Supplemental Digital Content is Available in the Text.
Epigenetic regulations of P2X3 receptors play a crucial role in cancer pain. Targeting p65 binding to demethylated P2X3 receptor gene suppresses cancer pain.
Keywords: Caner pain, Dorsal root ganglion, p65, P2X3 receptors, DNA demethylation
Abstract
Nuclear factor-kappa B (NF-κB) signaling is implicated in both cancer development and inflammation processes. However, the roles and mechanisms of NF-κB signaling in the development of cancer-induced pain (CIP) remain unknown. This study was designed to investigate the roles of the p65 subunit of NF-κB in regulation of the purinergic receptor (P2X3R) plasticity in dorsal root ganglion (DRG) of CIP rats. We showed here that tumor cell injection produced mechanical and thermal hyperalgesia, and an enhanced body weight–bearing difference, which was correlated with an upregulation of p65 and P2X3R expression in lumber DRGs and a potentiation of ATP-evoked responses of tibia-innervating DRG neurons. Inhibition of NF-κB signaling using p65 inhibitor pyrrolidine dithiocarbamate, BAY-11-7082, or lentiviral-p65 short-hairpin RNA significantly attenuated CIP and reversed the activities of P2X3R. Interestingly, tumor cell injection led to a significant demethylation of CpG island in p2x3r gene promoter and enhanced ability of p65 to bind the promoter of p2x3r gene. Our findings suggest that upregulation of P2X3R expression was mediated by the enhanced binding capability of p65 with demethylated promoter of p2x3r gene, thus contributing to CIP. NF-κBp65 might be a potential target for treating CIP, a neuropathic pain generated by tumor cell–induced injury to nerves that innervate the skin.
1. Introduction
Cancer pain resulting from primary tumors or metastatic carcinoma is one of the most severe and intractable types of chronic pain, which severely compromises the quality of life of cancer patients and imposes a huge burden on society.7,23,26 Despite the availability of bisphosphonates, nonsteroidal anti-inflammatory drugs and opioids, many patients with cancer pain report limited pain relief and adverse side effects, such as neuropsychiatric symptoms and gastric bleeding.1,43 No new pharmacotherapy has emerged, and there is an urgent need for new treatments for patients with cancer pain. Recent studies using rodent models of cancer-induced pain (CIP) suggested that sensitization of primary afferent neurons innervating tumor tissue likely contributes to mechanical allodynia and thermal hyperalgesia.42,46 However, the mechanism underlying the development of CIP remains largely unknown.
Purinergic P2X receptors are preferentially expressed on dorsal root ganglion (DRG) neurons and have been implicated in neuropathic,2,3,39 inflammatory,28,38 and visceral pain hypersensitivity.16,40 Relatively few studies have reported on the roles of purinergic P2X receptors in cancer pain conditions. Recent reports have shown an increase in P2X3 receptor (P2X3R) expression on primary sensory afferents.10,19 However, the mechanism underlying P2X3R upregulation under CIP conditions is not fully understood. The nuclear factor-κB (NF-κB) might be a contributor because it is considered to control numerous genes encoding inflammatory and nociceptive mediators.21,32,35 It also controls the expression of genes linked with tumorigenesis and metastasis in breast cancer cells.18,30,37 Recent studies show that expression of NF-κBp65 subunits is upregulated in DRG neurons after partial sciatic nerve injury9,20 and diabetic gastric hypersensitivity.44 Moreover, intraperitoneal (i.p.) injection of pyrrolidine dithiocarbamate (PDTC) against p65 subunit of NF-κB significantly alleviated diabetic mechanical allodynia and thermal hyperalgesia.31,44 However, whether p65 is involved in the regulation of P2X receptor sensitization in cancer pain conditions remains unknown.
In this study, we tested whether the selective inhibition of p65 using PDTC or BAY-11-7082 can attenuate pain behaviors associated with CIP. In addition, we constructed a lentiviral (LV) vector encoding a specially designed short-hairpin RNA (shRNA) against NF-κBp65 gene (LV-p65 shRNA) and observed the inhibitory effect of in vivo intrathecal (i.t.) delivery of LV-shRNA on a CIP model. To understand the molecular mechanisms potentially involved in upregulation of p65 and P2X3Rs in CIP, a chromatin immunoprecipitation (ChIP) assay of p65-binding ability with p2x3r gene promoter in DRG neurons was performed. Our data demonstrate for the first time that tumor cell injection promoted the ability of p65 binding to the demethylated p2x3r gene promoter. Selective blockade of p65 by PDTC or shRNA attenuates CIP behavior in both reversal and preventative paradigms through inhibition of P2X3R activities of lumber DRG neurons that innervate the tibia. Therefore, selective inhibition of p65 represents a novel target for CIP therapy.
2. Methods
2.1. Animals
Female Sprague-Dawley (SD) rats weighing 160 to 180 g were used in the present studies. They were housed in temperature (24 ± 1°C) and light-controlled (12:12-hour alternating light–dark cycle) room with free access to food and water. All experiments were approved by the Institutional Animal Care and Use Committee at Soochow University and the Association of laboratory animals in Jiangsu Province, China.
2.2. Induction of cancer-induced pain model
The Walker 256 mammary gland carcinoma cell line was syngenic with the SD rat as described previously.24,36,41 In brief, a total of 1 mL Walker 256 cells (2 × 107 cells/mL) was injected into the abdominal cavity of SD rats (weighing ∼60 g). Seven days later, ascites (approximately 10-30 mL) were collected. The cells were collected by centrifugation for 3 minutes at 1200 rpm, and the pellet was washed 3 times with 10 mL normal saline (NS) and recentrifuged for 3 minutes at 1200 rpm. The cells were counted using a haemocytometer and diluted to a final concentration of 1 × 108 cells per milliliter with NS and kept on ice until injected into rats. Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) for surgery. Four microliters of carcinoma cells (4 × 105) or the same volume of NS (vehicle group) was slowly injected into the tibia cavity by using a 10-μL microinjection syringe with a 23-gauge needle. The syringe was left in place for an additional 2 minutes to prevent the carcinoma cells from leaking out along the injection track. All animals were allowed to recover from the surgery for 3 to 4 days before any experimentation.
To assess the tibia bone destruction from the inoculated tumor cells, each rat was placed on a clear plane plexiglass, exposed to an x-ray source under sodium pentobarbital anesthesia on day 14 after Walker 256 inoculation. Tibia bone radiographs were performed using E-COM Digital Radiographer System (ECOM Technology Co LTD, Guangdong, China). Radiological images of tibia taken at the different end point of the study revealed bone destruction caused by carcinoma cells compared with control rats (Supplementary Figure S1A, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A108).
For bone histology, rats were transcardially perfused with 300 mL of 0.9% NS followed by 300 mL 4% paraformaldehyde (PFA) after being anesthetized with an overdose of sodium pentobarbital at the end of the experiment. Tibia bones were removed and decalcified in decalcifying solution for 24 hours. The bones were rinsed, dehydrated, and then embedded in paraffin, cut into 4-μm cross-sections using a rotary microtome (Reichert-Jung 820; Cambridge Instruments GmbH, Nussloch, Germany), and stained with hematoxylin and eosin to visualize the extent of tumor infiltration and bone destruction. The Walker 256–inoculated tibia bone showed that the surface of bone was roughened, and hematoxylin–eosin staining pictures showed significant tumor cells growth in Walker 256–inoculated rats when compared with age- and sex-matched controls (Supplementary Figure S1B and C, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A108).
2.3. Pain behavioral assessments
Experiments were performed on tumor cell–injected rats and the age- and sex-matched control rats. Mechanical allodynia was measured as the hind paw withdrawal response to von Frey hair stimulation according to the up–down method. To quantify the mechanical sensitivity of the hind paw, rats were placed in individual plastic boxes and allowed to acclimate for at least 30 minutes. Testing was performed by an observer in a blinded manner. The rat hind paw withdrawal threshold (PWT) in response to stimulation of von Frey filaments was determined as described previously.4,29,31 A series of calibrated von Frey filaments (ranging from 0.4 to 15.0 g) were applied perpendicularly to the plantar surface of the hind paw with sufficient force to bend filaments for 1 to 2 seconds. A trial began with the application of the 2.0 g of hair. In the presence of a response, a filament of the next greater force was applied. In the absence of a response, a filament of the next lower force was applied. To avoid unnecessary tissue damage during tests, the cutoff strength of von Frey filament was set at 15.0 g. The tactile stimulus producing a 50% likelihood of withdrawal was determined by means of the “up–down” calculating method. Each trial was repeated 2 to 3 times at an approximately 10-minute intervals, and the mean value was used as the force to produce withdrawal response.
Thermal hyperalgesia of the hind paws was tested as described by a previous report.11 Rats were allowed to acclimate for 30 minutes within acrylic enclosures on a clear glass plate. A radiant heat source was focused onto the plantar surface of the hind paw. Measurements of paw withdrawal latency (PWL) were taken by a timer that was started by activation of a heat source and stopped when withdrawal of the paw was detected with a photodetector. A maximal cutoff time of 20 seconds was used to prevent unnecessary tissue damage. Three measurements of PWL were taken for each rat and were averaged as the result of each test session. The hind paw was tested alternately with greater than 5-minute intervals between consecutive tests.
Hind limb body weight–bearing difference (WBD) was also measured using the incapacitance tester. The measurement was recorded as an average of 3 trials, with each trial measuring the weight over 3 seconds and expressed as contralateral–ipsilateral readings.
2.4. Cell retrograde labeling
The origin of the primary afferent innervation of the tibia was determined by retrograde tracing using 1,19-dioleyl-3,3,39,3-tetramethylindocarbocyanine methanesulfonate (DiI; Invitrogen, Carlsbad, CA). Experiments were performed on adult female SD rats (160 to 180 g). Animals were anesthetized with chloral hydrate (360 mg/kg). Then, DiI (25 mg in 0.5 mL methanol) was slowly injected in 5 μL volumes into the tibia cavity by using a 10-μL microinjection syringe with a 23-gauge needle 7 days after injection of tumor cells or NS. The syringe was left in place for an additional 2 minutes to prevent the DiI from leaking out along the injection track. One week later, lumbar L2-L5 DRGs were dissected out for an excitability study using patch-clamp recordings or immunostaining.
