ABSTRACT
Psoriasis is a chronic, immune-mediated disease characterized by itchy, scaly, and often painful plaques in the skin. Psoriasis can have significant psychosocial burdens and increased risks for numerous comorbidities, including diabetes, hypertension, and cardiovascular disease, particularly in patients with moderate-to-severe disease. Dermatology nurse practitioners and physician assistants are an important part of the healthcare team, contributing to all aspects of psoriasis management. This review reinforces the unique aspects of care that nurse practitioners and physician assistants provide to patients with psoriasis, such as facilitating conversations about managing disease, setting appropriate expectations, and considering treatment options, including when treatment response or tolerability is suboptimal. The importance of relationship building is stressed. Patient management topics discussed include helpful tips about assessing treatment options, initiating biologic therapy, optimizing patient adherence, and managing comorbidities. Also reviewed are how to deal with common barriers including lack of knowledge about psoriasis or making healthy lifestyle changes, fear of injections or side effect risks, lack of health insurance, and concerns about treatment costs. Overall, by forming meaningful relationships and engaging patients in their psoriasis care, nurse practitioners and physician assistants can help to optimize clinical efficacy outcomes and consistently manage moderate-to-severe psoriasis and its comorbidities over the patient’s life course.
Key words: Biologics, Nurse Practitioner, Patient Management, Physician Assistant, Plaque Psoriasis, Psoriasis Therapy

Psoriasis is a chronic, immune-mediated disease that affects roughly 7.4 million adults in the United States (~3% of the U.S. population; Rachakonda, Schupp, & Armstrong, 2014). Psoriasis is associated with physical symptoms including red, scaly, itchy, and painful skin lesions (Langley, Krueger, & Griffiths, 2005), and many patients experience adverse psychological effects, including poor body image, stress, embarrassment, and depression (Feldman, Behnam, Behnam, & Koo, 2005; Young, 2005). Patients with moderate-to-severe psoriasis are also at increased risk for numerous comorbidities, likely because of increased systemic levels of inflammation and the chronic nature of the disease, including psoriatic arthritis, metabolic syndrome, diabetes, obesity, hypertension, and cardiovascular disease (Armstrong, Harskamp, & Armstrong, 2012, 2013a, 2013b; Armstrong, Schupp, & Bebo, 2012; Gottlieb, Chao, & Dann, 2008).
To effectively manage psoriasis, patients and healthcare providers must work together to identify treatment goals that take into account psoriasis severity (both in terms of skin involvement and effects on quality of life), comorbidities, potential side effects of medications, treatment costs, and patient preferences (Baker et al., 2013). When such goals are not well defined or followed, patient dissatisfaction is high, and adherence to treatment is low, resulting in suboptimal clinical outcomes (Armstrong, Robertson, Wu, Schupp, & Lebwohl, 2013; Baker et al., 2013). On the basis of these findings, it has been recognized that increased patient advocacy and education are needed to ensure that patients can make informed decisions about their psoriasis and how best to manage it, along with having access to their preferred methods of treatment (Armstrong, Robertson, et al., 2013).
Given that demands on physicians are numerous and time is limited, nurse practitioners (NPs) and physician assistants (PAs) are valuable assets to healthcare teams because they may be able to spend more time with patients (Courtenay, Carey, Stenner, Lawton, & Peters, 2011). It is well established that incorporation of NPs and PAs into dermatology practices can reduce wait times and increase access to care. In addition, in our experience, most NPs and PAs see fewer patients per day than their physician colleagues, allowing expanded time and consultation options for patients. Thus, NPs and PAs have the opportunity to provide patients with both increased access to dermatologic care as well as care related to comorbidities associated with psoriasis. Because NPs and PAs are often perceived to be approachable and easy to talk with, they are excellent candidates to provide patients and their families with the resources necessary to make informed treatment choices, adopt healthy practices, and ultimately achieve significant, lasting improvements in their psoriasis symptoms and overall well-being (Courtenay et al., 2011).
The objective of this review is to discuss the wide-ranging roles that NPs and PAs can play in caring for patients with moderate-to-severe psoriasis including considerations for selecting the best treatment options. In addition, other key contributions made by NPs and PAs, including development of ongoing patient–provider relationships, facilitation of conversations about disease management, and setting appropriate expectations, are discussed.
RELATIONSHIP BUILDING: THE KEY TO SUCCESS
Evidence from studies evaluating nurse-led care in dermatology has shown that patients generally have very positive experiences when visiting a nurse, including reporting that the visits increased their knowledge about their condition and improved their ability to cope with their disease (Courtenay & Carey, 2006). Such findings suggest that, by patients and their caregivers establishing and maintaining a trusting relationship with an NP or a PA, the patient’s quality of care can be improved. These relationships should start with discussions of the patient’s short-term goals for disease improvement, skin clearance, the importance of maintaining good health and wellness habits, and awareness of other lives, issues that can affect individuals with chronic diseases.
All parties, including the patient’s family members and caregivers, should be actively involved in such discussions, as the negative impact of psoriasis can affect all aspects of life, potentially impairing patients’ overall well-being and the well-being of everyone around them (Martínez-García et al., 2014). Thus, NPs and PAs must take time to get to know patients and their families and make a point of inviting family members to periodic clinic visits. Practitioners also need to establish how psoriasis affects patients’ relationships with their close companions, both in the short term and over the course of their life. While building these interpersonal relationships, healthcare providers should work to establish a long-term treatment program that meets a patient’s goals for sustained skin clearance, disease management, preventing or managing comorbidities, and side effect minimization. When discussing long-term goals, healthcare providers need to maintain awareness that psoriasis is affecting their patients’ lives at all times over the course of their lives, not only for the few moments they meet at each clinic visit.
When NPs and PAs successfully establish a trusting environment, patients feel more comfortable having open, honest dialogues about sensitive topics related to psoriasis, such as pain, itching, effects on interpersonal/sexual relationships, impact on work environment, mental health issues (e.g., depression), and the financial impact of the disease and/or treatments (Kimball, Jacobson, Weiss, Vreeland, & Wu, 2005). In turn, NPs and PAs can develop an in-depth knowledge of their patients and can begin to recognize nonverbal cues, which can elicit more probing to help improve overall patient management. With each visit, it is critical for providers to continue to foster trust and to let patients know that the providers are committed to caring for all aspects of psoriasis including its physical and psychological comorbidities.
During each visit, NPs and PAs should ask patients about signs and symptoms of psoriasis, including arthralgia. They should examine a patient’s skin thoroughly, evaluate the patient’s overall health, and explain the reasoning behind ordering any tests, procedures, changes in therapy, or referrals. NPs and PAs must also promote patient engagement and self-care by continually educating patients and their families about psoriasis and its ongoing management. Patients and their supporters should be provided with literature that is suited to their educational background, level of interest in learning about psoriasis, and personal preferences for receiving information depending on their stage of disease. For example, some patients may prefer to read brochures or articles in print, whereas others may appreciate being directed to useful Web sites (e.g., The National Psoriasis Foundation: www.psoriasis.org). NPs and PAs should always ensure that the guidance and educational materials they are providing are consistent with current findings from the psoriasis literature. By staying abreast of new research and sharing timely information with patients, NPs and PAs can reinforce their knowledge and credibility as competent, if not expert, caregivers.
In addition to using technology to communicate key educational messages, many dermatology practices are using electronic communications (e.g., email and text messaging) and periodic telephone calls to follow up on patients and remind them about treatment schedules. Such reminders can improve adherence and reinforce with patients that their healthcare team is there to support them. In turn, patients and their caregivers should be encouraged to contact their NP or PA should any concerns arise.
