Endotoxin-induced systemic immune activation leads to visceral and musculoskeletal hyperalgesia in healthy humans, irrespective of biological sex.
Keywords: Sex differences, Pain, Sickness behaviour, Endotoxin, Lipopolysaccharide, Inflammation, Cytokines, Pressure pain thresholds, Visceral pain thresholds
Abstract
A role of the innate immune system is increasingly recognized as a mechanism contributing to pain sensitization. Experimental administration of the bacterial endotoxin lipopolysaccharide (LPS) constitutes a model to study inflammation-induced pain sensitization, but all existing human evidence comes from male participants. We assessed visceral and musculoskeletal pain sensitivity after low-dose LPS administration in healthy men and women to test the hypothesis that women show greater LPS-induced hyperalgesia compared with men. In this randomized, double-blind, placebo-controlled crossover study, healthy men (n = 20) and healthy women using oral contraceptives (n = 20) received an intravenous injection of 0.4 ng/kg body weight LPS or placebo. Pain sensitivity was assessed with established visceral and musculoskeletal pain models (ie, rectal pain thresholds; pressure pain thresholds for different muscle groups), together with a heartbeat perception (interoceptive accuracy) task. Plasma cytokines (tumor necrosis factor-α and interleukin-6) were measured along with state anxiety at baseline and up to 6-hour postinjection. Lipopolysaccharide application led to significant increases in plasma cytokines and state anxiety and decreased interoceptive awareness in men and women (P < 0.001, condition effects), with more pronounced LPS-induced cytokine increases in women (P < 0.05, interaction effects). Although both rectal and pressure pain thresholds were significantly decreased in the LPS condition (all P < 0.05, condition effect), no sex differences in endotoxin-induced sensitization were observed. In summary, LPS-induced systemic immune activation leads to visceral and musculoskeletal hyperalgesia, irrespective of biological sex. These findings support the broad applicability of experimental endotoxin administration as a translational preclinical model of inflammation-induced pain sensitization in both sexes.
1. Introduction
Sex differences in the prevalence of virtually all types of chronic pain conditions are well documented.17,20 Although epidemiologic findings consistently document a female preponderance, mechanistic studies comparing men and women with respect to pain-related measures have provided inconclusive results both in patients and in healthy cohorts.50,58 The role of inflammatory mediators has been prominently pointed out as 1 key mechanism of both peripheral and central pain sensitization,6,19,43,45,61,64 supported by accumulating evidence that immune mechanisms may promote the initiation and/or maintenance of various chronic inflammatory and functional pain conditions.15,18,36,44,45,47 Interestingly, there is first evidence suggesting that inflammatory mediators may contribute to sex differences in pain sensitivity, involving several possible peripheral and central pathways.34,35,43,44,58 For example, recent animal work, reviewed in Ref. 44 showed that sex differences in Toll-like receptor (TLR) 4-dependent neuroimmune pathways could explain sex-specific differences in pain sensitivity. In humans, data are scarce calling for more experimental work comparing healthy men and women in clinically relevant pain models to address the possible contribution of pro-inflammatory mediators to sex differences in pain sensitization.
We have previously studied pain sensitization in healthy humans during transient systemic immune activation using an experimental low-dose endotoxemia model.4,65 Lipopolysaccharide (LPS), the major component of the outer membrane of gram-negative bacteria, is a prototypic pathogen-associated molecular pattern that stimulates through TLR-4-dependent pathways the synthesis and release of pro-inflammatory cytokines.44,53,55 Our own data4,65 and findings from other groups13,31,32 have documented that pain sensitivity is increased during LPS-induced immune activation. Based on these convergent findings, the potential of experimental endotoxemia as a preclinical model to study inflammation-mediated sensitization in the context of immune-targeted drugs has been proposed.2,30 Importantly, however, with the exception of 1 recent study,32 all existing pain-related human findings during LPS-induced endotoxemia thus far have come from studies conducted exclusively in male participants.4,13,31,65 Therefore, our goal in this follow-up study was to compare LPS-induced pain sensitization in men and women. Based on previous evidence that low-dose LPS (0.4 ng/kg) effectively increased visceral4 and musculoskeletal pain sensitivity in male subjects,13,65 we implemented this randomized, double-blind, placebo-controlled, crossover study using 2 established clinically relevant pain models, that is, rectal distension and pressure pain models, respectively, in healthy men and in healthy women on oral contraceptives. Given the female preponderance of chronic pain conditions, and evidence of sex differences in pain-related neuroimmune communication,35,44 we hypothesized greater LPS effects on pain thresholds in women compared with men. As a secondary aim, we conducted correlative analyses addressing associations between pain sensitivity and inflammatory mediators during LPS-induced endotoxemia separately for men and women. Finally, given that systemic inflammation reportedly increased the activity in brain regions that are involved in interoception25 and higher interoceptive sensitivity was recently shown to be associated with decreased somatic pain thresholds,49 we explored LPS effects on heartbeat perception accuracy using an established task54 to address interoceptive awareness as a possible mediator of sex differences in inflammation-induced hyperalgesia.
2. Methods
2.1. Participants and safety routine
Healthy men and healthy women using hormonal contraceptives aged 18 to 45 years were recruited by public advertisement. Of note, participants were newly recruited for this study, and data do not overlap with any of our previously reported results.4,21,22,24,37,38,65 The screening and safety procedures consisted of a physical examination, a personal interview conducted by a physician, and laboratory assessments (ie, complete blood cell count, liver enzymes, renal parameters, electrolytes, coagulation factors, and C-reactive protein [CRP]), which were conducted before, 24 hours after endotoxin administration, and up to 1 week after completion of the study. General exclusion criteria were pre-existing or current medical or psychological conditions, body mass index <18 or ≥29 kg/m2, current medications, smoking, regular high alcohol use (>4 drinks per week), or anxiety and/or depression scores exceeding published cutoffs of the Hospital Anxiety and Depression Scale.28 Complaints suggestive of any functional or organic gastrointestinal condition were assessed by semistructured interview and a standardized questionnaire.39 To strictly exclude pregnancy, only women using hormonal contraceptives were included as per our ethics committee, and a commercially available pregnancy test was conducted on each study day. Female volunteers were scheduled for both study days at a time period outside of the phase of withdrawal bleeding (ie, shedding of the uterine lining that is triggered by the drop in estrogen levels that occurs while taking the placebo pills). For safety reasons, participants were instructed to refrain from strenuous exercise 48 hours before and 24 hours after the study days and were not allowed to drive a vehicle. Participants were monitored up to at least 6 hours after LPS injections. Follow-up examinations, including physical examination and laboratory analyses of CRP levels, were completed 24 hours after each session and 7 days after the final session. The study protocol was approved by the ethics committee of the University Hospital Essen (Permit No. 09-4271). All subjects provided written informed consent and were paid for their participation.
