Offset analgesia could be activated with a feedback-controlled near-infrared laser system. Larger and delayed response to temperature decrease than to temperature increase was observed.
Keywords: Offset analgesia, Laser heat pain, Temporal filtering of pain perception, Mathematical modeling
Abstract
Physical stimuli are subject to pronounced temporal filtering during afferent processing such that changes occurring at certain rates are amplified and others are diminished. Temporal filtering of nociceptive information remains poorly understood. However, the phenomenon of offset analgesia, where a disproportional drop in perceived pain intensity is caused by a slight drop in noxious heat stimulation, indicates potent temporal filtering in the pain pathways. To develop a better understanding of how dynamic changes in a physical stimulus are constructed into an experience of pain, a transfer function between the skin temperature and the perceived pain intensity was modeled. Ten seconds of temperature-controlled near-infrared (970 nm) laser stimulations above the pain threshold with a 1°C increment, decrement, or constant temperature were applied to the dorsum of the hand of healthy human volunteers. The skin temperature was assessed by an infrared camera. Offset analgesia was evoked by laser heat stimulation. The estimated transfer functions showed shorter latencies when the temperature was increased by 1°C (0.53 seconds [0.52-0.54 seconds]) than when decreased by 1°C (1.15 seconds [1.12-1.18 seconds]) and smaller gains (increase: 0.89 [0.82-0.97]; decrease: 2.61 [1.91-3.31]). The maximal gain was observed at rates around 0.06 Hz. These results show that temperature changes occurring around 0.06 Hz are best perceived and that a temperature decrease is associated with a larger but slower change in pain perception than a comparable temperature increase. These psychophysical findings confirm the existence of differential mechanisms involved in temporal filtering of dynamic increases and decreases in noxious stimulus intensity.
1. Introduction
Temporal filtering of afferent information, ie, changes in a sensory perception depending on the frequency of the stimulation, is a feature of many sensory modalities.12 The sensory information is filtered in a way that amplifies changes occurring at certain stimulation frequencies while faster and slower changes are attenuated. In the visual system, changes occurring around 10 Hz are most readily perceived.19,25 In the tactile system, the highest sensitivity is present for vibrations occurring at frequencies of approximately 250 Hz,52 and in the auditory system, vibrations around 2000 Hz are most easily perceived.33 This phenomenon resembles a “band-pass” filtering effect. Most experiments relating to temporal aspects of pain processing have investigated repeated transient stimuli. Nociceptive amplification is often observed and has been related to wind-up, temporal summation, or long-term potentiation.31,32,41,56 However, a decrease in the nociceptive response,20,21,43,44 habituation of the cortical potentials,2 and brain metabolism5 have been observed depending on stimulation paradigm and the context.
Several biophysical and neural mechanisms are involved in temporal filtering of temporal changes in the perception of heat pain. When heat is applied by a contact thermode or a far-infrared laser, the energy is conducted through the upper epidermal layers to the thermal nociceptors located at depths of 50 μm below the skin surface.13,18,49 Near-infrared photons penetrate deeper into the skin and are absorbed closer to the nociceptors, minimizing the heat conduction delay.4 As the nociceptor endings are heated, heat-sensitive ion channels start opening through a stochastic process. The transduction and conduction delays have been shown to differ between afferent fiber types,49,50 resulting in a complex temporal barrage of the spinal ascending neurons. Both peripheral and central processes may be subject to habituation or sensitization, resulting in complex “filtering” of the heat stimulus–related information.14 Although only few studies on the temporal aspects of pain perception are available, the sensory experience of pain substantially upon the distinct temporal dynamics of the noxious stimulus.15–17,58 Such dynamic processes are exemplified by offset analgesia. Offset analgesia is the phenomenon that when constant painful heat stimulation is applied and is followed by a small decrease of 1°C, a disproportionately large reduction in perceived pain intensity occurs.15,58 The mechanisms governing offset analgesia are not clear; however, several brain regions including the periaqueductal gray and rostral ventral medulla are activated, indicating a dynamic activation of the endogenous pain inhibitory system.57
The aim of the study was to investigate and model temporal filtering of nociceptive thermal stimulation. Therefore, a skin temperature feedback-controlled near-infrared laser system was developed to evoke the offset analgesia phenomenon. Moreover, temporal filtering was quantified by estimating the transfer function between perceived pain intensity and superficial skin temperature.
2. Methods
2.1. Subjects
Twelve healthy volunteers (10 men and 2 women) between the ages of 22 and 29 years participated in the study. All subjects gave written informed consent, acknowledging that they would experience experimental painful stimuli and that they were free to withdraw from the experiment at any time. The volunteer and the experimenter wore protective goggles during the experiment. The volunteer was sitting in an office chair with arms resting on a table, in a temperature-controlled room at 22°C. All procedures were approved by the local ethics committee (Reference No. N-20080026).
