Findings from a 5-day observation study link high levels of self-reported overactivity to repeated periods of prolonged activity engagement followed by significant pain increases.
Keywords: Chronic pain, Overactivity, Objective activity, Avoidance, Accelerometer
Abstract
Overactivity is a frequently used term in chronic pain literature. It refers to the phenomenon whereby individuals engage in activity in a way that significantly exacerbates pain, resulting in periods of incapacity. Overactivity, as a construct, has been derived solely from patients' self-reports, raising concerns about the legitimacy of the construct. Self-reported overactivity reflects an individual's “belief,” collected retrospectively, that their earlier activity levels have resulted in increased levels of pain. This may be different to an individual actually engaging in activity in a way that significantly exacerbates pain. In this study, a 5-day observational study design was used to investigate the validity of overactivity as a construct by examining the relationship between a self-report measure of overactivity, patterns of pain, and objectively measured physical activity over time. A sample of 68 adults with chronic pain completed a questionnaire investigating self-reported habitual engagement in overactivity and activity avoidance behaviour, before commencing 5 days of data collection. Over the 5-day period, participants wore an activity monitor and recorded their pain intensity 6 times a day using a handheld computer. Associations were found between (1) high levels of pain and both high overactivity and high avoidance, (2) high levels of overactivity and more variation in pain and objective activity across days, and (3) high levels of overactivity and the reoccurrence of prolonged activity engagement followed by significant pain increases observed in data sets. These results offer some preliminary support for the validity of overactivity as a legitimate construct in chronic pain.
1. Introduction
Overactivity is a behaviour that is commonly referred to in chronic pain literature. The construct was originally introduced as part of Fordyce's operant model of chronic pain16,17,28 and has frequently featured in the pain literature since. Overactivity can be defined as engagement in activity in a way that significantly exacerbates pain, which results in periods of incapacity.10,33,37 Individuals who habitually engage in overactivity behaviour are thought to have a “yo-yo” activity and pain pattern whereby periods of prolonged activity are followed by significant pain increases, resulting in extended rest periods where pain decreases.11,19 Recent empirical investigations suggest that overactivity is an enduring pattern of behaviour, with evidence that habitually overactive individuals have premorbid patterns of engaging in high levels of work and productive tasks.3,7
Avoidance (decreasing activity levels to minimise pain escalation) is another behaviour originally described in Fordyce's operant model16 and which remains a frequently used concept in pain literature.4 Clinicians have reported that a combination of high levels of overactivity and avoidance may simultaneously manifest in the same person with chronic pain.33,37 These observations suggest that some individuals who initially engage in overactivity begin to avoid certain pain-provoking activities as pain exacerbations become more severe over time. The notion that all these behaviours (ie, overactivity, avoidance, or a combination of both) result in increased pain and disability over time is outlined in pain education books.10,37
A number of self-report measures have been developed to measure the extent to which individuals habitually engage in avoidance and/or overactivity behaviour.4 The validity of these measures has, however, been questioned,43 as they have failed to explain individual differences in mean or total objective physical activity levels in some observational studies.20,24 As overactivity as a construct has been derived from patients' self-reports, the legitimacy of the construct is also uncertain. Individuals may, retrospectively, attribute pain exacerbations to increased activity levels based on their beliefs about the relationship between activity and pain. Thus, high scores on overactivity measures might reflect an individual's inaccurate belief about the cause of increased pain rather than an individual having engaged in activity in a way that significantly exacerbated pain.
In this study, the construct validity of a self-report measure of overactivity was examined, by investigating the relationship between an individual's self-reported habitual approach to activity engagement and patterns of both pain and objective physical activity over a 5-day period. The following hypotheses were made:
(1) Self-reported higher levels of both overactivity and avoidance would be associated with higher levels of pain on average;
(2) Individuals reporting higher levels of overactivity would have more variation in their pain and objective physical activity (ie, a larger difference in values over time secondary to the theorised yo-yo activity and pain pattern) irrespective of their level of activity avoidance;
(3) Patterns of prolonged activity engagement followed by significant increases in pain would be observed more often in the data of individuals who self-report high levels of overactivity.
2. Methods
2.1. Participants
Participants were recruited from a cohort of patients attending a multidisciplinary pain centre (MPC) at a large tertiary hospital in Australia. Inclusion criteria were (1) outpatient of the MPC, (2) persistent non-cancer pain for at least 3 months, (3) generalised pain distribution impacting on the participant's gross movement (ie, gross movement patterns increase the participant's pain), (4) English literate, (5) 18 years and over, (6) residing in the metropolitan area where the MPC was located, and (7) able to provide written informed consent. As the activity monitors used in this study measure an individual's gross movement, only individuals who had generalised pain in body parts associated with gross movement (ie, the lower limb(s) and/or torso) were recruited to ensure that the relationship between gross movement and pain was similar across participants. A member of the multidisciplinary treatment team assessed each patient's pain distribution before recruitment to determine their suitability for the study. Ninety-three patients were invited to participate in the study, with 20 declining because of other commitments, resulting in a sample size of 73. Of these 73 participants, 5 ceased the study before completing the fourth day of data collection, resulting in more than 20% of missing data for these participants. Therefore, only the data from the remaining 68 participants were used. The demographic information for these participants is reported in Tables 1 and 2. Participants were predominantly female, married, Australian, and unemployed due to pain, with an age range of 25 to 73 years. The majority of participants reported having pain for an extended period of time (M = 5.11 years) and across numerous pain sites (M = 6.99). The main pain complaint was lower back pain (78%).
Table 1.
Descriptive information for categorical demographic variables, N = 68.
