Abstract
Tissue factor (TF) is a critical mediator of direct acute lung injury (ALI) with global TF deficiency resulting in increased airspace inflammation, alveolar-capillary permeability, and alveolar hemorrhage after intra-tracheal lipopolysaccharide (LPS). In the lung, TF is expressed diffusely on the lung epithelium and intensely on cells of the myeloid lineage. We recently reported that TF on the lung epithelium, but not on myeloid cells, was the major source of TF during intra-tracheal LPS-induced ALI. Because of a growing body of literature demonstrating important pathophysiologic differences between ALI caused by different etiologies, we hypothesized that TF on myeloid cells may have distinct contributions to airspace inflammation and permeability between direct and indirect causes of ALI. To test this, we compared mice lacking TF on myeloid cells (TF∆mye, LysM.Cre+/−TFflox/flox) to littermate controls during direct (bacterial pneumonia, ventilator-induced ALI, bleomycin-induced ALI) and indirect ALI (systemic LPS, cecal ligation and puncture). ALI was quantified by weight loss, bronchoalveolar lavage (BAL) inflammatory cell number, cytokine concentration, protein concentration, and BAL procoagulant activity. There was no significant contribution of TF on myeloid cells in multiple models of experimental ALI, leading to the conclusion that TF in myeloid cells is not a major contributor to experimental ALI.
The acute respiratory distress syndrome (ARDS), a severe form of acute lung injury (ALI), causes more than 75,000 deaths annually in the United States1 and has no specific therapies aside from use of low tidal volume ventilation and conservative fluid management2,3. Most commonly, ARDS occurs in the setting of direct lung injury from bacterial pneumonia or in the setting of indirect lung injury from non-pulmonary sepsis4,5. The mechanisms underlying development of ALI and ARDS remain unclear.
Tissue factor (TF) is a trans-membrane protein critical for activation of the coagulation cascade during hemostasis as well as in response to tissue injury. In the lung, TF is expressed primarily on the lung epithelium and on cells of the myeloid lineage (monocytes, macrophages, and neutrophils)6,7,8,9,10. In a model of direct ALI caused by intra-tracheal delivery of lipopolysaccharide (LPS), global reduction of TF resulted in increased airspace inflammation and increased alveolar-capillary barrier permeability11, suggesting that TF is protective during direct ALI. In contrast, other studies using animal models of indirect ALI have suggested that TF deficiency or systemic TF inhibition reduced tissue injury12,13,14,15. We recently identified that TF expressed on the lung epithelium was the predominant source of TF in the lung during direct LPS-induced ALI16, with no significant contribution of TF from myeloid cells in this model. This finding was in contrast to prior data showing that TF expressed on cells in the circulation was critical for the systemic response to LPS in a model of endotoxemia17. Because of these apparent differences in the role of TF in direct ALI compared to indirect ALI and because of the critical role of macrophages and neutrophils in pulmonary host defense, we sought to better understand the role of myeloid TF in different models of ALI. Using mice with a targeted genetic deletion of TF only in cells of the myeloid lineage, we tested the hypothesis that TF on myeloid cells would be protective in models of direct ALI but detrimental in models of indirect ALI.
Results
Myeloid TF in direct lung injury
To test the impact of myeloid cell TF in acute lung injury, mice with myeloid cell TF deletion (LysM.Cre+/−TFflox/flox, referred to as TF∆mye) and littermate controls (LysM.Cre−/−TFflox/flox, wild-type, WT) were subjected to multiple models of ALI. Circulating blood cells from TF∆mye mice have been previously shown to have abrogated expression of TF mRNA during endotoxemia and peripheral blood cells from TF∆mye mice had a 93% reduction in pro-coagulant activity after in vitro LPS stimulation17. We independently confirmed deletion of myeloid TF in our colony of TF∆mye mice. Alveolar macrophages isolated from TF∆mye mice that were stimulated ex vivo with LPS had TF mRNA levels that were 90% lower than in cells isolated from wild-type littermates (p = 0.019). Similarly, thioglycollate-induced peritoneal macrophages isolated from TF∆mye mice had TF mRNA levels 89.5% lower after LPS stimulation than cells isolated from wild-type littermates (p = 0.019). These studies confirm that myeloid cells in our mouse model have a functional defect in TF expression and activity.