2.5. Dissociation of DRG neurons and patch-clamp recording
Isolation of DRG neurons from adult female SD rats has been described previously.44 Briefly, 2 weeks after injection of tumor cells or NS, rats were killed by cervical dislocation, followed by decapitation. Lumber DRGs (L2-L5) were dissected out and transferred to an ice-cold oxygenated fresh dissecting solution containing (in millimolars) 130 NaCl, 5 KCl, 2 KH2PO4, 1.5 CaCl2, 6 MgSO4, 10 glucose, and 10 HEPES, pH = 7.2, osmolarity = 305 mOsm. After the removal of the connective tissue, DRGs were transferred to a tube with 5 mL dissecting solution containing collagenase D (1.8-2.0 mg/mL; Roche, Indianapolis, IN) and trypsin (1.2-1.5 mg/mL; Sigma, St Louis, MO) and incubated for 1.5 hours at 34.5°C. Dorsal root ganglions were taken from enzyme solution, washed, and transferred to 2 mL of dissecting solution containing DNase (0.5 mg/ml; Sigma). A cell suspension was subsequently obtained by repeated trituration through a series of flame-polished glass pipettes. Cells were plated onto acid-cleaned glass coverslips. Coverslips containing adherent DRG cells were then put in a small recording chamber (0.5 mL) and attached to the stage of an inverting microscope (Olympus IX71; Olympus, Tokyo, Japan). The microscope is fitted with both fluorescent and phase objectives. DiI-labeled neurons were identified as tibia-innervating neurons by a fluorescence microscope. Neurons were perfused at room temperature with normal external solution containing (in millimolars) 130 NaCl, 5 KCl, 2 KH2PO4, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH = 7.2, adjusted with NaOH, osmolarity = 295-300 mOsm). For patch-clamp recording experiments, cells were continuously superfused with normal external solution. Recording pipettes were pulled from borosilicate glass tubing using a horizontal puller (P-97; Sutter Instruments, Novato, CA) and typically had a resistance of 3.0 to 5.0 MΩ when filled with the pipette solution containing (in millimolars): 140 potassium gluconate, 10 NaCl, 10 HEPES, 10 glucose, 5 BAPTA, and 1 CaCl2 (pH = 7.25 adjusted with KOH, osmolarity = 295 mOsm). The resting membrane potential and action potentials (APs) were recorded under current-clamp conditions. Tip potentials were zeroed before the membrane–pipette giga seal was formed. Capacitive transient was corrected with capacitive cancellation circuitry on the amplifier that yielded whole-cell capacitance. Up to 90% of series resistance was compensated electronically. Currents were filtered at 2 to 5 kHz and sampled at 50 or 100 μs per point. Whole-cell voltages and currents were acquired with an EPC10 patch-clamp amplifier and were stored on a computer for later analysis using FitMaster (HEKA, Lambrecht, Germany). Patch-clamp recordings were carried out at room temperature (∼22°C).
2.6. Western blotting analysis
The expressions of NF-κBp65 and P2X receptor in L2-L5 DRGs from control and tumor cell–injected rats were measured using Western blotting analysis. The rats were killed by an overdose of chloral hydrate. L2-L5 DRGs were quickly dissected out and lysed and centrifuged at 12,000 rpm for 20 minutes at 4°C. A total of 20 μg aliquot of protein was fractionated on 10% polyacrylamide gels (Bio-Rad, Hercules, CA). Proteins were then transferred to polyvinylidenedifluoride membranes (Bio-Rad) for 1.5 hours at 200 mA. The membranes were blocked for 2 hours in TBS (50 mM Tris-HCl, 133 mM NaCl, pH = 7.4) containing a 5% dilution of nonfat milk powder at room temperature and then incubated with primary antibody (anti-P2X3R at 1:1000, Neuromics Cat# RA10109-150 or NF-κBp65 at 1:200, Santa Cruz Biotechnology, Inc, Cat# sc-8008) in TBS containing 1% milk at 4°C overnight. After washing in TBST (0.5% Tween-20), membranes were incubated with horseradish peroxidase–conjugated secondary antibodies (1:5000, Santa Cruz Biotechnology, Inc, Cat# sc-2313 or Santa Cruz Biotechnology, Inc, Cat# sc-2096) in TBS containing 1% milk for 2 hours at room temperature. Bands were visualized using an enhanced chemiluminescence kit (Amersham, Buckinghamshire, United Kingdom) and appropriate exposure to Kodak x-ray film. Membranes were subsequently stripped and reprobed for actin (anti-β-actin 1:5000; Abnova Corporation Cat# MAB1445). The densities of protein bands were analyzed using NIH image software. P2X3R and NF-κB protein expression was normalized to β-actin.
2.7. Real-time quantitative polymerase chain reaction for mRNA
Total RNA was extracted from L2-L5 DRGs from control and tumor cell–injected rats with Trizol (Invitrogen). cDNA was synthesized from total RNA using an Omniscript RT kit 50 (QIAGEN, Valencia, CA) following the supplier's instructions. The sequences of the primers for P2X3R, P2X2R, P2X1R, DNMT3a, DNMT3b, TDG, Gadd45a, MBD2, MBD4, and β-actin (as an internal control) used in quantitative polymerase chain reaction are shown in Table 1. Control reactions were performed in the absence of cDNA templates. The Ct value was defined as the cycle number at which fluorescence intensity reached a certain threshold where amplification of each target gene was within the linear region of the reaction amplification curves. The relative expression level for each target gene was normalized by the Ct value of β-actin using a 2ΔΔCt relative quantification method.
Table 1.
Oligonucleotides used in the study.

2.8. Immunofluorescence study
One week after DiI injection, the control or tumor cell–injected rats were perfused transcardially with 300 mL phosphate-buffered saline (PBS) followed by 300 mL ice-cold 4% PFA in PBS. L2-L5 DRGs were removed and postfixed for 4 hours in PFA and cryoprotected overnight in 20% sucrose in PBS. For triple labeling, 10-μm sections of DRG were simultaneously incubated with P2X3R (1:200) and p65 (1:100) antibodies for overnight at 4°C and then incubated with secondary antibody with Alexa Fluor 488 and 355 for 2 hours at room temperature. Negative control (NC) was performed by omitting primary antibody. Sections were viewed with filter cubes appropriate for DiI (rhodamine filter), Alexa 355, and 488. Images were captured and analyzed using Metaview software as described previously.44
2.9. Methylation-specific PCR and bisulfite sequencing
The methylation status of CpG island of all samples was initially screened at p2x3r gene promoter regions by methylation-specific PCR (MSP) as described previously.29 Genomic DNA extracted from DRGs (L2-L5) was modified with bisulfite reagents following the manufacturer's instructions (Zymo Research, Irvine, CA). This modification converts unmethylated cytosine to thymine, whereas methylated cytosine remained unchanged. A total of 20 ng of bisulfite-modified DNA was subjected to PCR amplification and directly sequenced using the ABI 3700 automated sequencing system (Applied Biosystems, Foster City, CA). When the CpG sites in the region analyzed by MSP are methylated, the methylated (M) band would show up. However, the unmethylated (U) band would be present when the sites are unmethylated. Occasionally, both bands could be present if the sites are partially methylated. The MSP primers designed for P2X3R were as follows: methylated: forward 5′-GAATTTGGGATCGGTTTAGAGAGC-3′ and reverse 5′-CGCGAAACGATTAAAATAAATCGAT-3′; unmethylated: forward 5′-AATTTGGGATTGGTTTAGAGAGTGT-3′ and reverse 5′-CCCCACAAAACAATTAAAATAAATCAAT-3′.
The methylation status was further validated by bisulfite sequencing as described previously.8,29 The following primers were designed to amplify CpG-rich regions within P2X3R: forward 5′-GAGAGGAAGATTTGGTTAGAAA-3′ and reverse 5′-CTAAAAAACAAACACAATCACC-3′.
2.10. Chromatin immunoprecipitation assay
A ChIP assay was performed using the Upstate-kit (Millipore, Charlottesville, VA) according to the manufacturer's instructions.44 In brief, DRGs from tumor cell–injected rats and age- and sex-matched control healthy rats were lysed for 10 minutes at 4°C and were sonicated 8 times for 15 seconds each. One third of the lysate was used as DNA input control. The remaining two-thirds were diluted 10-fold with ChIP dilution buffer supplied within the commercial kit followed by incubation with an anti-p65 antibody (Santa-Cruz Biotechnology) or mouse immunoglobulin G (IgG; NC) overnight at 4°C. Immunoprecipitated complexes were collected using protein G-agarose beads. The precipitates were extensively washed and then incubated in elution buffer (1% SDS and 0.1 M NaHCO3) at room temperature for 15 minutes. Cross-linking of protein–DNA complexes was reversed at 65°C for 4 hours, followed by treatment with 10 mg/ml proteinase K for 1 hour at 45°C. DNA was extracted with phenol/chloroform and precipitated with ethanol. Pellets were resuspended in TE buffer and subjected to PCR amplification using NF-κB consensus site specific primer 1 (forward: CTTACATCTCAACCCGACACC; reverse: GGGACGATTGGGATGGA), specific primer 2 (forward: AATGGATTCGCCCACTGC; reverse: TTCCCCCGTATCCCTATCC), specific primer 3 (forward: ATAGGGATACGGGGGAAGG; reverse: TCTTCCTCTCTATGGTTTGTGC), specific primer 4 (forward: GCACAAACCATAGAGAGGAAGA; reverse: CCTCGCTTTAGACGACCAG) in p2x3 promoter and specific primer 5 (forward: GTGCCCCTGGTCGTCTAA; reverse: CTTTGAAGAGCAGCCTGTAACT) in p2x3 promoter (Table 1). The resulting product was separated by 2% agarose gel electrophoresis.
