Abstract
Objective
Obesity is one of the primary healthcare challenges of the 21st century. Signals relaying information regarding energy needs are integrated within the brain to influence body weight. Central among these integration nodes are the brain pro-opiomelanocortin (POMC) peptides, perturbations of which disrupt energy balance and promote severe obesity. However, POMC neurons are neurochemically diverse and the crucial source of POMC peptides that regulate energy homeostasis and body weight remains to be fully clarified.
Methods
Given that a 5-hydroxytryptamine 2c receptor (5-HT2CR) agonist is a current obesity medication and 5-HT2CR agonist's effects on appetite are primarily mediated via POMC neurons, we hypothesized that a critical source of POMC regulating food intake and body weight is specifically synthesized in cells containing 5-HT2CRs. To exclusively manipulate Pomc synthesis only within 5-HT2CR containing cells, we generated a novel 5-HT2CRCRE mouse line and intercrossed it with Cre recombinase-dependent and hypothalamic specific reactivatable PomcNEO mice to restrict Pomc synthesis to the subset of hypothalamic cells containing 5-HT2CRs. This provided a means to clarify the specific contribution of a defined subgroup of POMC peptides in energy balance and body weight.
Results
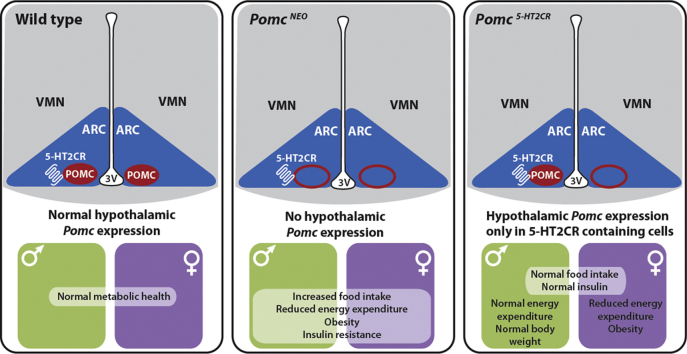
Here we transform genetically programed obese and hyperinsulinemic male mice lacking hypothalamic Pomc with increased appetite, reduced physical activity and compromised brown adipose tissue (BAT) into lean, healthy mice via targeted restoration of Pomc function only within 5-HT2CR expressing cells. Remarkably, the same metabolic transformation does not occur in females, who despite corrected feeding behavior and normalized insulin levels remain physically inactive, have lower energy expenditure, compromised BAT and develop obesity.
Conclusions
These data provide support for the functional heterogeneity of hypothalamic POMC neurons, revealing that Pomc expression within 5-HT2CR expressing neurons is sufficient to regulate energy intake and insulin sensitivity in male and female mice. However, an unexpected sex difference in the function of this subset of POMC neurons was identified with regard to energy expenditure. We reveal that a large sex difference in physical activity, energy expenditure and the development of obesity is driven by this subpopulation, which constitutes approximately 40% of all POMC neurons in the hypothalamic arcuate nucleus. This may have broad implications for strategies utilized to combat obesity, which at present largely ignore the sex of the obese individual.
Keywords: Pro-opiomelanocortin (Pomc), 5-HT2c receptor, Obesity, Energy expenditure, Brown adipose tissue, Hyperinsulinemia, Sexual dimorphism, Hypothalamus
Graphical abstract
Highlights
-
•
Generation of 5-HT2CRYFP mouse line revealed 40% of hypothalamic POMC neurons co-express 5-HT2CRs in male and female mice.
-
•
Pomc expressed exclusively within 5-HT2CR cells modulates energy intake and insulin sensitivity in male and female mice.
-
•
An unexpected sex difference in physical activity and energy expenditure is modulated by hypothalamic Pomc.
-
•
Pomc expressed exclusively within 5-HT2CR cells differentially impacts body weight accumulation in male and female mice.
1. Introduction
When energy intake exceeds energetic demands, energy is stored primarily as fat. Excess adipose accumulation is the hallmark feature of obesity. Though men and women are exposed to the same environmental conditions, the World Health Organization (WHO) reports higher rates of obesity in women worldwide, reaching twice the prevalence of men in some regions of the world [1]. Obesity has a significant and widespread impact on human health, placing it at the forefront of healthcare priorities and challenges of this century. Obesity medications are in general prescribed without attention to the sex of the obese individual, implying the absence of sex-based differences in the molecular regulation of energy balance.
Insights from genetic research have led to the discovery of key regulators of energy balance [2], such as the melanocortin peptides encoded by the pro-opiomelanocortin gene (Pomc) [3]. Humans and animals unable to synthesize melanocortins or the receptor through which they primarily signal to influence energy balance, the melanocortin4 receptor (MC4R/Mc4r), have dramatically increased food intake, reduced physical activity or energy expenditure and develop profound obesity [4], [5]. POMC/Pomc function also has broader application to common obesity; in both high fat diet-induced obesity and middle-age associated obesity, POMC neuron activity within the arcuate nucleus of the hypothalamus (ARC) is diminished, which has been proposed to have a causal role in the increased acquisition of body weight and adiposity [6], [7], [8]. Treatment with a 5-hydroxytryptamine 2c receptor (5-HT2CR) agonist, such as the new obesity medication lorcaserin (Arena Pharmaceuticals), restores diminished POMC neuron function and improves obesity [9], [10], [11]. Furthermore, inactivating 5-HT2CRs specifically on POMC neurons in mice, a genetic strategy employed to manipulate 5-HT2CR expression, prevents the anorectic effect of 5-HT2CR agonists [12], thereby revealing that 5-HT2CR agonists modulate food intake via POMC neurons. Thus, POMC peptides are an important driver of body weight and POMC expressing neurons are amenable to pharmacological manipulation. Here, we sought to clarify the source of POMC peptides that critically mediate body weight using a newly developed genetic approach.
