Supplemental Digital Content is Available in the Text.
CB1R and CB2R differentially modulate the affective manifestations but not the cognitive impairment associated with osteoarthritis pain.
Keywords: Animal model, Cannabinoid receptor 1, Cannabinoid receptor 2, Anandamide, 2-arachidonoylglycerol, Anxiety
Abstract
In this study, we investigated the role of the endocannabinoid system (ECS) in the emotional and cognitive alterations associated with osteoarthritis pain. The monosodium iodoacetate model was used to evaluate the affective and cognitive manifestations of osteoarthritis pain in type 1 (CB1R) and type 2 (CB2R) cannabinoid receptor knockout and wild-type mice and the ability of CB1R (ACEA) and CB2R (JWH133) selective agonists to improve these manifestations during a 3-week time period. The levels of the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) were measured in plasma and brain areas involved in the control of these manifestations. Patients with knee osteoarthritis and healthy controls were recruited to evaluate pain, affective, and cognitive symptoms, as well as plasma endocannabinoid levels and cannabinoid receptor gene expression in peripheral blood lymphocytes. The affective manifestations of osteoarthritis were enhanced in CB1R knockout mice and absent in CB2R knockouts. Interestingly, both ACEA and JWH133 ameliorated the nociceptive and affective alterations, whereas ACEA also improved the associated memory impairment. An increase of 2-AG levels in prefrontal cortex and plasma was observed in this mouse model of osteoarthritis. In agreement, an increase of 2-AG plasmatic levels and an upregulation of CB1R and CB2R gene expression in peripheral blood lymphocytes were observed in patients with osteoarthritis compared with healthy subjects. Changes found in these biomarkers of the ECS correlated with pain, affective, and cognitive symptoms in these patients. The ECS plays a crucial role in osteoarthritis and represents an interesting pharmacological target and biomarker of this disease.
1. Introduction
Osteoarthritis is the most prevalent joint disorder with a social cost of up to 0.5% of the gross domestic product in developed countries.47 Osteoarthritis is characterized by pain and physical disability, problems that are often associated with anxiety, depression,3,15 and alterations of certain cognitive functions including mental flexibility and memory,28,41 all of which have a negative impact on the quality of life. A treatment designed to improve these emotional and cognitive alterations would be essential for an effective management of the disease. The endocannabinoid system (ECS) has recently emerged as a possible therapeutic target for osteoarthritis.31,32 The ECS is composed of at least 2 cannabinoid receptors, CB1R and CB2R, their endogenous ligands (endocannabinoids), mainly AEA and 2-AG, and the enzymes responsible for endocannabinoid biosynthesis and inactivation. The ECS regulates several pathophysiological processes, including articular metabolism, pain, emotions, and memory functions,32,38 and a therapeutic intervention on this system could offer the advantage to target multiple aspects of osteoarthritis. The ECS participates in the nociceptive manifestations of osteoarthritis,31,32 although the specific involvement in the emotional and memory alterations has not been yet investigated. The ECS is widely distributed in corticolimbic structures, including the paraventricular nucleus (PVN) of hypothalamus, prefrontal cortex (PFC), amygdala, and hippocampus,17,19,21,35,51 that are involved in the regulation of the behavioral responses to stress through hypothalamic–pituitary–adrenal (HPA) axis activity.24 Endocannabinoid signaling is altered in these brain areas after stress exposure.19–21,56 Chronic pain is a form of stress4 that may produce maladaptive changes within these limbic circuits, leading to affective and memory dysfunctions.27,42 Osteoarthritis has been reported to modify ECS activity in the affected joint and spinal cord,5,31,49,50 although possible alterations of this system in limbic and other supraspinal areas remain still unknown.
In this study, we evaluated the specific involvement of the ECS in anxiety and memory alterations associated with knee osteoarthritis in mice. We also analyzed the possible changes induced by osteoarthritis in endocannabinoid levels in mouse corticolimbic areas and plasma and explored the potential usefulness of endocannabinoids as biomarkers for human osteoarthritis.
2. Materials and methods
2.1. Animal experimental conditions
Swiss albino (Charles River, Lyon, France), CB1R and CB2R constitutive knockout (CB1KO and CB2KO, respectively), and wild-type (WT) mice, all on a CD1 genetic background, were used. The generation of mice lacking CB1R and CB2R was previously described.31,33 Mice were 2 to 3 months old at the beginning of the experiments and were housed in groups of 3 to 4 with ad libitum access to water and food. The housing conditions were maintained at 21 ± 1°C and 55 ± 10% relative humidity in a controlled light/dark cycle (lights on between 8 am and 8 pm). All experimental procedures and animal husbandry were conducted according to standard ethical guidelines (European Community Guidelines on the Care and Use of Laboratory Animals 86/609/EEC) and approved by the local ethical committee (Comité Etico Experimental Animal–Instituto Municipal de Asistencia Sanitaria/Universitat Pompeu Fabra). Only male mice were used, and all experiments were performed under blind conditions with treatments randomized between groups.
2.2. Drugs and treatments
The selective CB1R agonist, ACEA (Sigma-Aldrich, Madrid, Spain), and CB2R agonist, JWH133 (Tocris Bioscience, Bristol, United Kingdom), were diluted in a vehicle composed of 5% ethanol, 5% Cremophor EL (Sigma-Aldrich), and 90% saline and administered intraperitoneally in a volume of 10 mL/kg. Both ACEA and JWH133 were administered at doses of 1 and 5 mg/kg based on the antinociceptive effects found for these drugs in previous studies.5,54
2.3. Intra-articular injection of monosodium iodoacetate
Osteoarthritis pain was induced in mice briefly anaesthetized with isoflurane by the intra-articular injection of monosodium iodoacetate ([MIA]; Sigma-Aldrich) (5 μL of 5 mg/mL MIA in sterile saline, 0.9%) into the knee joint, as previously described.31 Control mice received the intra-articular injection of vehicle (sterile saline, 0.9%).
2.4. Nociceptive behavior
Mechanical allodynia was quantified by measuring the hind paw withdrawal response to von Frey filament stimulation. Briefly, the von Frey calibrated filaments (North Coast Medical, Gilroy, CA) were applied using the up–down paradigm, as previously reported.7 The threshold of the response was then calculated by the up–down Excel program provided by Dr A. Basbaum (University of California, San Francisco, CA). Both ipsilateral and contralateral hind paws were tested.
2.5. Affective behavior
The elevated plus-maze (EPM) test was used to evaluate anxiety and performed in a black Plexiglas apparatus with 4 arms (29 cm long × 5 cm wide), 2 open and 2 closed, set in cross from a neutral central square (5 × 5 cm) elevated 40 cm above the floor. Five-minute test sessions were performed, and the percentage of entries and time spent in the open arms was determined. The total entries in the open and closed arms of the EPM were measured as a control for locomotor activity.8
2.6. Cognitive behavior
Object recognition memory (ORM) was performed in the V-maze (Panlab, Barcelona, Spain) to measure cognitive performance, as previously described.46 This task consists of 3 sessions (habituation, training, and test). On day 1, mice were habituated for 9 minutes to the V-maze. On the second day, mice were put back in the maze for 9 minutes, 2 identical objects were presented, and the time that mice spent exploring each object was recorded. Mice were again placed in the maze 24 hours later for 9 minutes, one of the familiar objects was replaced with a novel object, and the time spent exploring each of the 2 objects (novel and familiar) was computed, and a discrimination index was calculated.46 The total time of exploration of the 2 objects was used as a measure of locomotor activity.46
2.7. Behavioral protocol in mice
The affective (EPM) and cognitive (ORM) manifestations induced by osteoarthritis were evaluated in CB1KO, CB2KO, and WT at 2 different time points: 1 week and 3 weeks after the intra-articular injection of MIA or saline (Supplemental methods, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A113).
