Patients suffering from persistent pain after endodontic treatment have less efficient pain modulation system compared with healthy controls.
Keywords: Posttraumatic trigeminal neuropathy, Neuropathic pain, Pain modulation, Temporal summation, Conditioned pain modulation, Postendodontic pain, Orofacial pain
Abstract
Persistent pain may follow nerve injuries associated with invasive therapeutic interventions. About 3% to 7% of the patients remain with chronic pain after endodontic treatment, and these are described as suffering from painful posttraumatic trigeminal neuropathy (PTTN). Unfortunately, we are unable to identify which patients undergoing such procedures are at increased risk of developing PTTN. Recent findings suggest that impaired endogenous analgesia may be associated with the development of postsurgical chronic pain. We hypothesized that patients with PTTN display pronociceptive pain modulation, in line with other chronic pain disorders. Dynamic (conditioned pain modulation, temporal summation) and static (response to mechanical and cold stimulation) psychophysical tests were performed intraorally and in the forearm of 27 patients with PTTN and 27 sex- and age-matched controls. The dynamic sensory testing demonstrated less efficient conditioned pain modulation, suggesting reduced function of the inhibitory endogenous pain-modulatory system, in patients with PTTN, mainly in those suffering from the condition for more than a year. The static sensory testing of patients with PTTN demonstrated forearm hyperalgesia to mechanical stimulation mainly in patients suffering from the condition for less than a year and prolonged painful sensation after intraoral cold stimulus mainly in patients suffering from the condition for more than a year. These findings suggest that PTTN is associated more with the inhibitory rather than the facilitatory arm of pain modulation and that the central nervous system has a role in PTTN pathophysiology, possibly in a time-dependent fashion.
1. Introduction
Persistent pain due to trigeminal nerve injury has been approached under a number of names as atypical odontalgia,32 “phantom tooth pain,”28 or atypical facial pain.10,34 Recently, the term “painful posttraumatic trigeminal neuropathy” (PTTN)7 has been adopted by the International Headache Society.36
Painful posttraumatic trigeminal neuropathy is characterized by moderate-to-severe continuous burning pain in an area that has a history of trauma. Rarely, the pain may cross the midline, spread to other areas of the face,27,28,39,52 or affect more than 1 site.27,30,37,39,52 Similar to other peripheral painful neuropathies, a local anesthetic block to the painful area gives ambiguous results and is not completely efficient at eliminating the pain,9,21 suggesting central changes in pain perception induced by peripheral injury.
When affecting a dentate region, the pain may mimic a toothache and may lead to irreversible and unnecessary dental procedures, with no resolution of the pain. Analgesics or narcotics do not relieve the symptoms,28,37 and the effect of antineuropathic pain pharmacotherapy is disappointingly poor.11
Painful posttraumatic trigeminal neuropathy often follows common dental procedures such as pulp extirpation, apicoectomy, or tooth extraction, and after routine endodontics, pain may persist in 3% to 8% of patients.23,29,33,39 Long preoperative pain duration, previous chronic pain problems, a history of painful treatment, and female gender are all considered risk factors for PTTN.17,20,33,39 Notwithstanding, the reason why some patients develop persistent pain after mild nerve injury remains unclear. One candidate is a faulty endogenous pain-modulatory system. Pain modulation is altered in patients with various chronic pain conditions,18,19 and it has been suggested that the pain-modulatory system can define susceptibility to develop chronic pain disorders.42,48,51,55
Although psychophysical studies in patients with PTTN have been reported,2,4,5,22,59 their modulatory pain system has not been examined. Dynamic psychophysical testing can assess pain facilitation or inhibition and provide information on the status of the modulation system. The 2 available tests in the laboratory are temporal summation (TS) and conditioned pain modulation (CPM). Temporal summation tests the facilitatory modulation process, usually performed by measurement of the change in pain perception as a result of a series of repeated, constant noxious stimuli.13 It is believed to be the psychophysical correlate of windup of second-/third-order neurons in the spinal cord and higher up, which may contribute to central sensitization.41,45,49
Conditioned pain modulation represents the inhibitory modulation process; it reflects the efficiency of endogenous analgesia exerted through the descending pain-modulatory system.55 It can be studied in the laboratory using 2 remote noxious stimuli, the “conditioning” stimulus that typically inhibits the “test” stimulus.55
We hypothesized that patients with PTTN display pronociceptive pain modulation (less efficient CPM and enhanced TS), in line with other chronic pain disorders. To test this, we studied the pain modulation profile of patients with PTTN associated with endodontic treatment in comparison with healthy subjects.
2. Materials and methods
The study was conducted at the Rutgers School of Dental Medicine (at the study time, the University of Medicine and Dentistry of New Jersey, New Jersey Dental School). The Institutional Review Board approved the study (IRB Protocol Number: 0120050296) and before inclusion in the study, informed consent was obtained from each participant.
2.1. Study overview
Patients diagnosed with PTTN and healthy subjects underwent dynamic and static sensory testing performed intraorally and at the volar surface of the dominant forearm. The dynamic sensory testing included TS and CPM; the static sensory testing included responses to noxious mechanical and cold stimulation. Two examiners who underwent several sessions of training performed the sensory testing. All subjects included in the study underwent a training session before the actual examination that included detailed explanation and exposure to various stimuli and tests performed in the study.
2.2. Exclusion and inclusion criteria
All patients with PTTN included in the study developed persistent pain after an endodontic treatment and were not under pharmacological treatment for chronic pain. The PTTN group comprised patients suffering from continuous, aching, or burning pain in a treated tooth for more than 3 months and based on current classifications.7,36 The pain was not related to a clinically detectable local (dental) cause as confirmed by radiographic evaluation. The affected area showed clinical sensory disturbance such as sensitivity to pressure. To avoid inclusion of pain due to endodontic failures, only patients with treatments that were evaluated as successful by board-certified endodontists (C.H. and G. H.) were included in the study. This evaluation included clinical and radiographic examinations. The teeth had been well treated from an endodontic perspective, and the patient's symptoms were not judged to be of endodontic origin. Additionally, the possible endodontic involvement of other teeth in the same quadrant arising after treatment of the study tooth was excluded. Subjects under 21 years and subjects suffering from other chronic pain conditions or neurological diseases were not included in the study.
The control group comprised age- and gender-matched healthy volunteers. Subjects included in this group were free of dental or oral pathologies, without orofacial pain or discomfort and did not have dental treatment in the last 6 months (with the exception of periodontal maintenance).
