Supplemental Digital Content is Available in the Text.
Treatment with ABT-639 100 mg for 6 weeks did not significantly reduce pain in patients with diabetic peripheral neuropathy.
Keywords: Diabetic neuropathic pain, ABT-639, T-type calcium channels
Abstract
T-type Cav3.2 calcium channels represent a novel target for neuropathic pain modulation. Preclinical studies with ABT-639, a peripherally acting highly selective T-type Cav3.2 calcium channel blocker, showed dose-dependent reduction of pain in multiple pain models. ABT-639 also demonstrated an acceptable safety profile at single- and multiple-dose levels evaluated in a clinical phase 1 study in healthy volunteers. The primary objective of this phase 2, multicenter, randomized, double-blind, placebo-controlled, and active-controlled study was to compare the analgesic efficacy and safety of ABT-639 with placebo in the treatment of diabetic neuropathic pain. Pregabalin, an approved treatment for painful diabetic neuropathy, was included as a positive control. A total of 194 patients were randomized and treated for 6 weeks; 62 patients received ABT-639 (100 mg twice daily), 70 patients received pregabalin (150 mg twice daily), and 62 patients received placebo. When assessing the mean changes from baseline in patient-recorded pain scores at the end of week 6, there was no significant difference observed for ABT-639 compared with placebo (−2.28 vs −2.36; P = 0.582). Pregabalin treatment resulted in a transient improvement in pain compared with placebo, which did not persist throughout the study. There were no significant safety issues identified with ABT-639. A majority of adverse events were considered mild to moderate in intensity. In conclusion, treatment with the highly selective T-type Cav3.2 calcium channel blocker ABT-639 100 mg twice daily for 6 weeks showed no safety signals that would preclude further investigation but did not reduce neuropathic pain in patients with diabetes (ClinicalTrials.gov identifier: NCT01345045).
1. Introduction
Diabetes affects approximately 246 million people worldwide and 8.3% of the population in the United States.12,23 It is estimated that painful diabetic neuropathy affects 10% to 26% of patients with diabetes.23 Patients with painful diabetic neuropathy have reduced quality of life, with a negative effect on physical, emotional, social functioning, and sleep.6
The management of patients with chronic neuropathic pain remains challenging despite the current treatment options, which include anticonvulsants and antidepressant therapies in addition to analgesics, such as opioids.11,12,18 The efficacy of these available agents is limited, as a majority of patients experience less than a 50% pain reduction.18,23 The number of patients who need to be treated to observe a clinically relevant effect (at least 50% pain reduction) in 1 patient ranges from 4.2 to 9.4 in some studies.23 In addition, many of the currently available treatment options have undesirable side effects such as dizziness and nausea.11,12,23 Furthermore, although it is established that strong opioids have efficacy in peripheral neuropathic pain, the risk of abuse with long-term use is a concern.11,12 As a result, there is a major unmet need for treatment options that provide superior efficacy for a greater proportion of patients, with improved tolerability.
T-type calcium channels represent a novel target for pain modulation. Over the last several years, a body of evidence has emerged indicating that activation of these channels contributes to ongoing chronic pain.20,21 Cav3.2 is the predominant T-type calcium channel in sensory nerves that modulates nociception.3 Cav3.2 is expressed primarily in dorsal root ganglion neurons and peripheral receptive fields, as well as in the spinal cord dorsal horn and the brain.19 Preclinical pharmacology, animal intrathecal Cav3.2 antisense oligonucleotide administration, and genetic knockdown studies support the role of Cav3.2 channels in pain.2,21
ABT-639 is a peripherally acting highly selective T-type Cav3.2 calcium channel blocker.4 It is significantly less active at other calcium channels (eg, Cav1.2 and Cav2.2) and shows only weak or no activity on other calcium channels, including L-type and P/Q-type channels.4 Preclinically, ABT-639 produced a dose-dependent reduction of nociception and increased tactile allodynia thresholds in multiple neuropathic pain models, including mechanical hypersensitivity in a spinal nerve ligation model, a chronic constriction injury model, a vincristine chemotherapy–induced neuropathic pain model, and a monoiodoacetic acid–induced joint pain model.4 In a phase 1 first-in-human study, ABT-639 demonstrated an acceptable safety profile at all single- and multiple-dose levels evaluated.1
The primary objective of this phase 2 proof-of-concept study was to compare the analgesic efficacy and safety of investigational drug ABT-639 with placebo in the treatment of diabetic neuropathic pain. Pregabalin, approved for the management of neuropathic pain associated with diabetic peripheral neuropathy, was included in this study as a comparator.