In a second experiment, the effects of CB1R (ACEA) and CB2R (JWH133) agonists or vehicle were evaluated in nociceptive (von Frey model), affective (EPM), and cognitive (ORM) behaviors (Supplemental methods). Briefly, after the establishment of nociceptive baseline responses with the von Frey paradigm, osteoarthritis was induced as described above and osteoarthritis symptoms were evaluated after 1 and 3 weeks. At both time points, mice were first habituated and trained in the ORM. On the following day, mice received the acute injection of ACEA (1 or 5 mg/kg), JWH133 (1 or 5 mg/kg), or vehicle and were tested in the following sequence: ORM, EPM, and von Frey model at 30, 45, and 60 minutes after administration, respectively.
Behavioral evaluation at 1 week and 3 weeks after MIA or saline injection was performed on independent sets of animals to reduce mice adaptation to the different paradigms.
2.8. Human subjects
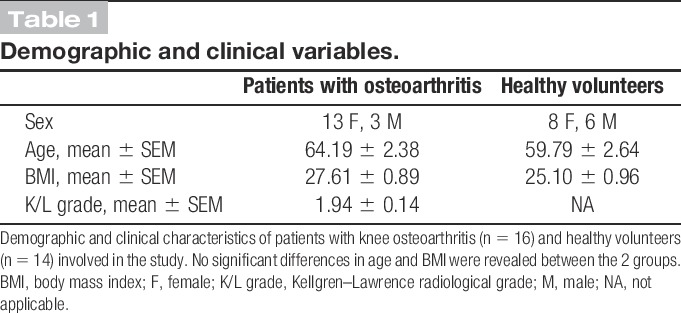
A total of 16 patients with knee osteoarthritis from the Osteoarthritis Unit of Rheumatology Department (Hospital del Mar, Barcelona, Spain) and 14 healthy volunteers participated in the study (Supplemental methods). Demographic and clinical characteristics of these subjects are summarized in Table 1. X-ray radiographies were used to determine Kellgren–Lawrence radiological grade in patients with osteoarthritis.29 The study was approved by the local ethical committee (Clinical Research Ethical Committee of the Parc de Salut Mar, CEIC-Parc de Salut Mar, Barcelona, Spain), in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). A written informed consent was obtained from participants.
Table 1.
Demographic and clinical variables.
2.9. Clinical assessments
The Huskisson scale,25 a self-administered visual analog pain rating scale, was used to record subjective estimates of pain intensity. The scale conventionally consists of a straight line 10 cm long that is marked at each end with labels indicating the range being considered: “pain as bad as it could be” at one end and “no pain” at the other.
The PainDETECT questionnaire9 was used to screen the presence of neuropathic pain components. This questionnaire assesses pain characteristics (ie, the presence of burning, tingling, prickling, or numbness sensations and pain in response to light touching, pressure, cold, or heat) and pain behavior patterns along time. The score range is between −1 and 38. Higher scores indicate higher presence of neuropathic pain components.
The mood state was evaluated by the Hospital Anxiety–Depression (HAD) scale.57 The HAD scale is a 14-item self-report scale containing 2 subscales, one for anxiety (HAD_Anxiety) and one for depression (HAD_Depression), each with 7 items, designed to screen for nonpsychiatric mood disorders in general. It focuses on subjective disturbances of mood rather than physical signs and aims at distinguishing depression from anxiety. Higher scores obtained with the 2 subscales indicate a higher level of subjective mood disturbances.
Health-related quality of life was evaluated by the SF-36 questionnaire,55 consisting of a multipurpose and Short-Form Health Survey with 36 questions. It yields an 8-scale profile to measure functional health and well-being scores as well as psychometrically based physical and mental health summary measures. This questionnaire represents a generic measure, as opposed to those targeting a specific age, disease, or treatment group. Lower scores indicate worst self-perceived health-related quality of life.
Visual memory abilities were explored through the Rey-Osterrieth Complex Figure.44 The test consists of a copy trial followed by a recall trial of a complex figure. The measures include a copy score (which reflects the accuracy of the original copy and is a measure of visual–spatial constructional ability), the time required to copy the figure, and 30-minute delayed recall scores. The figure is divided into 18 scored elements. Between 0.5 and 2 points are awarded for each element depending on the accuracy, distortion, and location of its reproduction. The maximum score is 36, which indicates perfect accuracy.
All neuropsychological measures were corrected for age and school education.44 Once the data were collected, blood samples were obtained from each participant for quantification of 2-AG and AEA plasmatic levels and CB1R and CB2R gene expression in peripheral blood lymphocytes.
2.10. Endocannabinoid analysis
Mice were killed by decapitation at the end of the third week (day 26) after receiving the intra-articular injection of MIA or saline to collect trunk blood and extract the brain. The endocannabinoid profile in mouse brain tissues (PFC, amygdala, and hippocampus) and mouse or human plasma was determined using liquid chromatography–mass spectrometry method (Supplemental methods).
2.11. Gene expression analysis by real-time polymerase chain reaction
At the end of the experiments, corticotropin-releasing hormone (CRH) and glucocorticoid receptor (GR) gene expression were evaluated in PVN and PFC of CB1KO, CB2KO, and WT. CB1R and CB2R gene expression were evaluated in the lymphocytes of patients with osteoarthritis and healthy controls (Supplemental methods).
2.12. Statistical analysis
Data from behavioral and gene expression studies in mice were compared using 2-way analysis of variance (ANOVA) between groups (intra-articular injection and genotype or dose as factors of variance), followed by Fisher Least Significant Difference (LSD) post hoc analysis when appropriate. Data from human subjects and endocannabinoid quantification were analyzed by Student t test. Correlations were determined with Pearson correlation analysis. STATISTICA 6.0 (StatSoft, Inc., Tulsa, OK) software was used. The differences were considered statistically significant when the P value was below 0.05.
3. Results
3.1. Role of CB1R and CB2R in anxiety and memory alterations associated with osteoarthritis pain in mice
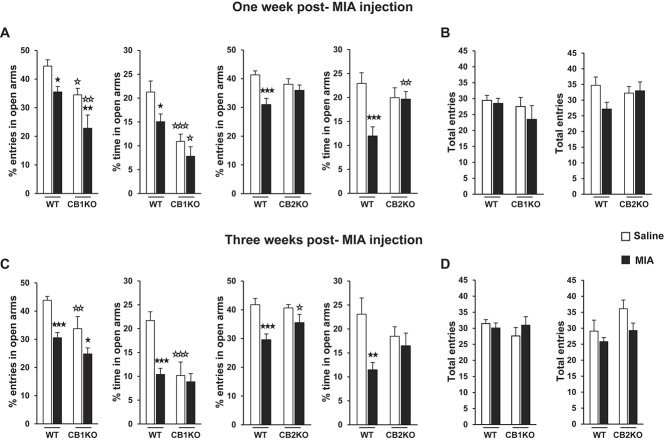
Osteoarthritis pain induced by MIA intra-articular injection31 increased the anxiety-like behavior in WT and CB1KO. At 1 week after injection, 2-way ANOVA revealed a significant effect of intra-articular injection (F(1,59) = 13.405; P < 0.001) and genotype (F(1,59) = 16.139; P < 0.001) in the percentage of entries in the open arms and a significant effect of intra-articular injection (F(1,59) = 5.571; P < 0.05) and genotype (F(1,59) = 19.938; P < 0.001) in the percentage of time spent in the open arms. At 3 weeks after injection, 2-way ANOVA also revealed a significant effect of intra-articular injection (F(1,48) = 21.676; P < 0.001) and genotype (F(1,48) = 10.943; P < 0.01) in the percentage of entries in the open arms and a significant effect of intra-articular injection (F(1,48) = 11.288; P < 0.01) and genotype (F(1,48) = 12.111; P < 0.01) and a significant interaction between these factors (F(1,48) = 7.047; P < 0.05) in the percentage of time spent in the open arms. Subsequent post hoc analysis indicated that MIA intra-articular injection produced an increase in the anxiety-like behavior in WT, as revealed by a significant decrease of the percentage of entries and time spent in the EPM open arms compared with saline at both 1 and 3 weeks after injection (Fig. 1A and C). Monosodium iodoacetate injection also decreased the open arm entries in CB1KO at both time points (Fig. 1A and C). However, CB1KO that already presented an anxiogenic phenotype at basal level (saline group, Fig. 1A and C)16 was even more anxious compared with WT 1 week after MIA injection (Figure 1A).
