fiber density is a result of modest effects on the entire NAG population, or an outsized impact on a discrete subpopulation is an important area for future research.
4.3. Postnatal undernutrition delays the maturation of pre- and postsynaptic inhibitory systems in NAG neurons
The impact of postnatal UN on KATP channel subunit expression at P30 was dramatic – Sur2 and Kir6.2 expression were reduced by 90% and 70% respectively (Figure 7A) and pinacidil failed to elicit a direct electrophysiological response in NAG neurons (Figure 7F). The observation that reductions in pinacidil-induced hyperpolarization of NAG neurons from LL offspring were pronounced when recordings were performed in the presence of TTX and bicuculline (Figure 7E,F) supports the idea that that NAG neurons are preferentially sensitive to the impacts of postnatal UN.
The effect on SUR1 was more modest – the 20% decrease detected by PCR was not significant (Figure 7A). The electrophysiological response to diazoxide was not altered in LL offspring when recordings were performed in the absence of TTX and bicuculline (Figure 7C), but was reduced in their presence (Figure 7D), consistent with the idea that the main effect of postnatal UN on SUR1-containing channels is mediated directly on NAG neurons, as is the case for SUR2-containing channels. The discrepancy between our PCR-based expression studies and electrophysiological recordings could be explained by SUR1 expression in non-NAG neurons that are included in the microdissected ARH region [61]. The modest impact of LL on SUR1 expression is also consistent with observations that regulation of hepatic glucose production, an important function of ARH SUR1-containing KATP channels [62], [63], is not impaired in LL offspring [25].
In addition to direct impacts on intrinsic firing properties, postnatal UN also influenced synaptic inputs to NAG neurons. Both excitatory and inhibitory inputs were decreased in LL offspring (Figure 5). Inhibitory currents were reduced by similar degree whether recordings were performed in the presence or absence of TTX (Figure 5A,B), consistent with a broad-based effect. In contrast, the impact of LL on excitatory currents was more pronounced in recordings that blocked presynaptic action potentials (Figure 5C,D). The very low frequency of mEPSCs (<0.2 Hz) in NAG neurons from LL offspring supports the idea that most excitatory signals are transmitted via inputs from action potentials (Figure 5C,D).
The timing of the maturation of pre- and postsynaptic inhibitory systems in NAG neurons is closely linked to the onset of homeostatic regulation of food intake in control and LL offspring (this paper and [30], [45]). In theory, deficits in intrinsic (Figure 7) and/or extrinsic (Figure 5) inhibitory signals to NAG neurons could contribute to the failure of leptin to suppress food intake at P30 in LL offspring (Figure 2). Evidence that impacts of leptin on food intake are primarily mediated through effects on presynaptic GABAergic neurons [47] supports the idea that delays in the establishment of inhibitory inputs are responsible for deficits in homeostatic regulation of feeding circuits in UN offspring. Though leptin-induced hyperpolarization of NAG neurons via KATP channel activation may not be the primary mechanism for food intake suppression in the post-weaning period, it likely marks the terminal differentiation of NAG neurons and the end of leptin's trophic actions on axonal outgrowth. While “developmental delay” normally has negative connotations, suppression of KATP channel expression due to postnatal UN may be beneficial if it extends the window during which the normal developmental program can be reinstated and homeostatic regulation of feeding restored.
4.4. Maturation of inhibitory systems for NAG neurons is linked to growth
Because inhibitory systems for NAG neurons do not fully mature until the post-weaning period [30], [45], signals that stimulate feeding circuits during lactation are largely unopposed [64]. In theory, delaying the onset of leptin-mediated inhibition of NAG neurons could promote food intake needed to support catch-up growth in LL offspring. However, as studies in ob/ob mice provide compelling evidence that leptin signals per se are not required for catch-up growth [65], other factors must be involved. In theory, delays in KATP channel expression and/or reduced inhibitory inputs could limit the ability of other homeostatic signals (i.e. glucose and insulin) or neuronal circuits to suppress the orexigenic actions of NAG neurons. However, it is also possible that increased NAG neuronal activity is not necessary for catch-up growth. The elucidation of factors and circuits regulating catch-up growth is an important area for future research.
