Supplemental Digital Content is Available in the Text.
Keywords: Analgesics, Animals, Meta-analysis, Neoplasm metastasis, Systematic review
Abstract
Analgesics are commonly used to manage pain in cancer patients. It has been suggested that there might be a relation between analgesics and the outgrowth of metastases. Opioids might increase and non-steroidal anti-inflammatory drugs decrease the risk of metastasis. Robust analysis of all preclinical evidence, however, has so far been lacking. Therefore, we conducted a systematic review and meta-analysis on the effect of treatment with analgesics on metastasis in experimental animal models. One hundred forty-seven studies met the inclusion criteria. Study characteristics, outcome data on the number, and incidence of metastases were extracted, and methodological quality was assessed. In the meta-analysis, we included 215 (±4000 animals) and 137 (±3000 animals) comparisons between analgesic vs control treatment, respectively, on the number and incidence of metastases. Overall, treatment with analgesics significantly decreases the number and risk of metastasis. This effect appears mainly to be the consequence of the efficacy of NSAIDs. Other factors that modify the efficacy are species, type of NSAIDs administered, timing, and duration of treatment. There is no evidence indicating that treatment with any analgesics increases the occurrence of metastases. Our findings appear robust for the various animal models and designs included in this review, which increases our confidence in the result and translatability to the clinical situation.
1. Introduction
Distant metastasis or local recurrence after primary tumour resection is a major clinical problem that also has major financial consequences. The presence of circulating cancer cells due to suboptimal tumour resection might be one of the reasons, but the biology of the tumour also seems to play an important role. Factors that modify the risk of metastasis and thus reduce mortality, therefore, are an important research topic. One of these factors that received growing attention over the past few years concerns the effects of analgesic and anaesthetic treatments on cancer outcomes especially since anaesthesiologists see cancer patients virtually on a daily basis not only for resection of tumours but also for pain management and palliative care during the various stages of the disease.
Some retrospective clinical studies suggest that there may be a relation between specific analgesics and the outgrowth of metastases, but in general the scarce evidence is conflicting and a clear scientific underpinning is missing.3,4 For now, there appears to be no hard clinical evidence to change clinical practice.3,9
Nevertheless, several mechanisms have been proposed by which analgesics influence cancer outcomes. There are studies showing that opioids increase the risk of cancer reoccurrence and metastasis as they might overexpress and activate μ-receptors located on the surface of certain cancers, triggering proliferation and invasion.2,8 Non-steroidal anti-inflammatory drugs (NSAIDs, or COX inhibitors) are suggested to decrease the risk of reoccurrence and metastasis, as COX is overexpressed in many cancers, and COX inhibitors reduce prostaglandin synthesis, which are believed to promote cancer cell adhesion, migration, and invasion.25 As a consequence of some studies showing that opioids had a negative effect on tumour reoccurrence, the research focus has shifted more towards the benefits of regional anaesthesia to limit opioid exposure.
This is quite surprising, as to our knowledge there is no substantial evidence for this shift. First of all, the evidence from clinical studies is conflicting. Secondly, the clinical studies that have been conducted so far are retrospective studies that suffer from confounding factors (eg, combination therapy such as anaesthesia during surgery and postoperative analgesic treatment). Prospective randomized clinical trials on the effects of specific treatments with analgesics on tumour metastasis, which could prove a causal link, are currently ongoing, and no results have yet been published.
Finally, there are substantial numbers of studies on the effects of treatment with analgesics in experimental animals, but the results seem to be conflicting. In addition, as far as we know, these preclinical studies have never been systematically analysed and robust preclinical evidence is lacking.
In conclusion, therefore, there is a great need for systematically summarizing the evidence for the effects of treatment with analgesics on tumour metastasis. In this review, we focus on experimental animal studies.
Systematic reviews and meta-analyses of animal studies can and have previously been used to improve translation of animal research to humans.14 For example, information about safety and efficacy of treatments, ie, hard to obtain from individual studies can be made transparent, decision making on whether to start a clinical trial, or improve the design of subsequent clinical trials can be optimized.21,24,26 Furthermore, a systematic review of preclinical animal studies may help to identify the knowledge gap, guide future animal studies, and reduce unnecessary duplication of often expensive animal experiments.23
This report presents the first systematic review and meta-analysis on the effect of treatment with analgesics on metastasis in experimental cancer models. We will provide (1) a complete and systematic overview of all animal studies on this topic, (2) insight into the efficacy of treatment with analgesics overall and in subgroups (such as opioids and NSAIDs), and (3) an overview of various factors that modify the efficacy of treatment with analgesics on tumour metastasis in experimental cancer models.
2. Methods
This systematic review investigates the effects of treatment with analgesic drugs on number of metastases or metastasis incidence in animals with experimental cancer. The inclusion criteria and method of analysis were specified in advance and documented in a protocol and put online on the SYRCLE Web site (www.syrcle.nl).
2.1. Search strategy and paper selection
We searched MEDLINE through the PubMed interface and EMBASE for original articles concerning “the effects of treatment with analgesic and anaesthetic drugs on” metastasis in experimental cancer published until January 23, 2014. To design the most optimal comprehensive search strategy, we used SYRCLE's step by step guide.19 The search strategy involved the following 4 search components: analgesics, anaesthetics, metastasis, and animals5,16 (for our complete search strategy, see Table 1). No language or date restrictions were applied. As others in our department were already investigating the effects of a treatment with anaesthetic drugs on metastasis in experimental cancer, the search and first steps of the selection process were combined. When necessary, articles in languages other than English were translated by scientists who were native speakers of that particular language. Reference lists of the selected relevant articles were screened by hand for potentially relevant new articles. No language or data restriction was used. Studies were included in this systematic review when they met all of the following criteria: (1) the study assessed the effect of an analgesic drug used in clinical practice on the number or incidence of metastasis in animal models with experimental cancer, (2) the study was performed in animals in vivo, (3) the study included an appropriate control group, and (4) the study was an original full paper that presented unique data. Studies were excluded when (1) animals underwent any cointervention or (2) animals suffered from co-morbidities.
Table 1.
Search strategy.