2.2. Study protocol
This randomized, double-blind, placebo-controlled, crossover study comprised 2 identical study days (Fig. 1), on which subjects received an intravenous injection of either LPS (0.4 ng of Escherichia coli endotoxin per kilogram of body weight dissolved in sterile water; LPS condition) or the same amount of saline (placebo condition) as previously described in detail.4,24 The LPS used (reference standard endotoxin, lot H0K354; United States Pharmacopeia, Rockville, MD) had been subjected to a microbial safety testing routine approved by the German Federal Agency for Sera and Vaccines (Paul Ehrlich Institute, Langen, Germany). On both study days, subjects were injected between 9 am and 11 am by an intravenous catheter placed in an antecubital forearm vein. The time interval between both study days was 5 to 7 days, and subjects were injected at identical time points on both days. Assessments of interoceptive sensitivity, visceral, and pressure pain sensitivity (see below) were conducted 2 to 3.5 hours after injection when cytokines are demonstrably increased.4,23,24,65 Because of the temporal dynamics of the LPS-induced cytokine response, the order of interoceptive and pain sensitivity assessments was not counterbalanced to ensure interindividual comparability of plasma cytokine levels during assessments as previously accomplished.4 Blood for cell counts and cytokine analyses was collected in EDTA-coated tubes 15 minutes before the injection (baseline) and 1, 2, 3, 4, and 6 hours after injection. Plasma was separated by centrifugation and was stored at −80°C until analysis. After each blood draw, body temperature (with an intra-aurical thermometer), blood pressure (with a blood pressure cuff), heart rate (palpated at radial artery), and state anxiety (with a validated questionnaire, see section 2.8) were assessed. Both participants and investigators involved in sensitivity testing were blinded to the condition. Because the outcome of pain assessments may be affected by investigator × subject sex interaction effects,1 20 participants (10 men and 10 women) were assessed by a male investigator, and the remaining 20 participants (10 men and 10 women) were assessed by a female investigator. Participants were randomized to one of the 2 investigators before the first study day. Assessments on both study days were conducted then by the same male or female investigator.
Figure 1.
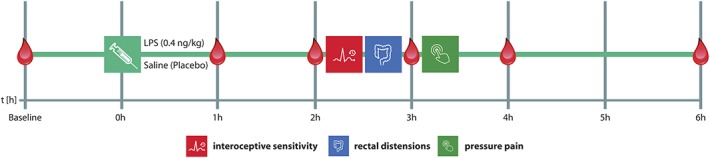
Study design: In this randomized, double-blind, placebo-controlled crossover study, healthy men (N = 20) and women using hormonal contraceptives (N = 20) received an intravenous injection of either lipopolysaccharide (LPS; 0.4 ng/kg body weight) or the same volume of saline (placebo) on 2 separate study days in a randomized order. Assessments of interoceptive sensitivity (accuracy of heartbeat perception), visceral thresholds (pressure-controlled rectal distensions), and musculoskeletal pain sensitivity (pressure pain thresholds) were conducted 2 to 3.5 hours after injection of LPS or placebo. Plasma samples for cytokine and cortisol analyses were collected at baseline as well as 1, 2, 3, 4, and 6 hours after injection, followed by assessment of state anxiety.
2.3. Visceral pain sensitivity (rectal distensions)
Pressure-controlled distension of the viscera such as the esophagus, stomach, or rectum constitutes an established visceral pain model in animal and human research and is widely applied in mechanistic studies addressing altered sensory functions including pain in the context of the functional gastrointestinal disorders such as irritable bowel syndrome.33 Herein, rectal distensions were carried out with a pressure-controlled barostat system (modified ISOBAR 3 device; G & J Electronics, Ontario, Canada), as previously described.3,4,56,57 Perception and pain thresholds were determined using double-random staircase distensions with random pressure increments of 2 to 8 mm Hg. Subjects were prompted to rate the sensation as follows: 1 = no perception, 2 = doubtful perception, 3 = sure perception, 4 = little discomfort, 5 = severe discomfort, still tolerable, 6 = pain. The sensory threshold was defined as the pressure when the rating changed from 2 to 3, and the pain threshold was defined as pressure at which the rating changed from 5 to 6. The maximal distension pressure was set at 50 mm Hg.
2.4. Musculoskeletal pain sensitivity (pressure pain thresholds)
Pressure pain thresholds (PPTs) are a commonly used and reliable method to evaluate somatic pain sensitivity.52 Pressure pain thresholds assess deep muscle pain sensitivity; most likely mediated by muscle C-fibers and Aδ-fibers.52 Pressure pain thresholds were measured using a digital algometer (FDX 50; Wagner Instruments, Greenwich, CT), as previously described in detail.65 Briefly, a 1 cm2 rubber tip was placed directly on the skin, and pressure was gradually increased by 1 kilogram force per square centimeter (kgf/cm2). Pressure pain threshold was defined as the pressure at which the participant first indicated that the stimulus becomes painful. To avoid a potential response bias, the display of the algometer was not visible to participants, and no feedback was given regarding the PPT values. Both investigators involved in PPT testing were trained before the study and achieved a good inter-rater reliability (rtt = 0.86) in a prestudy in N = 10 healthy volunteers (data not included in this study). Pressure pain thresholds were assessed bilaterally for 4 body parts in a fixed order, that is, left/right lower back (5 cm from spinous process of L3), calf (one-third of total gastrocnemius muscle length below the popliteal space), insertion of the deltoid muscle, and trapezius muscle (central). To avoid unreliable PPT measurements, PPTs exceeding 20 kg/cm2 were excluded from further analysis according to established procedures.52 Each PPT measurement was repeated 4 times within the respective muscle group, and the mean PPT values of the second to fourth measurement were merged for both body sites to provide an overall PPT value for the respective muscle group, as suggested by a recent methodological algometry study.40
2.5. Interoceptive sensitivity (accuracy of heartbeat perception)
Given evidence suggesting a role of interoceptive sensitivity (or accuracy) in pain perception49 and a lack of knowledge regarding possible effects of inflammation on this measure, we assessed interoceptive accuracy with an established heartbeat perception task (ie, mental tracking method54). Interoceptive accuracy was always assessed first as it was the least invasive method and so as to avoid pain-induced interference with results of this measure. Participants were seated in a comfortable chair in a silent room, fitted with an electrocardiogram system (Task Force Monitor; CNSystems, Graz, Austria). After a short resting period, participants were asked to silently count their heartbeat by concentrating on their body sensations, without taking their pulse or other manipulations allowing to objectively detect their cardiac rhythm (according to Refs. 49 and 54). Notably, the display of the Task Force Monitor was not visible for participants, and no feedback was given on the accuracy of perceived heartbeat. Three consecutive sessions of varying length (ie, 25, 35, and 45 seconds) were conducted with the beginning and end of each session announced from outside the room. Participants were instructed to report the number of perceived heartbeats immediately after each session. According to Pollatos et al.,49 the accuracy of heartbeat perception was calculated as accuracy score = 1/3 Σ (1 − [| heartbeatrecorded − heartbeatperceived |]/heartbeatrecorded), which can range between 0 and 1, with higher scores indicating higher perception accuracy. Scores were computed separately for the study conditions (LPS, placebo).
2.6. White blood cell counts
White blood cell counts were determined using an automated cell counter (Sysmex KX-21N, Norderstedt, Germany).
2.7. Cytokines, cortisol, and C-reactive protein
Plasma concentrations of tumor necrosis factor (TNF)-α and interleukin (IL)-6 were analyzed using commercial enzyme-linked immunosorbent assays (Quantikine IL-6 and high-sensitive TNF-α ELISA; R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The sensitivity of the assays was 0.70 pg/mL for IL-6 and 0.11 pg/mL for TNF-α. Total plasma cortisol was analyzed with commercial enzyme-linked immunosorbent assay according to the manufacturer's instructions (Cortisol ELISA; IBL International, Hamburg, Germany; detection limit 0.138 nmol/L). C-reactive protein was assessed before and 24-hour postinjection of LPS and up to 1 week after study after completion of the study. C-reactive protein was analysed with a polyethylene glycol–enhanced immunoturbidimetric assay by the Division of Laboratory Research of the University Hospital Essen (Germany).
2.8. Questionnaires
State anxiety was assessed at baseline, and 1, 2, 3, 4, and 6 hours after injection using the state version of the State-Trait-Anxiety-Inventory.60 Higher sum scores indicate higher state anxiety. Retrospective assessment of side effects was accomplished 6 hours after application of LPS or placebo with an adapted version of a validated questionnaire (Generic Assessment of Side Effects, GASE51). Briefly, subjects were asked to rate the severity of 30 different LPS-related side effects (eg, headache, nausea, sweating) from 0 (not present) to 3 (severe), and a sum score was calculated. Ratings of each symptom and the GASE sum score were compared between men and women.