Figure 1.
Anxiety-like behavior associated with knee osteoarthritis in WT, CB1KO, and CB2KO. The percentage of entries and time spent in the open arms and the total entries in the open and closed arms of the elevated plus-maze were evaluated 1 (A and B) and 3 weeks (C and D) after the intra-articular injection of monosodium iodoacetate or saline. Data are expressed as mean ± SEM (n = 12-18 per group). ★P < 0.05, ★★P < 0.01, ★★★P < 0.001 vs saline injection (Fisher LSD test); ☆P < 0.05, ☆☆P < 0.01, ☆☆☆P < 0.001 vs WT (Fisher LSD test). WT, wild type.
In contrast, MIA injection did not modify the anxiety-like behavior in CB2KO at any time point. At 1 week after injection, 2-way ANOVA revealed in these mice a significant effect of intra-articular injection (F(1,44) = 11.428; P < 0.01) and a significant interaction between intra-articular injection and genotype (F(1,44) = 4.971; P < 0.05) in the percentage of entries in the open arms and a significant effect of intra-articular injection (F(1,44) = 8.297; P < 0.01) and a significant interaction between intra-articular injection and genotype (F(1,44) = 7.438; P < 0.01) in the percentage of time spent in the open arms. At 3 weeks after injection, 2-way ANOVA also revealed a significant effect of intra-articular injection in the percentage of entries (F(1,38) = 16.300; P < 0.001) and percentage of time spent in the open arms (F(1,38) = 7.277; P < 0.05). Subsequent post hoc analysis indicated that MIA intra-articular injection in WT produced a significant decrease of the percentage of entries and time spent in the EPM open arms compared with saline at both 1 and 3 weeks after injection (Fig. 1A and C). In contrast, no effects of MIA were revealed in CB2KO (Fig. 1A and C).
Monosodium iodoacetate injection similarly impaired memory functions in WT, CB1KO, and CB2KO. Two-way ANOVA only revealed a significant effect of intra-articular injection in the discrimination index evaluated in the ORM in these mice at both 1 week (WT/CB1KO: F(1,46) = 42.722, P < 0.001; WT/CB2KO: F(1,41) = 45.001, P < 0.001) and 3 weeks after injection (WT/CB1KO: F(1,42) = 28.737, P < 0.001; WT/CB2KO: F(1,38) = 56.650, P < 0.001). Thus, MIA injection produced a decrease in the ORM discrimination index in a similar way in WT, CB1KO, and CB2KO at both time points (Fig. 2A and C). Monosodium iodoacetate injection did not modify locomotor activity in any of the experimental groups, as no significant effects were revealed by 2-way ANOVA in the total entries of the EPM (Fig. 1B and D) or the total time of exploration in the ORM (Fig. 2B and D), discarding any possible bias due to motor disruption in the different responses evaluated. These results reveal that CB1R and CB2R differentially modulate the alterations in anxiety-like behavior but do not influence the memory impairment induced by MIA.
Figure 2.
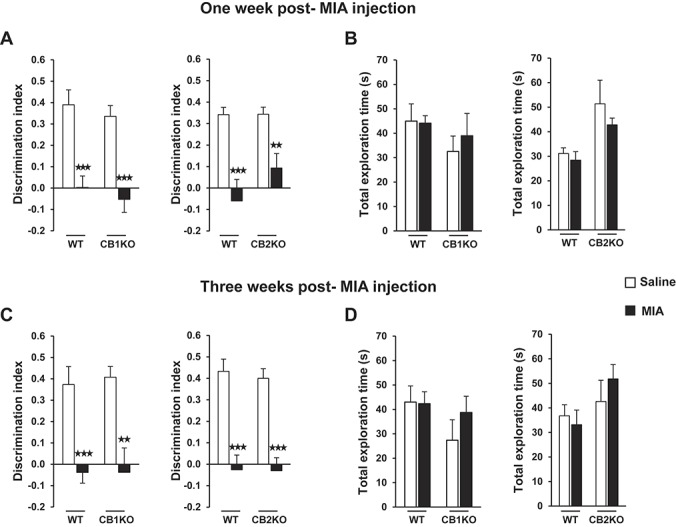
Memory impairment associated with knee osteoarthritis in WT, CB1KO, and CB2KO. The discrimination index and the total time of exploration in the object recognition memory were evaluated 1 (A and B) and 3 weeks (C and D) after the intra-articular injection of monosodium iodoacetate or saline. Data are expressed as mean ± SEM (n = 12-18 per group). ★★P < 0.01, ★★★P < 0.001 vs saline injection (Fisher LSD test). WT, wild type.
3.2. CB1R and CB2R regulation of corticotropin-releasing hormone and glucocorticoid receptor gene expression in osteoarthritic mice
Several studies indicate that ECS signaling in PVN and PFC is critical for the behavioral responses and the glucocorticoid-mediated adaptation of the HPA axis activity after stress exposure.21,24 As the alterations promoted by osteoarthritis in the anxiety-like behavior were differentially modulated in CB1KO and CB2KO in this study, CRH and GR gene expression were measured in the PVN and PFC of these mice to evaluate the role of CB1R and CB2R in the regulation of these key HPA axis components during osteoarthritis. Two-way ANOVA for CRH gene expression in the PVN of WT and CB1KO only revealed a significant effect of intra-articular injection (F(1,20) = 59.029; P < 0.001), suggesting that MIA intra-articular injection similarly reduced CRH gene expression in the PVN of WT and CB1KO (Fig. 3A). In contrast, 2-way ANOVA for CRH gene expression in the PVN of WT and CB2KO revealed a significant effect of genotype (F(1,32) = 90.038; P < 0.001) and a significant interaction between genotype and intra-articular injection (F(1,32) = 15.047; P < 0.001). Indeed, post hoc analysis indicated that MIA produced a further increase of the higher basal levels of CRH gene expression in CB2KO (Fig. 3A).
Figure 3.
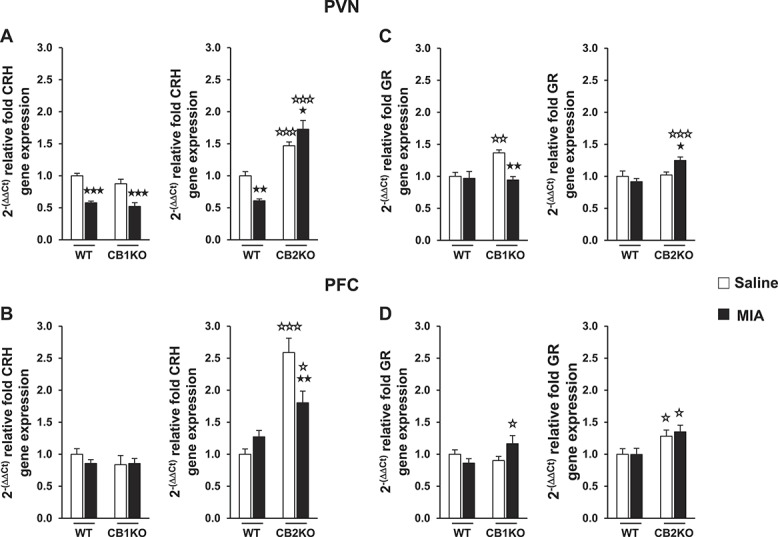
Relative corticotropin-releasing hormone (A and B) and glucocorticoid receptor (C and D) gene expression analysis in paraventricular nucleus and prefrontal cortex of WT, CB1KO, and CB2KO after receiving monosodium iodoacetate or saline injection. Data are expressed as mean ± SEM (n = 5-9 per group). ★P < 0.05, ★★P < 0.01, ★★★P < 0.001 vs saline injection (Fisher LSD test); ☆P < 0.05, ☆☆P < 0.01, ☆☆☆P < 0.001 vs WT (Fisher LSD test). WT, wild type.