2.2. Monte Carlo simulation and heat transfer model
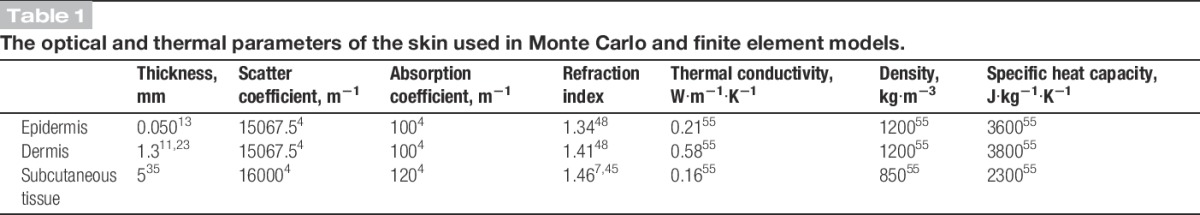
Mathematical models were applied to estimate the delay of temperature changes at the location of the heat nociceptors. This delay may be caused by heat transfer from the location of photon absorption to the location of nociceptor nerve endings. The 970 nm photons from the diode laser penetrate into the skin, and the energy is therefore absorbed deeper in the skin than when heat is applied by thermodes and CO2 lasers. The location of photon absorption was modeled by an implicit photon capture Monte Carlo simulation of photon scatter and absorption.40,54 A beam radius of 4 mm was simulated to mimic the laser system used. A multilayered approach was applied where the dermal and epidermal layers were explicitly described. Absorption, scattering, reflection, and refraction were modeled similar to previously described models.40,54 The geometry and optical parameters used for each layer were adopted from the literature and shown in Table 1.
Table 1.
The optical and thermal parameters of the skin used in Monte Carlo and finite element models.
The temperature distribution caused by photon absorption and the subsequent heat transport were estimated by a finite element model of the heat equation.13 The geometry used in the finite element model was the same as in the Monte Carlo simulation, and the thermal properties were adopted from the literature (Table 1).
2.3. Thermal stimulation and pain assessment
Thermal stimuli were delivered to the dorsum of the left hand by a 20 W near-infrared diode laser with a wavelength of 970 nm (DL-20; IPG Laser, Burbach, Germany). The laser beam was guided through an optical fiber into a beam expander, providing a 0.5-cm2 area collimated beam. The temperature of the skin was measured by an infrared camera (FLIR A40, Sweden, sensitive between 7.5 and 13 μm, thus not sensitive to photons reflected by the 970 nm diode laser). The maximum temperature in the irradiated skin area was used to control the laser through a proportional–integral–derivative (PID) control loop (LabVIEW PID toolbox; National Instruments, Austin, TX). The PID controller minimized the difference between the target temperature and the recorded temperature by regulating the intensity of the laser. The PID controller settings were optimized in a pilot study. The skin temperature was recorded at 10 Hz and stored.
A visual analog scale (VAS) was used continuously throughout the experiments and sampled at 10 Hz to assess the pain intensity of the radiant heat stimulations. The scale was anchored by 0 indicating “no pain” and 10 indicating “most intense pain imaginable.”
2.4. Experiment 1: perception of pain intensity
In this experiment, the “step-up” sequence was investigated by increasing the temperature from baseline skin temperature (approximately 34°C) to plateaus of 35°C to 45°C in steps of 1°C. The temperature was maintained at each step for 15 seconds. This stimulation paradigm was used to investigate the pain intensity rating to stepwise increasing heat pain stimuli. For analysis, these ratings were fit to a power function. According to Stevens power law, a physical stimulus (S) relates to the perceived intensity (ψ) as ψ = kSn,46,47 where k is a proportionality constant and n is the exponent that differs between senses. Nonlinear least squares was used to fit the data to Stevens power law as VAS = k (Temp-34°C)n, ie, the baseline skin temperature (34°C) was subtracted from the assessed skin temperature. The temperature was sampled immediately before a temperature rise.
2.5. Experiment 2: thermal offset analgesia
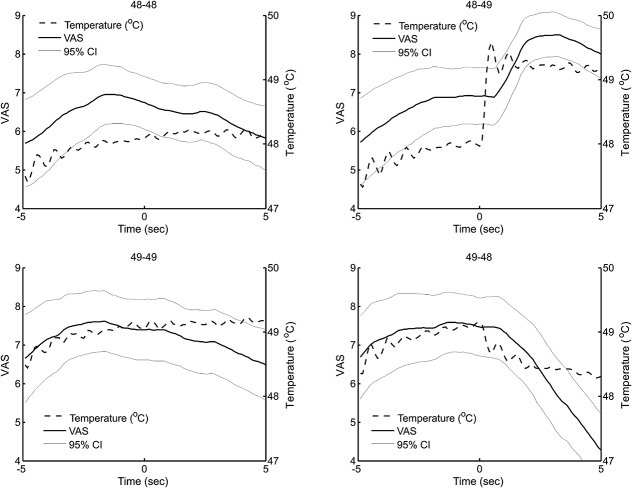
Heat was applied for a duration of 10 seconds excluding the rise and fall times. Four stimulation paradigms were applied: “48-49” where the temperature was kept at 48°C for 5 seconds and then increased to 49°C and maintained for another 5 seconds, “49-48” where the temperature was kept at 49°C for 5 seconds and then decreased to 48°C and maintained for another 5 seconds, “48-48” where the temperature was kept at 48°C for 10 seconds, and “49-49” where the temperature was maintained at 49°C for 10 seconds.
