Supplemental Digital Content is Available in the Text.
Inhibition of dimethylarginine dimethylaminohydrolase 1 attenuates pain-related behavior and hyperexcitability in pain conditions associated with excessive nitric oxide production, representing a novel therapeutic target.
Keywords: Spinal hyperexcitability, Nitric oxide, Neuronal nitric oxide synthase, Asymmetric dimethylargenine, Dimethylarginine dimethylaminohydrolase inhibition
Abstract
Activation of neuronal nitric oxide synthase, and consequent production of nitric oxide (NO), contributes to spinal hyperexcitability and enhanced pain sensation. All NOS isoforms are inhibited endogenously by asymmetric dimethylarginine, which itself is metabolised by dimethylarginine dimethylaminohydrolase (DDAH). Inhibition of DDAH can indirectly attenuate NO production by elevating asymmetric dimethylarginine concentrations. Here, we show that the DDAH-1 isoform is constitutively active in the nervous system, specifically in the spinal dorsal horn. DDAH-1 was found to be expressed in sensory neurons within both the dorsal root ganglia and spinal dorsal horn; L-291 (NG–[2-Methoxyethyl]-l-arginine methyl ester), a DDAH-1 inhibitor, reduced NO synthesis in cultured dorsal root ganglia neurons. Spinal application of L-291 decreased N-methyl-d-aspartate–dependent postdischarge and windup of dorsal horn sensory neurons—2 measures of spinal hyperexcitability. Finally, spinal application of L-291 reduced both neuronal and behavioral measures of formalin-induced central sensitization. Thus, DDAH-1 may be a potential therapeutic target in neuronal disorders, such as chronic pain, where elevated NO is a contributing factor.
1. Introduction
Activation of neuronal nitric oxide synthase (nNOS), and consequent generation of nitric oxide (NO), is coupled to upstream stimulation of N-methyl-d-aspartate (NMDA) receptors.9–11 In the spinal dorsal horn, NO contributes to sensitization of nociceptive signalling pathways in both acute and chronic pain states, although it is not thought to be involved in basal pain perception.4,11,12,15,20,22,30
A key endogenous inhibitor of NOS is asymmetric dimethylarginine (ADMA), which is liberated after posttranslational methylation of arginine residues within proteins and subsequent proteolysis.14,21 Structurally similar to l-arginine, ADMA competes for the active site of NOS and thus regulates NO formation. Physiological concentrations of ADMA in the brain are sufficient to modulate nNOS function and suppress NO-mediated excitotoxicity.3 Other evidence suggests that ADMA modulates various processes in the central nervous system (CNS), including the response to nerve injury and in nociception.25,14 Asymmetric dimethylarginine itself is actively regulated by dimethylarginine dimethylaminohydrolase (DDAH), of which there are 2 isoforms, DDAH-1 and DDAH-2; this supports the concept that ADMA confers an important physiological function.18,32 DDAH metabolises ADMA to l-citrulline and dimethylamine, regulating the inhibitory influence of ADMA on NOS (Supplementary Figure 1A, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A115). Accordingly, pharmacologic inhibition of DDAH increases levels of ADMA sufficiently to attenuate NO synthesis.29,17
We have developed a competitive inhibitor of DDAH, NG-[2-methoxyethyl] arginine methyl ester (L-291),29 selective for DDAH-1.26 L-291 indirectly reduces NO signalling through accumulation of ADMA.17,26,29 Inhibition of DDAH-1 within the cardiovascular system was found to be protective in rodent models of septic shock, in which NO reaches pathological levels and contributes to sustained circulatory collapse.17,26,34 Targeting DDAH isoforms may be beneficial in other pathologies in which increased NO signalling is implicated, particularly within the CNS.
The distribution of DDAH mRNA correlates well with NOS expression in a number of tissues; in fact, DDAH-1 is found predominantly in tissues expressing nNOS.18,32 Both DDAH and nNOS mRNA are upregulated in axotomized motoneurons.25 The functional contribution of DDAH in the nervous system, however, remains largely unknown. Here, we have investigated the role of DDAH-1 in the nervous system, specifically in the spinal cord, by administering L-291 in 2 models of spinal nociceptive plasticity.
2. Materials and methods
2.1. Animals
Experimental procedures in adult male Sprague-Dawley rats (220-250 g) or C57BL/6 mice (∼25-30 g), Central Biological Services—University College London, King's College London or Harlan, United Kingdom, were approved by the UK Home Office and were conducted in accordance with the guidelines of the International Association for the Study of Pain.35
2.2. DDAH-1-specific inhibitor, L-291, and control inactive enantiomer, L-456
L-291 (NG-[2-methoxyethyl] arginine methyl ester) (Supplementary Figure 1B, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A115) is a selective competitive inhibitor of DDAH-1, whereas L-456 is its inactive d-enantiomer and serves as a control. Both compounds were dissolved in 0.9% saline. Full details of their synthesis and characterization have been described previously.29,17
2.3. Dorsal root ganglia culture and fluorometric detection of nitrite and asymmetric dimethylarginine
Dorsal root ganglia from adult male rodents were removed into sterile Ham's F12 medium (Gibco 21765-029, Life Sciences, Paisley, United Kingdom) and DRG neurons were isolated as described previously.27,1 Neurons were plated into 96-well plates, coated with poly-l-lysine (100 μg/mL; P8920, Sigma-Aldrich, Gillingham, United Kingdom) and laminin (10 μg/mL; Sigma L-2020). After 24 hours, the medium was replaced with customised (100 μM l-arginine) Dulbecco's Modified Eagle Medium (Gibco) containing L-291 (0-10 mM) for a further 24 hours, after which medium was acquired for either nitrite or ADMA measurement.