2. Methods
2.1. Patients
Male and female patients between the ages of 18 and 75 years with diabetes mellitus type 1 or type 2 with a diagnosis of distal symmetric diabetic polyneuropathy and the presence of pain due to diabetic peripheral neuropathy for at least 6 months were included in this study. Patients were enrolled from approximately 40 sites in North America and Europe. Patients must have had a score of ≥2.5 on the physical assessment portion of the Michigan Neuropathy Screening Instrument (MNSI) at screening, a score of ≥4 on the Brief Pain Inventory 24-hour average pain at screening, and an average score of ≥4 and <10 on the 24-hour average pain score (0-10 numerical rating scale) collected from the daily electronic diary over approximately 7 consecutive (minimum of 5) days before the baseline visit. Patients had to have been taking a stable antidiabetic medication regimen for 30 days before randomization.
Patients were excluded if they had any clinically significant neuropathic pain condition other than painful diabetic peripheral neuropathy. Patients were excluded if they had a history of hypoglycemia with unconsciousness, ketoacidosis, any changes in diabetes therapy (during the 3 months before the study), or a lower-extremity amputation (other than toes) due to diabetes. Patients were also excluded if they had any history of major depressive episodes within the past 2 years, a history of major psychiatric disorders, or any significant cardiovascular abnormalities. The use of anticonvulsants, antidepressants (eg, tricyclic antidepressants, serotonin–norepinephrine reuptake inhibitors, or norepinephrine reuptake inhibitors [patients may have been eligible if on a stable dose of selective serotonin reuptake inhibitors]), opioids, tramadol, or topical analgesics containing lidocaine or capsaicin were not permitted throughout the course of the study. All patients voluntarily signed an informed consent form, approved by an independent ethics committee or institutional review board, before the conduct of any study-specific procedures. Patients were enrolled in this study between April 27, 2011, and October 26, 2011.
2.2. Study design and treatment
This was a phase 2, multicenter, randomized, double-blind, placebo-controlled, active-controlled, parallel group study. The study included a 4-week screening/washout phase, a 6-week treatment phase, and a 1-week follow-up phase. During the washout period, patients discontinued all current medications for the treatment of neuropathic pain including, but not limited to, anticonvulsants, tricyclic antidepressants, serotonin–norepinephrine reuptake inhibitors, norepinephrine reuptake inhibitors, tramadol, opioids, steroids, topical analgesics containing lidocaine or capsaicin, and other prohibited medications.
Patients were randomized through the computer-generated IVRS/IWRS system in a 1:1:1 ratio to 1 of the 3 treatment groups. At the baseline visit (day 1), patients received a blinded kit of study drug (ABT-639, placebo, or pregabalin) and were instructed to take the evening dose of 2 capsules, with or without food. On days 2 through 7, patients self-administered the study drug twice daily, approximately 12 hours apart, as follows: ABT-639 100 mg (2 × 50-mg capsules), pregabalin 75 mg (1 × 75-mg capsule and 1 placebo capsule), or placebo (2 capsules). Patients treated with pregabalin were titrated from 75 mg twice daily to 150 mg twice daily at the end of the first week of study drug administration. The use of acetaminophen was permitted as a rescue medication and was limited to 3000 mg per day and recorded in the daily diary. Dose selection of ABT-639 was based on the results from a phase 1 study along with the data obtained from preclinical pharmacology and toxicology studies.1,4 A 100-mg twice daily dose of ABT-639 was selected for this study as this dose was tolerated in the phase 1 study and was expected to be safe and to demonstrate an adequate tolerability profile in this 6-week outpatient study.1 In addition, this dose was projected to be efficacious based on preclinical animal pain model data.4 For pregabalin, the dose of 300 mg/d was chosen in accordance with the approved dosage for diabetic neuropathic pain.9 The investigators, study coordinator, and patients remained blinded to each patient's treatment throughout the course of the study. The placebo capsules for ABT-639 were identical in appearance to the ABT-639 capsules. Pregabalin tablets were overencapsulated into capsules that were identical in appearance to ABT-639 capsules.