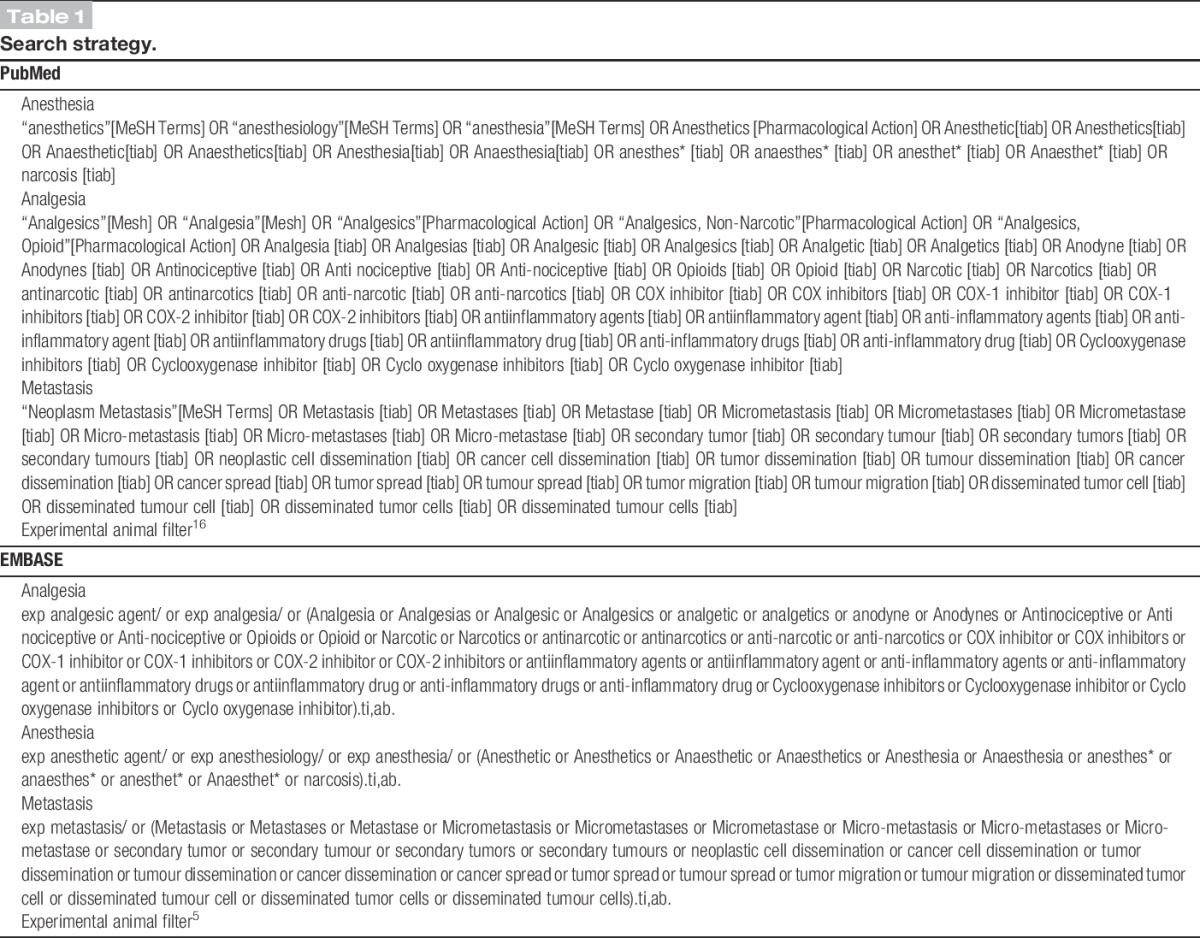
We used Early Review Organising Software (EROS; Institute of Clinical Effectiveness and Health Policy, Buenos Aires, Argentina) to randomly allocate each reference to 2 independent reviewers, who screened it for inclusion on the basis of its title and abstract (C.H. and M.B.). In case of doubt, the whole publication was evaluated. Full-text copies of all publications eligible for inclusion were subsequently assessed by 2 independent reviewers (C.H. and M.E.) and included when they met our prespecified inclusion criteria. Disagreement was solved by discussion or by consulting a third investigator (G.J.S.).
2.2. Study characteristics and data extraction
From the included studies, we registered bibliographic data such as authors, year of publication, journal of publication, and language. We also extracted data on study design (number of animals in experimental and control groups, presence of control group), animal model characteristics (animal species, strain, age, weight, and gender), cancer model (transgenic or induced, type of cells/drugs used to induce cancer, type of cancer, number of cells, location of injection of tumour cells, and type of anaesthetics used to create model), intervention characteristics (type of analgesics, route of administration, dose, frequency, timing relative to tumour cell injection, duration of treatment and type of control group), and outcome measures (either number of metastases or incidence of metastasis, region of metastasis count, age, and anaesthetics used at the time of outcome assessment).
In each of the included publications, we identified all independent comparisons of the number or incidence of metastases in animals with experimental cancer receiving analgesic or control treatment. Data on the number or incidence of metastases were extracted when raw data or group averages (mean, median, or incidence), SD, SE, or ranges and number of animals per group (n) were reported or could be recalculated. When there were 2 or more replications of the same experiment, they were analysed separately. When outcome measure data were missing, we attempted to contact authors for additional information. When the data could not be obtained, a conservative estimate was used if possible. When the group size was reported as a range (eg, 6-9), the lowest number of animals was used in our meta-analysis. When no conservative estimate could be made, the comparison was excluded from the meta-analysis. When data were only presented graphically, they were measured using Universal Desktop Ruler software (http://avpsoft.com/products/udruler/) by 2 independent reviewers. When multiple experimental groups were compared with the same control group, the group size of the control group was corrected for the number of comparisons made (n/number of comparisons).
2.3. Assessment of the methodological quality and risk of bias
We used the SYRCLE Risk of Bias tool15 to assess the risk of bias in the included studies. Two independent reviewers assessed the risk of bias in each included article (S.G., M.S., C.H., F.G.). Regarding the risk of attrition bias, we assumed that there had been no exclusion of animals when the number of animals per group mentioned in the materials and methods section was identical to the number stated in the figure legends or results section. A “yes” score indicates low risk of bias, a “no” score indicates high risk of bias, and a “?” score indicates unknown risk of bias.
To overcome the problem of judging too many items as “unclear risk of bias” because reporting of experimental details on animals, methods, and materials is very poor,18 we added 2 items on reporting: reporting of any measure of randomization and reporting of any measure of blinding. For these 2 items, a “yes” score indicates “reported” and a “no” score indicates “unreported.”
2.4. Data synthesis and statistical analyses
Data were analysed using Comprehensive Meta-Analysis (CMA version 2.0). For the outcome measure “number of metastases,” the standardized mean difference (SMD) was calculated (the mean of the experimental group − the mean of the control group divided by the pooled SDs of the 2 groups). When data were presented as median and percentiles, they were converted to mean and SD. For the outcome measure incidence of metastases, the risk ratio (RR) was calculated. When one of the cells contained a 0-value or the risk in either the control or experimental group was 100%, we added 0.5 to each cell to calculate the RR. In the number of tumours data set, comparisons with an SD of 0 were excluded from meta-analysis.