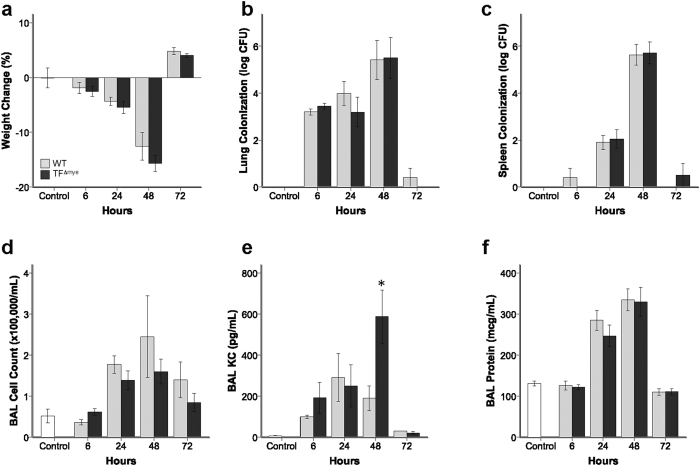
We previously demonstrated that TF on myeloid cells had no significant impact on ALI due to IT LPS administration16. However, because of the critical role of myeloid cells in coordination of pulmonary innate immunity in response to bacterial infection, we hypothesized that TF on myeloid cells may have a prominent role during bacterial pneumonia. To test this possibility, mice were infected with Klebsiella pneumoniae, a common cause of bacterial pneumonia. WT mice developed significant weight loss (Fig. 1a) associated with increased bacterial colonization in the lung and spleen (Fig. 1b,c), which resolved after 72 hours. BAL inflammatory cells (Fig. 1d), KC/CXCL-1 expression (Fig. 1e), and total BAL protein (Fig. 1f) peaked after 48 hrs in WT mice. Deletion of myeloid TF had no impact in this model system. WT and TF∆mye animals had indistinguishable bacterial colonization in the lung and similar bacterial dissemination to the spleen. Mice lacking myeloid TF had similar neutrophilic BAL inflammation throughout the experiment (data not shown). Expression of KC in BAL of TF∆mye mice was variable over time, with mice lacking myeloid TF having more BAL KC only at the 48 hr assessment, a finding of uncertain biological significance (Fig. 1e). In addition, isolated peritoneal macrophages from WT and TF∆mye mice had similar phagocytosis and bacterial killing of K. pneumoniae in vitro (Fig. 2a). Furthermore, peritoneal macrophages isolated from WT and TF∆mye mice induce pro-inflammatory M1 macrophage markers, but not M2 macrophage markers, in response to LPS in vitro (Fig. 2b). These data confirm that lack of TF had no effect on antimicrobial macrophage functions.
Figure 1. Effect of myeloid tissue factor deletion during acute Klebsiella pneumoniae infection.
There were no significant differences between wild-type mice (gray bars) and mice lacking myeloid tissue factor (TF∆mye) (black bars) in (a) weight change, (b) bacterial colonization in the lung, (c) bacterial colonization in the spleen, and (d) total inflammatory cell count in bronchoalveolar lavage (BAL) fluid. There was a statistical difference in (e) BAL KC/CXCL-1 between genotypes only at the 48hr time point (*p = 0.023). There were no significant differences between WT and TF∆mye mice in (f) BAL total protein. Analysis includes 4–21 mice in each genotype at each time point. Statistical comparisons were performed with Student’s t tests comparing genotypes at each time point. Data from uninfected controls (white bars) are included for reference.
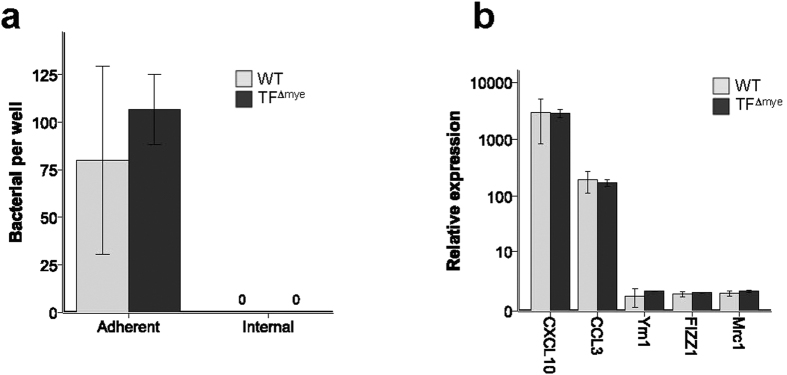
Figure 2. Effect of deletion of myeloid TF on macrophage phagocytosis and cytokine production.
(a) Thioglycollate-induced peritoneal macrophages from mice lacking myeloid TF (TF∆mye) and littermate controls have similar attachment, phagocytosis, and killing of Klebsiella pneumoniae at multiplicity of infection (MOI) of 10. Similar results were obtained at MOI of 100, 500, and 1000. (b) Thioglycollate-induced peritoneal macrophages from TF∆mye mice and littermate controls had increased expression of M1 macrophage markers (CXCL10, CCL3) with no upregulation of M2 markers (Ym1, FIZZ1, Mrc1) in response to ex vivo LPS stimulation, with no difference between WT cells and those lacking TF (results normalized to PBS-treated controls).