2.3. Sensory testing
All tests were performed in random order on the volar part of the dominant forearm, the nonkeratinized gingiva apical to the painful tooth, and the nonkeratinized gingiva apical to the contralateral tooth, with 10-minute interval between tests. In the control group, the intraoral tests were performed randomly either in premolar or molar areas. To clarify randomization, all the sensory tests were performed first in randomized order, and subsequently, all tests involving immersions were performed in randomized order with 10-minute interval between tests. Experimental session lasted approximately 2 hours.
2.3.1. Dynamic sensory testing (temporal summation and conditioned pain modulation)
Mechanical TS was assessed with a 5.46 von Frey filament (Stoelting Ltd, Wood Dale, IL), inducing 26g of force. A single stimulus and then a train of 30 successive stimuli were applied with a frequency of 1 Hz. Patients were asked to rate the resulting sensation on a numerical pain scale (NPS) of 0 to 20, where 20 is the maximum possible sensation and 0 is no sensation.12 Numerical pain scale scores were obtained after the first and then at the end of every 10 stimuli delivered. Two TS scores were calculated, one after 10 stimuli and the other after 30 stimuli. The 10 stimuli TS is commonly used to compare study and control groups.41,45 The 30 stimuli TS induced higher pain level and therefore was used to evaluate CPM.56 The differences between the scores after the 10th and the 30th stimuli and the score after a single stimulus were calculated and represented TS (ie, last minus first score).
For assessment of CPM, immersion of the nondominant hand to the wrist level in hot water bath served as the conditioning stimulus and the mechanical TS protocol as above as the test stimulus. The nondominant hand was immersed up to the wrist in water held at 46.5°C for 60 seconds (Water Bath; Boekel, Feasterville-Trevose, PA). The hot water was circulated during the test to maintain homogenous water temperature. Thirty-one seconds after the hand was immersed in the water, TS stimuli were applied using a 5.46 von Frey filament as a single stimulus, then followed by a train of 30 stimuli. The subjects were asked to report a number reflecting the level of stimulus intensity on a NPS as above. Data were collected after a single stimulus and then at the end of every 10th stimulus delivered. The difference between the 30 stimuli mechanical TS before immersion of the hand into water bath and the 30 stimuli TS during the hand immersion was calculated and represents CPM; negative values indicate more efficient pain reduction.
2.3.2. Static sensory testing
“Mechanical stimuli” were applied with 2 calibrated von Frey monofilaments, 5.46 and 4.31, inducing 26g and 2g, respectively. For each stimulus, the subjects were asked to rate the stimulus intensity using a 0 to 20 NPS, where 20 is the maximal possible pain sensation and 0 is no pain. The response was calculated as the average of 3 stimuli applied by each filament to slightly different sites.
The tests were performed on the volar part of the dominant forearm, the nonkeratinized gingiva apical to the painful tooth, and the nonkeratinized gingiva apical to the contralateral tooth, in random order with 10-minute interval between tests. In the control group, the intraoral tests were performed randomly either in premolar or molar areas.
“Cold stimulus” was applied to the alveolar mucosa of the tested area for 3 seconds with a cotton swab (5 mm in diameter) sprayed with ethyl chloride (Gebauer, Cleveland, OH). The subject was asked to report the type of sensation felt, whether either painful or non-painful. Pain sensations were rated using a 0 to 20 NPS. The durations of the cold pain sensations after removal of the stimulus were recorded using a stopwatch.
The tests were performed in the nonkeratinized gingiva apical to the painful tooth and in the nonkeratinized gingiva apical to the contralateral tooth, in random order with 10-minute interval between tests.59 In the control group, the intraoral tests were performed randomly either in premolar or molar areas.
2.4. Data analysis
Mechanical TS for each tested site was calculated as the score in response to the 10th stimulus minus the score after the first stimulus (TS = Response to the 10th stimulus − Response to the first stimulus) and the score in response to the 30th stimulus minus the score after the first stimulus (TS = Response to the 30th stimulus − Response to the first stimulus). Conditioned pain modulation was calculated as the 30 stimuli mechanical TS during the hand immersion in hot water minus the 30 stimuli mechanical TS performed without hand immersion (CPM = TS with conditioning − TS without conditioning). Conditioned pain modulation negative values indicate more efficient pain reduction.
The Shapiro–Wilk method was used to test the normality of the distribution of outcomes. The results indicated that the outcomes are highly skewed. Therefore, we used nonparametric approaches to compare the difference between study groups. The exact Wilcoxon rank sum test was used to compare the medians of 2 groups (PTTN and control), and the exact Kruskal–Wallis test was used to compare the medians across multiple groups (PTTN > 1 year, PTTN < 1 year, and controls) with Dwass, Steel, Critchlow, and Fligner (DSCF) multiple comparison analysis to control the overall type I error in pairwise comparisons. Effect sizes were calculated for all analyses. The significance level was set at 0.05. All statistical analyses were implemented with SAS 9.3 (SAS Institute Inc, Cary, NC).
3. Results
A total of 54 subjects were included in this study, 27 in the PTTN group and 27 in the control group, 20 females and 7 males in each group. The mean age of the patients in the PTTN group was 55.33 ± 11.80 years (range, 32-78) and 53.81 ± 10.50 years (range, 32-74) in the control group (P = 0.62). Complete data were obtained for all patients.