PATIENT MANAGEMENT: SETTING REASONABLE EXPECTATIONS
In our experience, patients tend to fall into one of three main groups, and NPs and PAs may need to tailor their psoriasis management approaches differently for patients in each group. The first group consists of the increasing number of patients who are highly educated and motivated to manage their psoriasis. Typically, these patients do research before and after each visit and often have literature in hand when they arrive at the clinic. Although it is rewarding to work with such engaged patients, it is important for providers to make sure these individuals are guided toward reliable online information, are aware of myths, and are steered away from sources, such as blogs, that can sometimes give misinformation and/or create unrealistic expectations regarding efficacy, safety, and appropriateness of different treatments.
The second group of patients is cautious and/or reluctant about adopting new treatment regimens. These patients are generally hopeful that they can work with providers to improve their symptoms, but they are likely to need more reassurance and professional guidance about the treatment approach that is being recommended. These patients may benefit from extra information sessions with healthcare providers or patient groups geared toward sharing findings from current research.
The third patient group tends to rely exclusively on their medical team for guidance. They typically want to have minimal involvement in decisions related to their disease and its treatment. Such individuals tend to be more trusting of their providers and less risk-averse. However, it is still important for healthcare providers to give these patients clear explanations and to encourage them to be involved in their own care.
Regardless of the patient group, it is essential for NPs and PAs to ensure that all patients and their families have realistic expectations about the disease and its management. From the outset, patients should be counseled as to whether complete clearance is likely to be achievable using different treatment strategies. For example, most patients with psoriasis will not achieve satisfactory clear skin with traditional treatments (e.g., topical agents, phototherapy, retinoids, and methotrexate; Al-Suwaidan & Feldman, 2000), whereas newer biologic agents may provide substantially higher rates of clearance for patients with moderate-to-severe psoriasis with a less intensive administration schedule compared with older therapeutic options. For example, application of topical therapies is time consuming, and patient dissatisfaction can lead to poor compliance (Augustin, Holland, Dartsch, Langenbruch, & Radtke, 2011). In addition, phototherapy can require the patient to travel to a treatment center several times a week. Thus, therapy with oral or injectable agents reinstates patients with time that they would otherwise have had to use for self-care. Overall, it is important to review all treatment options throughout the course of care and ensure that patients understand the rationale behind making changes to treatment (e.g., because of side effects, suboptimal efficacy, or other concerns) and allow them opportunities to ask questions and express concerns.
With any treatment, it is important for NPs and PAs to emphasize the need for long-term follow-up and adherence. It should be made clear to patients that treatment approaches may change over time for a variety of reasons, including availability of better treatments, loss of efficacy, development of side effects, changing disease course, or changes in insurance coverage/life situation. This is a natural part of managing any disease where a cure is not yet available, and switching therapy has been shown to be a common and effective strategy for patients when their psoriasis is not optimally controlled (Lecluse, de Groot, Bos, & Spuls, 2009; Leman & Burden, 2012; Norlin, Steen Carlsson, Persson, & Schmitt-Egenolf, 2012; Yeaw et al., 2014). In our experience, it is estimated that, about 70% of the time, healthcare providers (including dermatologists, NPs, PAs, primary care physicians, and consultant specialists [e.g., rheumatologists]) are the ones who initially suggest changing therapy. The other 30% of the time, it is a joint discussion between healthcare providers, the patient, and his or her family or caregivers.
Once any psoriasis therapy is initiated, adherence can be a major challenge, with recent literature estimating nonadherence rates of up to 40%–50% (Bewley & Page, 2011; Reich & Daudén, 2014; Richards, Fortune, & Griffiths, 2006). Nonadherence to therapy can occur either unintentionally, because of forgetfulness or practical difficulties with administering treatment, or intentionally, because patients become frustrated with factors such as hassles of use, obtaining refills, access to convenient appointments, side effects, lack of efficacy, and cost (Figure 1; Bewley & Page, 2011; Richards et al., 2006; Zschocke et al., 2014). Patients with more severe forms of psoriasis are often less adherent than patients with less severe disease, possibly because these patients have tried several therapies in the past that were ineffective (Augustin et al., 2011). This can lead to a cycle of suboptimal care: as patients lose confidence in the efficacy of treatment, they are more likely to become nonadherent, thereby reducing the efficacy of treatment (Bewley & Page, 2011).
FIGURE 1.
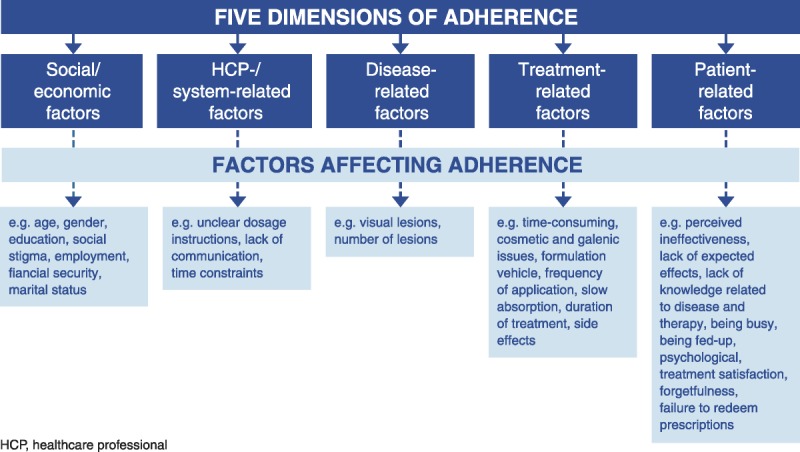
Barriers to adherence in dermatology (reprinted from Zschocke, Mrowietz, Karakasili, & Reich, 2014, with permission from John Wiley & Sons, Inc.).
NPs and PAs should be aware of factors that influence adherence and discuss them with patients and their families during early follow-up visits after initiation of any new treatment (Davis, Lin, Yu, Balkrishnan, & Feldman, 2014). Early and often during the course of treatment, providers should assess whether patients are satisfied with their treatment by asking how they are doing and whether their skin is improving. To minimize the risk of nonadherence, NPs and PAs should be proactive in discussing potential life events that could interrupt psoriasis treatment, such as pregnancy, changes in insurance or employment status, and relocation. If patients are experiencing psoriasis flares, it is important to determine whether this is because of nonadherence or other factors, such as increased stress, hormonal changes, or illness (e.g., strep throat or other infection; Gudjonsson, Thorarinsson, Sigurgeirsson, Kristinsson, & Valdimarsson, 2003; Xhaja, Shkodrani, Frangaj, Kuneshka, & Vasili, 2014). Once these factors have been ruled out, the possibility of treatment failure should be considered, but not assumed, if the patient continues to experience flares. Overall, developing and maintaining a mutually trusting patient–provider relationship has been shown to contribute to improved adherence, clinical outcomes, and quality of life for patients with psoriasis (Bewley & Page, 2011).
ASSESSING TREATMENT OPTIONS
When assessing the severity of psoriasis and determining the best treatment options for a patient, it is important to consider not only the physical manifestations of psoriasis (e.g., body surface area affected or Psoriasis Area and Severity Index [PASI] score) but also the psychosocial impact of the disease and the effects of both physical and psychosocial symptoms on the patient’s overall life course (Finlay, 2005; Mrowietz et al., 2011). Patients with mild disease should be treated, or at least started, with topical therapy (Menter et al., 2009a), whereas patients with moderate-to-severe disease are candidates for phototherapy (Menter et al., 2010), traditional systemic agents (e.g., methotrexate, cyclosporine, acitretin; Menter et al., 2009b), newer systemic agents (apremilast; Papp, Kaufmann, et al., 2013), and biologics (Menter et al., 2008). In 2013, the American Academy of Dermatology issued a position statement that advised “psoriasis patients with moderate-to-severe psoriasis and, thus, candidates for systemic therapy, should be placed on the appropriate therapy from the beginning, i.e., phototherapy, or systemic therapy including biologic therapy” (American Academy of Dermatology and AADA, 2013).