The order of the stimulation paradigms was randomized, and stimuli were applied with an interstimulation interval of at least 60 seconds. The stimulation site was moved slightly between each of the 4 paradigms to avoid habituation by stimulating the same skin area twice. Each stimulus was applied twice in each subject.
2.6. Transfer function
Active cooling is not possible when applying radiant heat. Therefore, the temperature fall time cannot be controlled and is therefore often longer than the rise time. A transfer function relating the superficial skin temperature and perceived pain intensity (VAS ratings) was estimated. A second-order transfer function H(s) was used:
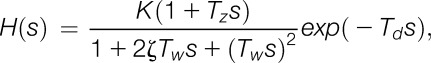 |
where K is the gain, Td is the latency, s is the sample time, Tz is a time constant that in this application describes the rise time and overshoot after a temperature change, and Tω is the time constant of a possible oscillation that is dampened by the coefficient ζ (the inverse of the frequency).26 The step responses (time domain) and the frequency responses (Bode plots) were estimated based on the findings for the paradigms with a 1°C increase or decrease in superficial skin temperature (48°C-49°C and 49°C-48°C).
2.7. Statistical analysis
The perception of pain intensity of increasing heat stimulation as fitted Stevens power law was estimated using a least sum of squares algorithm.
To assess offset analgesia, the reported VAS score at the end of the 10-second thermal stimulus of the 48°C to 48°C, 49°C to 49°C, 48°C to 49°C, 49°C to 49°C paradigms were compared using a mixed linear model (MLM) with “paradigm” as main factor. Bonferroni adjustments were used for pairwise multiple comparisons. Linear regression was performed on the VAS ratings during the constant temperature stimulation paradigms (48°C-48°C and 49°C-49°C) to test for habituation.
The estimated parameters describing the step and frequency response curves of the transfer functions were compared and considered significantly different when 95% confidence intervals (CIs) were nonoverlapping.10 Data are presented as mean values with 95% CI.
3. Results
3.1. Skin temperature at receptor depth
The Monte Carlo simulation showed that absorption of photons was highest in the epidermis and dermis (Fig. 1A), and some absorption also occurred wider than the beam radius due to scattering of the near-infrared photons.
Figure 1.
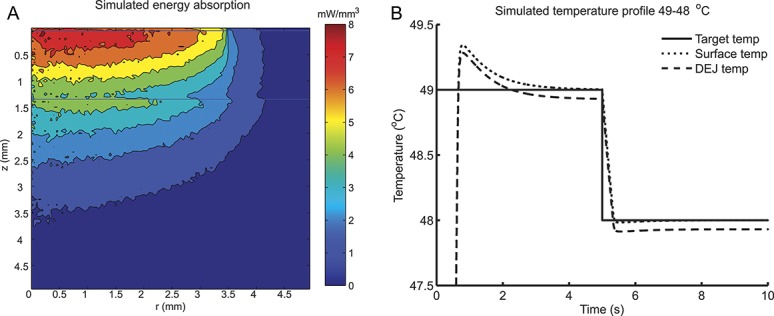
Simulated laser stimulation. A, Absorption of 100,000 Monte Carlo simulated photon packages normalized to 1 W. Photon absorption is highest at the dermal–epidermal junction (DEJ) (z = 0.050). Furthermore, photons are scattered and absorbed outside the beam radius (r = 3.5 mm). The horizontal line at z = 0.050 mm indicates the DEJ, and the horizontal line at z = 1.305 mm indicates the dermis–subcutaneous junction. B, Simulation of the temperature profile at the beam center, r = 0, during a simulated 49°C to 48°C stimulation. The temperature was estimated at the surface (dotted line) and the DEJ (dashed line) by combining a Monte Carlo model of photon absorption and a finite element model for heat transfer.
The combined Monte Carlo simulation and finite element model showed that the difference in the temperature profiles was less than 0.1°C at the skin surface and dermal–epidermal junction where the heat-sensitive nociceptors are most abundant.18 Moreover, the temporal delay was less than 12 milliseconds (Fig. 1B). Therefore, it is valid to use the temperature measured at the skin surface for controlling the temperature at the nociceptor level.
3.2. Experiment 1: perception of pain intensity
An overshoot in the skin temperature occurred at each step when applying the temperature increase from 35°C to 45°C in steps of 1°C (Fig. 2A). The simultaneously sampled pain intensity showed an even more pronounced overshoot in relation to each step. The pain intensity followed Stevens power law with an adjusted R2 of 0.48 and an exponent of 2.9 (2.3-3.4; Fig. 2B).