This study was conducted in accordance with the Good Clinical Practice Guideline as defined by the International Conference on Harmonisation, The Declaration of Helsinki, and all applicable federal and local regulations and institutional review board.
2.3. Assessments
2.3.1. Daily pain assessments
The primary efficacy end point was the change from baseline to the final weekly mean of the 24-hour average pain score derived from the patient's daily pain diary. The pain score was based on an 11-point numeric rating scale (NRS), where 0 = no pain and 10 = worst pain imaginable. Patients recorded the pain score daily on an electronic patient-reported outcomes device (diary). Secondary end points included the worst pain during the day and nighttime pain, both of which were based on an 11-point NRS; pain intensity in the morning, which was based on a 5-point verbal scale; daily sleep score interference scale, which was based on an 11-point NRS; rescue medication usage; Brief Pain Inventory (short form [BPI-SF]) Interference only score, which was used to capture patients' interference by pain; and the Neuropathic Pain Symptom Inventory total intensity score, which was calculated from 10 questions regarding the severity of common neuropathic pain qualities (ie, burning, pressure, squeezing, electric shock, stabbing, tingling, and pins and needles) and from pain evoked by brushing, pressure, and cold. In addition, the number and proportion of patients with ≥30% or ≥50% pain reduction were calculated.
2.3.2. Quality-of-life assessments
At scheduled study visits, several quality-of-life assessments were completed, including the Patients Global Impression of Change, in which patients evaluated their overall general impression of how they felt since beginning the study medication; Neuropathic Pain Impact on Quality of Life, which is a questionnaire designed to assess the neuropathic pain and the effect it has on the quality of daily life; and the EuroQuol-5D-5L (EQ-5D-5L), which is a 5-dimensional tool capturing patient's mobility, self-care, usual activity, pain/discomfort, and anxiety/depression.
2.3.3. Pharmacokinetic assessments
Blood samples for the assay of ABT-639 were collected at weeks 1, 2, 4, and 6. Plasma concentrations of ABT-639 were determined under the supervision of the drug analysis department at Abbott (North Chicago, IL) using a validated liquid chromatography/mass spectrometry (laser diode thermal desorption with tandem mass spectrometry). In brief, the analytical method was validated over the concentration range of 1.11 to 3450 ng/mL for the plasma analyses, with all calibration curves having r2 values greater than 0.993. The lower limit of quantitation value for ABT-639 was 1.1 ng/mL. The coefficient of variation values ranged from 2.3% to 26.8% and mean bias values ranged from −4.5% to 5.8%.
2.3.4. Safety assessments
Safety was assessed using observed and spontaneously reported adverse events (AEs) that were classified, according to the investigator's opinion, by severity and relationship to study treatment and were coded using the Medical Dictionary for Regulatory Activities (version 14.0). All AEs reported from the time of study drug administration until 30 days after the last dose of study drug administration were collected. Findings on patients' physical examinations, vital sign measurements, 12-lead electrocardiograms, and results from clinical laboratory tests were assessed throughout the study, at baseline, weeks 1, 2, 4, and 6, 1-week posttreatment follow-up, and on any discontinuation of treatment.
2.4. Statistical analysis
Efficacy analyses were performed on a modified intent-to-treat (mITT) data set. The mITT data set included all randomized patients who took at least 1 dose of the study drug, except that patients treated at 1 site were excluded due to data quality issues at that site. Demographic and safety analyses were performed on the ITT and mITT data sets (mITT data not presented). Statistical tests were evaluated at a significance level of 0.05, using 1-sided tests for efficacy analyses and 2-sided tests for all other analyses. For all efficacy analyses, the primary comparison was between the ABT-639 treatment group and the placebo treatment group in the mITT population. Patients who were missing the 24-hour average pain baseline score or were missing all postbaseline 24-hour average pain scores were excluded from the primary efficacy analysis, and data for these patients were not imputed. Secondary analyses evaluated by weekly intervals were conducted using the last observation carried forward and observed case methodologies. Secondary efficacy variables used the last nonmissing observation at or before the baseline visit, and the “final” refers to the last nonmissing observation no more than 3 days after the last dose of the study drug.
The sample size calculation was based on the primary efficacy variable, weekly mean of the 24-hour average pain score, and the primary comparison between the ABT-639 treatment group and the placebo group. The sample size was sufficient to detect a difference of 1.25 points (effect size of 0.5) between the ABT-639 and placebo groups with at least 80% power and type-1 error at 0.05 for a 1-sided test.