In conjunction with initiating pharmacologic treatment for psoriasis, NPs and PAs should encourage patients to make lifestyle and behavioral changes, such as quitting smoking, decreasing alcohol consumption, following a healthy diet, and increasing physical activity (Aldridge, 2014). Making such changes can be especially important for patients with moderate-to-severe psoriasis given its strong association with comorbidities including obesity, diabetes, renal disease, hypertension, and cardiovascular disease (Yeung et al., 2013). These comorbidities can have a substantial impact on a patient’s well-being, can impact the choice of pharmacologic intervention, can provide the patient with the opportunity to increase their self-care participation, and can increase healthcare costs (Aldridge, 2014; Schmieder et al., 2012).
When discussing lifestyle changes, it is important for providers to recognize that patients can face many barriers that make it difficult to adopt healthier behaviors. Common barriers include fear of change, lack of knowledge about healthy eating, limited availability of healthy foods, low income, lack of time, physical disability, depression, and cultural beliefs (Aldridge, 2014). NPs and PAs should discuss possible barriers and work to minimize feelings of guilt patients may experience related to the notion that they have somehow caused or contributed to the disease process by making poor lifestyle choices in the past. Rather, providers should try to keep patients focused on the potential benefits of improved wellness, such as expanding their treatment options and reducing the sequelae of events from uncontrolled inflammation. NPs and PAs should also encourage families to be supportive of the patient’s decisions to make lifestyle changes. One approach is to remind them that, although psoriasis is genetically driven, which cannot be changed, there are some steps they can take to improve their overall health and the impact of their disease. Patients often ask what they can do with their diet, supplements, and so forth, and this is the perfect segue into self-care with healthy lifestyles. Other members of the healthcare team such as primary care providers, rheumatologists, dieticians, mental health practitioners, and other specialists can also provide consultation and support as patients work to develop lasting healthy lifestyles. For more information on healthy lifestyle choices, patients should be referred to the National Psoriasis Foundation Web site (www.psoriasis.org).
Overall, the healthcare team needs to work with patients and their families to identify the best treatment option(s) that can provide desired efficacy with good tolerability, without creating unmanageable burdens associated with administration or cost. To help reduce management burdens, NPs and PAs should provide patients with a personalized written treatment plan.
In our experience, patients with moderate-to-severe psoriasis can achieve the greatest efficacy and life impact when they are treated with biologics under the care of an experienced dermatology provider who can monitor treatment response, overall well-being, and safety parameters associated with these therapies.
PRESCRIBING BIOLOGICS
The clinical acceptance of biologic therapies has provided clinicians with safe and reliable options for the treatment of moderate-to-severe psoriasis. Biologic agents act through targeted inhibition of cytokines involved in the pathophysiology of psoriasis, and because of this specificity of action, biologics are not associated with toxicities that are commonly found with broad-spectrum therapies such as methotrexate and cyclosporine (Mrowietz et al., 2014). Five biologic agents are currently approved for the treatment of moderate-to-severe psoriasis in the United States; three of these agents (adalimumab, etanercept, and infliximab) target tumor necrosis factor alpha (TNF-α), one agent (ustekinumab) targets interleukin (IL)-12/23, and one agent (secukinumab) targets IL-17A (Langley et al., 2014; Sivamani et al., 2013). The efficacy of these therapies have been shown in large randomized clinical trials, and long-term safety monitoring, when available, has shown these agents to be well tolerated (Reich, Burden, Eaton, & Hawkins, 2012; Rustin, 2012).
When initiation of or transition to a biologic agent is being considered for the treatment of moderate-to-severe psoriasis, NPs and PAs should have detailed discussions with patients and their caregivers regarding the rationale for why a biologic agent may be appropriate. Biologics may be considered as a first-line systemic therapy for patients with moderate-to-severe psoriasis based on clinical need (Hsu et al., 2012). In addition, biologic agents should be considered for patients with moderate-to-severe psoriasis who have failed to achieve their treatment goals on other therapies, are unresponsive to topical therapies, are unable to adhere to phototherapy regimens because of personal life circumstances, or are experiencing significantly impaired quality of life (Heller et al., 2012; Mrowietz et al., 2011; Zeichner, 2012). Table 1 provides a summary of the properties of currently available biologic agents (Cosentyx [secukinumab] prescribing information, 2015; Enbrel [etanercept] solution prescribing information, 2013; Humira [adalimumab] injection prescribing information, 2014; Langley et al., 2014; Nast et al., 2013; Reich et al., 2012; Remicade [infliximab] lyophilized concentrate for injection prescribing information, 2013; Stelara [ustekinumab] injection prescribing information, 2014), which can serve as a starting point for provider-led discussions intended to prepare patients for what they can expect in terms of efficacy and potential side effects or other issues based on current data. NPs and PAs should also work to develop knowledge about the systemic immunologic nature of the psoriatic disease process and how the mechanisms of action of different biologic agents (Figure 2) target these underlying inflammatory processes in more specific ways than traditional systemic agents (Johnson-Huang et al., 2012).
TABLE 1.
Summary of Available Biologics for the Treatment of Moderate-to-Severe Plaque Psoriasis
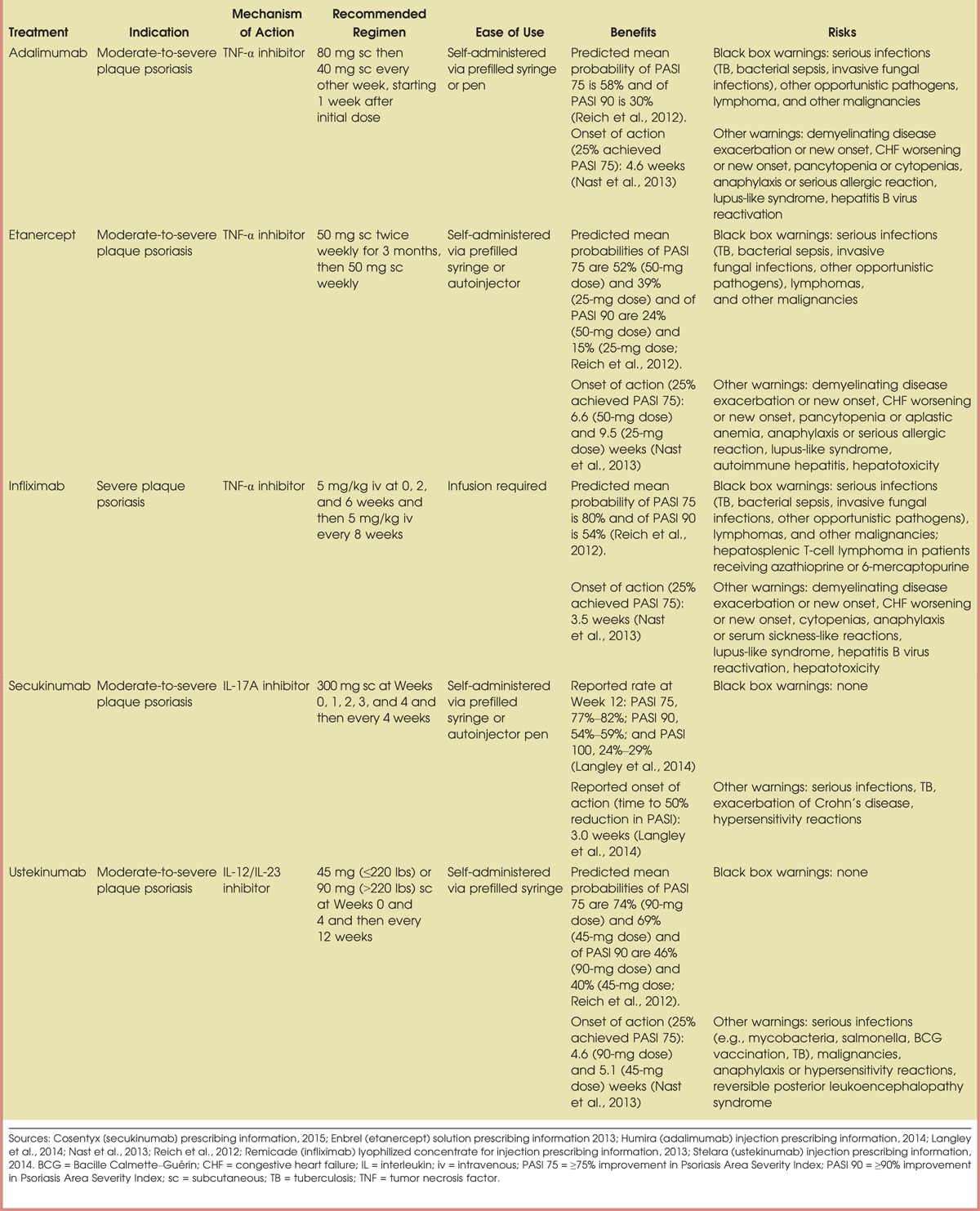
FIGURE 2.
