Abstract
The ability of nanoparticles (NPs) to be promptly uptaken by the cells makes them both dangerous and useful to human health. It was recently postulated that some NPs might cross the plasma membrane also by a non-endocytotic pathway gaining access to the cytoplasm. To this aim, after having filled mature Xenopus oocytes with Calcein, whose fluorescence is strongly quenched by divalent metal ions, we have exposed them to different cobalt NPs quantifying quenching as evidence of the increase of the concentration of Co2+ released by the NPs that entered into the cytoplasm. We demonstrated that cobalt oxide NPs, but not cobalt nor cobalt oxide NPs that were surrounded by a protein corona, can indeed cross plasma membranes.
It is well known that nanoparticles (NPs) readily enter cells1 by different endocytotic mechanisms2,3,4,5,6. The capability of NPs to be promptly uptaken by the cells, as well as that of crossing biological barriers7,8,9, makes them at the same time potentially dangerous and useful to human health. Dangerous, as NPs might exert their toxicity, once inside the cell, very close to target organelles as nuclei and mitochondria, a phenomenon which is referred to as Trojan horse effect10,11,12. Useful, as they can be directed to exert their toxicity toward cancer cells, used for drug delivery, injected as a contrast agent for diagnostic and even for theranostic purposes13, and assumed for food supplementation.
Recently, it has been considered the possibility that some NPs might also cross the plasma membrane by a non-endocytotic pathway14,15,16,17,18,19 gaining a direct access to the cytoplasm. This pathway is usually poorly considered as it challenges the idea of non-permeability of membranes to large hydrophilic molecules. To verify this possibility, we have set up a new protocol that has proved capable to follow the NP-plasma membrane dynamics and we have demonstrated that cobalt oxide NPs, but not cobalt nor cobalt oxide NPs that were surrounded by a protein corona, can cross plasma membranes.
Cobalt NPs have a large use in industrial and biomedical applications. They efficiently catalyse the combustion of various hydrocarbons20 and the degradation of water pollutants21 providing a cheap candidate to replace noble metals. Cobalt NPs are also used in electrocatalysis for the oxygen evolution reaction22, important in hydrogen generation. Recently, it has been shown that cobalt NPs can self-assemble to constitute photonic hyper-crystals23, which might have a strong potential in biological and chemical sensing. Moreover, cobalt NPs are magnetic and this property allows to manipulate them in a chemical or biological system using an external magnet. Moreover, magnetic NPs can be easily conjugated to biologically important constituents such as DNA, peptides, antibodies24 as well as enzymes25,26 and sugars27,28 to construct versatile bio-nano hybrids.
Results and Discussion
Calcein as “Metal Detector” in Xenopus laevis oocytes
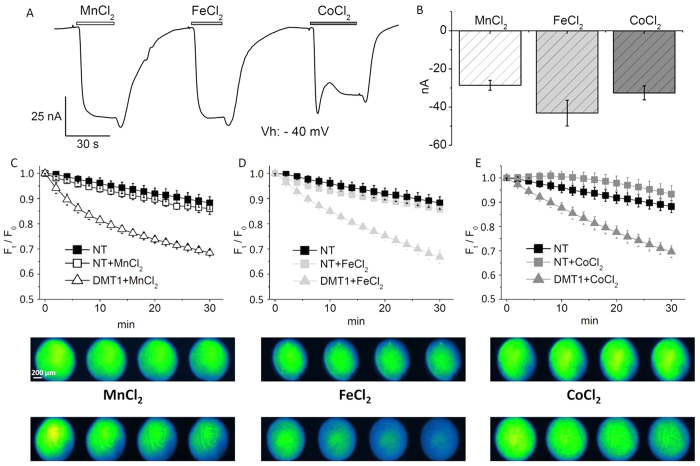
As a preliminary step, we have tested the ability of Calcein to detect cobalt uptake in fully grown Xenopus laevis oocytes. Oocytes are naturally arrested for prolonged period of time at prophase of meiosis I during which time the oocyte grows and stores macromolecular components that are necessary for future development. They exhibit different sizes that reflect different stages of growth. Fully grown oocytes, which have been used in the present paper, have a diameter of about 1.2 mm and provide a simple system for membrane transport characterization. For this purpose, we firstly needed to set up a system capable to consistently transport divalent metal ions across the plasma membrane from the extracellular milieu to the cytoplasm. We have, therefore, prepared transfected Xenopus oocytes by injecting them with the cRNA of the Divalent Metal ion Transporter 1 from rat (rDMT1). This membrane protein is a proton and voltage dependent transporter of divalent metal ions such as Fe2+ and Mn2+, as well as Co2+, Ni2+and Cd2+29,30,31,32. In mammals, it is mostly expressed in duodenum enterocytes, but it can be also found in kidney, brain, testis and placenta. By a two electrode voltage-clamp with a holding potential of −40 mV, we have recorded the currents generated by the exposure to manganese, iron and cobalt ions at pH 5.5. In non transfected (i.e., not injected with DMT1 cRNA) Xenopus laevis oocytes, the perfusion of ions in the bath solution did not elicit currents indicating the absence of electrogenic endogenous transporters in their plasma membrane. Conversely, in rDMT1 transfected oocytes, all the three substrates elicited, as expected33, inward currents in the range of −40 to −50 nA (Fig. 1A,B). As shown in Fig. 1B, iron, the physiological substrate, resulted slightly more efficiently transported than cobalt and manganese. With these experiments, we have confirmed that rDMT1 transfected oocytes, but not non-transfected ones, were able to transport iron, cobalt and manganese ions across their plasma membrane.
Figure 1. Calcein as a cytoplasmic “metal detector”.