2.9. Statistical analyses
All data were tested for normal distribution with Kolmogorov–Smirnov test, and nonnormally distributed data were log transformed to achieve normal distribution before analysis (ie, cytokine data). Sociodemographic and psychological data obtained from men and women were compared using independent samples t tests or χ2 tests. Ratings of single GASE items (indicating symptom severity of different LPS-related side effects) were compared with Bonferroni-corrected Mann–Whitney U tests. For all repeated measures, analyses of variance (ANOVAs) with the between-subject factor sex (men, women) and the repeated factors condition (ie, LPS, placebo) and time (ie, measurement points) were computed, with effect sizes reported as partial η2 (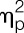 ). If ANOVA revealed significant sex × time or sex × condition effects for repeated measures (ie, cytokine and cortisol levels, body temperature), independent samples t tests were computed to compare men and women within the LPS condition. Post hoc paired t tests were computed separately in men and women to compare conditions (ie, LPS vs placebo) at single measurement points in case of significant ANOVA condition or condition × time effects. Post hoc paired t tests (1-tailed) were also computed to additional explore LPS effects on pain measures separately in men and women. Correlation analyses were carried out by computing Spearman's ρ. The alpha level was set at 0.05. All data are presented as mean and standard error of the mean unless indicated otherwise.
). If ANOVA revealed significant sex × time or sex × condition effects for repeated measures (ie, cytokine and cortisol levels, body temperature), independent samples t tests were computed to compare men and women within the LPS condition. Post hoc paired t tests were computed separately in men and women to compare conditions (ie, LPS vs placebo) at single measurement points in case of significant ANOVA condition or condition × time effects. Post hoc paired t tests (1-tailed) were also computed to additional explore LPS effects on pain measures separately in men and women. Correlation analyses were carried out by computing Spearman's ρ. The alpha level was set at 0.05. All data are presented as mean and standard error of the mean unless indicated otherwise.
3. Results
3.1. Sociodemographic and psychological characteristics
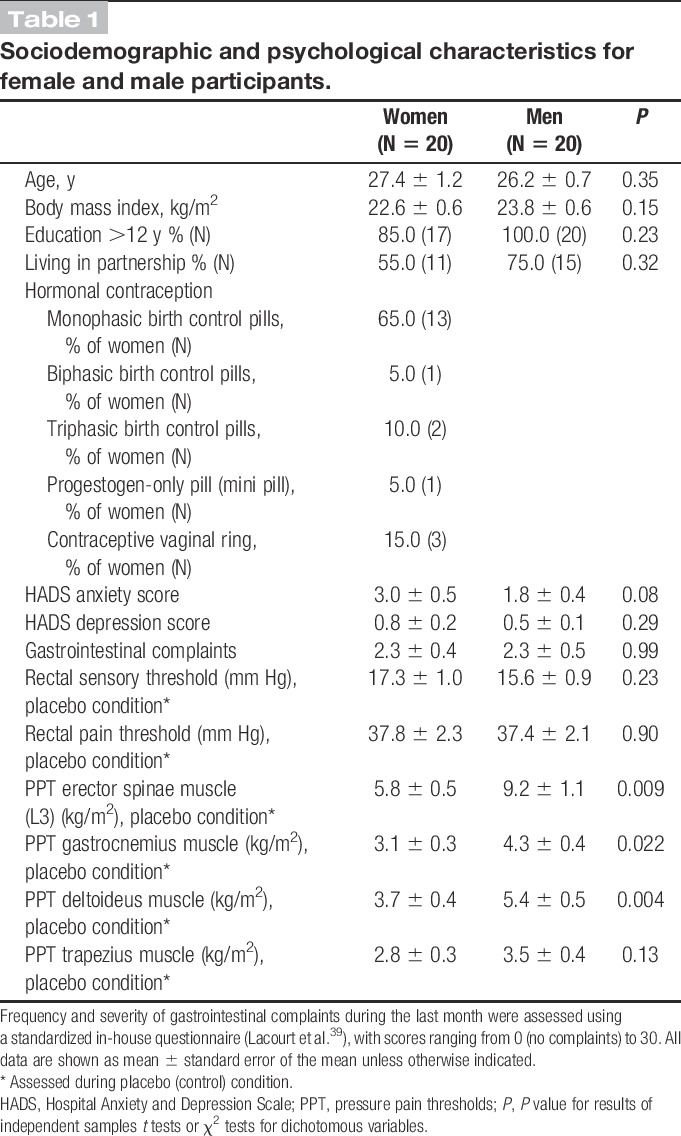
Forty healthy volunteers (20 men and 20 women on hormonal contraceptives) with a mean age of 27.6 ± 0.5 years participated in this study. Men and women did not differ significantly in age, body mass index, education, or partnership status (Table 1). Hospital Anxiety and Depression Scale anxiety and depression scores as well as frequency and severity of gastrointestinal complaints during the preceding month were well within the normal range, without evidence of any sex differences (Table 1).
Table 1.
Sociodemographic and psychological characteristics for female and male participants.
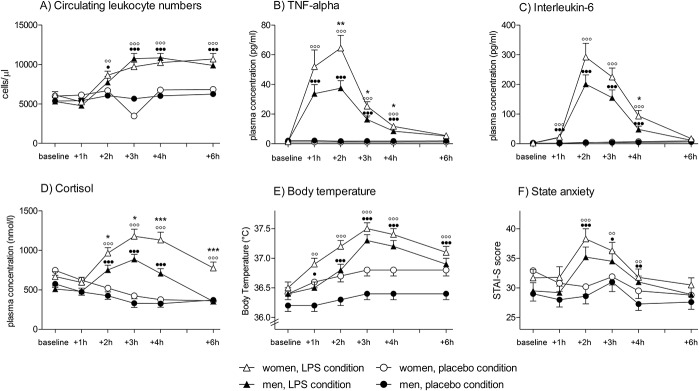
3.2. White blood cell counts, plasma cytokines, cortisol, body temperature, and C-reactive protein
Endotoxin administration led to an acute and transient systemic immune activation as reflected by increases in white blood cell counts, pro-inflammatory cytokine levels, and body temperature. Specifically, number of circulating leukocytes (F = 69.4, P < 0.001, 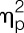 = 0.65), plasma concentrations of TNF-α (F = 208.4, P < 0.001,
= 0.65), plasma concentrations of TNF-α (F = 208.4, P < 0.001, 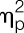 = 0.97), and IL-6 (F = 122.5, P < 0.001,
= 0.97), and IL-6 (F = 122.5, P < 0.001, 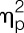 = 0.95) significantly increased in response to LPS injection (all condition × time interaction effects; Fig. 2A-C). Women showed more pronounced increases in TNF-α (F = 6.4, P = 0.016,
= 0.95) significantly increased in response to LPS injection (all condition × time interaction effects; Fig. 2A-C). Women showed more pronounced increases in TNF-α (F = 6.4, P = 0.016, 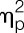 = 0.15; sex × condition interaction effect) in response to LPS, and IL-6 levels were higher across all time points (F = 4.9, P = 0.034,
= 0.15; sex × condition interaction effect) in response to LPS, and IL-6 levels were higher across all time points (F = 4.9, P = 0.034, 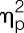 = 0.11, sex effect), along with a delayed increase in circulating leukocyte number (F = 3.1, P = 0.01,
= 0.11, sex effect), along with a delayed increase in circulating leukocyte number (F = 3.1, P = 0.01, 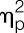 = 0.08, sex × condition × time interaction effect) (for results of post hoc tests, see Fig. 2). Plasma cortisol concentrations significantly increased (F = 38.1, P < 0.001,
= 0.08, sex × condition × time interaction effect) (for results of post hoc tests, see Fig. 2). Plasma cortisol concentrations significantly increased (F = 38.1, P < 0.001, 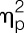 = 0.85; condition × time interaction; Fig. 2D), with women demonstrating a significantly enhanced and prolonged response (F = 5.2, P = 0.001,
= 0.85; condition × time interaction; Fig. 2D), with women demonstrating a significantly enhanced and prolonged response (F = 5.2, P = 0.001, 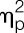 = 0.44, sex × condition × time interaction effect). Body temperature also increased slightly in response to LPS, with a maximum of 37.5 ± 0.1°C in women and 37.3 ± 0.1°C in men 3 hours after injection (F = 11.9, P < 0.001,
= 0.44, sex × condition × time interaction effect). Body temperature also increased slightly in response to LPS, with a maximum of 37.5 ± 0.1°C in women and 37.3 ± 0.1°C in men 3 hours after injection (F = 11.9, P < 0.001, 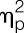 = 0.64; condition × time interaction effect) without evidence of sex differences (Fig. 2E). Twenty-four hours after injection of LPS, CRP concentrations were slightly higher in women (2.32 ± 0.2 mg/dL) compared with men (1.86 ± 0.1 mg/dL), but this difference failed to reach statistical significance (t = 2.0, P = 0.056). C-reactive protein concentrations were below detection level (ie, <0.5 mg/dL) before and 7 days after completion of the study in all subjects.