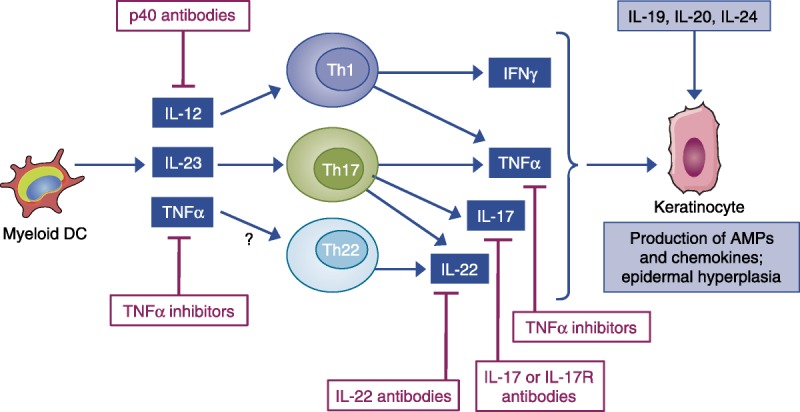
The targets of approved or emerging drugs in the pathophysiology of psoriasis (reprinted from Johnson-Huang, Lowes, & Krueger, 2012). Myeloid DCs produce cytokines that induce IFN-γ production by Th1 cells and IL-17 production by Th17 cells. IL-23 induces production of IL-22 by Th17 and possibly IL-22 cells. IFN-γ, IL-17A, and TNF-α induce production of AMPs and chemokines by keratinocytes, thereby enhancing immune-cell recruitment and inflammation in lesions. Drugs that are currently approved target upstream molecules in this pathway (the p40 subunit of IL-12/IL-23 and TNF-α), whereas drugs in the pipeline are directed against downstream molecules (IL-17A, IL-17RA). AMP = antimicrobial peptide; DC = dendritic cell; IFN = interferon; IL = interleukin; TNF = tumor necrosis factor.
Up to 80% of patients with psoriasis receive no treatment or only topical therapy (Lebwohl et al., 2014). Many individuals who have received suboptimal treatment regimens (such as moisturizing creams or low-potency steroids) have low expectations when initiating new treatments and tend to underestimate the improvement they can achieve with a biologic. Therefore, it is important for NPs and PAs to highlight findings from clinical studies of biologic therapies, all of which have shown that more than half of all patients treated with these agents can achieve significant reductions in the size and severity of psoriatic lesions as reported by at least a 75% improvement in PASI score by following the recommended dosing schedule (Table 1; Cosentyx [secukinumab] prescribing information, 2015; Enbrel [etanercept] solution prescribing information, 2013; Humira [adalimumab] injection prescribing information, 2014; Langley et al., 2014; Nast et al., 2013; Reich et al., 2012; Remicade [infliximab] lyophilized concentrate for injection prescribing information, 2013; Stelara [ustekinumab] injection prescribing information, 2014). Results from meta-analyses and comparative efficacy studies suggest that infliximab has the greatest efficacy, followed by ustekinumab, adalimumab, and etanercept (Reich et al., 2012; Schmitt et al., 2014). However, in our experience, because of cost considerations and the fact that infliximab can only be administered via intravenous infusion, it is generally not used as first-line biologic therapy. Newer agents have not yet been studied to the same extent; however, secukinumab was superior to both etanercept and ustekinumab in two head-to-head comparisons (Langley et al., 2014; Thaçi et al., 2015).
Although PASI 75 has typically been the benchmark for measuring improvement in clinical studies, it is also noteworthy that, by using approved biologic agents, some patients are able to achieve almost-complete skin clearance as denoted by PASI 90 responses and complete skin clearance as denoted by PASI 100 responses (Langley et al., 2014; Reich et al., 2012; Schmitt et al., 2014). It is important for NPs and PAs to be aware of new agents that recently have been added (secukinumab; Langley et al., 2014) or may be added (brodalumab and ixekizumab; Leonardi et al., 2012; Papp, Leonardi, et al., 2012) to the armamentarium of choices for the treatment of moderate-to-severe psoriasis, which could help patients reach their treatment goals of complete or almost-complete skin clearance. Studies have shown that patients who achieve high levels of skin clearance, including PASI 90 or Physician Global Assessment of 0 (clear skin) or 1 (almost clear skin), have experienced significantly greater improvements in health-related quality of life and less quality-of-life impairment than patients who achieved PASI 75 or Physician Global Assessment of 1.5 (Takeshita et al., 2014; Torii, Sato, Yoshinari, Nakagawa, & Japanese Infliximab Study Investigators, 2012).
Convenience of therapy is another factor that should not be underestimated when determining the appropriate course of treatment for patients with moderate-to-severe psoriasis. For example, in a recent study of patient-reported outcomes in patients with psoriasis who achieved clear or almost clear skin, up to 20% of patients rated their treatment as a failure, in part because treatment was inconvenient (Takeshita et al., 2014). Patients generally consider conventional systemic and biologic therapies to be much more convenient than topical therapies (Callis Duffin et al., 2014; Takeshita et al., 2014); however, there are differences between the available biologic agents that may affect patient perceptions of convenience. For patients who express a preference for less frequent dosing, infliximab, secukinumab, or ustekinumab may be appropriate choices because they are administered less often than adalimumab and etanercept (Langley et al., 2014; Sivamani et al., 2013). Route of administration may also influence patient preferences (Scarpato et al., 2010). Adalimumab, etanercept, secukinumab, and ustekinumab are all administered via subcutaneous injection, which offers patients the flexibility of performing self-injections at home or receiving doses at the clinic (e.g., if they prefer not to self-inject or if they would rather not store medication at home in their refrigerator). Infliximab, on the other hand, must be administered intravenously and therefore requires an infusion center. Agents under development (brodalumab and ixekizumab) are both administered by subcutaneous injection.
Safety is an important consideration when selecting any psoriasis therapy. Although long-term safety data are limited for biologics, studies published to date indicate that the approved biologics have been generally well tolerated for up to 1 year or longer (Langley et al., 2014; Papp, Griffiths, et al., 2013; Papp, Leonardi, et al., 2012; Rustin, 2012). Although there were initial concerns about a possible relationship between IL-12/IL-23 inhibition and increased rates of major adverse cardiovascular events, recent evidence suggests no increased risk and possibly a reduced risk with each of the available biologics (Hugh et al., 2014). These findings are supported by data from psoriasis registries, which provide a means to continually monitor the safety of conventional and biologic therapies. Results from recent psoriasis registry analyses suggest that, overall, cardiovascular adverse event rates are lower with biologics than with conventional therapies (Bissonnette et al., 2013; Carretero et al., 2015).