3.1. Temporal summation
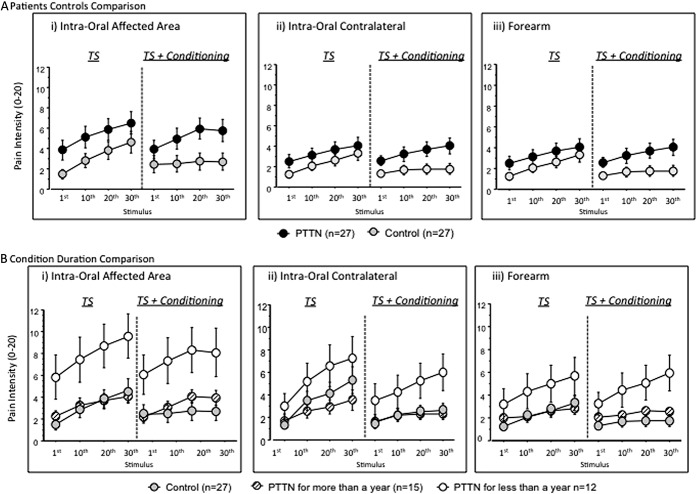
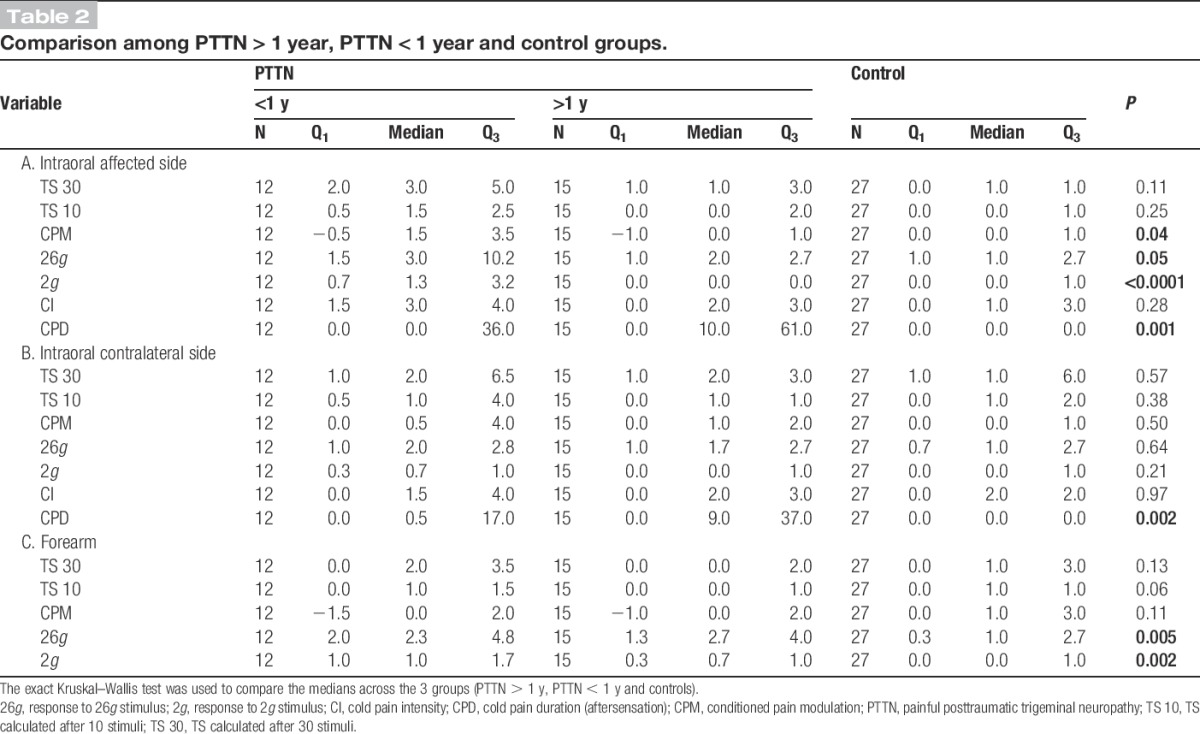
Temporal summation was present in both groups in all sites (Friedman test, P < 0.0001). However, no significant differences were found between the TS scores after 10 or 30 stimuli of the PTTN and control groups (Figs. 1B and 2A) in the affected area (Table 1A; T10: P = 0.30, TS 30: P = 0.18), contralateral side (Table 1B; T10: P = 0.79, TS 30: P = 0.36), or the forearm (Table 1C; T30: P = 0.23, T10: P = 0.10). No significant differences were found between the TS scores of patients with PTTN who suffered from the condition for more than a year, patients who suffered from the condition for less than a year, and control group subjects (Fig. 1D) in the affected area (Table 2A; T30: P = 0.11, T10: P = 0.25), contralateral side (Table 2B; T30: P = 0.57, T10: P = 0.38), or the forearm (Table 2C; T30: P = 0.13, T10: P = 0.06).
Figure 1.
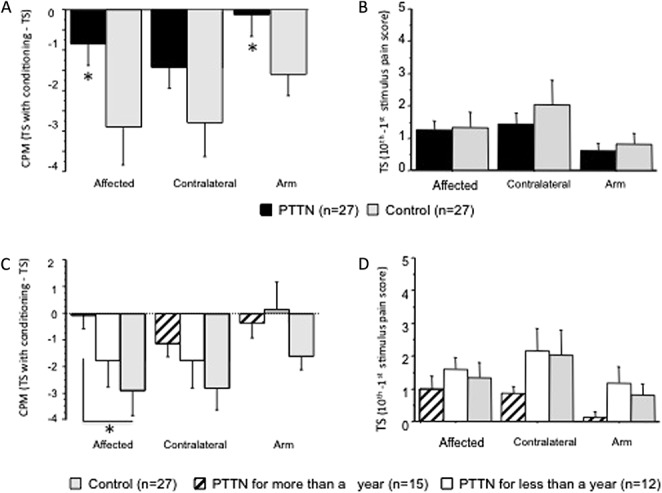
Conditioned pain modulation in 27 patients suffering from painful posttraumatic trigeminal neuropathy (PTTN) and 27 healthy controls. Results show intraoral evaluations in the subjects' affected (injured) and contralateral trigeminal sites and on the dominant forearm. Data are presented as mean ± SD. Nonparametric analysis was used to compare the difference between study groups. The exact Wilcoxon rank sum test was used to compare the medians of 2 groups (PTTN and control), and the exact Kruskal–Wallis test was used to compare the medians across multiple groups (PTTN > 1 year, PTTN < 1 year, and controls). (A) Conditioned pain modulation was significantly (*) less efficient in patients with PTTN compared with the control group in the affected (injured) site and in the arm. Although similar pattern was observed in the contralateral trigeminal site, this was not statistically significant. (B) No significant differences in temporal summation were found between patients with PTTN and healthy controls. (C) Patients with PTTN with longer disease duration (more than a year) demonstrated significantly (*) less efficient conditioned pain modulation compared with the control group when tested intraorally in the affected area. (D) No significant differences in temporal summation were found between patients with PTTN suffering from the condition for more than a year, less than a year, and healthy controls.
Figure 2.
The raw data used to calculate temporal summation and conditioned pain modulation (presented in Fig. 1) of patients with PTTN and healthy control subjects are presented. Resulting sensations on a numerical pain scale of 0 to 20 in response to 26g of force stimulation were recorded after the first and then at the end of every 10 stimuli out of a train of 30 successive stimuli. The stimuli were applied intraorally to the affected (injured) and contralateral sites and to the dominant forearm of the subjects. The data were recorded with and without conditioning pain stimulus (panel A) and presented in patients suffering from the condition for more or less than a year (panel B).
Table 1.
Comparison between the PTTN and control groups.