= 0.64; condition × time interaction effect) without evidence of sex differences (Fig. 2E). Twenty-four hours after injection of LPS, CRP concentrations were slightly higher in women (2.32 ± 0.2 mg/dL) compared with men (1.86 ± 0.1 mg/dL), but this difference failed to reach statistical significance (t = 2.0, P = 0.056). C-reactive protein concentrations were below detection level (ie, <0.5 mg/dL) before and 7 days after completion of the study in all subjects.
Figure 2.
Indicators of lipopolysaccharide (LPS)-induced systemic inflammation: White blood cell counts (A), plasma levels of tumor necrosis factor (TNF)-α (B), interleukin (IL)-6 (C), and cortisol (D) as well as body temperature (E) and state anxiety (State-Trait-Anxiety-Inventory) (F) were measured at baseline and 1, 2, 3, 4, and 6 hours after injection of either LPS (0.4 ng/kg body weight) or saline (placebo). All parameters showed significant increases in response to LPS compared with placebo, indicating a systemic immune activation. Black dots indicate results of post hoc paired t tests comparing the LPS vs placebo condition within male subjects (●P < 0.05, ●●P < 0.01, ●●●P < 0.001), and white dots indicate results of post hoc paired t tests comparing the LPS vs placebo condition within female subjects (○○P < 0.01, ○○○P < 0.001). Women showed significantly greater increases in plasma TNF-α, IL-6, and cortisol in responses to LPS application (*P < 0.05, **P < 0.01, ***P < 0.001, results of post hoc t tests comparing men and women within the LPS condition). For results of analysis of variance, see text. Only women using hormonal contraceptives were included in this study.
3.3. Visceral pain sensitivity
Men and women did not differ in rectal sensory thresholds (t = 1.2, P = 0.23) or pain thresholds (t = 0.1, P = 0.90) in the placebo condition (Table 1). Lipopolysaccharide administration significantly lowered rectal pain thresholds (F = 24.8, P < 0.001, 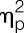 = 0.40, condition effect), with similar reductions in women and men (F = 1.5, P = 0.24,
= 0.40, condition effect), with similar reductions in women and men (F = 1.5, P = 0.24, 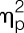 = 0.04, sex × condition effect; Fig. 3A). Additional exploratory analyses conducted separately within men and women confirmed that both men (t = −3.2, P = 0.002) and women (t = −3.8, P < 0.001) showed significant decreases in pain rectal thresholds after LPS application. Rectal sensory thresholds did not differ between men and women and did not change in response to LPS administration (Fig. 3B).
= 0.04, sex × condition effect; Fig. 3A). Additional exploratory analyses conducted separately within men and women confirmed that both men (t = −3.2, P = 0.002) and women (t = −3.8, P < 0.001) showed significant decreases in pain rectal thresholds after LPS application. Rectal sensory thresholds did not differ between men and women and did not change in response to LPS administration (Fig. 3B).
Figure 3.
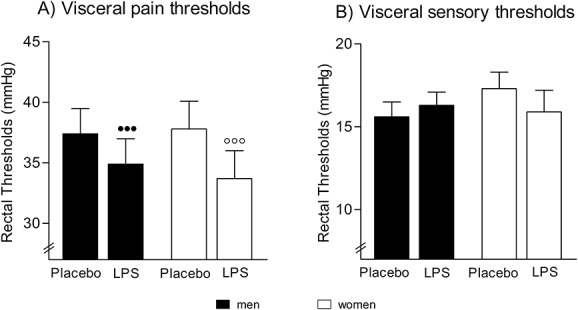
Rectal thresholds: Rectal pain (A) and sensory (B) thresholds assessed 2 hours after injection of lipopolysaccharide (LPS; 0.4 ng/kg body weight) or saline (placebo) in healthy men and in healthy women using hormonal contraceptives. Lipopolysaccharide application led to significantly reduced rectal pain thresholds in men (●●●P < 0.001) and women (○○○P < 0.001) (results of post hoc paired t tests), whereas sensory thresholds remained unchanged. No significant sex differences or sex × condition interactions were observed. For results of analysis of variance, see text.
3.4. Musculoskeletal pain sensitivity
Across both study conditions, women displayed lower pain thresholds for the erector spinae (F = 8.7, P = 0.005, 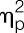 = 0.19), deltoideus (F = 11.0, P = 0.002,
= 0.19), deltoideus (F = 11.0, P = 0.002, 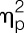 = 0.23), and gastrocnemius muscle (F = 4.5, P = 0.041,
= 0.23), and gastrocnemius muscle (F = 4.5, P = 0.041, 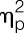 = 0.11) (all main effects of sex; for post hoc tests, see Fig. 4). After LPS administration, significantly decreased PPTs were observed for the lower back (ie, erector spinae muscle: F = 6.0, P = 0.019,
= 0.11) (all main effects of sex; for post hoc tests, see Fig. 4). After LPS administration, significantly decreased PPTs were observed for the lower back (ie, erector spinae muscle: F = 6.0, P = 0.019, 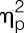 = 0.14), the shoulder region (ie, deltoid muscle: F = 18.8, P < 0.001,
= 0.14), the shoulder region (ie, deltoid muscle: F = 18.8, P < 0.001, 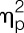 = 0.34; trapezius muscle: F = 14.8, P < 0.001,
= 0.34; trapezius muscle: F = 14.8, P < 0.001, 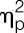 = 0.29), and for the lower legs (gastrocnemius muscle: F = 5.8, P = 0.021,
= 0.29), and for the lower legs (gastrocnemius muscle: F = 5.8, P = 0.021, 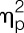 = 0.13) (all condition effects), without evidence of sex differences (for all muscle groups: P > 0.1, sex × condition effects; Fig. 4). In additional exploratory analyses, the effects of LPS on pain thresholds were assessed separately in men and women. In men, pain thresholds of all muscle groups were significantly decreased after LPS application (erector spinae muscle: t = −2.0, P = 0.029; gastrocnemius muscle: t = −3.0, P = 0.003; deltoid muscle: t = −4.1, P < 0.001; trapezius muscle: t = −3.8, P < 0.001). In women, significant LPS-induced decreases in pain thresholds were only observed for the deltoid muscle (t = −2.3, P = 0.016) and trapezius muscle (t = −2.0, P = 0.03) but not for the erector spinae muscle (t = −1.5, P = 0.07) and the gastrocnemius muscle (t = −0.7, P = 0.26).