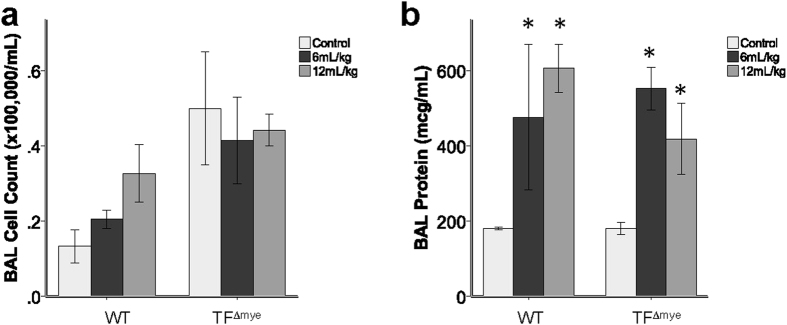
Next, we tested whether mice lacking TF on myeloid cells had altered susceptibility to mechanical lung injury caused by mechanical ventilation. As expected, mechanical ventilation with both low (6 mL/kg) and high (12 mL/kg) tidal volumes induced a significant increase in BAL protein with limited BAL inflammation in WT mice. There was no significant impact of myeloid TF on BAL inflammation (Fig. 3a) or BAL protein (Fig. 3b) in this model of direct lung injury. BAL levels of cell-free hemoglobin were below the limit of detection (10 mg/dL) in both WT and TF∆mye animals (data not shown).
Figure 3. Effect of myeloid tissue factor deletion during mechanical ventilation.
There were no significant differences between wild-type (WT) mice and mice lacking myeloid tissue factor (TF∆mye) in (a) total inflammatory cell counts in bronchoalveolar lavage (BAL) fluid or (b) BAL total protein after 2 hours of mechanical ventilation at either low (6 mL/kg) or high (12 mL/kg) tidal volumes. *indicates p < 0.05 compared to control treated animals in each genotype. The differences between ventilated and non-ventilated control mice are significant showing that this model induces lung injury in WT and TF∆mye mice. Analysis includes 2–5 mice in each genotype. Statistical comparisons were performed by two-way ANOVA.
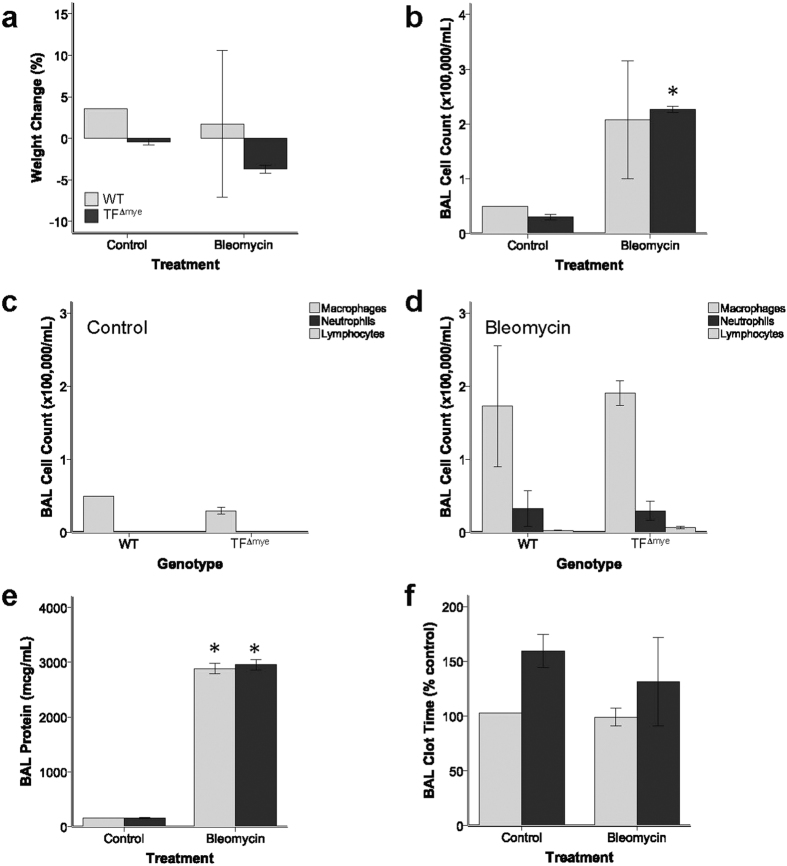
We also tested whether myeloid TF had a more prominent role during prolonged inflammation using a model of lung injury associated with fibrosis. Mice were treated with intra-tracheal bleomycin and samples collected after 7 days, which coincides with the peak of lung inflammation in this model. WT and TF∆mye mice had similar weight changes (Fig. 4a), BAL inflammatory cell counts (Fig. 4b), BAL cell differentials (Fig. 4c,d), BAL protein (Fig. 4e), and BAL clot times (Fig. 4f). BAL levels of cell-free hemoglobin were below the limit of detection (10 mg/dL) in both WT and TF∆mye animals (data not shown).