For nitrite measurements, 100 μL medium was removed from each well and transferred to a black-bottomed 96-well plate. Nitrite levels were measured by 2,3-diaminonaphthalene fluorometry, as per Misko et al.23 Fluorescence was recorded using a spectrofluorometer equipped with Xenon lamp source (excitation 365 nm; emission 410 nm). Nitrite levels were calculated from linear calibration curves of known nitrite concentrations (0-5 μM) made up in the same medium.
Asymmetric dimethylarginine was measured from the cell culture media using LC-MS/MS as previously described.2
2.4. Western immunoblotting of DDAH-1 in neuronal tissues
Urethane-anesthetized rats were killed by decapitation and tissues (spinal dorsal horn, DRG, and hippocampus) were dissected and snap frozen. Tissues were homogenized in RIPA (radioimmunoprecipitation assay) buffer (50 mM Tris HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.1% SDS + 0.5% deoxycholic acid + complete protease inhibitor cocktail) using a glass homogenizer. Homogenates were centrifuged at 14,000 rpm for 10 minutes at 4°C, and supernatants containing whole-cell tissue lysates were collected. Protein concentrations were determined using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Cramlington, United Kingdom). Laemmli loading buffer was added to protein lysates (40 μg) and samples were incubated at 70°C for 30 minutes, before loading onto 8% gels, and separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis.
Proteins were transferred to nitrocellulose membranes and probed overnight at 4°C with goat anti-DDAH-1 (1:500; ab2231; Abcam, Cambridge, United Kingdom) or rabbit anti-neuronal β-III Tubulin (1:3000; ab18207; Abcam), which served as a loading control. Membranes were incubated with IRDye-linked donkey anti-goat 680 or donkey anti-rabbit 800CW secondary antibody (1:15,000) for 1 hour at RT. Proteins were revealed using the Odyssey fluorescence detection system (Licor, Cambridge, United Kingdom).
2.5. Immunohistochemical detection of DDAH-1
Rats were anaesthetized using sodium pentobarbital (60 mg/kg) and perfused transcardially with 100 mL of saline followed by 500 mL of 4% paraformaldehyde in 0.2 M phosphate buffer pH 7.4 (PB). The spinal cord and lumbar L5 DRG were dissected and postfixed at 4°C overnight (spinal cord) or for 2 hours (DRG). Tissues were cryoprotected overnight at 4°C in 30% sucrose in 0.1 M phosphate-buffered saline (PBS) and then frozen in optimal cutting temperature (OCT) embedding medium. Dorsal root ganglia sections (20 μm) were cut using a cryostat and collected directly on slides; spinal cord sections (30 μm) were collected in PBS for “free-floating” immunostaining.
All sections were incubated overnight with goat anti-DDAH-1 antibody (1:100; ab2231; Abcam) in PBS-T-Azide (0.1 M PBS + 0.3% Triton X100 + 0.002% sodium azide) at RT, followed by donkey anti-goat Alexa Fluor 488 secondary antibody in PBS-T (1:1000; Invitrogen, CA) for 2 hours. After washes in PBS, sections were mounted in Vectashield medium (Vector Laboratories, CA).
2.6. Double immunofluorescent staining of DDAH-1 with NeuN, CGRP, IB4, β-III Tubulin, and NF200
Sections were incubated overnight at RT in goat anti-DDAH-1 antibody (1:100; ab2231; Abcam) plus one of the following primary antibodies/lectin: mouse anti-NeuN (1:1000; VMA377; Abcys, Paris, France), IB4-FITC (1:700; L2895; Sigma Aldrich), rabbit anti-CGRP (1:4000; Sigma Aldrich), mouse anti-β-III Tubulin (1:1000; Promega, Charbonnieres, France), or mouse anti-NF200 (1:1000; N0142; Sigma Aldrich). After washing in PBS, tissues were incubated for 2 hours at RT in a mixture of secondary antibodies: donkey anti-goat Alexa Fluor 568 (1:1000) and donkey anti-mouse Alexa Fluor 488 (1:1000). Tissues were washed in PBS and mounted in Vectashield medium (Vector Laboratories).
2.7. Specificity of DDAH-1 immunostaining
Goat anti-DDAH-1 primary antibody (1:100; ab2231; Abcam) was preabsorped with 10 times excess (weight/weight) of the peptide used to generate the antibody (ab99047; Abcam) overnight at 4°C. The mixture was then centrifuged for 20 minutes at 10,000 rpm and the top half of the solution was collected and applied to tissue sections. A positive control (antibody alone at the same dilution) and a negative control (lack of primary antibody) were run in parallel.
2.8. In vivo electrophysiology—setup
Rats were anesthetized using 4% to 5% isoflurane (66% N2O and 33% O2) and a tracheal cannula was inserted. Rats were placed in a stereotaxic frame, and core body temperature was maintained at 37°C using a feedback heating blanket. Anesthesia was reduced to 2.5% isoflurane and a laminectomy was performed at the L1-L3 vertebral level to expose the L4-L5 segments of the spinal cord. Anesthesia was then maintained at 1.5% isoflurane.
Extracellular recordings from single convergent deep dorsal horn (>600 μm) wide dynamic range (WDR) neurons were made using parylene-coated tungsten electrodes (A-M Systems, Sequim, WA). Wide dynamic range neurons respond to both innocuous and noxious stimulation in a graded manner and can respond to mechanical, thermal, electrical, and chemical stimuli. Data were captured by a CED 1401 interface coupled to a Pentium computer with Spike 2 software (Cambridge Electronic Design, UK; PSTH and rate functions).
2.9. Electrical stimulation of the rat hind paw to induce windup
A train of 16 transcutaneous electrical stimuli (2-millisecond wide pulses, 0.5 Hz) was applied at 3 times the threshold current for C-fibers through 2 stimulating needles inserted under the skin of the hind paw. A poststimulus histogram was constructed, and Aβ- (0-20 milliseconds), Aδ- (20-90 milliseconds), and C-fiber- (90-300 milliseconds) evoked responses were separated and quantified by latency. Responses occurring after the C-fiber latency band were taken to be the postdischarge (PD) of the cell (300-800 milliseconds). Input (nonpotentiated response) was calculated as response after the first stimulus × 16. Windup was calculated as (total number of action potentials after 16 stimuli) − (input).