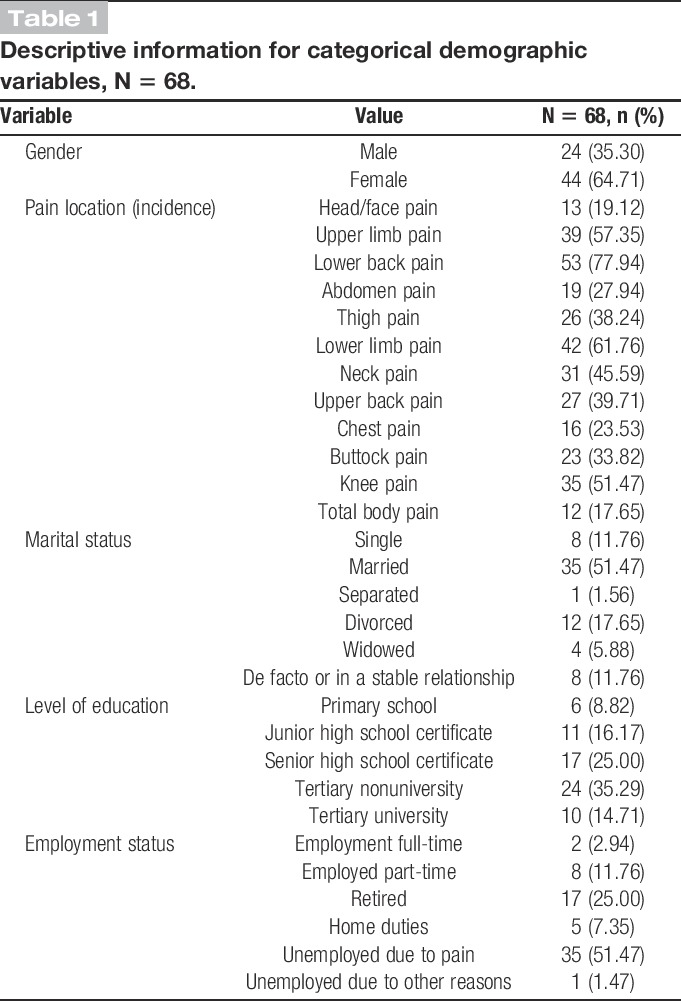
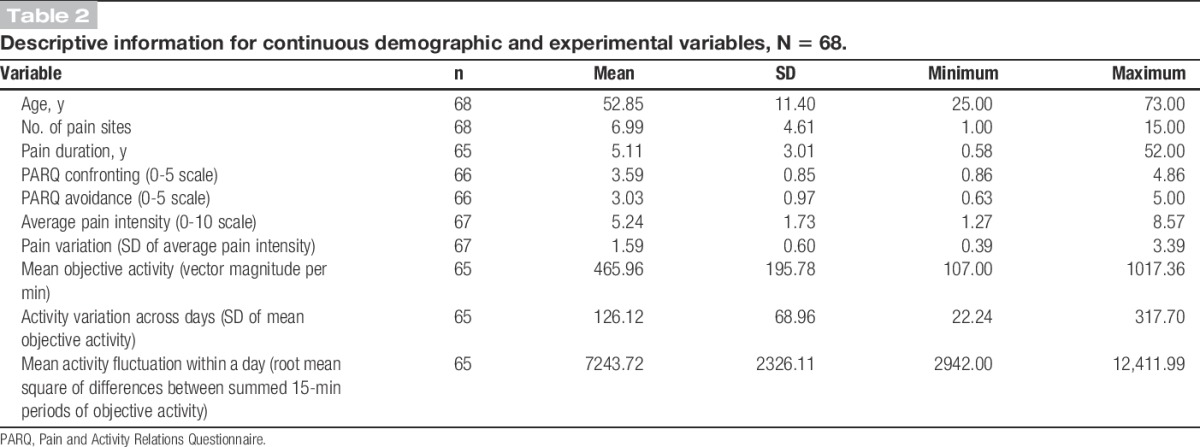
Table 2.
Descriptive information for continuous demographic and experimental variables, N = 68.
2.2. Procedure
The Royal Brisbane and Women's Hospital Human Research Ethics Committee (Number: HREC/09/QRBW/365) and The University of Queensland Behavioural and Social Sciences Ethical Review Committee (Number: 2010000501) approved the study protocol. Over a 3-year period, participants meeting the selection criteria were identified by medical or allied health staff at the MPC. The study was explained to patients verbally, and written informed consent was obtained. Participants then completed a demographic questionnaire and the Pain and Activity Relations Questionnaire (PARQ)29 before commencing 5 days of data collection. This 5-day data collection period included at least 1 weekend day. Over the 5 days, participants wore an activity monitor and were given a Palm handheld computer, with installed software, that prompted participants to record the intensity of their pain 6 times a day and to fill in a paper diary detailing the activities they did throughout the day. On completion of data collection, participants received a $20 gift voucher for use in popular retail stores in Australia.
2.3. Measures
2.3.1. Demographic information
Data were collected in relation to gender, age, pain location, number of pain sites, pain duration, marital status, level of education, and employment status.
2.3.2. Self-reported habitual approach to activity engagement
The extent to which participants habitually engaged in avoidance and overactivity behaviour was assessed using the PARQ.29 The PARQ has 21 items divided into 3 subscales: avoidance, confrontation, and pacing. Participants rate the frequency with which they engage in certain behaviours on a 6-point Likert scale (0 = never to 5 = always). The PARQ confronting scale consists of 7 items. This subscale can be considered to be a measure of overactivity. Four of these items refer to patterns of activity and pain that are characteristic of overactivity behaviour: “I alternate between doing nothing and pushing too hard,” “I spend too much time on some activities and experience increased pain later,” “When my pain decreases I try to be as active as possible,” and “When my pain reduces I catch up on what I missed.” One item reflects perceptions of doing too much: “Considering my pain problem I do more than I should.” The remaining 2 items refer to activity persistence despite pain: “I push to get things done despite my pain level” and “I do what I need to regardless of the pain I feel.”
The avoidance subscale consists of 8 items that refer to avoiding activities or reducing activity engagement secondary to pain, eg, “I avoid activities that cause pain” and “When I feel pain, I try to stay as still as possible.” The pacing subscale was not used in this study because of confusion in the literature about whether self-report measures of pacing reflect quota-contingent pacing, as taught in chronic pain programmes, or pain-contingent pacing, which may be maladaptive.4,34 The internal consistency and validity of the confronting and avoidance subscales of the questionnaire have been shown to be adequate based on initial psychometric testing.29 Validity was supported using factor analysis and examination of the correlations between the scales and measures physical activity (ie, avoidance subscale of the Pain Anxiety Symptoms Scale, patients' estimated average daily uptime, physical disability composite score from the Sickness Impact Profile). Internal consistency ratings for these scales in this study were 0.82 (confronting) and 0.82 (avoidance).
2.3.3. Pain
Participants' pain intensity rating was measured using an electronic questionnaire. Using a Palm Pilot handheld computer (m100, Zire and Tungsten series), participants responded to a 10-point horizontal pain visual analogue scale at random intervals 6 times a day during waking hours, over the 5-day data collection period. The scale was anchored by 0 (no pain) and 10 (severe pain). The single-item visual analogue scale for pain intensity has been shown to have adequate validity (see review, Ref. 21). The electronic questionnaire was developed by the researchers using the Experience Sampling Program,8 which is an open-source software package for running questionnaires on a Palm Pilot. The Palm Pilot displays questions, receives responses, and records reaction times. The programme was configured to alert participants to indicate how much pain they were in directly before the computer prompt. Participants' mean pain scores over the 5 days were calculated to provide a measure of “average pain intensity.” The SD of the pain scores provided a measure of “pain variation.” The SD of a variable is considered a robust and widely used measure of variation because, unlike the range and interquartile range, it takes into account every value in an individual's data set.13
2.3.4. Objective physical activity
The GT3X ActiGraph activity monitor was chosen to objectively measure daytime physical activity. Participants were required to wear the activity monitor during waking hours and to remove it only for showering and swimming over the 5-day data collection period. The GT3X ActiGraph incorporates a triaxial accelerometer that collects changes in acceleration 30 times each second across 3 axes (vertical, horizontal, and perpendicular).1 The device translates this movement into a digital code that is stored in computerised form.1 In this study, activity counts per minute were recorded for each axis. This equates to the accumulation of filtered changes in acceleration measured during a 60-second period.1 The vector magnitude per minute (calculation of the magnitude of the vector that forms when combining activity counts per minute from all 3 axes) was then used to calculate physical activity variables. The vector magnitude per minute can be interpreted as the intensity of physical activity performed over the course of a minute.1
Three activity variables were calculated and used in analyses: mean objective activity, activity variation across days, and mean activity fluctuation within a day. “Mean objective activity” was calculated by first finding the average vector magnitude per minute between the time participants got out of bed and when they went to bed on a given day, as indicated in their diary. The mean of these average values for each participant then provided a measure of mean objective activity over the 5 days of data collection. Higher levels of mean objective activity indicated engagement in higher intensity activities over the 5-day data collection period.