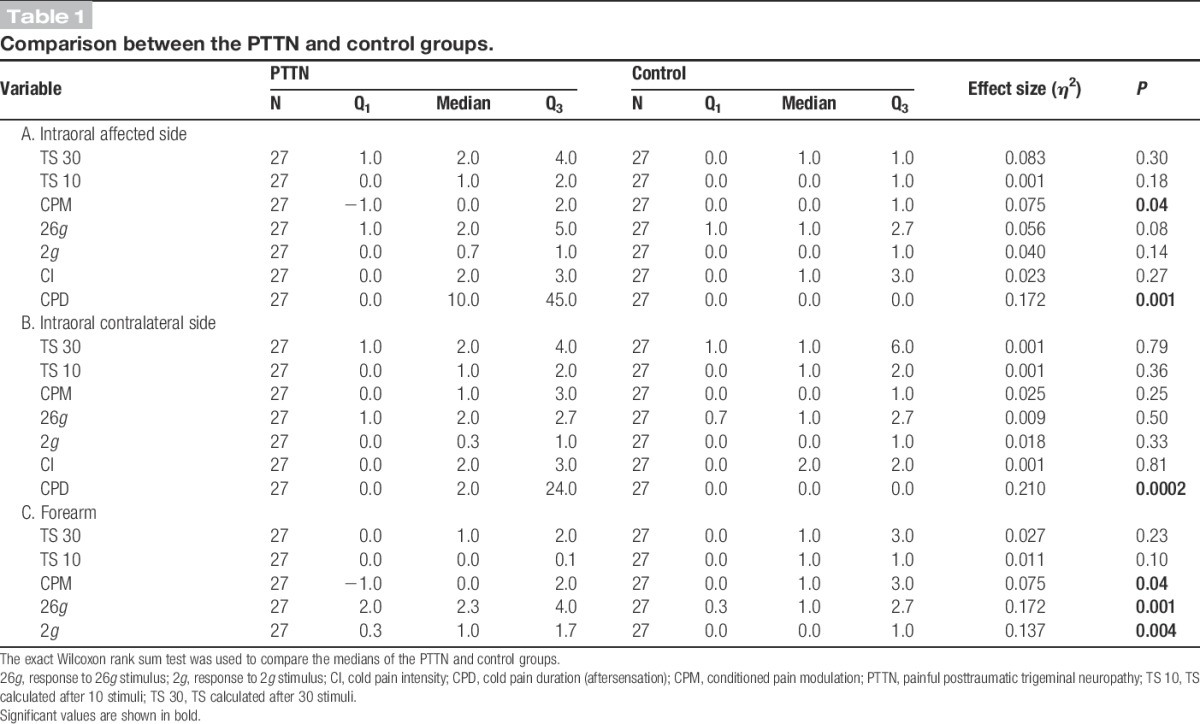
Table 2.
Comparison among PTTN > 1 year, PTTN < 1 year and control groups.
3.2. Conditioned pain modulation
Patients with PTTN demonstrated significantly less efficient CPM compared with the control group (Figs. 1A, 2A) in the affected area (Table 1A; P = 0.04, η2 = 0.075) and in the forearm (Table 1C; P = 0.04, η2 = 0.075), but not different in the contralateral side (Table 1B; P = 0.25). The comparison between patients suffering from the condition for more than a year, less than a year, and healthy controls (Fig. 1C, 2B) demonstrated that the intraoral CPM values in the affected side of the 3 groups are significantly different (Table 2A; P = 0.04, η2 = 0.113). The DSCF method shows that the significant result is due to the less efficient CPM in patients with PTTN suffering from the condition for more than 1 year compared with the patients in control group (Table 3; P = 0.02), while no significant difference is found between less than 1 year PTTN and controls.
Table 3.
P-value of pairwise comparison by the DSCF method.
3.3. Response to mechanical stimulation
3.3.1. Responses to 26g
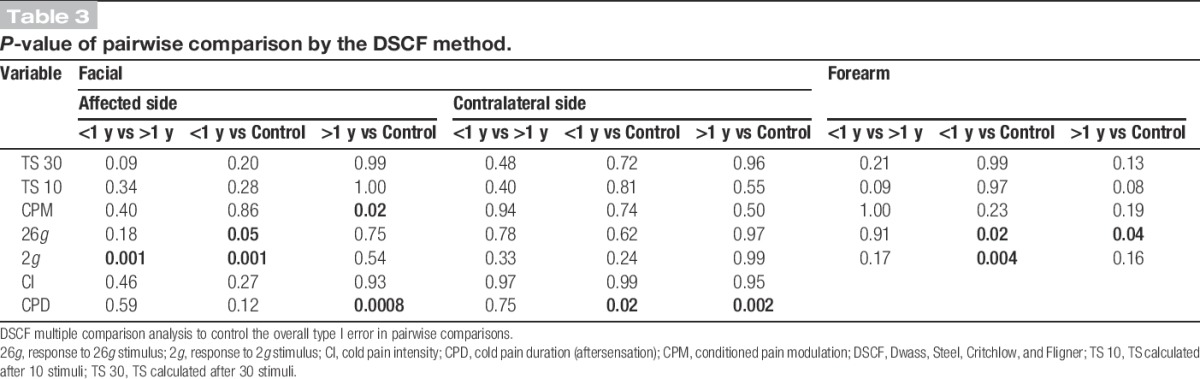
The PTTN and control groups' responses to 26g intraoral stimuli were not significantly different (Fig. 3A) in the affected side (Table 1A; P = 0.08) and in the contralateral side (Table 1B; P = 0.50). The PTTN group's response to forearm 26g stimuli (Fig. 3A) was significantly elevated compared with the control group (Table 1C; P = 0.001, η2 = 0.17).
Figure 3.