Secondly, we conducted meta-analyses. Despite anticipated heterogeneity, the individual effect sizes were pooled to obtain an overall SMD and RR and 95% confidence interval. We used the random effects model,6 which takes into account the precision of individual studies and the variation between studies and weighs each study accordingly. When the number or incidence of metastasis was measured in multiple regions in the same animals in a particular study, the data were pooled for the overall analyses. Subgroup analyses were predefined in the protocol (www.syrcle.nl) and performed to assess the influence of variables on effect size. The results from subgroup analyses were only interpreted when subgroups contained at least 10 studies, and they were assessed for type of drug (NSAID: opioids, paracetamol [acetaminophen], ketamine), species, gender, region of metastasis, timing (pretreatment of cells; before tumour cell injection, immediately after tumour cell injection [0-23 hours], after tumour cell injection [24 hours and more]), and duration of treatment (once, 1, 2, 3, 4 weeks, or more than 4 weeks). We expected the variance to be comparable within the subgroups; therefore, we assumed a common among-study variance across subgroups. For subgroup analyses, we adjusted our significance level according to the conservative Bonferroni method to account for multiple analyses (P × number of comparisons). However, differences between subgroups should be interpreted with caution and should only be used for constructing new hypotheses rather than for drawing final conclusions.
We assessed the possibility of publication bias by visually evaluating the possible asymmetry in the funnel plot for incidence of metastases, performing Duval and Tweedie's trim and fill analysis and Egger's regression analysis for small study effects. Heterogeneity was assessed using I2.
2.5. Sensitivity analyses
To assess the robustness of our findings and to further explain observed study heterogeneity, we performed sensitivity analyses. We assessed the impact of (1) induction of cancer vs using transgenic animals, (2) excluding studies in which injected cancer cells are pretreated with analgesics, (3) effect of including animal models with co-morbidities (immune-compromised animal models), and (4) recalculating median and ranges into means and SDs or adjusting cells for calculating RR.
3. Results
3.1. Study selection process
Figure 1 depicts a flow chart of the study selection process. Out of 2567 unique publications retrieved from PubMed or EMBASE, 337 were included after screening on title and abstract. Out of these 337 publications, 147 met our inclusion criteria; the remainder were excluded according to the criteria listed in Figure 1. The references of the included articles can be found in supplement file 1 (available online as Supplemental Digital Content at http://links.lww.com/PAIN/A128).
Figure 1.
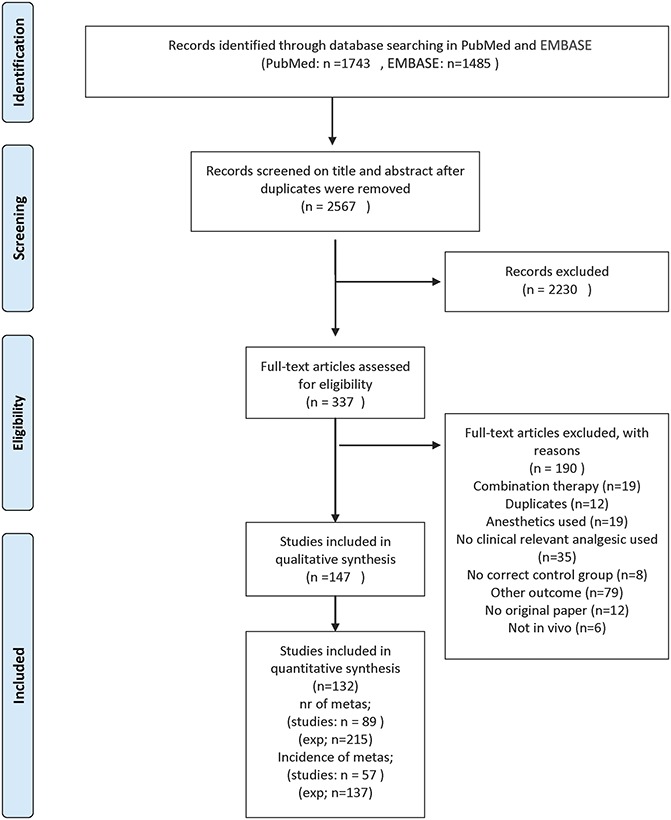
Flow chart of the study selection process. Exp, experiment.
3.2. Study characteristics
3.2.1. Number of metastases
Data on number of metastases could be retrieved from 89 studies, and 215 independent experiments from these 89 studies could be analysed in meta-analysis. Characteristics of all studies and comparisons are listed in supplement file 2 (available online as Supplemental Digital Content at http://links.lww.com/PAIN/A129).
Out of 215 comparisons, 73% represented experiments conducted in mice and 25% of the data were obtained from studies using rats. One study used rabbits, and only 2 experiments used hamsters.
Male and female animals were used in 44% and 39% of the comparisons, respectively. Only 4% (8 comparisons) used mixed sex groups, and 13% of the comparisons failed to report the sex of the animals used.
Most studies investigated the effects of NSAIDs on the number of metastases (81%). Opioids were administered in 14% of the comparisons. An α-agonist or ketamine was used in the remaining 5% of the comparisons.
Metastases of breast cancer were studied in 34% of the comparisons. Metastases of lung, skin, or colon cancer were studied in 18%, 11%, and 11% of the comparisons, respectively. The remainder of the comparisons studied the number of metastases after treatment with analgesic drugs in the stomach, pancreas, prostate, or bone. In 4% of the comparisons, it was unclear what type of cancer was studied.
Most studies investigated the effect of treatment with analgesic drugs either immediately before or after tumour inoculation (34% and 35%, respectively). No details on the timing of the treatment with analgesic drugs were reported in 7% of the comparisons, and 14% of the comparisons pretreated the cells with analgesics before inoculation in the animal model. Most animals were treated with analgesics between 1 and 4 weeks (65% of the comparisons). The treatment was administered only once in 7% of the experiments. In 15% of the total number of comparisons, it was unclear what the duration of the treatment with analgesic drugs was.
The number of metastases were studied in 7 regions of the body. The number of metastases in the lung were studied in 78% of the comparisons. The second most popular region was the liver (12% of the comparisons). The remaining 10% of the comparisons studied the number of metastases in the lymph nodes, bone, peritoneal cavity, or internal organs in general.