3. Results
3.1. Patient disposition and baseline characteristics
A total of 194 patients were randomized and treated; 62 patients received ABT-639, 70 patients received pregabalin, and 62 patients received placebo (Table 1). Overall, patients were, on average, 59 years of age and a majority of patients were white. Most patients had type 2 diabetes (94%), and the average duration of diabetes since diagnosis was 14 years (minimum-maximum [min-max], 0.5-63.6 years). The average duration of diabetic neuropathic pain was 5.9 years (min-max, 0.6-41.5 years). A majority of patients were taking metformin before initiation of the study to control their diabetes. There were no significant differences in baseline characteristics between groups.
Table 1.
Baseline demographics and characteristics (ITT population).

Most patients (177, 91%) completed the study. Seventeen patients discontinued the study prematurely, with AEs cited as the most common reason for discontinuation in the ABT-639 group (3 patients, 5%), the placebo group (2 patients, 3%), and the pregabalin group (4 patients, 6%). Other causes for discontinuation included the following (some patients may have cited more than 1 reason): 6 patients withdrew consent, 2 patients discontinued due to lack of efficacy, 1 patient was lost to follow-up, and 3 patients discontinued for other reasons (protocol deviation at baseline, use of excluded medication, out of town without study drug). For more detailed patient disposition, refer the CONSORT diagram and checklist for reporting randomized trials in the Appendix (available online as Supplemental Digital Content at http://links.lww.com/PAIN/A131).
3.2. Efficacy assessments
3.2.1. Primary end point: weekly mean of 24-hour average pain score
Assessment of pain intensity and quality of life at baseline are summarized by the treatment group in Table 2. The mean (±SD) pain intensity score was 6.5 (1.25) at baseline for the mITT data set. No significant differences among treatment groups were observed at baseline for efficacy assessment scores.
Table 2.
Baseline pain intensity and quality-of-life scores (mITT).

The final weekly mean (±SD) values of the 24-hour average pain diary scores were 4.25 (2.22) for ABT-639, 4.51 (2.17) for pregabalin, and 4.34 (2.35) for placebo. There was no statistically significant difference in the mean change from baseline to the final weekly mean of the 24-hour average pain score for patients treated with ABT-639 compared with patients receiving placebo (−2.28 vs −2.36, respectively; P = 0.582; Fig. 1). Patients treated with pregabalin also did not show significant improvement in pain compared with patients receiving placebo in the mean change from baseline to the final weekly mean of the 24-hour average pain score (−2.19 vs −2.36, respectively; P = 0.680). There was also no significant difference in the mean change from baseline to each postbaseline weekly mean of the 24-hour average pain score between patients treated with ABT-639 and those receiving placebo (P > 0.05 for all time points). However, when comparing pregabalin with placebo, there was a significant treatment effect at weeks 1 and 2 (P = 0.017 and 0.043, respectively). In a secondary analysis of the primary efficacy variable, the proportion of responders with at least 30% or 50% improvement in pain from baseline to week 6 for mean 24-hour average pain score was not significantly greater than placebo for patients receiving ABT-639 or pregabalin (Table 3).
Figure 1.
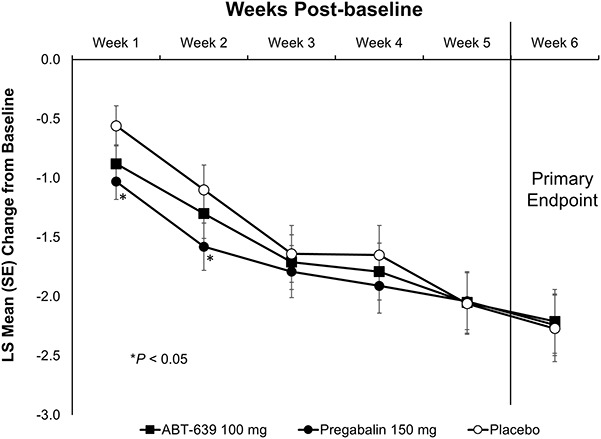
Least square mean change from baseline over time for 24-hour average pain daily score (mITT). Treatments included 100-mg ABT-639, 150-mg pregabalin, or placebo, each administered twice daily. Significance derived from an analysis of variance comparing ABT-639 or pregabalin with placebo. mITT, modified intent-to-treat.