2. Materials and methods
2.1. Mice
5-HT2CRCRE line. 5.6 kb of genomic DNA containing portions of the final exon and the 3′ UTR of the murine Htr2c gene was amplified by PCR from R1 ES cells [(129X1/SvJ × 129S1)F1 genetic background] and cloned into a plasmid for insertion of a FRT-NEO-FRT-IRES-CRE cassette between the STOP codon and the polyadenylation site, as previously described [17]. The targeting construct was linearized using NotI and electroporated into R1 mouse embryonic stem cells at the University of Michigan Transgenic Animal Model Core. Neomycin-resistant clones were analyzed by quantitative real-time PCR [18] for copy number of the native Htr2c allele and further confirmed by Southern blotting using an external probe. Correctly targeted ES cells were injected into C57BL/6J blastocysts to generate chimeras. Male chimeras were then bred to C57BL/6J females, and pups were genotyped to confirm insertion of IRES-Cre into the appropriate locus. These 5-HT2CRCRE pups were then bred to a germline FlpO deleter strain (129S4/SvJae-Gt(ROSA)26Sortm2(FLP*)Sor/J; Jackson Laboratory) to remove the Neo cassette. Pups positive for FlpO and 5-HT2CRCRE were genotyped for loss of the neo cassette in 5-HT2CRCRE and further bred away from the FlpO allele.
5-HT2CRCRE mice were then intercrossed with either ROSA26-stop-enhanced yellow fluorescent protein (YFP) (B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J; Jackson Laboratory) to create a reporter 5-HT2CRYFP line or PomcNEO mice [13] to generate wild type, 5-HT2CRCRE, ARC Pomc null (PomcNEO), and restored Pomc specifically in 5-HT2CR expressing cells (Pomc5-HT2CR) littermates.
All mice were group housed and maintained on a 12 h light/dark cycle with ad libitum access to water and standard laboratory chow diet. All experiments were in accordance with guidelines and approvals of the University of Michigan or the U.K. Animals (Scientific Procedures) Act 1986.
2.2. Immunohistochemistry (IHC)
Tissue was processed for endogenous POMC and for 5-HT2CRYFP as previously described [9], [10], [11]. Briefly, under deep terminal anesthesia, mice were transcardially perfused with phosphate buffered saline (PBS) followed by 10% neutral buffered formalin (Sigma). Brains were extracted, post-fixed in 10% neutral buffered formalin at 4 °C, cryoprotected in 20% sucrose at 4 °C and then sectioned coronally on a freezing sliding microtome at 25 μm. Tissue was processed for POMC-immunoreactivity (IR) and 5-HT2CRYFP (GFP-IR) as previously described [14], [15] using rabbit anti-POMC primary antibody (1:1000; H-029-30, Phoenix Pharmaceuticals, Burlingame, CA, USA), chicken anti-GFP (1:500; ab13970, AbCam, Cambridge, UK) and Alexa Fluor secondary antibodies (1:500 A-11012, Life Technologies, Paisley, UK), respectively. Single and dual-labeled POMC-IR and GFP-IR cells were counted in the ARC [16]. Analysis was carried out on 7 levels of ARC (−1.46 to −2.18 from Bregma) for each mouse (n = 4/sex).
2.3. Quantitative PCR
Total RNA was purified from whole hypothalamus, brainstem and interscapular brown adipose tissue (BAT) using RNA STAT 60 (AMS Biotechnology, Abington, UK) according to the manufacturer's instructions and as previously described (9 months of age; n = 5–9/genotype/sex) [13]. cDNA was obtained by reverse transcription of 500 ng hypothalamic RNA, 1000 ng brainstem RNA and 500 ng BAT RNA. Real-time PCR analysis of cDNA was performed in duplicate on an ABI Prism 7900 sequence detection system using Taqman or Sybr assays for Pomc (ABI Taqman Gene expression assay Mm00435874_m1), elongation of very long fatty acids-like 3 (Elovl3) and peroxisome proliferator-activated receptor gamma coactivator-1alpha (Pgc-1a). Data for levels of target gene mRNAs are expressed in arbitrary units corrected to the geometric average of four housekeeping genes: 18s, 36β4, βactin and glyceraldehyde-3-phosphate dehydrogenase (Gapdh). Sequences of primers and probes used are listed in Supplementary Table 1.
2.4. Metabolic profile
Body weight was measured from weaning up to 1 year of age (n = 7–17/genotype/sex). Home cage 24-h food intake was measured up to 6 months of age (n = 5–9/genotype/sex). At 9 months of age, a more detailed energy balance profile was performed, including light and dark cycle food intake, locomotor activity and energy expenditure assessment using indirect calorimetry in a Metabolic-Trace (Meta-Trace) system (Ideas Studio, UK; n = 5–9/genotype/sex). Body composition was also analyzed at 7–9 months of age using dual-energy X-ray absorptiometry (DEXA) Lunar PIXImus2 mouse densitometer (General Electric Medical Systems, Fitchburg, WI, USA; n = 5–11/genotype/sex).
Gonadal white adipose tissue (WAT) and interscapular BAT was dissected, fixed in 10% neutral buffered formalin, embedded in paraffin, cut into 5 μm sections and stained with hematoxylin and eosin. WAT cell diameter (μm) and BAT lipid droplet size (% of total area) was measured on an inverted light microscope (Olympus BX41, Olympus UK Ltd, Southend-on-Sea, UK) using CellˆD Olympus Software (Shinjuku, Tokyo, Japan). Analysis was carried out on 5-9 sections for each mouse (9 months of age; n = 4–6/genotype/sex).
Blood samples were taken from the left ventricle in 6 h light cycle fasted terminally anesthetized mice (9 months of age; n = 5–9/genotype/sex) (phenobarbital sodium (Dolethal); Vétoquinol, UK). Insulin and leptin were assayed using a two-plex electrochemical luminescence microtiter plate immunoassay (MesoScale Discovery, Gaithersburg, MD, USA).
2.5. Insulin tolerance test
Mice (6–8 months of age; n = 5–9/genotype/sex) were fasted for 6 h during the light cycle. Blood was sampled from tail vein immediately prior to insulin (1.1 U/kg IP, males; 0.8 U/kg IP, females) bolus, and 15, 30, 45, 60 and 90 min following bolus administration. Blood glucose was analyzed using AlphaTrak glucometer (Chicago, IL, USA).
2.6. Statistics
Data were analyzed with One-way, Two-way or Repeated Measures ANOVA or ANCOVA followed by Tukey's or Bonferroni post hoc tests. General linear models were also performed for energy expenditure analysis. For all analyses, significance was assigned at P < 0.05. Data are presented as mean ± SEM.