= 0.13) (all condition effects), without evidence of sex differences (for all muscle groups: P > 0.1, sex × condition effects; Fig. 4). In additional exploratory analyses, the effects of LPS on pain thresholds were assessed separately in men and women. In men, pain thresholds of all muscle groups were significantly decreased after LPS application (erector spinae muscle: t = −2.0, P = 0.029; gastrocnemius muscle: t = −3.0, P = 0.003; deltoid muscle: t = −4.1, P < 0.001; trapezius muscle: t = −3.8, P < 0.001). In women, significant LPS-induced decreases in pain thresholds were only observed for the deltoid muscle (t = −2.3, P = 0.016) and trapezius muscle (t = −2.0, P = 0.03) but not for the erector spinae muscle (t = −1.5, P = 0.07) and the gastrocnemius muscle (t = −0.7, P = 0.26).
Figure 4.
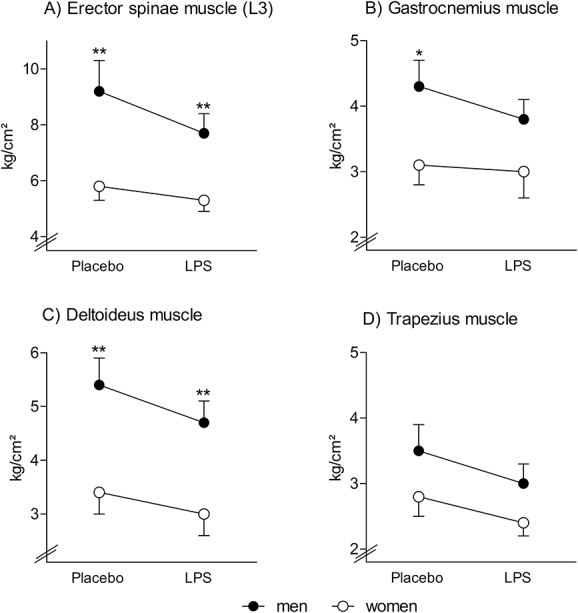
Pressure pain thresholds: Pressure pain thresholds of the low back (A, erector spinae muscle), leg (B, gastrocnemius muscle), and shoulder region (C, deltoid muscle; D, trapezius muscle) were assessed using a handheld algometer 3 hours after injection of either lipopolysaccharide (LPS; 0.4 ng/kg body weight) or saline (placebo) in healthy men and in healthy women on hormonal contraceptives. Lipopolysaccharide application led to significant decreased pressure pain thresholds (for results of analysis of variance, see text). Women displayed significantly lower pressure pain thresholds compared with men (*P < 0.05, **P < 0.01, results of post hoc independent samples t tests), whereas no evidence of sex × condition interactions was observed. For results of analysis of variance and results from additionally computed exploratory within group comparisons, see text.
3.5. Interoceptive sensitivity
Accuracy of heartbeat perception was significantly decreased in the LPS condition (F = 8.3, P < 0.01, 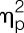 = 0.19, condition effect). Although no main effect of sex was observable (F = 1.3, P = 0.26,
= 0.19, condition effect). Although no main effect of sex was observable (F = 1.3, P = 0.26, 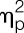 = 0.04; group effect), men showed a tendency towards greater LPS-induced decreases in accuracy scores (F = 3.2, P = 0.079,
= 0.04; group effect), men showed a tendency towards greater LPS-induced decreases in accuracy scores (F = 3.2, P = 0.079, 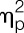 = 0.08, sex × condition interaction) (men: 0.73 ± 0.15, placebo condition; 0.62 ± 0.11, LPS condition; women: 0.74 ± 0.17, placebo condition; 0.71 ± 0.20, LPS condition).
= 0.08, sex × condition interaction) (men: 0.73 ± 0.15, placebo condition; 0.62 ± 0.11, LPS condition; women: 0.74 ± 0.17, placebo condition; 0.71 ± 0.20, LPS condition).
3.6. State anxiety, side effects
State anxiety scores (State-Trait-Anxiety-Inventory) were significantly increased after LPS administration (F = 10.2, P < 0.001, 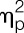 = 0.60, condition × time interaction effect), without evidence of sex differences or sex × condition interaction effects (Fig. 2F). Retrospectively self-reported side effects of LPS were overall comparable for men and women (GASE sum scores for men: 9.2 ± 2.0; for women: 11.3 ± 2.1; t = 0.7, P = 0.43). Comparisons of single GASE items revealed that women reported significantly more severe symptoms of nausea compared with men (women: 1.2 ± 0.2; median [25th, 75th percentile] = 1 [0, 2]; men: 0.2 ± 0.1; median [25th, 75th percentile] = 0 [0, 0]; Z = 3.2, P = 0.001), whereas no significant differences were observed for the remaining symptoms after Bonferroni correction for multiple testing (data not shown).
= 0.60, condition × time interaction effect), without evidence of sex differences or sex × condition interaction effects (Fig. 2F). Retrospectively self-reported side effects of LPS were overall comparable for men and women (GASE sum scores for men: 9.2 ± 2.0; for women: 11.3 ± 2.1; t = 0.7, P = 0.43). Comparisons of single GASE items revealed that women reported significantly more severe symptoms of nausea compared with men (women: 1.2 ± 0.2; median [25th, 75th percentile] = 1 [0, 2]; men: 0.2 ± 0.1; median [25th, 75th percentile] = 0 [0, 0]; Z = 3.2, P = 0.001), whereas no significant differences were observed for the remaining symptoms after Bonferroni correction for multiple testing (data not shown).
3.7. Correlational analyses
Within the LPS condition, correlations between sensitivity measures (rectal and PPTs, interoceptive sensitivity), state anxiety, and peak plasma cytokine and cortisol concentrations were computed for the time point 2-hour postinjection. This time point was chosen given its proximity to the beginning of sensitivity testing in light of the well-established time course of cytokine responses to low-dose LPS.4,23,24,65 Within male but not female subjects, higher peak TNF-α concentrations correlated with increased pain sensitivity (ie, lower pain thresholds) for both modalities but reached statistical significance only for PPTs for all body regions (Fig. 5; Table 2). No consistent correlative findings were observed for other parameters (Table 2).
Figure 5.
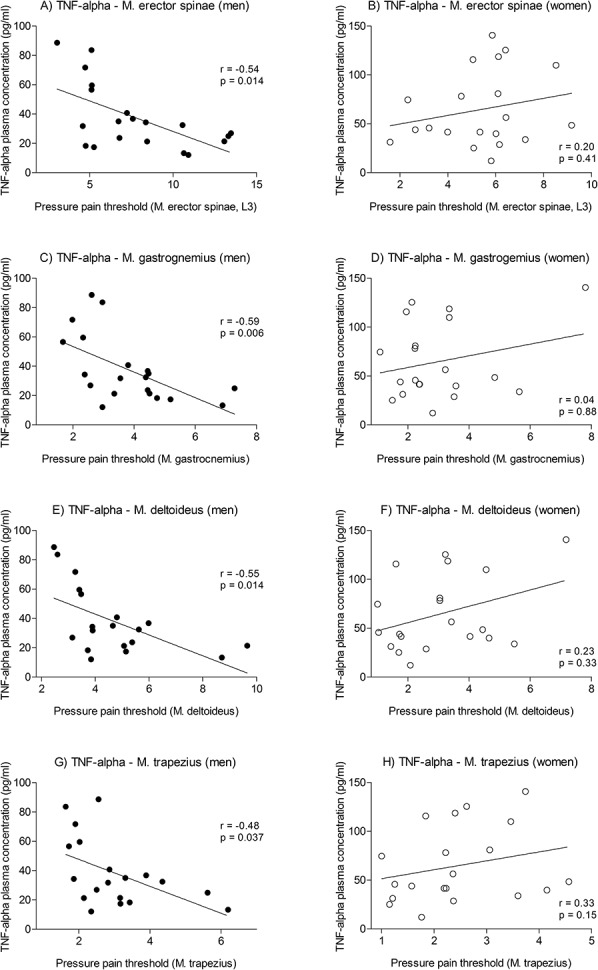
Associations between plasma tumor necrosis factor (TNF)-α and pressure pain thresholds in men and women: In men (left panel), pressure pain thresholds of the low back (A, erector spinae muscle), leg (C, gastrocnemius muscle), and shoulder region (E, deltoid muscle; G, trapezius muscle) showed significant negative associations with plasma levels of TNF-α (assessed 2 hours after injection of 0.4 ng/kg body weight lipopolysaccharide, LPS). In women (right panel), no significant correlations were observed for any muscle group (B, erector spinae muscle; D, gastrocnemius muscle; F, deltoid muscle; H, trapezius muscle). Only women using hormonal contraceptives were included in this study.