Table 3.
Proportion of responders with improvement from baseline to week 6 in weekly mean of the 24-hour average pain diary score (mITT).

3.2.2. Secondary end points
Baseline scores for secondary end points are summarized in Table 2, and the results of secondary efficacy end points are summarized in Table 4. There were no clinically meaningful improvements from baseline in pain reduction or quality of life based on the findings of the secondary end points after treatment with ABT-639 or pregabalin when compared with placebo. In addition, there were no significant differences between treatment groups in the proportion of patients by response category of the PGI-C at the final visit compared with baseline.
Table 4.
Secondary end points: change from baseline to final weekly mean (mITT).

A smaller proportion of patients in the ABT-639 treatment group (25 patients, 44%) took at least 1 dose of rescue medication during the treatment period compared with patients in the placebo group (58%); however, the difference was not significant (Table 5). The same proportion of patients in the pregabalin and placebo treatment groups took at least 1 dose of rescue medication during the treatment period. No significant difference between the ABT-639 and placebo groups or between the pregabalin and placebo groups was observed for the mean number of days rescue medication was used during the treatment period.
Table 5.
Rescue medication use (mITT).

3.2.3. Pharmacokinetic assessments
The mean plasma concentration of ABT-639 varied minimally, from 2862 ± 1940 ng/mL at week 1, 3139 ± 1993 ng/mL at week 2, 2706 ± 1802 ng/mL at week 4, and 2932 ± 1971 ng/mL at week 6. These observations indicate that steady-state concentrations were within the concentration range observed in the phase 1 study for the same dose.1
3.2.4. Tolerability and safety
Overall, 51% of patients had at least 1 treatment-emergent AE (TEAE) during the study. The proportion of patients who experienced AEs was lower in the ABT-639 group (44%) compared with the placebo group (55%), although the difference was not significant. In the pregabalin group, 54% of patients reported TEAEs. In addition, the proportion of patients who experienced TEAEs that were possibly or probably related to the study drug was lower in patients receiving ABT-639 (16%) than in those patients receiving pregabalin (24%) or placebo (24%). Regardless of treatment, most AEs were considered mild or moderate in intensity, with severe AEs reported by 8 patients: 2 (3%) patients in the placebo group, 3 (5%) patients in the ABT-639 group, and 3 patients (4%) in the pregabalin group.
The most frequently reported TEAEs (≥3%) in the ABT-639 treatment group, and at rates numerically higher than those reported with placebo, were abdominal distension (5%), muscle spasms (5%), viral gastroenteritis (3%), insomnia (3%), nasopharyngitis (3%), rash (3%), and sinusitis (3%) (Table 6). There were no significant differences between the ABT-639 and placebo groups based on incidence of any specific AE. Patients treated with pregabalin experienced AEs commonly reported for this medication, including 7 (10%) patients experiencing peripheral edema, 3 (4%) patients reporting somnolence, and 2 (3%) patients reporting dizziness. In comparison, only 1 (2%) patient treated with ABT-639 reported either peripheral edema or dizziness. There was a significant difference between the pregabalin and placebo groups based on incidence of peripheral edema (10% vs 0%; P < 0.05) and constipation (0% vs 6.5%; P < 0.05). Five patients experienced a serious AE: 1 patient (a 64-year-old white female in the ABT-639 group) experienced angina pectoris with onset on day 11 and resolved on day 15, which was not considered related to ABT-639 treatment by the investigator, 1 patient in the placebo group had pneumonia, and 3 patients in the pregabalin group experienced 9 serious AEs: 1 patient developed acute cholecystitis and postoperative wound infection, 1 patient developed intraocular melanoma, and 1 patient developed myocardial infarction, nausea, pyrexia, renal failure, lung infiltration, and urticaria.
Table 6.
Treatment-emergent adverse events reported in 3% or more patients in the ABT-639 group (ITT).

In general, there were no statistically significant differences from baseline to final assessment in laboratory values between ABT-639 and placebo, with the exception of a decrease in mean change from baseline to final uric acid for ABT-639 compared with placebo, which was not deemed to be clinically meaningful. There were no significant or clinically meaningful differences in vital sign measurements between ABT-639 and placebo from baseline to the final measurement, except for a significant decrease in diastolic blood pressure (76.2 vs 77.2 mm Hg, respectively; P = 0.040). Electrocardiographic measurements indicated that there were no statistically significant differences in PR interval, QRS duration, and QT/QTc interval between ABT-639 and placebo from the baseline to the final measurement. There was a statistically significant increase in heart rate obtained from electrocardiograms in the ABT-639 group from baseline to weeks 1, 4, 6, and the final evaluation when compared with placebo, ranging from 2.7 beats per minute to 3.6 beats per minute (all P < 0.05).