Common side effects with biologics, including nasopharyngitis, upper respiratory tract infection, headache, and injection- or infusion-site reactions, are generally mild; however, in rare cases, serious side effects have been reported (see black box warnings in Table 1). When discussing safety with patients and their families, NPs and PAs should focus on providing education on how to identify and manage the more common side effects and on communicating the low risk for more serious adverse events. It is important for NPs and PAs to try to put the risk of serious adverse events into perspective for patients, because many patients have heard about these risks through direct-to-consumer advertising and misinformation they found online. It should be explained to patients that they will need to be monitored for these rare, yet serious, side effects (Cosentyx [secukinumab] prescribing information, 2015; Enbrel [etanercept] solution prescribing information, 2013; Humira [adalimumab] injection prescribing information, 2014; Menter et al., 2008; Remicade [infliximab] lyophilized concentrate for injection prescribing information, 2013; Stelara [ustekinumab] injection prescribing information, 2014), perhaps most notably for tuberculosis (TB) and other infections, even if a latent TB test is negative. This monitoring consists of baseline and yearly TB skin tests and complete blood counts. In addition, patients who have been infected with hepatitis B should be monitored for reactivation during and for several months after treatment with any of the TNF-α inhibitors (i.e., adalimumab, etanercept, or infliximab; Enbrel [etanercept] solution prescribing information, 2013; Humira [adalimumab] injection prescribing information, 2014; Remicade [infliximab] lyophilized concentrate for injection prescribing information, 2013). Hepatic function should also be assessed periodically during and after treatment with TNF-α inhibitors. Patients with signs of hepatitis (e.g., loss of appetite, fatigue, nausea, pruritus, jaundice) or very elevated liver enzyme levels should be referred to a hepatologist (Papp, Dekoven, et al., 2012).
On the basis of our experience from initiating biologic therapy, we recommend starting with a 1-month prescription and two refills. Follow-up visits should be scheduled at least once every 3 months (or more frequently, if needed) until tolerability and efficacy have been established. Over time, the prescribed duration of therapy can be increased, and follow-up visits can be less frequent if patients are doing well. NPs and PAs can give patients or their caregivers the opportunity to contact them by telephone, email, or text message to provide reassurance and support until the patient becomes more familiar with a biologic therapy. When efficacy and/or tolerability is not optimal with the initial choice of biologic therapy, international consensus guidelines for managing moderate-to-severe psoriasis recognize switching to a different biologic as accepted practice and provide recommended transition dosing schedules (Mrowietz et al., 2014). With the availability of ustekinumab, an IL-12/IL-23 inhibitor, and secukinumab, an IL-17 inhibitor, it is now possible to switch from a TNF-α inhibitor to an agent with a different mechanism of action. It is important for NPs and PAs to continually research advances in psoriasis treatment to keep abreast of new therapies with a different mechanism of action, such as inhibition of IL-17 by secukinumab, ixekizumab, and brodalumab (Langley et al., 2014; Leonardi et al., 2012; Papp, Leonardi, et al., 2012) and inhibition of phosphodiesterase-4 by apremilast (Papp, Kaufmann, et al., 2013) that may improve the available options for disease management and to keep patients informed about these promising new therapies.
SPECIAL CONSIDERATIONS FOR PATIENT CARE AND HELPFUL TIPS
Health Insurance and Cost
With the evolving landscape of health insurance policies and procedures in the United States, it can be challenging for patients and practitioners to navigate the system to ensure that patients have access to recommended treatments at costs that are manageable. Results of National Psoriasis Foundation surveys conducted between 2003 and 2011 found that approximately 8% of patients had not sought any medical care for their psoriasis and more than 20% of patients were not receiving specialist care, in large part because of prohibitive costs and/or lack of insurance (Bhutani, Wong, Bebo, & Armstrong, 2013).
It is our hope that, under the Affordable Care Act, Americans will have access to quality health coverage that cannot be denied because of preexisting diseases, such as psoriasis. However, even with health insurance, the cost of biologics can still be prohibitive for some patients, especially those with large deductibles. The National Psoriasis Foundation estimates that average copayments for a biologic are $1500 per year and that total out-of-pocket costs can be more than $2500 per year for patients with insurance (National Psoriasis Foundation, 2015). In addition, new healthcare laws have increased yearly deductibles, which can make some treatment options financially out of reach unless assistance programs are available. Fortunately, several savings and rebate programs are available to help in this regard. All of the biologics manufacturers offer programs for qualifying patients with commercial insurance that reduce costs to about $5–$10 per dose with the Enbrel Support Card, Humira Protection Plan, or Stelara Support Instant Savings Program and to $50 per infusion with the infliximab RemiStart Program. Uninsured patients may also be eligible for assistance through the Amgen ENcourage Foundation (etanercept), the AbbVie Patient Assistance Foundation (adalimumab), the Janssen Patient Assistance Foundation (infliximab and ustekinumab), or other nonbranded foundations that provide medications at no cost. It is important for NPs and PAs to educate patient on the availability of these programs that may offer financial assistance and make biologics attainable.
Although prescription costs are a sizeable component of the overall out-of-pocket cost of psoriasis, it is also important to consider the tremendous benefits that patients can experience when treated with biologics. With improved overall health, patients on biologic therapy can often improve their productivity, reduce their disability, and reduce their need for outpatient care (Kimball et al., 2012; Larsen et al., 2013; Reich et al., 2011), which can have a substantial positive economic impact. For example, a recent Swedish study found that, although initiating treatment with a biologic increased the direct costs of managing psoriasis, the economic benefits of increased productivity, including reduced long-term sick leave and disability pension payments, significantly outweighed the increased direct costs (Norlin, Steen Carlsson, Persson, & Schmitt-Egenolf, 2015). Such findings highlight the importance of communicating openly with patients to weigh the benefits versus costs of available treatments. For many patients, the long-term improvements in productivity, physical functioning, and quality of life resulting from treatment with biologics may be worth higher costs, especially for individuals with widespread disease in sensitive body locations or a high psychosocial disease burden.
Fear of Injections With Biologic Therapies
Some patients are reluctant to try biologic therapy because of a fear of injection, regardless of how motivated they are to treat their psoriasis. In an effort to allay such fears, NPs and PAs should explain the injection procedures (subcutaneous for adalimumab, etanercept, secukinumab, and ustekinumab and intravenous for infliximab) and describe the practical differences between administration techniques. Some patients may feel more comfortable using an autoinjector (available with adalimumab, etanercept, and secukinumab; Cosentyx [secukinumab] prescribing information, 2015; Enbrel [etanercept] solution prescribing information, 2013; Humira [adalimumab] injection prescribing information, 2014), which is held up to the skin and injected by pushing a button, rather than using a prefilled syringe, which is the only administration option for ustekinumab (Stelara [ustekinumab] injection prescribing information, 2014). It should be made clear to patients that biologics (excluding infliximab) can be administered at home, either via self-injection or by a caregiver, whichever is preferable.
NPs and PAs should work with patients and their caregivers to ensure that they feel comfortable with the injection training they have received, continually showing sensitivity about any fears of needles or injections. We strongly recommend documenting the patient’s improvement by taking photographs before and during treatment. These photographs can be taken at each visit by the clinic staff, or the patient can take photographs at home with a smartphone or camera if he or she is sensitive about being photographed at the clinic. Visually documenting what is often a dramatic skin improvement with biologic therapy may help patients see the extent of the treatment benefit over time, which may increase their willingness to tolerate injections.
Visit Adherence
The importance of attending office visits should be stressed to all patients. For patients who do not live near the clinic or for those who do not have the time and/or transportation resources to make frequent visits to the clinic, teledermatology utilizing smartphone cameras can be used to reduce the number of required face-to-face visits, while still providing effective monitoring for patients on biologic therapy (Frühauf et al., 2010; Koller et al., 2011). Teledermatology can also be useful to address any sudden issues that may develop between scheduled office visits (Schreier et al., 2008). By using such technology, patients who prefer to minimize time at the clinic may find that biologics are preferable to other treatments, such as phototherapy, which requires multiple clinic visits per week during the initiation phase. Consistent with the meaningful use requirements of the Affordable Care Act, electronic communications can also be used to remind patients about upcoming office visits and the need for blood work or other testing (King, Patel, & Furukawa, 2012).