Table 2.
Correlations of visceral and musculoskeletal pain thresholds with plasma cytokine and cortisol concentrations, state anxiety, and interoceptive sensitivity in men and women on hormonal contraceptives within the lipopolysaccharide condition.
3.8. Investigator × subject sex interaction effects
Given evidence that pain assessments may be affected by investigator × subject sex interaction effects,1 half of our participants were assessed by a male investigator and vice versa. In a supplementary analysis, we addressed possible investigator × subject sex interactions using ANOVAs, which revealed no significant results (data not shown).
4. Discussion
The innate immune system is increasingly recognized in the mediation of acute and chronic pain, particularly with respect to TLR-4-dependent pathways.19,42–45,53,61 Experimental administration of the bacterial endotoxin LPS offers a reliable model to study inflammation-induced pain sensitization.2,30,55 However, with 1 recent exception,32 virtually all existing knowledge about LPS-induced pain responses in humans is based on data from male volunteers, underscoring the need for studies comparing men and women. Therefore, in this randomized, double-blind, placebo-controlled crossover study, we assessed visceral and musculoskeletal pain sensitivity after low-dose LPS administration in healthy men and in women on hormonal contraceptives. Overall, our results revealed increased pain sensitivity, reflected by decreased pain thresholds, for both the visceral and the musculoskeletal pain modality during experimental endotoxemia in both sexes. Of note, we could not replicate our earlier finding of decreased visceral sensory thresholds after LPS application,4 neither in this study nor in an independent sample of healthy males.5 Thus, our earlier conclusion supporting LPS-induced visceral allodynia in addition to hyperalgesia4 must be interpreted in caution in the light of our more recent larger data sets. Nevertheless, the results from this study confirm decreased visceral4 and pressure pain65 thresholds in response to low-dose LPS in healthy men and are in line with data from other groups,13,31,32 including a recent report indicating reduced PPTs in response to 0.6 ng LPS in men and women.32 Together, these findings support the broad applicability of experimental endotoxin administration as a translational preclinical model of inflammation-induced hyperalgesia30 in both sexes.
Given the female preponderance of chronic pain conditions such as IBS26,46 and musculoskeletal pain syndromes,41,43,66 which are typically characterized by increased pain sensitivity,15,58 we hypothesized greater LPS-induced pain sensitization in women. Although women revealed increased musculoskeletal pain sensitivity per se, in line with existing evidence,50 LPS-induced changes in pain thresholds were nearly similar in men and women for both pain modalities. Only in exploratory post hoc tests within each group was there evidence indicating that LPS affected fewer muscle groups in women. It remains to be clarified whether this was due to the low PPTs in women at baseline (ie, floor effect) or to the possibility that not all muscle groups are responsive to LPS effects in women. Nevertheless, the largely negative results for visceral and somatic pain thresholds are particularly striking given pronounced sex differences in cytokine and cortisol responses to LPS administration: Specifically, women revealed a more pronounced pro-inflammatory response and a higher rise in plasma cortisol. These findings are congruent with the general observations that women display a greater number and activity of immune cells and a more pronounced activation of the innate immune system compared with men.34,35 Only a few studies have analyzed sex-related differences in immune responses to experimental endotoxemia. Although 1 report of greater TNF-α responses to comparably high-dose LPS (2 ng/kg) in women62 was consistent with our results, other studies using varying endotoxin doses did not reveal sex differences in immune parameters.9,14,16 Clearly, more experimental work is needed to clarify the putative role of sex in peripheral neuroendocrine and immune responses to experimental endotoxemia. Of note, because of safety reasons, we only assessed women on hormonal contraceptives. Because the use of hormonal contraceptives has been linked to (low-grade) pro-inflammatory states48 and increased TNF-α production after in vitro stimulation of monocyte-derived macrophages with LPS,7 our results may not be transferable to naturally cycling or postmenopausal women. Nevertheless, given the present focus on pain sensitivity, the question arises how to reconcile our findings of divergent peripheral cytokine and cortisol responses in women on hormonal contraceptives with the absence of sex differences in pain sensitization. After all, the proalgesic effects of immune mediators such as TNF-α or IL-6 are reasonably well established both in animals and humans.19,61,64 First, it is conceivable that the association between peripheral cytokine responses and pain sensitization is nonlinear in women. Indeed, our correlational analyses revealed a linear association between TNF-α concentrations and PPTs only in men. Second, it is important to consider that pain sensitization during experimental endotoxemia likely involves central processes,31 as previously shown by our group using functional magnetic resonance imaging in a cohort of men.5 Indeed, peripheral pro-inflammatory mediators (eg, TNF-α, IL-6) can activate multiple peripheral and central pathways relevant to the processing of painful stimuli.2,19,42,64 First and foremost, cytokines and other inflammatory mediators released during states of inflammation sensitize vagal and spinal afferent neurons and hence increase afferent immune-to-brain processing.19,42,44 In addition, peripheral cytokine release can trigger the production of inflammatory mediators and activation of glial cells within the brain through TLR-4-dependent pathways.19,44 Recent data from animal studies suggest that TLR-4 receptor sensitivity may be influenced by sex hormones,44 and more intriguingly, effects of TLR-4 activation on pain sensitivity were only found in male but not in female mice.59 Together, these data would suggest that pain sensitization during systemic inflammation might be driven by different processes in men and women, both likely involving central mechanisms. Interestingly, a recent study provided evidence of impaired conditioned pain modulation in women, which may indicate that pronounced cytokine responses to LPS may primarily affect central processes of endogenous pain inhibition in women.32 Based on the experimental endotoxemia model, studies implementing brain imaging or immunomodulatory drugs are warranted to further disentangle sex-specific mechanisms of pain sensitization in the context of inflammation.
Given evidence supporting a positive association between cardiac interoceptive accuracy and somatic pain perception,49 we also analyzed interoceptive accuracy with an established heartbeat perception task as a possible mediator of putative sex differences in inflammation-induced hyperalgesia. Although we did not find evidence of sex differences in interoceptive accuracy during endotoxemia, our data revealed significantly reduced interoceptive sensitivity in response to LPS. The observed reduction in cardiac interoceptive accuracy during endotoxemia was unexpected and counterintuitive if one assumes that sensitivity to (or awareness of) interoceptive signals arising from different bodily parts are intercorrelated within 1 individual. However, although brain imaging studies clearly support that various interoceptive signals (eg, visceral pain, cardiac signals, immune activation, etc.) are processed by overlapping and closely related brain circuits (for review, see Ref. 10), experimental studies designed to compare interoceptive sensitivity across different modalities are rare and have revealed conflicting results,27,29 critically discussed in Ref. 8. Moreover, the conscious perception of pain during acute systemic inflammation is obviously adaptive and may protect the organism from potential harm, whereas other rather nonconscious interoceptive signals (eg, hunger, thirst) might be less important. Indeed, it has been pointed out that sickness behavior induced by systemic inflammation is a protective motivational state, which “competes with other internally or externally driven motivational states and takes precedence unless competing motivational stimuli become more important for survival.”11 In conclusion, there does not appear to be a general improvement of interoceptive capability during endotoxemia in either sex.