After stable control responses at 10-minute intervals, L-291 was injected directly onto the surface of the spinal cord (after removal of residual cerebrospinal fluid) in cumulative doses of 1.2, 12, and 120 μg in a volume of 50 μL (0.1, 1, and 10 mM, respectively) using a Hamilton syringe. Electrical tests were continued at 10-minute intervals with the effect of each dose followed for 1 hour. Control values were obtained by averaging the responses of the 3 neuronal tests immediately before initial drug administration. A separate group of animals received 120 μg (10 mM) of the control L-456 for comparison.
2.10. Neuronal formalin test
Wide dynamic range neurons were characterized before formalin administration. Transcutaneous stimulating needles delivered electrical stimuli to the receptive field. Next, thermal stimulation was applied using a constant jet of water onto the center of the receptive field to indicate a strong C-fiber input to the WDR neuron, which is required for the response to subcutaneous formalin.8
Rats were then pretreated by topical spinal administration of 12 μg of L-291 (1 mM) or 120 μg of L-456 (10 mM), 20 minutes before the injection of formalin. Both drugs were delivered in a volume of 50 μL using a Hamilton syringe. Formalin (5%, 50 μL) was prepared from a 37% formaldehyde solution and injected subcutaneously into the hind paw receptive field. Firing responses of WDR neurons were recorded for 70 minutes. Activity was displayed as a rate recording and quantified in 10-minute time bins.
2.11. Behavioral formalin test
Rats (220-250 g) were placed in a Plexiglas box and acclimatized for 30 minutes. Next, rats were lightly anesthetized with isoflurane and injected intrathecally with 20 μL of either 24 μg of L-291 or 24 μg of L-456 (both 5 mM) using a 25-gauge needle and syringe inserted into the subarachnoidal space between the L5 and L6 spinal processes. Experimenters were blind to the treatment for the whole testing period. Twenty minutes later, rats received a subcutaneous injection of 50 μL of formalin (5%) into the plantar surface of the right hind paw. The time spent lifting, flinching, licking, and biting the injected paw was recorded in seconds during the 60-minute period after formalin administration. Data were captured in 5-minute time bins.
2.12. Statistical analysis
Effects of L-291 on nitrite accumulation and after hind paw electrical stimulation were compared using 1-way repeated-measure analysis of variance (ANOVA), followed by Bonferroni's multiple comparison posttests. Cell characteristics were compared between treatment groups by the Student unpaired t tests. Formalin time courses were compared between treatment groups by 2-way repeated-measure ANOVA, followed by Bonferroni's posttests. Total activity in the first and second phases was compared by quantifying the area under each curve and analyzed by 1-way ANOVA, followed by Bonferroni's posttests. Statistical analyses were conducted using GraphPad Prism v.4 software (GraphPad Software, San Diego, CA).
3. Results
3.1. DDAH-1 is found in sensory neurons within the dorsal root ganglia and spinal dorsal horn
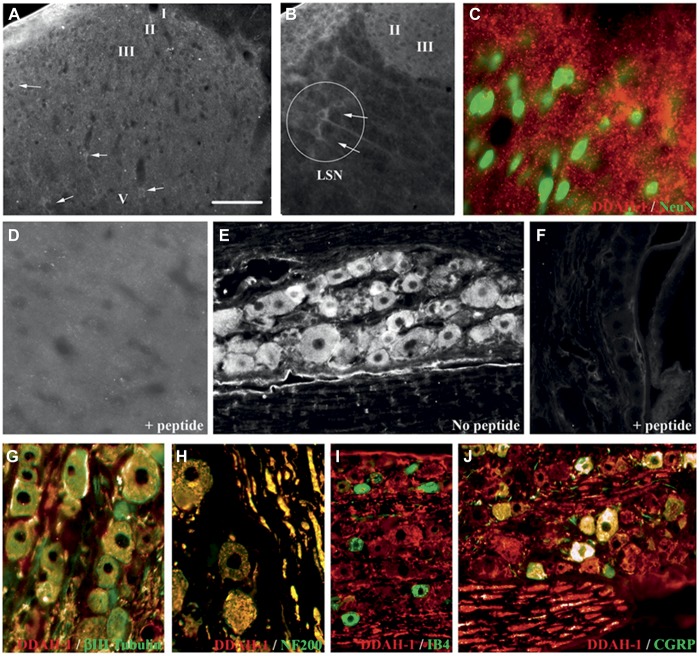
Western blot analysis revealed DDAH-1 protein expression in rat hippocampus, DRG, and the dorsal horn (Fig. 1A). DDAH-1 immunostaining in the dorsal horn was present in scattered neuronal profiles in all laminae (Fig. 2A). The intensity of staining was relatively weak, although neurons in the lateral spinal nucleus (LSN) were more strongly stained (Fig. 2B). Double staining with NeuN, a marker of neuronal nuclei, did not show overlap with DDAH-1 but rather DDAH-1 staining was observed around NeuN, suggesting that DDAH-1 is located in neuronal soma (Fig. 2C). In the DRG, DDAH-1 immunoreactivity was observed in neurons of all sizes (Fig. 2E), as confirmed by double staining with the neuronal marker β-III Tubulin (Fig. 2G; 100% of β-III Tubulin neurons were DDAH1 positive). Levels of expression varied between different DRG neurons as demonstrated by the strong colocalization of DDAH-1 in large-sized myelinated neurons (Fig. 2H; 100% of NF 200–positive neurons were DDAH1 positive) and small-sized peptidergic neurons (Fig. 2J) but relative absence in small-sized nonpeptidergic neurons (Fig. 2I). Double staining with IB4, a marker of small-sized nonpeptidergic neurons, showed that only 11.5% of IB4-positive neurons expressed DDAH1. Double staining with the neuropeptide CGRP showed that a large proportion (76.9%) of CGRP-positive neurons expressed DDAH1. Specificity of the staining was confirmed by preincubation of the primary antibody with a DDAH-1-specific peptide that prevented all staining in spinal cord and DRG (Figs. 2D, F).