3. Results and discussion
3.1. Generation of 5-HT2CRCRE line
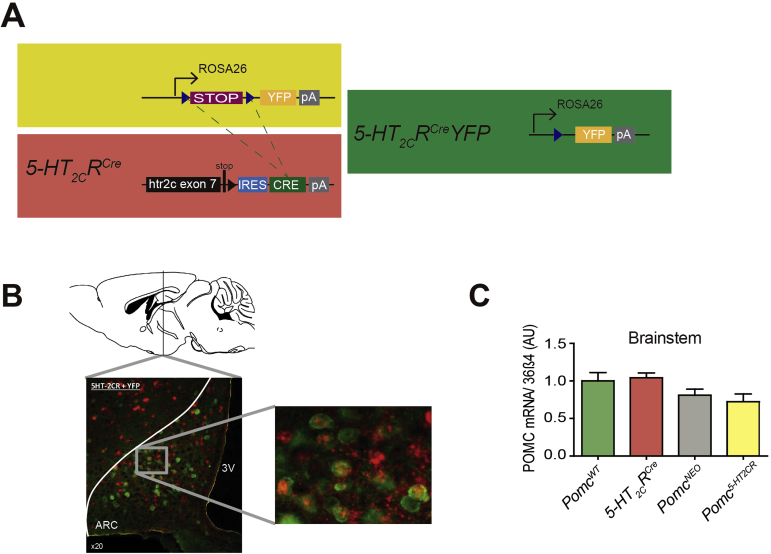
To clarify the source of POMC peptides that underpin energy balance and body weight regulation, we utilized a Cre recombinase-dependent and ARC specific reactivatable PomcNEO line [13] to restore Pomc synthesis within the discrete subset of cells expressing 5-HT2CRs. To achieve this, we first generated a 5-HT2CRCRE line. To confirm that 5-HT2CRCRE is contained in cells expressing endogenous 5-HT2CR, 5-HT2CRCRE mice were intercrossed with B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J (Rosa26YFP) mice, which have a loxP-flanked STOP sequence followed by an YFP gene inserted into the Gt(ROSA)26Sor locus. Intercrossing with 5-HT2CRCRE mice removes the STOP sequence and YFP is visualized in 5-HT2CRCRE expressing cells (Figure S1A). Performing immunofluorescent staining in the ARC for YFP-immunoreactivity (IR) and fluorescent in situ hybridization (FISH) to label endogenous 5-HT2CR mRNA revealed that the majority of Cre containing cells expressed endogenous 5-HT2CR mRNA (Figure S1B).
3.2. Anatomical localization and genetic manipulation of subset of hypothalamic Pomc co-expressing 5-HT2CRs
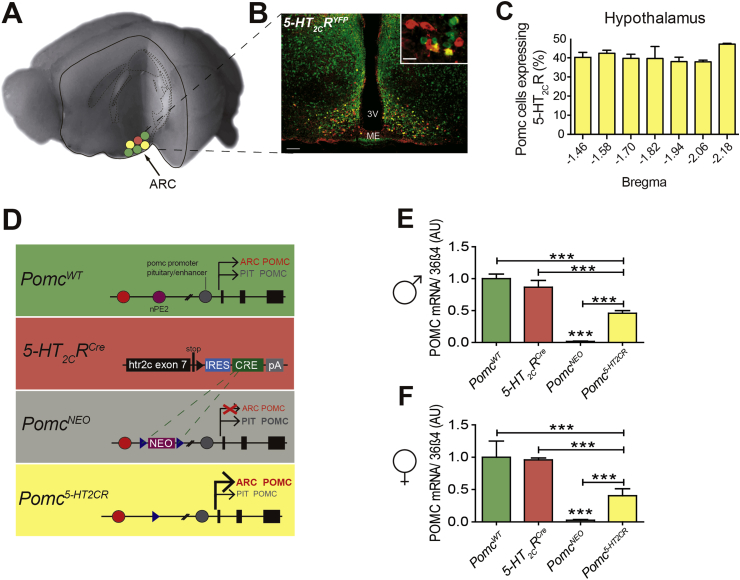
To determine the anatomical localization of the subset of ARC POMC neurons specifically co-expressing 5-HT2CRs, dual-immunofluorescent analysis was performed for GFP-IR and POMC-IR in the 5-HT2CRYPF line. This analysis revealed that approximately 40% of ARC POMC neurons co-express 5-HT2CRs in male and female mice (Figure 1A–C). This co-expression profile is similar to that previously observed in rats [9]. Further analysis of anatomical co-localization indicated that this 40% co-expression rate was consistent across the rostral-caudal extent of the ARC (Figure 1C).
Figure 1.
Generation of mice with restored ARC Pomc function in 5-HT2CR expressing cells. (A,B) ARC POMC neurons (POMC-IR, red) co-express 5-HT2CRs (GFP-IR, green; co-labeled, yellow). Scale bar, 50 μm (inset) and 100 μm. (C) POMC-IR and 5-HT2CR (GFP-IR) co-expression by ARC bregma level. (D) Schematic of wild-type allele (PomcWT) containing both neuronal Pomc enhancers, nPE1 and nPE2 (green); 5-HT2CRCRE inserted after ht2rc exon 7 (red); a disrupted PomcNEO knockout allele carrying nPE2 deletion and a loxP-flanked-mediated disruption of nPE1 transcriptional activation function (gray); and a re-activated Pomc/5-HT2C allele (Pomc5−HT2CR) (yellow). (E)Pomc re-expression in 5-HT2CR neurons in male (F3,19 = 22.40, P < 0.0001) and (F) female mice (F3,15 = 18.40, P < 0.0001) normalized to 36β4 mRNA, relative to PomcWT, in arbitrary units (AU). *P < 0.05, **P < 0.01, ***P < 0.001 as indicated.
To investigate the physiological importance of this specific source of POMC peptides in the regulation of energy balance and body weight, we intercrossed a Cre recombinase-dependent and ARC specific reactivatable PomcNEO line to restore Pomc expression only within cells expressing 5-HT2CRs (Figure 1D). As expected, PomcNEO mice had no detectable hypothalamic Pomc mRNA, whereas Pomc was restored by 44% in male and 41% in female Pomc5-HT2CR mice (Figure 1E,F). This Pomc reactivation level is consistent with the anatomical co-localization determined above. No differences in Pomc expression among genotypes were detected in the brainstem, which includes the nucleus tractus solitarius (Figure S1C). Therefore, we created a genetic means to probe the specific function of a subset of ARC POMC neurons in the regulation of whole body energy balance and body weight.