(A): two electrode voltage clamp of a representative rDMT1 transfected oocyte; inward currents are induced by 100 μM MnCl2, FeCl2 and CoCl2 (Vh = −40 mV, pH 5.5). (B): means and standard errors of the transport currents obtained from 40 oocytes, five batches. (C–E): Plots of fluorescence decay (Ft/F0) with corresponding images of Calcein-injected oocytes (upper series: non-transfected (NT) and lower series: rDMT1 transfected) exposed to 100 μM MnCl2 (C), FeCl2 (D), and CoCl2 (E) at pH 5.5 from 3 to 10 oocytes, from 2 to 4 oocytes batches.
We have, then, filled transfected and not transfected oocytes with Calcein and monitored their fluorescence decay with an inverted fluorescence microscope. We have controlled that, before Calcein injection and at the used wavelengths, oocytes were not fluorescent (data not shown). We have also monitored the decay of the fluorescence signal in non-transfected Calcein-injected oocytes. Fluorescence decreased about 11.8% ± 2.5% in a 30 min interval (Fig. 1C–E, black squares) and, in the pH range 5.5–7.6, the decrease was pH-independent. Therefore, at our experimental conditions, only minimal photo-bleaching phenomena occurred. Similarly, non-transfected Calcein-injected oocytes which were exposed to 100 μM MnCl2, FeCl2, and CoCl2 (Fig. 1C–E, squares) underwent a moderate fluorescence decay not dissimilar to that occurring in the absence of the tested divalent metal ions.
Fluorescence decay was, instead, evident in transfected oocytes, i.e., in oocytes expressing rDMT1. We measured a 31.5% ± 1.1% decay for Mn2+ (empty triangles), a 33.2% ± 2.7% decay for Fe2+ (light grey triangles) and a 30.4% ± 2.4% for Co2+ (grey triangles). This indicates that the entry of the divalent metal ions into the cell caused the quenching of Calcein and, consequently, that Calcein can be used to monitor divalent metal ion concentration changes in the cytoplasm of Xenopus oocytes.
In this context, we further investigated metal-Calcein interactions by spectrofluorimetry measuring quenching in cuvettes at pH 7.6, close to the intracellular value, and at pH 5.5, value at which rDMT1 performs optimally. Values at the emission peak wavelength (i.e., 512 nm) were recorded for each concentration of Fe2+, Mn2+ and Co2+. The data revealed that Calcein quenching is higher for Co2+ and Fe2+, with K0.5 of 7.6 ± 0.7 and 5.3 ± 0.4 μM at pH 5.5 and 9 ± 3 and 0.9 ± 0.04 μM at pH 7.6. Quenching is lower for Mn2+ with a K0.5 of 53.6 ± 27 μM at pH 5.5 and of 21 ± 5.7 μM at pH 7.6. These spectrofluorometric data confirm that Calcein can be used to evaluate changes in the intracellular concentration of Mn2+, Fe2+ and Co2+.
NPs cross the plasma membrane of Xenopus laevis oocytes
After having verified that we were able to detect an increase of divalent metal ions in the cytoplasm of Xenopus oocytes filled with Calcein, we have used them to reveal the possible permeation of NPs inside the cell. To this aim, we have chosen cobalt NPs in two different forms, metallic (Co0) and oxide (Co3O4). Both NP forms undergo dissolution1,11,34,35 releasing cobalt ions that can be detected by Calcein quenching. Therefore, we have exposed Calcein-filled oocytes to cobalt NPs and, as a control, to the corresponding ion.
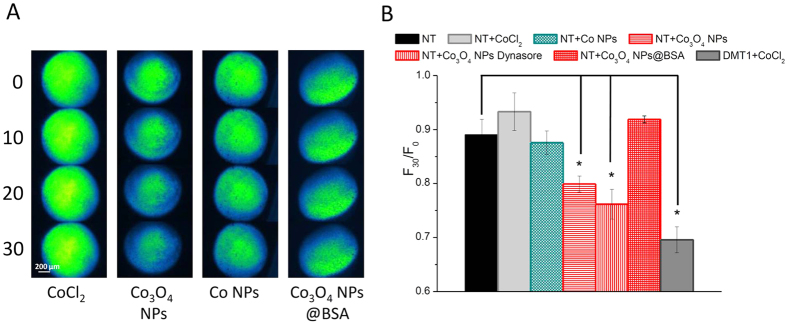
In oocytes from different batches, Co3O4 NPs consistently induced a quenching of Calcein fluorescence (Fig. 2). This fluorescence decrease, although lower than that occurring in rDMT1 expressing oocytes exposed to CoCl2 (Fig. 2B), was significantly higher than that occurring in non-transfected oocytes either exposed or not exposed to CoCl2. These results suggest that Co3O4 NPs interact with the plasma membrane of the oocyte, succeed in crossing it and, once in the cytoplasm, their partial dissolution causes the observed quenching activity. Indeed, cobalt ions and not NPs are able to interact with Calcein and quench its fluorescence. Co NPs, instead, did not cause a reduction of fluorescence (Fig. 2B) suggesting to be unable to pass through the plasma membrane of the oocyte. This different behavior of cobalt and cobalt oxide NPs could be ascribed to different chemical and physical characteristics of their surfaces. The importance of the surface structure of NPs in their interactions with cell membranes has been demonstrated comparing membrane penetration of two NPs that were coated with the same molecules, but arranged differently19. In our case, the normal spinel structure Co2+ Co23+ O22− of Co3O4 NPs36 might present a surface charge distribution capable to electrostatically interact with the negative charges which are present on the plasma membrane surface; indeed, Co3O4 NPs firmly bind, through electrostatic interactions, to negatively charged biomolecules such as heparin and carboxymethylchitosan27,28. Different cationic NPs have been shown to interact with lipid bilayers and cause their disruption37, cationic gold NPs can enter cells by a non-endocytotic, energy-independent pathway16 and cationic polystyrene NPs electrostatically interact with lipid bilayers causing deformation and poration, while anionic polystyrene NPs do not14,38. In this context, Lin and Alexander-Katz18, with a coarse-grained simulation, have described the dynamics of cationic NP translocation through cell membranes and Nolte and colleagues15 have modelled the transport of spherical metal oxide NPs across a lipid bilayer.