Figure 4. Effect of myeloid tissue factor deletion after intra-tracheal bleomycin administration.
There were no significant differences between wild-type (WT) mice and mice lacking myeloid tissue factor (TF∆mye) in (a) weight change, (b) total inflammatory cell counts in bronchoalveolar lavage (BAL) fluid, (c,d) BAL differential cell counts, (e) BAL total protein, or (f) BAL clot time at 7 days after bleomycin or control. *indicates p < 0.05 compared to control treated animals in each genotype. Analysis includes 2–3 mice in each genotype. Statistical comparisons were performed with Student’s t tests.
Myeloid TF during indirect acute lung injury
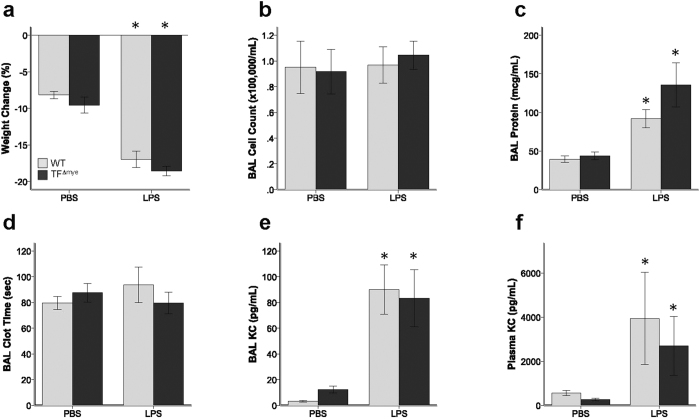
Indirect ALI during sepsis is predominantly an endothelial injury, with secondary acute lung injury in part due to vascular leak into the alveolar space5. We hypothesized that myeloid TF may have a more prominent role during this injury because of activation of circulating inflammatory cells during sepsis. Systemic delivery of LPS over 48 hours in WT mice resulted in significant increases in weight loss (Fig. 5a), BAL protein (Fig. 5c), BAL KC (Fig. 5e), and plasma KC (Fig. 5f) as compared to PBS-treated controls, demonstrating that this model causes significant systemic inflammation, increased lung permeability, and elevated airspace chemokines. Contrary to our hypothesis, there were no differences between WT and TF∆mye mice in weight loss (Fig. 5a), BAL cell counts (Fig. 5b), BAL protein (Fig. 5c), BAL KC (Fig. 5e), or plasma KC (Fig. 5f) levels. There was no significant activation of airspace coagulation (Fig. 5d) in this model. BAL levels of cell-free hemoglobin were below the limit of detection (10 mg/dL) in both WT and TF∆mye animals (data not shown).
Figure 5. Effect of myeloid tissue factor deletion during systemic lipopolysaccharide administration.
Mice were treated with continuous LPS infusion (8 μg/hr) via implanted osmotic pump and samples collected after 48 hours. There were no significant differences between wild-type (gray bars) mice and mice lacking myeloid tissue factor (black bars) in (a) weight change, (b) total inflammatory cell count in bronchoalveolar lavage (BAL) fluid, (c) BAL total protein, (d) BAL clot time, (e) BAL KC/CXCL-1, or (f) plasma KC. *indicates p < 0.05 compared to control treated animals in each genotype. Analysis includes 4–21 mice in each genotype at each time point. Statistical comparisons were performed by Student’s t test.