4. Discussion
This phase 2 proof-of-concept study compared the analgesic efficacy and safety of ABT-639 with placebo in patients with diabetes with peripheral neuropathic pain for 6 weeks. There was no significant difference from baseline to the final weekly mean of the 24-hour average pain score when comparing patients treated with ABT-639 and patients receiving placebo. In addition, there were no clinically meaningful or significant differences for the other secondary end points between the ABT-639 and placebo treatment groups.
It is unlikely that the absence of analgesic efficacy of ABT-639 observed in this trial resulted from trial design or conduct because pregabalin, a positive control for assay sensitivity, demonstrated pain reduction for the first 2 weeks. Although the analgesic effect of pregabalin did not persist throughout the study, the current trial is not the first study to show the inconsistent performance of pregabalin in neuropathic pain trials.13,14,22 Pregabalin does have a dose-dependent effect; and perhaps at higher doses, a greater reduction in pain and improvement in quality of life would be obtained.8,9,22 However, because 300 mg/d pregabalin is the highest approved dose in the United States for the treatment of neuropathic pain associated with diabetes, a higher dose could not be used in this trial. Nevertheless, the lack of a persistent effect of pregabalin treatment throughout the 6-week treatment period is one of the limitations of this study. Thus, the possibility of a false-negative result for ABT-639 cannot be completely ruled out. Perhaps, another active comparator such as duloxetine could have been used in this study; however, given the mechanisms of action of pregabalin and ABT-639, pregabalin was considered a better option. Another limitation of the trial is that it may have been too short to detect efficacy of ABT-639. However, based on the mechanism of ABT-639, it should not have taken more than 6 weeks to see an effect or at least a trend in efficacy.
It is conceivable that the ABT-639 dose of 100-mg twice daily was not high enough to produce an analgesic effect of ABT-639. This is particularly noteworthy given that the tolerability and safety profile from a phase 1 study and the current trial was benign and there were no significant safety issues identified.1 However, based on the findings from animal toxicology studies,4 it was determined that 100-mg twice daily ABT-639 was the appropriate highest dose with an adequate safety margin for this proof-of-concept study. In addition, the dose used in this study was predicted to provide an exposure level above what was required for efficacy in humans based on the projection from preclinical animal models.1 Indeed, ABT-639 pharmacokinetic analysis indicated that plasma concentrations obtained from this study in patients with diabetic neuropathic pain were consistent with previous predictions and exposures observed in healthy adults for the same dosing regimen1 and were within the preclinically predicted plasma level range required for human efficacy, suggesting that lack of efficacy of the 100-mg twice daily dose is unlikely due to underexposure of ABT-639. Furthermore, the relatively high placebo response observed in this study and others may obscure any potential reduction in pain that may occur at this dose of ABT-639.21 Nevertheless, whether a higher dose of ABT-639 would exert an acceptable efficacy in neuropathic pain remains uncertain.
In this study, ABT-639 demonstrated an acceptable safety profile. The proportion of patients who experienced TEAEs in the ABT-639 treatment group was lower than that experienced by patients in the placebo group, although this difference was not significant. Although more than 51% of patients experienced at least 1 TEAE, most TEAEs were considered to be mild to moderate in intensity, with TEAEs considered to be possibly or probably related to study drug occurring in 16% of patients receiving ABT-639, 24% of patients receiving pregabalin, and 24% of patients receiving placebo. In general, there were very few AEs related to the central nervous system in the ABT-639 treatment group. There was a significant difference in mean change from baseline in uric acid levels at all visits and the final measurement in this study; however, this was not considered to be clinically meaningful and had been previously observed in the completed phase 1 study.1 Overall, ABT-639 100-mg given twice daily demonstrated an acceptable safety and tolerability profile in patients with diabetic neuropathic pain. For patients in the pregabalin treatment group, the observed incidences of the most frequently reported AEs, dizziness and somnolence, were lower in this study compared with other clinical trials.10,15 Although the exact reason is unclear, this may be explained by the fact that some patients enrolled in this study were treated previously with pregabalin and gabapentin and, therefore, may have developed a tolerance to these pregabalin-associated AEs.