Figure 2. Calcein quenching in oocytes exposed to cobalt NPs.
(A): Representative image series of non-transfected (NT) Calcein-injected oocytes exposed to CoCl2 or different cobalt NPs for 0, 10, 20 and 30 min. (B): Means of the fluorescence decay of 5 to 25 oocytes (obtained from 2 to 5 different batches). Decay is expressed as the fluorescence intensity at time 30 min over fluorescence intensity at time 0 (F30/F0). Note that quenching is statistically significant in NT oocytes exposed to bare Co3O4 NPs and in rDMT1 expressing oocytes exposed to CoCl2 (positive control); moreover, the endocytosis blocker Dynasore does not change the quenching effect of Co3O4 NPs. Bars are ± SE; stars indicate a statistically significant (One-way ANOVA, P < 0.05) difference with non exposed oocytes.
Protein corona impedes plasma membrane crossing
The importance of the surface characteristics of NPs in their interactions with the biological matter is well documented and is cardinal for their toxicity as well as for their use in nanomedicine. We have, therefore, modified the surface characteristics of Co3O4 NPs by letting them to adsorb bovine serum albumin (BSA). This is known to create, around the NP, a protein corona, which is capable to modify NP-membrane interaction39,40 as also suggested by computer simulations17. In our experiments, BSA coated Co3O4 NPs (Co3O4 NP@BSA) do not cause fluorescence quenching (Fig. 2).
These results suggest that a “protein corona” effect can prevent or significantly reduce the interactions between NPs and the oocyte membrane blocking or limiting the passage of NPs into the cytoplasm. These findings are in agreement with experiments where the interaction of cationic polystirene NPs with artificial lipid bilayers were eliminated with serum proteins38. Alternatively, rather than impeding NP entry into the oocytes, the protein corona could have stabilized NPs against dissolution, preventing them, once in the cytoplasm, from releasing Calcein-quenching ions. To rule out this possibility, we have performed in vitro experiments where NPs, which were previously exposed to BSA, were tested for their ability to quench Calcein. Since no significant differences were observed, we think that BSA treatment of NPs, although capable to generate a protein corona, was not able to prevent dissolution.
Endocytosis does not seem to be responsible of Co3O4 NP entry
Some of our results could also be explained by endocytosis followed by NP escape from the endosomal compartment to the cytoplasm. To exclude this possibility, we have used two different approaches: in the first one, we have repeated quenching experiments on oocytes treated with Dynasore, an endocytosis inhibitor that has been used several times to block membrane recycle in Xenopus oocytes41; in the second one, we have verified whether or not NP exposure might have elicited endocytosis by optical and electron microscopy.
Dynasore is a cell‐permeable molecule that inhibits the GTPase activity of dynamin which in turn blocks dynamin-dependent endocytosis42. Quenching experiments were repeated in oocytes that were previously incubated for 24 h in 40 μM Dynasore. As shown in Fig. 2B, there are no significant differences in the quenching activity of Co3O4 NPs between oocytes that were treated with Dynasore and oocytes that were not.
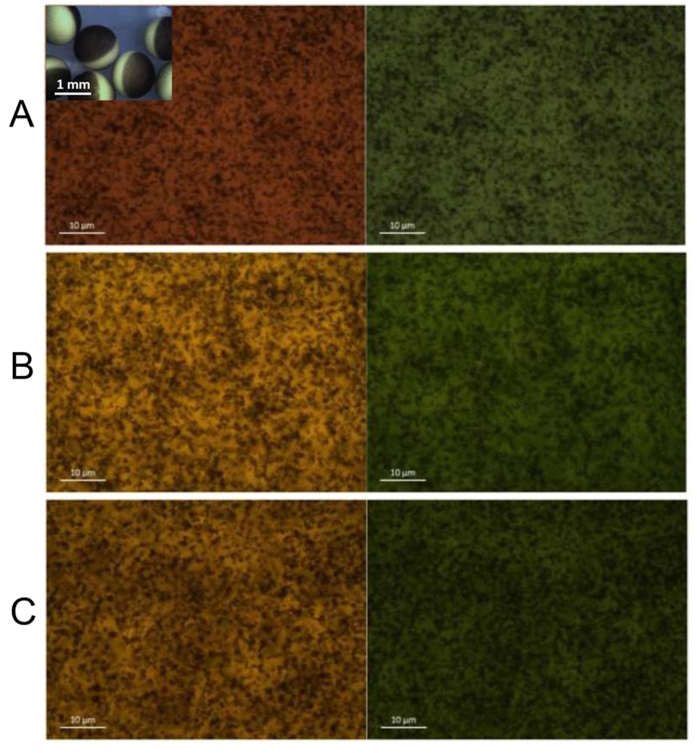
Dynasore, however, does not halt all the endocytotic routes. Therefore, to reveal the possible formation of endocytotic vesicles after NP exposure, we have used Lucifer Yellow CH, a water-soluble and membrane-impermeable fluorescent dye; it contains a carbohydrazide (CH) group that allows it to be covalently linked to the surrounding biomolecules by aldehyde fixation. Fully grown oocytes were exposed to the tracer dye in presence and in absence of NPs for 30 min, fixed and observed under a fluorescence microscope with a 63X oil immersion objective. As shown in Fig. 3A–C, no fluorescent vesicles are visible indicating that there is minimal or no endocytosis in the tested conditions. Lucifer Yellow CH had been previously shown to be effective in tracing endocytosis occurring immediately after cortical granule exocytosis during Xenopus egg fertilization43,44. Likewise, treated samples were prepared also for transmission electron microscopy and, notwithstanding a careful observation of the oocyte cortex, we could not find any NP carrying endocytotic vesicle among the pigment granules and the tightly packed cortical granules which characterise the oocyte cortex (data not shown).