3.3. Hypothalamic Pomc expressed exclusively within 5-HT2CR containing cells modulates energy intake in male and female mice
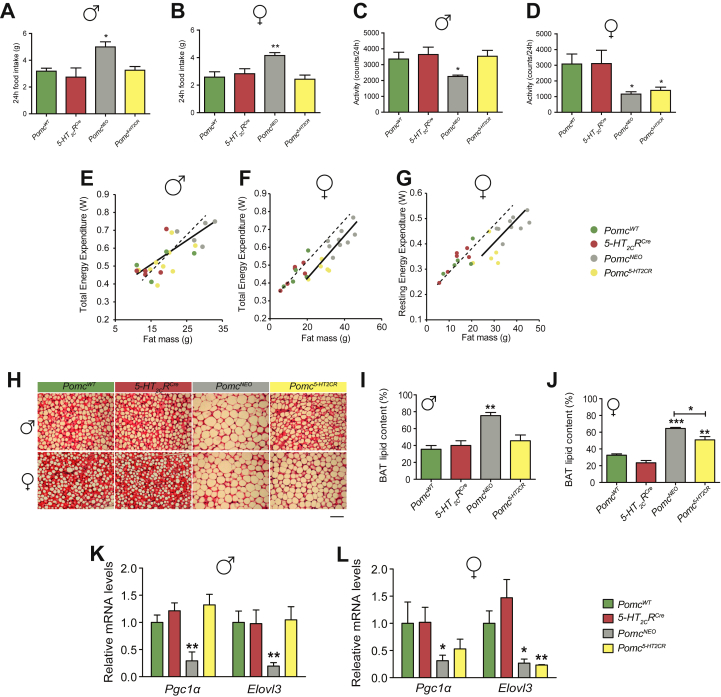
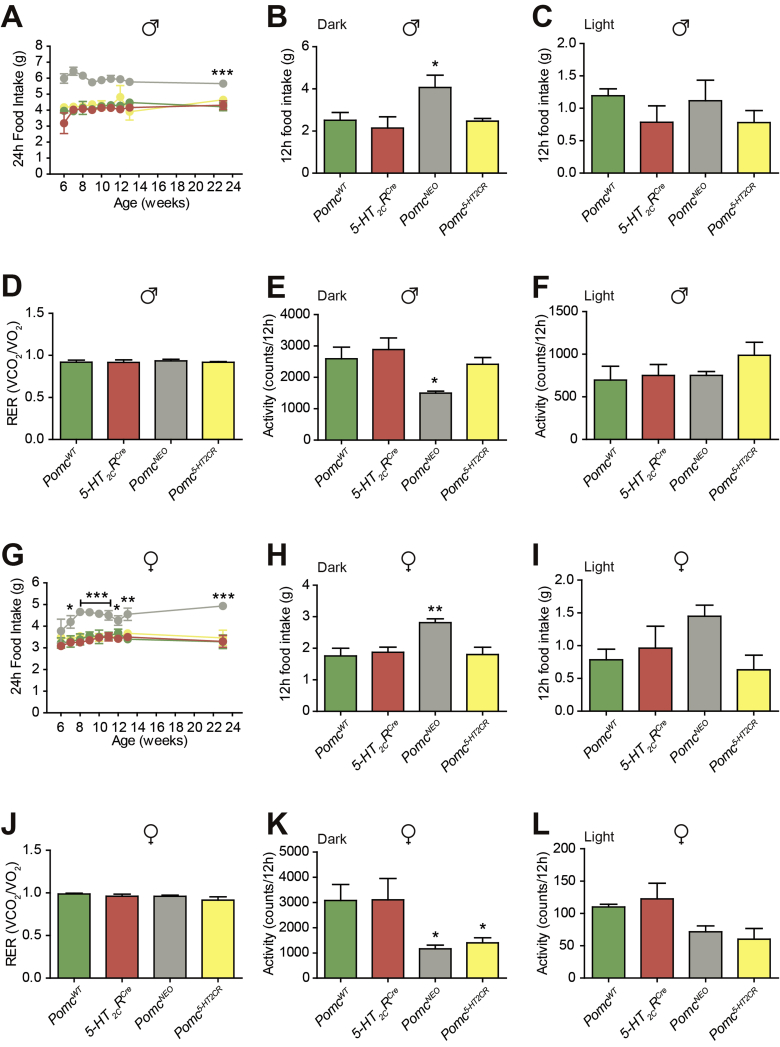
Given that current obesity medication 5-HT2CR agonist lorcaserin improves obesity by influencing appetite, but not energy expenditure [19], and that 5-HT2CR agonists reduce food intake via increased activity of POMC neurons [9], [20], we surmised that POMC peptides synthesized exclusively in neurons expressing 5-HT2CRs perform an essential role in the regulation of energy intake. To investigate this, we compared 24-hour food intake in PomcNEO, 5-HT2CRCRE, wild type and mice with restored Pomc expression only in cells expressing 5-HT2CRs (Pomc5-HT2CR). As expected, male and female PomcNEO mice exhibited significant hyperphagia (Figure 2A,B; Figure S2A–C, S2G-I), consistent with previous reports [13]. Restoration of hypothalamic Pomc expression only within 5-HT2CR cells normalized 24-h food intake in both male and female mice (Figure 2A,B). Additional circadian analysis revealed that PomcNEO energy intake was significantly higher during the dark cycle in male (Figure S2B,C) and female (Figure S2H,I) mice, and this was normalized by the restoration of Pomc expression in 5-HT2CR cells in both sexes. Next, we tracked 24-h food intake in male and female mice by genotype until 6 months of age. We observed that PomcNEO hyperphagia persisted with age, whereas Pomc5-HT2CR mice continued to consume food at a level consistent with control 5-HT2CRCRE and wild type littermates (Figure S2A,G). Consequently, these data indicate that POMC peptides synthesized exclusively in neurons expressing 5-HT2CRs are sufficient to mediate POMC's effects on food intake.
Figure 2.