We previously reported that the initiation of KATP channel subunit expression, the switch in leptin's effect on NAG neurons, and the ingrowth of inhibitory inputs to NAG neurons coincided with the development of homeostatic feedback to feeding circuits. Here we found that this close relationship is maintained even when the maturation of NAG neurons is delayed by postnatal UN. The observation that KATP channel expression is initiated at the point when body weight and adiposity of LL offspring attained the levels exhibited by controls at weaning raises the possibility that a critical threshold of growth- or fat-related signal(s) might be required to initiate the final stages of the maturation of pre- and postsynaptic inhibitory systems for NAG neurons.
4.5. Implications for other circuits impacted by UN
The idea that postnatal UN delays the maturation of circuits is not unique to feeding, as it has been reported in the context of both reproduction and cognition. Postnatal UN leads to impairments in the development of hypothalamic circuits regulating reproduction and delays in the onset of puberty [59], [66]. Similarly, UN is associated with deficits in cognitive function at weaning, which can be recovered following catch-up growth [6], [8]. There is evidence to support the hypothesis that leptin acts a permissive signal for the maturation of circuits regulating growth, puberty and cognitive development [59], [67], [68], [69], [70], [71]. As the maturation of hippocampal neurons in the peri-weaning period is associated with a switch in leptin's effects on excitatory synaptic transmission [72], [73], [74], [75], it raises the possibility that delayed cognitive development due to UN might be also be mediated by effects on the acquisition of membrane properties of adult neurons. If a common mechanism governs the maturation of circuits regulating feeding, reproductive and cognitive behaviors, it could provide a means to ensure that energetically-costly processes do not divert an organism from growth when food supplies are scarce.
5. Conclusion
Elucidation of the mechanism responsible for the developmental delay caused by undernutrition and the identification of signals that can reinstate the normal maturation program are important areas for future research. This knowledge could lead to the development of effective strategies support the healthy growth and development of small for gestational age babies.
Acknowledgments
This work was supported by American Diabetes Association Grant 7-11-BS179 (L.M.Z.) and 7-13-MI-06 (A.F.B. and K.L.G.) and NIH-Grants R01 DK089038 (L.M.Z.), P30 DK063608 Columbia Diabetes and Endocrinology Research Center (P30 DK063608), P51 OD011092 Oregon National Primate Research Center, and RO1 DK079194 (K.L.G.).
Contributor Information
Alain Juan De Solis, Email: aj2495@cumc.columbia.edu.
Arian F. Baquero, Email: arbq@novonordisk.com.
Camdin M. Bennett, Email: bennecam@ohsu.edu.
Kevin L. Grove, Email: kvgo@novonordisk.com.
Lori M. Zeltser, Email: lz146@cumc.columbia.edu.
Conflict of interest
None declared.