3.2.2. Incidence of metastasis
From 57 studies, 137 independent comparisons on the incidence of tumour metastasis could be retrieved (supplement file 2 available online as Supplemental Digital Content at http://links.lww.com/PAIN/A129). Mice and rats were used in 83% and 15% of the comparisons, respectively. Most studies used male animals (48%). Females or mixed gender groups were used in 28% and 4% of the studies, respectively. Gender was unreported in 20% of the comparisons.
Most studies studied the effects of NSAIDs (93%) for incidence of metastases. The effects of opioids were investigated in 5% of the experiments.
The incidence of metastasis was studied in 12 regions of the body. The 4 most popular regions were the colon, lung, prostate, and breast (23%, 20%, 19%, and 12%, respectively). The timing of treatment was unreported in 18% of the comparisons. Thirty-five percent studied the effect of treatment with analgesics after (24 hours or more) tumour inoculation, whereas 29% studied the effect on metastases incidence immediately before or immediately after inoculation. Two experiments pretreated the inoculated cells, and in 17% of the experiments the analgesics were administered before tumour inoculation.
Ninety percent of the comparisons administered analgesics for 1 week or more, whereas 8% did not provide any details on treatment duration. Although 90% of the comparisons for number of metastases were determined in the lung and liver, the lymph nodes and total body were also very popular sites for outcome incidence of metastases.
3.2.3. Study quality and risk of bias
Because reporting of experimental details on animals, methods, and materials was poor,18 we scored 2 items on reporting: reporting of any measure of randomization and reporting of any measure of blinding. In 41% (61/147 publications), it was reported that the experiment had been randomized in some way. Blinding of the experiment at any level was reported in only 17% (25/147) of cases.
Figure 2 also shows the overall results of our risk of bias assessment of the 149 studies included in this systematic review. In many cases, poor reporting led to an unclear risk of bias. For example, none of the authors described the allocation sequence or whether this sequence had been concealed. Fortunately, the groups appeared to be similar at baseline in 63% of the publications.
Figure 2.
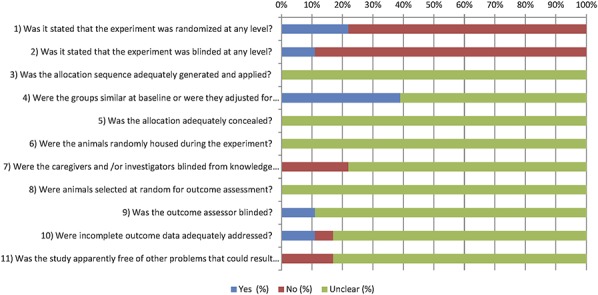
Results of the risk of bias assessment of the 147 studies included in this systematic review. The first 2 items assess study quality by scoring reporting, a “yes” score indicating reported and a “no” score indicating unreported. The other items assessed risk of bias, with “yes” indicating low risk of bias, “no” high risk of bias, and “?” unclear risk of bias.
Measures to reduce performance bias (items 6 and 7) were reported in only one publication, where staff had been blinded during the intervention and the animals had been randomly housed across the cages and room.
Concerning the risk of detection bias (items 8 and 9), the outcome assessor had been blinded for animal allocation in only 12% of the studies. No details about outcome assessor blinding were provided in 88% of the studies. None of the articles reported on random outcome assessment.
There was a high risk of attrition bias in 7% of the studies. Incomplete outcome data were not adequately addressed. Data to assess the risk of attrition bias were incomplete in 69% of the studies, leading to an unclear risk of bias.
When assessing other potential sources of bias, we found that the control treatment procedure was not identical to that of the experimental group in 8 studies. In addition, there was clearly selective outcome reporting in 2 studies, as the outcomes described in the materials and methods were not all discussed in the results.
Probably as a consequence of poor reporting of essential details of the methodology to reduce bias in animal experiments, most studies had to be scored as an unclear risk of bias. Consequently, the subgroups of studies that scored either a high or low risk of bias were too small to be included in meta-analysis for drawing reliable conclusion.
3.3. Meta-analysis of the efficacy of treatment with analgesic drugs
3.3.1. Number of metastases: efficacy of administration with analgesics
A total of 215 experiments (over 4000 animals) investigating the effects of analgesics on tumour metastasis in experimental animal models could be included in the meta-analysis. Overall, administration of analgesics significantly reduces the number of metastases (SMD, −0.82 [−0.99 to −0.65]). Between-study heterogeneity was high (I2 = 81.7%).
3.3.2. Number of metastases: effects of study characteristics on efficacy of administration with analgesics
Subgroup analyses were only conducted in groups with 10 or more experiments. Subgroup analysis revealed that the effect varied across species (P = 0.003). The effect of treatment with analgesic drugs was significantly larger in mice (SMD, −0.98 [−1.18 to −0.79], n = 159, I2 = 79%) than in rats (SMD, −0.35 [−0.70 to −0.02], n = 54, I2 = 87%) (P = 0.002). Gender did not appear to alter the results (Fig. 3A).
Figure 3.
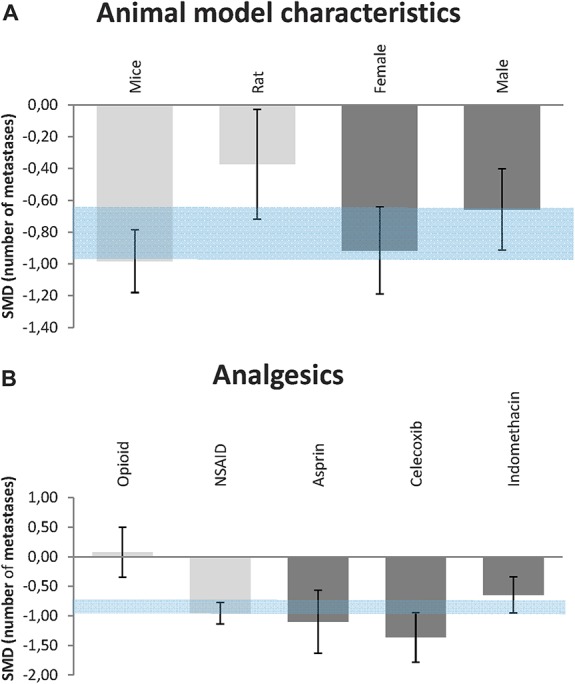
Efficacy of treatment with analgesics on the number of metastases. (A) Impact of animal model characteristics. (B) Impact of various subtypes of analgesics. The gray bars represent the 95% confidence interval of the pooled effect estimate. The columns indicate the effect estimate with the 95% confidence interval of the subgroups (species and gender in [A]; subtypes of analgesics and subtypes of NSAIDs in [B]). The results from subgroup analyses were only interpreted when subgroups contained at least 10 studies. SMD, standardized mean difference.