Our results should be discussed in the light of several limitations: First, we chose 2 established and clinically relevant pain models rather than using more refined protocols such as the Quantitative Sensory Testing protocol.52 This choice was made in light of the considerable time demands of more extensive testing and was based on our previous findings comparing the sensitivity of different somatic pain modalities to LPS65 and our proof-of-concept study in the visceral domain.4 First and foremost, however, the temporal dynamics of the inflammatory and neuroendocrine responses to LPS pose limitations regarding the feasibility of more extensive testing protocols. This was also the primary reason for our decision not to counterbalance the order of sensitivity testing herein, although the fixed order does not control for possible carryover effects. Furthermore, we cannot exclude the possibility that sex differences in pain sensitization exist for other pain modalities and/or at other time points with respect to LPS administration. Another aspect is the fact that we only included women on hormonal contraceptives. As per our ethics committee, excluding any possibility of pregnancy was mandatory, and sample sizes did not allow for subgroup analyses addressing possible effects of different types of contraceptive medications. Finally, our findings are based on a model of acute and transient immune activation in healthy young adults. Clearly, in patients with chronic inflammatory or pain conditions, altered neuroendocrine-immune axes, for example, chronic activation and blunted feedback loops of the HPA axis and negative affectivity,63 may contribute to symptom exacerbation.12 Thus, more studies are needed to understand sex differences in the interaction between pain sensitivity and immune activation in acute and chronic pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
This study was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft; DFG) (BE 5173/2-1; EL 236/11-1). A. Wegner received an IFORES stipend for clinicians of the Medical Faculty, University Duisburg Essen. The funding organizations were not involved in study design, in collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Acknowledgements
The authors express their gratitude to Alexandra Kornowski, Bettina Loeschner, and Magdalene Vogelsang for excellent technical support.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Aslaksen PM, Myrbakk IN, Hoifodt RS, Flaten MA. The effect of experimenter gender on autonomic and subjective responses to pain stimuli. PAIN 2007;129:260–8. [DOI] [PubMed] [Google Scholar]
- [2].Benson S, Engler H, Schedlowski M, Elsenbruch S. Experimental endotoxemia as a model to study neuroimmune mechanisms in human visceral pain. Ann N Y Acad Sci 2012;1262:108–17. [DOI] [PubMed] [Google Scholar]
- [3].Benson S, Kattoor J, Kullmann JS, Hofmann S, Engler H, Forsting M, Gizewski ER, Elsenbruch S. Towards understanding sex differences in visceral pain: enhanced reactivation of classitally-conditioned fear in healthy women. Neurobiol Learn Mem 2014;109:113–21. [DOI] [PubMed] [Google Scholar]
- [4].Benson S, Kattoor J, Wegner A, Hammes F, Reidick D, Grigoleit J-S, Engler H, Oberbeck R, Schedlowski M, Elsenbruch S. Acute experimental endotoxemia induces visceral hypersensitivity and altered pain evaluation in healthy humans. PAIN 2012;153:794–9. [DOI] [PubMed] [Google Scholar]
- [5].Benson S, Rebernik L, Wegner A, Kleine-Borgmann J, Engler H, Schlamann M, Forsting M, Schedlowski M, Elsenbruch S. Neural circuitry mediating inflammation-induced central pain amplification in human experimental endotoxemia. Brain Behav Immun 2015. 10.1016/j.bbi.2015.03.017; pii: S0889–1591(15)00111-7. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [6].Bingham B, Ajit SK, Blake DR, Samad TA. The molecular basis of pain and its clinical implications in rheumatology. Nat Clin Pract Rheumatol 2009;5:28–37. [DOI] [PubMed] [Google Scholar]
- [7].Campesi I, Sanna M, Zinellu A, Carru C, Rubattu L, Bulzomi P, Seghieri G, Tonolo G, Palermo M, Rosano G, Marino M, Franconi F. Oral contraceptives modify DNA methylation and monocyte-derived macrophage function. Biol Sex Differ 2012;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ceunen E, Van Diest I, Vlaeyen JWS. Accuracy and awareness of perception: related, yet distinct (commentary on Herbert, 2012). Biol Psychol 2013;92:426–7. [DOI] [PubMed] [Google Scholar]
- [9].Coyle SM, Calvano SE, Lowry SF. Gender influences in vivo human responses to endotoxin. Shock 2006;26:538–43. [DOI] [PubMed] [Google Scholar]
- [10].Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron 2013;77:624–38. [DOI] [PubMed] [Google Scholar]
- [11].Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L. The neuroimmune basis of fatigue. Trends Neurosci 2014;37:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008;9:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].de Goeij M, van Eijk LT, Vanelderen P, Wilder-Smith OH, Vissers KC, van der Hoeven JG, Kox M, Scheffer GJ, Pickkers P. Systemic inflammation decreases pain threshold in humans in vivo. PLoS One 2013;8:e84159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage 2009;47:881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Elsenbruch S. Abdominal pain in irritable bowel syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun 2011;25:386–94. [DOI] [PubMed] [Google Scholar]
- [16].Ferguson JF, Patel PN, Shah RY, Mulvey CK, Gadi R, Nijjar PS, Usman HM, Mehta NN, Shah R, Master SR, Propert KJ, Reilly MP. Race and gender variation in response to evoked inflammation. J translational Med 2013;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10:447–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Generaal E, Vogelzangs N, Macfarlane GJ, Geenen R, Smit JH, Dekker J, Penninx BW. Basal inflammation and innate immune response in chronic multisite musculoskeletal pain. PAIN 2014;155:1605–12. [DOI] [PubMed] [Google Scholar]
- [19].Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol 2014;14:217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: a consensus report. PAIN 2007;132(suppl 1):S26–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grigoleit J-S, Kullmann JS, Oberbeck R, Schedlowski M, Engler H. Salivary alpha-amylase response to endotoxin administration in humans. Psychoneuroendocrinology 2013;38:1819–23. [DOI] [PubMed] [Google Scholar]
- [22].Grigoleit J-S, Kullmann JS, Winkelhaus A, Engler H, Wegner A, Hammes F, Oberbeck R, Schedlowski M. Single-trial conditioning in a human taste-endotoxin paradigm induces conditioned odor aversion but not cytokine responses. Brain Behav Immun 2012;26:234–8. [DOI] [PubMed] [Google Scholar]
- [23].Grigoleit J-S, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, Engler H, Gizewski E, Oberbeck R, Schedlowski M. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One 2011;6:e28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Grigoleit J-S, Oberbeck JR, Lichte P, Kobbe P, Wolf OT, Montag T, del Rey A, Gizewski ER, Engler H, Schedlowski M. Lipopolysaccharide-induced experimental immune activation does not impair memory functions in humans. Neurobiol Learn Mem 2010;94:561–7. [DOI] [PubMed] [Google Scholar]
- [25].Hannestad J, Subramanyam K, Dellagioia N, Planeta-Wilson B, Weinzimmer D, Pittman B, Carson RE. Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. J Nucl Med 2012;53:601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Heitkemper M, Jarrett M. Irritable bowel syndrome: does gender matter? J Psychosom Res 2008;64:583–7. [DOI] [PubMed] [Google Scholar]