References
- 1.Warren M.P. Effects of undernutrition on reproductive function in the human. Endocrine Reviews. 1983;4(4):363–377. doi: 10.1210/edrv-4-4-363. [DOI] [PubMed] [Google Scholar]
- 2.Lumey L.H., Stein A.D., Susser E. Prenatal famine and adult health. Annual Review of Public Health. 2011;32:237–262. doi: 10.1146/annurev-publhealth-031210-101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ojha S., Robinson L., Symonds M.E., Budge H. Suboptimal maternal nutrition affects offspring health in adult life. Early Human Development. 2013;89(11):909–913. doi: 10.1016/j.earlhumdev.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Georgieff M.K., Brunette K.E., Tran P.V. Early life nutrition and neural plasticity. Development and Psychopathology. 2015;27(2):411–423. doi: 10.1017/S0954579415000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown J.L., Pollitt E. Malnutrition, poverty and intellectual development. Scientific American February. 1996:38–43. doi: 10.1038/scientificamerican0296-38. [DOI] [PubMed] [Google Scholar]
- 6.Castro C.A., Tracy M., Rudy J.W. Early-life undernutrition impairs the development of the learning and short-term memory processes mediating performance in a conditional-spatial discrimination task. Behavioural Brain Research. 1989;32(3):255–264. doi: 10.1016/s0166-4328(89)80058-0. [DOI] [PubMed] [Google Scholar]
- 7.Pongcharoen T., Ramakrishnan U., DiGirolamo A.M., Winichagoon P., Flores R., Singkhornard J. Influence of prenatal and postnatal growth on intellectual functioning in school-aged children. Archives of Pediatrics and Adolescent Medicine. 2012;166(5):411–416. doi: 10.1001/archpediatrics.2011.1413. [DOI] [PubMed] [Google Scholar]
- 8.Akitake Y., Katsuragi S., Hosokawa M., Mishima K., Ikeda T., Miyazato M. Moderate maternal food restriction in mice impairs physical growth, behavior, and neurodevelopment of offspring. Nutrition Research. 2015;35(1):76–87. doi: 10.1016/j.nutres.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Desai M., Gayle D., Babu J., Ross M.G. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2005;288(1):R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 10.Ong K.K., Loos R.J. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatrica. 2006;95(8):904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez-Chillaron J.C., Patti M.E. To catch up or not to catch up: is this the question? Lessons from animal models. Current Opinion in Endocrinology, Diabetes and Obesity. 2007;14(1):23–29. doi: 10.1097/MED.0b013e328013da8e. [DOI] [PubMed] [Google Scholar]
- 12.Taveras E.M., Rifas-Shiman S.L., Sherry B., Oken E., Haines J., Kleinman K. Crossing growth percentiles in infancy and risk of obesity in childhood. Archives of Pediatrics and Adolescent Medicine. 2011;165(11):993–998. doi: 10.1001/archpediatrics.2011.167. [DOI] [PubMed] [Google Scholar]
- 13.Widdowson E.M., McCance R.A. The effect of finite periods of undernutrition at different ages on the composition and subsequent development of the rat. Proceedings of the Royal Society B: Biological Sciences. 1963;158:329–342. doi: 10.1098/rspb.1963.0051. [DOI] [PubMed] [Google Scholar]
- 14.Oscai L.B., McGarr J.A. Evidence that the amount of food consumed in early life fixes appetite in the rat. American Journal of Physiology. 1978;235(3):R141–R144. doi: 10.1152/ajpregu.1978.235.3.R141. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez-Chillaron J.C., Hernandez-Valencia M., Lightner A., Faucette R.R., Reamer C., Przybyla R. Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia. 2006;49(8):1974–1984. doi: 10.1007/s00125-006-0311-7. [DOI] [PubMed] [Google Scholar]
- 16.Prior L.J., Velkoska E., Watts R., Cameron-Smith D., Morris M.J. Undernutrition during suckling in rats elevates plasma adiponectin and its receptor in skeletal muscle regardless of diet composition: a protective effect? International Journal of Obesity (London) 2008;32(10):1585–1594. doi: 10.1038/ijo.2008.141. [DOI] [PubMed] [Google Scholar]