Figure 1.
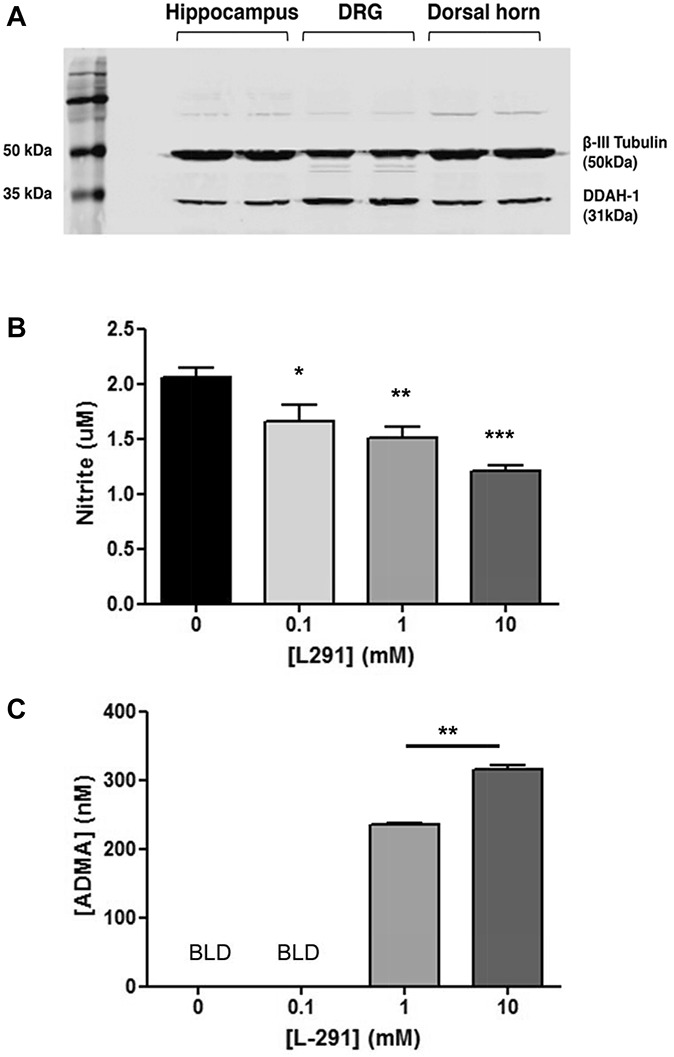
Expression of DDAH-1 in the dorsal root ganglion (DRG) and dorsal horn and effect of DDAH-1 inhibitor L-291 on nitric oxide production in DRG neurons. (A) Western immunoblot showing expression of DDAH-1 protein in hippocampus (2 left lanes), DRG (2 middle lanes), and spinal dorsal horn (2 right lanes). β-III Tubulin was used as a loading control. Measurement of (B) nitrite production or (C) asymmetric dimethylarginine levels in isolated DRG neurons treated with L-291 (0-10 mM) (BLD indicates values fall below the limit of detection). Data are expressed as mean nitrite concentration (±SEM) of 4 to 10 wells from 3 to 5 animals; *P < 0.05, **P < 0.01, and ***P < 0.001 vs control (0 mM L-291), 1-way repeated-measure analysis of variance followed by Bonferroni's post hoc tests or by Student paired t test.
Figure 2.
DDAH-1 is expressed in sensory neurons within the DRG and dorsal horn. (A-J) Expression of DDAH-1 in the rat spinal cord and DRG. (A) Immunofluorescent staining of DDAH-1 in the lumbar spinal dorsal horn is localized in weakly stained neuronal soma in the grey matter. (B) Neurons stained in the lateral spinal nucleus (LSN) display stronger staining in the dorsal horn. (C) Merged double staining image of DDAH-1 (red) and NeuN (green, marker of neuronal nuclei) in the dorsal horn. (D, F) Specificity of the anti-DDAH-1 antibody demonstrated by preabsorption with DDAH-1 peptide and subsequent loss of staining in (D) spinal cord and (F) DRG. (E) Immunofluorescent staining of DDAH-1 in DRG neurons. (G-J) Merged double staining images of DDAH-1 (red) in DRG with (G) β-III Tubulin (green, neuronal marker); (H) with NF200 (green, marker of large myelinated DRG neurons); (I) with IB4 (green, marker of small nonpeptidergic DRG neurons); (J) with CGRP (green, marker of small peptidergic DRG neurons). Scale bar = (A): 70 μm, (B): 78 μm, (C, D): 28 μm, (E, F): 70 μm, (G): 42 μm, (H): 55 μm, (I): 97 μm, (J): 61 μm.
3.2. Selective DDAH-1 inhibitor L-291 reduces nitric oxide synthesis in sensory neurons
To demonstrate a functional role for DDAH-1 in NO production in sensory neurons, we applied L-291 to cultured DRG neurons and measured nitrite accumulation, a marker for NO synthesis. L-291 caused a significant and concentration-dependent reduction in nitrite levels (Fig. 1B) consistent with a concentration-dependent increase in ADMA levels (Fig. 1C). Our results confirm the presence of DDAH-1 in sensory neurons of the DRG and spinal cord and suggest that DDAH-1 contributes to NO signaling in neurons.