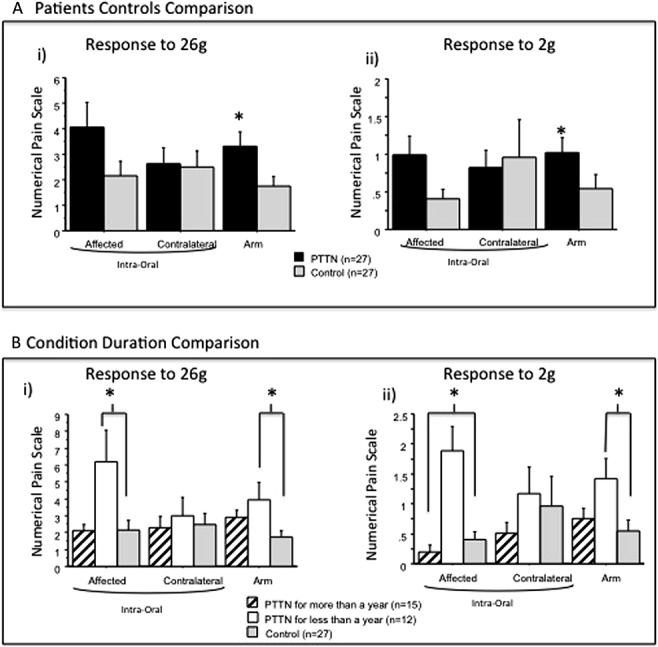
Responses to 2g and 26g stimulation on a 0 to 20 numerical pain scale were recorded from painful posttraumatic trigeminal neuropathy (PTTN) patients and healthy control subjects. Results show evaluations in the subjects' affected (injured) and contralateral intraoral sites and on the dominant forearm. Data are presented as mean ± SD. Nonparametric analysis was used to compare the difference between study groups. The exact Wilcoxon rank sum test was used to compare the medians of 2 groups (PTTN and control), and the exact Kruskal–Wallis test was used to compare the medians across multiple groups (PTTN > 1 year, PTTN < 1 year, and controls). (A) The arm response rating to 26g and 2g stimuli was significantly (*) elevated in the PTTN group compared with the control group. In the affected and contralateral sides, no significant differences were found between the PTTN and control groups. (B) Patients with PTTN for less than a year had a significantly (*) elevated response to 26g and 2g stimuli in the affected side compared with patients with PTTN who suffer from the condition for more than a year and controls. In the arm, patients with PTTN for less than a year had a significantly (*) elevated response to 26g and 2g stimuli compared with controls but not compared with patients with PTTN who suffer from the condition for more than a year.
The comparison between patients suffering from the condition for more than a year, less than a year, and healthy controls (Fig. 3B) demonstrated that the responses of the 3 groups to 26g stimuli are marginally significantly different in the intraoral affected side (Table 2A; P = 0.05, η2 = 0.10) and in the forearm (Table 2C; P = 0.05, η2 = 0.16). The DSCF method shows that in the forearm, the significant results are due to the elevated response in patients with PTTN suffering from the condition for more than a year (Table 3; P = 0.04) and patients suffering from the condition for less than a year (Table 3; P = 0.02) compared with patients in the control group. The marginally significant results in the intraoral affected side are due to the elevated response in patients suffering from the condition for less than a year compared with patients in the control group (Table 3; P = 0.05).
3.3.2. Responses to 2g
The PTTN and control groups' responses to 2g intraoral stimuli were not significantly different (Fig. 3A) in the affected side (Table 1A; P = 0.14) and in the contralateral side (Table 1B; P = 0.33). The PTTN group's response to forearm 2g stimuli was significantly elevated compared with the control group (Table 1C; P = 0.004, η2 = 0.14).
The comparison between patients suffering from the condition for more than a year, less than a year, and healthy controls (Fig. 3B), demonstrated that the responses of the 3 groups to 2g stimuli are significantly different intraorally in the affected side (Table 2A; P < 0.0001, η2 = 0.26) and in the forearm (Table 2C; P = 0.002, η2 = 0.18). The DSCF method shows that in the forearm, the significant results are due to the elevated response in patients with PTTN suffering from the condition for less than a year compared with the control group (Table 3; P = 0.02). The significant results in the intraoral affected side are due to the elevated response in patients suffering from the condition for less than a year compared with patients suffering from the condition for more than a year (Table 3; P = 0.001) and patients in the control group (Table 3; P = 0.001).
3.4. Response to cold stimulation
There were no statistically significant differences in pain intensity scores in response to cold application between the PTTN and control groups (Table 1A and 1B).
The cold pain duration after removal of the cold stimulus (time for the sensation to subside) was significantly longer in patients with PTTN (Fig. 4A) compared with the control group in the affected (Table 1A; P = 0.001, η2 = 0.17) and contralateral (Table 1B; P = 0.0002, η2 = 0.21) sides.
Figure 4.
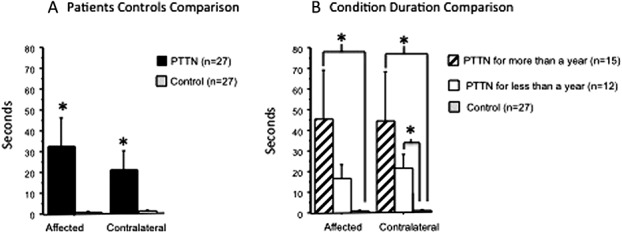
Responses to cold application in patients with painful posttraumatic trigeminal neuropathy (PTTN) and healthy controls. Results show evaluations in the subjects' affected (injured) and contralateral trigeminal sites. Data are presented as mean ± SD. Nonparametric analysis was used to compare the difference between study groups. The exact Wilcoxon rank sum test was used to compare the medians of 2 groups (PTTN and control), and the exact Kruskal–Wallis test was used to compare the medians across multiple groups (PTTN > 1 year, PTTN < 1 year, and controls). (A) The duration of pain after cold application in patients with PTTN was significantly (*) longer at both the affected (injured) and contralateral sites relative to controls. (B) Patients with PTTN who suffered from the condition for more than a year had significantly (*) increased duration of cold pain sensation in the affected and contralateral sides relative to controls.
The comparison between patients suffering from the condition for more than a year, less than a year, and healthy controls (Fig 4B) demonstrated that the cold pain duration of the 3 groups after removal of the stimulus is significantly different intraorally in the affected side (Table 2A; P = 0.001, η2 = 0.20) and in the contralateral side (Table 2B; P = 0.002, η2 = 0.19). The DSCF method shows that in the affected side, the significant results are due to significant (Table 3; P < 0.001) prolonged aftersensation in patients with PTTN suffering from the condition for more than a year compared with the control group. In the contralateral side, the significant results are due to significant prolonged aftersensation in patients with PTTN suffering from the condition for either more (Table 3; P = 0.002) or less (Table 3; P = 0.02) than a year compared with controls.
4. Discussion
To our knowledge, this study is the first comprehensive measurement of both static and dynamic sensory changes in patients with persistent pain after endodontic treatment or PTTN. The static psychophysical profile largely confirmed previous findings that show a mix of sensory gain and loss in patients with PTTN,5,22,46 which is typically seen in neuropathic pains. Evaluation based on the condition duration demonstrated that mechanohypersensitivity is significant in patients suffering from the condition for less than a year, whereas prolonged painful sensation after cold stimulus is increased in patients suffering from the condition for more than a year. The dynamic sensory testing demonstrated reduced function of the endogenous pain-modulatory system mainly in patients with PTTN suffering from the condition for more than a year.