The SD of the average vector magnitude per minute values for each participant provided a measure of “activity variation across days.” The SD was once again chosen to provide a measure of variation because, unlike the range and interquartile range, it takes into account every value in an individual's data set. Activity variation across days can be interpreted as the degree to which an individual's activity on a given day differs from their mean or “typical” physical activity level with higher scores indicating a greater variation in activity across the 5 days.
To calculate “mean activity fluctuation within a day,” an activity fluctuation value for each day of data collection was obtained by adding the vector magnitude per minute over 15-minute periods from the time participants got out of bed to the time they went to bed on a given day. Next, the difference between these summed 15-minute periods was found by subtracting each summed 15-minute time period from the 15-minute time period directly before it. The root mean square of these difference values was then calculated to express the magnitude of these differences, and the mean activity fluctuation value for each participant was then calculated. This method of calculating fluctuations in physical activity was chosen because it has been used in previous studies.5,23,25 Higher values indicate greater fluctuation in activity levels within a day over the 5-day data collection period whereby periods of low-intensity movement are directly followed by periods of high-intensity movement or vice versa.
Actigraph devices have been shown to provide a valid measure of physical activity, with the data from the device shown to (1) be effective in differentiating between various physical and sedentary activities in healthy adults and (2) correlate significantly with oxygen uptake and heart rate.36 A study investigating the feasibility of actigraphy in home-based settings found that it is easily used and well tolerated by participants.35 Two types of accelerometers are commonly used in research: uniaxial and triaxial. A uniaxial accelerometer measures movement in only 1 dimension and is therefore likely to detect less movement when compared with a triaxial accelerometer that measures movement in 3 dimensions.44 While some studies have shown strong reliability between triaxial and uniaxial accelerometers in the measurement of physical activity,27,44 other studies have shown that a triaxial accelerometer is more precise than a uniaxial accelerometer in the assessment of physical activity.14,38 Thus, a triaxial accelerometer was used in this study, with vector magnitudes (ie, the triaxial measurement) favoured over activity counts (ie, the uniaxial measurement) as a measure of objective physical activity.
2.3.5. Self-reported activity
Participants detailed the activities in which they engaged in throughout the day over the 5-day collection period in a paper diary. Participants were instructed to fill in this diary as often as they could throughout the day to ensure its accuracy. They also received a written prompt to fill in the diary 6 times a day through the screen of their handheld computer after they had entered their pain score. Information from this diary was used to assist with determining objective periods of overactivity as described below.
2.3.6. Objective period of overactivity
Recently, Andrews et al.6 outlined a way to incorporate objective measures of physical activity to measure overactivity behaviour in observational studies. They suggested that, as overactivity (operationalised by Fordyce's operant model of chronic pain) implies engagement in “excessive” amounts of physical activity that significantly exacerbates pain,16,37 this could be determined by observing physical activity levels that are a “certain level” above a person's average activity level, which is then followed by an increase in pain that escalates to “a point” that is above an individual's average pain intensity. To examine this, participants' pain scores and objective activity values (vector magnitudes per minute) were first converted into z-scores. As pain scores were obtained at random intervals throughout the day, cubic splines were used to interpolate these data to create a pain score for every minute of the 5-day sampling period. Cubic spline is a process that fits a series of unique cubic polynomials between each of the data points to form a curve that is continuous and appears smooth.30 SRS1 Cubic Spline for Excel39 was used to interpolate pain data. Periods of significant increases in pain were then identified. This was classified as an increase in pain that escalated to a z-score value of 1.65 or higher (ie, a pain value that is in the top 5% of all possible values given pain is normally distributed). The objective activity data in the 2 hours before this significant pain increase were then examined to identify periods of high activity. A period of high activity was classified as objective activity z-scores that were consistently above zero for at least 10 minutes. An example of this is shown in Figure 1.
Figure 1.
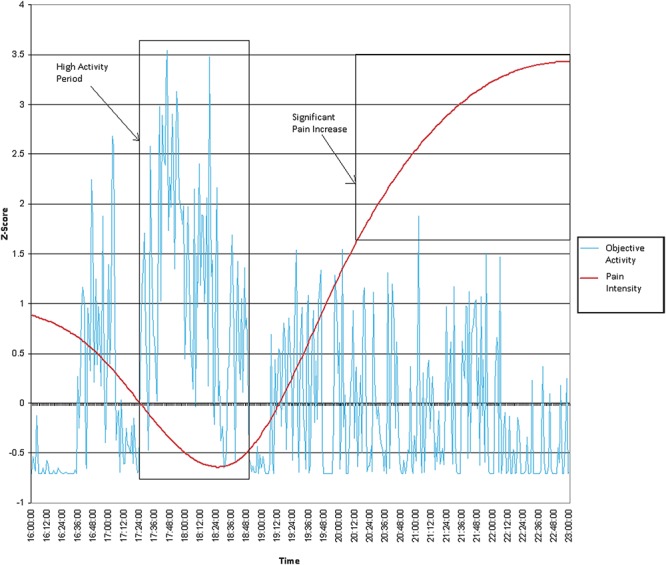
An example of a period of high activity.
The mean z-score for these high activity periods was then examined. An individual high activity period was labelled as a period of objective overactivity if the mean z-score was 1.65 or higher for time periods under an hour (ie, activity values that averaged in the top 5% of all possible values given activity is normally distributed) or the mean z-score was 1.28 or higher for time periods over an hour (ie, activity values over a period of time longer than an hour that averaged in the top 10% of all possible values given activity is normally distributed). In clinical settings, individuals with chronic pain often report exacerbating their pain by spending too long on sedentary activities that require a sustained spinal position, and this is accepted by clinicians as being a form of overactivity.10,33 As such, participants' diary entries were also examined to determine whether periods of significant increases in pain could be attributed to prolonged periods of time spent on sedentary activities. A period of objective overactivity was labelled if participants had spent longer than 1 hour on a sedentary activity that required a sustained spinal position in the 2 hours before the pain increase. The first author (N.E.A.) determined whether activities before a pain increase were both sedentary and required a sustained spinal posture. The third author (P.J.M.) then checked this coding. P. J. Meredith is a senior lecturer in occupational therapy, and N. E. Andrews is a senior occupational therapist with over 5 years of clinical experience. Sedentary activities that were determined to result in a significant pain increase in this study included folding newsletters, sitting at a computer, travelling in a car, filling in paperwork, and ironing. Thus, for the purpose of this study, the occurrence of an objective period of overactivity can be interpreted as a prolonged period of activity engagement followed by a significant increase in pain.