2.11. Drug application
To investigate the role of p65, PDTC or BAY-11-7082 was used in this study. One week after tumor cell injection, 29 rats were divided into 5 groups and received an i.p. injection of NS (n = 4), or PDTC 5 (n = 5), PDTC 25 (n = 5), PDTC 50 mg/kg (n = 5), once a day for consecutive 7 days, respectively. Paw withdrawal threshold, PWL, and WBD were recorded after PDTC treatment. Two weeks after tumor cell injection, rats received PDTC injection (25 mg/kg, i.p., n = 6). Paw withdrawal threshold, PWL, and WBD were recorded 2 hours, 12 hours, 2 days, 4 days, 6 days, and 8 days after PDTC treatment. Two weeks after tumor cell injection, 25 rats were divided into 4 groups and received an i.t. injection of NS (n = 6), suramin at the doses of 5 μg (n = 6), 50 μg (n = 7), and 500 μg (n = 6), respectively. Age- and sex-matched control rats received suramin injection (50 μg/kg, i.t., n = 6). Paw withdrawal threshold and PWL were recorded 0.5, 1, 2, and 4 hours after suramin treatment. To further determine which subunit of P2 receptors mediated cancer pain, A317491 was tested. A317491 is a selective P2X3R and P2X2R/P2X3R antagonist.13 Two weeks after tumor cell injection, 25 rats were divided into 4 groups and received an i.t. injection of NS (n = 6), A317491 at the doses of 3 nmol (n = 6), 30 nmol (n = 7), and 100 nmol (n = 6), respectively. Age- and sex-matched control rats received A317491 injection (30 nmol, i.t., n = 6). Paw withdrawal threshold and PWL were recorded 0.5, 1, 2, and 4 hours after A317491 treatment.
2.12. Lentiviral vectors production and intrathecal injection
According to a previously published article,5 shRNA targeting cDNA sequence of rat NF-κBp65 (GenBank Accession NM_199267) was used for this experiment. The sequence of shRNA was 5′-GCA GTT CGA TGC TGA TGA ATT-3′. An additional scrambled sequence was also designed as a NC (5′-TTC TCC GAA CGT GTC ACG T-3′). Replication-deficient self-inactivating LV expressing vector pFU-GW-shRNA-GFP (LV-p65 shRNA and LV-NC) was generated as follows. The cDNAs corresponding to shRNA and NC were subcloned into the vector pFU-GW-RNAi-GFP (Shanghai Gene Chem Co, Ltd). The resulting recombinant LV vectors were designated as LV-p65 shRNA and LV-NC. To produce lentivirus, 293T cells were transfected with 20 μg of pFU-GW-shNF-κBp65 plasmid together with 15 μg of pHelper1.0 and 10 μg of pHelper2.0 packaging plasmids.6 The culture medium was collected 48 hours after transfection, concentrated by ultracentrifugation, aliquoted, and stored at −80°C until used. The titer of LV was determined by hole-by-dilution titer assay.6 Four days after a single exposure of 293T cells to LV, strong green fluorescence was shown in more than 90% of cells, indicating a high and stable transduction of the LV vector system. The final titer of LV-p65 shRNA and LV-NC was 1 × 109 TU/mL. The lentiviruses were injected intrathecally. In brief, after anesthesia with chloral hydrate (360 mg/kg body weight), the spinal column was exposed. A guiding needle (18 G) was passed between the lumbar vertebrae 5 and 6 to enter the i.t. space and 10 μL volume of lentivirus was slowly injected.
2.13. Data analysis
All data are expressed as mean ± standard error of the mean. Statistical analyses were conducted using OriginPro 8 (OriginLab, Northampton, MA) and SPSS statistics 17.0 software. Normality was checked for all data before analysis. The Student t or χ2 test was used to determine significance of changes between 2 groups. Two-way repeated-measures analysis of variance (ANOVA) followed by Tukey's post hoc test were performed where appropriate. A P value <0.05 was considered statistically significant.
3. Results
3.1. Tumor cell injection sensitizes P2X3 receptors in tibia-specific DRG neurons
Consistent with previous reports,15,25,34 rats injected with Walker 256 tumor cells into the tibia canal showed no signs of pain behavior up to day 14 postinoculation, at which point significant mechanical allodynia (Supplementary Figure S1D, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A108), thermal allodynia (Supplementary Figure S1E, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A108), and WBDs (Supplementary Figure S1F, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A108) started to be observed. Intrathecal injection of nonselective P2 receptor inhibitor significantly enhanced the PWT, PWL, and attenuated WBD in dose- and time-dependent manners in bone cancer (BC) rats (Supplementary Figure S2A-C [available online as Supplemental Digital Content at http://links.lww.com/PAIN/A109], *P < 0.05 as compared with PRE, #P < 0.05 compared with corresponding NS group, 2-way repeated-measures ANOVA followed by Tukey's post hoc test, n = 6, 6, 7, and 6 for NS, 5, 50 and 500 μg, respectively). The maximal inhibitory effects were observed at the dose of 500 μg for suramin. The inhibitory effect started at 0.5 hour and lasted for 4 hours after injection of suramin within our observation time period. However, suramin at the dose of 50 μg did not produce any effect on PWT, PWL, and WBD in age- and sex-matched healthy rats (Supplementary Figure S2D-F, n = 5 for each group, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A108). Furthermore, we used a selective P2X3 and P2X2/3 receptor antagonist A317491 to determine whether P2X3Rs were involved in the development of tumor-induced hyperalgesia. Consistent with the above data, i.t. application of A317491 dramatically enhanced the PWT and PWL and attenuated WBD time and dose dependently in BC rats (Figs. 1A-C, *P < 0.05 as compared with PRE, #P < 0.05 compared with corresponding NS group, 2-way repeated-measures ANOVA followed by Tukey's post hoc tests, n = 6, 6, 7, and 6 for NS, A317491 30, 100, and 300 nmol, respectively). The maximal inhibitory effect was observed at the dose of 100 nmol for A317491. The inhibitory effect started at 0.5 hour and lasted for 4 hours after injection of A317491. However, A317491 at the dose of 100 nmol did not produce any effect on PWT, PWL, and WBD in age- and sex-matched healthy rats (supplementary Figure S2G, H, and I, n = 5 for each group available online as Supplemental Digital Content at http://links.lww.com/PAIN/A108). These data demonstrate that P2X3Rs were only involved in rats with tumor-induced hyperalgesia but not involved in rats under healthy conditions. To test the hypothesis that ATP signaling is enhanced in Walker 256–inoculated rats, currents evoked by ATP in single tibia-specific DRG neuron were measured using whole-cell patch-clamp recording techniques. Tibia bone–specific DRG neurons were labeled by fluorescent dye DiI (Fig. 1D), which was injected into the marrow cavity of the tibia bone. Some DiI-labeled neurons were present in L1, L6 DRG (Supplementary Figure S3A, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A110). However, the majority of the DiI-labeled neurons were located in L2-L5 DRG (Supplementary Figure S3A, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A110). Thus, DRG (L2-L5) neurons were isolated 14 days after tumor cell injection. To exclude the possibility that DiI may leak from the bone marrow cavity and enter the blood circulation, we have performed additional experiments to determine whether contralateral ganglia and cervical ganglia neurons were also labeled by DiI. As shown in Supplementary Figure S3B (available online as Supplemental Digital Content at http://links.lww.com/PAIN/A110), there were no DiI-labeling neurons in ipsilateral cervical DRG (C5) ganglia and in contralateral lumbar DRG (L3), indicating that the dye might not be in the general circulation. Additionally, to characterize the nature of these Dil-labeled primary afferents that innervate the tibia, we have performed experiments to show that DiI was colocalized with IB4 (a marker for nonpeptide-containing DRG neurons with unmyelinated fibers), calcitonin gene–related peptide (a marker for peptide containing DRG neurons with unmyelinated fibers), or NF-200 (a marker for DRG neurons with myelinated fibers) by immunohistochemistry. The majority of DiI-labeled neurons were IB4- and calcitonin gene–related peptide–positive DRG neurons and a few were NF-200-positive neurons (Supplementary Figure S4, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A111).
Figure 1.
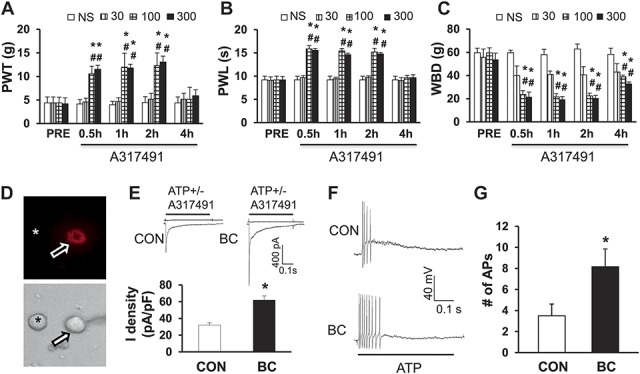
Involvement of P2X3 receptors of DRG neurons in cancer pain rats. (A) Intrathecal application of A317491 dramatically enhanced the mechanical PWT in dose- and time-dependent manners in cancer rats. * denotes P = 0.008 on 0.5 hours, 0.027 on 1 hour, 0.014 on 2 hours in the 100 nmol group, 0.01 on 0.5 hours and 0.013 on 1 hour, 0.002 on 2 hours in the 300 nmol group as compared with PRE; # denotes P = 0.005 on 0.5 hours, 0.022 on 1 hour, 0.021 on 2 hours in the 100 nmol group, 0.011 on 0.5 hours, 1 hour, 0.006 on 2 hours in the 300 nmol group as compared with the corresponding NS group, 2-way repeated-measures ANOVA followed by Tukey's post hoc test, n = 6, 6, 7, and 6 for NS, 30, 100, and 300 nmol, respectively. (B) Intrathecal application of A317491 dramatically enhanced the thermal threshold (PWL) in dose- and time-dependent manners in cancer rats. * denotes P < 0.0001 on 0.5 hours, 1 hour, 0.001 on 2 hours in the 100 nmol group, <0.0001 on 0.5 hours and 0.001 on 1 hour, 2 hours in the 300 nmol group as compared with PRE; # denotes P < 0.0001 on 0.5 hours, 1 hour, 0.001 on 2 hours in the 100 nmol group, <0.0001 on 0.5 hours, 1 hour, 0.001 on 2 hours in the 300 nmol group as compared with the corresponding NS group, 2-way repeated-measures ANOVA followed by Tukey's post hoc test, n = 6, 6, 7, and 6 for NS, 30, 100, and 300 nmol, respectively. (C) Intrathecal application of A317491 dramatically attenuated the WBD in dose- and time-dependent manners in cancer rats. * denotes P = 0.018 on 0.5 hours, 0.023 on 1 hour, 0.017 on 2 hours in the 30 nmol group, <0.001 on 0.5 hours and <0.0001 on 1 hour, 2 hours, <0.001 on 4 hours in the 100 nmol group, 0.002 on 0.5 hours and <0.001 on 1 hour, 2 hours, 0.021 on 4 hous in the 300 nmol group as compared with PRE; # denotes P < 0.001 on 0.5 hours, 0.009 on 1 hour, 0.002 on 2 hours, 0.037 on 4 hours in the 30 nmol group, <0.0001 from 0.5 hours through 2 hours, <0.001 on 4 hours in the 100 nmol group, <0.0001 from 0.5 hours through 2 hours, 0.005 on 4 hours in the 300 nmol group as compared with corresponding data point in the NS group, 2-way repeated-measures ANOVA followed by Tukey's post hoc test, n = 6, 6, 7, and 6 for NS, 30, 100, and 300 nmol, respectively. (D) An example of a DiI-labeled neuron, which is innervating the tibia, was used to do patch-clamp recordings in this study, indicated by an arrow. A star showing a neuron, which is DiI negative. (E) An example of ATP (20 μM) evoked inward currents in DiI-labeled neurons under voltage-clamp conditions. Addition of A317491 greatly inhibited ATP-induced inward currents. Bar graph showing an average of ATP-induced current density of DiI-labeled DRG neurons from control and CIP rats. * denotes P < 0.001 as compared with CON, 2-sample t test, n = 15 cells for each group. (F) An example of ATP-evoked action potentials (APs) of DiI-labeled neurons under current-clamp conditions. (G) The number of ATP-evoked APs was greater in CIP rats than in control rats. * denotes P = 0.034 as compared with CON, 2-sample t test, n = 16 cells for each group.