4.1. Pain modulation in patients with painful posttraumatic trigeminal neuropathy
Less efficient CPM was found in patients with PTTN at both injury sites and in the arm demonstrating more widespread extrasegmental inhibitory pronociceptive changes in somatosensory processing. Although a trend of the same pattern of reduced CPM in patients with PTTN was present at the contralateral trigeminal site, this was not significant. A dysfunction of the incoming afferents or a dampening effect at the brainstem level was demonstrated in studies of brain stem reflexes in PTTN consistently showing abnormal blink reflexes.3,15 Less efficient CPM have been reported in various chronic pain conditions such as fibromyalgia, tension headache, temporomandibular disorders, migraine, and irritable bowel syndrome,16,18,26,35,38 suggesting that impaired CPM might have a role in the pathogenesis and maintenance of chronic pain.
It is unclear whether patients with PTTN are endowed with deficient CPM profile and subsequently develop pain or whether this dysfunctional CPM profile was driven by the chronic pain state. Possibly, both may occur so that deficient CPM may be a risk for long-lasting disease, a perpetuating factor, and a result of long-standing pain. Analyzing the study data by disease duration demonstrated that the CPM in the intraoral affected side in patients suffering from the condition for more than a year is significantly lower than controls, whereas the CPM in the same site in patients suffering from this condition for less than a year did not. The condition duration did not have a significant role on the forearm CPM scores, pointing to possible differences between painful and non-painful sites or between the CPM processing of the trigeminal system and spinal nerves.
Bigger sample size studies should use regression analysis to study the condition duration effect; however, the findings of this study suggest a potential association between the condition duration (or ongoing pain) and CPM efficiency.
Faulty CPM makes a target for therapeutic intervention, and some of the medications commonly used for chronic pain have the potential to augment pain inhibition. These should hypothetically be more effective in patients with decreased ability to inhibit pain. Based on this premise, recent research has shown that in patients with painful diabetic neuropathy, less efficient CPM is associated with greater benefit from duloxetine treatment.58 Duloxetine is a serotonin–norepinephrine (NE) reuptake inhibitor that enhances descending pain inhibition by inhibiting the reuptake of NE and serotonin.14,47,58 It has been shown that among the various drugs available for treating neuropathic pain, patients with PTTN benefit significantly from tricyclic antidepressants (TCAs).1,31 Like duloxetine, TCAs increase synaptic levels of both serotonin and NE and therefore increase the activity of the descending modulation system. Tricyclic antidepressants do not work evenly for all patients suffering from PTTN or from other forms of neuropathic pain, and currently, we cannot predict who will benefit from treatment.11 In view of recent findings, TCAs or serotonin–NE reuptake inhibitors should be considered as drugs of choice for patients with PTTN with less efficient CPM.
It has been suggested that reduced CPM is a risk factor for the development of chronic pain following surgical procedures.48,51,55 If so, measuring CPM preoperatively might help identify patients “at risk” of developing chronic posttraumatic pain. Indeed, a recent study has shown that patients undergoing thoracotomy who present preoperatively with altered pain modulation are more prone to develop chronic postsurgical pain.57
A previous study on patients with similar chronic intraoral pain (atypical odontalgia) demonstrated increased intraoral windup to pinprick stimuli and dynamic measures of allodynia using brush and vibratory stimuli,22 while this study did not find significant changes in the TS of patients with PTTN. As TS most likely reflects excessive activation of N-methyl-d-aspartate (NMDA) receptors, the fact that in this study TS is not increased in patients with PTTN might suggest that the ongoing/persistent pain in patients with PTTN may involve a different mechanism. Interestingly, infusion of the NMDA antagonist S-ketamine failed to produce an analgesic effect on patients with PTTN.2 Taken together, this suggests that “windup” and activation of NMDA receptors may not be the main mechanism in patients with PTTN.2 Inconsistent data of either increased8,41,43 or unaltered40 TS have been reported in other chronic pain conditions as well.
4.2. Static psychophysical profile
Peripheral traumatic neuropathies, by definition, present after a lesion of afferent neurons that results in partial or complete loss of sensation and a heterogeneous mix of sensory signs as discussed above.5–7,22 A mix of an elevated detection threshold (hypesthesia) combined with increased responses to painful stimuli (hyperalgesia) is typical of patients with PTTN.7,44,50
The response to the 26g monofilament in a healthy individual is typically a mildly painful and unpleasant sensation, both intraorally and on the arm. This is in line with the mean ratings of 2 of 20 on the NPS reported in this study by controls. The hyperalgesic response seen in patients with PTTN was extratrigeminal (forearm) and may be associated at least in part to a generalized faulty CPM. The hyperalgesic profile observed after mechanical stimulation in patients with PTTN is due mainly to increased sensitivity in patients suffering from the condition for less than a year. Patients with PTTN longer than 1 year had response scores similar to controls.
The 2g monofilament induces a very mild response; controls rated this as 0.5 of 20 on the NPS at all sites. However, a profile similar to 26g stimulus was observed. A significantly increased response in the forearm and intraorally in the affected side of patients suffering from PTTN for less than a year. It is possible that patients with PTTN for less than 1 year largely contributed to the allodynic profile observed at the affected site in previous studies. The presence of mechanohypersensitivity in patients suffering from the condition for less than a year but not in patients who suffer from the condition for more than a year may suggest a change in the central nervous system (CNS) as the disease progresses.
Local cold application to the painful gingivae resulted in significantly longer cold pain sensation in patients with PTTN. This phenomenon has been demonstrated previously on a similar cohort of patients.59 It was suggested that prolonged cold sensation after cold stimulus points to central involvement. In contrast to the time-dependent improvement in the response to mechanical stimulation, the cold response becomes significantly longer (worsens) in patients with PTTN for more than a year.
When considering signs such as allodynia and hyperalgesia, it is important to appreciate that evidence from imaging experiments in human spinal nerve neuropathies suggests a complex CNS involvement. Hyperalgesia is associated with neuronal activity that spreads beyond the pain neuromatrix25,60 involving cognitive and emotional responses and shows significant differences in the pattern of neuronal activity vis-a-vis dynamic mechanical allodynia.54 Furthermore, thermal hyperalgesia demonstrated a different activation pattern than mechanical hyperalgesia.24 This clearly shows that mechanical hyperalgesia has a distinct cerebral activation pattern, suggesting differential control for the sensory features of neuropathic pain.
4.3. Pathophysiology of painful posttraumatic trigeminal neuropathy
Both peripheral and central mechanisms are probably involved in the pathophysiology of PTTN. The fact that topical anesthetic agents can sometimes reduce PTTN-related pain21,53 might emphasize the role of the peripheral nervous system in this specific subgroup. However, a dysfunctional modulatory system and prolonged painful sensation after intraoral cold stimulus as shown here suggest a significant role for the CNS component. The findings of this study also suggest that PTTN is more associated with inhibitory pronociception rather than the facilitatory arm of pain modulation.