Subpopulation of Pomc differentially modulates physical activity and energy expenditure in male and female mice. 24 h food intake was significantly elevated in (A) male (F3,12 = 13.98, P < 0.001) and (B) female (F3,11 = 6.55, P < 0.001) ARC Pomc null (PomcNEO) mice, and this was normalized by restoration of Pomc in 5-HT2CR neurons in Pomc5−HT2CR mice. 24 h locomotor activity was significantly reduced in (C) male (F3,17 = 3.68, P < 0.05) and (D) female (F3,18 = 5.36, P < 0.05) PomcNEO mice, and this was normalized in male, but not female, Pomc5-HT2CR mice. General linear model in (E) male and (F) female mice illustrating reduced total daily energy expenditure in PomcNEO and Pomc5-HT2CR mice (solid line) compared to control siblings (dashed line) in females (group effect P < 0.01) but not in males (group effect P > 0.05). (G) General linear model revealing PomcNEO and Pomc5-HT2CR females (solid line) display reduced resting metabolic rate compared to control siblings (dashed line) (group effect P < 0.01). (H,I) Lipid accumulation in interscapular brown adipose tissue (BAT; F3,17 = 11.61, P < 0.001) and relative expression of mitochondrial genes important for BAT thermogenesis, (K)Pgc-1α (F3,18 = 8.638, P < 0.001) and Elovl3 (F3,18 = 4.147, P < 0.05), were fully normalized by restoration of Pomc in 5-HT2CR neurons (Pomc5-HT2CR) in male mice. By contrast, Pomc5-HT2CR female mice display an increase in (H,J) BAT lipid content (F3,10 = 40.00, P < 0.0001) and a downregulation in (L)Pgc-1α(F3,12 = 8.941, P < 0.01) and Elovl3 (F3,18 = 8.941, P < 0.01) compared to littermate controls. Data expressed as arbitrary units and expression of target genes corrected to the geometric average of four housekeeping genes:18s, 36β4, Gapdh and βactin. Scale bar, 2000 μm *P < 0.05, **P < 0.01, ***P < 0.001 compared to all other genotypes, except in D, J and L compared to PomcWT and 5-HT2CRCre mice.
3.4. Unexpected sex difference in physical activity and energy expenditure modulated by hypothalamic Pomc exclusively expressed within 5-HT2CR containing cells
Analysis of energy homeostasis in Pomc5-HT2CR mice uncovered a substantial and unexpected sex difference in the molecular regulation of physical activity and energy expenditure. In both males and females, PomcNEO mice exhibited significantly reduced 24-h locomotor activity, which was accounted for by a reduction in activity within the dark, but not light cycle (Figure 2C,D; Figure S2E,F,K,L). However, restoration of Pomc5-HT2CR function fully normalized physical activity only in male mice (Figure 2C; Figure S2E,F). While a significant effect of body fat mass on total daily energy expenditure was detected in males (Figure 2E), this did not vary by genotype. Conversely, in female mice, restoration of Pomc5-HT2CR function had no impact on reduced physical activity (Figure 2D, Figure S2K,L) or total daily energy expenditure (Figure 2F). Thus, in females, a significant genotype effect of fat mass on total daily energy expenditure was observed. A general linear model using both fat and lean mass explained 80% of the variance in energy expenditure in pooled female wild type and 5-HT2CRCRE mice. Both Pomc5-HT2CR and PomcNEO female mice displayed significantly reduced total daily energy expenditure compared to wild type and 5-HT2CRCRE female siblings (Tukey's post hoc P < 0.01) but did not differ from each other (P > 0.05). We next explored the impact of genotype and body composition on resting metabolic rate in female mice (Figure 2G). A general linear model using both fat and lean mass explained 91.8% of the variance in energy expenditure in pooled female wild type and 5-HT2CRCRE mice. These results reveal that both PomcNEO and Pomc5-HT2CR female mice had significantly reduced resting energy expenditure compared to wild type and 5-HT2CRCRE siblings (Tukey's post hoc P < 0.01) but did not differ from each other (P > 0.05).
Brown adipocytes contain a large number of mitochondria for thermogenesis, and nutrient oxidation in BAT can account for up to 60% of the total energy expenditure of mice [21], [22]. Melanocortinergic regulation of BAT thermogenesis and energy expenditure has been reported via Mc4rs expressed by cholinergic preganglionic sympathetic neurons within the intermediolateral nucleus of the thoracic spinal cord (IML) [23], [24], [25]. The IML is directly innervated by ARC POMC neurons [26], [27] and postganglionic neurons innervating brown adipose tissue receive projections from the IML [28], [29], [30], suggesting a pathway through which POMC neurons may participate in the regulation of BAT thermogenesis and energy expenditure.
We therefore next examined whether Pomc exclusively synthesized within 5-HT2CR expressing neurons influences BAT function. In male and female mice, genetic inactivation of ARC Pomc was associated with increased lipid accumulation in BAT (Figure 2H–J) and reduced expression of Pgc-1a and Elolv3, mitochondrial genes important for BAT thermogenesis (Figure 2K–L). In male mice, restoration of Pomc5-HT2CR function normalized BAT lipid accumulation (Figure 2H,I) and Pgc-1a and Elolv3 expression (Figure 2K). In contrast, Pomc5-HT2CR female mice displayed an increase in BAT lipid content (Figure 2H,J) and a downregulation in thermogenic gene expression (Figure 2L) compared to control littermates. These data suggest that impaired BAT thermogenesis in female PomcNEO and Pomc5-HT2CR mice contributes to the observed reduction in whole body energy expenditure.
Taken together, these data signify that ARC POMC regulates both physical activity related energy demands and resting metabolism and that restoration of POMC function within the subset of 5-HT2CR expressing neurons is not sufficient to appropriately regulate energy expenditure or BAT function in female mice. No differences in respiratory exchange ratio (RER) were detected by genotype in either sex, and, in the combined data set, there was no sex effect on the RER (Figure S2D,J). However, we uncovered an unexpected sex difference in the molecular underpinnings driving physical activity and determined that Pomc5-HT2CR have a broader, sex-specific, function in the regulation of energy usage.