Figure 2.
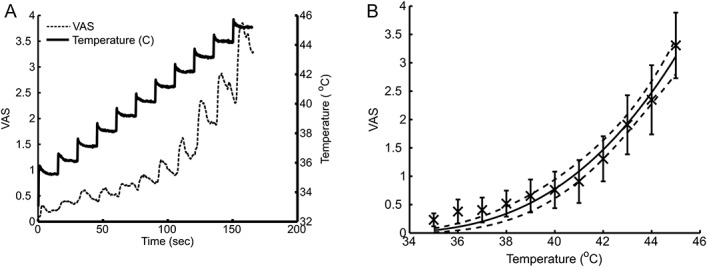
Subjects rated a stepwise increasing thermal stimulation. A, The skin temperature was increased from 35°C to 45°C in steps of 1°C (solid line). The subjects rated the perceived pain intensity on a continuously sampled visual analog scale (dashed line). B, The perceived pain intensity was fitted to Stevens power law and showed a fitted power of 2.9 (2.3-3.4).
3.3. Experiment 2: thermal offset analgesia
The pain intensity at the end of the 10-second stimulation was significantly different between the 4 paradigms (MLM, P < 0.001; Fig. 3). In particular, the VAS score of the “49-48” stimulation of 4.2 (3.1-5.4) was significantly lower than the VAS score of the “48-48” stimulation of 5.8 (4.7-7.0) and the VAS score of the “48-49” stimulation of 8.0 (7.0-9.0) was significantly higher than the VAS score of the “49-49” stimulation of 6.2 (5.0-7.3) (MLM, Bonferroni P < 0.05). The difference between the “48-48” and the “49-49” stimulation was not found to be statistically significant (MLM, Bonferroni nonsignificant). At constant temperature (“48-48” and “49-49”), habituation to the painful heat stimulus was observed as a decrease in VAS ratings during the 10-second stimulation (slope = −0.154/s [−0.164/s to −0.145/s] at 48°C and −0.150/s [−0.159/s to −0.142/s] at 49°C).
Figure 3.
Grand mean of the continuous visual analog scale score (solid line, left axis) and superficial skin temperature (dashed line, right axis) during the 4 stimulation paradigms: Constant temperature of 48°C (48-48) and 49°C, temperature increase from 48°C to 49°C (48-49), and temperature decrease from 49°C to 48°C (49-48).
3.4. Transfer function
The estimated transfer functions showed a latency, Td, of 0.53 seconds (0.52-0.54 seconds) when the temperature was increased by 1°C, which was significantly less than the latency of 1.15 seconds (1.12-1.18 seconds) when the temperature was decreased by 1°C. Furthermore, the transfer function predicts that the absolute change, K, in the pain intensity to an infinitely steep change in skin temperature is significantly larger when the temperature is decreased (2.61 [1.91-3.31]) compared with an increase (0.89 [0.82-0.97]; Fig. 4A). The frequency responses of estimated transfer functions for the “48-49” and “49-48” experiments were not significantly different (overlapping 95% CI) and peaked around 0.06 Hz (Fig. 4B).
Figure 4.
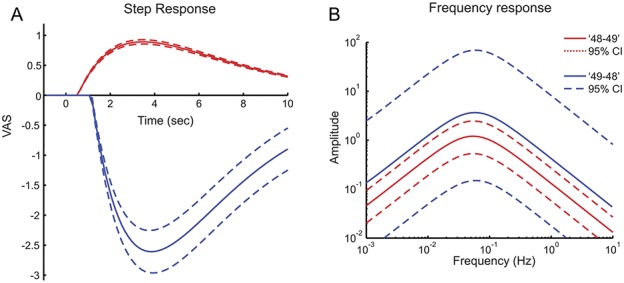
A, The estimated pain perception to a theoretical 1°C instantaneous increase (“48-49”; red) and a 1°C decrease (“49-48”; blue) of the skin temperature. This theoretical 1°C instantaneous step response was estimated by a transfer function describing the “transfer” between skin temperature and perception of pain intensity. B, The frequency characteristics of the transfer functions show which rates of skin temperature changes are transferred to changes in pain perception. The responses and frequency characteristics are indicated in solid lines with 95% confidence intervals (dashed lines).
4. Discussion
Offset analgesia was activated by applying a decrease in superficial skin temperature with a feedback-controlled near-infrared laser system. These findings importantly indicate that passive cooling of the skin is sufficient to evoke offset analgesia and confirm that offset analgesia can occur in naturalistic situations that are not associated with active cooling of the skin. The simulated laser stimulation showed that the temperature at the surface does not differ significantly from the temperature at the dermal–epidermal junction where the cutaneous nociceptors are most abundant. The fit to Stevens power law in the step-up paradigm showed that perceptual coding of skin temperature is maintained despite substantial temporal filtering. Moreover, the transfer function between skin temperature and pain perception showed a larger and delayed response for a temperature decrease than for a temperature increase, indicating that different mechanisms may be involved.