Figure 3. Endocytosis is not involved in NP quenching activity.
In the inset, few fully grown oocytes are visible; note the presence of a pigmented pole (denominated animal pole) and of an unpigmented one (denominated vegetal pole). After fixation, a spherical cap is hand sliced with a razor blade, placed on a glass slide under a coverslip and observed with a 63X oil immersion objective from above. (A–C): Bright field (left) and the corresponding FITC filter (right) images of oocytes exposed to 1 mg/mL Lucifer Yellow CH. (A) control oocyte; (B) oocyte exposed to 0.1 mg/mL Co3O4 NPs and (C) oocyte incubated 24 h with 40 μM Dynasore and exposed to 0.1 mg/mL Co3O4 NPs. In bright field images, pigment granules, which are present in the cortex of the oocyte, are clearly visible as dark brown dots indicating that we are observing the oocyte animal pole. In the corresponding FITC filter images, no fluorescent vesicles are visible indicating no endocytotic activity.
No endogenous divalent metal ion transporters are present on the oocyte plasma membrane
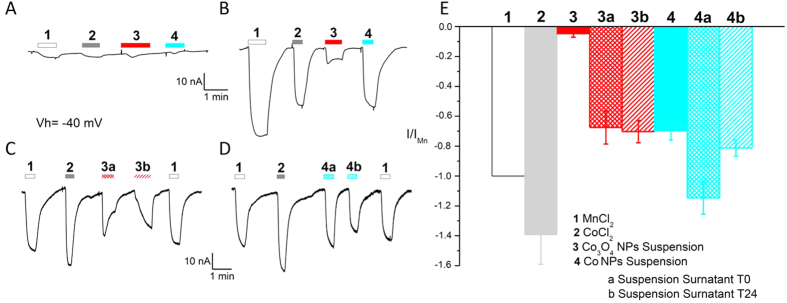
To better characterize our system, and also to rule out possible unpredicted artefacts, we have performed further experiments which are shown in Fig. 4. We have confirmed by electrophysiology that no endogenous divalent metal ion transporters are present on the oocyte plasma membrane. Indeed, as shown in Fig. 4A, with two electrode voltage clamp no currents were recorded in the presence of CoCl2, MnCl2; similarly, no currents were recorded also in the presence of Co3O4 and Co NPs, which are known to readily dissolve releasing ions. This is in agreement with the results shown in Fig. 2 where there was not fluorescence reduction in non-transfected oocytes placed in solution containing CoCl2.
Figure 4. Two electrode voltage-clamp of Xenopus oocytes.
Inward currents elicited by solutions and NP suspensions in NT (A) and rDMT1 transfected (B–D) representative oocytes. Oocytes were clamped at a holding potential of −40 mV and exposed to MnCl2 (1) and CoCl2 (2) solutions, to Co3O4 (3) and Co (4) NP suspensions and to their surnatants obtained at time 0 h (a) and 24 h (b). (E): Current mean values (±SE) obtained by subtracting from the current in the presence of the substrate, the current in its absence and normalizing to the Mn2+ current, a reference for divalent metal transporters in electrophysiological studies 6 to 12 oocytes from 4 batches.
Co, Co3O4 and BSA coated Co3O4 NPs release ions
Conversely, when rDMT1 transfected oocytes were tested in the presence of CoCl2 and MnCl2, inward currents were recorded (Fig. 4B), indicating an electrogenic transport of ions across the plasma membrane. Similarly, inward currents were recorded also in the presence of Co3O4 and Co NPs, indicating that both NPs, although in a different amount, were releasing ions. To confirm the release of Co ions from NPs, we have performed a set of experiments (Fig. 4C,D) exposing rDMT1 transfected oocytes to the surnatants of suspensions of Co3O4 and Co NPs and obtaining the expected inward currents. Moreover, we added BSA exposed NPs to rDMT1 expressing oocytes and, in all the tested conditions, we observed a current similar to that evoked by the same NPs that were not exposed to BSA (data not shown). These data rule out the possibility that BSA might have a role in preventing dissolution in our experiments.