CONCLUSIONS
NPs and PAs can play an instrumental role in evaluating and managing patients with dermatologic diseases, including psoriasis. They are well positioned to optimize psoriasis care by building strong, long-term relationships with their patients. Patients who have trusting relationships with their providers are more likely to listen and adhere to healthcare advice and are more likely to play an active role in managing their psoriasis. Such engaged patients are likely to have fewer complications with their disease or therapy as well as more realistic expectations about the benefits and risks of their treatment and how their disease and its treatment may change over time. Patients can benefit from the increased understanding of psoriasis that NPs and PAs can provide. As providers continue to build on their relationship with patients, they can instill an appreciation for the need to adopt lasting healthy behaviors and adhere to therapy and increase patient awareness of new options for care.
Figure.

No caption available.
Footnotes
Technical assistance with editing and styling of the manuscript for submission was provided by Oxford PharmaGenesis, Inc., and was funded by Novartis Pharmaceuticals Corporation.
The authors were fully responsible for all content and editorial decisions and received no financial support or other form of compensation related to the development of this manuscript.
The opinions expressed in the manuscript are those of the authors, and Novartis Pharmaceuticals had no influence on the contents.
LMA serves as speaker and/or consultant for AbbVie, Amgen, Celgene, Galderma, Janssen-Ortho Inc, LEO Pharma Inc, Lilly ICOS LLC, Merck, Novartis, and Pfizer. MSY serves as speaker and/or consultant for AbbVie, Celgene, Janssen, and Lilly, and as a clinical investigator for Amgen, Janssen, Novartis, Merck, Celgene, Pfizer, Galderma, and Lilly.
References
- Aldridge A. (2014). The role of the community nurse in psoriatic comorbidities interventions. British Journal of Community Nursing, 19(1), 38– 42. [DOI] [PubMed] [Google Scholar]
- Al-Suwaidan S. N., Feldman S. R. (2000). Clearance is not a realistic expectation of psoriasis treatment. Journal of the American Academy of Dermatology, 42(5 Pt. 1), 796– 802. [DOI] [PubMed] [Google Scholar]
- American Academy of Dermatology and AADA. (2013). Position statement on treatment of psoriatic patients. Retrieved from https://www.aad.org/Forms/Policies/Uploads/PS/PS%20on%20Treatment%20of%20Psoriatic%20Patients.pdf [Google Scholar]
- Armstrong A. W., Harskamp C. T., Armstrong E. J. (2012). The association between psoriasis and obesity: A systematic review and meta-analysis of observational studies. Nutrition & Diabetes, 2, e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong A. W., Harskamp C. T., Armstrong E. J. (2013a). The association between psoriasis and hypertension: A systematic review and meta-analysis of observational studies. Journal of Hypertension, 31(3), 433– 442. [DOI] [PubMed] [Google Scholar]
- Armstrong A. W., Harskamp C. T., Armstrong E. J. (2013b). Psoriasis and metabolic syndrome: A systematic review and meta-analysis of observational studies. Journal of the American Academy of Dermatology, 68(4), 654– 662. [DOI] [PubMed] [Google Scholar]
- Armstrong A. W., Robertson A. D., Wu J., Schupp C., Lebwohl M. G. (2013). Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: Findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatology, 149(10), 1180– 1185. [DOI] [PubMed] [Google Scholar]
- Armstrong A. W., Schupp C., Bebo B. (2012). Psoriasis comorbidities: Results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology, 225(2), 121– 126. [DOI] [PubMed] [Google Scholar]
- Augustin M., Holland B., Dartsch D., Langenbruch A., Radtke M. A. (2011). Adherence in the treatment of psoriasis: A systematic review. Dermatology, 222(4), 363– 374. [DOI] [PubMed] [Google Scholar]
- Baker C., Mack A., Cooper A., Fischer G., Shumack S., Sidhu S., Foley P. (2013). Treatment goals for moderate to severe psoriasis: An Australian consensus. The Australasian Journal of Dermatology, 54(2), 148– 154. [DOI] [PubMed] [Google Scholar]
- Bewley A., Page B. (2011). Maximizing patient adherence for optimal outcomes in psoriasis. Journal of the European Academy of Dermatology and Venereology, 25(Suppl. 4), 9– 14. [DOI] [PubMed] [Google Scholar]
- Bhutani T., Wong J. W., Bebo B. F., Armstrong A. W. (2013). Access to health care in patients with psoriasis and psoriatic arthritis: Data from National Psoriasis Foundation survey panels. JAMA Dermatology, 149(6), 717– 721. [DOI] [PubMed] [Google Scholar]
- Bissonnette R., Naldi L., Gottlieb A. B., Kerdel F., Fakharzadeh S., Calabro S., … PSOLAR Steering Committee. (2013). Major adverse cardiovascular events (MACE) in the Psoriasis Longitudinal Assessment and Registry (PSOLAR) study: Current status of observations [poster P4.31]. Poster presented at the Canadian Dermatology Association 2013 Annual Conference, 2013June26–30, Quebec City, Canada. [Google Scholar]
- Callis Duffin K., Yeung H., Takeshita J., Krueger G. G., Robertson A. D., Troxel A. B., Gelfand J. M. (2014). Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. The British Journal of Dermatology, 170(3), 672– 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero G., Ferrandiz C., Dauden E., Vanaclocha Sebastián F., Gómez-García F. J., Herrera-Ceballos E., … BIOBADADERM Study Group. (2015). Risk of adverse events in psoriasis patients receiving classic systemic drugs and biologics in a 5-year observational study of clinical practice: 2008–2013 results of the Biobadaderm registry. Journal of the European Academy of Dermatology and Venereology, 29(1), 156– 163. [DOI] [PubMed] [Google Scholar]
- Cosentyx (secukinumab) injection [prescribing information]. (2015). East Hanover, NJ: Novartis Pharmaceuticals Corporation. [Google Scholar]
- Courtenay M., Carey N. (2006). Nurse-led care in dermatology: A review of the literature. The British Journal of Dermatology, 154(1), 1– 6. [DOI] [PubMed] [Google Scholar]
- Courtenay M., Carey N., Stenner K., Lawton S., Peters J. (2011). Patients’ views of nurse prescribing: Effects on care, concordance and medicine taking. The British Journal of Dermatology, 164(2), 396– 401. [DOI] [PubMed] [Google Scholar]
- Davis S. A., Lin H. C., Yu C. H., Balkrishnan R., Feldman S. R. (2014). Underuse of early follow-up visits: A missed opportunity to improve patients’ adherence. Journal of Drugs in Dermatology, 13(7), 833– 836. [PubMed] [Google Scholar]
- Enbrel (etanercept) solution [prescribing information]. (2013). Thousand Oaks, CA: Immunex Corporation. [Google Scholar]
- Feldman S., Behnam S. M., Behnam S. E., Koo J. Y. (2005). Involving the patient: Impact of inflammatory skin disease and patient-focused care. Journal of the American Academy of Dermatology, 53(1 Suppl. 1), S78– S85. [DOI] [PubMed] [Google Scholar]
- Finlay A. Y. (2005). Current severe psoriasis and the rule of tens. The British Journal of Dermatology, 152(5), 861– 867. [DOI] [PubMed] [Google Scholar]
- Frühauf J., Schwantzer G., Ambros-Rudolph C. M., Weger W., Ahlgrimm-Siess V., Salmhofer W., Hofmann-Wellenhof R. (2010). Pilot study using teledermatology to manage high-need patients with psoriasis. Archives of Dermatology, 146(2), 200– 201. [DOI] [PubMed] [Google Scholar]