4.1. Perception of static skin temperature
In the classical report of the relation between radiant heat stimulation and heat pain perception, Adair et al. showed an exponent of Stevens power law of 1,1 whereas this report showed a significantly larger exponent of 2.9 (2.3-3.4). The difference may relate differences in methods; Adair et al. used the Dol scale, whereas the present study used the VAS, and Adair et al. used the irradiance, and the present study used the skin temperature to quantify the stimulation intensity.
4.2. Transfer function, delay, and rise time frequency response
The temporal profile of a noxious thermal stimulus affects the perception of pain so that increasing skin temperature enhances several aspects of pain perception compared with constant temperature.16,17 Pain perception to dynamic and transient changes in skin temperature occurs slowly enough to be continuously rated by the subjects in a predictable manner that can be modeled by a second-order differential equation.9 In this study, we showed a frequency response with maximal gain for temperature changes occurring around 0.06 Hz. This clearly indicates that the nociceptive system mediates information changing at substantial slower rates than other sensory modalities (eg, 10 Hz in the visual system,19,25,27 250 Hz In the tactile system,52 and 2000 Hz in the auditory system33).
The present study showed a delay in the transfer function of 0.53 seconds during a temperature increase of 1°C and 1.15 seconds during a temperature decrease. The latency for detection of the first heat pain has been shown to range between 0.2 and 0.8 seconds for a rapid temperature increase from skin temperature to 48°C during 200 to 250 milliseconds.8 The temporal step response appears to have similar shape and merely differs in delay and amplitude. The delays in perception of temperature changes and the low maximal gain in the frequency response are caused both by heat transfer delay and neural mechanisms. For example, when a heat stimulation stops or is removed, heat must dissipate passively away from the nociceptors for the temperature to drop. The temperature decrease is therefore not instantaneous, and the perception of temperature decrease is therefore delayed longer than a forced temperature increase.
4.3. Contact vs radiant heat stimulation
In the present investigation, VAS ratings of pain intensity during laser stimulation were markedly higher than those typically seen during contact heat stimuli, particularly for those stimuli in the low end of the noxious range. For example, a stimulus temperature of approximately 42°C was sufficient to produce average VAS ratings greater than 1. In previous studies using contact thermodes, concurrent mechanical afferent input from the probe itself is likely mixed with the afferent activity evoked by the thermal stimuli. It is unclear to what extent this non-noxious mechanical input diminishes nociceptive processing in a gate control–like way.53
The dynamic component of the stimulus increase during the rapid (∼5°C/s) rise of laser induced skin temperatures may also be a significant driver of these relatively high pain ratings. Previous work by Koyama et al.22 showed that relatively weak noxious stimuli (43°C, 45°C) delivered with a contact probe elicited ratings that peaked early but rapidly waned over time. This suggests that the 6°C/s stimulus temperature increase was more effective at recruiting nociceptive activity than the sustained delivery of those stimulus temperatures. Furthermore, thermal energy is deposited at the skin surface when heat is applied by contact thermodes. The thermal energy must be conducted through the cornified layers of the epidermis to the vial layers where thermal and nociceptive afferents terminate.13,18 In the present investigation, the detection latencies are quite short (∼0.53 seconds). The near-infrared (eg, 970 nm) photons from the diode laser penetrate the skin and deposit the energy closer to the thermal receptors.3 The delay caused by heat transport through the epidermis may therefore be minimized by near-infrared laser stimulation and may result in more synchronized activation of nociceptors. With both types of stimulation, thermal nociceptor activation and conduction velocity differ between fiber types49,50 and therefore add significantly to perception delay.
During stimulus decreases, contact thermodes actively cool the skin and may potentially recruit distinct populations of afferents that respond to such decreases. Indeed, the response latency of 2-4 seconds to a temperature decrease reported by Yelle et al.58 is substantially longer than the neural and motor delay.8 Despite the passive cooling that occurred during laser stimulation, latencies to detect temperature decreases were shorter (∼1.15 seconds) than those previously observed for contact stimuli. It is remarkable that offset analgesia can still occur during passive cooling of the skin that occurs during/after laser stimulation. This observation underscores how offset analgesia can occur during more natural stimuli.
4.4. Possible neural mechanisms of temporal filtering
Although the frequency responses were similar during temperature increase and decrease, differences were found in the temporal delay and amplitude of the temporal response. This indicates that different mechanisms may be involved in temporal filtering of thermal increase and decrease of painful heat perception.
In the present study, habituation was significant during constant stimulation paradigms (“48-48” and “49-49”). Primary afferents are known to adapt to constant stimulations.24 However, offset analgesia is a mechanism that goes beyond adaptation because pain perception during the “49-48” paradigm drops significantly below the “48-48” paradigm. Therefore, other peripheral and/or central mechanisms must be responsible for offset analgesia.