3.5. Unexpected sex difference in body weight regulation modulated by hypothalamic Pomc exclusively expressed within 5-HT2CR containing cells
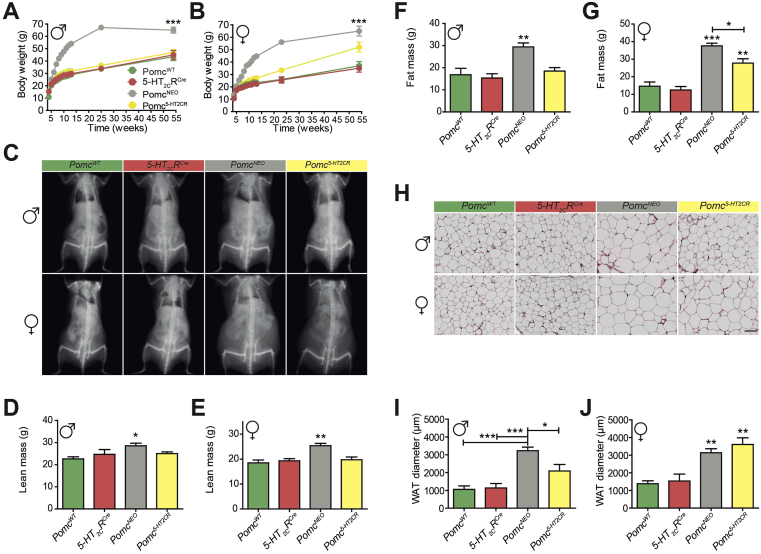
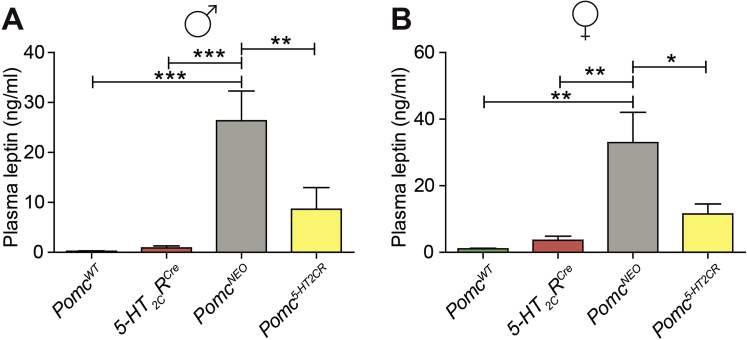
We next investigated the ramifications of this discrete source of ARC POMC peptides on body weight and adiposity. Consistent with the metabolic consequence of restored energy balance in male Pomc5-HT2CR mice, a prevention of obesity in male Pomc5-HT2CR mice was observed compared to PomcNEO littermates (Figure 3A). Furthermore, male Pomc5-HT2CR mice displayed levels of lean mass (Figure 3C,D), fat mass (Figure 3F) and leptin levels (Figure S3A) that were comparable to control 5-HT2CRCRE and wild type littermates. Likewise, male PomcNEO mice had larger white adipocytes and this phenotype was normalized in male Pomc5-HT2CR mice (Figure 3H,I).
Figure 3.
Subpopulation of Pomc differentially modulates body weight and adiposity in male and female mice. (A,B) Body weight (male, F3,20 = 114.35, P < 0.001; female F3,19 = 60.80, P < 0.001), (C, F, G) fat mass (male F3,20 = 9.43, P < 0.001; female F3,22 = 42.04, P < 0.001), (H–J) gonadal white adipocyte diameter (male F3,14 = 13.98, P < 0.001; female F3,13 = 11.87, P < 0.001) were increased in ARC Pomc null (PomcNEO) mice and normalized by restoration of Pomc in 5-HT2CR neurons (Pomc5-HT2CR) in male but not female mice. However, female PomcNEO mice still exhibited significantly greater body weight and fat mass, but not adipocyte size, compared with Pomc5-HT2CR mice. (D, E) Both male and female PomcNEO mice exhibited significantly greater lean mass compared to Pomc5-HT2CR mice and controls. Scale bar, 2000 μm *P < 0.05, **P < 0.01, ***P < 0.001 compared to control PomcWT and 5-HT2CRCRE siblings, except where noted in D, E, G and I.
In contrast, ARC Pomc synthesized within neurons expressing 5-HT2CRs was not sufficient to regulate adiposity in female mice. Female Pomc5-HT2CR mice were genetically predisposed to develop obesity compared to wild type and 5-HT2CRCRE female siblings (Figure 3B), with increased fat mass (Figure 3C,G) and displayed significantly larger white adipocytes (Figure 3H,J). This elevation in body weight and fat mass was less severe than that produced by full ARC Pomc nulls, though both PomcNEO and Pomc5-HT2CR mice showed comparable increases in white adipocyte size. However, only PomcNEO mice exhibited statistically increased lean mass (Figure 3E) and leptin levels (Figure S3B) and this was corrected in Pomc5-HT2CR mice. These data reveal that the subpopulation of ARC POMC expressed within 5-HT2CR containing neurons is sufficient to regulate whole body energy balance, body weight and adiposity in male, but not female, mice.
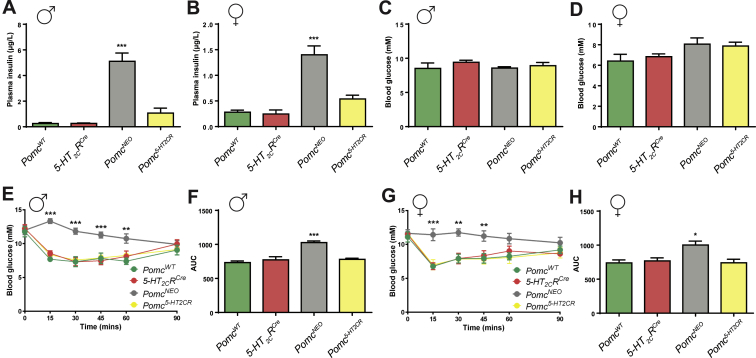
3.6. Hypothalamic Pomc expressed exclusively within 5-HT2CR containing cells modulates insulin sensitivity in male and female mice
Recent reports revealed that though PomcNEO mice exhibit insulin resistance, they display normal glucose levels and improved glucose tolerance, primarily by increasing glycosuria [31], [32]. We next considered whether Pomc synthesized exclusively within 5-HT2CR expressing cells is sufficient to normalize PomcNEO hyperinsulinemia and impaired insulin tolerance. As expected, severely hyperphagic and obese male and female PomcNEO mice exhibited pronounced hyperinsulinemia (Figure 4A,B) with normal fasting blood glucose (Figure 4C,D), a pattern indicating insulin resistance compensated by increased insulin secretion from pancreatic beta cells. Consistent with the metabolic consequence of normalized energy balance and adiposity in male Pomc5-HT2CR mice, hyperinsulinemia was corrected in male Pomc5-HT2CR mice compared to PomcNEO littermates (Figure 4A). Despite disrupted energy balance and pronounced obesity, female Pomc5-HT2CR mice also displayed insulin levels comparable to wild type and 5-HT2CRCRE siblings (Figure 4B). Examining insulin sensitivity further, we found that both male (Figure 4E,F) and female (Figure 4G,H) PomcNEO mice exhibited impaired responses in an insulin tolerance test compared with control littermates, and this was normalized by restoring Pomc within 5-HT2CR expressing cells. Taken together, these results indicate that despite a sex difference in energy balance and obesity, Pomc synthesized in 5-HT2CR expressing cells is sufficient to mediate POMC's effects on insulin sensitivity in both male and female mice.