NPs do not impair oocyte plasma membrane integrity
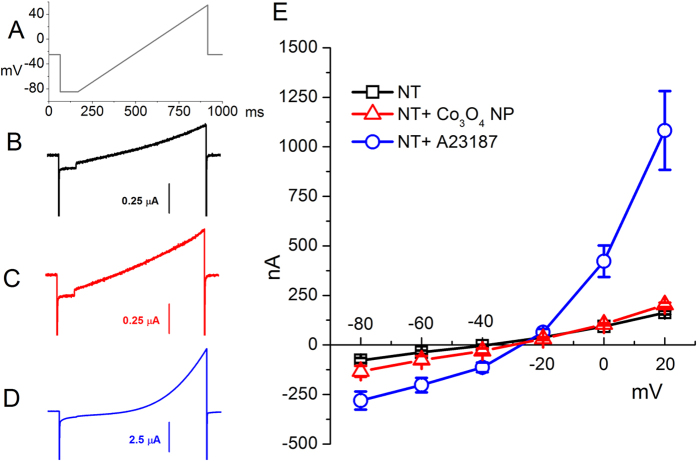
To understand whether or not Co3O4 NPs damage oocyte plasma membrane, we have measured membrane resistence by two electrode voltage clamp. If the NPs damaged the oocyte membrane, we would expect a change in membrane permeability and the entrance of Ca2+ ions that, in Xenopus oocytes, activate Ca2+-gated chloride channels45 which, at potentials more positive than the chloride reversal potential, give rise to an outward current46. As shown in Fig. 5, we applyed a voltage ramp to oocytes which were previously exposed to Co3O4 NPs for 30 min and we did not observe a change in membrane resistance. Conversely, after A23187 ionophore addition45,47, a large chloride current appeared, expecially at more positive potentials, due to the activation of the Ca2+-gated chloride channels. Therefore, we think that NP entry does not cause injury to the plasma membrane.
Figure 5. Measure of membrane resistence of Xenopus oocytes.
Ramp (from −85 to + 55 mV) protocols (A) were applied in non transfected oocytes starting from the holding potential of −25 mV. Representative currents elicited by the protocol in not-exposed oocytes (black line in B), exposed to Co3O4 NP suspensions (red line in C) and perfused with ionophore A23187 (blue line in D). Current mean values at −80, −60, −40, −20, 0 and +20 mV (± SE) obtained from at least 12 oocytes (from 2 different batches) are plotted in (E).
In conclusion, we have demonstrated that NPs can cross cytomembranes with no evident damage to cell integrity. The canonical way of NPs to be uptaken by cells is endocytosis that makes NPs to gain access to the endosomal compartment. The NP capability to cross lipid bilayers exposes further cellular compartments to NPs. We have learned that the capability of Co3O4 NPs to cross the oocyte plasma membrane is not paralleled by that of Co NPs and that the crossing of Co3O4 NPs can be prevented by a protein corona. Moreover, we have set up a system that can be of help in evaluating the effects of different functionalizations on NP ability to cross cytomembranes. Finally, we have confirmed that Co NPs and, to a less extent, Co3O4 NPs release ions in the environment where they are present, i.e., in the extracellular solution as well as in the cytoplasm. Dissolution, indeed, is a phenomenon one should take into account not only for nanotoxicological studies, but also in nanomedicine or in food and feed fortification.
Materials and Methods
Solutions
ND96 solution had the following composition (in mM): NaCl 96, KCl 2, MgCl2 1, HEPES 5, pH 7.6; modified Barth’s saline (MBS) solution had the following composition (in mM): NaCl 88, KCl 1, NaHCO3 2.4, HEPES 15, Ca(NO3)2 0.30, CaCl2 0.41, MgSO4 0.82, sodium penicillin 10 μg/mL, streptomycin sulphate 10 μg/mL, gentamycin sulphate 100 μg/mL, pH 7.6; external control solution contained (in mM): NaCl 98; MgCl2, 1; CaCl2, 1.8, HEPES or MES 5, pH 7.6 or 5.5; intracellular solution contained (in mM): KCl 130, NaCl 4, MgCl2 1.6, EGTA 5, HEPES 10, glucose 5, pH 7.6. The final pH values of 5.5 or 7.6 were adjusted with HCl and NaOH.
Oocytes collection and preparation
Oocytes were obtained from adult Xenopus laevis females. Animals were anaesthetised in 0.1% (w/v) MS222 (tricaine methansulfonate) solution in tap water and portions of the ovary were removed through an incision on the abdomen. The oocytes were treated with 1 mg/mL collagenase (Sigma Type IA) in ND96 calcium free for at least 1 h at 18 °C. Healthy and fully grown oocytes were selected and stored at 18 °C in MBS solution48. The oocytes to be transfected with the cDNA coding for rDMT1 were injected with 25 ng of cRNA in 50 nl of water, the day after the removal, using a manual microinjection system (Drummond Scientific Company, Broomall, PA) and incubated at 18 °C for 3–4 days before electrophysiological or fluorescence experiments. The experiments were carried out according to the institutional and national ethical guidelines (permit nr. 05/12).
NP preparation
Oocytes were exposed to zerovalent (Co, 28 nm, IOLITEC, Salzstrasse 184, D-74076 Heilbronn) and oxide (Co3O4, <50 nm TEM determined, Sigma-Aldrich) cobalt NPs. 1 mg/mL Stock suspensions were prepared in deionised water. 0.1 mL of stock suspension was added to the test chamber containing 0.9 mL of external control solution (pH 7.6). Suspensions were carefully sonicated before addition to the test chamber.
For dissolution experiments, stock suspensions of Co and Co3O4 NPs were prepared to have a 10 mM concentration in terms of cobalt (i.e., 5.9 mg/10 mL for Co NPs and 8 mg/10 mL for Co3O4 NPs). Stock suspensions were sonicated for 15 min and 0.5 mL of each suspension was added to a Petri dish containing 24.5 mL of deionised water or of 1 mg/mL BSA to reach a final cobalt concentration of 200 μM.
After 1 and 24 h, 15 mL were collected from each Petri dish and centrifuged for 5 min at 8000 g at 10 °C. The surnatant was transferred in a new tube and the procedure was repeated for 4 times. Finally, 8 mL of surnatant from the last centrifugation were ultracentrifuged at 300 000 g at 4 °C for 2 h. The surnatant was collected and filtered (0.22 μm syringe filter). The resulting surnatant was diluted 1:1 in a 2X external control solution at pH 5.5 and used in electrophysiological experiments.