The pronounced decrease in pain perception after a relatively small decrement in the physical stimulus intensity indicates a potent endogenous analgesic mechanism. The temporal filtering mechanism underlying offset analgesia appears to differ from the conditioned pain modulation as substantially different brain mechanisms are involved.36 Moreover, different pharmacologic mechanisms are engaged.38 Conditioning pain modulation facilitates pain perception after ketamine injection, whereas offset analgesia remains unchanged.39 However, offset analgesia has been shown to be reduced or disrupted in chronic neuropathic pain patients.37 This may indicate that the neural mechanism behind offset analgesia becomes deficient during neuropathic pain and/or that peripheral mechanisms are responsible for offset analgesia. Therefore, it cannot be excluded that the mechanism also involves a peripheral mechanism related to the transduction process. However, offset analgesia remained intact in capsaicin and heat-sensitized skin.28
Some indications to a possible spinal mechanism behind offset analgesia were shown by McGaraughty and Henry.29 A subgroup of spinal wide dynamic range neurons in both intact and spinalized rats was inhibited during hind paw immersion into noxiously hot water and displayed an after discharge when the receptive field was removed from the water.29 The inhibition during immersion could be abolished by strychnine, indicating that spinal glycine receptors were responsible for silencing the neuron. Furthermore, administration of ketamine decreased the after discharge, indicating that spinal NMDA receptors were involved in the diminished firing after hind paw immersion.30 If information from such neurons is interpreted as inhibitory during cognitive processes, their response properties may be the neural basis for offset analgesia.38
Cortical mechanisms may also be involved in temporal filtering of pain perception. Habituation of repeatedly evoked cortical potentials to stimuli applied at different sites has been shown, indicating a central component of habituation.14 Furthermore, habituation to repeated heat stimuli has been shown as a decreased metabolism in classical pain processing areas such as the thalamus, insula, SII, and putamen, but with an increase in the subgenual anterior cingulate cortex indicating an active antinociceptive brain process,6 a mechanism that appears not to involve the endogenous opioid system.42 The pivotal role of the superspinal processes in temporal filtering of nociceptive information is further supported by the activation of the periaqueductal gray during offset analgesia.57 Still, it is not fully understood how differences in pain perception between habituation (“48-48” paradigm with an end VAS score of 5.8) and the offset analgesia mechanism (“49-48” paradigm with an end VAS score of 4.2) emerge.
4.5. Behavioral significance of offset analgesia
The phenomenon of offset analgesia is one example among others like graphesthesia34 and saltation illusion51 that the nociceptive system is capable of processing rather complex sensory information. The functional significance of mediating and processing complex noxious sensory information may be the foundation for pain-avoiding behavior, such as shifting between the “fight-or-flight” states or appropriate withdrawal motions. Deeper understanding of the spatiotemporal processing may be a way towards an understanding of the nociceptive system and the multidimensional aspects of pain perception. Eventually, such an understanding may lead to better treatments of chronic pain syndromes.
Conflict of interest statement
The authors have no conflicts of interest to declare.
The study was supported by a traveling grant from IASP funded by ScanDesign by Bruun Foundation and the National Institutes of Health Grant NS039426.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Adair ER, Stevens JC, Marks LE. Thermally induced pain, the Dol scale, and the psychophysical power law. Am J Psychology 1968;81:147–64. [PubMed] [Google Scholar]
- [2].Angel RW, Quick WM, Curtis Boylls C, Weinrich M, Rodnitzky RL. Decrement of somatosensory evoked potentials during repetitive stimulation. Electroencephalogr Clin Neurophysiol 1985;60:335–42. [DOI] [PubMed] [Google Scholar]
- [3].Arendt-Nielsen L, Chen AC. Lasers and other thermal stimulators for activation of skin nociceptors in humans. Neurophysiol Clin 2003;33:259–68. [DOI] [PubMed] [Google Scholar]
- [4].Bashkatov AN, Genina EA, Kochubey V, Tuchin V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J Phys D Appl Phys 2005;38:2543. [Google Scholar]
- [5].Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med 1999;41:1044–57. [DOI] [PubMed] [Google Scholar]