The small- and medium-sized DRG neurons were studied in this study because they are the primary sensory neurons responsible for pain sensation.12,38 At −60 mV holding potential, ATP (20 μM) evoked fast inactivating currents in the majority of DiI-labeled DRG neurons from control rats (Fig. 1E, left). Because the slow inactivating current was evoked in <10% of neurons and this figure did not change after tumor cell injection, only the fast inactivating currents were included in this study. The average peak current density obtained from control rats was 31.2 ± 3.0 pA/pF (n = 15) in DiI-labeled DRG neurons. In BC rats, however, these values were increased by a factor of more than 2-fold in DiI-labeled DRG neurons (Fig. 1E, *P < 0.001 as compared with the control group (CON), 2-sample t test, n = 15 cells for each group). These currents were completely blocked by application of A317491 (Fig. 1E), indicating an involvement of P2X3 and P2X2/3 subtypes of purinergic receptors. We also compared the effect of ATP (20 μM) on membrane depolarization of tibia bone–specific DRG neurons from control and BC rats under current-clamp conditions. Application of ATP to recorded DiI-labeled neurons evoked a significantly greater number of APs in BC rats than in controls (Figs. 1F, G, *P = 0.034 as compared with CON, 2-sample t test, n = 16 cells for each group), indicating an enhanced function of P2X3Rs after tumor cell injection.
3.2. Expression of P2X3R and p65 is increased after tumor cell injection
To determine whether the expression of P2X3Rs was increased in L2-L5 DRGs after tumor cell injection, quantitative RT-PCR and western blotting assays were performed 7, 14, and 21 days after tumor cell injection. The mRNA level of P2X3Rs in L2-L5 DRGs was significantly increased at days 14 and 21 after tumor cell injection when compared with control (Fig. 2A, * P = 0.022 on day 14, 0.021 on day 21 as compared with CON, Student t test, n = 5 for each group). We also measured the expression of P2X1R, P2X2R, and the endogenous hydrogen sulfide–producing enzymes cystathionine beta-synthetase (CBS), as a NC, in L2-L5 DRGs. By contrast, expression of P2X1R, P2X2R, and CBS was not altered at day 14 after tumor cell injection (Figs. 2B, C, n = 5 for each group). The protein level of P2X3R expression in L2-L5 DRGs was also significantly increased at days 14 and 21 when compared with control (Fig. 2D, *P < 0.001 on days 14 and 21 as compared with CON, Student t test, n = 4 for each group). Tumor cell injection did not alter the expression of CBS at the protein level (Supplementary Figure S5A, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A112). Taken together, these data suggest that P2X3R expression is significantly upregulated both at protein and mRNA levels in rat DRGs after tumor cell injection, which is correlated with the changes in pain behavior in BC rats.
Figure 2.
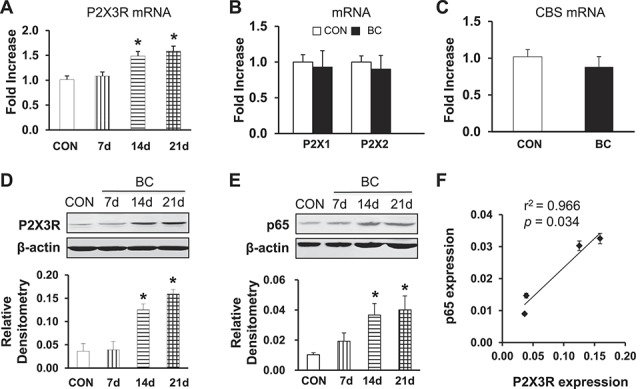
Upregulation of P2X3R and p65 expression in cancer pain rat DRGs. (A) Expression of P2X3 receptors at mRNA levels in L2-L5 DRGs was greatly enhanced at 14 and 21 days after tumor cell injection. * denotes P = 0.022 on day 14, 0.021 on day 21 as compared with CON, Student t test, n = 5 for each group. (B) Expression of P2X1 and P2X2 receptors was not significantly altered after tumor cell injection, n = 5 for each group. (C) Expression of the endogenous hydrogen sulfide producing enzymes cystathionine beta-synthetase (CBS) at mRNA level was not significantly altered after tumor cell injection. (D) Expression of P2X3 receptors at protein levels in L2-L5 DRGs was drastically enhanced at 14 and 21 days after tumor cell injection. * denotes P < 0.001 on days 14 and 21 as compared with CON, Student t test, n = 4 for each group. (E) Expression of nuclear factor kappa B (NF-κB) subunit p65 in L2-L5 DRGs was significantly enhanced at 14 and 21 days after tumor cell injection. * denotes P = 0.02 on day 14, 0.033 on day 21 as compared with CON, Student t test, n = 5 for each group. (F) Correlation analysis showed that the alterations of P2X3R and p65 had a significant positive correlativity, r2 = 0.966, P = 0.034, n = 4.
To determine whether NF-κB plays a role in the development of CIP, we also examined expression of p65 in L2-L5 DRGs postinjection. The expression of p65 in L2-L5 DRGs was greatly enhanced at days 14 and 21 postinjection of tumor cells when compared with age- and sex-matched controls (Fig. 2E, CON, *P = 0.02 on day 14, 0.033 on day 21 as compared with CON, Student t test, n = 5 for each group), which approximately paralleled the upregulation of P2X3Rs on the time line (Fig. 2D). Correlation analysis showed that the alterations of P2X3Rs and p65 had a significant positive correlativity (r2 = 0.966, P = 0.034; Fig. 2F). Furthermore, the expression of phosphorylated p65 (p-p65) was determined. Tumor cell injection significantly enhanced the expression of p-65 (Supplementary Figure S5E, n = 4 rats for each group, *P < 0.05, compared with CON, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A112). However, expression of RelB (Supplementary Figure S5B, n = 4 rats for each group), RANKL (Supplementary Figure S5C, n = 4 rats for each group, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A112), and p52 (Supplementary Figure S5D, n = 4 rats for each group, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A112) was not significantly altered after tumor cell inoculation.
3.3. Treatment of NF-κB inhibitor pyrrolidinedithiocarbamate or BAY-11-7082 reverses upregulation of P2X3R and attenuates hyperalgesia
To further determine whether there is a correlation between p65 and P2X3R, we then examined whether the injection of PDTC, an inhibitor of NF-κB, reverses P2X3R expression. PDTC was injected intraperitoneally at different doses once a day for consecutive 7 days in BC rats from day 7 to day 14 after tumor cell injection. The same volume of NS was used as control. PDTC treatment markedly suppressed P2X3R expression in BC rats at 14 days after tumor cell injection (Fig. 3A, #P = 0.036 as compared with CON. *P = 0.041 for 5 mg PDTC, 0.02 for 25 mg PDTC, 0.019 for 50 mg PDTC as compared with BC; Tukey's post hoc test after one-way ANOVA, n = 4 for NS group; n = 5 for each of the rest groups). Intraperitoneal injection (once) of PDTC attenuated the PWT in tumor cell–injected rats (Fig. 3B). The maximal inhibitory effects were observed at the dose of 25 mg/kg for PDTC (Fig. 3B). Similarly, PDTC at doses of 25 and 50 mg significantly attenuated the PWL and WBD in CIP rats (Figs. 3C, D). We then observed the effect of PDTC at a dose of 25 mg/kg (i.p.) once daily for 7 consecutive days. The inhibitory effect of PDTC at the dose of 25 mg/kg on PWT lasted for up to 4 days (Fig. 3E). Similarly, the inhibitory effect of PDTC at the dose of 25 mg/kg on PWL and WBD lasted for up to 4 days (Figs. 3F, G). However, PDTC did not produce any significant effect on PWT PWL and WBD in age- and sex-matched healthy control rats. These data suggest an involvement of NF-κBp65 in rats with CIP. To further confirm the role of p65, another selective inhibitor, BAY-11-7082,17 was used. Similarly, administration of BAY-11-7082 at the doses of 1 and 3 mg/kg body weight significantly enhanced the PWT (Fig. 3H) and PWL (Fig. 3I) and reduced the WBD (Fig. 3J). The effect lasted for about 2 hours (n = 8 rats for each group).
Figure 3.