A recent qualitative study found that some individuals who have an optimal approach to activity engagement (ie, they pace activity effectively and have low levels of activity avoidance and low levels of overactivity), still, on occasion, spend prolonged periods of time on certain activities and aggravate their pain.7 What distinguishes habitually overactive individuals from these individuals is that they aggravate their pain frequently by engaging in high levels of physical activity or spending prolonged periods of time on sedentary activities, which results in large fluctuations in pain and activity.7 This is reflected in our third hypothesis (ie, patterns of prolonged activity engagement followed by significant increases in pain would be observed more often in the data of individuals who self-report high levels of overactivity). To investigate this hypothesis, 2 categorical variables were then created to be used in analyses: (1) whether or not a period of objective overactivity was observed in the participant's data (labelled “occurrence of an objective period of overactivity”) and (2) whether or not a period of objective activity was repeatedly observed (ie, occurred more than once) in the participant's data (labelled “reoccurrence of an objective period of overactivity”).
2.4. Statistical analysis
The Statistical Package for Social Sciences (SPSS) GradPack version 18.0 (SPSS Inc, Chicago, IL) was used to analyse the results of this study. Variables were initially assessed for normality and outliers. The overactivity variable was negatively skewed and activity fluctuation across days was positively skewed. Box–Cox transformation, a procedure that identifies the most appropriate exponent to use to transform data into a normal shape, was used to transform skewed variables. The data were also assessed to identify missing data patterns. If participants did not have at least 4 complete days of diary entries, objective activity, or pain data (ie, 80%) the variables that related to these measurements was classified as missing. The amount of data missing for each variable is presented in Table 2. As illustrated in Table 2, missing data were minimal (maximum 3 participants for any 1 variable) and, upon inspection, there was no observable pattern. As such, missing data resulted in exclusion of that case from analyses.
The association between individuals' self-reported habitual approach to activity engagement and measures of pain and objective physical activity were examined through a series of linear regression and analysis of covariance (ANCOVA) models. Linear regression analyses were first conducted to examine additive and possible interaction effects of continuous measures of overactivity and avoidance on pain and objective activity. Overactivity and avoidance was first centred before creating the interaction term. Centring reduces multicollinearity among predictor variables and makes regression coefficients more meaningful (ie, the intercept reflects the value for average scores of the dependent variables as opposed to a score of zero for these variables).2 As age and gender have been shown to (1) impact on pain perception,31,45 and (2) explain a large amount of variability in objectively measured physical activity in healthy populations,18,42 both age and gender were entered as covariates in the models. One linear regression model was produced for each of the dependent variables (ie, average pain intensity, pain variation, mean objective activity, activity variation across days, mean activity fluctuation within a day) with age, gender, overactivity, avoidance, and the interaction term entered as independent variables. If an interaction term was not significant it was then removed from the model to allow for the interpretation of main effects.
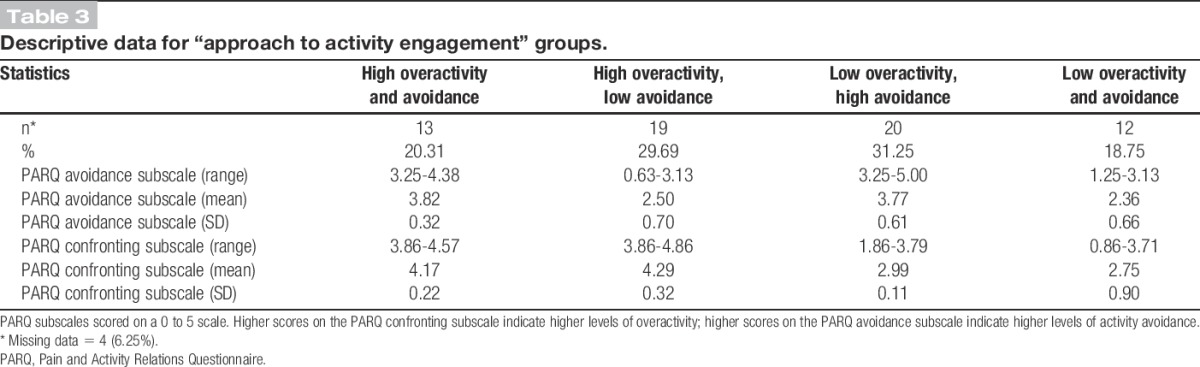
To provide further insight into nature of the relationship between overactivity and dependent variables, ANCOVA models were also produced to illustrate differences between 4 different “approach to activity engagement” subgroups. “Approach to activity engagement” subgroups were calculated by placing participants into 1 of 4 categories using a median split (those high in overactivity and avoidance; those high in avoidance but low in overactivity; those high in overactivity but low in avoidance; and those low in both overactivity and avoidance). The median value for overactivity and avoidance was classified as high when categorising participants. Descriptive data for approach to activity categories are presented in Table 3. One model was again produced for each of the dependent variables, and age and gender were controlled for. The “low overactivity, low avoidance” subgroup was chosen as the reference category for statistical comparisons in the models and coded accordingly. Cohen's d was calculated for each comparison to provide an effect size index. This was done by dividing each mean difference (B) by the square root of the mean square error from the ANCOVA model.22 A Levene test was performed for each ANCOVA model to test for homogeneity of variances. Residuals were also saved and checked for normality and homoscedasticity for all linear regression and ANCOVA models.
Table 3.
Descriptive data for “approach to activity engagement” groups.
A χ2 test was conducted to determine whether an individual's “approach to activity engagement” category was related to whether or not the occurrence of an objective period of overactivity (ie, a prolonged period of activity engagement followed by a significant increase in pain) was observed in participants' data. The reoccurrence of an objective period of overactivity in participants' data was rare, resulting in less than 80% of cells having an expected frequency of 5 or greater. As such, a Fisher exact test was conducted to explore whether or not the distribution of the reoccurrence of an objective period of overactivity significantly differed across “approach to activity engagement” categories. As Fisher exact test for 2 × 4 contingency tables is not available on standard SPSS packages, VassarStats online Fisher exact probability test, 2 × 426 was used for this analysis.
The final sample size in this study was determined by pragmatics. Based on a priori power calculations using G*Power,15 the study had adequate power (over 0.80) to detect large effect sizes at a significance level of 0.05 in the statistic test used. The study is, however, slightly underpowered in terms of the ability to detect conventional medium effect sizes at a significance level of 0.05 in some statistical test (eg, 0.68 for linear regression and 0.67 for χ2 tests). Because of this, effect size indices, point estimates, and precision estimates are reported for all associations tested using regression, and ANCOVA modelling and effect sizes are commented on to facilitate the interpretation of results. A significance level of 0.05 was set for statistical tests, and Cohen's12 cutoff points for small, medium, and large effect sizes were used when interpreting results. As recommended by Streiner and Norman,40 the P value (0.05) was not adjusted to account for multiple analyses due to the exploratory nature of this study.