Two-way ANOVA for CRH gene expression in the PFC of WT and CB1KO did not reveal any significant effect, suggesting that MIA injection did not modify CRH expression in the PFC of these animals. In contrast, 2-way ANOVA for CRH gene expression in the PFC of WT and CB2KO revealed a significant effect of genotype (F(1,20) = 44.659; P < 0.001) and a significant interaction between genotype and intra-articular injection (F(1,20) = 11.105; P < 0.01). Post hoc analysis showed that MIA injection significantly reduced the higher basal expression of CRH gene in the PFC of CB2KO (Fig. 3B).
Two-way ANOVA for GR gene expression in the PVN of WT and CB1KO revealed a significant effect of intra-articular injection (F(1,12) = 10.249; P < 0.01) and genotype (F(1,12) = 5.731; P < 0.05) and a significant interaction between these factors (F(1,12) = 7.566; P < 0.05). Two-way ANOVA for GR gene expression in the PVN of WT and CB2KO also revealed a significant effect of genotype (F(1,20) = 8.548; P < 0.01) and a significant interaction between genotype and intra-articular injection (F(1,20) = 6.680; P < 0.05). Subsequent post hoc analysis indicated that no changes in GR gene expression were induced by MIA in the PVN of WT groups. However, MIA reversed the higher basal GR expression in CB1KO in this brain area and produced an opposite increase of this gene in CB2KO (Fig. 3C).
Two-way ANOVA for GR gene expression in the PFC revealed a significant interaction between genotype and intra-articular injection (F(1,12) = 5.387; P < 0.05) in WT and CB1KO and a significant effect of genotype (F(1,20) = 10.850; P < 0.01) in WT and CB2KO. Post hoc analysis indicated that MIA did not change GR gene expression in the PFC of any of the genotypes, although significant differences between MIA CB1KO and WT and higher basal GR expression levels in CB2KO were observed in this brain area (Fig. 3D). These results reveal that CB1R and CB2R have an opposite role in the control of CRH and GR gene expression in brain areas involved in the regulation of HPA axis activity during osteoarthritis.
3.3. Effects of CB1R (ACEA) and CB2R (JWH133) agonists in nociceptive, anxiety, and memory alterations in osteoarthritic mice
The effects produced by acute CB1R (ACEA) and CB2R (JWH133) agonist administration were evaluated in the nociceptive, anxiety, and memory alterations induced by MIA. Thus, mice receiving ACEA (1 or 5 mg/kg), JWH133 (1 or 5 mg/kg), or vehicle were tested at both 1 and 3 weeks after MIA injection in the following sequence: ORM, EPM, and von Frey model at 30, 45, and 60 minutes after administration, respectively. These drugs did not produce any significant alteration in locomotor activity at the tested doses, as no significant effects were revealed by 2-way ANOVA in the total entries of the EPM or the total time of exploration in the ORM in these experiments (data not shown).
Baseline values in the nociceptive behavior (von Frey stimulation model) were similar in the different groups before intra-articular injection (data not shown). However, 2-way ANOVA for ACEA in the withdrawal threshold of the ipsilateral paw (von Frey model) revealed a significant effect of intra-articular injection (F(1,89) = 112.051; P < 0.001), dose (F(2,89) = 3.791; P < 0.05), and interaction between these factors (F(2,89) = 8.413; P < 0.001) at 1 week after injection and a significant effect of intra-articular injection (F(1,89) = 80.775; P < 0.001), dose (F(2,89) = 6.385; P < 0.01), and interaction between these factors (F(2,89) = 5.718; P < 0.01) at 3 weeks after injection. Two-way ANOVA for JWH133 in the withdrawal threshold of the ipsilateral paw also revealed a significant effect of intra-articular injection (F(1,90) = 109.74; P < 0.001), dose (F(2,90) = 5.418; P < 0.01), and interaction between these factors (F(2,90) = 6.481; P < 0.01) at 1 week after injection and a significant effect of intra-articular injection (F(1,90) = 147.562; P < 0.001), dose (F(2,90) = 8.323; P < 0.001), and interaction between these factors (F(2,90) = 6.008; P < 0.01) at 3 weeks after injection. Subsequent post hoc analysis indicated that both ACEA (1 and 5 mg/kg) and JWH133 (1 and 5 mg/kg) administration did not modify the nociceptive responses in the ipsilateral paw of control saline–injected mice in comparison with vehicle at 1 and 3 weeks (Fig. 4A and D). However, both doses of ACEA and JWH133 improved the mechanical allodynia by increasing the mechanical withdrawal threshold in the ipsilateral paw of MIA mice when compared with vehicle at 1 and 3 weeks (Fig. 4A and D). Nevertheless, the mechanical allodynia in MIA mice was not completely abolished by these doses of ACEA and JWH133 compared with saline mice (Fig. 4A and D). In contrast, these compounds did not produce any modification in the contralateral nociceptive responses, as revealed by 2-way ANOVA in the contralateral paw (Supplemental Figure S1, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A113).
Figure 4.
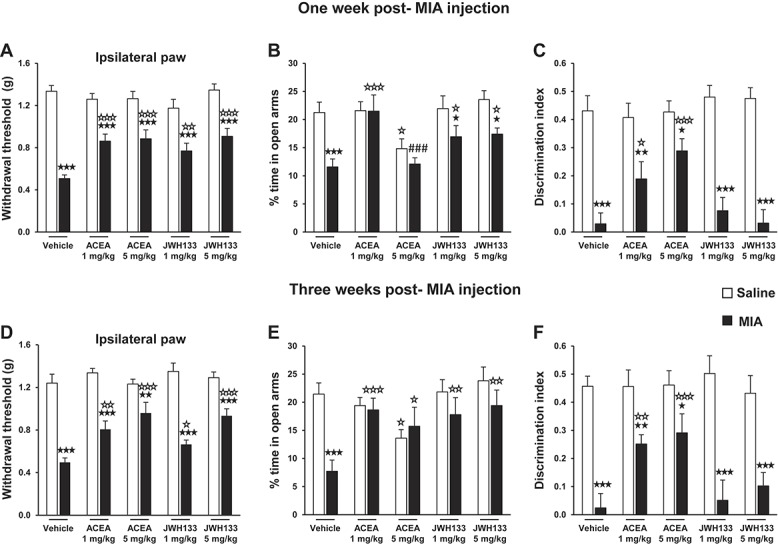
Effects of ACEA and JWH133 in nociceptive, affective, and cognitive behaviors in osteoarthritic mice. Mechanical nociceptive responses in the ipsilateral paw (A and D), anxiety-like behavior (B and E), and memory (C and F) were evaluated 60, 45, and 30 minutes, respectively, after the intraperitoneal administration of ACEA or JWH133, at 1 and 3 weeks after monosodium iodoacetate or saline injection. Data are expressed as mean ± SEM (n = 15-20 per group). ★P < 0.05, ★★P < 0.01, ★★★P < 0.001 vs saline injection (Fisher LSD test); ☆P < 0.05, ☆☆P < 0.01, ☆☆☆P < 0.001 vs vehicle administration (Fisher LSD test); ###P < 0.001 vs 1 mg/kg dose (Fisher LSD test).