- Gottlieb A. B., Chao C., Dann F. (2008). Psoriasis comorbidities. The Journal of Dermatological Treatment, 19(1), 5– 21. [DOI] [PubMed] [Google Scholar]
- Gudjonsson J. E., Thorarinsson A. M., Sigurgeirsson B., Kristinsson K. G., Valdimarsson H. (2003). Streptococcal throat infections and exacerbation of chronic plaque psoriasis: A prospective study. The British Journal of Dermatology, 149(3), 530– 534. [DOI] [PubMed] [Google Scholar]
- Heller M. M., Wong J. W., Nguyen T. V., Lee E. S., Bhutani T., Menter A., Koo J. Y. (2012). Quality-of-life instruments: Evaluation of the impact of psoriasis on patients. Dermatologic Clinics, 30(2), 281– 291. [DOI] [PubMed] [Google Scholar]
- Hsu S., Papp K. A., Lebwohl M. G., Bagel J., Blauvelt A., Duffin K. C., … National Psoriasis Foundation Medical Board. (2012). Consensus guidelines for the management of plaque psoriasis. Archives of Dermatology, 148(1), 95– 102. [DOI] [PubMed] [Google Scholar]
- Hugh J., Van Voorhees A. S., Nijhawan R. I., Bagel J., Lebwohl M., Blauvelt A., Weinberg J. M. (2014). From the Medical Board of the National Psoriasis Foundation: The risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. Journal of the American Academy of Dermatology, 70(1), 168– 177. [DOI] [PubMed] [Google Scholar]
- Humira (adalimumab) injection [prescribing information]. (2014). North Chicago, IL: AbbVie Inc. [Google Scholar]
- Johnson-Huang L. M., Lowes M. A., Krueger J. G. (2012). Putting together the psoriasis puzzle: An update on developing targeted therapies. Disease Models & Mechanisms, 5(4), 423– 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball A. B., Jacobson C., Weiss S., Vreeland M. G., Wu Y. (2005). The psychosocial burden of psoriasis. American Journal of Clinical Dermatology, 6(6), 383– 392. [DOI] [PubMed] [Google Scholar]
- Kimball A. B., Yu A. P., Signorovitch J., Xie J., Tsaneva M., Gupta S. R., Mulani P. M. (2012). The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. Journal of the American Academy of Dermatology, 66(2), e67– e76. [DOI] [PubMed] [Google Scholar]
- King J., Patel V., Furukawa M. F. (2012). Physician adoption of electronic health record technology to meet meaningful use objectives: 2009–2012. ONC Data Brief No. 7. National Coordinator for Health Information Technology. Retrieved from http://www.healthit.gov/sites/default/files/onc-data-brief-7-december-2012.pdf [Google Scholar]
- Koller S., Hofmann-Wellenhof R., Hayn D., Weger W., Kastner P., Schreier G., Salmhofer W. (2011). Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Dermato-Venereologica, 91(6), 680– 685. [DOI] [PubMed] [Google Scholar]
- Langley R. G., Elewski B. E., Lebwohl M., Reich K., Griffiths C. E., Papp K., … FIXTURE Study Group. (2014). Secukinumab in plaque psoriasis—Results of two phase 3 trials. The New England Journal of Medicine, 371(4), 326– 338. [DOI] [PubMed] [Google Scholar]
- Langley R. G., Krueger G. G., Griffiths C. E. (2005). Psoriasis: Epidemiology, clinical features, and quality of life. Annals of the Rheumatic Diseases, 64(Suppl. 2), ii18– ii23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. G., Andersen P. H., Lorentzen H., Zachariae C., Huldt-Nystrøm T., Dotterud L. K., Qvitzau S. (2013). Clinical and economic impact of etanercept in real-life: A prospective, non-interventional study of etanercept in the treatment of patients with moderate to severe plaque psoriasis in private dermatologist settings (ESTHER). European Journal of Dermatology, 23(6), 774– 781. [DOI] [PubMed] [Google Scholar]
- Lebwohl M. G., Bachelez H., Barker J., Girolomoni G., Kavanaugh A., Langley R. G., van de Kerkhof P. C. (2014). Patient perspectives in the management of psoriasis: Results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. Journal of the American Academy of Dermatology, 70(5), 871– 881. [DOI] [PubMed] [Google Scholar]
- Lecluse L. L., de Groot M., Bos J. D., Spuls P. I. (2009). Experience with biologics for psoriasis in daily practice: Switching is worth a try. The British Journal of Dermatology, 161(4), 948– 951. [DOI] [PubMed] [Google Scholar]
- Leman J., Burden A. D. (2012). Sequential use of biologics in the treatment of moderate-to-severe plaque psoriasis. The British Journal of Dermatology, 167(Suppl. 3), 12– 20. [DOI] [PubMed] [Google Scholar]
- Leonardi C., Matheson R., Zachariae C., Cameron G., Li L., Edson-Heredia E., Banerjee S. (2012). Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. The New England Journal of Medicine, 366(13), 1190– 1199. [DOI] [PubMed] [Google Scholar]
- Martínez-García E., Arias-Santiago S., Valenzuela-Salas I., Garrido-Colmenero C., García-Mellado V., Buendía-Eisman A. (2014). Quality of life in persons living with psoriasis patients. Journal of the American Academy of Dermatology, 71(2), 302– 307. [DOI] [PubMed] [Google Scholar]
- Menter A., Gottlieb A., Feldman S. R., Van Voorhees A. S., Leonardi C. L., Gordon K. B., Bhushan R. (2008). Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. Journal of the American Academy of Dermatology, 58(5), 826– 850. [DOI] [PubMed] [Google Scholar]
- Menter A., Korman N. J., Elmets C. A., Feldman S. R., Gelfand J. M., Gordon K. B., Bhushan R. (2009a). Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. Journal of the American Academy of Dermatology, 60(4), 643– 659. [DOI] [PubMed] [Google Scholar]
- Menter A., Korman N. J., Elmets C. A., Feldman S. R., Gelfand J. M., Gordon K. B., Bhushan R. (2009b). Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. Journal of the American Academy of Dermatology, 61(3), 451– 485. [DOI] [PubMed] [Google Scholar]
- Menter A., Korman N. J., Elmets C. A., Feldman S. R., Gelfand J. M., Gordon K. B., Bhushan R. (2010). Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. Journal of the American Academy of Dermatology, 62(1), 114– 135. [DOI] [PubMed] [Google Scholar]
- Mrowietz U., de Jong E. M., Kragballe K., Langley R., Nast A., Puig L., Warren R. B. (2014). A consensus report on appropriate treatment optimization and transitioning in the management of moderate-to-severe plaque psoriasis. Journal of the European Academy of Dermatology and Venereology, 28(4), 438– 453. [DOI] [PubMed] [Google Scholar]
- Mrowietz U., Kragballe K., Reich K., Spuls P., Griffiths C. E., Nast A., Yawalkar N. (2011). Definition of treatment goals for moderate to severe psoriasis: A European consensus. Archives of Dermatological Research, 303(1), 1– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nast A., Sporbeck B., Rosumeck S., Pathirana D., Jacobs A., Werner R. N., Schmitt J. (2013). Which antipsoriatic drug has the fastest onset of action? Systematic review on the rapidity of the onset of action. The Journal of Investigative Dermatology, 133(8), 1963– 1970. [DOI] [PubMed] [Google Scholar]
- National Psoriasis Foundation. (2015). Advocacy toolkit. Psoriasis and psoriatic arthritis (PsA): Serious autoimmune diseases that affect more than skin and joints. Retrieved from https://www.psoriasis.org/toolkit/the-burden-of-psoriatic-diseases