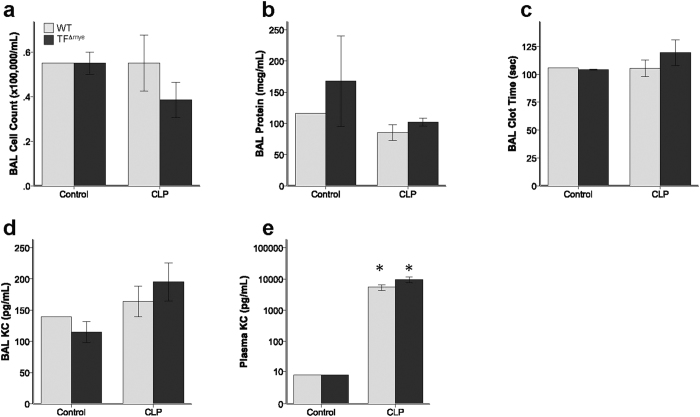
Finally, we tested whether myeloid TF had a significant role in the response to polymicrobial sepsis caused by cecal ligation and puncture (CLP), a common model of sepsis-associated end-organ injury. Although CLP does not cause a robust acute lung injury18, we hypothesized that loss of myeloid TF would exacerbate lung injury in this model. Both WT and TF∆mye mice had limited BAL inflammation (Fig. 6a), BAL protein (Fig. 6b), BAL procoagulant activity (Fig. 6c), and BAL KC expression (Fig. 6d), with no differences between groups. Both groups of mice had increased plasma KC levels compared to sham treated mice (Fig. 6e) with no difference between genotypes in this measure of systemic inflammation. BAL levels of cell-free hemoglobin were below the limit of detection (10 mg/dL) in both WT and TF∆mye animals (data not shown).
Figure 6. Effect of myeloid tissue factor deletion after cecal ligation and puncture.
Wild-type (WT) mice and mice lacking myeloid tissue factor (TF∆mye) had similar (a) total inflammatory cell counts in bronchoalveolar lavage (BAL) fluid, (b) BAL total protein, (c) BAL clot time, (d) BAL KC/CXCL-1, and (e) plasma KC. *indicates p < 0.05 compared to control treated animals in each genotype. Analysis includes 1–2 sham mice and 11–14 CLP mice in each genotype. Statistical comparisons were performed by Student’s t tests.
Discussion
In this study, we demonstrate that deletion of TF on cells of the myeloid lineage has no detectable impact during acute lung injury caused by multiple models of direct or indirect lung injury. While these findings are consistent with our previous report of a limited effect of myeloid TF during intra-tracheal LPS-induced acute lung injury, the lack of any effect of myeloid TF is very interesting given the robust upregulation of TF in myeloid cells in models of lung injury. Several studies have shown a major role for TF in mediating coagulant and inflammatory responses in sepsis8,12,13,14,19,20,21. TF deficiency or inhibition reduced indirect acute lung injury and improved mortality in experimental models of sepsis14,22,23. Baboons with experimental sepsis treated with site-activated factor VII (which prevents TF-induced coagulation) developed less ALI14. In a similar study, baboons given TF-pathway-inhibitor (TFPI) in the setting of bacteremia had reduced mortality22, suggesting a key role for TF in outcomes of sepsis. However, it should be pointed out that TFPI administration likely has additional effects that differ from genetic deletion of TF so it is difficult to make a direct comparison. In addition, after a single injection of LPS, mice with relative TF deficiency had less cytokine induction and increased survival23. These preclinical data were so compelling that two clinical trials of systemic TF inhibition in human sepsis and in community-acquired pneumonia were performed, each failing to show clinical benefit with one study showing higher mortality with TF inhibition during pneumonia24,25. Although these studies suggest that TF is pro-inflammatory and pro-injurious, none of them studied cell-specific effects of TF. Our study of myeloid TF in multiple different direct and indirect ALI models convincingly establishes that myeloid TF does not play a significant role in ALI pathogenesis.
Another distinction is that many of these studies focused on the impact of TF inhibition on systemic illness rather than addressing the role of TF specifically in the injured lung. TF activation is necessary for fibrin deposition, which may attenuate alveolar permeability. Pawlinski et al. showed that TF on circulating hematopoietic cells was sufficient for activation of coagulation in plasma after exposure to systemic LPS17. In contrast, our group has previously shown that mice that are globally-deficient in TF (low TF mice) have increased airspace inflammation and increased lung permeability after direct LPS-mediated ALI11. This result prompted our additional investigation of the role of myeloid TF during lung injury. However, our current studies demonstrate no major effect of myeloid TF in multiple models of either direct or indirect lung injury, even when the injury is non-infectious. Furthermore, the lack of difference in systemic inflammation during polymicrobial sepsis between WT and myeloid TF-deficient animals is surprising and is contrary to our hypothesis that loss of myeloid TF would cause lung inflammation in these models that do not typically result in significant lung inflammation. Our results suggest that other cellular sources of TF are the primary mediators of coagulation and inflammation in response to tissue injury. Consistent with this concept, loss of TF on the lung epithelium exacerbates direct ALI16, whereas TF on the vascular endothelium may be most critical in models of sepsis as suggested by Pawlinski et al.17.