- [6].Bingel U, Schoell E, Herken W, Büchel C, May A. Habituation to painful stimulation involves the antinociceptive system. PAIN 2007;131:21–30. [DOI] [PubMed] [Google Scholar]
- [7].Bolin FP, Preuss LE, Taylor RC, Ference RJ. Refractive index of some mammalian tissues using a fiber optic cladding method. Appl Opt 1989;28:2297–303. [DOI] [PubMed] [Google Scholar]
- [8].Campbell JN, Lamotte RH. Latency to detection of 1st pain. Brain Res 1983;266:203–8. [DOI] [PubMed] [Google Scholar]
- [9].Cecchi GA, Huang L, Hashmi JA, Baliki M, Centeno MV, Rish I, Apkarian AV. Predictive dynamics of human pain perception. PLoS Comput Biol 2012;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cumming G. Inference by eye: reading the overlap of independent confidence intervals. Stat Med 2009;28:205–20. [DOI] [PubMed] [Google Scholar]
- [11].Fornage BD, McGavran MH, Duvic M, Waldron CA. Imaging of the skin with 20-MHz US. Radiology 1993;189:69–76. [DOI] [PubMed] [Google Scholar]
- [12].Fortune ES, Rose GJ. Short-term synaptic plasticity as a temporal filter. Trends Neurosci 2001;24:381–5. [DOI] [PubMed] [Google Scholar]
- [13].Frahm K, Andersen OK, Arendt-Nielsen L, Mørch CD. Spatial temperature distribution in human hairy and glabrous skin after infrared CO2 laser radiation. Biomed Eng Online 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Greffrath W, Baumgartner U, Treede RD. Peripheral and central components of habituation of heat pain perception and evoked potentials in humans. PAIN 2007;132:301–11. [DOI] [PubMed] [Google Scholar]
- [15].Grill JD, Coghill RC. Transient analgesia evoked by noxious stimulus offset. J Neurophysiol 2002;87:2205–8. [DOI] [PubMed] [Google Scholar]
- [16].Hashmi JA, Davis KD. Effect of static and dynamic heat pain stimulus profiles on the temporal dynamics and interdependence of pain qualities, intensity, and affect. J Neurophysiol 2008;100:1706–15. [DOI] [PubMed] [Google Scholar]
- [17].Hashmi JA, Davis KD. Effects of temperature on heat pain adaptation and habituation in men and women. PAIN 2010;151:737–43. [DOI] [PubMed] [Google Scholar]
- [18].Hilliges M, Wang LX, Johansson O. Ultrastructural evidence for nerve-fibers within all vital layers of the human epidermis. J Invest Dermatol 1995;104:134–137. [DOI] [PubMed] [Google Scholar]
- [19].Holliday IE, Ruddock KH. Two spatio-temporal filters in human vision. Biol Cybern 1983;47:173–90. [DOI] [PubMed] [Google Scholar]
- [20].Jung K, Rottmann S, Ellrich J. Long-term depression of spinal nociception and pain in man: influence of varying stimulation parameters. Eur J Pain 2009;13:161–70. [DOI] [PubMed] [Google Scholar]
- [21].Klein T, Magerl W, Treede RD. Perceptual correlate of nociceptive long-term potentiation (LTP) in humans shares the time course of early-LTP. J Neurophysiol 2006;96:3551–5. [DOI] [PubMed] [Google Scholar]
- [22].Koyama Y, Koyama T, Kroncke AP, Coghill RC. Effects of stimulus duration on heat induced pain: the relationship between real-time and post-stimulus pain ratings. PAIN 2004;107:256–66. [DOI] [PubMed] [Google Scholar]
- [23].Krackowizer P, Brenner E. Thickness of the human skin: 24 points of measurement. Phlebologie 2008;37:83–92. [Google Scholar]
- [24].Lamotte RH, Thalhammer JG, Robinson CJ. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: a comparison of neural events in monkey with sensory judgments in human. J Neurophysiol 1983;50:1–26. [DOI] [PubMed] [Google Scholar]
- [25].Lehky SR. Temporal properties of visual channels measured by masking. J Opt Soc Am A 1985;2:1260–72. [DOI] [PubMed] [Google Scholar]
- [26].Ljung L. Identification for control: simple process models. Proceedings of the IEEE Conference on Decision and Control. 2002;4:4652–7. [Google Scholar]
- [27].Mandler MB, Makous W. A three channel model of temporal frequency perception. Vision Res 1984;24:1881–7. [DOI] [PubMed] [Google Scholar]
- [28].Martucci KT, Eisenach JC, Tong C, Coghill RC. Opioid-independent mechanisms supporting offset analgesia and temporal sharpening of nociceptive information. PAIN 2012;153:1232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McGaraughty S, Henry JL. Relationship between mechano-receptive fields of dorsal horn convergent neurons and the response to noxious immersion of the ipsilateral hindpaw in rats. PAIN 1997;70:133–40. [DOI] [PubMed] [Google Scholar]
- [30].McGaraughty S, Henry JL. The effects of strychnine, bicuculline, and ketamine on “immersion-inhibited” dorsal horn convergent neurons in intact and spinalized rats. Brain Res 1998;784:63–70. [DOI] [PubMed] [Google Scholar]
- [31].Mendell LM. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol 1966;16:316–32. [DOI] [PubMed] [Google Scholar]
- [32].Mendell LM, Wall PD. Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature 1965;206:97–9. [DOI] [PubMed] [Google Scholar]