3. Results
Results are presented in Tables 4-6 and detailed in the text below. Tables 4 and 5 include both the standardised and unstandardised regression coefficients, as well as the 95% confidence intervals for all parameters in regression models. Table 6 reports the 95% confidence intervals, Cohen's d, and the point estimates for mean differences between “approach to activity engagement” subgroups after adjusting for the effects of age and gender. Significant associations are indicated in these tables. The text below provides more detailed statistics for the significant and nonsignificant associations that relate to the study hypotheses including t values, degrees of freedom, and specific P values. Results for the χ2 and Fisher exact tests are also detailed in the text below. All regression and ANCOVA models met normality and homoscedasticity assumptions. However, the Levene test for equality of variance was statically significant for the pain variation ANCOVA model, suggesting that this model violates homogeneity of variance assumptions. Thus, this model is not valid and was not interpreted.
Table 4.
Results of linear regression analyses examining the effects of continuous measures of overactivity and avoidance on pain.
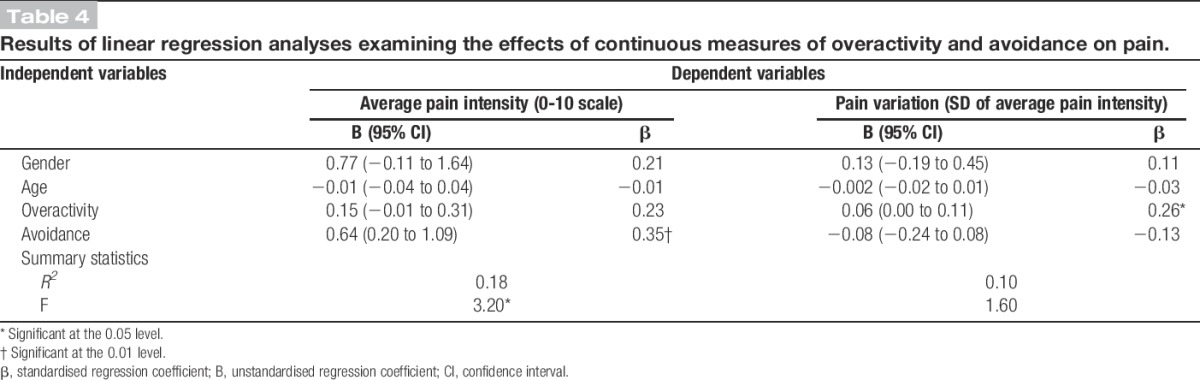
Table 6.
Means differences between “approach to activity engagement” subgroups after adjusting for the effects of age and gender.
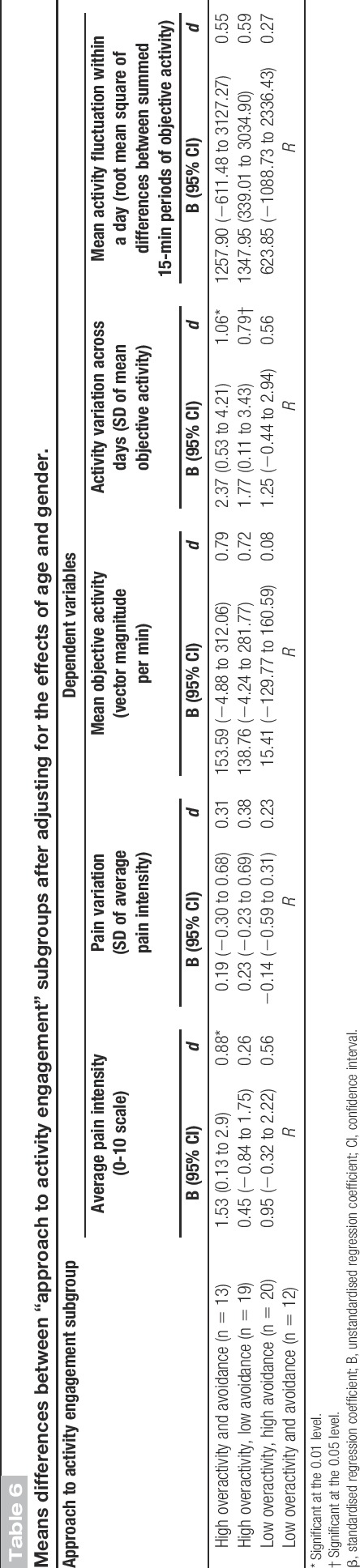
Table 5.
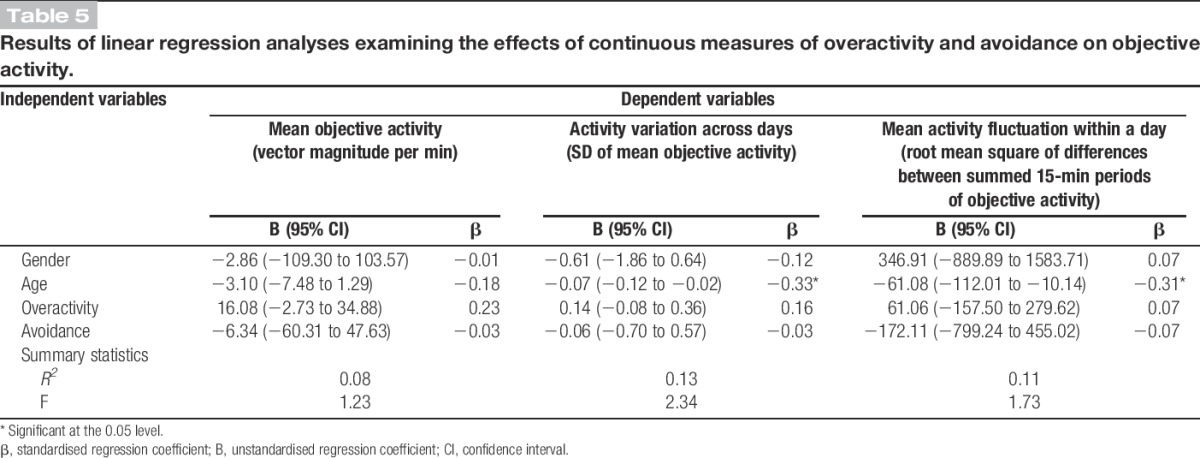
Results of linear regression analyses examining the effects of continuous measures of overactivity and avoidance on objective activity.