3.3. Spinal L-291 inhibits C-fiber-evoked responses, postdischarge, and windup of deep dorsal horn wide dynamic range neurons
We next assessed the function of DDAH-1 in sensory and nociceptive processing in the dorsal horn. Spinal administration of L-291 (1.2 μg, n = 9; 12 μg, n = 9; 120 μg, n = 6) produced selective and significant dose-dependent inhibition of C-fiber-evoked responses (Fig. 3C), PD (Fig. 3D), and windup (Figs. 3F, G) of WDR neurons. No changes were seen in Aβ-fiber- or Aδ-fiber-evoked responses (Fig. 3A, B respectively) nor in input (Fig. 3E). The control drug, L-456 (120 μg, n = 8), had no effect (Figs. 3A-H). Peak inhibitory effects of L-291 were evident by 40-minute postadministration and persisted for the remainder of the recording period. Predrug control responses did not differ between treatment groups (Supplementary Table 1, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A115).
Figure 3.
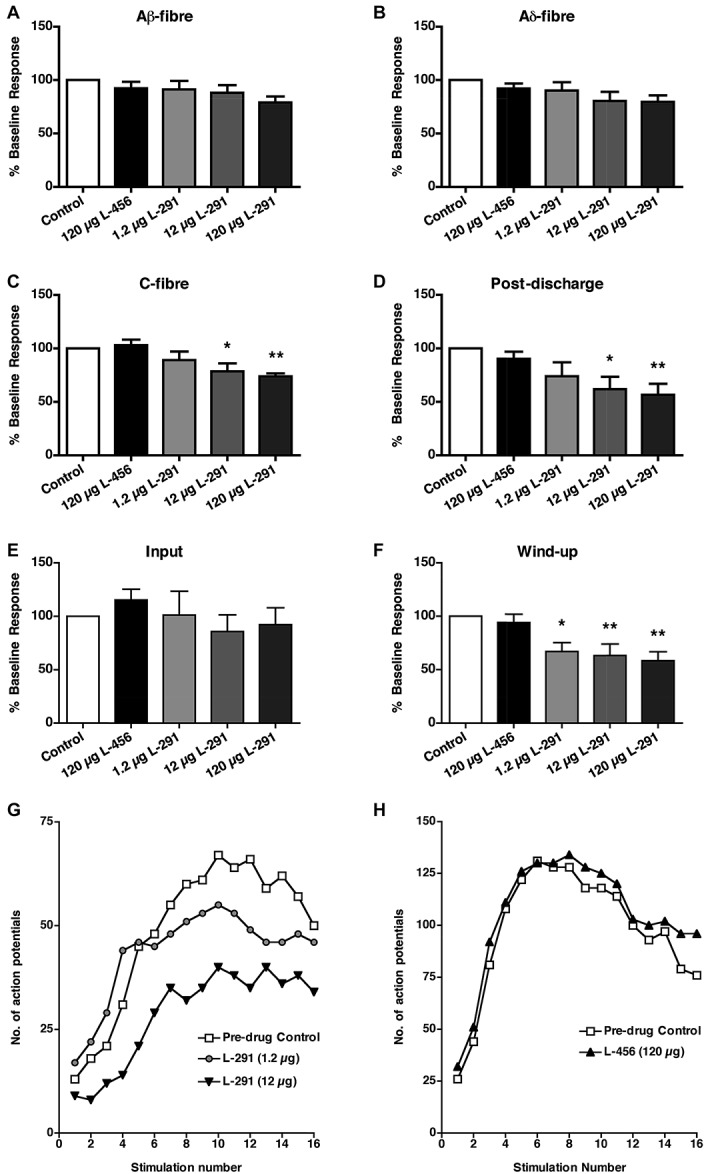
Spinal L-291 decreases C-fiber-evoked responses and postdischarge of wide dynamic range (WDR) neurons. (A-H) Effects of spinal application of L-291 (1.2 μg (0.1 mM), n = 9; 12 μg (1 mM), n = 9; 120 μg (10 mM), n = 6) or L-456 (120 μg, n = 8) on (A-C) afferent-evoked responses, (D) postdischarge, (E) input, and (F) windup of WDR neurons, induced by transcutaneous electrical stimulation of the hind paw. White bars represent predrug control. Data are presented as mean ± SEM of predrug control responses; *P < 0.05, **P < 0.01 vs predrug control, 1-way repeated-measure analysis of variance followed by Bonferroni's posttests. (G, H) Examples of windup of single WDR neurons after repetitive electrical stimulation in the presence of spinal (G) L-291 (1.2 and 12 μg) or (H) L-456 (120 μg).
Thus, spinal application of L-291 selectively reduces C-fiber-evoked responses, PD, and NMDA-dependent windup of WDR neurons, indicating a role for DDAH-1 in nociceptive signaling and plasticity within the dorsal horn rather than basal sensory transmission. No changes were seen in A-fiber-evoked responses and input, suggesting a predominantly postsynaptic effect of L-291 and DDAH-1 inhibition.
3.4. Formalin-induced central sensitization of deep dorsal horn wide dynamic range neurons is reduced by spinal pretreatment with L-291
In electrophysiological recordings in rats pretreated spinally with control drug L-456 (120 μg, n = 14), formalin injection into the hind paw induced a characteristic biphasic neuronal firing response (Figs. 4A-C). In comparison, spinal pretreatment with L-291 (12 μg, n = 10) significantly and selectively reduced second phase neuronal firing (Figs. 4A, B, D). No change in first-phase activity was observed. Neurons were characterized before injection of drugs and were comparable between treatment groups (Supplementary Table 2, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A115).
Figure 4.