- [33].Moore BCJ, Glasberg BR, Plack CJ, Biswas AK. The shape of the ear's temporal window. J Acoust Soc Am 1988;83:1102–16. [DOI] [PubMed] [Google Scholar]
- [34].Mørch CD, Andersen OK, Quevedo AS, Arendt-Nielsen L, Coghill RC. Exteroceptive aspects of nociception: insights from graphesthesia and two-point discrimination. PAIN 2010;151:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mørch CD, Hennings K, Andersen OK. Estimating nerve excitation thresholds to cutaneous electrical stimulation by finite element modeling combined with a stochastic branching nerve fiber model. Med Biol Eng Comput 2011;49:385–95. [DOI] [PubMed] [Google Scholar]
- [36].Nahman-Averbuch H, Martucci KT, Granovsky Y, Weissman-Fogel I, Yarnitsky D, Coghill RC. Distinct brain mechanisms support spatial vs temporal filtering of nociceptive information. PAIN 2014;155:2491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Niesters M, Hoitsma E, Sarton E, Aarts L, Dahan A. Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. Anesthesiology 2011;115:1063–71. [DOI] [PubMed] [Google Scholar]
- [38].Niesters M, Dahan A, Swartjes M, Noppers I, Fillingim RB, Aarts L, Sarton EY. Effect of ketamine on endogenous pain modulation in healthy volunteers. PAIN 2011;152:656–63. [DOI] [PubMed] [Google Scholar]
- [39].Niesters M, Proto PL, Aarts L, Sarton EY, Drewes AM, Dahan A. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anaesth 2014;113:148–56. [DOI] [PubMed] [Google Scholar]
- [40].Prahl S, Keijzer M, Jacques S, Welch AJ. A Monte Carlo model of light propagation in tissue. SPIE Proceedings of Dosimetry Laser Radiat Med Biol 1989;IS 5:102–11. [Google Scholar]
- [41].Price DD, Hayes RL, Ruda M, Dubner R. Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensations. J Neurophysiol 1978;41:933–47. [DOI] [PubMed] [Google Scholar]
- [42].Rennefeld C, Wiech K, Schoell ED, Lorenz J, Bingel U. Habituation to pain: further support for a central component. PAIN 2010;148:503–8. [DOI] [PubMed] [Google Scholar]
- [43].Rottmann S, Jung K, Vohn R, Ellrich J. Long-term depression of pain-related cerebral activation in healthy man: an fMRI study. Eur J Pain 2010;14:615–24. [DOI] [PubMed] [Google Scholar]
- [44].Sandkuhler J, Chen JG, Cheng G, Randic M. Low-frequency stimulation of afferent A delta-fibers induces long-term depression at primary afferent synapses with substantia gelatinosa neurons in the rat. J Neurosci 1997;17:6483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schmitt JM, Zhou GX, Walker EC, Wall RT. Multilayer model of photon diffusion in skin. J Opt Soc Am A 1990;7:2141–53. [DOI] [PubMed] [Google Scholar]
- [46].Stevens SS. On the psychophysical law. Psychol Rev 1957;64:153–81. [DOI] [PubMed] [Google Scholar]
- [47].Stevens SS. Neural events and the psychophysical law. Science 1970;170:1043–50. [DOI] [PubMed] [Google Scholar]
- [48].Tearney GJ, Brezinski ME, Southern JF, Bouma BE, Hee MR, Fujimoto JG. Determination of the refractive index of highly scattering human tissue by optical coherence tomography. Opt Lett 1995;20:2258–60. [DOI] [PubMed] [Google Scholar]
- [49].Tillman DB, Treede RD, Meyer RA, Campbell JN. Response of C-fiber nociceptors in the anesthetized monkey to heat stimuli: estimates of receptor depth and threshold. J Physiol 1995;485:753–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Treede RD, Meyer RA, Campbell JN. Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J Neurophysiol 1998;80:1082–93. [DOI] [PubMed] [Google Scholar]
- [51].Trojan J, Stolle AM, Kleinbohl D, Morch CD, Arendt-Nielsen L, Holzl R. The saltation illusion demonstrates integrative processing of spatiotemporal information in thermoceptive and nociceptive networks. Exp Brain Res 2006;170:88–96. [DOI] [PubMed] [Google Scholar]
- [52].Verrillo RT. Effect of contactor area on vibrotactile threshold. J Acoust Soc Am 1963;35:1962. [Google Scholar]
- [53].Wall PD. Gate control-theory of pain mechanisms: re-examination and re-statement. Brain 1978;101:1–18. [DOI] [PubMed] [Google Scholar]
- [54].Wang L, Jacques SL, Zheng L. MCML-Monte Carlo modeling of light transport in multi-layered tissues. Comput Methods Programs Biomed 1995;47:131–46. [DOI] [PubMed] [Google Scholar]
- [55].Wilson and SB. A tissue heat transfer model for relating dynamic skin temperature changes to physiological parameters. Phys Med Biol 1988;33:895. [DOI] [PubMed] [Google Scholar]
- [56].Woolf CJ. Windup and central sensitization are not equivalent. PAIN 1996;66:105–8. [PubMed] [Google Scholar]
- [57].Yelle MD, Oshiro Y, Kraft RA, Coghill RC. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J Neurosci 2009;29:10264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yelle MD, Rogers JM, Coghill RC. Offset analgesia: a temporal contrast mechanism for nociceptive information. PAIN 2008;134:174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]