3.1. The association between self-reported habitual approach to activity engagement and measures of pain
The interaction between avoidance and overactivity was not significant in any of the linear regression models and was removed to allow for the interpretation of main effects. Higher levels of avoidance were associated with more intense pain, on average, over the 5 days of data collection (β [avoidance] = 0.35; t(62) = 2.88; P = 0.01), but were not associated with pain variation (β [avoidance] = −0.13; t(62) = −1.00; P = 0.32). Higher levels of overactivity were associated with more pain variation (β [overactivity] = 0.26; t(62) = 2.00; P = 0.05), and a small- to medium-sized positive association was found between overactivity and average pain that failed to reach significance (β [overactivity] = 0.23, t(62) = 1.93, P = 0.06). Gender and age did not make a significant contribution to the prediction of pain variables. ANCOVA modelling revealed that individuals reporting high levels of overactivity and avoidance had higher average pain intensity ratings compared with individuals reporting low levels of overactivity and avoidance (d [high overactivity and avoidance] = 0.88; t(57) = 2.19; P = 0.03). Both the “high overactivity, low avoidance” and “low overactivity, high avoidance” subgroups had higher average pain intensity ratings compared with the “low overactivity, low avoidance” subgroup; however, these differences were not statistically significant (d [high overactivity, low avoidance] = 0.26; t(57) = 0.70; P = 0.49 and d [low overactivity, high avoidance] = 0.56; t(57) = 1.50; P = 0.14). The pain variation ANCOVA model was determined to be not valid, and hence the results are not detailed for this model.
3.2. The association between self-reported habitual approach to activity engagement and measures of objective activity
The interaction between avoidance and overactivity was not significant in any of the linear regression models and was hence removed. A positive small- to medium-sized association was found between overactivity and mean objective activity that failed to reach significance (β [overactivity] = 0.23; t(60) = 1.71; P = 0.09). No relationship was found between avoidance and mean objective activity levels (β [avoidance] = −0.03; t(60) = −0.24; P = 0.82). ANCOVA modelling revealed that both the “high overactivity and avoidance” and “high overactivity, low avoidance” subgroups had higher mean objective activity levels compared with the “low overactivity and avoidance” subgroup. However, the confidence intervals for these estimates were relatively wide, and these moderate–large differences failed to reach statistical significance (d [high overactivity and avoidance] = 0.79; t(55) = 1.94; P = 0.06 and d [high overactivity, low avoidance] = 0.72; t(55) = 1.95; P = 0.06).
The continuous measure of overactivity was not associated with the 2 activity variation variables: activity variation across days (β [overactivity] = 0.16, t(60) = 1.24, P = 0.22) and mean activity fluctuations (β [overactivity] = 0.07, t(60) = 0.56, P = 0.58). Age was the only variable to make a significant contribution in these models: activity variation across days (β [age] = −0.33, t(60) = −2.67, P = 0.01) and mean activity fluctuation within a day (β [age] = −0.31, t(60) = −2.40, P = 0.02). However, ANCOVA modelling revealed that individuals reporting low levels of overactivity and avoidance had less fluctuation in their mean objective activity across days compared with the 2 subgroups reporting high levels of overactivity: “high overactivity and avoidance” (d [high overactivity and avoidance] = 1.06; t(55) = 2.58; P = 0.01) and “high overactivity and low avoidance” (d [high overactivity, low avoidance] = 0.79; t(55) = 2.13; P = 0.04). There were no significant differences between groups for the mean activity fluctuation model. It should be noted that the SD for mean activity fluctuation was large and the confidence intervals for the activity fluctuation model were relatively wide.
3.3. The association between self-reported habitual approach to activity engagement and the occurrence of a period of objective overactivity
An objective period of overactivity was observed 26 times across all participants. Twelve of these observations related to a period of high objective activity directly followed by a significant increase in pain. The remaining 14 observations related to prolonged sedentary task engagement that required a sustained spinal posture, which directly preceded a significant pain exacerbation. These 26 observations were found in the data of 18 participants. These 18 participants were relatively evenly distributed across “approach to activity engagement” categories and closely matched expected counts. The χ2 test confirmed that there was no relationship between the occurrence of an objective period of overactivity in participants' data and their “approach to activity engagement”category (χ2(3,N = 61) = 0.42, P = 0.94). The reoccurrence of a period of objective activity was observed in the data of 6 participants. Five of these 6 participants reported a combination of high levels of overactivity and low levels of avoidance. The Fisher exact test revealed that the relationship between the reoccurrence of an objective period of overactivity in participant's data and an individual's “approach to activity engagement” category was significant (P = 0.03), with individuals reporting high levels of overactivity but low levels of avoidance more likely to have a period of objective overactivity repeatedly observed in their data.
4. Discussion
This study used a 5-day observational study design to examine the construct validity of a self-report measure of overactivity by investigating the relationship between an individual's self-reported habitual approach to activity engagement and patterns of pain and objective physical activity over a 5-day study period. The results provided some support for our first hypothesis that higher levels of both overactivity and avoidance would be associated with higher levels of pain averaged over the 5 days. Group comparisons revealed that individuals reporting a combination of high levels of overactivity and avoidance had the highest average pain intensity ratings of all subgroups over the 5-day period and the difference between this subgroup and the “low overactivity and avoidance” reference group was statistically significant. This finding supports hypothesis 1 and is consistent with that of a previous study.24
As individuals who habitually engage in overactivity behaviour are thought to have a yo-yo activity and pain pattern, the second hypothesis was that individuals reporting high levels of overactivity would have a larger variation in their pain and objective physical activity irrespective of their level of activity avoidance. The results of this study provided support for an association between high levels of overactivity and more pain variation. A significant positive association was found between the continuous measure of overactivity and pain variation, while the continuous measure of avoidance and the interaction between overactivity and avoidance were not significantly associated with pain variation in the same model. This suggests that higher levels of overactivity were associated with more pain variation (ie, a larger difference in pain intensity ratings over the course of 5 days), and this was not affected by an individual's level of avoidance, supporting hypothesis 2.
Results relating to the association between overactivity and objective activity variation were, however, mixed. While the continuous measure of overactivity was not significantly associated with activity variation across days, the 2 subgroups reporting high levels of overactivity (ie, “high overactivity and avoidance” and “high overactivity, low avoidance”) had significantly more variation in their mean objective activity across days compared with individuals reporting low levels of overactivity and avoidance. An examination of the scatter plot of the continuous measure of overactivity and activity variation across days suggests that there is a threshold effect as opposed to the relationship being linear (ie, there is a certain point on the overactivity scale that results in larger values for activity variation as opposed to values gradually getting larger with increases in overactivity), which explains the observed associations. This should be taken into consideration in future studies of this nature.
Additionally, there was no significant association found between overactivity and activity fluctuation within a day. This complements the results of a previous study.24 It may be that individuals who are habitually overactive only display a large fluctuation in their activity within a day (ie, periods of low-intensity movement directly followed by periods of high-intensity movement or vice versa) on the day when their pain significantly increases or when they recommence activity after the significant pain increase. This could explain the small nonsignificant associations observed. A measure of activity fluctuation within a day may be more useful in validating the occurrence of a period of overactivity in within-person study designs as opposed to cross-sectional comparisons.