At 1 week after injection, 2-way ANOVA for ACEA in the EPM revealed a significant effect of intra-articular injection (F(1,100) = 10.248; P < 0.01), dose (F(2,100) = 6.093; P < 0.01), and interaction between these factors (F(2,100) = 4.476; P < 0.05) in the percentage of entries in the open arms and a significant effect of intra-articular injection (F(1,100) = 8.185; P < 0.01), dose (F(2,100) = 10.118; P < 0.001), and interaction between these factors (F(2,100) = 3.822; P < 0.05) in the percentage of time spent in the open arms. At 3 weeks after injection, 2-way ANOVA for ACEA in the EPM also revealed a significant effect of intra-articular injection (F(1,66) = 8.746; P < 0.01) and interaction between intra-articular injection and dose (F(2,66) = 4.178; P < 0.05) in the percentage of entries in the open arms and a significant effect of intra-articular injection (F(1,66) = 5.422; P < 0.05) and interaction between intra-articular injection and dose (F(2,66) = 7.551; P < 0.01) in the percentage of time spent in the open arms. Moreover, at 1 week after injection, 2-way ANOVA for JWH133 in the EPM revealed a significant effect of intra-articular injection (F(1,95) = 15.819; P < 0.001) and dose (F(2,95) = 3.576; P < 0.05) in the percentage of entries in the open arms and a significant effect of intra-articular injection (F(1,95) = 24.221; P < 0.001) and dose (F(2,95) = 3.127; P < 0.05) in the percentage of time spent in the open arms. At 3 weeks after injection, 2-way ANOVA for JWH133 in the EPM also revealed a significant effect of intra-articular injection (F(1,64) = 6.452; P < 0.05), dose (F(2,64) = 3.282; P < 0.05), and interaction between these factors (F(2,64) = 3.254; P < 0.05) in the percentage of entries and a significant effect of intra-articular injection (F(1,64) = 13.714; P < 0.001) and dose (F(2,64) = 4.534; P < 0.05) in the percentage of time spent in the open arms. Post hoc analysis indicated that ACEA (1 mg/kg) and JWH133 (1 and 5 mg/kg) administration did not modify the responses evaluated in the EPM in control saline–injected mice in comparison with vehicle at 1 and 3 weeks after injection (Fig. 4B and E; Supplemental Figure S2, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A113), although ACEA at 5 mg/kg produced an anxiogenic-like effect, as revealed by a decrease in the percentage of entries and time spent in the EPM open arms in these mice at both time points (Fig. 4B and E; Supplemental Figure S2), as previously described with high doses of CB1R agonists.40 Moreover, ACEA at 1 mg/kg produced a reversion of the altered anxiety-like behavior induced by MIA at both time points (Fig. 4B and E; Supplemental Figure S2). No further modifications were observed at 1 week in the anxiety-like behavior of MIA mice administered with ACEA at 5 mg/kg as compared with vehicle (Fig. 4B; Supplemental Figure S2), although a significant difference was revealed in these mice at 3 weeks as compared with vehicle (Fig. 4E; Supplemental Figure S2). The altered anxiety-like behavior of MIA mice was improved by both doses of JWH133 at 1 week (Fig. 4B) and was reversed at 3 weeks (Fig. 4E).
Two-way ANOVA for ACEA in the discrimination index of ORM revealed a significant effect of intra-articular injection (F(1,109) = 40.869; P < 0.001), dose (F(2,109) = 4.104; P < 0.05), and interaction between these factors (F(2,109) = 4.445; P < 0.05) at 1 week after injection and a significant effect of intra-articular injection (F(1,66) = 41.567; P < 0.001), dose (F(2,66) = 4.059; P < 0.05), and interaction between these factors (F(2,66) = 3.899; P < 0.05) at 3 weeks after injection. In contrast, 2-way ANOVA for JWH133 in the discrimination index of ORM only revealed a significant effect of intra-articular injection at 1 week (F(1,94) = 123.070; P < 0.001) and 3 weeks after injection (F(1,65) = 76.539; P < 0.001). Post hoc analysis showed that both ACEA doses improved the memory impairment by increasing the discrimination index in MIA mice compared with vehicle at 1 and 3 weeks, although it was not completely reversed compared with saline mice (Fig. 4C and F). In contrast, JWH133 did not modify the memory impairment of MIA mice at any of the doses and time points tested (Fig. 4C and F). Thus, both CB1R and CB2R agonists ameliorated the alterations in the nociceptive and anxiety-like behaviors, but only the CB1R agonist improved the memory deficit induced by MIA.
3.4. Increased endocannabinoid levels in prefrontal cortex and plasma of osteoarthritic mice
We investigated the possible modulation of the endocannabinoid tone during osteoarthritis at the peripheral level and in brain areas involved in pain, emotional, and cognitive processing. The levels of 2-AG and AEA were measured in the PFC, amygdala, hippocampus, and plasma of MIA and saline mice. No significant differences between MIA and control groups were found in AEA levels in the different tissues evaluated. Similarly, no significant changes in 2-AG content were induced by MIA in amygdala and hippocampus. However, 2-AG levels were significantly higher in the PFC and plasma of MIA mice compared with control (Fig. 5C and D). Therefore, 2-AG tone was increased at both central (PFC) and peripheral (plasma) levels in osteoarthritic mice.
Figure 5.
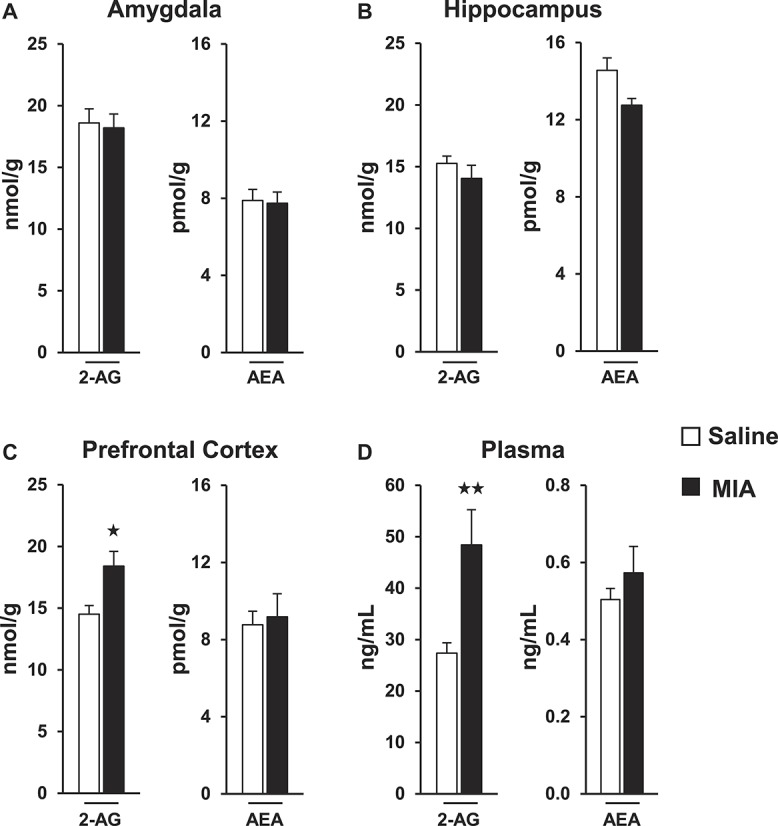
2-AG and AEA quantification in the amygdala (A), hippocampus (B), prefrontal cortex (C), and plasma (D) of mice receiving monosodium iodoacetate or saline injection. Data are expressed as mean ± SEM (n = 8-10 per group). ★P < 0.05, ★★P < 0.01 vs saline injection (Student t test).