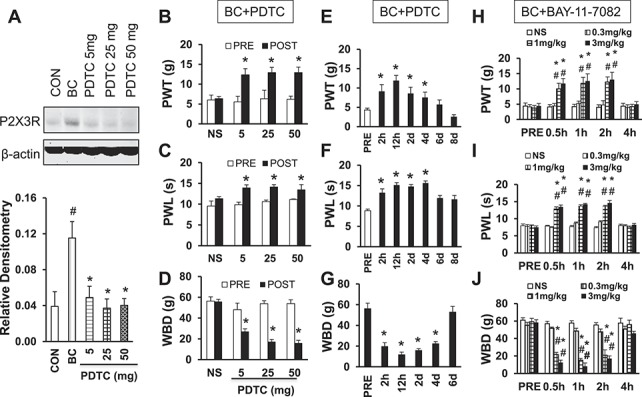
Reversed upregulation of P2X3 receptor expression and cancer pain hypersensitivity by PDTC. (A) Intraperitoneal injection of NF-κB inhibitor PDTC once daily for consecutive 7 days markedly reversed the upregulation of P2X3 receptors in CIP rats. # denotes P = 0.036 as compared with CON. * denotes P = 0.041 for 5 mg PDTC, 0.02 for 25 mg PDTC, 0.019 for 50 mg PDTC as compared with BC, n = 4 for NS group, n = 5 for the rest each group. (B) Intraperitoneal injection of PDTC greatly enhanced the PWT in cancer-induced pain rats. * denotes P = 0.01 for 5 mg PDTC, 0.038 for 25 mg PDTC, 0.002 for 50 mg PDTC as compared with PRE, n = 6 for each group. (C) Intraperitoneal injection of PDTC greatly enhanced the PWL in cancer-induced pain rats. * denotes P = 0.001 for 5 mg PDTC, <0.001 for 25 mg PDTC, 0.046 for 50 mg PDTC as compared with PRE, n = 6 for each group. (D) Intraperitoneal injection of PDTC significantly reduced the WBD in cancer-induced pain rats. * denotes P = 0.011 for 5 mg PDTC, <0.001 for 25 mg and 50 mg PDTC as compared with PRE, n = 6 for each group. (E) Intraperitoneal injection of PDTC dramatically enhanced the PWT in time-dependent manner in cancer rats. * denotes P = 0.014 on 2 hours, <0.001 on 12 hours, 0.012 on day 2, and 0.026 on day 4 as compared with PRE, n = 6 for each group. (F) Intraperitoneal injection of PDTC dramatically enhanced the PWL in time-dependent manners in cancer rats. * denotes P < 0.001 from 2 hours through day 4 as compared with PRE, n = 6 for each group. (G) Intraperitoneal injection of PDTC dramatically attenuated the WBD in time-dependent manners in cancer rats. * denotes P < 0.001 from 2 hours through day 4 as compared with PRE, n = 6 for each group. The inhibitory effect of PDTC at the dose of 25 mg/kg on PWT, PWL, and WBD lasted for up to 4 days. In all panels, statistical significance was tested by Tukey's post hoc test after one-way ANOVA. Intraperitoneal administration of BAY-11-7082 at the doses of 1 and 3 mg/kg body weight significantly enhanced the PWT (H) and PWL (I) and reduced the WBD (J). The effect lasted for about 2 hours (n = 8 rats for each group). BAY-11-7082 at the dose of 0.3 mg/kg body weight did not alter the pain sensitivity of CIP rats. * denotes P < 0.05 compared with PRE. # denotes P < 0.05 compared with NS, n = 8 for each group.
3.4. Lentiviral vector–derived shRNA approach against NF-κBp65 suppresses P2X3R expression and function and attenuates cancer-induced pain behavior
To further determine whether p65 is involved in the regulation of P2X3R expression, we next determined whether pretreatment with LV-p65 shRNA suppressed P2X3R expression and affected the initiation of mechanical hyperalgesia induced by tumor cell injection. We have developed a highly efficient method of lentivirus-mediated delivery of shRNA targeting NF-κBp65 for gene silencing as described previously.32 We then transfected the LV-p65 shRNA or LV-NC. The inhibitory doses for LV-p65 shRNA and LV-NC were 1 × 107 TU used in this experiment. Rats were pretreated with LV-p65 shRNA or LV-NC 30 minutes before injection of tumor cells. Fourteen days after transfection, L2-L5 DRGs were dissected out for triple labeling and western blotting analysis. As described above, tibia-innervating DRG neurons were labeled by DiI (Fig. 4A, red). Green signals indicated that the LV-p65 shRNA was successfully transduced into these neurons (Fig. 4B). P2X3R-positive neurons were labeled in blue (Fig. 4C). Arrows indicated that these tibia-innervating neurons were DiI and GFP double positive but P2X3R negative, indicating suppression of P2X3R expression by transduced LV-p65 shRNA. Arrowheads indicated that these neurons were GFP negative but P2X3R positive, indicating a normal expression of P2X3R if there is no transduced LV-p65 shRNA. The percentage of numbers of P2X3R-positive neurons in DiI-labeled neurons was significantly decreased (Fig. 4G). In addition, delivery of shRNA targeting NF-κBp65 for gene silencing greatly suppressed expression of P2X3Rs in BC rats (Fig. 4H, P = 0.039 as compared with NC group, 2-sample t test, n = 3 for each group). As expected, the expression of p65 was significantly reduced in L2-L5 DRGs (Fig. 4I, *P = 0.034 as compared with NC group, 2-sample t test, n = 3 for each group).
Figure 4.
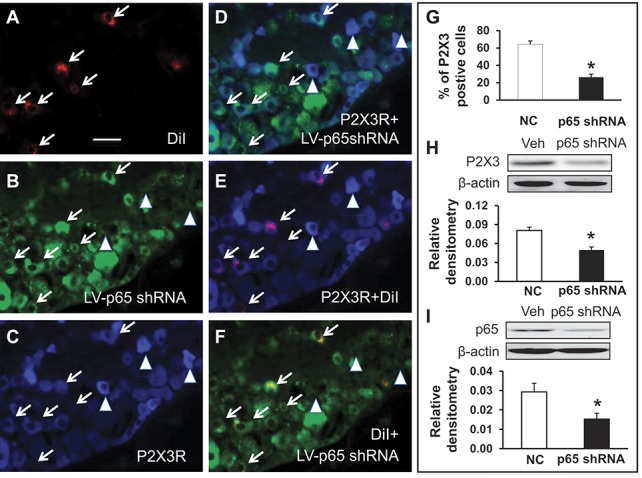
Suppression of P2X3R by p65 knockdown with lentiviral (LV)-p65 shRNA. (A) Neurons in red (arrows) are DiI-labeled L4 DRG neurons innervating the tibia. (B) Neurons in green are LV-GFP-p65 shRNA-transduced neurons. (C) Neurons in blue are P2X3 receptor–positive DRG neurons. (D) Merge of double labeling of P2X3R and LV-GFP-p65 shRNA. (E) Merge of DiI and P2X3R labeling. (F) Merge of DiI and LV-GFP-p65 shRNA. Scale bar, 50 μm. Neurons indicated by arrowhead are DiI and GFP double-negative neurons. These neurons are strongly stained with P2X3 receptor antibodies. Because of LV-GFP-p65 shRNA transduction, DiI-labeled neurons are negative or weak stained with P2X3 receptor antibodies (C, arrows) when compared with double-negative neurons indicated by arrowheads (C). (G). The percentage of numbers of P2X3R and DiI double-positive neurons in total DiI-labeled DRG neurons was dramatically reduced after transfection of LV-GFP-p65 shRNA when compared with negative control shRNA (LV-GFP-NC). * denotes P < 0.001 as compared with NC group, 2-sample t test, n = 9 for each group. (H) The delivery of shRNA targeting NF-κBp65 for gene silencing significantly decreased expression of P2X3 receptors in L2-L5 DRGs of CIP rats. * denotes P = 0.039 as compared with NC group, 2-sample t test, n = 3 for each group. (I) The expression of p65 was significantly reduced in L2-L5 DRGs of CIP rats after the delivery of shRNA targeting NF-κBp65. * denotes P = 0.034 as compared with NC group, 2-sample t test, n = 3 for each group.
We next determined whether pretreatment with LV-p65 shRNA reduced ATP-induced responses of tibia-innervating DRG neurons of BC rats. Under the voltage-clamp condition, the ATP-induced inward current was drastically decreased after injection of LV-p65 shRNA (Figs. 5A, B). The average current density was 23.52 ± 2.38 pA/pF (n = 9) in LV-p65 shRNA-injected group and was 53.61 ± 5.58 pA/pF (n = 9) in LV-NC group (Fig. 5B). Under a current-clamp configuration, the number of APs evoked by ATP (20 μM) application was significantly reduced after injection of LV-p65 shRNA when compared with NC (Fig. 5C). The average number of APs was 2.71 ± 0.79 (n = 14) in the LV-p65 shRNA-injected group and was 6.57 ± 1.45 (n = 14) in the NC group (Fig. 5D, *P = 0.027 as compared with the NC group, 2-sample t test, n = 14 cells for each group). More importantly, delivery of shRNA targeting NF-κBp65 prevented the reduction in mechanical withdraw threshold (Fig. 5E) and WBDs (Fig. 5G) when compared with the NC shRNA. This inhibitory effect lasted for 10 days in PWL and for 21 days in WBD within our observation time period. However, delivery of shRNA targeting NF-κBp65 did not alter the thermal behavior of BC rats (Fig. 5F).
Figure 5.