Most experiments administered NSAIDs (n = 175), and 14% of the studies administered opioids (n = 32). Reduction of the number of metastases as a consequence of treatment with analgesic drugs appears to be due to NSAID administration (Fig. 3B). NSAIDs significantly reduce the number of metastases (SMD, −0.94 [−1.14 to −0.77], I2 = 81%), but this effect was no longer present in the animals that received opioids (SMD, 0.076 [−0.35 to 0.50], I2 = 83%). The other subgroups of analgesic drugs (α-agonists or ketamine) were too small to draw any reliable conclusions.
The duration of treatment with analgesic drugs does not appear to affect treatment efficacy much (supplement file 3, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A130). However, to have an effect, the animals need to be treated more than once (once: SMD, −0.23 [−0.82 to 0.36], n = 16, I2 = 77%).
Furthermore, pretreatment of cancer cells with analgesics does not appear to be effective (supplement file 3, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A130). Administration of analgesics before, immediately, or after tumour cell inoculation is effective (before: SMD, −1.01 [−1.49 to −0.62], n = 32, I2 = 85%; immediately: SMD, −0.97 [−1.26 to −0.67], n = 74, I2 = 82%; after: SMD, −0.90 [−1.18 to −0.61], n = 78, I2 = 74%).
Most animal models studied the effect of analgesics on tumour metastases in the lung (78%) and liver (12%). The efficacy of treatment with analgesic drugs appears to be similar in both regions (supplement file 3, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A130) (liver: SMD, −1.26 [−1.77 to −0.76], n = 26, I2 = 72%; lung: SMD, −0.73 [−0.93 to 0.53], n = 168, I2 = 83%). There were too few studies on other regions to be able to draw reliable conclusions. The type of cancer induced in the animal models does not appear to influence efficacy significantly (P = 0.39) (supplement file 3, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A130).
3.3.3. Number of metastases: sub subgroup interactions
The finding that administration of NSAIDs reduces the number of metastases whereas administration of opioids does not appears to be similar in rats and mice separately (data not shown).
Figure 3B shows that there appears to be a difference in efficacy between the type of NSAIDs administered (P = 0.02). Supplemental file 2 (available online as Supplemental Digital Content at http://links.lww.com/PAIN/A129) described the type of NSAIDs used in each study. Indomethacin was administered in 37.7% of the studies (SMD, −0.65 [−0.95 to −0.34], n = 66, I2 = 83%), 21.1% Celecoxib (SMD, −1.36 [−1.78 to −0.95], n = 37, I2 = 78%) and 13.1% Aspirin (SMD, −1.1 [−1.63 to −0.57], n = 23, I2 = 90%). The effect of Celecoxib is significantly larger than that of Indomethacin treatment (P = 0.02).
Of the 32 experiments investigating the efficacy of opioids, 46% (n = 12) studied morphine (supplemental file 2, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A129). Other types of opioids were much less frequently studied (less than 10 experiments per subtype). Like opioids in general, morphine administration did not decrease the number of metastases (SMD, 0.16 [−0.86 to 1.17], I2 = 89%).
3.3.4. Incidence of metastasis: efficacy of administration with analgesics
The efficacy of administration with analgesics on the incidence of metastasis was assessed in 137 independent comparisons (over 3000 animals) in 60 original studies. Overall, administration of analgesics significantly reduced the incidence of metastasis (RR = 0.73 [0.68-0.78]), and between-study heterogeneity was only moderate (I2 = 41%).
3.3.5. Incidence of metastasis: effects of study characteristics on efficacy of administration with analgesics
Subgroup analysis (of groups with 10 or more experiments) revealed that the reducing effect of treatment with analgesics was only significant in mice (RR = 0.72 [0.67-0.77], n = 114, I2 = 35%). In rats (n = 20, I2 = 50%), the direction of effects was similar but no longer significant.
Regarding the number of metastases, gender did not influence the efficacy of administration with analgesics (Fig. 4A).
Figure 4.
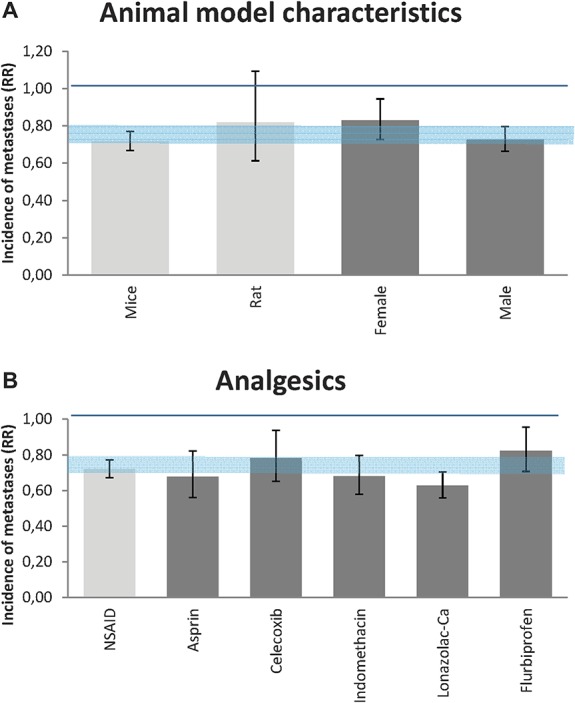
Efficacy of treatment with analgesics on the incidence of metastases. (A) Impact of animal model characteristics. (B) Impact of various subtypes of analgesics. The gray bars represent the 95% confidence interval of the pooled effect estimate. The columns indicate the effect estimate with the 95% confidence interval of the subgroups (species and gender in [A]; subtypes of analgesics and subtypes of NSAIDs in [B]). The results from subgroup analyses were only interpreted when subgroups contained at least 10 studies. RR, risk ratio.
Most experiments administered NSAIDs (Fig. 4B; n = 127). NSAIDs significantly reduce the incidence of metastasis (RR = 0.72 [0.67-0.77], I2 = 35%). The other subgroups (opioids, paracetamol, and combinations of various analgesics) concerned fewer than 10 studies, but we will here provide the estimation of the 7 studies administering opioids, the second largest group of analgesics in this analysis. Opioids did not show a significant effect on the incidence of metastasis (RR = 0.89 [0.61-1.31], I2 = 71%). Because of the low number of studies, these results should be interpreted with caution.