3.5. Involvement of the endocannabinoid system in human osteoarthritis
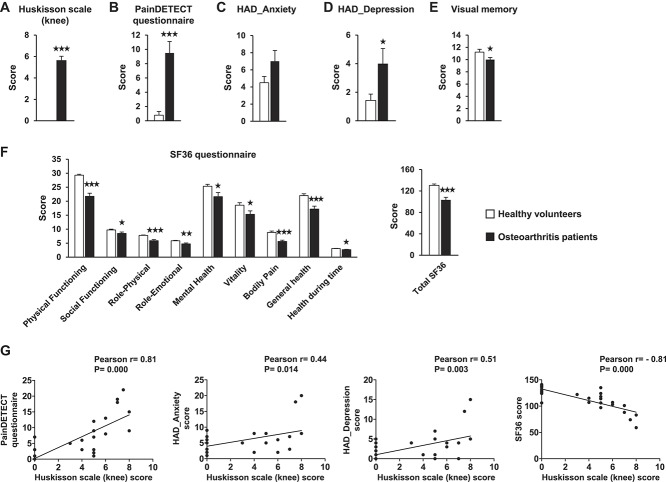
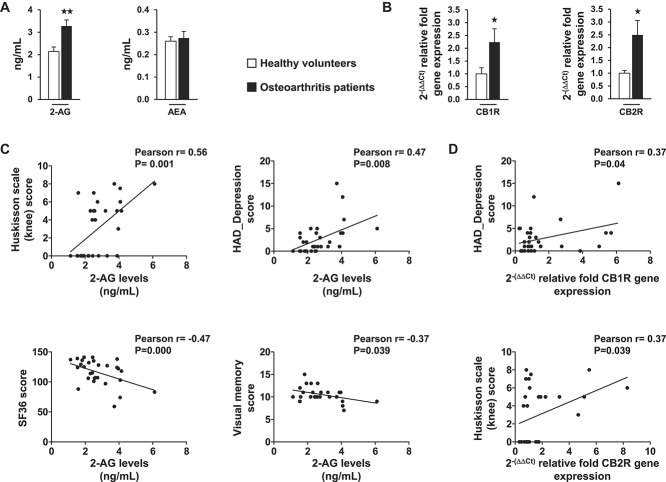
We investigated whether similar changes to those found in endocannabinoid levels in the MIA mouse model occur in human osteoarthritis and the possible correlation of these changes with clinical symptoms. As expected, significant differences between patients with knee osteoarthritis and healthy subjects were observed in the scores obtained with the Huskisson scale for knee pain and the PainDETECT questionnaire for pain characteristics (Fig. 6A and B). A significant higher score for the depressive state (HAD_Depression) and a nonsignificant trend for the anxiety score (HAD_Anxiety) were found in patients compared with healthy controls (Fig. 6C and D). Patients also displayed more difficulties in performing the visual memory task (Rey-Osterrieth Complex Figure test; Fig. 6E) and lower scores in the quality of life SF-36 questionnaire (Fig. 6F) compared with healthy volunteers. Significant correlations were observed between knee pain scores and the scores of PainDETECT, HAD_Anxiety, HAD_Depression, and SF-36 questionnaires in these subjects (Fig. 6G). Interestingly, a significant increase in 2-AG plasmatic levels, but not AEA, was revealed in patients with osteoarthritis in comparison with healthy controls (Fig. 7A), replicating the findings obtained in osteoarthritic mice. Moreover, an upregulation of CB1R and CB2R gene expression in blood lymphocytes was observed in patients with osteoarthritis compared with control subjects (Fig. 7B). Significant positive correlations were found between 2-AG levels and knee pain and HAD_Depression scores, as well as significant negative correlations with SF-36 and memory performance scores (Fig. 7C). In addition, significant positive correlations were obtained between the gene expression levels of CB1R in lymphocytes and HAD_Depression scores and between the expression levels of CB2R and knee pain scores (Fig. 7D). Therefore, key components of the ECS were upregulated in human osteoarthritis with significant correlations with clinical symptoms.
Figure 6.
Clinical assessment of pain (Huskisson scale [A] and PainDETECT questionnaire [B]), emotional state (Hospital Anxiety–Depression scale [C and D]), visual memory (E), and health-related quality of life (SF-36 questionnaire [F]) in patients with osteoarthritis and healthy volunteers. Data are expressed as mean ± SEM (n = 14-16 per group). ★P < 0.05, ★★P < 0.01, ★★★P < 0.001 vs healthy volunteers (Student t test). Pearson correlations between knee pain scores (Huskisson scale) and PainDETECT, Hospital Anxiety–Depression scale, and SF-36 scores are presented (G).
Figure 7.
Plasmatic endocannabinoid quantification (A) and CB1R and CB2R gene expression in the lymphocytes (B) of patients with osteoarthritis and healthy volunteers. Data are expressed as mean ± SEM (n = 14-16 per group). ★P < 0.05, ★★P < 0.01 vs healthy volunteers (Student t test). Pearson correlations between 2-AG levels and knee pain (Huskisson scale), HAD_Depression, SF-36, and visual memory scores (C) as well as correlations between CB1R or CB2R gene expression and HAD_Depression and pain scores (Huskisson scale), respectively, are presented (D). HAD, Hospital Anxiety–Depression scale.
4. Discusssion
In this study, we validated a new model to evaluate the affective and cognitive alterations associated with knee osteoarthritis in mice and identified for the first time the involvement of the ECS in the affective symptoms that are crucial for the management of this chronic pain state. Osteoarthritis pain in mice was associated with increased anxiety-like behavior in the EPM and reduced memory functions in the ORM, as previously reported in other chronic pain models.34 The EPM was the most sensitive paradigm to reveal the alterations in the anxiety-like behavior during osteoarthritis pain under our experimental conditions. Alternative versions of the ORM paradigm have been used in other studies2 and could also be potentially applied to evaluate the cognitive impairment produced by osteoarthritis pain. A limitation of this study is represented by the lack of histological data to make correlations between the joint lesions induced by MIA and the behavioral findings obtained 1 and 3 weeks after MIA intra-articular injection. However, previous studies demonstrated that the histopathological changes already appear within the first week and increase progressively over time in the MIA model.10,12,53
Although chronic pain could be considered a stressor producing similar effects to those observed in other stress-related disorders, the precise contribution of CRH and other components of the HPA axis remains unclear. Here, we found a downregulation of CRH gene expression in PVN of osteoarthritic mice that may represent an adaptive modification to limit HPA axis activity under osteoarthritis pain and may underlie the absence of HPA neuroendocrine alterations in patients with osteoarthritis.30 In agreement, CRH signaling in the limbic system seems to contribute to nociceptive, affective, and cognitive alterations in rodent chronic pain paradigms that were not associated with HPA axis dysfunctions.27,52 In our study, osteoarthritis pain was associated with increased levels of 2-AG in PFC, a crucial brain area involved in pain, cognitive, and emotional processing, that constitutes one of the primary targets of HPA axis hormones.37 The augmented PFC endocannabinoid tone may represent a compensatory mechanism to maintain proper neuroendocrine and behavioral functions in response to persistent pain. An increase in PFC 2-AG levels with similar functional consequences has also been demonstrated in chronic stress animal models.19,37 Indeed, the stress-induced increase of PFC endocannabinoid signaling through CB1R mediates a feedback mechanism to suppress HPA activity by disinhibiting output neurons on subcortical structures that regulate CRH secretion in PVN.21 The same mechanisms may be responsible for the CRH downregulation found in PVN of osteoarthritic mice.