The disconnection between human data in the current trial and preclinical animal data represents one of the greatest challenges in analgesic drug development, particularly with ion channel blockers. There are no validated biomarkers that can demonstrate target engagement. In parallel with this phase 2 proof-of-concept study, a microneurography study and a human experimental pain study were conducted with ABT-639 in an attempt to bridge preclinical and clinical findings.16 Microneurography is a technique that directly assesses abnormal spontaneous activity in C-nociceptors as a marker for spontaneous pain; however, in that study, ABT-639 did not show the reduction of spontaneous activity of nociceptive C-fibers in patients with diabetic neuropathic pain.16 In the capsaicin experimental pain study, ABT-639 failed to show an effect on pain induced by intradermal injection of capsaicin in healthy human volunteers.16 However, ABT-639 demonstrated antinociceptive effects in multiple animal pain models (eg, spinal nerve ligation, vincristine-induced, and capsaicin-induced secondary hypersensitivity).4,5 Although the data from this phase 2 proof-of-concept study are consistent with findings from the above 2 studies, further analysis of the data and understanding of the discrepancies between preclinical and clinical outcomes is warranted.
The failure of achieving proof-of-concept with ABT-639 in this study does not support T-type calcium channel Cav3.2 as a potential target for treating diabetic neuropathic pain. However, another T-type calcium channel blocker, Z944, which may be less selective and more central-acting, has shown reduction in pain scores in human experimental pain models and in preclinical studies.7,16 Therefore, targeting of T-type calcium channel blockers for the treatment of chronic pain should not be discarded without further investigation.
Conflict of interest statement
The authors have no conflicts of interest to declare.
W. R. Duan and J. W. Thomas are full-time employees of AbbVie Inc and hold AbbVie stock or stock options. W. Nothaft and G. An are former employees of Abbott and hold Abbott stock or stock options. W. Nothaft is now employed by Shire, Lexington, MA. G. An is now employed at the Division of Pharmaceutics and Translational Therapeutics, College of Pharmacy, University of Iowa, Iowa City, IA. D. Ziegler received honoraria for consulting activities from Eli Lilly, Pfizer, Wörwag, Meda, Glenmark, Daiichi-Sankyo, Roche, Takeda, and Astellas; for speaking activities from Eli Lilly, Pfizer, Meda, Wörwag, Takeda, Berlin-Chemie, Eisai, Shire, and Menarini; and research support from NeuroMetrix and AbbVie.
This study was funded by AbbVie Inc, North Chicago, IL. AbbVie was involved in the study design. D. Ziegler, W. R. Duan, G. An, J. W. Thomas, and W. Nothaft were responsible for collecting and interpreting data. All authors were involved in the writing process and critically reviewed the manuscript and approved the final version for submission. All authors accept responsibility for the accuracy of the data and its analysis.
Acknowledgements
The authors wish to thank Kelly Cameron, PhD, of The JB Ashtin Group, Inc., who provided medical writing support in the development of this manuscript, and this support was funded by AbbVie Inc.