There were no significant associations found between either overactivity or avoidance and mean objective activity levels. Andrews et al.6 have previously suggested that mean objective activity levels may not be a good indicator of activity avoidance or overactivity in cross-sectional comparisons due to the large variation in this variable in healthy populations. This study did, however, link overactivity to predictable patterns of activity and pain as per our third hypothesis. Individuals who reported high levels of overactivity but low levels of avoidance were significantly more likely to have a pattern of prolonged activity engagement (ie, either a period of high-intensity activity or an extended period of time spent on sedentary activities that required a sustained spinal posture), followed by significant increases in pain, observed more than once in their data. This result provides support for hypothesis 3 and the idea that individuals who reported high levels of overactivity but low levels of avoidance are more likely to frequently engage in activity in a way that significantly exacerbated pain, as opposed to these individuals inaccurately attributing the cause of pain exacerbations to their activity levels. It should be noted, however, that the incidence of a reoccurrence of a pattern of prolonged activity engagement followed by a significant increase in pain was very low across data sets, which does complicate the interpretation of this result. Future studies of this nature may consider collecting data over a longer period or use less conservative cutoff points to address this issue.
This study also provided some insight into individuals who simultaneously report high levels of overactivity and avoidance. A combination of high levels of overactivity and high avoidance is thought to result when individuals who are initially overactive after pain onset (ie, those who report high levels of overactivity but low levels of avoidance) avoid certain pain-provoking activities over time as pain exacerbations, secondary to overactivity behaviour, become progressively more severe.33,37 In this study, the subgroup of individuals who reported high levels of avoidance and high overactivity displayed the features of people who are overactive (ie, larger variations in pain and objective physical activity) and also reported significantly higher levels of pain. These results support the notion that a combination of high overactivity and high avoidance may be the outcome of “high overactivity, low avoidance” where increased pain that has developed over time contributes to increased levels of avoidance. The reoccurrence of prolonged periods of activity engagement followed by significant increases in pain was, however, not observed across the data of this subgroup. It is unclear why these individuals displayed more pain and objective activity variation but not predictable patterns of activity and pain using cutoff points. One possible explanation is that the pain exacerbations associated with this “high overactivity and avoidance” group may be linked to certain activities that these individuals have decreased due to avoidance behaviour. These pain-aggravating activities may not be as easily identified using cutoffs for time and objective activity across data sets. An investigation of the nature of pain exacerbations in this group of individuals is an avenue for future research.
The results of this study should be interpreted cautiously. While some evidence was found to support each hypothesis, expected associations were not always significant, and there were inconsistencies across the analyses using continuous measures of overactivity and avoidance vs the categorical “approach to activity engagement” variable. The study was slightly underpowered, which increases the chances of results that may be clinically relevant failing to reach statistical significance. The SDs for objective activity variables were relatively large, and the confidence intervals in some of the objective activity models were wide. Although age and gender were controlled for, a number of additional variables are known to impact on physical activity levels in healthy populations.6 Additional studies may consider using a larger sample size and controlling for additional variables to increase confidence in the effect sizes observed.
In addition, social desirability responding was possible due to the self-report nature of some measures, and the cutoff points used to categorise approach to activity engagement and determine an objective period of overactivity were somewhat arbitrary. Finally, the number of statistical tests conducted increases the chance of making a type I error. A priori hypotheses were stated to address the issue of alpha inflation; however, the results require replication.40
Despite these limitations, this is the first known study to examine the construct validity of a self-report measure of overactivity by comprehensively investigating the relationship between an individual's self-reported habitual approach to activity engagement and patterns of pain and objective physical activity. With some support found for each hypothesis, the results of this study offer some preliminary support for the validity of overactivity as a construct. This is important to know, as overactivity is the target of a highly endorsed treatment strategy taught in pain management programmes around the world (ie, activity pacing).9,41 Murphy et al.32 have previously used activity variation across days as an outcome measure for a tailored activity pacing intervention. The results of this study support the use of patterns of objective activity and pain as outcome measures in studies investigating the effectiveness of activity pacing for individuals who are habitually overactive. Longitudinal research designs investigating the associations considered in this study would be useful, particularly in increasing our understanding of how a combination of overactivity and avoidance develops. Additional recommendations for future research include (1) the replication of this study using larger more representative samples and (2) the examination of optimal cutoff points for the procedures used in this study, to increase confidence in the associations observed in this study and to improve our understanding of overactivity as a construct.
Conflict of interest statement
The authors have no conflicts of interest to declare.
The equipment used in the study was funded by The Professor Tess Cramond Multidisciplinary Pain Centre.
N. E. Andrews was supported by a Royal Brisbane and Women's Hospital Foundation Scholarship, an Occupational Therapy Board of Queensland Novice Researcher Grant, and the Cramond Fellowship in Occupational Therapy and Pain Management at The Royal Brisbane and Women's Hospital.
Acknowledgements
The authors thank the staff and patients of The Professor Tess Cramond Multidisciplinary Pain Centre for their contribution to data collection and Dr Asad Khan, The University of Queensland, for his assistance with the statistical processes of some parts of this research.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
URL: http://www.shrs.uq.edu.au/ (N. E. Andrews).
References
- [1].Actigraph. ActiLife 6 manual. Available at: http://www.actigraphcorp.com/support/downloads/#manuals. Accessed March 6, 2013. [Google Scholar]
- [2].Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Thousand Oaks: Sage Publications, 1991. [Google Scholar]
- [3].Andrews NE, Meredith PJ, Strong J, Donohue GF. Adult attachment and approaches to activity engagement in chronic pain. Pain Res Manag 2014;19:317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Andrews NE, Strong J, Meredith PJ. Activity pacing, avoidance, endurance, and associations with patient functioning in chronic pain: a systematic review and meta-analysis. Arch Phys Med Rehabil 2012;93:2109–21.e2107. [DOI] [PubMed] [Google Scholar]
- [5].Andrews NE, Strong J, Meredith PJ, D'Arrigo RG. Association between physical activity and sleep in adults with chronic pain: a momentary, within-person perspective. Phys Ther 2014;94:499–510. [DOI] [PubMed] [Google Scholar]
- [6].Andrews NE, Strong J, Meredith PJ. Avoidance or incapacitation: a discussion on the definition and validity of objective measures of avoidance, persistence, and overactivity. Clin J Pain 2015;31:670–2. [DOI] [PubMed] [Google Scholar]
- [7].Andrews NE, Strong J, Meredith PJ, Gordon K, Bagraith KS. “It's very hard to change yourself”: an exploration of overactivity in people with chronic pain using interpretative phenomenological analysis. PAIN 2015;156:1215–31. [DOI] [PubMed] [Google Scholar]
- [8].Barrett L, Barrett D. Experience Sampling Program version 4.0. 2006. Available at: http://www.experience-sampling.org. Accessed July 10, 2010.