Figure 4.
Subpopulation of Pomc modulates insulin sensitivity in male and female mice. (A,B) Plasma insulin (male, F3,21 = 63.20, P < 0.0001; female F3,23 = 20.33, P < 0.0001) was increased in ARC Pomc null (PomcNEO) mice and normalized by restoration of Pomc in 5-HT2CR neurons (Pomc5-HT2CR). (C,D) Fasting blood glucose was not statistically different among genotypes (male, F3,15 = 0.70, P > 0.05; female F3,18 = 2.30, P > 0.05). Both male (E,F) and female (G,H)PomcNEO mice exhibited impaired responses in an insulin tolerance test compared with control littermates, which were normalized by restoration of Pomc in 5-HT2CR neurons (Pomc5-HT2CR); (E,G) Insulin tolerance tests (male, F3,104 = 33.09, P < 0.0001; female F3,102 = 24.39, P < 0.0001) and (F,H) their respective areas under the curve (AUC) (male, F3,18 = 18.06, P < 0.0001; female F3,17 = 6.56, P < 0.01). *P < 0.05, **P < 0.01, ***P < 0.001 compared to all other genotypes.
These data reveal a discrete and specific sex difference in the regulation of body weight, fat accumulation and adipocyte size driven by the subpopulation of hypothalamic POMC peptides exclusively synthesized in neurons expressing 5-HT2CRs. Interestingly, POMC within the subpopulation of ARC leptin receptor expressing neurons was sufficient to normalize energy balance and body weight in both male and female PomcNEO mice [32]. Thus, these data reveal a specific and functionally distinct role of POMC within the subset of cells examined here, providing further support for the functional heterogeneity of ARC derived POMC peptides.
The sex difference in energy storage is consistent with significantly reduced energy usage in female Pomc5-HT2CR mice. Given that food intake is normalized in female Pomc5-HT2CR mice, this genetic model reflects a new example of the cumulative impact of reduced physical activity and energy expenditure on body weight and adiposity with time. It may be noted that in the PomcNEO mice, the impact on energy expenditure was much greater in the females compared to males (compare Figure 2E,F) and the corresponding fat mass also much greater (compare Figure 3F–G). Therefore, the localized synthesis of POMC peptides within a subset of neurons within the discrete brain region the ARC produces a substantial difference in the magnitude of adiposity accumulation in male and female mice. This phenotype cannot simply be explained by impairing POMC activity by estrogens, because the inactivation of estrogen receptor specifically within ARC POMC neurons does not impact fat mass or reduce energy expenditure [33].
4. Conclusions
These findings support the functional heterogeneity of ARC POMC, revealing that the source synthesized within 5-HT2CR expressing neurons is sufficient to regulate energy intake and insulin sensitivity in male and female mice. Moreover, these data provide evidence for a specific neurochemical basis for levels of reduced physical activity and reveal that the molecular underpinnings of the impetus to engage in physical activity are differentially modulated in males and females. However, physical activity comprises only one means of utilizing energy and our data further show that the same neuronal population plays a key role, modulated by sex, in the regulation of resting rates of expenditure. Consequently, these data uncovered an unexpected sex difference, mediated by POMC, in total energy expenditure, thermogenic activity of BAT and adiposity. These findings provide evidence that males and females are hardwired differently in their regulation of energy balance. Given the reported reduction of POMC neuron activity in middle age in mice [7], these data may have translational relevance by providing a potential molecular explanation for the global sex differences in obesity prevalence. Finally, these data may have broad implications for future sex-specific strategies in treating overweight and obesity.
Acknowledgments
Work was supported by the Wellcome Trust (WT098012; WT081713) and Biotechnology and Biological Sciences Research Council (BB/K001418/1) to LKH, Wellcome Trust (093566/Z/10/A) to LKB/LKH, the Diabetes UK (13/0004680) to MLE, the Genomics/Transcriptomics core, Disease Model Core, Bespoke Mouse Models, Imaging, Proteomics Wellcome Trust Strategic Award [100574/Z/12/Z] and Genomics/Transcriptomics Core Facilities, Disease Model Core, CBAL MRC Metabolic Diseases Unit [MRC_MC_UU_12012/5] to LKH, MLE, BD and LKB, the Neuroscience Graduate Program, University of Michigan to MG-Y, the National Institute of Health (DK066604) to MJL and (DK068400) to MJL and MR, and National Institute of Health (DK056731) and the Marilyn H. Vincent Foundation to MGM. The University of Michigan Transgenic Core facility is partially supported by the NIH-funded University of Michigan Center for Gastrointestinal Research (DK034933). The authors would like to thank Dr Samuel Virtue for technical training in BAT lipid accumulation quantification.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.01.005.
Contributor Information
Mark L. Evans, Email: mle24@cam.ac.uk.
Lora K. Heisler, Email: lora.heisler@abdn.ac.uk.