Appendix A. Supplemental Digital Content
Supplemental Digital Content associated with this article can be found online at http://links.lww.com/PAIN/A131.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].An G, Liu W, Duan WR, Nothaft W, Awni W, Dutta S. Erratum to: population pharmacokinetics and exposure-uric acid analyses after single and multiple doses of ABT-639, a calcium channel blocker, in healthy volunteers. AAPS J 2015;17:481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bourinet E, Alloui A, Monteil A, Barrere C, Couette B, Poirot O, Pages A, McRory J, Snutch TP, Eschalier A, Nargeot J. Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J 2005;24:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science 2003;302:1416–18. [DOI] [PubMed] [Google Scholar]
- [4].Jarvis MF, Scott VE, McGaraughty S, Chu KL, Xu J, Niforatos W, Milicic I, Joshi S, Zhang Q, Xia Z. A peripherally acting, selective T-type calcium channel blocker, ABT-639, effectively reduces nociceptive and neuropathic pain in rats. Biochem Pharmacol 2014;89:536–44. [DOI] [PubMed] [Google Scholar]
- [5].Jarvis MF, Scott VE, McGaraughty S, Niforatos W, Milicic I, Joshi S, Zhang S, Xia Z. A peripherally acting, selective T-type calcium channel blocker, ABT-639, effectively reduces nociceptive and neuropathic pain in rats. Presented at the Society for Neuroscience, November 13, 2013; Poster 829.01. [DOI] [PubMed]
- [6].Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology 2007;68:1178–82. [DOI] [PubMed] [Google Scholar]
- [7].Lee M. Z944: a first in class T-type calcium channel modulator for the treatment of pain. J Periph Nerv Sys 2014;19(suppl 2):S11–12. [DOI] [PubMed] [Google Scholar]
- [8].Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology 2004;63:2104–10. [DOI] [PubMed] [Google Scholar]
- [9].Lyrica. [package insert]. New York, NY: Parke-Davis, division of Pfizer Inc., 2013. [Google Scholar]
- [10].Moon DE, Lee DI, Lee SC, Song SO, Yoon DM, Yoon MH, Kim HK, Lee YW, Kim C, Lee PB. Efficacy and tolerability of pregabalin using a flexible, optimized dose schedule in Korean patients with peripheral neuropathic pain: a 10-week, randomized, double-blind, placebo-controlled, multicenter study. Clin Ther 2010;32:2370–85. [DOI] [PubMed] [Google Scholar]
- [11].Moulin D, Boulanger A, Clark AJ, Clarke H, Dao T, Finley GA, Furlan A, Gilron I, Gordon A, Morley-Forster PK, Sessle BJ, Squire P, Stinson J, Taenzer P, Velly A, Ware MA, Weinberg EL, Williamson OD. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manage 2014;19:328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Peltier A, Goutman SA, Callaghan BC. Painful diabetic neuropathy. BMJ 2014;348:g1799. [DOI] [PubMed] [Google Scholar]
- [13].Pfizer A placebo-controlled trial of pregabalin and amitriptyline for treatment of painful diabetic peripheral neuropathy. PhRMA Web Synopsis, 2007. Protocol 1008–040. [Google Scholar]
- [14].Pfizer A randomized double-blind, placebo-controlled, parallel-group, multi-center trial of pregabalin versus placebo in the treatment of neuropathic pain associated with diabetic peripheral neuropathy. PhRMA Web Synopsis, 2007. Protocol A0081071: NCT00113456. [Google Scholar]
- [15].Satoh J, Yagihashi S, Baba M, Suzuki M, Arakawa A, Yoshiyama T. Efficacy and safety evaluation of pregabalin treatment over 52 weeks in patients with diabetic neuropathic pain extended after a double-blind placebo-controlled trial. J Diabetes Investig 2011;2:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Serra J, Duan WR, Locke C, Solá R, Lin W, Nothaft W. Effects of a T-type calcium channel blocker, ABT-639, on spontaneous activity in c-nociceptors in patients with painful diabetic neuropathy: a randomized controlled trial. Pain 2015; published online ahead of print. 10.1097/j.pain.0000000000000249. [DOI] [PubMed] [Google Scholar]
- [17].Short G, Lee M, Snutch T. Z944: A first in-class T-type calcium channel blocker effective in nonclinical models of acute and inflammatory pain. J Pain 2013;14:S71. [Google Scholar]
- [18].Snedecor SJ, Sudharshan L, Cappelleri JC, Sadosky A, Mehta S, Botteman M. Systematic review and meta-analysis of pharmacological therapies for painful diabetic peripheral neuropathy. Pain Pract 2014;14:167–84. [DOI] [PubMed] [Google Scholar]
- [19].Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci 1999;19:1895–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Todorovic SM, Jevtovic-Todorovic V. Regulation of T-type calcium channels in the peripheral pain pathway. Channels (Austin) 2007;1:238–45. [DOI] [PubMed] [Google Scholar]
- [21].Todorovic SM, Jevtovic-Todorovic V. T-type voltage-gated calcium channels as targets for the development of novel pain therapies. Br J Pharmacol 2011;163:484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tolle T, Freynhagen R, Versavel M, Trostmann U, Young JP., Jr Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain 2008;12:203–13. [DOI] [PubMed] [Google Scholar]
- [23].Ziegler D, Fonseca V. From guideline to patient: a review of recent recommendations for pharmacotherapy of painful diabetic neuropathy. J Diabetes Complications 2015;29:146–56. [DOI] [PubMed] [Google Scholar]