Single Oocyte Fluorescence Assay (SOFA)
Untransfected oocytes and oocytes transfected with cRNA encoding rDMT1 were injected with a 50 nL drop of a 25 μM Calcein in intracellular solution. The nominal volume of a 1.2 mm diameter oocyte is 1 μL; therefore, a 50 nL injected drop will be diluted 20 times. The exact dilution factor is, however, difficult to establish, since not all the theoretical volume may be available for free diffusion49. Following Calcein injection, the oocytes were placed in external control solution solution at pH 5.5 or 7.6 containing or not, divalent metals at a final concentration of 0.1 mM.
For NP experiments, Co NPs and Co3O4 NPs were added to the testing solution to a final concentration of 0.1 mg/mL (pH 7.6). All experiments were carried out at room temperature. To block the endocytotic pathway, oocytes were incubated in 40 μM Dynasore (Sigma-Aldrich) for 24 h before the experiment.
Images of single oocytes were acquired every 2 min for 30 min with a fluorescence microscope (AxioVert 200, Carl Zeiss with a 4x objective, COLIBRI fluorescence filters, 470 nm excitation −515 to 565 nm emission) equipped with CCD camera (Axiocam ICM1, Carl Zeiss).
Fluorescence and transmission electron microscopy
To assess endocytosis, oocytes were incubated for 30 min in external control solution at pH 7.6 with 1 mg/mL Lucifer Yellow CH (Sigma-Aldrich). Negative controls, oocytes exposed to 0.1 mg/mL Co3O4 NP, and oocytes pretreated with Dynasore and exposed to 0.1 mg/mL Co3O4 NPs were washed 3 times in cold (4 °C) external control solution at pH 7.6 and fixed in 4% paraformaldehyde for two days. Oocytes were washed 3 times in cold external control solution, cut in 2 halves which were placed on a slide and covered with slips. Samples were observed with a fluorescence microscope (Axiophot, Carl Zeiss) with a 63x oil ojective. Bright field and FITC filter images were taken using a CCD camera (Discovery C30, TiEsselab).
For TEM, oocytes were fixed in 4% paraformaldehide and 2% glutaraldehide in 0.1 M sodium cacodylate buffer (pH 7.4) for 24h at 4 °C and stored in 0.1 M sodium cacodylate buffer (pH 7.4) at 4 °C. Specimens were postfixed in 1% OsO4 in 0.1 M sodium cacodylate buffer (pH 7.4), dehydrated in ethanol and embedded in Epon-Araldite. For ultrastructural studies, thin sections were cut using a Reichert Ultracut-S-Ultratome ultramicrotome (Leica, Nussloch, Germany), collected on 300 mesh copper grids, counterstained with uranyl acetate and lead citrate and examined under a Jeol 1010 electron microscope operating at 90 KV (Jeol, Tokyo, Japan).
Spectrofluorometry
Calcein quenching by divalent metal ions were measured in external control solution at pH 5.5 and 7.6 using a Jasco FP-750 fluorometer with excitation at 470 nm. The spectra of 2.5 μM Calcein alone or mixed with iron, manganese and cobalt ions at different concentrations were acquired in the 490 to 560 nm range. Experiments at pH 7.6 with FeCl2 were performed in presence of 1 mM ascorbic acid to maintain iron in a reduced form.
Electrophysiology
The two-electrode voltage-clamp technique was performed with an Oocyte Clamp OC-725B (Warner Instruments, Hamden, CT, USA). Intracellular glass microelectrodes, filled with 3 M KCl, had tip resistances in the 0.5–4 MΩ range. Agar bridges (3% agar in 3 M KCl) connected the bath electrodes to the experimental chamber. The holding potential (Vh) was −40 mV for the recording of transport currents and −25 mV for the measurements of membrane resistance. Currents associated to membrane transport of divalent ions were recorded in oocytes perfused with external control solution at pH 5.5 in the absence or in the presence of the indicated divalent metal ions, NPs and NP surnatants. To check for the possible presence of membrane damage, the oocyte conductances were tested applying a 1-s long protocol with a voltage ramp from −85 to + 55 mV. Positive controls of membrane conductance alteration were obtained perfusing oocytes with 10 μM ionophore A-23187 (Sigma-Aldrich). WinWCP version 4.4.6 (J. Dempster, University of Strathclyde, UK) or Clampex 10.2 (Molecular Devices, Sunnyvale, CA, USA, www.moleculardevices.com) were used to run the experiments.
Data analysis
Data were analysed using Clampfit 10.2 software (Molecular Devices, Sunnyvale, CA, USA, www.moleculardevices.com) while OriginPro 8.0 (OriginLab Corp., Northampton, MA, USA, www.originlab.com) was used for statistics and figure preparation. Transport currents were determined by subtracting the records in the absence of a substrate from the corresponding ones in its presence. Fluorescence decay images were analysed with ImageJ (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2015). For Ft/F0 quantification, the fluorescence intensity at time 0 (F0) and at subsequent times (Ft) was calculated in the entire area of the oocyte.
Additional Information
How to cite this article: Bossi, E. et al. Cobalt oxide nanoparticles can enter inside the cells by crossing plasma membranes. Sci. Rep. 6, 22254; doi: 10.1038/srep22254 (2016).
Acknowledgments
This work was supported by Fondazione Cariplo (2013-1052). We thank Dr. Raffella Cinquetti and Ms Arianna Parnigoni for their help in performing experiments.
Footnotes
Author Contributions E.B. and R.G. planned and designed the experiments, D.Z. performed the experiments and prepared the figures, E.B. and G.B. wrote the manuscript. All authors reviewed the manuscript.