- 17.Aponte Y., Atasoy D., Sternson S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature Neuroscience. 2011;14(3):351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krashes M.J., Koda S., Ye C., Rogan S.C., Adams A.C., Cusher D.S. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. Journal of Clinical Investigation. 2011 doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cone R.D., Cowley M.A., Butler A.A., Fan W., Marks D.L., Low M.J. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. International Journal of Obesity and Related Metabolic Disorders. 2001;25(Suppl. 5):S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 20.Cowley M.A., Cone R.D., Enriori P., Louiselle I., Williams S.M., Evans A.E. Electrophysiological actions of peripheral hormones on melanocortin neurons. Annals of the New York Academy of Sciences. 2003;994:175–186. doi: 10.1111/j.1749-6632.2003.tb03178.x. [DOI] [PubMed] [Google Scholar]
- 21.Grove K.L., Grayson B.E., Glavas M.M., Xiao X.Q., Smith M.S. Development of metabolic systems. Physiology & Behavior. 2005;86(5):646–660. doi: 10.1016/j.physbeh.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 22.Bouret S.G. Nutritional programming of hypothalamic development: critical periods and windows of opportunity. International Journal of Obesity Supplements. 2012;2:S19–S24. doi: 10.1038/ijosup.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plagemann A., Harder T., Rake A., Waas T., Melchior K., Ziska T. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. Journal of Neuroendocrinology. 1999;11(7):541–546. doi: 10.1046/j.1365-2826.1999.00357.x. [DOI] [PubMed] [Google Scholar]
- 24.Yura S., Itoh H., Sagawa N., Yamamoto H., Masuzaki H., Nakao K. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metabolism. 2005;1(6):371–378. doi: 10.1016/j.cmet.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Patterson C.M., Bouret S.G., Park S., Irani B.G., Dunn-Meynell A.A., Levin B.E. Large litter rearing enhances leptin sensitivity and protects selectively bred diet-induced obese rats from becoming obese. Endocrinology. 2010;151(9):4270–4279. doi: 10.1210/en.2010-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahima R.S., Prabakaran D., Flier J.S. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. Journal of Clinical Investigation. 1998;101(5):1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mistry A.M., Swick A., Romsos D.R. Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. American Journal of Physiology. 1999;277(3 Pt 2):R742–R747. doi: 10.1152/ajpregu.1999.277.3.R742. [DOI] [PubMed] [Google Scholar]
- 28.Bouret S.G., Draper S.J., Simerly R.B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 29.Bouyer K., Simerly R.B. Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice. Journal of Neuroscience. 2013;33(2):840–851. doi: 10.1523/JNEUROSCI.3215-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baquero A.F., de Solis A.J., Lindsley S.R., Kirigiti M.A., Smith M.S., Cowley M.A. Developmental switch of leptin signaling in arcuate nucleus neurons. Journal of Neuroscience. 2014;34(30):9982–9994. doi: 10.1523/JNEUROSCI.0933-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bautista C.J., Boeck L., Larrea F., Nathanielsz P.W., Zambrano E. Effects of a maternal low protein isocaloric diet on milk leptin and progeny serum leptin concentration and appetitive behavior in the first 21 days of neonatal life in the rat. Pediatric Research. 2008;63(4):358–363. doi: 10.1203/01.pdr.0000304938.78998.21. [DOI] [PubMed] [Google Scholar]
- 32.Delahaye F., Breton C., Risold P.Y., Enache M., Dutriez-Casteloot I., Laborie C. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149(2):470–475. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- 33.Spanswick D., Smith M.A., Groppi V.E., Logan S.D., Ashford M.L. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390(6659):521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- 34.van den Top M., Lee K., Whyment A.D., Blanks A.M., Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nature Neuroscience. 2004;7(5):493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- 35.van den Pol A.N., Yao Y., Fu L.Y., Foo K., Huang H., Coppari R. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. Journal of Neuroscience. 2009;29(14):4622–4639. doi: 10.1523/JNEUROSCI.3249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogt M.C., Paeger L., Hess S., Steculorum S.M., Awazawa M., Hampel B. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell. 2014 doi: 10.1016/j.cell.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez M., Seoane L.M., Tovar S., Garcia M.C., Nogueiras R., Dieguez C. A possible role of neuropeptide Y, agouti-related protein and leptin receptor isoforms in hypothalamic programming by perinatal feeding in the rat. Diabetologia. 2005;48(1):140–148. doi: 10.1007/s00125-004-1596-z. [DOI] [PubMed] [Google Scholar]