- [9].Brown CA. Occupational therapists' beliefs regarding treatment options for people with chronic pain. Br J Occu Ther 2002;65:398–404. [Google Scholar]
- [10].Butler D, Moseley G. Explain pain. Adelaide: Noigroup Publications, 2013. [Google Scholar]
- [11].Cane D, Nielson WR, McCarthy M, Mazmanian D. Pain-related activity patterns: measurement, interrelationships, and associations with psychosocial functioning. Clin J Pain 2013;29:435–42. [DOI] [PubMed] [Google Scholar]
- [12].Cohen J. A power primer. Psychol Bull 1992;112:155–9. [DOI] [PubMed] [Google Scholar]
- [13].Diekhoff G. Statistics for the social and behavioral sciences: univariate, bivariate, multivariate. Dubuque: Wm. C. Brown Publishers, 1992. [Google Scholar]
- [14].Eston RG, Rowlands AV, Ingledew DK. Validity of heart rate, pedometry, and accelerometry for predicting the energy cost of children's activities. J Appl Physiol 1998;84:362–71. [DOI] [PubMed] [Google Scholar]
- [15].Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- [16].Fordyce WE. Behavioral methods for chronic pain and illness. Saint Louis: The C.V. Mosby Company, 1976. [Google Scholar]
- [17].Gatzounis R. Operant learning theory in pain and chronic pain rehabilitation. Curr Pain Headache Rep 2012;16:117–26. [DOI] [PubMed] [Google Scholar]
- [18].Hagstromer M, Oja P, Sjostrom M. Physical activity and inactivity in an adult population assessed by accelerometry. Med Sci Sports Exerc 2007;39:1502–8. [DOI] [PubMed] [Google Scholar]
- [19].Hanson R, Gerber K. Coping with chronic pain: a guide to patient self management. New York: Guilford Press, 1990. [Google Scholar]
- [20].Hasenbring MI, Plaas H, Fischbein B, Willburger R. The relationship between activity and pain in patients 6 months after lumbar disc surgery: do pain-related coping modes act as moderator variables? Eur J Pain 2006;10:701–9. [DOI] [PubMed] [Google Scholar]
- [21].Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain. Arthritis Care Res 2011;63:S240–52. [DOI] [PubMed] [Google Scholar]
- [22].Horn RA. Understanding analysis of covariance (ANCOVA). Available at: http://oak.ucc.nau.edu/rh232/courses/eps625/handouts/ancova/understanding%20ancova.pdf. Accessed April 27, 2015.
- [23].Huijnen IPJ, Kindermans HPJ, Seelen HAM, Peters ML, Smeets RJEM, Serroyen J, Roelofs J, Goossens M, Verbunt JA. Effects of self-discrepancies on activity-related behaviour: explaining disability and quality of life in patients with chronic low back pain. PAIN 2011;152:2165–72. [DOI] [PubMed] [Google Scholar]
- [24].Huijnen IPJ, Verbunt JA, Peters ML, Smeets RJEM, Kindermans HPJ, Roelofs J, Goossens M, Seelen HAM. Differences in activity-related behaviour among patients with chronic low back pain. Eur J Pain 2011;15:748–55. [DOI] [PubMed] [Google Scholar]
- [25].Huijnen IPJ, Verbunt JA, Roelofs J, Goossens M, Peters M. The disabling role of fluctuations in physical activity in patients with chronic low back pain. Eur J Pain 2009;13:1076–9. [DOI] [PubMed] [Google Scholar]
- [26].Lowry R. VassarStats' Fisher exact probability test: 2 × 4. Available at: http://vassarstats.net/fisher2x4.html. Accessed October 28, 2014.
- [27].Macfarlane DJ, Lee CCY, Ho EYK, Chan KL, Chan D. Convergent validity of six methods to assess physical activity in daily life. J Appl Physiol 2006;101:1328–34. [DOI] [PubMed] [Google Scholar]
- [28].Main CJ, Keefe FJ, Jensen MP, Vlaeyen JWS, Vowles KE. Fordyce's behavioral methods for chronic pain and illness. Philadelphia: Lippincott Williams and Wilkins, 2014. [Google Scholar]
- [29].McCracken LM, Samuel VM. The role of avoidance, pacing, and other activity patterns in chronic pain. PAIN 2007;130:119–25. [DOI] [PubMed] [Google Scholar]
- [30].McKinley S, Levine M. Cubic spline interpolation. Available at: http://online.redwoods.cc.ca.us/instruct/darnold/laproj/Fall98/SkyMeg/Proj.PDF. Accessed October 21, 2014.
- [31].Molton IR, Terrill AL. Overview of persistent pain in older adults. Am Psychol 2014;69:197–207. [DOI] [PubMed] [Google Scholar]
- [32].Murphy SL, Smith DM, Lyden AK. Type of activity pacing instruction affects physical activity variability in adults with symptomatic knee or hip osteoarthritis. J Phys Act Health 2012;9:360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nicholas M, Molloy A, Tonkin L, Beeston L. Manage your pain. Sydney: ABC Books, 2011. [Google Scholar]
- [34].Nielson WRP, Jensen MPP, Karsdorp PAP, Vlaeyen JWSP. Activity pacing in chronic pain: concepts, evidence, and future directions. Clin J Pain 2013;29:461–8. [DOI] [PubMed] [Google Scholar]
- [35].Noor ZM, Smith AJ, Smith SS, Nissen L. Feasibility and acceptability of wrist actigraph in assessing sleep quality and sleep quantity: a home-based pilot study in healthy volunteers. Health 2013;05:63–72. [Google Scholar]
- [36].Patterson SM, Krantz DS, Montgomery LC, Deuster PA, Hedges SM, Nebel LE. Automated physical activity monitoring: validation and comparison with physiological and self-report measures. Psychophysiol 1993;30:296–305. [DOI] [PubMed] [Google Scholar]
- [37].Philips C. The psychological management of chronic pain. New York: Springer, 1988. [Google Scholar]
- [38].Plasqui G, Joosen AMCP, Kester AD, Goris AHC, Westerterp KR. Measuring free-living energy expenditure and physical activity with triaxial accelerometry. Obes Res 2005;13:1363–9. [DOI] [PubMed] [Google Scholar]
- [39].SRS1 Software LLC. SRS1 Cubic Spline for Excel. Available from: http://www.srs1software.com/SRS1CubicSplineForExcel.aspx. Accessed October 1, 2014.
- [40].Streiner DL, Norman GR. Correction for multiple testing: is there a resolution? Chest 2011;140:16–18. [DOI] [PubMed] [Google Scholar]
- [41].Torrance N, Smith BH, Elliott AM, Campbell SE, Chambers WA, Hannaford PC, Johnston M. Potential pain management programmes in primary care. A UK-wide questionnaire and Delphi survey of experts. Fam Pract 2011;28:41–8. [DOI] [PubMed] [Google Scholar]
- [42].Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40:181–8. [DOI] [PubMed] [Google Scholar]
- [43].Van Damme S, Kindermans H. A self-regulation perspective on avoidance and persistence behavior in chronic pain: new theories, new challenges? Clin J Pain 2015;31:115–22. [DOI] [PubMed] [Google Scholar]
- [44].Vanhelst J, Béghin L, Duhamel A, Bergman P, Sjöström M, Gottrand F. Comparison of uniaxial and triaxial accelerometry in the assessment of physical activity among adolescents under free-living conditions: the HELENA study. BMC Med Res Methodol 2012;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med 2005;2:137–45. [DOI] [PubMed] [Google Scholar]