- Norlin J. M., Steen Carlsson K., Persson U., Schmitt-Egenolf M. (2012). Switch to biological agent in psoriasis significantly improved clinical and patient-reported outcomes in real-world practice. Dermatology, 225(4), 326– 332. [DOI] [PubMed] [Google Scholar]
- Norlin J. M., Steen Carlsson K., Persson U., Schmitt-Egenolf M. (2015). Resource use in patients with psoriasis after the introduction of biologics in Sweden. Acta Dermato-Venereologica, 95(2), 156– 161. [DOI] [PubMed] [Google Scholar]
- Papp K. A., Dekoven J., Parsons L., Pirzada S., Robern M., Robertson L., Tan J. K. (2012). Biologic therapy in psoriasis: Perspectives on associated risks and patient management. Journal of Cutaneous Medicine and Surgery, 16(3), 153– 168. [DOI] [PubMed] [Google Scholar]
- Papp K. A., Griffiths C. E., Gordon K., Lebwohl M., Szapary P. O., Wasfi Y., … ACCEPT Investigators. (2013). Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: Final results from 5 years of follow-up. The British Journal of Dermatology, 168(4), 844– 854. [DOI] [PubMed] [Google Scholar]
- Papp K. A., Kaufmann R., Thaçi D., Hu C., Sutherland D., Rohane P. (2013). Efficacy and safety of apremilast in subjects with moderate to severe plaque psoriasis: Results from a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison study. Journal of the European Academy of Dermatology and Venereology, 27(3), e376– e383. [DOI] [PubMed] [Google Scholar]
- Papp K. A., Leonardi C., Menter A., Ortonne J. P., Krueger J. G., Kricorian G., Baumgartner S. (2012). Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. The New England Journal of Medicine, 366(13), 1181– 1189. [DOI] [PubMed] [Google Scholar]
- Rachakonda T. D., Schupp C. W., Armstrong A. W. (2014). Psoriasis prevalence among adults in the United States. Journal of the American Academy of Dermatology, 70(3), 512– 516. [DOI] [PubMed] [Google Scholar]
- Reich K., Burden A. D., Eaton J. N., Hawkins N. S. (2012). Efficacy of biologics in the treatment of moderate to severe psoriasis: A network meta-analysis of randomized controlled trials. The British Journal of Dermatology, 166(1), 179– 188. [DOI] [PubMed] [Google Scholar]
- Reich K., Daudén E. (2014). Treatment adherence: A hurdle for real-life effectiveness in psoriasis? Journal of the European Academy of Dermatology and Venereology, 28(Suppl. 2), 1– 3. [DOI] [PubMed] [Google Scholar]
- Reich K., Schenkel B., Zhao N., Szapary P., Augustin M., Bourcier M., Langley R. G. (2011). Ustekinumab decreases work limitations, improves work productivity, and reduces work days missed in patients with moderate-to-severe psoriasis: Results from PHOENIX 2. The Journal of Dermatological Treatment, 22(6), 337– 347. [DOI] [PubMed] [Google Scholar]
- Remicade (infliximab) lyophilized concentrate for injection [prescribing information]. (2013). Horsham, PA: Janssen Biotech, Inc. [Google Scholar]
- Richards H. L., Fortune D. G., Griffiths C. E. (2006). Adherence to treatment in patients with psoriasis. Journal of the European Academy of Dermatology and Venereology, 20(4), 370– 379. [DOI] [PubMed] [Google Scholar]
- Rustin M. H. (2012). Long-term safety of biologics in the treatment of moderate-to-severe plaque psoriasis: Review of current data. The British Journal of Dermatology, 167(Suppl. 3), 3– 11. [DOI] [PubMed] [Google Scholar]
- Scarpato S., Antivalle M., Favalli E. G., Nacci F., Frigelli S., Bartoli F., … RIVIERA co-authors. (2010). Patient preferences in the choice of anti-TNF therapies in rheumatoid arthritis. Results from a questionnaire survey (RIVIERA study). Rheumatology, 49(2), 289– 294. [DOI] [PubMed] [Google Scholar]
- Schmieder A., Schaarschmidt M. L., Umar N., Terris D. D., Goebeler M., Goerdt S., Peitsch W. K. (2012). Comorbidities significantly impact patients’ preferences for psoriasis treatments. Journal of the American Academy of Dermatology, 67(3), 363– 372. [DOI] [PubMed] [Google Scholar]
- Schmitt J., Rosumeck S., Thomaschewski G., Sporbeck B., Haufe E., Nast A. (2014). Efficacy and safety of systemic treatments for moderate-to-severe psoriasis: Meta-analysis of randomized controlled trials. The British Journal of Dermatology, 170(2), 274– 303. [DOI] [PubMed] [Google Scholar]
- Schreier G., Hayn D., Kastner P., Koller S., Salmhofer W., Hofmann-Wellenhof R. (2008). A mobile-phone based teledermatology system to support self-management of patients suffering from psoriasis. Annual International Conference of the IEEE Engineering in Medicine and Biology Society; (pp. 5338– 5341). [DOI] [PubMed] [Google Scholar]
- Sivamani R. K., Goodarzi H., Garcia M. S., Raychaudhuri S. P., Wehrli L. N., Ono Y., Maverakis E. (2013). Biologic therapies in the treatment of psoriasis: A comprehensive evidence-based basic science and clinical review and a practical guide to tuberculosis monitoring. Clinical Reviews in Allergy & Immunology, 44(2), 121– 140. [DOI] [PubMed] [Google Scholar]
- Stelara (ustekinumab) injection [prescribing information]. (2014). Horsham, PA: Janssen Biotech, Inc. [Google Scholar]
- Takeshita J., Callis Duffin K., Shin D. B., Krueger G. G., Robertson A. D., Troxel A. B., Gelfand J. M. (2014). Patient-reported outcomes for psoriasis patients with clear versus almost clear skin in the clinical setting. Journal of the American Academy of Dermatology, 71(4), 633– 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaçi D., Blauvelt A., Reich K., Tsai T. F., Vanaclocha F., Kingo K., Milutinovic M. (2015). Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. Journal of the American Academy of Dermatology, 73(3), 400– 439. [DOI] [PubMed] [Google Scholar]
- Torii H., Sato N., Yoshinari T., Nakagawa H., & Japanese Infliximab Study Investigators. (2012). Dramatic impact of a Psoriasis Area and Severity Index 90 response on the quality of life in patients with psoriasis: An analysis of Japanese clinical trials of infliximab. The Journal of Dermatology, 39(3), 253– 259. [DOI] [PubMed] [Google Scholar]
- Xhaja A., Shkodrani E., Frangaj S., Kuneshka L., Vasili E. (2014). An epidemiological study on trigger factors and quality of life in psoriatic patients. Materia Socio-Medica, 26(3), 168– 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaw J., Watson C., Fox K. M., Schabert V. F., Goodman S., Gandra S. R. (2014). Treatment patterns following discontinuation of adalimumab, etanercept, and infliximab in a US managed care sample. Advances in Therapy, 31(4), 410– 425. [DOI] [PubMed] [Google Scholar]
- Yeung H., Takeshita J., Mehta N. N., Kimmel S. E., Ogdie A., Margolis D. J., Gelfand J. M. (2013). Psoriasis severity and the prevalence of major medical comorbidity: A population-based study. JAMA Dermatology, 149(10), 1173– 1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. (2005). The psychological and social burdens of psoriasis. Dermatology Nursing, 17(1), 15– 19. [PubMed] [Google Scholar]
- Zeichner J. A. (2012). Considerations when initiating psoriasis patients on biologic therapy. Journal of Drugs in Dermatology, 11(5 Suppl.), s11– s14. [PubMed] [Google Scholar]
- Zschocke I., Mrowietz U., Karakasili E., Reich K. (2014). Nonadherence and measures to improve adherence in the topical treatment of psoriasis. Journal of the European Academy of Dermatology and Venereology, 28(Suppl. 2), 4– 9. [DOI] [PubMed] [Google Scholar]