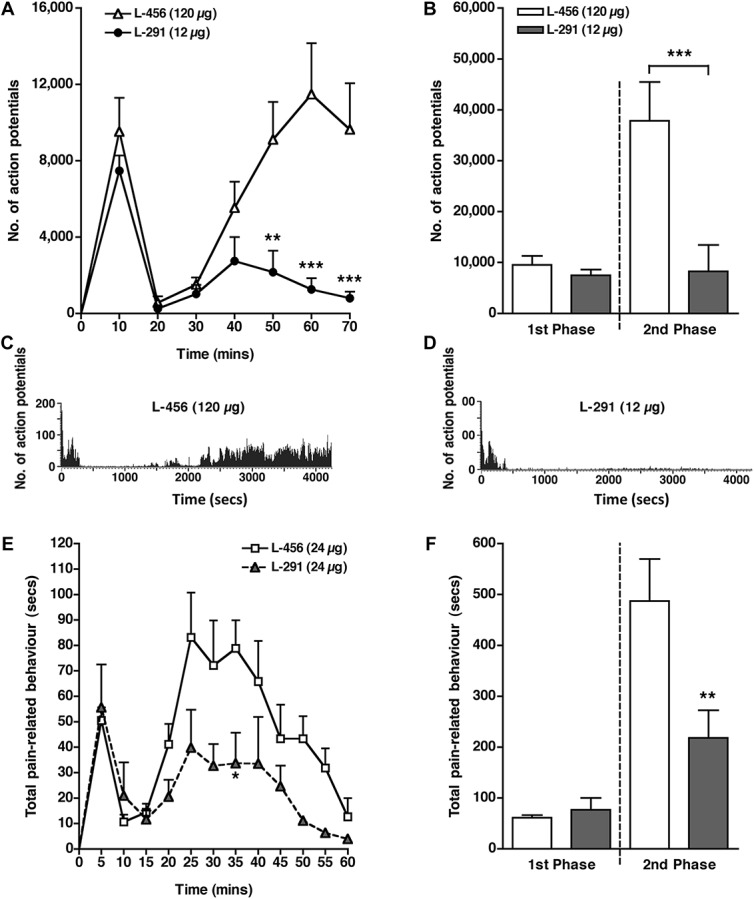
Spinal L-291 reduces formalin-induced central sensitization. (A) Time course of wide dynamic range (WDR) firing response to subcutaneous formalin (5%, 50 μL) injection into the hind paw after spinal pretreatment with control L-456 (120 μg/10 mM, n = 14) or L-291 (12 μg/1 mM, n = 10; **P < 0.01 at 50 minutes, ***P < 0.001 at 60 minutes, ***P < 0.001 at 70 minutes vs L-456; 2-way repeated-measure analysis of variance (ANOVA) followed by Bonferroni's posttests). (B) Total neuronal activity during the first (0-10 minutes) and second phases (10-70 minutes) of the formalin response with spinal L-456 or L-291 (second phase: ***P < 0.001 vs L-456, 1-way ANOVA followed by Bonferroni's posttests). (C, D) Representative rate recordings of firing responses of WDR neurons to formalin after spinal pretreatment with (C) L-456 or (D) L-291. (E) Time course of pain-related behaviors induced by intraplantar injection of formalin (5%, 50 μL) after intrathecal pretreatment with L-456 (24 μg) or L-291 (24 μg, *P < 0.05 at 35 minutes vs L-456; 2-way repeated-measure ANOVA followed by Bonferroni's posttests). (F) Total pain-related behavior during the first phase (0-10 minutes) and second phase (10-60 minutes) of the response to formalin after intrathecal pretreatment with L-456 or L-291 (second phase: **P < 0.01 vs L-456; 1-way ANOVA followed by Bonferroni's posttests). All data are presented as mean ± SEM, n = 6 in each group.
3.5. Spinal L-291 inhibits pain-related behaviors because of formalin-induced central sensitization
Effects of spinal inhibition of DDAH-1 were next assessed in the formalin behavioral test. Pain-related behaviours were divided into 2 categories: reflexive lifting and flinching behaviours; and active licking and biting of the injured paw. In rats pretreated intrathecally with the control compound L-456 (24 μg, n = 6), formalin induced a biphasic pain–related behavioral response (Figs. 4E, F). In contrast, pretreatment with L-291 (24 μg, n = 6) significantly and selectively decreased pain-related behavior during the second phase (Figs. 4E, F). No difference was seen during the first phase between treatment groups. The inhibitory effect of L-291 during the second phase of the response to formalin reduced both types of behavior (Supplementary Figure 2, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A115).
Thus, spinal administration of L-291 reduces both second-phase neuronal firing and pain-related behaviors induced by intraplantar formalin injection, suggesting that DDAH-1 has a role in NMDA-dependent central sensitization of deep dorsal horn WDR neurons.
4. Discussion
The results of this study suggest that DDAH-1, an enzyme that metabolizes asymmetric methylarginines, contributes to NMDA-dependent plasticity of spinal dorsal horn sensory neurons after noxious peripheral stimulation. We have demonstrated the presence of DDAH-1 protein in the spinal cord and DRG, and, importantly, that pharmacologic inhibition of DDAH-1, with a selective inhibitor, L-291, elevates ADMA and reduces NO synthesis, neuronal hyperexcitability, and pain-related behavior after formalin-induced spinal central sensitization.
Biochemical analyses demonstrated protein expression of DDAH-1 in the soma of DRG and dorsal horn neurons. In the DRG, expression tended to be higher in larger myelinated cell bodies but was also visualized in small, probably nociceptive, peptidergic cells. In the dorsal horn, cell bodies and axons were stained in LSN, reinforcing the idea that DDAH-1 is found in both afferent and intrinsic spinal neuronal systems. Interestingly, the LSN receives deep inputs from tissue suggesting a role of ADMA in their regulation and the cutaneous-evoked activity in this study.
Despite strong expression in large myelinated cells, A-fiber-evoked responses and input were unaffected by L-291, suggesting that DDAH-1 in these cells may not contribute to normal sensory signalling.