The alterations induced by osteoarthritis in the anxiety-like behavior appeared more pronounced in CB1KO and were absent in CB2KO, revealing an opposite role of CB1R and CB2R in the control of these manifestations. The role of CB1R in these emotional responses resembled that observed in other types of chronic stress. Thus, CB1KO displayed an increased sensitivity to develop anxiety and depressive-like states following repetitive stress procedures,18,36 in agreement with the expression of CB1R in corticolimbic circuits related to stress responses.17,19,21,35,51 Despite the protective role of CB2R in pain modulation previously described in the MIA model,31 an opposite regulation by this receptor was observed in the anxiety-like behavior. CB2R has been proposed to participate in emotional responses.43 In line with our findings, chronic CB2R blockade produced anxiolysis and antidepressant-like effects after stress.13,14 The opposite role of CB1R and CB2R in these affective manifestations could be related to their different role in regulating HPA axis components during osteoarthritis pain. Thus, MIA-induced CRH downregulation was not modified in CB1KO and was fully reversed in CB2KO. A similar opposite regulation in the absence of these cannabinoid receptors was observed for the expression of GR gene in PVN of osteoarthritic mice. The lack of CB1R and the concomitant deregulation of GR gene expression in PVN may interfere with the ability of endocannabinoids to exert the glucocorticoid-dependent control of HPA axis,24 resulting in the altered affective responses observed in CB1KO. The lack of CB2R together with the basal CRH and GR gene expression modifications in PVN and PFC may facilitate adaptive responses during osteoarthritis pain to prevent the affective alterations in CB2KO. The PFC seems to be particularly involved in the modulation of these responses in CB2KO. Thus, the higher basal expression of GR in PFC of CB2KO could promote the downregulation of CRH gene expression by a glucocorticoid-mediated mechanism,39 which would limit the excitatory influence of cortical CRH on HPA axis and the anxiety-like behavior.26 Therefore, the endocannabinoid signaling through CB1R and CB2R seems to be crucial for the emotional and stress-related responses produced by osteoarthritis pain.
In contrast, the memory impairment induced by osteoarthritis was not modified in mice lacking CB1R or CB2R, suggesting that these receptors do not participate in these cognitive manifestations. Interestingly, CB1R (ACEA) or CB2R (JWH133) pharmacological activation improved the alterations in the nociceptive and anxiety-like behaviors, whereas only ACEA improved the memory impairment. Notably, ACEA and JWH133 were previously found to produce antinociception devoid of central side effects,54 which supports the interest of cannabinoid agonists for chronic pain treatment. The improvement of the affective and cognitive alterations observed in our osteoarthritis model could be a direct consequence of pain relief produced by these cannabinoid agonists. However, the lack of effects of JWH133 in the memory task suggests that the amelioration of these symptoms is more likely to depend on a direct effect in emotional and cognitive processes. Accordingly, ACEA and JWH133 produce anxiolysis6,40 and cannabinoid effects on memory highly depend on the experimental conditions.1 Both ACEA and JWH133 present a very high affinity for CB1R (ACEA Ki value = 1.24 nM; JWH133 Ki value = 677 nM) and CB2R (ACEA Ki value = 195 nM; JWH133 Ki value = 3.4 nM), respectively.45 The selectivity of these compounds suggests a specific activation of CB1R and CB2R under our experimental conditions.
Taken together, our results revealed that corticolimbic endocannabinoid signaling is a key modulator of different osteoarthritis pain manifestations and participates in adaptive mechanisms that would help to maintain appropriate functions during osteoarthritis. The PFC endocannabinoid changes were reflected at periphery by a similar increase of 2-AG plasmatic levels in osteoarthritic mice. These peripheral endocannabinoid changes can provide a useful tool to investigate potential peripheral biomarkers of human osteoarthritis. Interestingly, a significant increase in the plasmatic levels of 2-AG was also revealed in patients with osteoarthritis, replicating the findings obtained in the mouse model. Pain in patients with osteoarthritis was associated with mood, cognitive, and psychosocial alterations, as previously reported.3,15,28 Significant correlations between knee pain scores and scores for anxiety and depressive state were observed in these subjects. Significant positive correlations between 2-AG plasmatic levels and scores of knee pain and depression, as well as significant negative correlations with memory performances and health status were observed in these subjects. In agreement, elevated 2-AG, but not AEA, serum content was found in individuals suffering from major depression and healthy individuals exposed to stress.22 In contrast, both endocannabinoids were elevated in the synovial fluid of patients with osteoarthritis,49 indicating a potentially distinct role of AEA (local structural alterations) and 2-AG (emotional and cognitive symptoms) during osteoarthritis. Although the source of circulating endocannabinoids remains unknown, immune cells could critically contribute to their release in the blood.48 An upregulation of CB1R and CB2R gene expression was also observed in lymphocytes of patients with osteoarthritis, suggesting a generalized adaptive response of ECS in the immune system during osteoarthritis. The different CB1R and CB2R correlations with emotional and pain scores, respectively, provide further evidence of a differential role of these 2 receptors in the control of osteoarthritis-related symptoms. Recent findings suggest that peripheral ECS changes could mirror similar alterations at central level and correlate with emotional and cognitive dysfunctions in depressive and schizophrenic patients.11,23
The preclinical and clinical results reported in this study reveal the important role of the ECS in the emotional manifestations of osteoarthritis pain and provide clinical evidence for the modification of key ECS components during this chronic pain state. The data described here also highlight the potential translational relevance of these results and suggest that the ECS may represent a novel biomarker for osteoarthritis and an interesting pharmacological target for the management of chronic pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
This work was supported by European Commission FP7 (#HEALTH-F2-2013-602891), RETICS-Instituto de Salud Carlos III (#RD12/0028/0023), MICINN-Ministerio de Ciencia e Innovación (#SAF2011-29864), AGAUR-Generalitat de Catalunya (#2009SGR00731, #2014-SGR-680, and #2014-SGR-1547), ISCIII-FIS-CAIBER (CAI08/01/0024), and CIBERobn (CB06/03/0028). CIBERobn is an initiative of the Instituto de Salud Carlos III, Madrid, Spain. FEDER cofunding is also acknowledged.
Appendix A. Supplemental Digital Content
Supplemental Digital Content associated with this article can be found online at http://links.lww.com/PAIN/A113.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Akirav I. The role of cannabinoids in modulating emotional and non-emotional memory processes in the hippocampus. Front Behav Neurosci 2011;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 2012;13:93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Axford J, Butt A, Heron C, Hammond J, Morgan J, Alavi A, Bolton J, Bland M. Prevalence of anxiety and depression in osteoarthritis: use of the Hospital Anxiety and Depression Scale as a screening tool. Clin Rheumatol 2010;29:1277–83. [DOI] [PubMed] [Google Scholar]
- [4].Blackburn-Munro G, Blackburn-Munro RE. Chronic pain, chronic stress and depression: coincidence or consequence? J Neuroendocrinol 2001;13:1009–23. [DOI] [PubMed] [Google Scholar]
- [5].Burston JJ, Sagar DR, Shao P, Bai M, King E, Brailsford L, Turner JM, Hathway GJ, Bennett AJ, Walsh DA, Kendall DA, Lichtman A, Chapman V. Cannabinoid CB2 receptors regulate central sensitization and pain responses associated with osteoarthritis of the knee joint. PLoS One 2013;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry 2011;70:479–86. [DOI] [PubMed] [Google Scholar]
- [7].Castañé A, Célérier E, Martín M, Ledent C, Parmentier M, Maldonado R, Valverde O. Development and expression of neuropathic pain in CB1 knockout mice. Neuropharmacology 2006;50:111–22. [DOI] [PubMed] [Google Scholar]