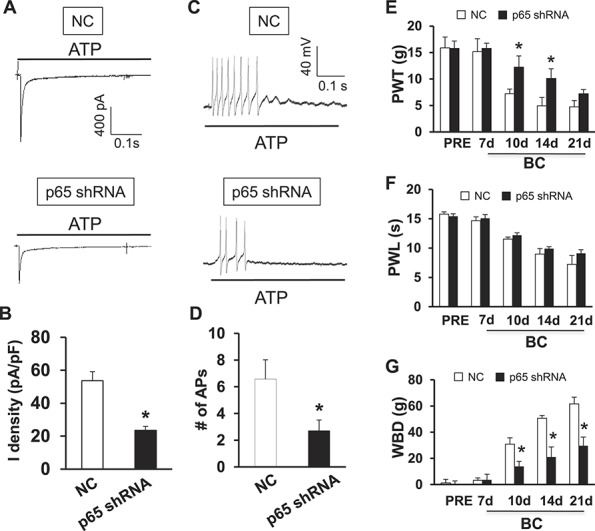
Reversal of ATP-evoked responses and attenuation of cancer pain hypersensitivity by p65 knockdown with LV-p65 shRNA. (A) An example of ATP-evoked inward current traces recorded from LV-shRNAp65 (lower) and NC shRNA-injected rats with CIP. (B) Bar graph showing a dramatical reduction in ATP-evoked current density after LV-p65 shRNA injection. * denotes P < 0.001 as compared with NC group, 2-sample t test, n = 9 cells for each group. (C) Under current-clamped condition, the ATP-evoked action potentials were observed from NC (top) and LV-p65 shRNA injection (bottom). (D) The number of ATP-evoked action potentials was markedly decreased in LV-p65 shRNA-injected group when compared with NC shRNA. * denotes P = 0.027 as compared with NC group, 2-sample t test, n = 14 cells for each group. (E) The delivery of shRNA targeting NF-κBp65 significantly enhanced mechanical withdrawal threshold (PWT) in cancer rats when compared with NC shRNA. This inhibitory effect lasted for 14 days in PWL within our observation time period. * denotes P = 0.031 on day 7 and 0.033 on day 14 as compared with corresponding NC group, Tukey's post hoc test following 1-way ANOVA, n = 8 rats for each group. (F) The delivery of shRNA targeting NF-κBp65 did not alter PWL to thermal stimulation in CIP rats. Tukey's post hoc test after 1-way ANOVA. (G) The delivery of shRNA targeting NF-κBp65 significantly attenuated the weight-bearing differences (WBD) in cancer rats when compared with NC shRNA. This inhibitory effect lasted for 21 days in WBD within our observation time period. * denotes P = 0.004 on day 7, 0.006 on day 14, and 0.002 on day 14 as compared with the corresponding NC group, Tukey's post hoc test after 1-way ANOVA, n = 8 rats for each group.
3.5. Tumor cell injection promotes binding ability of p65 with p2x3r gene promoter
The molecular mechanisms involved in the regulation of P2X3R expression occur mostly at a transcriptional level and seem to require activation of some transcription factors such as NF-κB. Based on this hypothesis, we first predicted whether p2x3r gene promoter CpG island contains transcription binding sites of p65 (TFSEARCH program, version 1.3; http://www.cbrc.jp/research/db/TFSEARCHJ.html). As indicated in Figure 6A, 5 transcription binding sites in p2x3r gene for p65 were found in the CpG island. We then used ChIP assay to further confirm these binding sites. The results of ChIP assay showed that nuclear extracts contain a protein complex that binds to p65 oligonucleotides from both the control and BC rats. Although there is modest binding in extracts isolated from age- and sex-matched controls, tumor cell injection dramatically increased p65-binding ability to the p2x3r gene promoter region for p65 binding site 1 in rats (Fig. 6B, *P = 0.011 as compared with CON, 2-sample t test, n = 3 samples for each group, 2 rats in each sample, n = 3 sample, 2 rats for each sample). However, the p65 binding site 4 (Fig. 6C) and 5 (Fig. 6D, n = 3 samples for each group, 2 rats in each sample) in tumor cell–injected rats were not remarkably altered when compared with age- and sex-matched controls. Of note is that the predicted p65 binding sites 2 and 3 were not confirmed by ChIP in this study (data not shown). Nevertheless, these data indicated that the interaction between the p65 and p2x3r gene promoter region was increased after tumor cell injection and that the predicted binding site 1 may play a vital role in CIP. We further examined whether p65 and P2X3Rs were colocalized in L2-L5 DRG neurons innervating the tibia bone. Triple-labeling techniques were used in this study. Tibia bone–innervating DRG neurons were retrogradely labeled by DiI. DRG sections containing DiI-labeled neurons were chosen for staining with P2X3R and p65 antibodies. About 90% of the tibia bone–innervating DRG neurons that were immunoreactive for P2X3Rs were also positive for p65 (Fig. 6E). Similarly, all tibia bone–specific DRG neurons that were immunoreactive for p65 also were positive for P2X3R (Fig. 6E).
Figure 6.
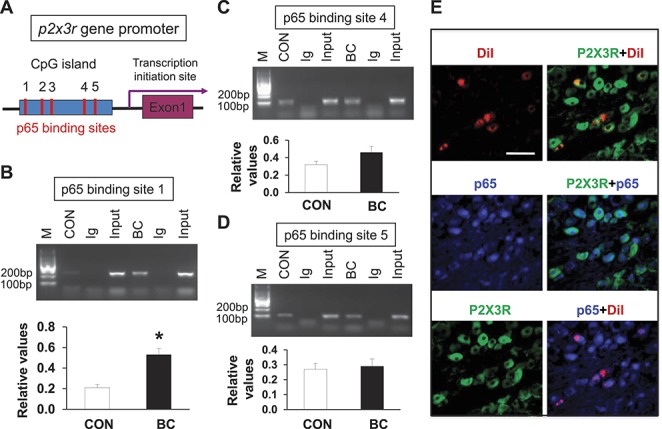
Promoted binding ability between p65 and p2x3r gene promoter in DRGs of cancer pain rat. (A) Schematic of p2x3r gene promoter showing locations of the 5 binding sites for NF-κB in CpG island. Potentiated interaction between NF-κB and p2x3r promoter regions. (B) The specific anti-p65 antibody or CON normal mouse IgG was used for immunoprecipitation. Chromatin immunoprecipitation assays indicated a significant increase in binding activity of p65 with the first binding site of promoter of p2x3r gene in CIP rats compared with age-matched CON rats (* denotes P = 0.011 as compared with CON, 2-sample t test, n = 3 sample, 2 rats for each sample). (C and D) Chromatin immunoprecipitation assays indicated no significant change in binding activity of p65 with the forth and fifth binding sites of promoter of p2x3r gene in CIP rats (n = 3 sample, 2 rats for each sample) compared with age-matched CON rats (n = 3, each for 2 rats). (E) Co-expression of P2X3R with p65 in tibia projection DRG neurons. Tibia-projecting DRG cells were labeled with DiI (red, top left) injected into the tibia. p65-positive cells are shown in blue (middle left). P2X3R-positive cells are shown in green (bottom left). Merge of double labeling of DiI and P2X3R (top right). Merge of p65-positive staining and P2X3R labeling (middle right). Merge of p65-positive staining and DiI labeling (bottom right). Scale bar, 50 μm.
3.6. Tumor cell injection leads to demethylation of CpG sites of p2x3r gene promoter region by downregulation of DNA methyltransferases
To determine whether an epigenetic mechanism contributes to the upregulation of P2X3R expression, DNA methylation status at the promoter region of the p2x3r gene was examined with MSP and bisulfite genomic sequence assay. The genomic structure of rat p2x3r gene contains 1 CpG island in the promoter region (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi) (Fig. 7A). We showed that all samples yielded methylated and unmethylated bands from control and BC groups by MSP assay (Fig. 7B). The ratio of methylated (M) to unmethylated (U) CpG sites for MSP primer was calculated. Tumor cell injection significantly reduced the M/U ratio of CpG sites (Fig. 7B, *P = 0.021 as compared with CON, 2-sample t test, n = 5 for each group), indicating for the first time that there is hypomethylation within CpG island of the p2x3r promoter region under CIP conditions. To further confirm the hypomethylation status of CpG sites within the p2x3r promoter, DNA sequencing was performed on PCR products of the 406-bp fragment (−1737 to −1332) obtained after treatment of genomic DNA samples with sodium bisulfite. As shown in Figure 7A, the 406-bp fragment contains 18 CpG sites indicated by red underlining. All DRG samples from the control and BC rats were successfully sequenced (Fig. 7C). The results indicate that the degree of methylation of CpG sites within the 406-bp fragment was significantly reduced in DRGs 14 days after tumor cell injection when compared with controls (Fig. 7D, *P = 0.012 compared with CON, χ2 test, n = 5 for each group), consistent with the results of the MSP assay. The hypomethylation of p2x3r gene implies that p2x3r gene transcription is enhanced, at least in part, by promoter demethylation, thus contributing to the upregulation of p2x3r expression under CIP conditions.
Figure 7.
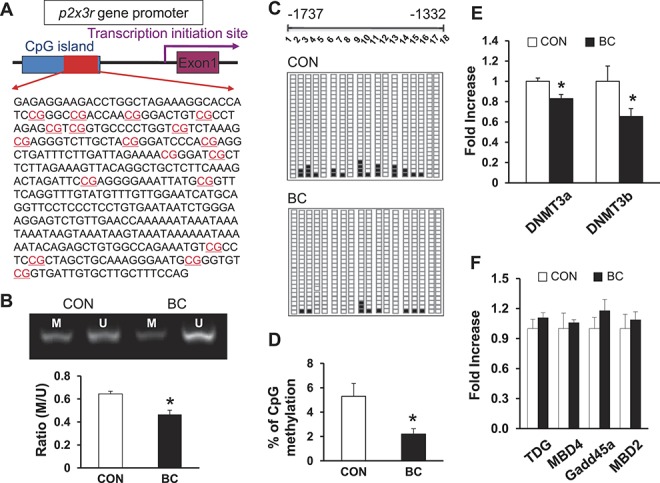
Enhanced demethylation of p2x3r gene promoter in cancer pain rats. (A) Schematic of CpG island showing locations of the 18 CpG sites in p2x3 receptor gene promoter area. (B) Representative PCR results of the p2x3 receptor promoter region using primers for methylation (M)- and unmethylation (U)-specific amplifications of genomic DNA from DRG samples prepared from control and tumor cell–treated rats. Bar graph below the gel images showed ratios of methylated (M) to unmethylated (U) products. Tumor cells injection significantly reduced the M/U ratio of CpG sites. * denotes P = 0.021 as compared with CON, 2-sample t test, n = 5 for each group. (C) Each square indicates the clones from control (top) and tumor cell–treated rats (bottom). Twenty-five clones were subjected to bisulfite sequencing. The methylated clones for individual CpG sites (indicated in C) are labeled in black. (D) Tumor cell injection significantly reduced the percentage of methylated CpG sites in this region. * denotes P = 0.012 compared with CON, χ2 test, n = 5 for each group. (E) Tumor cell injection led to a reduced expression of DNMT3a and DNMT3b in DRG samples when compared wit controls. * denotes P = 0.009 for DNMT3a and 0.041 for DNMT3b as compared with corresponding CON, Student t test, n = 5 for each group. (F) Tumor cell injection did not produce significant changes in the expression of TDG MBD4, Gadd45a, and MBD2 in DRG samples when compared with controls, n = 5 for each group.