Further research performed on bigger sample size will need to approach this finding; however, the transition from hypersensitivity to mechanical stimulation in patients suffering from the condition for less than a year to prolonged painful sensation after cold stimulation and less efficient CPM in patients suffering from the condition for more than a year may be a reflection of the CNS transition from more acute to more chronic phase of the disease.
Conflict of interest statement
The authors have no conflicts of interest to declare.
This research was supported by NIH Grant R21DE01790001A2.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Baad-Hansen L. Atypical odontalgia—pathophysiology and clinical management. J Oral Rehabil 2008;35:1–11. [DOI] [PubMed] [Google Scholar]
- [2].Baad-Hansen L, Juhl GI, Jensen TS, Brandsborg B, Svensson P. Differential effect of intravenous S-ketamine and fentanyl on atypical odontalgia and capsaicin-evoked pain. PAIN 2007;129:46–54. [DOI] [PubMed] [Google Scholar]
- [3].Baad-Hansen L, List T, Kaube H, Jensen TS, Svensson P. Blink reflexes in patients with atypical odontalgia and matched healthy controls. Exp Brain Res 2006;172:498–506. [DOI] [PubMed] [Google Scholar]
- [4].Baad-Hansen L, Pigg M, Ivanovic SE, Faris H, List T, Drangsholt M, Svensson P. Chairside intraoral qualitative somatosensory testing: reliability and comparison between patients with atypical odontalgia and healthy controls. J Orofac Pain 2013;27:165–70. [DOI] [PubMed] [Google Scholar]
- [5].Baad-Hansen L, Pigg M, Ivanovic SE, Faris H, List T, Drangsholt M, Svensson P. Intraoral somatosensory abnormalities in patients with atypical odontalgia–a controlled multicenter quantitative sensory testing study. PAIN 2013;154:1287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Benoliel R, Kahn J, Eliav E. Peripheral painful traumatic trigeminal neuropathies. Oral Dis 2012;18:317–32. [DOI] [PubMed] [Google Scholar]
- [7].Benoliel R, Zadik Y, Eliav E, Sharav Y. Peripheral painful traumatic trigeminal neuropathy: clinical features in 91 cases and proposal of novel diagnostic criteria. J Orofac Pain 2012;26:49–58. [PubMed] [Google Scholar]
- [8].Cathcart S, Winefield AH, Lushington K, Rolan P. Noxious inhibition of temporal summation is impaired in chronic tension-type headache. Headache 2010;50:403–12. [DOI] [PubMed] [Google Scholar]
- [9].de Leeuw JR. Orofacial pain: guidelines for assessment, diagnosis and management. American Academy of Orofacial Pain. Chicago: Quintessence, 2008. [Google Scholar]
- [10].Forssell H, Tenovuo O, Silvoniemi P, Jaaskelainen SK. Differences and similarities between atypical facial pain and trigeminal neuropathic pain. Neurology 2007;69:1451–9. [DOI] [PubMed] [Google Scholar]
- [11].Haviv Y, Zadik Y, Sharav Y, Benoliel R. Painful traumatic trigeminal neuropathy: an open study on the pharmacotherapeutic response to stepped treatment. J Oral Facial Pain Headache 2014;28:52–60. [DOI] [PubMed] [Google Scholar]
- [12].Herr KA, Spratt K, Mobily PR, Richardson G. Pain intensity assessment in older adults: use of experimental pain to compare psychometric properties and usability of selected pain scales with younger adults. Clin J Pain 2004;20:207–19. [DOI] [PubMed] [Google Scholar]
- [13].Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol 2000;61:169–203. [DOI] [PubMed] [Google Scholar]
- [14].Iyengar S, Webster AA, Hemrick-Luecke SK, Xu JY, Simmons RM. Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther 2004;311:576–84. [DOI] [PubMed] [Google Scholar]
- [15].Jaaskelainen SK, Forssell H, Tenovuo O. Electrophysiological testing of the trigeminofacial system: aid in the diagnosis of atypical facial pain. PAIN 1999;80:191–200. [DOI] [PubMed] [Google Scholar]
- [16].Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. PAIN 2005;114:295–302. [DOI] [PubMed] [Google Scholar]
- [17].Klasser GD, Kugelmann AM, Villines D, Bradford JR. The prevalence of persistent pain after nonsurgical root canal. Quintessence Int 2011;42:259–69. [PubMed] [Google Scholar]
- [18].Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. PAIN 1997;70:41–51. [DOI] [PubMed] [Google Scholar]
- [19].Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain 1997;13:189–96. [DOI] [PubMed] [Google Scholar]
- [20].Law AS, Nixdorf DR, Aguirre AM, Reams GJ, Tortomasi AJ, Manne BD, Harris DR; National Dental PBRN Collaborative Group. Predicting severe pain after root canal therapy in the National Dental PBRN. J Dent Res 2015;94: 37S–43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].List T, Leijon G, Helkimo M, Oster A, Svensson P. Effect of local anesthesia on atypical odontalgia–a randomized controlled trial. PAIN 2006;122:306–14. [DOI] [PubMed] [Google Scholar]
- [22].List T, Leijon G, Svensson P. Somatosensory abnormalities in atypical odontalgia: A case-control study. PAIN 2008;139:333–41. [DOI] [PubMed] [Google Scholar]
- [23].Lobb WK, Zakariasen KL, McGrath PJ. Endodontic treatment outcomes: do patients perceive problems? J Am Dent Assoc 1996;127:597–600. [DOI] [PubMed] [Google Scholar]
- [24].Maihofner C, Handwerker HO. Differential coding of hyperalgesia in the human brain: a functional MRI study. NeuroImage 2005;28:996–1006. [DOI] [PubMed] [Google Scholar]
- [25].Maihofner C, Handwerker HO, Birklein F. Functional imaging of allodynia in complex regional pain syndrome. Neurology 2006;66:711–17. [DOI] [PubMed] [Google Scholar]
- [26].Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. PAIN 1995;63:341–51. [DOI] [PubMed] [Google Scholar]
- [27].Marbach J. Phantom tooth pain. J Endod 1978;4:362–72. [DOI] [PubMed] [Google Scholar]
- [28].Marbach J. Orofacial phantom pain: theory and phenomenology. J Am Dent Assoc 1996;127:221–9. [DOI] [PubMed] [Google Scholar]
- [29].Marbach JJ, Hulbrock J, Hohn C, Segal AG. Incidence of phantom tooth pain: an atypical facial neuralgia. Oral Surg Oral Med Oral Pathol 1982;53:190–3. [DOI] [PubMed] [Google Scholar]