In the lung, TF is expressed on cells of the myeloid lineage, including monocytes, macrophages, and neutrophils6,8,11,16,26,27, albeit at total levels less than seen on the lung epithelium. Lung biopsies of patients with ARDS show strong staining for TF in alveolar macrophages6. In vitro, macrophages robustly upregulate TF expression after LPS stimulation. Similarly, neutrophils have been shown to immunostain for TF28,29. Thus, we were surprised that myeloid TF had no demonstrable effect during ALI. We add to the known literature of TF in macrophages by demonstrating that genetic deletion of TF from myeloid cells had no significant impact on macrophage phagocytosis or microbicidal functions (Fig. 2a) and no effect on cytokine production or macrophage polarization in response to LPS (Fig. 2b).
There are several potential explanations for our findings. The simplest explanation is that TF on other cell types contributes the majority of TF in the lungs. We recently showed that the lung epithelium contributes more than 70% of total lung TF both basally and in response to LPS16. Despite alveolar macrophages from TF∆mye mice having only 10% of WT TF mRNA levels, the overall amount of TF protein expressed in the injured lung of TF∆mye mice is indistinguishable from WT animals16. While macrophages often have intense TF staining on an individual cell, the majority of total TF in the lung is expressed on the lung epithelium. Another explanation for the lack of impact of myeloid TF in these models of ALI is that the importance of myeloid TF is obscured in the presence of TF on other cells such as the lung epithelium. In addition, while TF on the endothelium had no detected role in activation of coagulation in plasma after systemic LPS administration17, there are few data on the impact of TF on endothelial cells in other systems. Finally, because the model systems used cause relatively mild inflammatory lung injury, a role for myeloid TF in inflammation and coagulation during severe lung injury remains possible.
In conclusion, despite extensive data that supports a role for TF in acute lung injury and sepsis and the substantial body of literature showing strong induction of TF expression in myeloid cells, we were unable to demonstrate any biologically significant role for TF expressed on myeloid cells in multiple models of both direct and indirect acute lung injury. In the lung, further investigation of the role of TF during ALI should focus on its protective role on the lung epithelium and its effects on maintenance of alveolar-capillary barrier integrity during direct ALI.
Material and Methods
Transgenic mice
Mice with myeloid cell TF deletion (LysM.Cre+/−TFflox/flox, TF∆mye) and littermate controls (LysM.Cre−/−TFflox/flox, wild-type, WT) on a C57Bl/6 background were used for all experiments. Generation of TF∆mye animals has been previously described16,17. Male and female mice aged 7–18 weeks were used for these studies. All experiments were approved by the Vanderbilt Institutional Animal Care and Use Committee and performed in accordance with approved procedures (protocol M12–221).
Quantification of tissue factor expression
Alveolar macrophages were collected by bronchoalveolar lavage of untreated wild type and TF∆mye mice, adhered to tissue culture dishes for 2 hours, and washed. Peritoneal macrophages were collected from the abdomen in 10 mL cold PBS 3 days after intraperitoneal 3% thioglycollate administration. Alveolar and peritoneal macrophages were washed and then stimulated in vitro with 10 μg/mL LPS for 2 hours. RNA was collected with a PureLink RNA extraction kit (Ambion, Grand Island, NY) and cDNA generated using SuperScript VILO reagents (Invitrogen, Life Technologies, Grand Island, NY). TF mRNA expression was measured by quantitative real time PCR using primers for murine TF according to established methods11.
Measurements of acute lung injury
At each time point in each model of ALI, mice were euthanized and plasma was collected in citrate. Bronchoalveolar lavage (BAL) was performed with 900 μl PBS as previously described11,16. Lungs were removed and flash frozen. All samples were stored at −80 °C until further study. For bacterial pneumonia experiments, one lung was separated and homogenized in PBS for bacterial quantification prior to BAL of the remaining lung with 600 μl PBS. BAL clot time, cell counts, protein, and cytokine concentrations were measured as previously described11,16,30,31.
Bacterial pneumonia
Klebsiella pneumoniae serotype 2 (ATCC strain #43816, courtesy of R. Stokes Peebles, Vanderbilt University, Nashville, TN) was grown overnight in Luria Bertani medium, diluted in fresh medium, and grown for 1–2 hours prior to preparation of the inoculum. Mice were anesthetized with ketamine and xylazine and 2 × 103 colony forming units were administered intra-nasally32. Samples were collected after 6, 24, 48, or 72 hours.