The effect of treatment duration on the incidence of metastases could only be studied for the subgroups taking 1 to 4 weeks or more. There were too few studies for reliable analysis of subgroups taking single administration. There appeared to be no effect of treatment duration in the various subgroups (supplement file 4, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A130).
Administration of analgesics before, immediately, or after tumour cell inoculation all reduces the incidence of metastases (before: RR = 0.65 [0.54-0.76], n = 23, I2 = 65%; immediately: RR = 0.67 [0.59-0.77], n = 40, I2 = 30%; after: RR = 0.80 [−0.73 to 0.89], n = 48, I2 = 26%). Most animal models studied the effect of analgesics on tumour metastasis in the lung (45%), liver (25%), lymph nodes (9%), and total body (9%). Treatment with analgesic drugs significantly reduced the risk of metastases in all these 4 regions. However, the risk of metastases after treatment with analgesics was significantly lower in the lymph nodes than in the lung (P = 0.05) (supplement file 4 available online as Supplemental Digital Content at http://links.lww.com/PAIN/A130).
The type of cancer induced in the animal models influences the efficacy of treatment with analgesics on the incidence of metastases. The risk of metastases as a consequence of treatment decreased significantly in colon, lung, and prostate cancer (supplement file 4, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A130). However, no effect of treatment with analgesics on the incidence of metastases was observed in animal models with breast cancer (RR = 0.97 [0.87-1.08], n = 17, I2 = 0%).
3.3.6. Incidence of metastases: sub subgroup interactions
Figure 4 shows that there is no significant difference in efficacy between the type of NSAIDs administered. The number of experiments was too small to analyse possible differences between subtypes of opioids.
3.3.7. Sensitivity analysis
To assess the robustness of our findings and to further explain the observed study heterogeneity, we performed a sensitivity analysis for some of the decisions we made in the inclusion and exclusion criteria. A small number of studies used transgenic animals as a cancer model instead of injecting tumour cells. We included both types of studies. Excluding the studies that used transgenic animals did not alter our results significantly.
We also included immune-compromised animals/immune-deficient animals (SCID or athymic animals: number of metastases 8 experiments; incidence of metastases 21 experiments). Excluding these animal models from meta-analysis did not change our results.
Some studies preincubated tumour cells with analgesics before injection (number of metastases 16 experiments, incidence of metastases 2 experiments). These studies were also included. Excluding these studies from meta-analysis did not significantly influence our results.
In 21 experiments that were included in the meta-analysis on number of metastases, data that had been presented as median and percentiles were converted to mean and SD. Excluding these studies changed the results of the subgroup analysis regarding type of cancer. Animal models for skin cancer (as opposed to breast, colon, and lung cancer) no longer reduced the number of metastases significantly, although the direction of the effect did not alter. Adjusting cells that either contained a 0-value or had an estimated risk of 100% did not influence our conclusion in this review.
As most studies studying the effect of treatment with analgesics on the incidence of metastases administered NSAIDs (93%), we checked whether the subgroup conclusions appeared to be robust (according to the direction of effects) when including solely those studies that administered NSAIDs. All subgroup analyses showed comparable results.
3.3.8. Publication bias
The possible presence of publication bias was assessed for the outcome incidence of metastases. Inspection of the funnel plots suggested some asymmetry due to an underrepresentation of studies with moderate precision and increased risk of metastases as a consequence of treatment with analgesics. Duval and Tweedie's trim and fill analysis resulted in 32 extra data points, indicating the presence of publication bias and a small overestimation of the summary effect size (supplementary file 5, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A130).
4. Discussion
4.1. Main results
It has been suggested that there might be a relation between specific analgesics and the outgrowth of metastases. However, robust evidence-based preclinical evidence has been lacking so far. Therefore, we conducted a systematic review and meta-analysis on the effect of treatment with analgesics on metastasis in experimental cancer models, including the results of more than 7000 animals.
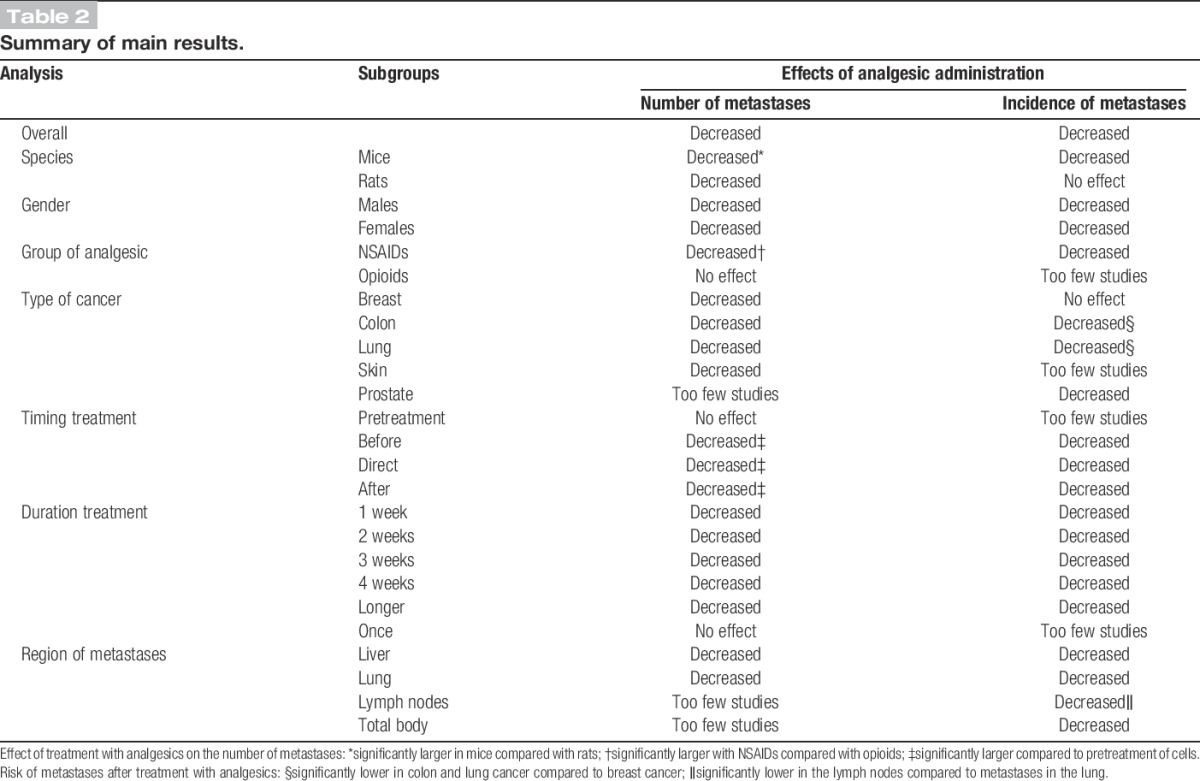
In this review, we provide robust evidence that treatment with analgesics significantly decreases both the number and the risk of metastases. This effect appears mainly to be the consequence of the efficacy of NSAIDs. Opioids do not appear to be effective. Other factors that modify the efficacy of treatment with analgesics are species, the type of NSAIDs administered, timing, and duration of treatment with analgesics (Table 2).