- 38.Hahn T.M., Breininger J.F., Baskin D.G., Schwartz M.W. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature Neuroscience. 1998;1(4):271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 39.Baskin D.G., Breininger J.F., Schwartz M.W. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes. 1999;48(4):828–833. doi: 10.2337/diabetes.48.4.828. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno T.M., Mobbs C.V. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology. 1999;140(2):814–817. doi: 10.1210/endo.140.2.6491. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K.A., Cone R.D. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146(3):1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- 42.Atasoy D., Betley J.N., Su H.H., Sternson S.M. Deconstruction of a neural circuit for hunger. Nature. 2012 doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betley J.N., Cao Z.F., Ritola K.D., Sternson S.M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155(6):1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaisse C., Halaas J.L., Horvath C.M., Darnell J.E., Jr., Stoffel M., Friedman J.M. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nature Genetics. 1996;14(1):95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 45.Baquero A.F., Kirigiti M.A., Baquero K.C., Lee S.J., Smith M.S., Grove K.L. Developmental changes in synaptic distribution in arcuate nucleus neurons. Journal of Neuroscience. 2015;35(22):8558–8569. doi: 10.1523/JNEUROSCI.0058-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinto S., Roseberry A.G., Liu H., Diano S., Shanabrough M., Cai X. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304(5667):110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 47.Vong L., Ye C., Yang Z., Choi B., Chua S., Jr., Lowell B.B. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71(1):142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van den Top M., Lyons D.J., Lee K., Coderre E., Renaud L.P., Spanswick D. Pharmacological and molecular characterization of ATP-sensitive K(+) conductances in CART and NPY/AgRP expressing neurons of the hypothalamic arcuate nucleus. Neuroscience. 2007;144(3):815–824. doi: 10.1016/j.neuroscience.2006.09.059. [DOI] [PubMed] [Google Scholar]
- 49.Shindo T., Yamada M., Isomoto S., Horio Y., Kurachi Y. SUR2 subtype (A and B)-dependent differential activation of the cloned ATP-sensitive K+ channels by pinacidil and nicorandil. British Journal of Pharmacology. 1998;124(5):985–991. doi: 10.1038/sj.bjp.0701927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Proulx K., Richard D., Walker C.D. Leptin regulates appetite-related neuropeptides in the hypothalamus of developing rats without affecting food intake. Endocrinology. 2002;143(12):4683–4692. doi: 10.1210/en.2002-220593. [DOI] [PubMed] [Google Scholar]
- 51.Kohno D., Gao H.Z., Muroya S., Kikuyama S., Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003;52(4):948–956. doi: 10.2337/diabetes.52.4.948. [DOI] [PubMed] [Google Scholar]
- 52.Yang Y., Atasoy D., Su H.H., Sternson S.M. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146(6):992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu T., Kong D., Shah B.P., Ye C., Koda S., Saunders A. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73(3):511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeltser L.M. Developmental influences on circuits programming susceptibility to obesity. Frontiers in Neuroendocrinology. 2015 doi: 10.1016/j.yfrne.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Padilla S.L., Carmody J.S., Zeltser L.M. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nature Medicine. 2010;16(4):403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunn-Meynell A.A., Rawson N.E., Levin B.E. Distribution and phenotype of neurons containing the ATP-sensitive K+ channel in rat brain. Brain Research. 1998;814(1–2):41–54. doi: 10.1016/s0006-8993(98)00956-1. [DOI] [PubMed] [Google Scholar]
- 57.Thomzig A., Laube G., Pruss H., Veh R.W. Pore-forming subunits of K-ATP channels, Kir6.1 and Kir6.2, display prominent differences in regional and cellular distribution in the rat brain. Journal of Comparative Neurology. 2005;484(3):313–330. doi: 10.1002/cne.20469. [DOI] [PubMed] [Google Scholar]