- [27].Herbert BM, Muth ER, Pollatos O, Herbert C. Interoception across modalities: on the relationship between cardiac awareness and the sensitivity for gastric functions. PLoS One 2012;7:e36646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Herrmann-Lingen C, Buss U, Snaith R. Hospital anxiety and depression scale—German version. Bern, Switzerland: Hans Huber, 2005. [Google Scholar]
- [29].Horing B, Kugel H, Brenner V, Zipfel S, Enck P. Perception and pain thresholds for cutaneous heat and cold, and rectal distension: associations and disassociations. Neurogastroenterol Motil 2013;25:e791–802. [DOI] [PubMed] [Google Scholar]
- [30].Hutchinson MR. Want more pain? Just add a dash of endotoxin to enhance your clinical pain model. Brain Behav Immun 2014;41:44–5. [DOI] [PubMed] [Google Scholar]
- [31].Hutchinson MR, Buijs M, Tuke J, Kwok YH, Gentgall M, Williams D, Rolan P. Low-dose endotoxin potentiates capsaicin-induced pain in man: evidence for a pain neuroimmune connection. Brain Behav Immun 2013;30:3–11. [DOI] [PubMed] [Google Scholar]
- [32].Karshikoff B, Lekander M, Soop A, Lindstedt F, Ingvar M, Kosek E, Olgart Hoglund C, Axelsson J. Modality and sex differences in pain sensitivity during human endotoxemia. Brain Behav Immun 2015;46:35–43. [DOI] [PubMed] [Google Scholar]
- [33].Keszthelyi D, Troost FJ, Simren M, Ludidi S, Kruimel JW, Conchillo JM, Masclee AA. Revisiting concepts of visceral nociception in irritable bowel syndrome. Eur J Pain 2012;16:1444–54. [DOI] [PubMed] [Google Scholar]
- [34].Klein SL. Immune cells have sex and so should journal articles. Endocrinology 2012;153:2544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis 2010;10:338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Koch A, Zacharowski K, Boehm O, Stevens M, Lipfert P, von Giesen HJ, Wolf A, Freynhagen R. Nitric oxide and pro-inflammatory cytokines correlate with pain intensity in chronic pain patients. Inflamm Res 2007;56:32–7. [DOI] [PubMed] [Google Scholar]
- [37].Kullmann JS, Grigoleit J-S, Lichte P, Kobbe P, Rosenberger C, Banner C, Wolf OT, Engler H, Oberbeck R, Elsenbruch S, Bingel U, Forsting M, Gizewski ER, Schedlowski M. Neural response to emotional stimuli during experimental human endotoxemia. Hum Brain Mapp 2013;34:2217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kullmann JS, Grigoleit J-S, Wolf OT, Engler H, Oberbeck R, Elsenbruch S, Forsting M, Schedlowski M, Gizewski ER. Experimental human endotoxemia enhances brain activity during social cognition. Soc Cogn Affect Neurosci 2014;9:786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lacourt TE, Houtveen JH, Doornen LJP, Benson S, Grigoleit JS, Cesko E, Elsenbruch S. Biological and psychological predictors of visceral pain sensitivity in healthy premenopausal women. Eur J Pain 2014;18:567–74. [DOI] [PubMed] [Google Scholar]
- [40].Lacourt TE, Houtveen JH, van Doornen LJP. Experimental pressure-pain assessments: test–retest reliability, convergence and dimensionality. Scand J Pain;3:31–7. [DOI] [PubMed] [Google Scholar]
- [41].Leveille SG, Zhang Y, McMullen W, Kelly-Hayes M, Felson DT. Sex differences in musculoskeletal pain in older adults. PAIN 2005;116:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. In: Caskey CT, editor. Annual review of medicine, Vol 62. Palo Alto: Annual Reviews, 2011. pp. 381–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci 2012;13:859–66. [DOI] [PubMed] [Google Scholar]
- [44].Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurol 2012;234:316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol 2010;7:163–73. [DOI] [PubMed] [Google Scholar]
- [46].Ouyang A, Wrzos HF. Contribution of gender to pathophysiology and clinical presentation of IBS: should management be different in women? Am J Gastroenterol 2006;101(12 suppl):S602–609. [DOI] [PubMed] [Google Scholar]
- [47].Parkitny L, McAuley JH, Di Pietro F, Stanton TR, O'Connell NE, Marinus J, van Hilten JJ, Moseley GL. Inflammation in complex regional pain syndrome: a systematic review and meta-analysis. Neurology 2013;80:106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pirkola J, Vaarasmaki M, Ala-Korpela M, Bloigu A, Canoy D, Hartikainen AL, Leinonen M, Miettola S, Paldanius M, Tammelin TH, Jarvelin MR, Pouta A. Low-grade, systemic inflammation in adolescents: association with early-life factors, gender, and lifestyle. Am J Epidemiol 2010;171:72–82. [DOI] [PubMed] [Google Scholar]
- [49].Pollatos O, Fustos J, Critchley HD. On the generalised embodiment of pain: how interoceptive sensitivity modulates cutaneous pain perception. PAIN 2012;153:1680–6. [DOI] [PubMed] [Google Scholar]
- [50].Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception—part 1: are there really differences between women and men? PAIN 2012;153:602–18. [DOI] [PubMed] [Google Scholar]
- [51].Rief W, Barsky AJ, Glombiewski JA, Nestoriuc Y, Glaesmer H, Braehler E. Assessing general side effects in clinical trials: reference data from the general population. Pharmacoepidemiol Drug Saf 2011;20:405–15. [DOI] [PubMed] [Google Scholar]
- [52].Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. PAIN 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- [53].Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 2009;89:707–58. [DOI] [PubMed] [Google Scholar]
- [54].Schandry R. Heart beat perception and emotional experience. Psychophysiology 1981;18:483–8. [DOI] [PubMed] [Google Scholar]
- [55].Schedlowski M, Engler H, Grigoleit JS. Endotoxin-induced experimental systemic inflammation in humans: a model to disentangle immune-to-brain communication. Brain Behav Immun 2014;35:1–8. [DOI] [PubMed] [Google Scholar]
- [56].Schmid J, Langhorst J, Gass F, Theysohn N, Benson S, Engler H, Gizewski ER, Forsting M, Elsenbruch S. Placebo analgesia in patients with functional and organic abdominal pain: a fMRI study in IBS, UC and healthy volunteers. Gut 2015;64:418–27. [DOI] [PubMed] [Google Scholar]
- [57].Schmid J, Theysohn N, Gass F, Benson S, Gramsch C, Forsting M, Gizewski ER, Elsenbruch S. Neural mechanisms mediating positive and negative treatment expectations in visceral pain: a functional magnetic resonance imaging study on placebo and nocebo effects in healthy volunteers. PAIN 2013;154:2372–80. [DOI] [PubMed] [Google Scholar]
- [58].Sluka KA, Berkley KJ, O'Connor MI, Nicolella DP, Enoka RM, Boyan BD, Hart DA, Resnick E, Kwoh CK, Tosi LL, Coutts RD, Kohrt WM. Neural and psychosocial contributions to sex differences in knee osteoarthritic pain. Biol Sex Differ 2012;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci 2011;31:15450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychology Press, 1970. [Google Scholar]
- [61].Tracey KJ. The inflammatory reflex. Nature 2002;420:853–9. [DOI] [PubMed] [Google Scholar]
- [62].van Eijk LT, Dorresteijn MJ, Smits P, van der Hoeven JG, Netea MG, Pickkers P. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit Care Med 2007;35:1464–9. [DOI] [PubMed] [Google Scholar]
- [63].Walker AK, Kavelaars A, Heijnen CJ, Dantzer R. Neuroinflammation and comorbidity of pain and depression. Pharmacol Rev 2014;66:80–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med 2005;257:139–55. [DOI] [PubMed] [Google Scholar]
- [65].Wegner A, Elsenbruch S, Maluck J, Grigoleit JS, Engler H, Jager M, Spreitzer I, Schedlowski M, Benson S. Inflammation-induced hyperalgesia: effects of timing, dosage, and negative affect on somatic pain sensitivity in human experimental endotoxemia. Brain Behav Immun 2014;41:46–54. [DOI] [PubMed] [Google Scholar]
- [66].Wijnhoven HA, de Vet HC, Picavet HS. Prevalence of musculoskeletal disorders is systematically higher in women than in men. Clin J Pain 2006;22:717–24. [DOI] [PubMed] [Google Scholar]