Table 2.
Summary of main results.
The finding that NSAIDs diminish the number of metastases in experimental cancer models whereas opioids do not appear to be effective is partly in line with previous research showing that COX is overexpressed in many cancers, and that COX inhibitors reduce prostaglandin synthesis, which is believed to promote cancer cell adhesion, migration, and invasion.25 In addition, a recent clinical study showed that the intraoperative use of NSAIDs (ketorolac or diclofenac) in conservative breast cancer surgery was associated with an improved disease-free survival.7
Opioids, however, have been suggested to increase the risk of cancer reoccurrence and metastasis, as they overexpress and activate μ-receptors located on the surface of certain cancers, triggering proliferation and invasion.2,8 Some other animal studies showed the opposite effect and indicated that opioids might have protective effects.1 In this review, however, we did not find evidence for these conclusions. For both outcomes, we observed no (significant) effect of opioids on the risk and number of metastases in this systematic review. Although the number of comparisons included in the number of metastases was large (n = 32; 660 animals), the number of comparisons investigating the effects of opioids on the incidence of metastases was relatively small (n = 7), which might be the cause for the observed nonsignificant effect. Nevertheless, none of our analyses showed evidence for an increase in either the number or risk of metastases. Based on the results of this evidence-based preclinical approach, therefore, there appears to be no robust evidence from animal studies to assume that opioids increase the risk of cancer reoccurrence.
It could not be ruled out that efficacy varies between different types of opioids, but this could not be investigated in detail in this review as, for all subtypes of opioids (but morphine), the number of experiments was too small to draw any conclusions.
We could show differences in efficacy between various subtypes of NSAIDs. Celecoxib appears to be more effective on reducing the number of metastases than Indomethacin. However, this difference in effect was not observed for the outcome incidence of metastases and should, therefore, be interpreted with some caution.
Further research into differences in efficacy between various NSAIDs and possible combinations of various NSAIDs is warranted, especially because this review showed that evidence is scarce for many subtypes of NSAIDs (eg, sulindac, rofecoxib, tilmacoxib, etodolac, etc).
This review also clearly shows that more research is needed into the effectiveness of other subgroups of analgesics such as ketamine and α-agonists, as the number of experimental animal studies was limited for these subgroups of analgesics.
Although we observed a reduction in the risk and number of metastases in both rats and mice, efficacy appears to be larger in mice. Therefore, when trying to use as few animals as possible in future experiments, mice might be more suitable than rats. When the magnitude of the effect is larger or, in other words, when the magnitude of the response to a treatment is larger, sample size can be reduced. In this way, scientists will directly be applying the 3Rs principle of Russell and Burch.22
Based on our results, we also recommend providing analgesics more than once in future experiments and not to pretreat the cancer cells that are inoculated, as these design characteristics appeared not to be effective. The use of males or females does not appear to influence the results much.
4.2. Limitations of this review
Our risk of bias analysis showed that most animal studies in this field are poorly reported. For each risk of bias items that was assessed, the risk of bias could not be estimated in at least 60% of the studies as a consequence of poor reporting. Although this is no exception in this field,11,20,26 it is worrying as the lack of reporting important methodological details will to some extent indicate neglected use of these methods to reduce bias causing skewed results.10
This seriously hampers drawing reliable conclusions from the included animal studies.
In addition, we observed a moderate level of between-study heterogeneity for the outcome measure incidence of metastases and a high level of between-study heterogeneity for the number of metastases. This is not very surprising as animal studies are often explorative and heterogeneous with respect to species, design, intervention protocols, and so on, compared with clinical trials. Exploring this heterogeneity is one of the added values of meta-analyses of animal studies and might help to inform the design of future animal studies and subsequent clinical trials.12 Some of our most important findings in this review are a consequence of the information we obtained from exploring the sources of heterogeneity, as in the subgroup analysis for type of analgesics. To account for anticipated heterogeneity, however, we used a random rather than fixed effects meta-analysis. The sensitivity analysis also increased our confidence in the results. We showed, eg, that some of the possible sources of heterogeneity, such as intravenous local inoculation of tumour cells or type of cancer induced, did not alter our conclusions.
Finally, we assessed the possible presence of publication bias. We identified some funnel plot asymmetry, and the Duval and Tweedie's trim and fill analysis predicts a small overestimation of the summary effect size.
4.3. Clinical relevance and recommendations
The most important findings of this review are that (1) treatment with analgesics reduces both the number and incidence of metastases in experimental animal models, (2) there is no evidence so far indicating that treatment with analgesics increases the risk or the number of metastases, and (3) the observed overall effect appears mainly to be the consequence of the efficacy NSAIDs. NSAIDs appear to have the highest efficacy in reducing tumour metastasis, and there is no evidence that opioids increase the risk and number of metastases. These results appear to be robust (eg, comparable direction of effects) for the various animal models (regarding species, gender, and type of cancer induced) identified in this review, which increases our confidence in the results and their translatability to the clinical situation. Based on this review, it appears to be safe to manage pain with analgesics in cancer patients. It is important, however, to remain cautious when trying to translate the results from animal studies to the clinical situation. Many species, eg, metabolize drugs differently compared with humans, and animal models vary in mimicking the clinical situation.1 Ideally, randomised clinical trials with cancer patients receiving either analgesic or placebo treatment is needed to confirm the results of our review. As this is obviously not ethically acceptable, we suggest to partly rely on the provided preclinical and retrospective evidence showing no clear evidence of harm and to focus on the variation in efficacy between various analgesic therapies in future animal studies and randomised clinical trials.
Based on the results in this review (NSAIDs diminish the incidence and number of metastases and opioids do not have an influence), we also recommend to focus on the combination of opioids and NSAIDs in future clinical trials because it is very important to unravel whether the combined administration of NSAIDs and opioids reduces the effects of NSAIDs alone. Large multicenter trials are recommended.