Ventilator-induced lung injury
Mice were anesthetized with repeated injections of pentobarbital (5 mg/g mouse). After undergoing tracheotomy, mice were either mechanically ventilated with either low (6 mL/kg) or high (12 mL/kg) tidal volumes, respiratory rate 40 breaths/min per L tidal volume, and positive end-expiratory pressure of 2 cm H2O or monitored without ventilation for 2 hours prior to sample collection.
Bleomycin-induced lung injury
Mice were anesthetized with isoflurane and 0.04 U bleomycin sulfate (Bristol-Myers Squibb Co., Princeton, NJ) in 100 μl PBS was administered IT as previously described11. Samples were collected after 7 days.
Systemic LPS-induced lung injury
Indirect LPS-induced lung injury was induced by continuous infusion of LPS (8 μg/hr for 24 hrs) (Escherichia coli, LPS, Sigma, St. Louis, MO) or equal volume PBS delivered by intra-peritoneal osmotic pump (Durect, Cupertino, CA) with a single intra-peritoneal dose of LPS (3 μg/mouse) or PBS at the time of pump implantation16,33. Samples were collected after 48 hours.
Cecal ligation and puncture
To induce indirect lung injury by cecal ligation and puncture (CLP), mice were sedated with isoflurane and a midline laparotomy performed. The cecum was identified, ligated with suture, and punctured once with a 23-gauge needle. The peritoneum and skin were closed with sutures. Mice were frequently monitored for discomfort and given narcotic pain medication preoperatively and as needed postoperatively. Samples were collected after 24 hours.
Macrophage phagocytosis and cytokine expression
Thioglycollate-elicited peritoneal macrophages were collected as described above and incubated with K. pneumoniae in vitro at a multiplicity of infection of 10. After 2 hours, cells were washed x 3 with PBS. Adherent bacteria were quantified by plating of cell lysates on LB agar. Internalized bacteria were quantified after treatment of triplicate wells with gentamicin for 1 hour prior to being washed x 3 with PBS. For cytokine expression, RNA was collected from peritoneal macrophages after 3 hours of incubation with LPS. Data for LPS-treated cells collected from individual mice are normalized to PBS-treated macrophages from the same mouse.
Statistical Analysis
All statistical analyses were done with SPSS version 22 for Macintosh with statistical consultation. P < 0.05 was considered statistically significant. Wild type and TF∆mye were compared in each treatment group by Student’s t tests. Non-normally distributed data were natural log transformed prior to analysis.
Additional Information
How to cite this article: Shaver, C. M. et al. Myeloid tissue factor does not modulate lung inflammation or permeability during experimental acute lung injury. Sci. Rep. 6, 22249; doi: 10.1038/srep22249 (2016).
Acknowledgments
This work was supported by grants NIH HL087738 to CMS, NIH HL103836 to LBW, American Heart Association Established Investigator Award to LBW, NIH HL090785 and HL126671 to JAB, and American Heart Association Clinical Research Award to JAB.
Footnotes
Author Contributions C.M.S. performed experiments, analyzed data, and wrote the manuscript. B.S.G. and J.K.C. performed experiments. N.M., L.B.W. and J.A.B. assisted with data analysis and edited the manuscript. All authors approved the final manuscript.
References
- Rubenfeld G. D. et al. Incidence and outcomes of acute lung injury. N Engl J Med 353, 1685–1693 (2005). [DOI] [PubMed] [Google Scholar]
- ARDSNet. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342, 1301–1308 (2000). [DOI] [PubMed] [Google Scholar]
- Wiedemann H. P. et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354, 2564–2575 (2006). [DOI] [PubMed] [Google Scholar]
- Ware L. B. & Matthay M. A. The acute respiratory distress syndrome. N Engl J Med 342, 1334–1349 (2000). [DOI] [PubMed] [Google Scholar]
- Shaver C. M. & Bastarache J. A. Clinical and Biological Heterogeneity in Acute Respiratory Distress Syndrome: Direct Versus Indirect Lung Injury. Clin Chest Med 35, 639–653, doi: 10.1016/j.ccm.2014.08.004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastarache J. A. et al. The alveolar epithelium can initiate the extrinsic coagulation cascade through expression of tissue factor. Thorax 62, 608–616, doi: 10.1136/thx.2006.063305 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich J., Fearns C., Mathison J., Ulevitch R. J. & Mackman N. Lipopolysaccharide induction of tissue factor expression in rabbits. Infect Immun 67, 2540–2546 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T. A., Cheng J., Chang A. & Taylor F. B. Jr. Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol 142, 1458–1470 (1993). [PMC free article] [PubMed] [Google Scholar]
- Wygrecka M. et al. Cellular origin of pro-coagulant and (anti)-fibrinolytic factors in bleomycin-injured lungs. Eur Respir J 29, 1105–1114, doi: 09031936.00097306 (2007). [DOI] [PubMed] [Google Scholar]