Conflicts of interest
The authors declare that no conflict of interest exists.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
2
3
References
- 1.World Health Organisation . 2014. Global Health Observatory (GHO) obesity situation and trends. [Google Scholar]
- 2.Williams K.W., Elmquist J.K. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nature Neuroscience. 2012 Oct;15(10):1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellacott K.L., Cone R.D. The role of the central melanocortin system in the regulation of food intake and energy homeostasis: lessons from mouse models. Philosophical Transactions of the Royal Society B: Biological Sciences. 2006 Jul;361(1471):1265–1274. doi: 10.1098/rstb.2006.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeo G.S., Heisler L.K. Unraveling the brain regulation of appetite: lessons from genetics. Nature Neuroscience. 2012 Oct;15(10):1343–1349. doi: 10.1038/nn.3211. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandrappa S., Farooqi I.S. Genetic approaches to understanding human obesity. Journal of Clinical Investigation. 2011 Jun;121(6):2080–2086. doi: 10.1172/JCI46044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mori H., Inoki K., Münzberg H., Opland D., Faouzi M., Villanueva E.C. Critical role for hypothalamic mTOR activity in energy balance. Cell Metabolism. 2009 Apr;9(4):362–374. doi: 10.1016/j.cmet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S.B., Tien A.C., Boddupalli G., Xu A.W., Jan Y.N., Jan L.Y. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron. 2012 Aug;75(3):425–436. doi: 10.1016/j.neuron.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newton A.J., Hess S., Paeger L., Vogt M.C., Fleming Lascano J., Nillni E.A. AgRP innervation onto POMC neurons increases with age and is accelerated with chronic high-fat feeding in male mice. Endocrinology. 2013 Jan;154(1):172–183. doi: 10.1210/en.2012-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heisler L.K., Cowley M.A., Tecott L.H., Fan W., Low M.J., Smart J.L. Activation of central melanocortin pathways by fenfluramine. Science. 2002 Jul;297(5581):609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- 10.Lam D.D., Przydzial M.J., Ridley S.H., Yeo G.S., Rochford J.J., O'Rahilly S. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008 Mar;149(3):1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke L.K., Doslikova B., D'Agostino G., Garfield A.S., Farooq G., Burdakov D. 5-HT obesity medication efficacy via POMC activation is maintained during aging. Endocrinology. 2014 Jul:en20141223. doi: 10.1210/en.2014-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berglund E.D., Liu C., Sohn J.W., Liu T., Kim M.H., Lee C.E. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. Journal of Clinical Investigation. 2013 Nov doi: 10.1172/JCI70338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bumaschny V.F., Yamashita M., Casas-Cordero R., Otero-Corchón V., de Souza F.S., Rubinstein M. Obesity-programmed mice are rescued by early genetic intervention. Journal of Clinical Investigation. 2012 Nov;122(11):4203–4212. doi: 10.1172/JCI62543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garfield A.S., Patterson C., Skora S., Gribble F.M., Reimann F., Evans M.L. Neurochemical characterization of body weight-regulating leptin receptor neurons in the nucleus of the solitary tract. Endocrinology. 2012 Oct;153(10):4600–4607. doi: 10.1210/en.2012-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam D.D., Leinninger G.M., Louis G.W., Garfield A.S., Marston O.J., Leshan R.L. Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metabolism. 2011 May;13(5):584–591. doi: 10.1016/j.cmet.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paxinos G., Watson C. 4th ed. 1998. The rat brain – in stereotaxic coordinates – preface. ix-+ p. [DOI] [PubMed] [Google Scholar]
- 17.Leinninger G.M., Opland D.M., Jo Y.H., Faouzi M., Christensen L., Cappellucci L.A. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metabolism. 2011 Sep;14(3):313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soliman G.A., Ishida-Takahashi R., Gong Y., Jones J.C., Leshan R.L., Saunders T.L. A simple qPCR-based method to detect correct insertion of homologous targeting vectors in murine ES cells. Transgenic Research. 2007 Oct;16(5):665–670. doi: 10.1007/s11248-007-9110-2. [DOI] [PubMed] [Google Scholar]
- 19.Martin C.K., Redman L.M., Zhang J., Sanchez M., Anderson C.M., Smith S.R. Lorcaserin, a 5-HT(2C) receptor agonist, reduces body weight by decreasing energy intake without influencing energy expenditure. Journal of Clinical Endocrinology and Metabolism. 2011 Mar;96(3):837–845. doi: 10.1210/jc.2010-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doslikova B., Garfield A.S., Shaw J., Evans M.L., Burdakov D., Billups B. 5-HT2C receptor agonist anorectic efficacy potentiated by 5-HT1B receptor agonist coapplication: an effect mediated via increased proportion of pro-opiomelanocortin neurons activated. Journal of Neuroscience. 2013 Jun;33(23):9800–9804. doi: 10.1523/JNEUROSCI.4326-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harms M., Seale P. Brown and beige fat: development, function and therapeutic potential. Nature Medicine. 2013 Oct;19(10):1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 22.Virtue S., Vidal-Puig A. Assessment of brown adipose tissue function. Frontiers in Physiology. 2013;4:128. doi: 10.3389/fphys.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi J., Balthasar N., Olson D., Scott M., Berglund E., Lee C.E. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metabolism. 2011 Feb;13(2):195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berglund E.D., Liu T., Kong X., Sohn J.W., Vong L., Deng Z. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nature Neuroscience. 2014 Jul;17(7):911–913. doi: 10.1038/nn.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sohn J.W., Harris L.E., Berglund E.D., Liu T., Vong L., Lowell B.B. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013 Jan;152(3):612–619. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias C.F., Lee C., Kelly J., Aschkenasi C., Ahima R.S., Couceyro P.R. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998 Dec;21(6):1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 27.Swanson L.W., Kuypers H.G. A direct projection from the ventromedial nucleus and retrochiasmatic area of the hypothalamus to the medulla and spinal cord of the rat. Neuroscience Letters. 1980 May;17(3):307–312. doi: 10.1016/0304-3940(80)90041-5. [DOI] [PubMed] [Google Scholar]
- 28.Bamshad M., Song C.K., Bartness T.J. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. American Journal of Physiology. 1999 Jun;276(6 Pt 2):R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 29.Rothwell N.J., Stock M.J. Effect of diet and fenfluramine on thermogenesis in the rat: possible involvement of serotonergic mechanisms. International Journal of Obesity. 1987;11(4):319–324. [PubMed] [Google Scholar]
- 30.Lowell B.B., Flier J.S. Brown adipose tissue, beta 3-adrenergic receptors, and obesity. Annual Review of Medicine. 1997;48:307–316. doi: 10.1146/annurev.med.48.1.307. [DOI] [PubMed] [Google Scholar]
- 31.Chhabra K.H., Adams J.M., Fagel B., Lam D.D., Qi N., Rubinstein M. Hypothalamic POMC-deficiency improves glucose tolerance despite insulin resistance by increasing glycosuria. Diabetes. 2015 Oct. doi: 10.2337/db15-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lam D.D., Attard C.A., Mercer A.J., Myers M.G., Rubinstein M., Low M.J. Conditional expression of Pomc in the Lepr-positive subpopulation of POMC neurons is sufficient for normal energy homeostasis and metabolism. Endocrinology. 2015 Apr;156(4):1292–1302. doi: 10.1210/en.2014-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y., Nedungadi T.P., Zhu L., Sobhani N., Irani B.G., Davis K.E. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metabolism. 2011 Oct;14(4):453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.