We next determine whether DNA methyltransferases 3a and 3b (DNMT3a and DNMT3b) are involved in the hypomethylation of p2x3r gene, because these 2 enzymes are the 2 primary enzymes responsive for DNA methylation. As shown in Figure 7E, the expression of DNMT3a and DNMT3b was remarkably reduced in BC rats when compared with age- and sex-matched controls (Fig. 7E, *P = 0.009 for DNMT3a and 0.041 for DNMT3b as compared with corresponding CON, 2-sample t test, n = 5 for each group). We also examined the expression of thymine DNA glycosylase, methyl-binding domain protein 2 and 4 (MBD2 and MBD4), and growth arrest and DNA damage-inducible protein alpha (Gadd45a) of L2-L5 DRGs in BC rats 14 days after tumor cell injection. None of these molecules was altered in expression after tumor cell injection (Fig. 7F).
4. Discussion
In this study, we attempt to elucidate a novel role for p65 signaling and an epigenetic regulation of p2x3r gene expression in female rats with cancer-induced pain hypersensitivity. We provide direct evidence for the first time to support the idea that p65 signaling contributes to the development of CIP by activation of P2X3 receptors. The development of mechanical hypersensitivity in CIP rats was reversed by NF-κB inhibitor, PDTC, and by LV-p65 shRNA, which suppressed p65 expression. Further studies showed that tumor cell injection increased expression of p65 in tibia-related DRGs. These data suggest that the p65 signaling pathway plays a critical role in CIP hypersensitivity and may be of importance in the clinic.
The injection of mammary gland carcinomas was used to establish an animal model of cancer pain. The clinical condition we are attempting to mimic is the pain induced by breast cancer bone metastasis. Because previous studies have shown that the repertoire of sensory nerve fibers that innervate the skin are very distinct from those that innervate the bone,14 and it is important for clinicians and basic scientists to understand the tissue the cancer pain is most likely arising from. Although we do not know the exact tissue that the tumor cells have invaded, the pathophysiological changes were observed in the primary sensory neurons, indicating the injury of sensory nerve fibers. Because the tumor cells were slowly injected into the tibia cavity by a microinjection syringe and the syringe was left in place for an additional 2 minutes and at last the pinhole was sealed with dental cement, it is least likely that the carcinoma cells could leak out along the injection track. So, we hold the view that in the early stage, tumor cells were limited in the tibia cavity and that the tumor cells invaded the bone tissue. In the later stage, however, the tumor cells destroyed the cortex of the bone and went into the surrounding tissues outside the tibia. It is likely that tumor cells induced injury to nerves that innervate the skin of the hind paw. Therefore, pain in the present setting is probably a neuropathic pain generated by tumor cell–induced injury to nerves that innervate the skin of the hind paw and not bone where the tumor cells were originally injected.
The pathophysiology of CIP is complex and multifactorial.23 Recent studies have demonstrated that increased purinergic receptor expression is implicated in the pathogenesis of CIP.19 In this study, we showed that tumor cell injection led to a significant upregulation of P2X3Rs without alteration in expression of P2X1 and P2X2 subtypes of purinergic receptors. Our findings confirm and extend previous reports that purinergic receptors play an important role in CIP through upregulation both at the protein and mRNA levels after tumor cell injection.19,36 In addition, we showed that tibia projection neurons from CIP rats are hyperactive to ATP stimulation. This conclusion was evidenced by our observations of a significant inward current density and enhanced firing frequencies of DRG neurons in responding to ATP application in CIP rats when compared with age- and sex-matched controls. Importantly, we showed that P2 receptor inhibitor suramin and selective P2X3 and P2X2/3 receptor inhibitor A317491 significantly attenuated behavioral responses to mechanical and noxious heat stimulation. Together with the findings that the expression of P2X1 and P2X2 receptors was not significantly altered, it is most likely that P2X3 receptors might be an effective facilitator of CIP hypersensitivity.
The most prominent finding in this study is that the molecular pathway involved in regulation of p2x3r gene expression might occur mainly at a transcriptional level. The mechanism by which tumor cell injection increases expression of p2x3r seems to involve NF-κB signaling because upregulation of p2x3r expression was markedly reduced by an NF-κB inhibitor PDTC. Using the retrograde neuronal tracer DiI, selective lumbar DRG neurons innervating the intramedullary space of the tibia were identified (Supplementary Figure S3A, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A110). Colocalization studies demonstrated that these DRG neurons express both P2X3R and p65, indicating a possible regulation of P2X3R expression by p65 signaling (Fig. 6E). However, how NF-κB regulates P2X3R expression remains largely unknown. In this study, we showed for the first time that p65-binding ability with p2x3r gene promoter was drastically enhanced in DRGs of CIP rats (Fig. 6B). Hence, the enhanced binding activity of p65 with p2x3r gene contributes to the upregulation of P2X3Rs after tumor cell injection in rats. Of note is that Liu et al.19 recently reported that visinin-like protein 1 is involved in the observed upregulation of P2X3Rs in DRG neurons. Thus, further investigation is merited to determine cross talk between these 2 pathways under CIP conditions.
The mechanism by which p65 binding to p2x3r gene was enhanced remains unknown. We propose a mechanism whereby a significant DNA demethylation of p2x3r promoter is a basis for the enhanced binding activity. Emerging evidence shows that epigenetic regulation (ie, DNA aberrant methylation) is involved in gene expression. We have previously reported that DNA demethylation of the cbs gene promoter area is involved in persistent inflammatory hyperalgesia and diabetic gastric hypersensitivity, which is consistent with upregulation of CBS protein and mRNA.29,44 We did not focus on the role of CBS gene in this study because cbs gene expression was not significantly altered in CIP (Fig. 2C and Supplementary Figure S5A, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A112). Very interestingly, we showed for the first time that the ratio of methylated and unmethylated DNA of p2x3r gene promoter was significantly reduced after tumor cell injection, indicating a significant DNA demethylation of p2x3r promoter after tumor cell injection. This would give rise to an enhanced biding activity of p65 with p2x3r gene and to an upregulation of P2X3R expression in CIP rats. It is well established that 4 members of DNMTs regulate DNA methylation in mammals. DNMT3a and DNMT3b are 2 major enzymes responsible for de novo methylation both in responses to stressors and in oncogenesis in mammals. Our observations of a significant decrease in DNMT3a and DNMT3b expression support the hypothesis that downregulation of DNMTs is a primary driving force for p2x3r gene demethylation after tumor cell-induced CIP. This is consistent with our previous report that DNMT3a and DNMT33b were drastically downregulated in gastric DRGs under diabetic neuropathic pain conditions.44 This is, however, different from our previously published study that showed an active mechanism is involved in persistent inflammatory pain.29 Although the detailed mechanism for this difference is unclear, it is possible that the mechanism of bone cancer–induced DNA demethylation may be different from that of inflammatory pain but may share a common mechanism with neuropathic pain. Our data suggest for the first time that an epigenetic regulation might be involved in development of CIP hypersensitivity by enhancing NF-κB-mediated p2x3r gene expression. Together with our previous reports29,44 and the reports of others,45 this study further supports a hypothesis that epigenetic regulation may be disease and/or organ specific.
A major hindrance has been the lack of suitable pharmacologic tools for the treatment of CIP. Although some selective P2X3 and P2X2/3 receptor antagonists have been recently reported to be efficacious in CIP,15 a significant drawback of these molecules is their pharmacokinetic attributes. This limits their value for in vivo model testing and forestalls any possible clinical application. Studies of the potential roles of NF-κB in pain behavior have been greatly advanced with the use of knockout mice and other molecular techniques, including small interfering RNA and oligonucleotides.9,32 Pan et al.27 demonstrated PDTC as the first potent and selective p65 antagonist to be effective in reducing chronic inflammatory and neuropathic pain behavior in animals. As a result of very poor oral bioavailability and CNS penetration, in addition to very high protein binding (>99.9%), the use of PDTC as an in vivo tool and its potential for further clinical development are limited. In this study, we have developed a highly efficient method of lentivirus-mediated delivery of shRNA targeting NF-κBp65 for gene silencing. Under the prophylactic regimen, p65 shRNA significantly altered CIP. The underlying mechanism is most likely by inhibition of P2X3R expression and function. This analgesic effect lasted for a relatively long time when compared with the effect induced by administration of PDTC. Of note is that some of the therapies used in previous reports might have disease-modifying actions in CIP.22,23,33 Future studies need to investigate the disease-modifying actions and the specificity of LV vector encoding shRNA against NF-κBp65 gene in this setting.
In summary, we have shown the analgesic efficacy of an i.p. injection of p65 inhibitor, PDTC, or an i.t. injection of LV-shRNA specifically targeting p65 for gene silencing in a clinically relevant chronic pain model. Particularly, an inhibition by LV-shRNA specifically targeting p65 for gene silencing may have therapeutic potential in alleviating CIP. Further studies are merited to investigate the effectiveness of this strategy. Our results also add to the growing body of evidence supporting an important role for the p65-P2X3 receptor signaling pathway by epigenetic regulations in cancer pain states.
Conflict of interest statement
The authors have no conflicts of interest to declare.
This work was supported by grants from National Natural Science Foundation of China (81070884 and 81471137 to GYX and 31271258 to XHJ) and from the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Author Contributions: Y.-L. Zhou researched, analyzed data, and wrote the manuscript. G.-Q. Jiang researched, analyzed data, and wrote the manuscript. J. Wei researched data. H.-H. Zhang researched and analyzed data. W. Chen researched data. H. Zhu researched data. S. Hu analyzed data. X. Jiang contributed to discussion and reviewed/edited manuscript. G.-Y. Xu designed and supervised the experiments and edited the manuscript.
Y.-L. Zhou, G.-Q. Jiang and J. Wei contributed equally to this work.