Spinal administration of L-291 inhibited PD and windup of WDR neurons induced by electrical stimulation of the hind paw. C-fiber-evoked responses were also reduced, though to a lesser extent. A-fiber-evoked responses and input were unaffected by L-291. These selective inhibitory effects of spinal DDAH-1 antagonism on NMDA-mediated neuronal events suggest a predominantly postsynaptic effect and are similar to those produced by spinal inhibition of nNOS,31,33 as well as drugs targeting spinal NMDA receptors.6,7 Targeting DDAH-1 may be a novel way of modulating the NMDA-NO signaling pathway through manipulation of an endogenous control, which may allow physiopathological signaling to be selectively modulated and so potentially increasing tolerability over direct NMDA receptor blockers. The selectivity of L-291 activity also suggests that altered local vascular effects do not influence spinal function, as these would be expected to alter all neuronal responses in a nonselective manner. Indeed, no obvious changes in the central blood vessel shape or size were observed during electrophysiological recordings after administration of L-291.
In both neuronal and behavioral formalin tests, spinal administration of L-291 reduced spinal central sensitization, as shown by a reduction of second phase activity. The lack of any effect on first-phase responses suggests that L-291 does not alter basal pain but rather attenuates potentiation of pain transmission. These findings are consistent with those of several studies investigating the role of the NOS pathway in the formalin test. Both systemic and spinal administration of NOS inhibitors selectively reduce second-phase pain-related behavior.5,20,24 Formalin injection into the hind paw has been shown to upregulate nNOS in the dorsal horn,13,16 whereas formalin-induced c-Fos expression in the superficial dorsal horn is reduced by spinal NOS inhibition.28 These studies establish a significant contribution of NO and NOS to spinal central sensitization and provide a context for the effects of DDAH-1 inhibition, which indirectly suppresses NO synthesis.19 Effects of L-291 on neuronal activity and pain-related behavior were produced at similar drug concentrations as effects on NO synthesis in DRG neurons.
This study is the first to demonstrate protein expression of DDAH-1 in the CNS and to suggest a functional role for DDAH-1 in neuronal signaling, as shown by reduction of NO synthesis in DRG neurons and modulation of NMDA-dependent spinal nociceptive plasticity by the DDAH-1 inhibitor L-291. DDAH-1 may therefore be a novel analgesic target in chronic pain. Furthermore, DDAH-1 inhibitors such as L-291 may prove to be effective therapies in various neuronal disorders, where nNOS activity and NO synthesis are elevated and causally linked to the underlying pathology, although consideration must be given to potential cardiovascular side effects.
Conflict of interest statement
The authors have no conflicts of interest to declare.
This work was supported by the Wellcome Trust-funded London Pain Consortium, the Centre National pour la Recherche Scientifique and British Heart Foundation programme Grant RG/02/005 and project Grant PG/09/073.
L-291 is an investigational product.
Appendix A. Supplemental Digital Content
Supplemental Digital Content associated with this article can be found online at http://links.lww.com/PAIN/A115.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Alexander R, Kerby A, Aubdool AA, Power AR, Grover S, Gentry C, Grant AD. 4alpha-phorbol 12,13-didecanoate activates cultured mouse dorsal root ganglia neurons independently of TRPV4. Br J Pharmacol 2013;168:761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Caplin B, Wang Z, Slaviero A, Tomlinson J, Dowsett L, Delahaye M, Salama A, Wheeler DC, Leiper J. Alanine-glyoxylate aminotransferase-2 metabolizes endogenous methylarginines, regulates NO, and controls blood pressure. Arterioscler Thromb Vasc Biol 2012;32:2892–900. [DOI] [PubMed] [Google Scholar]
- [3].Cardounel AJ, Zweier JL. Endogenous methylarginines regulate neuronal nitric-oxide synthase and prevent excitotoxic injury. J Biol Chem 2002;277:33995–4002. [DOI] [PubMed] [Google Scholar]
- [4].Chu YC, Guan Y, Skinner J, Raja SN, Johns RA, Tao YX. Effect of genetic knockout or pharmacologic inhibition of neuronal nitric oxide synthase on complete Freund's adjuvant-induced persistent pain. PAIN® 2005;119:113–23. [DOI] [PubMed] [Google Scholar]
- [5].Coderre TJ, Yashpal K. Intracellular messengers contributing to persistent nociception and hyperalgesia induced by L-glutamate and substance P in the rat formalin pain model. Eur J Neurosci 1994;6:1328–34. [DOI] [PubMed] [Google Scholar]
- [6].D'Mello R, Marchand F, Pezet S, McMahon SB, Dickenson AH. Perturbing PSD-95 interactions with NR2B-subtype receptors attenuates spinal nociceptive plasticity and neuropathic pain. Mol Ther 2011;19:1780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology 1987;26:1235–8. [DOI] [PubMed] [Google Scholar]
- [8].Dickenson AH, Sullivan AF. Subcutaneous formalin-induced activity of dorsal horn neurones in the rat: differential response to an intrathecal opiate administered pre or post formalin. PAIN® 1987;30:349–60. [DOI] [PubMed] [Google Scholar]
- [9].Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 1988;336:385–8. [DOI] [PubMed] [Google Scholar]
- [10].Garthwaite J, Garthwaite G, Palmer RM, Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol 1989;172:413–16. [DOI] [PubMed] [Google Scholar]