References
- Papis E. et al. Engineered cobalt oxide nanoparticles readily enter cells. Toxicology letters 189, 253–259, doi: 10.1016/j.toxlet.2009.06.851 (2009). [DOI] [PubMed] [Google Scholar]
- Contreras J. et al. Intracellular Uptake and Trafficking of Difluoroboron Dibenzoylmethane-Polylactide Nanoparticles in HeLa Cells. Acs Nano 4, 2735–2747, doi: 10.1021/nn901385y (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregar V. B., Lojk J., Sustar V., Veranic P. & Pavlin M. Visualization of internalization of functionalized cobalt ferrite nanoparticles and their intracellular fate. Int J Nanomedicine 8, 919–931, doi: 10.2147/IJN.S38749 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L., Nienhaus K. & Nienhaus G. U. Engineered nanoparticles interacting with cells: size matters. Journal of nanobiotechnology 12, 5–5, doi: 10.1186/1477-3155-12-5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. International journal of nanomedicine 7, 5577–5591, doi: 10.2147/IJN.S36111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickler F. M. et al. Tuning Nanoparticle Uptake: Live-Cell Imaging Reveals Two Distinct Endocytosis Mechanisms Mediated by Natural and Artificial EGFR Targeting Ligand. Nano Letters 12, 3417–3423, doi: 10.1021/nl300395q (2012). [DOI] [PubMed] [Google Scholar]
- Castellini C. et al. Long-term effects of silver nanoparticles on reproductive activity of rabbit buck. Systems biology in reproductive medicine 60, 143–150, doi: 10.3109/19396368.2014.891163 (2014). [DOI] [PubMed] [Google Scholar]
- Coccini T. et al. Gene Expression Changes in Rat Liver and Testes after Lung Instillation of a Low Dose of Silver Nanoparticles. Journal of Nanomedicine & Nanotechnology 5, doi: 10.4172/2157-7439.1000227 (2014). [DOI] [Google Scholar]
- Ye D. et al. Nanoparticle accumulation and transcytosis in brain endothelial cell layers. Nanoscale 5, 11153–11165, doi: 10.1039/c3nr02905k (2013). [DOI] [PubMed] [Google Scholar]
- Gliga A. R., Skoglund S., Wallinder I. O., Fadeel B. & Karlsson H. L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol 11, 11, doi: 10.1186/1743-8977-11-11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega R. et al. Low-solubility particles and a Trojan-horse type mechanism of toxicity: the case of cobalt oxide on human lung cells. Particle and fibre toxicology 11, 14–14, doi: 10.1186/1743-8977-11-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabella S. et al. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale 6, 7052–7061, doi: 10.1039/c4nr01234h (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A. G. et al. Nanotechnology and human health: risks and benefits. Journal of applied toxicology : JAT 30, 730–744, doi: 10.1002/jat.1609 (2010). [DOI] [PubMed] [Google Scholar]
- Li S. & Malmstadt N. Deformation and poration of lipid bilayer membranes by cationic nanoparticles. Soft Matter 9, 4969–4976, doi: 10.1039/c3sm27578g (2013). [DOI] [Google Scholar]
- Nolte T. M., Kettler K., Meesters J. A., Hendriks A. J. & van de Meent D. A semi-empirical model for transport of inorganic nanoparticles across a lipid bilayer: implications for uptake by living cells. Environmental toxicology and chemistry/SETAC 34, 488–496, doi: 10.1002/etc.2812 (2015). [DOI] [PubMed] [Google Scholar]
- Taylor U. et al. Nonendosomal cellular uptake of ligand-free, positively charged gold nanoparticles. Cytometry. Part A : the journal of the International Society for Analytical Cytology 77, 439–446, doi: 10.1002/cyto.a.20846 (2010). [DOI] [PubMed] [Google Scholar]
- Ding H.-m. & Ma Y.-q. Computer simulation of the role of protein corona in cellular delivery of nanoparticles. Biomaterials 35, 8703–8710, doi: 10.1016/j.biomaterials.2014.06.033 (2014). [DOI] [PubMed] [Google Scholar]
- Lin J. Q. & Alexander-Katz A. Cell Membranes Open “Doors” for Cationic Nanoparticles/Biomolecules: Insights into Uptake Kinetics. Acs Nano 7, 10799–10808, doi: 10.1021/nn4040553 (2013). [DOI] [PubMed] [Google Scholar]
- Verma A. et al. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat Mater 7, 588–595, doi: 10.1038/nmat2202 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. In situ oxidation of carbon-encapsulated cobalt nanocapsules creates highly active cobalt oxide catalysts for hydrocarbon combustion. Nature Communications 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal A., Adhikary B. & Mukherjee D. Room-temperature synthesis of air stable cobalt nanoparticles and their use as catalyst for methyl orange dye degradation. Colloid Surface A 482, 248–257 (2015). [Google Scholar]
- Wu L. H. et al. Stable Cobalt Nanoparticles and Their Monolayer Array as an Efficient Electrocatalyst for Oxygen Evolution Reaction. Journal of the American Chemical Society 137, 7071–7074 (2015). [DOI] [PubMed] [Google Scholar]
- Smolyaninova V. N., Yost B., Lahneman D., Narimanov E. E. & Smolyaninov I. I. Self-assembled tunable photonic hyper-crystals. Scientific Reports 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun Y.-W., Seo J.-W. & Cheon J. Nanoscaling laws of magnetic nanoparticles and their applicabilities in biomedical sciences. Accounts of chemical research 41, 179–189 (2008). [DOI] [PubMed] [Google Scholar]