- [30].Marbach JJ, Raphael KG. Phantom tooth pain: a new look at an old dilemma. Pain Med 2000;1:68–77. [DOI] [PubMed] [Google Scholar]
- [31].Melis M, Lobo SL, Ceneviz C, Zawawi K, Al-Badawi E, Maloney G, Mehta N. Atypical odontalgia: a review of the literature. Headache 2003;43:1060–74. [DOI] [PubMed] [Google Scholar]
- [32].Merskey H, Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definition of pain terms. Seattle: IASP, 1994. [Google Scholar]
- [33].Nixdorf DR, Moana-Filho EJ, Law AS, McGuire LA, Hodges JS, John MT. Frequency of persistent tooth pain after root canal therapy: a systematic review and meta-analysis. J Endod 2010;36:224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nobrega JC, Siqueira SR, Siqueira JT, Teixeira MJ. Diferential diagnosis in atypical facial pain: a clinical study. Arq Neuropsiquiatr 2007;65:256–61. [DOI] [PubMed] [Google Scholar]
- [35].Olesen J. Clinical and pathophysiological observations in migraine and tension-type headache explained by integration of vascular, supraspinal and myofascial inputs. PAIN 1991;46:125–32. [DOI] [PubMed] [Google Scholar]
- [36].Olesen J, Bendtsen L, Dodick D, Ducros A, Evers S, First M, Goadsby PJ, Hershey A, Katsarava Z, Levin M, Pascual J, Russell MB, Schwedt T, Steiner TJ, Tassorelli C, Terwindt GM, Vincent M, Wang SJ; Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- [37].Pertes RA, Bailey DR, Milone AS. Atypical odontalgia–a nondental toothache. J N J Dent Assoc 1995;66:29–31, 33. [PubMed] [Google Scholar]
- [38].Pielsticker A, Haag G, Zaudig M, Lautenbacher S. Impairment of pain inhibition in chronic tension-type headache. PAIN 2005;118:215–23. [DOI] [PubMed] [Google Scholar]
- [39].Polycarpou N, Ng YL, Canavan D, Moles DR, Gulabivala K. Prevalence of persistent pain after endodontic treatment and factors affecting its occurrence in cases with complete radiographic healing. Int Endod J 2005;38:169–78. [DOI] [PubMed] [Google Scholar]
- [40].Potvin S, Paul-Savoie E, Morin M, Bourgault P, Marchand S. Temporal summation of pain is not amplified in a large proportion of fibromyalgia patients. PAIN Res Treat 2012;2012:938595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. PAIN 2002;99:49–59. [DOI] [PubMed] [Google Scholar]
- [42].Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. PAIN 2009;144:16–19. [DOI] [PubMed] [Google Scholar]
- [43].Raphael KG, Janal MN, Anathan S, Cook DB, Staud R. Temporal summation of heat pain in temporomandibular disorder patients. J Orofac Pain 2009;23:54–64. [PMC free article] [PubMed] [Google Scholar]
- [44].Renton T, Yilmaz Z. Profiling of patients presenting with posttraumatic neuropathy of the trigeminal nerve. J Orofac Pain 2011;25:333–44. [PubMed] [Google Scholar]
- [45].Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Sex differences in temporal summation of pain and aftersensations following repetitive noxious mechanical stimulation. PAIN 2004;109:115–23. [DOI] [PubMed] [Google Scholar]
- [46].Siqueira SR, Siviero M, Alvarez FK, Teixeira MJ, Siqueira JT. Quantitative sensory testing in trigeminal traumatic neuropathic pain and persistent idiopathic facial pain. Arq Neuropsiquiatr 2013;71:174–9. [DOI] [PubMed] [Google Scholar]
- [47].Smith T, Nicholson RA. Review of duloxetine in the management of diabetic peripheral neuropathic pain. Vasc Health Risk Manag 2007;3:833–44. [PMC free article] [PubMed] [Google Scholar]
- [48].Staud R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev Neurother 2012;12:577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Staud R, Robinson ME, Vierck CJ, Jr, Cannon RC, Mauderli AP, Price DD. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. PAIN 2003;105:215–22. [DOI] [PubMed] [Google Scholar]
- [50].Teerijoki-Oksa T, Jaaskelainen SK, Soukka T, Virtanen A, Forssell H. Subjective sensory symptoms associated with axonal and demyelinating nerve injuries after mandibular sagittal split osteotomy. J Oral Maxillofac Surg 2011;69:e208–213. [DOI] [PubMed] [Google Scholar]
- [51].van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain 2010;11:408–19. [DOI] [PubMed] [Google Scholar]
- [52].Vickers ER, Cousins MJ. Neuropathic orofacial pain part 1–prevalence and pathophysiology. Aust Endod J 2000;26:19–26. [DOI] [PubMed] [Google Scholar]
- [53].Vickers ER, Cousins MJ, Walker S, Chisholm K. Analysis of 50 patients with atypical odontalgia. A preliminary report on pharmacological procedures for diagnosis and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85:24–32. [DOI] [PubMed] [Google Scholar]
- [54].Witting N, Kupers RC, Svensson P, Jensen TS. A PET activation study of brush-evoked allodynia in patients with nerve injury pain. PAIN 2006;120:145–54. [DOI] [PubMed] [Google Scholar]
- [55].Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 2010;23:611–15. [DOI] [PubMed] [Google Scholar]
- [56].Yarnitsky D, Bouhassira D, Drewes AM, Fillingim RB, Granot M, Hansson P, Landau R, Marchand S, Matre D, Nilsen KB, Stubhaug A, Treede RD, Wilder-Smith OH. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain 2015;19:805–6. [DOI] [PubMed] [Google Scholar]
- [57].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. PAIN 2008;138:22–8. [DOI] [PubMed] [Google Scholar]
- [58].Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. PAIN 2012;153:1193–8. [DOI] [PubMed] [Google Scholar]
- [59].Zagury JG, Eliav E, Heir GM, Nasri-Heir C, Ananthan S, Pertes R, Sharav Y, Benoliel R. Prolonged gingival cold allodynia: a novel finding in patients with atypical odontalgia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;111:312–19. [DOI] [PubMed] [Google Scholar]
- [60].Zambreanu L, Wise RG, Brooks JC, Iannetti GD, Tracey I. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. PAIN 2005;114:397–407. [DOI] [PubMed] [Google Scholar]