- Wygrecka M. et al. Compartment- and cell-specific expression of coagulation and fibrinolysis factors in the murine lung undergoing inhalational versus intravenous endotoxin application. Thromb Haemost 92, 529–540 (2004). [DOI] [PubMed] [Google Scholar]
- Bastarache J. A. et al. Low levels of tissue factor lead to alveolar haemorrhage, potentiating murine acute lung injury and oxidative stress. Thorax 67, 8, doi: 10.1136/thoraxjnl-2012-201781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welty-Wolf K. E. et al. Coagulation blockade prevents sepsis-induced respiratory and renal failure in baboons. Am J Respir Crit Care Med 164, 1988–1996 (2001). [DOI] [PubMed] [Google Scholar]
- Welty-Wolf K. E. et al. Blockade of Tissue Factor-Factor X binding attenuates sepsis-induced respiratory and renal failure. Am J Physiol Lung Cell Mol Physiol 290, 11 (2006). [DOI] [PubMed] [Google Scholar]
- Carraway M. S. et al. Blockade of tissue factor: treatment for organ injury in established sepsis. Am J Respir Crit Care Med 167, 1200–1209 (2003). [DOI] [PubMed] [Google Scholar]
- Enkhbaatar P. et al. Recombinant tissue factor pathway inhibitor reduces lipopolysaccharide-induced pulmonary vascular injury by inhibiting leukocyte activation. Am J Respir Crit Care Med 162, 1752–1759 (2000). [DOI] [PubMed] [Google Scholar]
- Shaver C. M. et al. Lung Epithelial Tissue Factor Regulates Alveolar Pro-coagulant Activity and Permeability in Direct Acute Lung Injury. Am J Respir Cell Mol Biol, doi: 10.1165/rcmb.2014-0179OC (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlinski R. et al. Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood 116, 806–814, doi: 10.1182/blood-2009-12-259267 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastarache J. A. & Matthay M. A. Cecal ligation model of sepsis in mice: new insights. Crit Care Med 41, 356–357, doi: 10.1097/CCM.0b013e318270e3ee (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor F. B. Jr. et al. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock 33, 127–134 (1991). [PubMed] [Google Scholar]
- Levi M. et al. Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. J Clin Invest 93, 114–120 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr C. et al. Recombinant E. coli-derived tissue factor pathway inhibitor reduces coagulopathic and lethal effects in the baboon gram-negative model of septic shock. Circ Shock 44, 126–137 (1994). [PubMed] [Google Scholar]
- Creasey A. A. et al. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J Clin Invest 91, 2850–2860 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlinski R. et al. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood 103, 1342–1347 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham E. et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. Jama 290, 238–247 (2003). [DOI] [PubMed] [Google Scholar]
- Wunderink R. G. et al. Recombinant tissue factor pathway inhibitor in severe community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med 183, 1561–1568, doi: 10.1164/rccm.201007-1167OC (2011). [DOI] [PubMed] [Google Scholar]
- Drake T. A., Morrissey J. H. & Edgington T. S. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol 134, 1087–1097 (1989). [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Sawdey M. S., Keeton M. R. & Loskutoff D. J. Murine tissue factor gene expression in vivo. Tissue and cell specificity and regulation by lipopolysaccharide. Am J Pathol 143, 76–84 (1993). [PMC free article] [PubMed] [Google Scholar]
- Higure A. et al. Macrophages and neutrophils infiltrating into the liver are responsible for tissue factor expression in a rabbit model of acute obstructive cholangitis. Thromb Haemost 75, 791–795 (1996). [PubMed] [Google Scholar]
- Todoroki H. et al. Neutrophils express tissue factor in a monkey model of sepsis. Surgery 127, 209–216, doi: 10 .1067/msy.2000.103027 (2000). [DOI] [PubMed] [Google Scholar]
- Bastarache J. A. et al. Accuracy and reproducibility of a multiplex immunoassay platform: a validation study. J Immunol Methods 367, 33–39, doi: 10.1016/j.jim.2011.01.005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastarache J. A., Fremont R. D., Kropski J. A., Bossert F. R. & Ware L. B. Procoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 297, L1035–1041, doi: 00214.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulek D. E. et al. Allergic airway inflammation decreases lung bacterial burden following acute Klebsiella pneumoniae infection in a neutrophil- and CCL8-dependent manner. Infect Immun 82, 3723–3739, doi: 10.1128/IAI.00035-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everhart M. B. et al. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J Immunol 176, 4995–5005 (2006). [DOI] [PubMed] [Google Scholar]