- Bava A. et al. D-amino acid oxidase-nanoparticle system: a potential novel approach for cancer enzymatic therapy. Nanomedicine (London, England) 8, 1797–1806, doi: 10.2217/nnm.12.187 (2013). [DOI] [PubMed] [Google Scholar]
- Cappellini F. et al. New synthesis and biodistribution of the D-amino acid oxidase-magnetic nanoparticle system. Future Science OA, doi: 10.4155/fso.15.67 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava A. et al. Heparin and carboxymethylchitosan metal nanoparticles: an evaluation of their cytotoxicity. BioMed research international 2013, 314091–314091, doi: 10.1155/2013/314091 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vismara E. et al. Non-covalent synthesis of metal oxide nanoparticle-heparin hybrid systems: a new approach to bioactive nanoparticles. Int J Mol Sci 14, 13463–13481, doi: 10.3390/ijms140713463 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H. et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482–488, doi: 10.1038/41343 (1997). [DOI] [PubMed] [Google Scholar]
- Au C., Benedetto A. & Aschner M. Manganese transport in eukaryotes: The role of DMT1. Neurotoxicology 29, 569–576, doi: 10.1016/j.neuro.2008.04.022 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie B. & Hediger M. A. SLC11 family of H + -coupled metal-ion transporters NRAMP1 and DMT1. Pflug Arch Eur J Phy 447, 571–579, doi: 10.1007/s00424-003-1141-9 (2004). [DOI] [PubMed] [Google Scholar]
- Bressler J. P., Olivi L., Cheong J. H., Kim Y. & Bannon D. Divalent metal transporter 1 in lead and cadmium transport. Ann Ny Acad Sci 1012, 142–152, doi: 10.1196/annals.1306.011 (2004). [DOI] [PubMed] [Google Scholar]
- Buracco S. et al. Dictyostelium Nramp1, which is structurally and functionally similar to mammalian DMT1 transporter, mediates phagosomal iron efflux. J Cell Sci 128, 3304–3316, doi: 10.1242/jcs.173153 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbioni E. et al. Interaction with culture medium components, cellular uptake and intracellular distribution of cobalt nanoparticles, microparticles and ions in Balb/3T3 mouse fibroblasts. Nanotoxicology 8, 88–99, doi: 10.3109/17435390.2012.752051 (2014). [DOI] [PubMed] [Google Scholar]
- Gornati R. et al. Zerovalent Fe, Co and Ni nanoparticle toxicity evaluated on SKOV-3 and U87 cell lines. Journal of applied toxicology : JAT, 36: 385–393, doi: 10.1002/jat.3220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J. et al. Surface structure dependent electrocatalytic activity of Co(3)O(4) anchored on graphene sheets toward oxygen reduction reaction. Sci Rep 3, 2300, doi: 10.1038/srep02300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroueil P. R. et al. Wide varieties of cationic nanoparticles induce defects in supported lipid bilayers. Nano Lett 8, 420–424, doi: 10.1021/nl0722929 (2008). [DOI] [PubMed] [Google Scholar]
- Lu B., Smith T. & Schmidt J. J. Nanoparticle-lipid bilayer interactions studied with lipid bilayer arrays. Nanoscale 7, 7858–7866, doi: 10.1039/c4nr06892k (2015). [DOI] [PubMed] [Google Scholar]
- Chen R. et al. Interaction of lipid vesicle with silver nanoparticle-serum albumin protein corona. Appl Phys Lett 100, 13703–137034, doi: 10.1063/1.3672035 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniak A. et al. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 6, 5845–5857, doi: 10.1021/nn300223w (2012). [DOI] [PubMed] [Google Scholar]
- Rousset M. et al. Regulation of neuronal high-voltage activated Ca(V)2 Ca(2+) channels by the small GTPase RhoA. Neuropharmacology 97, 201–209, doi: 10.1016/j.neuropharm.2015.05.019 (2015). [DOI] [PubMed] [Google Scholar]
- Kirchhausen T., Macia E. & Pelish H. E. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol 438, 77–93, doi: 10.1016/S0076-6879(07)38006-3 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres A. & Bernardini G. The effective membrane capacity of Xenopus eggs: Its relations with membrane conductance and cortical granule exocytosis. Pflugers Archiv European Journal of Physiology 404, 266–272 (1985). [DOI] [PubMed] [Google Scholar]
- Bernardini G., Ferraguti M. & Stipani R. Fertilization induces endocytosis in Xenopus eggs. Cell differentiation 21, 255–260 (1987). [DOI] [PubMed] [Google Scholar]
- Centinaio E., Bossi E. & Peres A. Properties of the Ca(2+)-activated Cl- current of Xenopus oocytes. Cell Mol Life Sci 53, 604–610 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram S., Cherubino F., Bossi E., Castagna M. & Peres A. GABA reverse transport by the neuronal cotransporter GAT1: influence of internal chloride depletion. American journal of physiology Cell physiology 301, C1064–1073 (2011). [DOI] [PubMed] [Google Scholar]
- Boton R., Dascal N., Gillo B. & Lass Y. Two calcium-activated chloride conductances in Xenopus laevis oocytes permeabilized with the ionophore A23187. The Journal of physiology 408, 511–534 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi E., Fabbrini M. S. & Ceriotti A. Exogenous protein expression in Xenopus oocytes: basic procedures. Methods in molecular biology 375, 107–131, doi: 10.1007/978-1-59745-388-2_6 (2007). [DOI] [PubMed] [Google Scholar]
- Zeuthen T., Zeuthen E. & Klaerke D. A. Mobility of ions, sugar, and water in the cytoplasm of Xenopus oocytes expressing Na(+)-coupled sugar transporters (SGLT1). The Journal of physiology 542, 71–87 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]