- [11].Guan Y, Yaster M, Raja SN, Tao YX. Genetic knockout and pharmacologic inhibition of neuronal nitric oxide synthase attenuate nerve injury-induced mechanical hypersensitivity in mice. Mol Pain 2007;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Haley JE, Dickenson AH, Schachter M. Electrophysiological evidence for a role of nitric oxide in prolonged chemical nociception in the rat. Neuropharmacology 1992;31:251–8. [DOI] [PubMed] [Google Scholar]
- [13].Herdegen T, Rudiger S, Mayer B, Bravo R, Zimmermann M. Expression of nitric oxide synthase and colocalisation with Jun, Fos and Krox transcription factors in spinal cord neurons following noxious stimulation of the rat hindpaw. Brain Res Mol Brain Res 1994;22:245–58. [DOI] [PubMed] [Google Scholar]
- [14].Kielstein A, Tsikas D, Galloway GP, Mendelson JE. Asymmetric dimethylarginine (ADMA)–a modulator of nociception in opiate tolerance and addiction? Nitric Oxide 2007;17:55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kitto KF, Haley JE, Wilcox GL. Involvement of nitric oxide in spinally mediated hyperalgesia in the mouse. Neurosci Lett 1992;148:1–5. [DOI] [PubMed] [Google Scholar]
- [16].Lam HH, Hanley DF, Trapp BD, Saito S, Raja S, Dawson TM, Yamaguchi H. Induction of spinal cord neuronal nitric oxide synthase (NOS) after formalin injection in the rat hind paw. Neurosci Lett 1996;210:201–4. [DOI] [PubMed] [Google Scholar]
- [17].Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O'Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med 2007;13:198–203. [DOI] [PubMed] [Google Scholar]
- [18].Leiper JM, Santa Maria J, Chubb A, MacAllister RJ, Charles IG, Whitley GS, Vallance P. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J 1999;343:209–14. [PMC free article] [PubMed] [Google Scholar]
- [19].MacAllister RJ, Parry H, Kimoto M, Ogawa T, Russell RJ, Hodson H, Whitley GS, Vallance P. Regulation of nitric oxide synthesis by dimethylarginine dimethylaminohydrolase. Br J Pharmacol 1996;119:1533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Malmberg AB, Yaksh TL. Spinal nitric oxide synthesis inhibition blocks NMDA-induced thermal hyperalgesia and produces antinociception in the formalin test in rats. PAIN® 1993;54:291–300. [DOI] [PubMed] [Google Scholar]
- [21].McBride AE, Silver PA. State of the arg: protein methylation at arginine comes of age. Cell 2001;106:5–8. [DOI] [PubMed] [Google Scholar]
- [22].Meller ST, Dykstra C, Gebhart GF. Production of endogenous nitric oxide and activation of soluble guanylate cyclase are required for N-methyl-D-aspartate-produced facilitation of the nociceptive tail-flick reflex. Eur J Pharmacol 1992;214:93–6. [DOI] [PubMed] [Google Scholar]
- [23].Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem 1993;214:11–16. [DOI] [PubMed] [Google Scholar]
- [24].Moore PK, Wallace P, Gaffen Z, Hart SL, Babbedge RC. Characterization of the novel nitric oxide synthase inhibitor 7-nitro indazole and related indazoles: antinociceptive and cardiovascular effects. Br J Pharmacol 1993;110:219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nakagomi S, Kiryu-Seo S, Kimoto M, Emson PC, Kiyama H. Dimethylarginine dimethylaminohydrolase (DDAH) as a nerve-injury-associated molecule: mRNA localization in the rat brain and its coincident up-regulation with neuronal NO synthase (nNOS) in axotomized motoneurons. Eur J Neurosci 1999;11:2160–6. [DOI] [PubMed] [Google Scholar]
- [26].Nandi M, Kelly P, Torondel B, Wang Z, Starr A, Ma Y, Cunningham P, Stidwill R, Leiper J. Genetic and pharmacological inhibition of dimethylarginine dimethylaminohydrolase 1 is protective in endotoxic shock. Arterioscler Thromb Vasc Biol 2012;32:2589–97. [DOI] [PubMed] [Google Scholar]
- [27].Pezet S, Spyropoulos A, Williams RJ, McMahon SB. Activity-dependent phosphorylation of Akt/PKB in adult DRG neurons. Eur J Neurosci 2005;21:1785–97. [DOI] [PubMed] [Google Scholar]
- [28].Roche AK, Cook M, Wilcox GL, Kajander KC. A nitric oxide synthesis inhibitor (L-NAME) reduces licking behavior and Fos-labeling in the spinal cord of rats during formalin-induced inflammation. PAIN® 1996;66:331–41. [DOI] [PubMed] [Google Scholar]
- [29].Rossiter S, Smith CL, Malaki M, Nandi M, Gill H, Leiper JM, Vallance P, Selwood DL. Selective substrate-based inhibitors of mammalian dimethylarginine dimethylaminohydrolase. J Med Chem 2005;48:4670–8. [DOI] [PubMed] [Google Scholar]
- [30].Schmidtko A, Tegeder I, Geisslinger G. No NO, no pain? The role of nitric oxide and cGMP in spinal pain processing. Trends Neurosci 2009;32:339–46. [DOI] [PubMed] [Google Scholar]
- [31].Stanfa LC, Misra C, Dickenson AH. Amplification of spinal nociceptive transmission depends on the generation of nitric oxide in normal and carrageenan rats. Brain Res 1996;737:92–8. [DOI] [PubMed] [Google Scholar]
- [32].Tran CT, Fox MF, Vallance P, Leiper JM. Chromosomal localization, gene structure, and expression pattern of DDAH1: comparison with DDAH2 and implications for evolutionary origins. Genomics 2000;68:101–5. [DOI] [PubMed] [Google Scholar]
- [33].Urch CE, Dickenson AH. Neuronal nitric oxide synthase modulation of dorsal horn neuronal responses in the rat: a developmental study. Dev Neurosci 2003;25:301–7. [DOI] [PubMed] [Google Scholar]
- [34].Wang Z, Lambden S, Taylor V, Sujkovic E, Nandi M, Tomlinson J, Dyson A, McDonald N, Caddick S, Singer M, Leiper J. Pharmacological inhibition of DDAH1 improves survival, haemodynamics and organ function in experimental septic shock. Biochem J 2014;460:309–16. [DOI] [PubMed] [Google Scholar]
- [35].Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. PAIN® 1983;16:109–10. [DOI] [PubMed] [Google Scholar]