Appendices. Supplemental Digital Content
Supplemental Digital Content associated with this article can be found online at http://links.lww.com/PAIN/A108, http://links.lww.com/PAIN/A109, http://links.lww.com/PAIN/A110, http://links.lww.com/PAIN/A111, and http://links.lww.com/PAIN/A112.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Bruera E, Kim HN. Cancer pain. JAMA 2003;290:2476–9. [DOI] [PubMed] [Google Scholar]
- [2].Burnstock G. Purinergic signalling: pathophysiology and therapeutic potential. Keio J Med 2013;62:63–73. [DOI] [PubMed] [Google Scholar]
- [3].Burnstock G, Kennedy C. P2X receptors in health and disease. Adv Pharmacol 2011;61:333–72. [DOI] [PubMed] [Google Scholar]
- [4].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- [5].Chen YL, Law PY, Loh HH. Sustained activation of phosphatidylinositol 3-kinase/Akt/nuclear factor kappaB signaling mediates G protein-coupled delta-opioid receptor gene expression. J Biol Chem 2006;281:3067–74. [DOI] [PubMed] [Google Scholar]
- [6].Coleman JE, Huentelman MJ, Kasparov S, Metcalfe BL, Paton JF, Katovich MJ, Semple-Rowland SL, Raizada MK. Efficient large-scale production and concentration of HIV-1-based lentiviral vectors for use in vivo. Physiol Genomics 2003;12:221–8. [DOI] [PubMed] [Google Scholar]
- [7].Delaney A, Fleetwood-Walker SM, Colvin LA, Fallon M. Translational medicine: cancer pain mechanisms and management. Br J Anaesth 2008;101:87–94. [DOI] [PubMed] [Google Scholar]
- [8].Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci U S A 1992;89:1827–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fu ES, Zhang YP, Sagen J, Candiotti KA, Morton PD, Liebl DJ, Bethea JR, Brambilla R. Transgenic inhibition of glial NF-kappa B reduces pain behavior and inflammation after peripheral nerve injury. PAIN 2010;148:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gilchrist LS, Cain DM, Harding-Rose C, Kov AN, Wendelschafer-Crabb G, Kennedy WR, Simone DA. Re-organization of P2X3 receptor localization on epidermal nerve fibers in a murine model of cancer pain. Brain Res 2005;1044:197–205. [DOI] [PubMed] [Google Scholar]
- [11].Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. PAIN 1988;32:77–88. [DOI] [PubMed] [Google Scholar]
- [12].Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci 2001;2:83–91. [DOI] [PubMed] [Google Scholar]
- [13].Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, van Biesen T, Cartmell J, Bianchi B. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci 2002;99:17179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jimenez-Andrade JM, Mantyh WG, Bloom AP, Xu H, Ferng AS, Dussor G, Vanderah TW, Mantyh PW. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone 2010;46306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kaan TK, Yip PK, Patel S, Davies M, Marchand F, Cockayne DA, Nunn PA, Dickenson AH, Ford AP, Zhong Y, Malcangio M, McMahon SB. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain 2010;133:2549–64. [DOI] [PubMed] [Google Scholar]
- [16].Kiyatkin ME, Feng B, Schwartz ES, Gebhart GF. Combined genetic and pharmacological inhibition of TRPV1 and P2X3 attenuates colorectal hypersensitivity and afferent sensitization. Am J Physiol Gastrointest Liver Physiol 2013;305:G638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kumar A, Negi G, Sharma SS. Suppression of NF-kappaB and NF-kappaB regulated oxidative stress and neuroinflammation by BAY 11-7082 (IkappaB phosphorylation inhibitor) in experimental diabetic neuropathy. Biochimie 2012;94:1158–65. [DOI] [PubMed] [Google Scholar]
- [18].Lin A, Karin M. NF-kappaB in cancer: a marked target. Semin Cancer Biol 2003;13:107–14. [DOI] [PubMed] [Google Scholar]
- [19].Liu M, Yang H, Fang D, Yang JJ, Cai J, Wan Y, Chui DH, Han JS, Xing GG. Upregulation of P2X3 receptors by neuronal calcium sensor protein VILIP-1 in dorsal root ganglions contributes to the bone cancer pain in rats. PAIN 2013;154:1551–68. [DOI] [PubMed] [Google Scholar]
- [20].Ma W, Bisby MA. Increased activation of nuclear factor kappa B in rat lumbar dorsal root ganglion neurons following partial sciatic nerve injuries. Brain Res 1998;797:243–54. [DOI] [PubMed] [Google Scholar]
- [21].Makarov SS. NF-kappaB as a therapeutic target in chronic inflammation: recent advances. Mol Med Today 2000;6:441–8. [DOI] [PubMed] [Google Scholar]
- [22].Mancini I, Lossignol DA, Body JJ. Opioid switch to oral methadone in cancer pain. Curr Opin Oncol 2000;12:308–13. [DOI] [PubMed] [Google Scholar]
- [23].Mantyh P. Bone cancer pain: causes, consequences, and therapeutic opportunities. PAIN 2013;154(suppl 1):S54–62. [DOI] [PubMed] [Google Scholar]
- [24].Mao-Ying QL, Zhao J, Dong ZQ, Wang J, Yu J, Yan MF, Zhang YQ, Wu GC, Wang YQ. A rat model of bone cancer pain induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells. Biochem Biophys Res Commun 2006;345:1292–8. [DOI] [PubMed] [Google Scholar]
- [25].Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M, Hattenberger M, Vaxelaire J, O'Reilly T, Wotherspoon G, Winter J, Green J, Urban L. A rat model of bone cancer pain. PAIN 2002;96:129–40. [DOI] [PubMed] [Google Scholar]
- [26].Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002;2:584–93. [DOI] [PubMed] [Google Scholar]
- [27].Pan YD, Guo QL, Wang E, Ye Z, He ZH, Zou WY, Cheng ZG, Wang YJ. Intrathecal infusion of pyrrolidine dithiocarbamate for the prevention and reversal of neuropathic pain in rats using a sciatic chronic constriction injury model. Reg Anesth Pain Med 2010;35:231–7. [DOI] [PubMed] [Google Scholar]
- [28].Prado F, Araldi D, Vieira A, Oliveira-Fusaro M, Tambeli C, Parada C. Neuronal P2X3 receptor activation is essential to the hyperalgesia induced by prostaglandins and sympathomimetic amines released during inflammation. Neuropharmacology 2013;67:252–8. [DOI] [PubMed] [Google Scholar]
- [29].Qi F, Zhou Y, Xiao Y, Tao J, Gu J, Jiang X, Xu GY. Promoter demethylation of cystathionine-beta-synthetase gene contributes to inflammatory pain in rats. PAIN 2013;154:34–45. [DOI] [PubMed] [Google Scholar]
- [30].Qu Y, Wang J, Ray PS, Guo H, Huang J, Shin-Sim M, Bukoye BA, Liu B, Lee AV, Lin X, Huang P, Martens JW, Giuliano AE, Zhang N, Cheng NH, Cui X. Thioredoxin-like 2 regulates human cancer cell growth and metastasis via redox homeostasis and NF-kappaB signaling. J Clin Invest 2011;121:212–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shi L, Zhang HH, Xiao Y, Hu J, Xu GY. Electroacupuncture suppresses mechanical allodynia and nuclear factor kappa B signaling in streptozotocin-induced diabetic rats. CNS Neurosci Ther 2013;19:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sun T, Luo J, Jia M, Li H, Li K, Fu Z. Small interfering RNA-mediated knockdown of NF-kappaBp65 attenuates neuropathic pain following peripheral nerve injury in rats. Eur J Pharmacol 2012;682:79–85. [DOI] [PubMed] [Google Scholar]
- [33].Syrjala KL, Jensen MP, Mendoza ME, Yi JC, Fisher HM, Keefe FJ. Psychological and behavioral approaches to cancer pain management. J Clin Oncol 2014;32:1703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tong Z, Luo W, Wang Y, Yang F, Han Y, Li H, Luo H, Duan B, Xu T, Maoying Q, Tan H, Wang J, Zhao H, Liu F, Wan Y. Tumor tissue-derived formaldehyde and acidic microenvironment synergistically induce bone cancer pain. PLoS One 2010;5:e10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang C, Ning LP, Wang YH, Zhang Y, Ding XL, Ge HY, Arendt-Nielsen L, Yue SW. Nuclear factor-kappa B mediates TRPV4-NO pathway involved in thermal hyperalgesia following chronic compression of the dorsal root ganglion in rats. Behav Brain Res 2011;221:19–24. [DOI] [PubMed] [Google Scholar]
- [36].Wu JX, Xu MY, Miao XR, Lu ZJ, Yuan XM, Li XQ, Yu WF. Functional up-regulation of P2X3 receptors in dorsal root ganglion in a rat model of bone cancer pain. Eur J Pain 2012;16:1378–88. [DOI] [PubMed] [Google Scholar]
- [37].Wu Z, Shen S, Zhang Z, Zhang W, Xiao W. Ubiquitin-conjugating enzyme complex Uev1A-Ubc13 promotes breast cancer metastasis through nuclear factor-кB mediated matrix metalloproteinase-1 gene regulation. Breast Cancer Res 2014;16:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xu GY, Huang LY. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci 2002;22:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xu GY, Li G, Liu N, Huang LY. Mechanisms underlying purinergic P2X3 receptor-mediated mechanical allodynia induced in diabetic rats. Mol Pain 2011;7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut 2008;57:1230–7. [DOI] [PubMed] [Google Scholar]
- [41].Xu Q, Zhang XM, Duan KZ, Gu XY, Han M, Liu BL, Zhao ZQ, Zhang YQ. Peripheral TGF-beta1 signaling is a critical event in bone cancer-induced hyperalgesia in rodents. J Neurosci 2013;33:19099–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ye Y, Dang D, Viet CT, Dolan JC, Schmidt BL. Analgesia targeting IB4-positive neurons in cancer-induced mechanical hypersensitivity. J Pain 2012;13:524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zech DF, Grond S, Lynch J, Hertel D, Lehmann KA. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. PAIN 1995;63:65–76. [DOI] [PubMed] [Google Scholar]
- [44].Zhang HH, Hu J, Zhou YL, Hu S, Wang YM, Chen W, Xiao Y, Huang LY, Jiang X, Xu GY. Promoted interaction of nuclear factor-kappaB with demethylated cystathionine-beta-synthetase gene contributes to gastric hypersensitivity in diabetic rats. J Neurosci 2013;33:9028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang Z, Cai YQ, Zou F, Bie B, Pan ZZ. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med 2011;17:1448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zheng Q, Fang D, Cai J, Wan Y, Han JS, Xing GG. Enhanced excitability of small dorsal root ganglion neurons in rats with bone cancer pain. Mol Pain 2012;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]