- [8].Cruz APM, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav 1994;49:171–6. [DOI] [PubMed] [Google Scholar]
- [9].De Andrés J, Pérez-Cajaraville J, Lopez-Alarcón MD, López-Millán JM, Margarit C, Rodrigo-Royo MD, Franco-Gay ML, Abejón D, Ruiz MA, López-Gomez V, Pérez M. Cultural adaptation and validation of the PainDETECT scale into Spanish. Clin J Pain 2012;28:243–53. [DOI] [PubMed] [Google Scholar]
- [10].Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, Kidd B, Bevan S, Winter J. Pain related behaviour in two models of osteoarthritis in the rat knee. PAIN 2004;112:83–93. [DOI] [PubMed] [Google Scholar]
- [11].Ferretjans R, de Campos SM, Ribeiro-Santos R, Guimarães FC, de Oliveira K, Cardoso ACA, Araújo MS, Teixeira-Carvalho A, Martins-Filho OA, Teixeira AL, Salgado JV. Cognitive performance and peripheral endocannabinoid system receptor expression in schizophrenia. Schizophr Res 2014;156:254–60. [DOI] [PubMed] [Google Scholar]
- [12].Gao X, Wang W, Wei S, Li W. Review of pharmacological effects of Glycyrrhiza radix and its bioactive compounds. Phytotherapy 2009;34:2695–700. [PubMed] [Google Scholar]
- [13].García-Gutiérrez MS, García-Bueno B, Zoppi S, Leza JC, Manzanares J. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABA(A) receptors. Br J Pharmacol 2012;165:951–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].García-Gutiérrez MS, Pérez-Ortiz JM, Gutiérrez-Adán A, Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. Br J Pharmacol 2010;160:1773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Goldenberg DL. The interface of pain and mood disturbances in the rheumatic diseases. Semin Arthritis Rheum 2010;40:15–31. [DOI] [PubMed] [Google Scholar]
- [16].Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci 2002;16:1395–8. [DOI] [PubMed] [Google Scholar]
- [17].Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 1991;11:563–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hill MN, Hillard CJ, McEwen BS. Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cereb Cortex 2011;21:2056–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TTY, Gray JM, Hillard CJ, Gorzalka BB, Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A 2010;107:9406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology 2009;34:2733–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TTY, Karatsoreos IN, Mackie K, Viau V, Pickel VM, McEwen BS, Liu Q, Gorzalka BB, Hillard CJ. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci 2011;31:10506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology 2009;34:1257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hill MN, Miller GE, Ho WSV, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry 2008;41:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J Neurosci 2010;30:14980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huskisson EC. Measurement of pain. Lancet 1974;2:1127–31. [DOI] [PubMed] [Google Scholar]
- [26].Jaferi A, Bhatnagar S. Corticotropin-releasing hormone receptors in the medial prefrontal cortex regulate hypothalamic-pituitary-adrenal activity and anxiety-related behavior regardless of prior stress experience. Brain Res 2007;1186:212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci 2010;30:5451–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Karp JF, Reynolds CF, Butters MA, Dew MA, Mazumdar S, Begley AE, Lenze E, Weiner DK. The relationship between pain and mental flexibility in older adult pain clinic patients. Pain Med 2006;7:444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Khoromi S, Muniyappa R, Nackers L, Gray N, Baldwin H, Wong KA, Matheny LA, Moquin B, Rainer A, Hill S, Remaley A, Johnson LL, Max MB, Blackman MR. Effects of chronic osteoarthritis pain on neuroendocrine function in men. J Clin Endocrinol Metab 2006;91:4313–8. [DOI] [PubMed] [Google Scholar]
- [31].La Porta C, Bura SA, Aracil-Fernández A, Manzanares J, Maldonado R. Role of CB1 and CB2 cannabinoid receptors in the development of joint pain induced by monosodium iodoacetate. PAIN 2013;154:160–74. [DOI] [PubMed] [Google Scholar]
- [32].La Porta C, Bura SA, Negrete R, Maldonado R. Involvement of the endocannabinoid system in osteoarthritis pain. Eur J Neurosci 2014;39:485–500. [DOI] [PubMed] [Google Scholar]
- [33].Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 1999;283:401–4. [DOI] [PubMed] [Google Scholar]
- [34].Liu MG, Chen J. Preclinical research on pain comorbidity with affective disorders and cognitive deficits: challenges and perspectives. Prog Neurobiol 2014;116:13–32. [DOI] [PubMed] [Google Scholar]
- [35].Malcher-Lopes R, Di S, Marcheselli VS, Weng F-J, Stuart CT, Bazan NG, Tasker JG. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci 2006;26:6643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) 2002;159:379–87. [DOI] [PubMed] [Google Scholar]
- [37].McLaughlin RJ, Hill MN, Gorzalka BB. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci Biobehav Rev 2014;42:116–31. [DOI] [PubMed] [Google Scholar]
- [38].Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol 2013;64:21–47. [DOI] [PubMed] [Google Scholar]
- [39].Meng QY, Chen XN, Tong DL, Zhou JN. Stress and glucocorticoids regulated corticotropin releasing factor in rat prefrontal cortex. Mol Cell. Endocrinol 2011;342:54–63. [DOI] [PubMed] [Google Scholar]
- [40].Moreira FA, Wotjak CT. Cannabinoids and anxiety. Curr Top Behav Neurosci 2010;2:429–50. [DOI] [PubMed] [Google Scholar]
- [41].Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol 2011;93:385–404. [DOI] [PubMed] [Google Scholar]
- [42].Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci 2012;32:5747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Onaivi ES, Ishiguro H, Gu S, Liu Q-R. CNS effects of CB2 cannabinoid receptors: beyond neuro-immuno-cannabinoid activity. J Psychopharmacol 2012;26:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Palomo R, Casals-Coll M, Sánchez-Benavides G, Quintana M, Manero RM, Rognoni T, Calvo L, Aranciva F, Tamayo F, Peña-Casanova J. Spanish normative studies in young adults (NEURONORMA young adults project): norms for the Rey-Osterrieth Complex Figure (copy and memory) and Free and Cued Selective Reminding Test. Neurologia 2013;28:226–35. [DOI] [PubMed] [Google Scholar]
- [45].Pertwee RG, Howlett AC, Abood ME, Alexander SPH, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 2010;62:588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci 2009;12:1152–8. [DOI] [PubMed] [Google Scholar]
- [47].Puig-Junoy J, Ruiz Zamora A. Socio-economic costs of osteoarthritis: a systematic review of cost-of-illness studies. Semin Arthritis Rheum 2014;44:531–41. [DOI] [PubMed] [Google Scholar]
- [48].Randall MD. Endocannabinoids and the haematological system. Br J Pharmacol 2007;152:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Richardson D, Pearson RG, Kurian N, Latif ML, Garle MJ, Barrett DA, Kendall DA, Scammell BE, Reeve AJ, Chapman V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther 2008;10:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sagar DR, Staniaszek LE, Okine BN, Woodhams S, Norris LM, Pearson RG, Garle MJ, Alexander SPH, Bennett AJ, Barrett DA, Kendall DA, Scammell BE, Chapman V. Tonic modulation of spinal hyperexcitability by the endocannabinoid receptor system in a rat model of osteoarthritis pain. Arthritis Rheum 2010;62:3666–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress 2011;14:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ulrich-Lai YM, Xie W, Meij JTA, Dolgas CM, Yu L, Herman JP. Limbic and HPA axis function in an animal model of chronic neuropathic pain. Physiol Behav 2006;88:67–76. [DOI] [PubMed] [Google Scholar]
- [53].van der Kraan PM, Vitters EL, van de Putte LB, van den Berg WB. Development of osteoarthritic lesions in mice by “metabolic” and “mechanical” alterations in the knee joints. Am J Pathol 1989;135:1001–14. [PMC free article] [PubMed] [Google Scholar]
- [54].Vera G, Cabezos PA, Martín MI, Abalo R. Characterization of cannabinoid-induced relief of neuropathic pain in a rat model of cisplatin-induced neuropathy. Pharmacol Biochem Behav 2013;105:205–12. [DOI] [PubMed] [Google Scholar]
- [55].Vilagut G, Ferrer M, Rajmil L, Rebollo P, Permanyer-Miralda G, Quintana JM, Santed R, Valderas JM, Ribera A, Domingo-Salvany A, Alonso J. The Spanish version of the Short Form 36 Health Survey: a decade of experience and new developments [in Spanish]. Gac Sanit 2005;19:135–50. [DOI] [PubMed] [Google Scholar]
- [56].Wamsteeker JI, Kuzmiski JB, Bains JS. Repeated stress impairs endocannabinoid signaling in the paraventricular nucleus of the hypothalamus. J Neurosci 2010;30:11188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]