- 58.Rocha M.L., Fernandes P.P., Lotufo B.M., Manhaes A.C., Barradas P.C., Tenorio F. Undernutrition during early life alters neuropeptide Y distribution along the arcuate/paraventricular pathway. Neuroscience. 2014;256:379–391. doi: 10.1016/j.neuroscience.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 59.Castellano J.M., Bentsen A.H., Sanchez-Garrido M.A., Ruiz-Pino F., Romero M., Garcia-Galiano D. Early metabolic programming of puberty onset: impact of changes in postnatal feeding and rearing conditions on the timing of puberty and development of the hypothalamic kisspeptin system. Endocrinology. 2011;152(9):3396–3408. doi: 10.1210/en.2010-1415. [DOI] [PubMed] [Google Scholar]
- 60.Coupe B., Amarger V., Grit I., Benani A., Parnet P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology. 2010;151(2):702–713. doi: 10.1210/en.2009-0893. [DOI] [PubMed] [Google Scholar]
- 61.Ibrahim N., Bosch M.A., Smart J.L., Qiu J., Rubinstein M., Ronnekleiv O.K. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology. 2003;144(4):1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- 62.Pocai A., Lam T.K., Gutierrez-Juarez R., Obici S., Schwartz G.J., Bryan J. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434(7036):1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 63.Lam T.K., Pocai A., Gutierrez-Juarez R., Obici S., Bryan J., Aguilar-Bryan L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nature Medicine. 2005;11(3):320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 64.Melnick I., Pronchuk N., Cowley M.A., Grove K.L., Colmers W.F. Developmental switch in neuropeptide y and melanocortin effects in the paraventricular nucleus of the hypothalamus. Neuron. 2007;56(6):1103–1115. doi: 10.1016/j.neuron.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 65.Cottrell E.C., Martin-Gronert M.S., Fernandez-Twinn D.S., Berends L.M., Ozanne S.E. Leptin independent programming of adult body weight and adiposity in mice. Endocrinology. 2011 doi: 10.1210/en.2010-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caron E., Ciofi P., Prevot V., Bouret S.G. Alteration in neonatal nutrition causes perturbations in hypothalamic neural circuits controlling reproductive function. Journal of Neuroscience. 2012;32(33):11486–11494. doi: 10.1523/JNEUROSCI.6074-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bray G.A. Regulation of energy balance: studies on genetic, hypothalamic and dietary obesity. Proceedings of the Nutrition Society. 1982;41(2):95–108. doi: 10.1079/pns19820018. [DOI] [PubMed] [Google Scholar]
- 68.Cheung C.C., Thornton J.E., Nurani S.D., Clifton D.K., Steiner R.A. A reassessment of leptin's role in triggering the onset of puberty in the rat and mouse. Neuroendocrinology. 2001;74(1):12–21. doi: 10.1159/000054666. [DOI] [PubMed] [Google Scholar]
- 69.Li X.L., Aou S., Oomura Y., Hori N., Fukunaga K., Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113(3):607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 70.Paz-Filho G.J., Babikian T., Asarnow R., Delibasi T., Esposito K., Erol H.K. Leptin replacement improves cognitive development. PLoS One. 2008;3(8):e3098. doi: 10.1371/journal.pone.0003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roa J., Garcia-Galiano D., Castellano J.M., Gaytan F., Pinilla L., Tena-Sempere M. Metabolic control of puberty onset: new players, new mechanisms. Molecular Cell Endocrinology. 2010;324(1–2):87–94. doi: 10.1016/j.mce.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 72.Shanley L.J., Irving A.J., Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. Journal of Neuroscience. 2001;21(24) doi: 10.1523/JNEUROSCI.21-24-j0001.2001. RC186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Durakoglugil M., Irving A.J., Harvey J. Leptin induces a novel form of NMDA receptor-dependent long-term depression. Journal of Neurochemistry. 2005;95(2):396–405. doi: 10.1111/j.1471-4159.2005.03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu L., Rensing N., Yang X.F., Zhang H.X., Thio L.L., Rothman S.M. Leptin inhibits 4-aminopyridine- and pentylenetetrazole-induced seizures and AMPAR-mediated synaptic transmission in rodents. Journal of Clinical Investigation. 2008;118(1):272–280. doi: 10.1172/JCI33009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oomura Y., Hori N., Shiraishi T., Fukunaga K., Takeda H., Tsuji M. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27(11):2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]