However, as such multicenter trials are often very expensive and time consuming, conducting some more animal experiments on this topic should be considered in the mean time.
As mentioned above, we identified some more gaps in knowledge in the preclinical literature. We suggest, therefore, that future animal experiments focus on the efficacy of (1) ketamine and α-agonists, (2) various subtypes of opioids, (3) various subtypes of NSAIDs (eg, sulindac, rofecoxib, tilmacoxib, etodolac, etc), and (4) combinations of various analgesics.
In addition, we recommend using mice over rats in these experiments, not pretreating the cancer cells that will be inoculated, and administering analgesics more than once.
Furthermore, we underline here that there is an urgent need for improving the reporting and methodological quality of animal studies. Adequate reporting (eg, using the ARRIVE guidelines17 and Gold Standard Publication Checklist13) is essential to allow researchers, clinicians, and policymakers to assess the quality of the evidence presented in animal studies and to improve the successful translation of animal research outcomes to humans in a clinical setting.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Acknowledgements
We gratefully acknowledge the valuable help of Moira Bruintjes with the search and title abstract screening, Marleen Egberink with the full text screening, Sandra Groot and Marieke Schouten with the risk of bias analysis, and Judith van Luijk with the submitting process. We are greatly indebted to all colleagues and fellow researchers who were willing to share their data sets with us. We would also like to thank Rikkert Stuve (www.textconsultant.nl) for copyediting services.
Author contributions: C. R. Hooijmans, conception and design, acquisition of data, analysis and interpretation of data, and drafting the article; F. J. Geessink, acquisition of data and analysis; M. R.-Hoitinga and G.-J. Scheffer, interpretation of data and revising the manuscript.
Appendices. Supplemental Digital Content
Supplemental Digital Content associated with this article can be found online at http://links.lww.com/PAIN/A128, http://links.lww.com/PAIN/A129, http://links.lww.com/PAIN/A130.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Afsharimani B, Doornebal CW, Cabot PJ, Hollmann MW, Parat MO. Comparison and analysis of the animal models used to study the effect of morphine on tumour growth and metastasis. Br J Pharmacol 2015;172:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, Ben-Eliyahu S. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol 2008;15:2042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Buggy DJ, Borgeat A, Cata J, Doherty DG, Doornebal CW, Forget P, Gottumukkala V, Gottschalk A, Gupta A, Gupta K, Hales TG, Hemmings HC, Hollmann MW, Kurz A, Ma D, Parat MO, Sessler DI, Shorten G, Singleton P. Consensus statement from the BJA Workshop on Cancer and Anaesthesia. Br J Anaesth 2014;114:2–3. [DOI] [PubMed] [Google Scholar]
- [4].Cata JP, Hernandez M, Lewis VO, Kurz A. Can regional anesthesia and analgesia prolong cancer survival after orthopaedic oncologic surgery? Clin Orthop Relat Res 2014;472:1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].de Vries RB, Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M. Updated version of the Embase search filter for animal studies. Lab Anim 2014;48:88. [DOI] [PubMed] [Google Scholar]
- [6].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [7].Forget P, Bentin C, Machiels JP, Berliere M, Coulie PG, De Kock M. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br J Anaesth 2014;113(suppl 1):i82–7. [DOI] [PubMed] [Google Scholar]
- [8].Fujioka N, Nguyen J, Chen C, Li Y, Pasrija T, Niehans G, Johnson KN, Gupta V, Kratzke RA, Gupta K. Morphine-induced epidermal growth factor pathway activation in non-small cell lung cancer. Anesth Analg 2011;113:1353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Heaney A, Buggy DJ. Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? Br J Anaesth 2012;109(suppl 1):i17–28. [DOI] [PubMed] [Google Scholar]
- [10].Hirst JA, Howick J, Aronson JK, Roberts N, Perera R, Koshiaris C, Heneghan C. The need for randomization in animal trials: an overview of systematic reviews. PLoS One 2014;9:e98856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hooijmans CR, de Vries RB, Rovers MM, Gooszen HG, Ritskes-Hoitinga M. The effects of probiotic supplementation on experimental acute pancreatitis: a systematic review and meta-analysis. PLoS One 2012;7:e48811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hooijmans CR, IntHout J, Ritskes-Hoitinga M, Rovers MM. Meta-analyses of animal studies: an introduction of a valuable instrument to further improve healthcare. ILAR J 2014;55:418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hooijmans CR, Leenaars M, Ritskes-Hoitinga M. A gold standard publication checklist to improve the quality of animal studies, to fully integrate the Three Rs, and to make systematic reviews more feasible. Altern Lab Anim 2010;38:167–82. [DOI] [PubMed] [Google Scholar]
- [14].Hooijmans CR, Ritskes-Hoitinga M. Progress in using systematic reviews of animal studies to improve translational research. Plos Med 2013;10:e1001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M. Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Lab Anim 2010;44:170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Plos Biol 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kilkenny C, Parsons N, Kadyszewski E, Festing MF, Cuthill IC, Fry D, Hutton J, Altman DG. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One 2009;4:e7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Leenaars M, Hooijmans CR, van Veggel N, ter Riet G, Leeflang M, Hooft L, van der Wilt GJ, Tillema A, Ritskes-Hoitinga M. A step-by-step guide to systematically identify all relevant animal studies. Lab Anim 2012;46:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Macleod MR, van der Worp HB, Sena ES, Howells DW, Dirnagl U, Donnan GA. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke 2008;39:2824–9. [DOI] [PubMed] [Google Scholar]
- [21].Pound P, Ebrahim S, Sandercock P, Bracken MB, Roberts I. Where is the evidence that animal research benefits humans? BMJ 2004;328:514–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Russell WMS, Burch RL. The Principles of Humane Experimental Technique London. Methuen & Co. Ltd., London, 1959. [Google Scholar]
- [23].Sena ES, Briscoe CL, Howells DW, Donnan GA, Sandercock PA, Macleod MR. Factors affecting the apparent efficacy and safety of tissue plasminogen activator in thrombotic occlusion models of stroke: systematic review and meta-analysis. J Cereb Blood Flow Metab 2010;30:1905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].van der Worp HB, Macleod MR, Kollmar R; European Stroke Research Network for H. Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials? J Cereb Blood Flow Metab 2010;30:1079–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer 2010;10:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wever KE, Menting TP, Rovers M, van der Vliet JA, Rongen GA, Masereeuw R, Ritskes-Hoitinga M, Hooijmans CR, Warle M. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS One 2012;7:e32296. [DOI] [PMC free article] [PubMed] [Google Scholar]


